Abstract
The mechanistic properties of two dietary antioxidants that are required by humans, vitamins C and E, are discussed relative to their biological effects. Vitamin C (ascorbic acid) is an essential cofactor for α-ketoglutarate-dependent dioxygenases. Examples are prolyl hydroxylases, which play a role in the biosynthesis of collagen and in down-regulation of hypoxia-inducible factor (HIF)-1, a transcription factor that regulates many genes responsible for tumor growth, energy metabolism, and neutrophil function and apoptosis. Vitamin C-dependent inhibition of the HIF pathway may provide alternative or additional approaches for controlling tumor progression, infections and inflammation. Vitamin E (α-tocopherol) functions as an essential lipid soluble antioxidant, scavenging hydroperoxyl radicals in lipid milieu. Human symptoms of vitamin E deficiency suggest that its antioxidant properties play a major role in protecting erythrocyte membranes and nervous tissues. As an antioxidant, vitamin C provides protection against oxidative stress-induced cellular damage by scavenging of reactive oxygen species, vitamin E-dependent neutralization of lipid hydroperoxyl radicals, and by protecting proteins from alkylation by electrophilic lipid peroxidation products. These bioactivities bear relevance to inflammatory disorders. Vitamin C plays also a role in the function of endothelial nitric oxide synthase (eNOS) by recycling the eNOS cofactor, tetrahydrobiopterin, which is relevant to arterial elasticity and blood pressure regulation. Evidence from plants supports a role for vitamin C in the formation of covalent adducts with electrophilic secondary metabolites. Mechanism-based effects of vitamin C and E supplementation on biomarkers and on clinical outcomes from randomized, placebo-controlled trials are emphasized in this review.
Keywords: Ascorbic acid, α-tocopherol, oxidative stress, antioxidant, biomarkers, collagen, lipid peroxidation
Introduction
The purpose of this review is to discuss two antioxidant nutrients, vitamins C and E, as well as to describe the mechanisms that make them necessary for humans to acquire in their diets. Further, we discuss the evidence for their potential benefits when consumed in amounts greater than required, for example as dietary supplements.
Vitamin C
Vitamin C (ascorbic acid) is a required nutrient for a variety of biological functions. Humans and other primates have lost the ability to synthesize ascorbic acid due to a defect in L-gulono-1,4-lactone oxidase, an enzyme that catalyzes the conversion of L-gulonolactone into ascorbic acid. Humans, primates and a few other animals (e.g., guinea pigs) depend on the diet as a source of vitamin C to prevent the vitamin C deficiency disease, scurvy, and to maintain general health. The health-promoting effects of vitamin C can be attributed to its biological functions as a co-factor for a number of enzymes, most notably hydroxylases involved in collagen synthesis, and as a water-soluble antioxidant. Vitamin C can also function as a source of the signaling molecule, hydrogen peroxide, and as a Michael donor to form covalent adducts with endogenous electrophiles in plants. These functions and the underlying mechanisms will be illustrated here with examples from the recent literature. This review focuses on chronic diseases and is not intended to provide an exhaustive account of the biological and clinical effects. Other authors have recently discussed the effects of vitamin C on cancer chemoprevention [1, 2] and in the treatment of cancer [3], sepsis [4], and neurodegenerative diseases [5, 6].
