Abstract
In many species social behaviors are dependent on integration of chemosensory and hormonal cues. Many chemosensory stimuli are detected by the vomeronasal organ, which projects to many regions that contain steroid receptors, including the medial amygdala. In male hamsters, testosterone is known to acutely increase in response to chemosensory stimulation; and can facilitate sexual behavior by direct action within the medial amygdala. Conspecific stimuli activate the anterior (MeA) and posterior (MeP) medial amygdala, while heterospecific stimuli activate only MeA. Chemosensory stimuli with different social significance differentially activate the dorsal and ventral subdivisions of MeA and MeP. Therefore, it is likely that steroids differentially facilitate stimulation of the medial amygdala by various chemosensory stimuli. We used Fos expression to examine activation of androgen receptor (AR)-containing cells in the medial amygdala by heterospecific and conspecific stimuli in intact male hamsters and castrated males with testosterone (T)-replacement. The number of AR-immunoreactive (-ir) cells was significantly different from control and between stimuli in intact males, but not in T-replaced castrates. Fos activation was similar in all animals. The results are consistent with a change in number of AR-ir cells in intact animals due to acute increases in testosterone caused by chemosignals.
Keywords: hamster, vomeronasal, conspecific, heterospecific, vaginal fluid, Fos
1. INTRODUCTION
Mating and other social behaviors are dependent in most mammals on both hormones and chemosensory input. In rodents, chemosensory signals are generally detected by the vomeronasal organ, which projects to the accessory olfactory bulb (AOB). The AOB projects to the medial part of the amygdala and few other areas, which send efferent connections to basal forebrain areas important for reproductive, defensive and other critical behaviors (Meredith, 1998; Canteras, 2002, Maras and Petrulis, 2010b). Regions along the accessory olfactory pathway, especially the medial amygdala, are rich in steroid receptors (Wood and Newman, 1993b). Lesions of these areas cause severe deficits in mating and other social behaviors (e.g., Lehman et al., 1980; Lehman and Winans, 1982; Liu et al., 1997; Kondo and Sachs, 2002) and local injections of testosterone can sustain mating in castrated animals (Wood and Coolen, 1997). Thus, these areas, especially the medial amygdala, integrate the endocrine and chemosensory signals necessary for mating and other social behaviors (e.g., Macrides et al., 1974; McGinnis and Dreifuss, 1989; Wood and Newman, 1995; Wood and Coolen, 1997; Maras and Petrulis, 2010c), as described in a comprehensive review by Hull et al. (2002).
The medial amygdala can be divided into anterior (MeA) and posterior (MeP) subnuclei, with a greater expression of androgen receptors in MeP (Wood and Newman, 1993b; Maras and Petrulis, 2010c). In male hamsters and mice both MeA and MeP are activated after exposure to chemical signals from the same species (conspecific; Fiber et al., 1993; Fernandez-Fewell and Meredith, 1994; Meredith et al., 2008, Maras and Petrulis, 2010b,c). Conversely, when exposed to most chemosensory stimuli from another species (heterospecific) only MeA is activated and MeP appears to be suppressed (Meredith and Westberry, 2004; Samuelsen and Meredith, 2009). MeA and MeP can be further subdivided into dorsal and ventral parts, which may be activated differently by chemosensory stimuli with different social implications (Choi et al., 2005; Meredith et al., 2008, Samuelsen and Meredith, 2009). For example, conspecific reproductively-related stimuli (female mouse urine for mice; hamster vaginal fluid, HVF, for hamsters) frequently activate cells strongly in the dorsal portion of MeP (MePd; although not exclusively: Baum and Everett, 1992; Fernandez-Fewell and Meredith, 1994; Meredith and Westberry, 2004; Choi et al., 2005; Samuelsen and Meredith, 2009). On the other hand, conspecific chemosignals from another male, (male flank gland secretion; mFGS for hamsters; male mouse urine, MMU for mice), activate cells located predominantly in ventral portion (MePv) (Meredith et al 2008, Samuelsen and Meredith 2009). MePv in mice and rats is also activated by chemosignals from cats, a potential predator (Dielenberg et al., 2001; Choi et al., 2005; Samuelsen and Meredith, 2009).
Medial amygdala subdivisions differentially project to areas of the hypothalamus known to be involved in reproductive (MePd) or defensive (MeAv, MePv) behavior (Canteras, 2002; Choi et al., 2005). Thus, MePd is implicated in processing of reproductive chemosignals, while MePv (and MeAv) may be involved in processing of competitor and/or defensive chemosensory stimuli.
Here we planned to investigate the participation of AR-expressing cells in these functionally correlated patterns of response. A number of studies have demonstrated that neurons in MePd that are activated during mating also contain androgen receptors (Wood and Newman, 1993b; Gréco et al., 1996, 1998a,b,c). Additionally, cells containing androgen receptors are activated in the dorsal medial amygdala in male rats exposed to a female, but not allowed to contact her (Gréco et al., 1998c). Testosterone (T) facilitates sexual behavior by direct action within the medial amygdala in hamsters (Wood and Coolen, 1997; Wood and Williams, 2001), and also increases nuclear binding of androgen receptors (ARs) in regions along the vomeronasal sensory pathway in rats (McGinnis and Dreifuss, 1989). An androgenic metabolite of testosterone, dihydrotestosterone (DHT), can also activate sexual behavior and increase AR density in regions like the medial amygdala in male hamsters (Romeo et al., 2001).
Steroid hormones and chemosensory input are also required for agonistic behavior in many species (Kollack-Walker and Newman, 1995; Choi et al., 2005; Gobrogge et al., 2007; Meredith et al., 2008; Cheng et al., 2008). So, Fos activation of steroid-receptor bearing cells in response to chemosignals that elicit agonistic behavior, perhaps in ventral medial amygdala, should be expected.
Fos expression is widely accepted as a measure of neuronal activation and has been widely used to study regions of the brain involved in chemosensory processing and social behavior (i.e., Brennan et al., 1992; Fiber et al., 1993; Fernandez-Fewell and Meredith, 1994; Swan, 1997; Meredith, 1998; Dudley and Moss, 1999; Westberry and Meredith, 2003; Blake and Meredith, 2010; Maras and Petrulis 2010b,c). Despite the evidence that testosterone in the medial amygdala is critical to mating behavior no study to date has examined activation of AR-expressing cells after chemosensory stimulation alone with and without control of testosterone level.
Therefore, the current experiments were designed to examine Fos protein expression in AR-containing cells in the medial amygdala in response to different chemosensory stimuli. Male hamsters were exposed to 3 conspecific stimuli (HVF, mFGS, and fFGS) and one heterospecific stimulus (MMU). Medial amygdala responses were measured by the expression of the immediate-early gene-protein, Fos, while also labeling for androgen receptors. In intact males, testosterone can acutely rise in response to chemosensory stimulation (e.g., by HVF; Pfeiffer and Johnston, 1994) and testosterone can influence the number of AR-ir cells (Wood and Newman, 1995). Therefore, additional groups of castrated males with testosterone-implants (i.e., fixed testosterone levels) were also tested with the same chemical stimuli to determine whether restricting changes in testosterone would affect activation of AR-ir cell in the medial amygdala. The stimuli tested have different social implications and may be specifically processed by different subnuclei of the medial amygdala (see above). Thus, we expect differential activation of amygdala subregions. Activated AR-ir cells will indicate a potential local integration of chemosensory and steroid influence on amygdala function. In general we would expect stimuli associated with androgen-dependent behaviors to increase activation of AR cells in brain regions already associated with steroid-dependent behaviors. Additionally, differences in activation of AR-ir cells between intact and fixed-T castrated/implanted animals would suggest a potential effect of changes in T levels on chemosensory processing in the brain.
2. Experimental Procedures
2.1 Animals
Adult (2-3 month) male golden hamsters (Mesocricetus auratus), from Charles River Laboratories used in these experiments were maintained on a long photoperiod (a partially reversed-14L /10D light cycle) with lights out at 9:30am. Temperature was maintained at approx 22°C and 60% relative humidity. Experiments were begun approximately 2 hours into the dark phase of the light/dark cycle. The animals were initially group-housed in clear plastic cages (44 cm × 21 cm × 18 cm) containing corn-cob bedding with food and water ad libitum. All were single-housed in similar cages at least 1 week prior to and throughout the experiment. Animals were either gonad-intact or castrated with testosterone-capsule replacement. All experimental procedures were approved by the Florida State University Institutional Animal Care and Use Committee.