Vitamin C as a cofactor for α-ketoglutarate-dependent dioxygenases
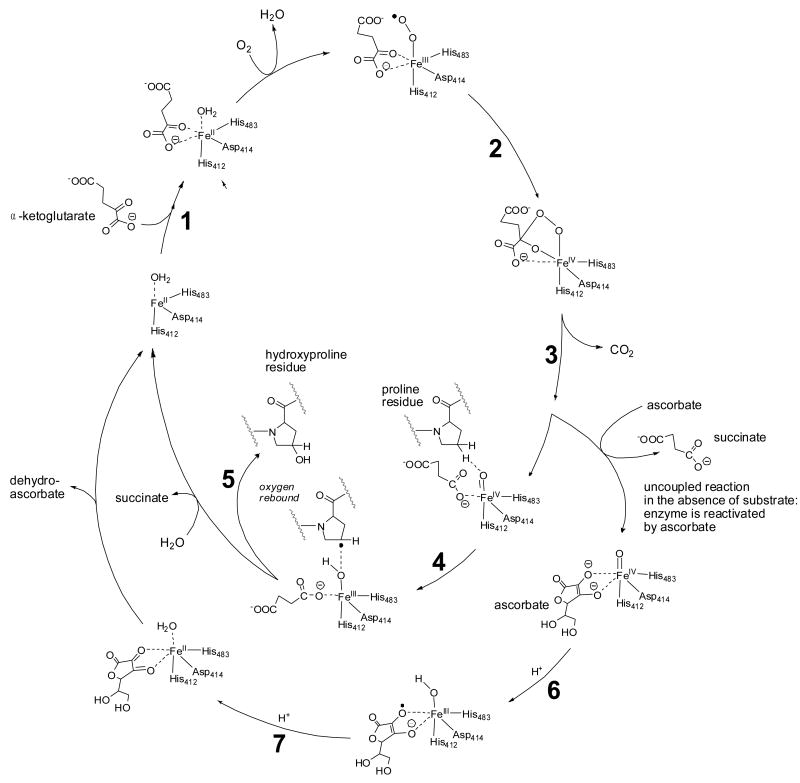
Vitamin C is required for collagen synthesis by acting as a cofactor for α-ketoglutarate-dependent nonheme iron dioxygenases such as prolyl 4-hydroxylase. Investigations into the mechanism of ascorbate-dependent dioxygenases have shown that ascorbate is not consumed in the catalytic cycle in which the co-substrate, α-ketoglutarate, undergoes oxidative decarboxylation to form succinate and a highly reactive iron oxo (FeIV= O) species. The formation of this FeIV= O species is coupled with homolytic cleavage of a C–H bond in the substrate molecule, e.g., proline (Figure 1). The reaction cycle reaches completion when the substrate is oxidized and the oxidation state of the enzyme-bound iron changes from +4 to +2 (Fig. 1). In the absence of a substrate molecule, the enzyme becomes uncoupled and then ascorbate reduces oxo-iron back to FeII, restoring the enzyme's activity. From competition studies with various inhibitors of prolyl 4-hydroxylase and ascorbate derivatives with modifications in the side chain and in the lactone ring, Majamaa et al. [7] concluded that ascorbate interacts directly with the enzyme-bound iron and acts as ‘an inner-sphere reductant in uncoupled reaction cycles’. These authors also concluded that the consumption of ascorbate in a Fenton-like reaction is precluded by the enzyme [7]. The direct interaction between ascorbate and the enzyme-bound iron is similar to Siegel's proposed mechanism of prolyl hydroxylase in which ascorbate coordinates with iron [8, 9]. Structural investigations have established that the iron in human prolyl 4-hydroxylase coordinates with His412, Asp414, and His483 in the catalytic site of the enzyme [10], leaving two coordination sites for binding of the co-substrate, α-ketoglutaric acid, and the last for binding to dioxygen. The cis-oriented oxygen atoms of ascorbate may bind to the enzyme-bound iron similar to α-ketoglutarate [10]. Coordination of ascorbate with enzyme-bound iron would provide the necessary electrons in uncoupled reaction cycles to reactivate the enzyme (Figure 1), consistent with the observation that ascorbate is consumed stoichiometrically in uncoupled reaction cycles [11]. Thus, the role of ascorbate is to keep the nonheme iron in the catalytically active, reduced state.
Fig. 1.
Vitamin C acts as a cofactor for prolyl 4-hydroxylase. The starting point for the catalytic cycle is where the co-substrate, α-ketoglutaric acid, coordinates with the enzyme-bound FeII (step 1). Activation of molecular oxygen (2) and subsequent decarboxylation of α-ketoglutaric acid (3) leads to the formation of the highly energetic FeIV=O reagent that hydroxylates proline residues in procollagen (steps 4 and 5, inner catalytic cycle) [217-219]. If decarboxylation of α-ketoglutaric acid and subsequent formation of the FeIV=O species takes place in the absence of a substrate molecule (proline residue), the FeIV=O species will oxidize a molecule of ascorbic acid in order to regain activity (steps 6 and 7, outer cycle). Thus, ascorbic acid is consumed stoichiometrically in the uncoupled reaction but is not consumed when substrate is available for oxidation. The iron is bound to prolyl 4-hydroxylase through interactions with amino acid residues, His 412, Asp414, and His 483 [10]. The co-substrate, α-ketoglutaric acid, binds to the enzyme-bound iron through two coordination sites as shown. These coordination sites can be occupied by ascorbate upon departure of succinate from the FeIV=O species in the uncoupled reaction (outer cycle).
Role of vitamin C in prolyl hydroxylation under pathological conditions
Scurvy is the prototypical deficiency disease that links insufficient intake of vitamin C to impaired collagen synthesis [12]. Collagen synthesis is required for maintaining normal vascular function but also for tumor angiogenesis. Tumor growth relies on angiogenesis to provide the cancerous tissue with metabolic substrates, growth factors, and oxygen. Low vitamin C levels would therefore be expected to limit tumor growth by compromising collagen synthesis. Telang et al. [13] tested this hypothesis in mice incapable of ascorbic acid synthesis (Gulo−/− mice with a deletion of the L-gulono-γ-lactone oxidase gene) by measuring the effect of vitamin C supplementation on growth of implanted Lewis lung carcinoma cells. They found that Gulo−/− mice with low plasma vitamin C levels (< 5 μM) developed smaller tumors when the animals consumed a vitamin C-depleted diet compared to partially or fully vitamin C-supplemented animals. The tumors from the scorbutic animals showed multiple areas of hemorrhage, poorly formed blood vessels, and decreased collagen synthesis [13]. The authors suggest that patients with existing cancer may not benefit from vitamin C supplementation; however, vitamin C deficiency is not likely to be beneficial for human cancer patients.
Arterial Tortuosity Syndrome (ATS) is associated with abnormal collagen and elastin synthesis. Twisting and lengthening of major arteries, as well as hypermobility of the joints and laxity for the skin, are characteristics of this rare and heritable disease, which is caused by defects in the gene SLCA10 that codes for GLUT-10 [14]. Since GLUT-10 is localized in the rough endoplasmic reticulum (ER) [15] where proline and lysine hydroxylation take place and where collagen is prepared for secretion by the Golgi apparatus, Segade [15] hypothesized that the defective GLUT-10 in ATS leads to a decrease in uptake of dehydroascorbic acid by the ER, inadequate availability of ascorbic acid for prolyl and lysyl hydroxylases inside the ER, and to synthesis and extracellular deposition of abnormal collagen and elastin. Segade [15] further hypothesized that a major source of ascorbic acid in the ER is dehydroascorbic acid that is taken up by GLUT-10 in the ER membrane and reduced by protein disulfide isomerase in the lumen of the ER [16]. The dependence of ascorbate availability in the ER on GLUT-10 activity and its relevance to ATS remains to be demonstrated.
It is estimated that a third of preterm births are due to premature rupture of the fetal membranes [17]. Mercer et al. [18] tested the hypothesis that fetal membrane strength can be improved by vitamin C and E supplementation to increase collagen synthesis and to inhibit ROS-induced fetal membrane weakening. In a placebo-controlled study, 13 women with a singleton pregnancy received a combination of vitamin C (1000 mg/day) and vitamin E (400 IU/day) from the second trimester until delivery. Vitamin supplementation had no effect on rupture strength, did not affect the normal fetal membrane remodeling process that leads to weakening and rupture at term, and did not alter protein levels or activity of matrix metalloproteinase-9 (MMP-9), a marker of fetal membrane remodeling [19]. Collagen content of the fetal membranes was not measured in this study. Thus, supplementation did not have any obvious benefits.