2.2 Castration and Testosterone Implantation
Forty animals remained gonad-intact and 44 animals were castrated. Hamsters to be castrated were anesthetized with Nembutal (sodium pentobarbital). A small incision was made in between the rectum and tail, and the testes were individually pulled through the incision. The main blood supply was then clamped and tied off, and the testicle was removed. After both testicles were removed, the incision was closed with 2-3 sutures. A silastic capsule (i.d. 1.98 mm.; o.d.3.18 mm.; Dow Corning, Midland, MI) packed with 10mm testosterone (Sigma), sufficient to maintain normal testosterone levels for at least one week (Wood and Newman, 1993a), was next implanted subcutaneously between the neck and back of the hamster (previously described by Wood and Newman,1993a; Grattan and Selmanoff, 1994). The animal was returned to its home cage and allowed 3 days for recovery. A radioimmunoassay (RIA) was conducted to ensure that these animals had normal baseline testosterone levels at the time of chemosensory stimulation (see Radioimmunoassy section below).
2.3 Chemosensory Stimuli
Hamsters were exposed to stimuli on clean “cotton” swabs (tips of Polyester-tipped Applicators; Puritan Medical Products) from one of three types: conspecific, heterospecific, or clean swab controls. None of the animals had encountered the other species or any of the conspecific individuals that were stimulus sources. Hamster vaginal fluid (HVF) was collected from three or more female hamsters in behavioral estrus using a smooth spatula. Samples were pooled and diluted 1:10 with distilled water, and then centrifuged to remove solids. MMU collected from three or more male mice in a metabolism cage and diluted 1:10 with distilled water. For HVF and MMU, 200 μl of diluted solution was pipetted on to a clean cotton swab immediately before exposure. mFGS and fFGS were collected by rubbing a clean swab on the flank of a donor hamster at least 10 times up and down. Each m- or f-FGS swab had FGS from 3 different donors. Stimuli were stored at -20° C before use.
2.4 Test Procedure and Stimulus Presentation
All hamsters were single housed at least 3 days prior to testing to minimize exposure to other male odorants. On the day of the test, hamsters were placed in a clean cage with clean corn cob bedding and allowed 1 minute to acclimate to the surroundings. Swab-tips, scented with stimuli as described above, or clean (control) swab-tips were presented in the middle of the cage and replaced every 3 minutes, five swabs per trial, for a total of 15 minutes. Investigation of stimulus swabs was recorded and was not significantly different between stimuli or between gonad-intact animals and castrates with testosterone-replacement. Animals were then placed back into their home cage for 30 min to allow production of FOS protein before they were perfused for immunocytochemistry. Peak Fos expression occurs at 45-60 min. after the beginning of stimulation in 5 brain areas, including medial amygdala, in a previous time-course study (Westberry and Meredith, unpublished).
2.5 Immunocytochemistry
Animals were deeply anesthetized with Nembutal and transcardially perfused with 0.1 M phosphate buffer (PB; pH 7.4) followed by 4% paraformaldehyde. Brains were removed and post-fixed overnight, cryoprotected in 30% sucrose, then sectioned serially on a freezing microtome at 25-μm thickness. Free-floating coronal sections were then processed for androgen receptor (AR) and Fos protein expression immunocytochemistry. Sections were washed twice for 10 min. each in 0.1 M PB and then incubated in primary solution containing 4% normal donkey serum, 0.4% Triton-X 100, polyclonal rabbit anti-AR (1:200;PG-21, Millipore) and goat anti c-fos primary antiserum (1:1,000; sc-52G, Santa Cruz Biotechnology, Inc.), for 48 h on a table shaker at 4°C. Sections were then washed twice for 10 min each in 0.1 M PB and incubated in Alexa 546-labeled fluorescent donkey anti-rabbit IgG and Alexa 488-labeled donkey anti-goat IgG secondary antisera (1:500; Invitrogen, Inc.) for 2 h at room temperature. Tissue was then washed twice for 10 min each in 0.1 M PB, mounted and coverslipped with Vectashield (Vector Labs).
AR- and Fos-positive nuclei were imaged on a Leica DMLB microscope (Leica Microsystems Inc) at 20x using a Diagnostic Instruments Inc RT-Slider SPOT camera in monochrome mode, and counted using computer image analysis software (MetaMorph version 5.0r1; Universal Imaging Corp., Downingtown PA, USA) by an experimenter blind to the treatment condition of each animal being counted. Regions were counted in a single tissue section for each brain-area of interest, carefully selected to be at the same anatomical position in each animal. The areas of interest included central projections of the VN and main olfactory pathway such as MeA, MeP, and their dorsal and ventral subdivisions (MeAd/v, MePd/v). In each area AR-IR, Fos-IR, and double-labeled nuclei were counted within the border of the neuroanatomical nucleus. Both sides of each area were counted and then averaged together for analysis.
For counting double-labeled nuclei, separate red and green images of the same brain region were analyzed to determine which nuclei were clearly labeled with each fluorphore. These nuclei were marked in the image and nuclei marked in both red and green images were counted as double-labeled. Double-labeled nuclei appear yellow in a merged image (e.g., Figure 4) but some yellow will also appear in nuclei that are superimposed on differently labeled but out of focus nuclei at a different depth in the tissue. Ambiguous double-label examples were checked in the tissue section by re-examining the red and green images at different focus depths. Only nuclei with clear superimposable labeled outlines in both images at the same depth were accepted.
Figure 4.
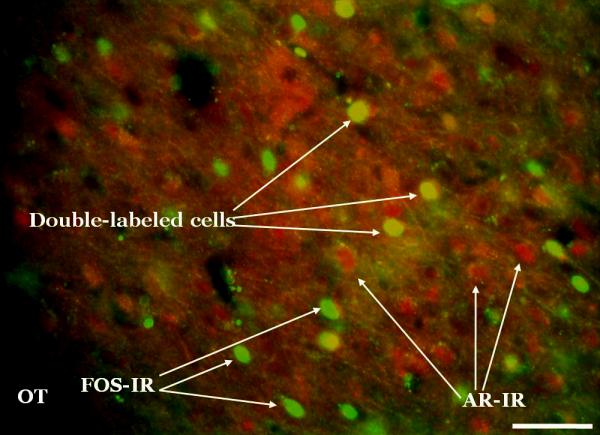
Fos and androgen receptor (AR) double-label sample photomicrograph. Fos and ARs were labeled with monoclonal primary antibodies which were then recognized by fluorescent secondary antibodies. This photomicrograph was taken at the level of the posterior medial amygdala. This image was captured at 20x by a high-resolution black and white camera, and then pseudo-colored (Fos = green, AR = red) using Metamorph software. Scale bar = 100μm.
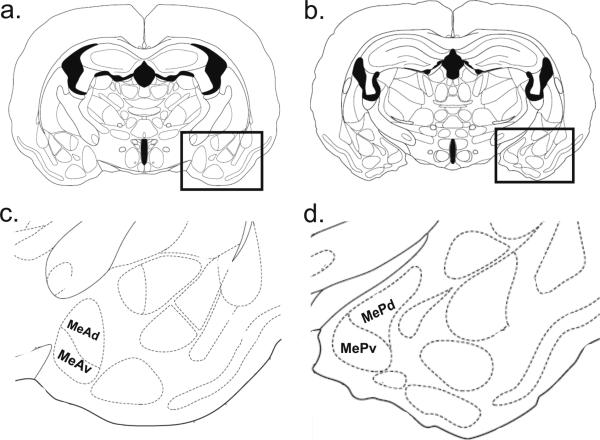
The anatomical positions of the sections chosen for counting were previously selected as representative areas of each nucleus in which Fos expression is increased in mating animals (Fernandez-Fewell and Meredith, 1994; see Blake and Meredith, 2010). The area of interest was identified on each side using specific local anatomical landmarks, including the shape and position of the optic tract and stria terminalis. Counts based on similar procedures using one section per brain-region of interest have been previously published by our lab and others (Fernandez-Fewell and Meredith, 1994; Kollack-Walker and Newman, 1997; Meredith and Westberry, 2004; Blake and Meredith, 2010) and have proved reliable from study to study. MeA counts were taken at the level of Fig. 25 and MeP counts were taken at the level of Fig. 28 in the hamster atlas by Morin and Wood (2001; see Figure 5).
Figure 5.
Schematic of anterior and posterior medial amygdala areas of interest. Coronal sections from the atlas of Morin and Wood (2001) at the level of anterior (a; Bregma: -1.2 mm.) and posterior (b; Bregma: -1.5 mm.) medial amygdala (boxed areas). The borders of the amygdala sub-regions used for counting are displayed in c and d (enlargements of the boxed regions of a and b, respectively).
2.6 Radioimmunoassay of serum testosterone
Immediately following Nembutal injection, and just prior to transcardial perfusion, 400 μL of blood were collected in heparinized syringes by cardiac puncture from castrated, T-replaced animals. Samples were stored at 4 °C until centrifuged (4000 g). Serum was collected and stored at −20 °C until analysis for T concentration by radioimmunoassay. The concentration of T in serum was determined in duplicate by radioimmunoassay (RIA) using a 125I RIA kit according to the method described by MP Biomedicals, Inc. (Solon, OH). Serum concentrations of T are expressed as ng/ml in terms of the hamster T standard (MP Biomedicals, Inc.). Assay sensitivity is 1 ng/ml and the intra-assay coefficient of variation was 5%. To prevent inter-assay variation, all samples were assayed in the same RIA per experiment.
Average serum testosterone level for castrated T-replaced males was 1.20 ng/ml.. The normal values reported for intact adult golden hamsters vary from study to study using different methods, but are generally within the range of 1.05-3.61 ng/ml (Bartke, 1985; Meredith, 1986; Tsuchiga and Horii, 1995), with some reports somewhat higher (e.g., Richardson et al., 2004). The lowest values are still considered adequate for normal reproductive behavior.
2.7 Statistical Analysis
Fos and AR counts were analyzed with repeated measures two-way analyses of variance (2-way RM ANOVA) comparing two factors: exposure (CS, MMU, HVF, mFGS, fFGS) and area (MeAd, MeAv, MePd, MePv). Additional 2-way RM ANOVAs were run comparing exposure (CS, MMU, HVF, mFGS, fFGS) and area (total MeA, total MeP) for the data within the total area of MeA (sum of MeAd and MeAv) and MeP (sum of MePd and MePv). These separate analyses provide data for comparison with previous published work that did not separate MeA and MeP into sub-regions (without using counts from the sub-regions twice in the same analysis). A Fisher LSD posthoc analysis was conducted to analyze differences within each subregion and for each individual stimulus. Significance level was set at p<0.05 (after adjustment within the Sigma Stat program when multiple comparisons were made). Repeated measures analysis takes into account the fact that all brain-areas are sampled in the same animal and are therefore not entirely independent. The two-way analysis ensures that we accept the response to a stimulus within a given area as significant only when there is an overall significant difference across all brain areas. Similar analyses for double-labeled cell counts indicate which stimuli significantly increased Fos activation in AR-ir cells. These analyses were run for intact animals and for castrated, T-replaced animals separately. These two groups were not compared within one statistical analysis because the two animal groups were not tested simultaneously. Finally, a 2-way RM ANOVA was used to compare the proportional increase over control in the Fos expression of AR-ir (double-labeled) cells compared to the proportional increase over control in Fos expression of all (Fos-ir) cells for each amygdala subregion. A repeated measures ANOVA is required because the double-label and the single label (Fos-ir) data are for the same areas and not independent. A separate ANOVA was run for each amygdala subregion (and for MeA-total and MeP-total). The two factors are (1) Phenotype (double label, single label) and (2) Exposure (HVF, mFGS, fFGS, MMU). Data for the main effect of phenotype and posthoc-test probabilities for each stimulus are shown in Table 1.
Table 1. Selective activation of AR-ir cells (greater than general activation in same area).
p-values for 2-way RM ANOVA for numbers of activated AR-ir cells compared with numbers of all activated cells, with different stimuli (Fisher LSD post-hoc analysis) and across medial amygdala subregions. ns= not significant.
| p-values for: | MeAtotal | MeAd | MeAv | MePtotal | MePd | MePv |
|---|---|---|---|---|---|---|
| Main Effect | F(1,63)=29.92, p<0.001 | F(1,63)=6.77, p=0.015 | F(1,63)=40.97, p<0.001 | F(1,63)= 27.83, p<0.001 | F(1,63)=19.78, p<0.001 | F(1,63)=5.99, p=0.021 |
| HVF | =0.005 | ns | <0.001 | <0.05 | <0.05 | <0.05 |
| fFGS | <0.001 | =0.004 | =0.001 | =0.002 | =0.007 | =0.003 |
| mFGS | <0.01 | ns | =0.002 | ns | <0.05 | ns |
| MMU | ns | ns | ns | <0.05 | ns | <0.02 |
3. Results
3.1 Fos expression
In gonad-intact and T-replaced castrates Fos expression was analyzed for effects of each of the chemosensory stimuli MMU, HVF, mFGS and fFGS and control (clean swab, CS) in MeAd, MeAv, MePd, and MePv. For comparison with earlier results (Meredith and Westberry, 2004), effects of each of the chemosensory stimuli were also compared in MeA-total and MeP-total.
3.1.1 Gonad-intact animals
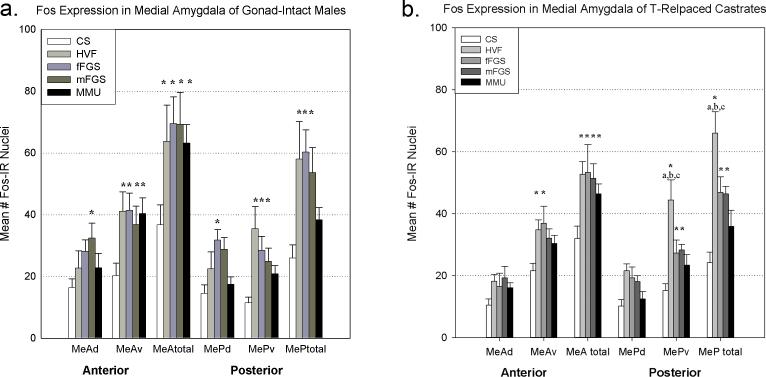
As previously seen in hamsters (Meredith and Westberry, 2004) and mice (Samuelsen and Meredith, 2009) conspecific stimuli (here HVF, mFGS, and fFGS) produced significantly more Fos expression in MeA-total and MeP-total than control (CS), while a heterospecific stimulus, MMU, activated MeA, but not MeP. There was a significant main effect of exposure (p<0.001; F(4,85)= 6.591). The analysis on dorsal and ventral subregions also revealed a significant main effect of exposure (p<0.001; F(4,171)= 11.051). Again, MMU failed to significantly activate either subregion of MeP (see Figure 1a). There was no significant interaction of the effects of exposure and area in either analysis.
Figure 1.
Fos expression in the medial amygdala after exposure to various chemosensory stimuli in gonad-intact (a) and testosterone-replaced (b) male hamsters. Conspecific stimuli activated both anterior (MeA) and posterior (MeP) medial amygdala, while the heterospecific stimulus only activated MeA (a and b). Asterisk = significantly greater than control ( p<.03), a,b,c= significantly greater than MMU, fFGS, and mFGS respectively (p<.001 for all). CS= clean swab, HVF= hamster vaginal fluid, fFGS= female flank gland secretion, mFGS= male flank gland secretion, MMU= male mouse urine, d= dorsal subnucleus, v=ventral subnucleus.
The effects in MeA appeared to be due mostly to responses to these stimuli in the ventral portion (MeAv; p<0.001 for all stimuli, Fisher LSD post-hoc analysis). However, male flank gland secretion (mFGS) also produced significantly more Fos expression than CS in MeAd (p= 0.008), MePd (p= 0.019) and MePv (p= 0.026). Female flank gland secretion (fFGS) produced significantly more Fos expression than CS in MePd (p= 0.005) and MePv (p= 0.005). HVF produced significantly more Fos expression than CS only in MePv (p<0.001; see Figure 1a).