Vitamin C as a regulator of hypoxia-inducible factor (HIF)-1
Vitamin C-dependent proline hydroxylation also plays a role in gene transcription mediated by hypoxia-inducible factor (HIF)-1 [20, 21]. Direct transcriptional targets of HIF-1 include genes that regulate growth and apoptosis, cell migration, energy metabolism, angiogenesis, vasomotor regulation, matrix and barrier functions, and transport of metal ions and glucose [21]. Binding of HIF-1 to DNA requires dimerization of α and β subunits. Under normoxic conditions, the HIF-1α subunit is targeted for degradation by HIF-specific prolyl hydroxylases that hydroxylate HIF-1α at proline residues 402 and 564 [22]. Prolyl hydroxylase domain (PHD)2 is the predominant form that regulates HIF activity in vivo [23]. Proline hydroxylation promotes HIF-1α binding to the von Hippel-Lindau tumor suppressor and its ubiquitin-dependent degradation [22], thereby repressing transcription of target genes. Under hypoxic conditions, such as exist in fast growing tumors, HIF-1α hydroxylation is repressed with the result that HIF-dependent gene transcription increases, thus promoting angiogenesis and tumor growth. Because HIF-1α prolyl hydroxylase is stimulated by ascorbic acid [24], low vitamin C levels would reduce HIF-1α hydroxylation and thus stabilize HIF-1α, thereby promoting HIF-dependent gene transcription and tumor growth. Kuiper et al. [25] investigated the impact of ascorbate levels in endometrial tumors, obtained from women undergoing hysterectomy, on HIF-1 activity and tumor pathology. They found lower ascorbate and increased HIF-1α protein levels in more aggressive tumor tissue (endometrioid adenocarcinoma) compared to less aggressive tumor tissue and normal tissue. A significant inverse correlation was observed between ascorbate levels in tumor tissue and markers of HIF-1 pathway activation such as VEGF, which, for the first time, supports the notion that adequate vitamin C levels inhibit tumor progression in humans through inhibition of the HIF-1 pathway [25].
Under normoxic conditions, HIF-1 can be induced by transition metal ions such as CoII, NiII and CrVI [26, 27]. The mechanism by with CoII and NiII activate the HIF-1 pathway is due to cellular depletion of ascorbate by metal-ion catalyzed air oxidation. It has also been suggested that NiII can inactivate prolyl hydroxylase by substitution of enzyme-bound FeII. CrVI showed a transient effect on HIF-1α stability and HIF-1 activity in cultured lung epithelial (1HAEo and A549) cells, which Kaczmarek et al. [27] attributed to ascorbate-mediated conversion of CrVI into inactive CrIII, allowing recovery of cellular ascorbate and restoration of HIF prolyl hydroxylase activity. These authors emphasized the importance of ascorbate levels in the lung to protect against toxicity of metal ions through activation of the HIF pathway.
A potent inducer of HIF-1 activity in vascular smooth muscle cells is angiotensin II, which was shown by Pagé et al. [28] to inhibit hydroxylation of proline 402 in HIF-1α. The mechanism was determined to be angiotensin II-mediated generation of hydrogen peroxide (H2O2) and depletion of cellular ascorbate. Alternatively or additionally, H2O2 and other ROS may also interfere with HIF prolyl hydroxylase by decreasing FeII in the catalytic site [29].