3.1.2 Castrates with testosterone replacement
As in gonad-intact animals, conspecific stimuli produced significantly more Fos expression in MeA-total and MeP-total than control (CS), while the heterospecific stimulus only activated MeA, but not MeP. There was a significant main effect of exposure (p<.001; F(4,81)= 7.750). The analysis on dorsal and ventral subregions also revealed a significant main effect of exposure (p<0.001; F(4,163)= 7.450). Again, MMU failed to significantly activate either subregion of MeP (see Figure 1b). There was no significant interaction of the effects of exposure and area for either analysis.
The effects in MeA and MeP appeared to be due mostly to effects of the stimuli in the ventral portion (MeAv: p= 0.011 for HVF and p= 0.022 for fFGS; MePv: p<0.001 for HVF, p= 0.029 for fFGS and p= 0.03 for mFGS; Fisher LSD post-hoc analysis).
HVF also yielded significantly more Fos expression than any other stimulus in MeP (p<0.001 for all stimuli), again due to Fos activation in MePv (p<0.001 for all stimuli; Fisher LSD post-hoc analysis; see Figure 1b).
3.2 Androgen receptor immunoreactivity
In gonad-intact and castrates with T-replacement, androgen receptor (AR) expression was analyzed for effects of each of the chemosensory stimuli: MMU, HVF, male mFGS, fFGS and control (CS) in MeAd, MeAv, MePd, and MePv. For comparison with earlier results (Meredith and Westberry, 2004), effects of each of the chemosensory stimuli were also compared in MeA-total and MeP-total.
3.2.1 Gonad-intact animals
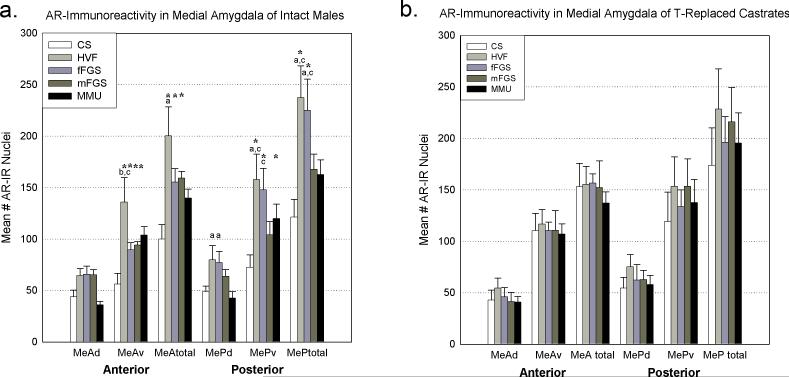
Animals exposed to different chemosensory stimuli had significantly different numbers of AR-immunoreactive (ir) cells in the medial amygdala. In the analysis of MeA-total and MeP-total there was a significant main effect of exposure (p<0.001; F(4,85)= 9.666). There was no significant interaction of the effects of exposure and area for either analysis.
In MeA-total, the conspecific stimuli (HVF, fFGS, mFGS) all yielded significantly more AR-ir cells than control (p<0.006, Fisher LSD post-hoc analysis). HVF also produced more AR-ir cells than MMU (p= 0.036). MMU did not significantly change the number of AR-ir cells in MeA-total.
In MeP-total, HVF and fFGS again produced significantly more AR-ir cells than CS (both p<0.001, Fisher LSD post-hoc analysis), but MMU and mFGS did not significantly change the number of AR-ir cells compared to CS. Comparing across stimuli, HVF exposure produced significantly more AR-ir cells than MMU (p= 0.01) and mFGS (p= 0.016). fFGS produced significantly more AR-ir cells than MMU(p= 0.031) and mFGS (p= 0.047;Fisher LSD post-hoc analysis, see Figure 2a).
Figure 2.
Androgen receptor (AR)-immunoreactivity (ir) in the medial amygdala after exposure to various chemosensory stimuli gonad-intact (a) and testosterone-replaced (b) male hamsters. Conspecific and heterospecific stimuli significantly altered the number of AR-ir cells in different areas across the medial amygdala in gonad-intact animals (a). Conversely, there were no significant changes in testosterone-replaced castrates (b). Asterisk = significantly greater than control ( p<.05), a,b,c= significantly greater than MMU, fFGS, and mFGS respectively (p<.001 for all). CS= clean swab, HVF= hamster vaginal fluid, fFGS= female flank gland secretion, mFGS= male flank gland secretion, MMU= male mouse urine, MeA= anterior medial amygdala, MeP= posterior medial amygdala, d= dorsal subnucleus, v=ventral subnucleus.
When analyzing the dorsal and ventral subregions of MeA and MeP there was also a significant effect of exposure (p<0.001; F(4,171)= 12.745) and a significant interaction between exposure and area (p=0.033; F(12,171)= 1.945). All changes in AR-ir (compared with control) occurred in the ventral subdivisions (see Figure 2a). There were no significant differences from control in MeAd or MePd.
In MeAv: animals exposed to HVF, fFGS, mFGS or MMU displayed more AR-ir than CS controls (p<0.001, p= 0.041, p= 0.021, p= 0.004, respectively, Fisher LSD posthoc analysis). Comparing across stimuli, HVF also produced significantly more AR-ir than fFGS and mFGS (p=0.009, p=0.018 respectively, Fisher LSD post-hoc analysis).
In MePd: no stimulus produced significantly more AR-ir than CS controls. However, the female conspecific stimuli both produced significantly more AR-ir cells than MMU (HVF: p= 0.034; fFGS: p= 0.049, Fisher LSD post-hoc analysis).
In MePv: HVF, fFGS and MMU each produced significantly more AR-ir cells than CS (p<0.001, p<0.001, p= 0.004, respectively, Fisher LSD post-hoc analysis). Comparing across stimuli, both female conspecific stimuli produced more AR-ir cells than mFGS (HVF: p= 0.003, fFGS: p= 0.013, Fisher LSD post-hoc analysis). HVF also produced significantly more AR-ir cells than MMU (p= 0.033, Fisher LSD post-hoc analysis, see figure 2a.)
3.2.2 Castrates with testosterone replacement
There were no significant differences in the number of AR-ir cells in any of the areas analyzed (see Figure 2b).
3.3 Double-labeled cells
In gonad-intact and T-replaced castrates the numbers of double-labeled cells (both Fos-ir and AR-ir) were analyzed for effects of each of the chemosensory stimuli listed above in MeAd, MeAv, MePd, MePv, and separately for MeA-total and MeP-total. (Examples of double-labeled and single-labeled cells are shown in Figure 4).
3.3.1 Gonad-intact animals
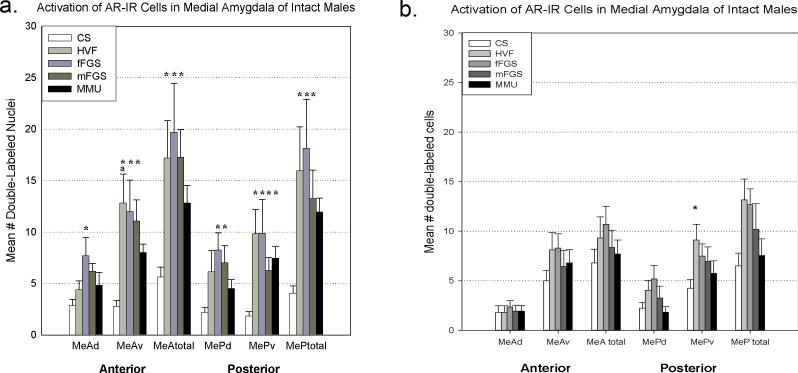
All of the conspecific stimuli (HVF, mFGS, and fFGS), but not the heterospecific stimulus (MMU), activated significantly more AR-ir cells (cells double-labeled with Fos and androgen receptor, AR) in MeA and MeP than did clean swab control (CS). There was a significant main effect of exposure (p<0.001; F(1,85)= 7.885). There was no significant interaction (see Figure 3a).
Figure 3.