Ascorbic acid has long been recognized as a key player in the ability of neutrophils to kill bacteria. In the presence of bacteria, ascorbate levels in neutrophils increase by up to 30-fold due to uptake of dehydroascorbic acid [30]. The accumulation of ascorbate in neutrophils is thought to protect these cells against damage by ROS that they produce [30]. As NADPH oxidase is the predominant source of ROS in neutrophils, it is conceivable that the protective effect of ascorbate is in part due to prevention of protein damage from reaction with ROS/lipid-derived 2-alkenals [31, 32]. In addition, ascorbate augments NO-mediated generation of ROS in polymorphonuclear leukocytes [33] and prolongs neutrophil NOS expression, NOS catalysis, and oxidative burst [34]. A moderate increase in NO itself leads to a decrease in HIF-1α accumulation [35, 36]. Neutrophil apoptosis and clearance, a normal physiological process in the resolution of inflammation, appears to be regulated by ascorbate through suppression of the HIF pathway [37]. The positive effects of vitamin C on neutrophil function and clearance could provide a rationale for vitamin C supplementation in individuals with low vitamin C status, e.g. in hospitalized, elderly patients who are at enhanced risk for being infected with bacteria.
Vitamin C and pre-eclampsia
Pre-eclampsia is a complication that develops in about 5% of pregnant women during the second half of gestation. The syndrome is characterized by hypertension and proteinuria. Reduced placental perfusion has been identified as a causal factor and has been associated with endothelial activation [38]. Several authors have hypothesized that oxidative stress and lipid peroxidation (LPO) play an important role in the development of pre-eclampsia [39, 40]. A recent systematic review and meta-analysis conducted by Gupta et al. [41] revealed increased levels of serum malondialdehyde, increased total serum thiobarbituric acid-reactive substances (TBARS), marginally decreased erythrocyte superoxide dismutase (SOD), and decreased serum vitamin C and E levels in pre-eclampsia cases compared to controls. The authors concluded that pre-eclampsia is associated with increased oxidative stress and decreased antioxidant vitamin levels [41]. Such findings have provided the rationale for a number of antioxidant trials aimed at reducing the rate and severity of pre-eclampsia. Supplementation with vitamin C and E proved a beneficial effect on the rate of pre-eclampsia in a randomized, placebo controlled trial with 283 women at risk [42]. Subsequent larger trials showed no beneficial effects of vitamin C and E supplementation on the rate of pre-eclampsia [43-49].
McCance et al. [50] studied the effect of vitamin C (1000 mg/day) and E (400 IU/day) supplementation in 379 pregnant women with type 1 diabetes, who were at higher risk for developing pre-eclampsia, presumably due to increased oxidative stress (DAPIT trial). These authors found no effect of vitamins C and E on the primary endpoint, pre-eclampsia, compared to 382 placebo-treated women (p = 0.20). However, the supplemented women had fewer preterm births (< 37 weeks, p = 0.046). In subgroup analysis, vitamin supplementation showed an effect on pre-eclampsia in two of 11 women with low antioxidant status at baseline. The authors concluded that vitamin supplementation may be beneficial in pregnant women with low antioxidant status. According to the authors, negative outcomes in previous vitamins C and E supplementation trials may be attributed to adequate vitamin C and E status of the women at baseline [50]. As pointed out by Talaulikar [51], the DAPIT supplementation study did not include measures of oxidative stress in order to determine whether the administered vitamin doses were effective in reducing oxidative stress.
A multi-center, randomized, double-masked, placebo-controlled vitamin C (1000 mg) and vitamin E (400 IU) supplementation trial conducted in the U.S. with 9,968 low-risk nulliparous women showed no effect in the prevention of spontaneous preterm birth at less than 37 and 35 weeks of gestation [52]. Preterm births due to premature rupture of membranes were less frequent before 32 weeks of gestation (0.3% vs. 0.6% adjusted OR 0.3-0.9) [52]. A Canadian study of the effects of vitamin C (1000 mg) plus E supplementation study (400 IU) with 2,363 women failed to reduce the rate of pre-eclampsia or gestational hypertension, but did increase the risk of fetal loss or perinatal death and premature rupture of membranes (INTAPP trial, [49]). A similar study conducted by Villar et al. [44] showed no effect of vitamin C (1000 mg/day) and E (400 IU/day) supplementation on pre-eclampsia, eclampsia, low birthweight, and perinatal death in at-risk pregnant women with low nutritional status from India, Peru, South Africa, and Vietnam (WHO trial). In this latter study, any effect of vitamin supplementation in women with low vitamin status may have remained undetected due to lack of measurements of vitamin levels and poor compliance [44]. Another study conducted in India with 140 normotensive pregnant women, however, revealed that preterm births were associated with oxidative stress, measured as malondialdehyde in maternal and in cord blood (p < 0.05), and with elevated vitamin C concentrations (p < 0.05) compared to at-term births [53].