Double-labeling of Fos and androgen receptors (AR) in the medial amygdala after exposure to various chemosensory stimuli gonad-intact (a) and testosterone-replaced (b) male hamsters. In gonad-intact males, conspecific stimuli yielded significantly more double-labeled cells than control (CS, clean swab) across the medial amygdala. The heterospecific stimulus (MMU, male mouse urine) produced significantly more double-labeled cells in the ventral portion of the posterior medial amygdala (MePv). Asterisk = significantly greater than control ( p<.05), a= significantly greater than MMU (p<.05). HVF= hamster vaginal fluid, fFGS= female flank gland secretion, mFGS= male flank gland secretion, MeA= anterior medial amygdala, MeP= posterior medial amygdala, d= dorsal subnucleus, v=ventral subnucleus.
When analysis was performed on the dorsal and ventral subdivisions of MeA and MeP there was also a significant main effect of exposure (p<0.001; F(4, 171)= 12.933) and no significant interaction between exposure and area.
In MeAd: Female flank gland secretion (fFGS) produced significantly more double-labeled cells than CS (p= 0.029, 2-way ANOVA, Fisher LSD post-hoc analysis).
In MeAv: all stimuli, including the heterospecific stimulus (MMU), produced significantly more double-labeled cells than CS (HVF, fFGS, mFGS: all p<0.001, MMU: p= 0.019, Fisher LSD post-hoc analysis). Comparing across stimuli, HVF produced significantly more double-labeled cells than MMU (p= 0.044, Fisher LSD post-hoc analysis).
In MePd only mFGS and fFGS produced significantly more double-labeled cells than CS controls (p= 0.030 and p= 0.007, respectively, Fisher LSD post-hoc analysis).
In MePv all conspecific stimuli provided significantly more double-labeled cells than CS controls: HVF (p<0.001), mFGS (p= 0.047), fFGS (p<0.001). Surprisingly, given that the heterospecific stimulus failed to activate MePv significantly when all activated cells were counted (see Figure 1a), MMU did significantly activate AR-ir cells above control in MePv (p= 0.012, Fisher LSD post-hoc analysis; see Figure 3a).
3.3.2 Castrates with testosterone replacement
The only significant difference in the number of double-labeled cells was seen in MePv; HVF generated significantly more double-labeled cells than clean swab control (p= 0.025; Fisher LSD post-hoc analysis, see Figure 3b).
4. Discussion
Fos expression in both gonad-intact and T-replaced castrated males confirmed previous results in hamsters and mice: Conspecific stimuli activated Fos-expression in both the anterior (MeA) and posterior (MeP) medial amygdala, while a heterospecific stimulus only activated Fos expression in MeA (Meredith and Westberry, 2004; Samuelsen and Meredith, 2009).
4.1 Summary of major new findings
As expected, AR-ir phenotype cells were differentially activated by different stimuli and in different sub-regions in intact animals. However, in animals with fixed testosterone levels, only one stimulus, HVF, activated AR-ir cells significantly more than in animals exposed to clean swabs. Unexpectedly, the numbers of AR-ir cells also changed with chemosensory stimulation. These changes disappeared in animals with fixed testosterone levels. The main difference between intact and castrated animals was that T levels were free to change in intact animals. Thus, observable nuclear AR expression could be influenced by changes in testosterone levels, resulting from chemosensory stimulation, which varied from stimulus to stimulus. In intact animals, AR-phenotype cells were also selectively activated (increased Fos protein expression) at levels above the average Fos activation of other cells in the same sub-region, for several stimuli (see below).
4.2 Differential effect of different stimuli on AR levels
Numbers of AR-ir cells were higher in some areas for all stimuli, but non-uniformly across sub-areas and stimuli. In intact animals, all conspecific stimuli increased Fos expression in AR-ir cells significantly above that for control swabs, in both MeA (-total) and MeP (-total). All conspecific stimuli also increased AR expression (except mFGS in MeP). All of these stimuli can facilitate behaviors that are also steroid dependent. Hamster vaginal fluid (HVF) attracts males and facilitates mating (Johnston 1974, 1975b; Singer et al., 1976; O'Connell and Meredith, 1984) in males with adequate testosterone levels. HVF can even induce males to attempt mating with inappropriate partners such as an anesthetized male scented with HVF (Johnston 1974, 1975b; Macrides et al., 1984; Meredith 1986). Normal behavioral responses to hamster flank gland secretion, both male (mFGS) and female (fFGS), have also previously been demonstrated to be dependent on adequate steroid levels (Johnston, 1975a). Here, they both activated AR-ir cells in both dorsal and ventral subdivisions of MeP.
Both female stimuli (HVF and fFGS) increased the overall number of AR-ir cells in MeA and MeP (in the ventral subdivisons) in intact males. The male stimulus, mFGS, increased the overall number of AR-ir cells in MeA, not MeP, but was able to selectively increase Fos expression in AR-ir cells in both areas. The ability of these stimuli to increase numbers of AR-ir cells in target areas, and to activate them, may facilitate the convergence of chemosensory and hormonal signals for behaviors where both are critical.
4.3 Possible mechanisms for changes in numbers of AR-ir cells
Conspecific chemosignals are known to induce testosterone surges in hamsters (Macrides et al., 1974) and mice (Coquelin et al., 1984), an effect dependent on an intact vomeronasal organ in both species (Pfeiffer and Johnston, 1994; Coquelin et al., 1984; Wysocki et al., 1983). The effects seen here in gonad-intact animals are consistent with an effect of increases in circulating testosterone in response to chemosensory stimuli; as these changes were not seen in animals with controlled testosterone levels (castrates with testosterone replacement).
The increase in AR-ir does not necessarily equate to a change in the total number of ARs. The relatively short time between stimulation and perfusion of the animals (45 min) makes upregulation of transcription and translation unlikely to be the mechanism for the observed changes in AR-ir. However, a change in observable AR-ir could be the result of a concentration of previously dispersed antigen molecules or a change in antigenicity. Thus, androgen receptors may be differentially bound and/or re-compartmentalized in response to certain chemosensory stimuli. The antibody used here is reported to bind primarily to occupied, nuclear-localized ARs (e.g., Gréco et al., 1996). Here the AR staining was indeed nuclear (Figure 4). Thus, these results likely reflect a change in the number of bound androgen receptors, suggesting an increase in available androgen, not necessarily a change in total AR expression.
Here, changes in AR-ir occurred within 45 min. In other studies, testosterone or non-aromatizable androgen treatment increased AR-ir within even shorter periods of time, 15-30 min. (Krey and McGinnis, 1990; Wood and Newman, 1993a; Handa et al., 1996; Lu et al., 1998). The ability of androgens to act quite rapidly, including perhaps in the medial amygdala, could be a key component in the brain's interpretation of chemosensory signals and production of an appropriate behavioral response. We believe the changes in AR-ir and Fos protein expression in AR-ir cells in chemosensory circuits of the amygdala is due to exposure to chemosensory stimuli.
4.4 Selective Fos activation of AR-phenotype cells for some stimuli
The numbers of double labeled cells (Fos-ir and AR-ir) are small in all regions. However, in intact animals, the Fos activation by some stimuli was greater in AR-phenotype cells than for other medial amygdala cells in the same region (increase over control levels for AR-phenotype cells compared to all cells, see Table 1). The increase in double-labeled cells in stimulated- compared to control- animals (e.g., Fig 3a; MeA-total, MeP-total) was significantly greater than the average increase for all Fos-labeled cells (e.g., Fig 1a; MeA-total, MeP-total). Thus, when testosterone is allowed to increase following stimulation, there was additional Fos protein expression in AR-ir cells, once the numbers of AR-ir cells had been increased as a consequence of stimulation. The selective Fos activation of AR-phenotype cells occurred for all conspecific stimuli used here, in both MeA-total and MeP-total as well as in MeAv and MePd. Only fFGS showed selective expression of Fos in both MeAd and in MePv. HVF was among the strongest activators of MePv, but did not selectively activate Fos in AR-ir cells above the level of Fos activation of all cells. However, in castrates with testosterone replacement, where testosterone levels were fixed, there was significant double labeling only for HVF stimulation in MePv, suggesting that MePv may be an important area for interpreting HVF signals in the absence of any change in testosterone. Increased Fos expression in MeP in response to HVF was also reported by Fiber and Swann (1996) and Swann (1997). Responses were similar in intact males and in both castrated males and castrated males with T-replacement indicating a robust chemosensory input to MeP not dependent on steroid levels, as also shown for conspecific stimuli in ferrets (Kelliher et al., 1998). Swann (1997) counted Fos expression in MeP-total and most Fos label in her figure is towards the ventral part of MeP, suggesting activation of MePv, as seen here.