Taken together, the majority of studies has failed to support the earlier stated hypothesis that pre-eclampsia and preterm births can be prevented by vitamin C or by combined vitamin C and E supplementation, despite the well-documented relationship between pre-eclampsia / preterm birth and oxidative stress. A mechanistic study into the effects of vitamins C and E on trophoblast apoptosis and authophagy, a common feature in placentas from pregnancies complicated by pre-eclampsia that results in impaired circulation, was conducted by Hung et al. [54] to shed light on the clinical findings. Using cultured trophoblasts and villous explants obtained from human term placentas, the authors observed reduced apoptosis and autophagy in trophoblasts exposed to 50 μM vitamin C and 50 μM vitamin E under normoxic conditions for 48 h. By contrast, they observed enhanced apoptosis and autophagy when the vitamin-supplemented cells were subjected to two cycles of hypoxia (8 h) and reoxygenation (16 h). The authors concluded that concomitant administration of vitamins C and E has differential effects on apoptosis and autophagy in placental cells under normoxia compared to hypoxia-reoxygenation, which may explain the adverse effects of vitamin C supplementation on placental function found in some of the clinical studies. Another explanation for the results from inconclusive supplementation trials was provided by Talaulikar and Manyonda [55] who argued in a reaction to the INTAPP publication (ref. [49]) that oxidative stress is unlikely to be the cause of defective trophoblast invasion which plays a key role in the pathophysiology of pre-eclampsia.
Scurvy in the elderly and in hospitalized patients
Scurvy is rare in the general population but is still prevalent today in populations at risk. In addition to poor diet, alcoholism [56], elderly age, socioeconomic deprivation [57], mental illness [58], malabsorption disorders, kidney failure, hemodialysis [59], and peritoneal dialysis [60] have been identified as risk factors for low vitamin C status and developing clinical symptoms of scurvy [61-63]. In a geriatric hospital in Paris, France, 18 elderly patients (12%), who showed clinical symptoms of scurvy, had low serum levels of ascorbic acid compared to control patients (6.2 ± 6.0 μM versus 28 ± 24 μM, p < 0.001) [64]. These data suggest that vitamin C status should be checked routinely in elderly patients admitted to geriatric institutions.
Plasma vitamin C levels can decrease rapidly as a result of acute inflammation produced by sepsis [4], myocardial infarction, cancer, medication, surgery [65] or use of a cardiopulmonary bypass machine [66]. Alexandrescu et al. [67] described a case of a 50 year old man who was treated for metastatic renal-cell carcinoma with high-dose interleukin-2 (IL-2), an inflammatory cytokine, and consequently developed acute symptoms of scurvy: petechiae and perifollicular hemorrhage on the arms and legs, and gingival bleeding. The patient's serum vitamin C decreased from 17 μM to 6 μM following treatment with IL-2, demonstrating that ‘symptoms of scurvy occur acutely whenever a state of chronic depletion of vitamin C reaches a critical threshold [67]’. The patient recovered from the clinical symptoms of acute scurvy after seven days of discontinuation of IL-2 treatment. The effect of IL-2 treatment on vitamin C status was earlier described by Marcus et al. [68, 69].
Vitamin C as an anti-oxidant
Role of vitamin C in lipid peroxidation
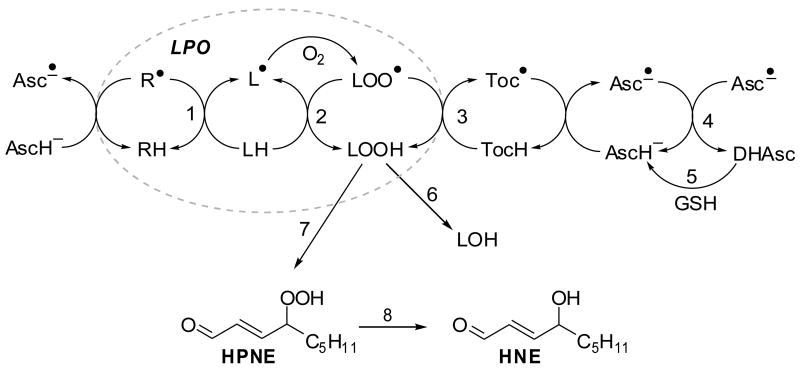
Lipid peroxidation (LPO) can be considered as an example of a radical chain reaction (Figure 2). Reactive oxygen species (ROS) produced by a variety of sources, such as the electron transport chain, xanthine oxidase, myeloperoxidase and NADPH oxidase, initiate the radical reaction through abstraction of hydrogen atoms from bisallylic C–H bonds thereby forming lipid radicals [12]. Lipids are often prime targets of oxygen radicals because many of the enzymes producing ROS are embedded in lipid bilayers and because the bisallylic C–H bond in polyunsaturated fatty acids (PUFAs) is relatively weak compared to other C–H bonds. Carbon-centered lipid radicals react with molecular oxygen to form peroxyl radicals that, if not neutralized by α-tocopherol in membranes, may participate in the radical propagation reaction. Lipid hydroperoxides are chemically unstable and, when not reduced by glutathione-dependent reductases to hydroxy-fatty acids, constitute a source of a variety of LPO products, including 2-alkenals, epoxides, and malondialdehyde. Vitamin C has the ability to protect against LPO by acting as a scavenger of ROS and by one-electron reduction of lipid hydroperoxyl radicals via the vitamin E redox cycle [12]. Furthermore, findings from our laboratory support a role for vitamin C in protection against cellular damage from LPO-derived 2-alkenals. Vitamin C-adequate cultured human THP-1 cells exposed to the LPO product, 4-hydroxy-2(E)-nonenal (HNE) showed a significant reduction in protein carbonylation compared to THP-1 cells that were not pre-incubated with vitamin C [31, 32]. The protective effects of ascorbate were associated with an increase in the formation of GSH-HNE conjugate and its phase I metabolites, measured by LC-MS/MS, and with increased transport of GSH conjugates from the cells into the medium [31].
Fig. 2.
Antioxidant effects of vitamins C and E on lipid peroxidation (LPO). The LPO chain reaction can be initiated by many radical species (indicated by R•) and converts LH into LOO•, which attacks another LH generating L• (paths 1 and 2, dotted oval). Ascorbic acid may scavenge the initiating radical species R• and reduce the tocopheroxyl radical, generating the ascorbyl radical, which can be reduced by glutathione dependent enzymes. Key to reaction steps: 1, initiating event; 2, radical propagation reaction; 3, termination of the radical reaction by tocopherol (TocH); 4, dismutation of ascorbyl radicals (Asc•–); 5, reduction of dehydroascorbate (DHAsc) by GSH-dependent dehydroascorbate reductase; 6, GSH peroxidase (GPx); 7, further oxygenation and non-enzymatic cleavage of carbon-carbon bonds yields 4-hydroperoxy-2(E)-nonenal (HPNE); 8, reduction yields 4-hydroxy-2(E)-nonenal (HNE).
Some authors have reported that vitamin C promotes the formation of LPO products from lipid hydroperoxides in vitro, even in the absence of catalytic iron [70]. The in vivo implications of a vitamin C-dependent conversion of lipid hydroperoxides into reactive 2-alkenals have been disputed by others who have argued that redox-active Fe2+ ions, considered necessary for homolytic cleavage of hydroperoxides via Fenton chemistry, are not freely available under physiological conditions [71]. We are not aware of any convincing evidence supporting the notion that vitamin C promotes formation of toxic 2-alkenals in vivo.