These results raise the possibility of a two stage response. The initial response at background (or very low) testosterone levels would be similar to that in castrated, T-replaced animals here (Figs. 1b, 3b). The later secondary response following a rise of testosterone would affect more cells expressing AR receptors. Although there appears to be a greater response overall in intact animals, it is not clear whether the increased Fos expression in AR-ir cells was due to the activation of additional cells (i.e., an amplified chemosensory response following T increase), or the expression of detectable nuclear AR-ir in cells that would have shown Fos-activation anyway. (Direct comparisons between intact and castrated-T-replaced animals were not made because these two groups were not tested at the same time and tissue from the two groups was not processed together.)
Testosterone itself can induce an increase in Fos expression in several brain areas, including MeP when a high dose (40 μg) is injected acutely (over 4 hrs) into the cerebral ventricles (Nagypal and Wood, 2007). This is evidence for a direct effect of T on neuronal activation, but whether all high-dose T-induced Fos expression is associated with increased neural activity is not clear. Although serum T levels can more than double with chemosensory stimulation (Richardson et al., 2004), it seems unlikely that intracerebral T-levels in our intact animals reached the levels produced in the Nagypal and Wood (2007) experiments. Nevertheless, there is a possibility that prolonged chemosensory exposure may have additional effects physiologically or behaviorally as a consequence of the rise in T levels. These effects could include either a secondary neural activation leading to increased Fos expression in cells already expressing AR, or a secondary susceptibility in some cells to modulation by steroids, as indicated by the appearance of AR expression, in addition to Fos expression.
4.5 Significance of subdivision Fos activation
There was a strong Fos response in MePv to HVF (see Figure 1). Conspecific female stimuli also activate both dorsal and ventral MeP in mice (Choi et al., 2005; Samuelsen and Meredith, 2009). Additionally, conspecific chemosensory stimuli from females (HVF, fFGS), but not from males (mFGS), increased AR-immunoreactivity in MePd above levels from a heterospecific stimulus (MMU).The expression of Fos protein in the dorsal subdivision of MeP (MePd) is strong in mating males indicating it's importance for reproductive behavior. This area has been proposed, mainly on the basis of such responses and its anatomical connections (Canteras, 2002; Choi et al., 2005), as an area specialized for processing chemosensory information on reproductive stimuli.
Maras and Petrulis (2010c) demonstrated that a higher proportion of MePd cells that project to MeA contain AR than is the case for cells projecting from MeA to MePd; but a higher proportion of cells projecting from MeA to MePd show increased Fos expression in response to conspecific chemosensory stimuli. These findings and the result of lesions in MeA and MeP (Maras and Petrulis, 2010a,b) suggest that chemosensory information flows predominantly from MeA to MeP; and that interactions between the two are important for onward information transmission and chemosensory driven behavior. Both MeA and MeP project more centrally and both appear to be important in the integration of chemosensory input and steroid influence (Maras and Petrulis, 2010c) as seen here. Lesions of MeA have a greater effect on attraction to opposite sex odors (Maras and Petrulis, 2010a) and on mating (Lehman and Winans, 1982); but MeA lesions would also cut off the inter-subregion flow of chemosensory information to MeP.
The heterospecific stimulus tested here (MMU) significantly increased the number of AR-ir cells in MeAv and MePv in intact males. MMU also activated AR-ir cells (double-labeled) significantly above control in MePv, and significantly above cells in general in that region (see Table 1). MePv is associated with the processing of chemosensory stimuli from other conspecific males (potential competitors) in both hamsters and mice. In mice and rats MePv is activated by cat stimuli (potential predator; Choi et al., 2005; Staples et al., 2008; Samuelsen and Meredith, 2009). Thus MePv functions appear to extend beyond purely social signal processing, and activation by heterospecific stimuli might also suppress social responses in inappropriate situations (Choi et al., 2005). Papes et al. (2010) show that heterospecific major urinary proteins can activate the vomeronasal system in mice, but it is not clear why heterospecific stimuli should increase AR expression here.
4.6 Conclusions
These results provide evidence for changes in immunoreactive AR-protein expression within 45 min of chemosensory stimulation, and for differences in Fos activation of AR-ir cells, depending on the signals to which the animal was exposed. We interpret these changes as a result of an acute rise in testosterone after chemosignal exposure, because similar changes do not occur in testosterone implanted castrates in which T-levels cannot rise after stimulation. Previous studies in rats have shown changes in AR expression in response to mating in regions along the vomeronasal sensory pathway, and other areas in the brain, including the amygdala, medial preoptic area , bed nucleus of the stria terminalis , and ventromedial hypothalamus (Fernandez-Guasti et al., 2003; Portillo et al., 2006), but none had tested AR expression in response to chemosensory stimuli alone. Our results suggest that immediate responses to chemosensory signals and responses to more prolonged stimuli may be different but both mediated by amygdala circuits. Changes in responses and/or circuit properties resulting from differential chemosignal-elicited hormonal responses may result in changes in behavioral responses to these same stimuli or to others.
Supplementary Material
Supplementary Table 1: p-values for 2-way RM ANOVA for numbers of activated AR-ir cells compared with numbers of all activated cells, with different stimuli (Fisher LSD post-hoc analysis) and across medial amygdala subregions (Fisher LSD post-hoc analysis). ns= not significant.
Research Highlights.
AR-ir was changed in Me by chemostimulation, but not in animals with fixed testosterone levels
AR-ir cells were differentially activated by various chemosignals in Me in intact male hamsters.
AR-+ cells were selectively activated by chemosignals at levels above average Fos activation in Me
Acknowledgements
The authors would like to acknowledge Dr. Cleyde Helena, Lindsey Silz, and Dr. Jessica Santollo for their assistance with this work. We would also like to thank Dr. Elaine Hull for the use of facilities and Dr. Marc Freeman for the use of the MetaMorph counting software.
Funding
This work was supported by the National Institutes of Health [DC005813].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartke A. Male reproductive endocrinology. In: Siegel Hl., editor. The hamster: reproduction and behavior. Plenum Press; New York: 1985. [Google Scholar]
- Baum MJ, Everitt BJ. Increased expression of c-fos in the medial preoptic area after mating in male rats: role of afferent inputs from the medial amygdala and midbrain central tegmental field. Neurosci. 1992;50:627–646. doi: 10.1016/0306-4522(92)90452-8. [DOI] [PubMed] [Google Scholar]
- Bisler S, Schleicher A, Gass P, Stehle G, Zilles K, Staiger JF. Expression of c-Fos, ICER, Krox-24 and Jun B in the whisker-to-barrel pathway of rats: Time course of induction upon whisker stimulation by tactile exploration of an enriched environment. J Chemical Neuroanat. 2002;23:187–198. doi: 10.1016/s0891-0618(01)00155-7. [DOI] [PubMed] [Google Scholar]
- Blake CB, Meredith M. Selective enhancement of main olfactory input to the medial amygdala by GnRH. Brain Res. 2010;1317:46–59. doi: 10.1016/j.brainres.2009.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras NS. The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol Biochem Behav. 2002;71:481–491. doi: 10.1016/s0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Zangenehpour S, Rahbar-Dehgan F, Ye F. Molecular maps of neural activity and quiescence. Act Neurobiol Exp. 2000;60:403–410. doi: 10.55782/ane-2000-1359. [DOI] [PubMed] [Google Scholar]
- Cheng SY, Taravosh-Lahn K, Delville Y. Neural circuitry of play fighting in golden hamsters. Neurosci. 2008;156(2):247–256. doi: 10.1016/j.neuroscience.2008.07.048. [DOI] [PubMed] [Google Scholar]
- Choi GB, Dong H, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Coquelin A, Clancy AN, Macrides F, Noble EP, Gorski RA. Pheromonally induced release of luteinizing hormone in male mice: involvement of the vomeronasal system. J Neurosci. 1984;4:2230–2236. doi: 10.1523/JNEUROSCI.04-09-02230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen LM, Peters HJ, Veening JG. Distribution of Fos immunoreactivity following mating versus anogenital investigation in the male rat brain. Neurosci. 1997;77:1151–1161. doi: 10.1016/s0306-4522(96)00542-8. [DOI] [PubMed] [Google Scholar]
- Dielenberg RA, Hunt GE, McGregor IS. ‘When a rat smells a cat’: the distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neurosci. 2001;104:1085–1097. doi: 10.1016/s0306-4522(01)00150-6. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fewell GD, Meredith M. c-fos expression in vomeronasal pathways of mated or pheromone-stimulated male golden hamsters: contributions from vomeronasal sensory input and expression related to mating performance. J Neurosci. 1994;14:3643–3654. doi: 10.1523/JNEUROSCI.14-06-03643.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Swaab D, Rodríguez-Manzo G. Sexual behavior reduces hypothalamic androgen receptor immunoreactivity. Psychoneuroendocrinol. 2003;28(4):501–512. doi: 10.1016/s0306-4530(02)00036-7. [DOI] [PubMed] [Google Scholar]
- Fewell GD, Meredith M. Experience facilitates vomeronasal and olfactory influence on Fos expression in medial preoptic area during pheromone exposure or mating in male hamsters. Brain Res. 2002;941(1-2):91–106. doi: 10.1016/s0006-8993(02)02613-6. [DOI] [PubMed] [Google Scholar]
- Fields RD, Eshete F, Dudek S, Ozsarac N, Stevens B. Regulation of gene expression by action potentials: dependence on complexity in cellular information processing. Novartis Found Symp. 1991;239:160–176. doi: 10.1002/0470846674.ch13. [DOI] [PubMed] [Google Scholar]
- Fiber JM, Swann JM. Testosterone differentially influences sex specific pheromone-stimulated Fos expression in limbic regions of Syrian hamster. Horm Behav. 1996;30:455–473. doi: 10.1006/hbeh.1996.0050. [DOI] [PubMed] [Google Scholar]
- Fiber JM, Adames P, Swann JM. Pheromones induce c-fos in limbic areas regulating male hamster mating behavior. Neuroreport. 1993;4:871–874. doi: 10.1097/00001756-199307000-00008. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Lombardo A, Pfaff DW. Sex steroids and fos expression in the CNS of prepubertal and newborn rats. Mol Cell Neurosci. 1991;1:250–261. doi: 10.1016/1044-7431(90)90007-q. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Jia X, Wang Z. Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J Comp Neurol. 2007;502:1109–1122. doi: 10.1002/cne.21364. [DOI] [PubMed] [Google Scholar]
- Grattan DR, Selmanoff M. Castration-induced decrease in the activity of medial preoptic and tuberoinfundibular GABAergic neurons is prevented by testosterone. Neuroendocrinol. 1994;60(2):141–149. doi: 10.1159/000126744. [DOI] [PubMed] [Google Scholar]
- Gréco B, Edwards DA, Michael RP, Clancy AN. Androgen receptor immunoreactivity and mating-induced Fos expression in forebrain and midbrain structures in the male rat. Neurosci. 1996;75:161–171. doi: 10.1016/0306-4522(96)00183-2. [DOI] [PubMed] [Google Scholar]
- Gréco B, Edwards DA, Michael RP, Clancy AN. Androgen receptors and estrogen receptors are colocalized in male rat hypothalamic and limbic neurons that express Fos immunoreactivity induced by mating. Neuroendocrinol. 1998a;67:18–28. doi: 10.1159/000054294. [DOI] [PubMed] [Google Scholar]
- Gréco B, Edwards DA, Zumpe D, Clancy AN. Androgen receptor and mating-induced fos immunoreactivity are co-localized in limbic and midbrain neurons that project to the male rat medial preoptic area. Brain Res. 1998b;781:15–24. doi: 10.1016/s0006-8993(97)01136-0. [DOI] [PubMed] [Google Scholar]
- Gréco B, Edwards DA, Zumpe D, Michael RP, Clancy AN. Fos induced by mating or noncontact sociosexual interaction is colocalized with androgen receptors in neurons within the forebrain, midbrain, and lumbosacral spinal cord of male rats. Horm Behav. 1998c;33:125–138. doi: 10.1006/hbeh.1998.1443. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Kerr JE, Don Carlos LL, McGivern RF, Hejna G. Hormonal regulation of androgen receptor messenger RNA in the medial preoptic area of the male rat. Mol Brain Res. 1996;39:57–67. doi: 10.1016/0169-328x(95)00353-t. [DOI] [PubMed] [Google Scholar]
- Hull EM, Meisel RL, Sachs BD. Male sexual behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, brain and behavior. Academic Press; San Diego: 2002. pp. 3–137. [Google Scholar]
- Johnson M, Hill CS, Chawla S, Treisman R, Bading H. Calcium controls gene expression via three distict pathways that can function independently of the Ras/mitogen-activated kinases (ERKs) signalling cascade. J Neurosci. 1997;17:6189–6202. doi: 10.1523/JNEUROSCI.17-16-06189.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RE. Sexual attraction function of golden hamster vaginal secretion. Behav Biol. 1974;12:111–117. doi: 10.1016/s0091-6773(74)91101-8. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Scent marking by male golden hamsters (Mesocricetus auratus) I. Effects of odors and social encounters. Z Tierpsychol. 1975a;37:75–98. doi: 10.1111/j.1439-0310.1975.tb01128.x. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Sexual excitation function of hamster vaginal secretion. Anim Learn Behav. 1975b;3:161–166. [PubMed] [Google Scholar]
- Johnston RE. Pheromones, the vomeronasal system, and communication. From hormonal responses to individual recognition. Ann N Y Acad Sci. 1998;855:333–348. doi: 10.1111/j.1749-6632.1998.tb10592.x. [DOI] [PubMed] [Google Scholar]
- Kelliher KR, Chang YM, Wersinger SR, Baum MJ. Sex difference and testosterone modulation of pheromone induced neuronal Fos in the Ferret's main olfactory bulb and hypothalamus. Biol Reprod. 1998;59:1454–1463. doi: 10.1095/biolreprod59.6.1454. [DOI] [PubMed] [Google Scholar]
- Kindo Y, Sachs BD. Disparate effects of small medial amygdala lesions on noncontact erection, copulation, and partner preference. Physiol Behav. 2002;76(4-5):443–447. doi: 10.1016/s0031-9384(02)00682-0. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neurosci. 1995;66(3):721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Newman SW. Mating induced expression of c-fos in the male syrian hamster brain: Role of experience, pheromones and ejaculations. J Neurobiol. 1997;32:481–501. doi: 10.1002/(sici)1097-4695(199705)32:5<481::aid-neu4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Krey LC, McGinnis MY. Time-courses of the appearance/disappearance of nuclear androgen + receptor complexes in the brain and adenohypophysis following testosterone administration/withdrawal to castrated male rats: relationships with gonadotropin secretion. J Steroid Biochem. 1990;35:403–408. doi: 10.1016/0022-4731(90)90247-p. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Winans SS. Vomeronasal and olfactory pathways to the amygdala controlling male hamster sexual behavior: audioradiographic and behavioral analyses. Brain Res. 1982;240(1):27–41. doi: 10.1016/0006-8993(82)90641-2. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Winans SS, Powers JB. Medial nucleus of the amygdala mediates chemosensory control of male hamster sexual behavior. Science. 1980;210:557–560. doi: 10.1126/science.7423209. [DOI] [PubMed] [Google Scholar]
- Liu YC, Salamone JD, Sachs BD. Lesions in medial preoptic are and bed nucleus of stria terminalis: differential effects on copulatory behavior and noncontact erection in male rats. J Neurosci. 1997;17(13):5245–5253. doi: 10.1523/JNEUROSCI.17-13-05245.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SF, McKenna SE, Cologer-Clifford A, Nau EA, Simon NG. Androgen receptor in mouse brain: sex differences and similarities in autoregulation. Endocrinol. 1998;139:1594–1601. doi: 10.1210/endo.139.4.5863. [DOI] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. Lesions that functionally disconnect the anterior and posterodorsal sub-regions of the medial amygdala eliminate opposite sex odor preference in male Syrian hamsters (Mesocricetus auratus). Neurosci. 2010a;165:1052–1062. doi: 10.1016/j.neuroscience.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. The anterior medial amygdala transmits sexual odor information to the posterior medial amygdala and related forebrain nuclei. Eur J Neurosci. 2010b;32:469–482. doi: 10.1111/j.1460-9568.2010.07289.x. [DOI] [PubMed] [Google Scholar]
- Maras PM, Petrulis A. Anatomical connections between the anterior and posterodorsal sub-regions of the medial amygdala: integration of odor and hormone signals. Neurosci. 2010c;170:610–622. doi: 10.1016/j.neuroscience.2010.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrides F, Bartke A, Fernandez F, D'Angelo W. Effects of exposure to vaginal odor and receptive females on plasma testosterone in the male hamster. Neuroendocrinol. 1974;15:355–364. doi: 10.1159/000122326. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Dreifuss RM. Evidence for a role of testosterone-androgen receptor interactions in mediating masculine sexual behavior in male rats. Endocrinol. 1989;124:618–626. doi: 10.1210/endo-124-2-618. [DOI] [PubMed] [Google Scholar]
- Meredith M. Vomeronasal organ removal before sexual experience impairs male hamster mating behavior. Physiol Behav. 1986;36:737–743. doi: 10.1016/0031-9384(86)90362-8. [DOI] [PubMed] [Google Scholar]
- Meredith M. Vomeronasal, olfactory, hormonal convergence in the brain: cooperation or coincidence. Ann NY Acad Sci. 1998;855:349–361. doi: 10.1111/j.1749-6632.1998.tb10593.x. [DOI] [PubMed] [Google Scholar]
- Meredith M, Samuelsen CL, Blake C, Westberry JW. Selective Response of Medial Amygdala Subregions to Reproductive and Defensive Chemosignals from Conspecific and Heterospecific Species. In: Hurst JL, Beynon RJ, Roberts SC, Wyatt T, editors. Chemical signals in vertebrates. Vol. 11. Springer Science+Business Media; New York: 2008. pp. 367–378. [Google Scholar]
- Meredith M, Westberry JW. Distinctive responses in the medial amygdala to same-species and different-species pheromones. J Neurosci. 2004;24:5719–5725. doi: 10.1523/JNEUROSCI.1139-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Wood RI. A stereotaxic atlas of the golden hamster brain. Academic Press; San Diego: 2001. [Google Scholar]
- Murphy TH, Worley PF, Baraban JM. L-type voltage sensitive calcium channels mediate synaptic activation of immediate early genes. Neuron. 1991;7:625–635. doi: 10.1016/0896-6273(91)90375-a. [DOI] [PubMed] [Google Scholar]
- O'Connell RJ, Meredith M. Effects of volatile and nonvolatile chemical signals on male sex behaviors mediated by the main and accessory olfactory systems. Behav Neurosci. 1984;98:1083–1093. doi: 10.1037//0735-7044.98.6.1083. [DOI] [PubMed] [Google Scholar]
- Papes F, Logan DW, Stowers L. The vomeronasal organ mediates interspecies defensive behaviors through detection of protein pheromone homologs. Cell. 2010;141:692–703. doi: 10.1016/j.cell.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer CA, Johnston RE. Hormonal and behavioral responses of male hamsters to females and female odors: roles of olfaction, the vomeronasal system, and sexual experience. Physiol Behav. 1994;55:129–138. doi: 10.1016/0031-9384(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Portillo W, Díaz NF, Cabrera EA, Fernández-Guasti A, Paredes RG. Comparative analysis of immunoreactive cells for androgen receptors and oestrogen receptor alpha in copulating and non-copulating male rats. J Neuroendocrinol. 2006;18:168–176. doi: 10.1111/j.1365-2826.2005.01401.x. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Cook-Wiens E, Richardson HN, Sisk CL. Dihydrotestosterone activates sexual behavior in adult male hamsters but not in juveniles. Physiol Behav. 2001;73:579–584. doi: 10.1016/s0031-9384(01)00499-1. [DOI] [PubMed] [Google Scholar]
- Riley LA, Bernstein JJ. Changes in c-fos expression in the dorsal column-medial lemniscal system following dorsal column lesions. J Neurosci Res. 1995;40:499–505. doi: 10.1002/jnr.490400409. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Nelson AL, Ahmed EI, Parfitt DB, Romero RD, Sisk CL. Female pheromones stimulate release of luteinizing hormone and testosterone without altering GnRH mRNA in adult male Syrian hamsters (Mesocricetus auratus). Gen Comp Endocrinol. 2004;138(3):211–217. doi: 10.1016/j.ygcen.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Samuelsen CL, Meredith M. Categorization of biologically relevant chemical signals in the medial amygdala. Brain Res. 2009a;1263:33–42. doi: 10.1016/j.brainres.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsen CL, Meredith M. The vomeronasal organ is required for the male mouse medial amygdala response to chemical-communication signals, as assessed by immediate early gene expression. Neurosci. 2009b;164(4):1468–1476. doi: 10.1016/j.neuroscience.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AG, Agosta WC, O'Connell RJ, Pfaffmann C, Bowen DV, Field FH. Dimethyl disulfide: an attractant pheromone in hamster vaginal secretion. Science. 1976;191(4230):948–950. doi: 10.1126/science.1251205. [DOI] [PubMed] [Google Scholar]
- Staples LG, McGregor IS, Apfelbach R, Hunt GE. Cat odor, but not trimethylthiazoline (fox odor), activates accessory olfactory and defense-related brain regions in rats. Neurosci. 2008;151(4):937–947. doi: 10.1016/j.neuroscience.2007.11.039. [DOI] [PubMed] [Google Scholar]
- Swann JM. Gonadal steroids regulate behavioral responses to pheromones by actions on a subdivision of the medial preoptic nucleus. Brain Res. 1997;750:187–194. doi: 10.1016/s0006-8993(96)01348-0. [DOI] [PubMed] [Google Scholar]
- Tsuchiga T, Horii I. Different effects of acute and chronic immobilization stress on plasma testosterone in male syrian hamsters. Psychoneuroendocrinol. 1995;20:95–102. doi: 10.1016/0306-4530(94)00047-6. [DOI] [PubMed] [Google Scholar]
- Westberry J, Meredith M. The influence of chemosensory input and gonadotropin releasing hormone on mating behavior circuits in male hamsters. Brain Res. 2003;974(1-2):1–16. doi: 10.1016/s0006-8993(03)02535-6. [DOI] [PubMed] [Google Scholar]
- Westberry JM. Doctoral Dissertation in Neuroscience. Florida State University; 2003. Categorical Responses to Chemosensory Input in the Amygdala. [Google Scholar]
- Wood RI, Coolen LM. Integration of chemosensory and hormonal cues is essential for sexual behavior in the male Syrian hamster: role of the medial amygdaloid nucleus. Neurosci. 1997;78:1027–1035. doi: 10.1016/s0306-4522(96)00629-x. [DOI] [PubMed] [Google Scholar]
- Wood RI, Newman SW. Intracellular partitioning of androgen receptor immunoreactivity in the brain of the male Syrian hamster: effects of castration and steroid replacement. J Neurobiol. 1993a;24:925–938. doi: 10.1002/neu.480240706. [DOI] [PubMed] [Google Scholar]
- Wood RI, Newman SW. Mating activates androgen receptor-containing neurons in chemosensory pathways of the male Syrian hamster brain. Brain Res. 1993b;614:65–77. doi: 10.1016/0006-8993(93)91019-o. [DOI] [PubMed] [Google Scholar]
- Wood RI, Newman SW. Androgen and estrogen receptors coexist within individual neurons in the brain of the Syrian hamster. Neuroendocrinol. 1995;62:487–497. doi: 10.1159/000127039. [DOI] [PubMed] [Google Scholar]
- Wood RI, Williams SJ. Steroidal control of male hamster sexual behavior in Me and MPOA: effects of androgen dose and tamoxifen. Physiol Beh. 2001;72:727–733. doi: 10.1016/s0031-9384(01)00427-9. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Katz Y, Bernhard R. Male vomeronasal organ mediates female-induced testosterone surges in mice. Biol Reprod. 1983;28(4):917–922. doi: 10.1095/biolreprod28.4.917. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: p-values for 2-way RM ANOVA for numbers of activated AR-ir cells compared with numbers of all activated cells, with different stimuli (Fisher LSD post-hoc analysis) and across medial amygdala subregions (Fisher LSD post-hoc analysis). ns= not significant.