LPO contributes to the development and progression of chronic diseases with an inflammatory component. Biomarkers of oxidative stress that are derived from LPO, such as F2-isoprostanes, HNE, and malondialdehyde, are often elevated in plasma, urine, or tissues obtained from patients diagnosed with cardiovascular diseases [72], neurodegenerative disease [72, 73], and diabetes [74]. These observations raise the question whether there is a causal relationship between oxidative stress and disease. If so, antioxidant therapy should prove beneficial in chronic inflammatory disease. Indeed, many clinical studies have been conducted using vitamins C to improve biomarker levels and clinical outcomes (reviewed in [75]). Disappointing results of antioxidant therapy in a variety of trials (see [76-78]) led some critics to hypothesize that antioxidant therapy may not be effective to provide secondary prevention in advanced stages of cardiovascular disease [79]. In a 2002 review of prospective studies on vitamin C status and cardiovascular disease, Loria [80] noted that outcomes were more likely to be positive when serum vitamin C concentrations were used to assess vitamin C status as opposed to vitamin C intake and when a wider definition of cardiovascular disease was used with inclusion of endpoints related and unrelated to oxidative stress. Others have argued that ‘overdosing’ with antioxidants proves ineffective in reducing oxidative stress due to suppression of normal physiological response systems following an oxidative stress insult, for example, physical exercise [81]. Our research group has investigated the effect of vitamin C supplementation (500 mg twice daily for 17 days) on urinary levels of metabolites of 4-hydroperoxy-2(E)-nonenal, as an indicator of oxidative stress [82, 83], in a double-blind, placebo-controlled, randomized crossover study in 22 young adults. Vitamin C supplementation was found to decrease the urinary concentrations of these LPO product metabolites by 20-30% [84], in support of the notion that vitamin C supplementation exerts antioxidant effects and reduces oxidative stress in vivo.
Harrison and May [85] have recently reviewed the cellular uptake, recycling, and neuroprotective functions of vitamin C in the brain. Tveden-Nyborg and Lykkesfeldt [86] suggested that the neonatal brain is particularly susceptible to low vitamin C concentrations and that vitamin C deficiency may adversely affect early brain development. A similar conclusion was reached by Harrison et al. [87] who studied the relationship between vitamin C deficiency and oxidative stress during development in Gulo–/– mice. The authors found that Gulo–/– mice pups had low ascorbic acid levels with accompanying elevations in liver malondialdehyde and brain F2-isoprostanes, both biomarkers of oxidative stress. The authors emphasized the critical role of ascorbic acid in preventing oxidative stress in the developing brain in animals that, like humans, cannot synthesize their own ascorbic acid.
Vitamin C, endothelial dysfunction and cardiovascular disease
Elevated levels of circulating low-density lipoprotein (LDL) constitute a risk factor for the development of cardiovascular disease. Uptake of LDL in peripheral tissues is restricted by LDL receptors but not when LDL is oxidatively modified by LPO-derived reactive aldehydes. Oxidized LDL can be taken up by macrophages residing in the vascular wall through scavenger receptors in an uncontrolled manner, leading to accumulation of oxidized LDL and cholesterol in these vascular macrophages and to their transformation into foam cells. Thus, oxidized LDL is more atherogenic than undamaged LDL. Furthermore, oxidized LDL induces cytokine production in monocytes, facilitating their transformation into macrophages, and induces expression of cell adhesion molecules in vascular endothelial cells, leading to recruitment of monocyte-macrophages to the vascular wall. Taken together, LPO and LDL oxidation play key roles in the early stages of vascular dysfunction and atherosclerosis which has become known as the ‘oxidative modification hypothesis of atherosclerosis [88-91].’ This hypothesis provides a rationale for antioxidant therapy to reduce LDL oxidation and oxidative stress-induced vascular dysfunction. Although several antioxidant trials have demonstrated beneficial effects on cardiovascular disease, the majority of the trials failed to show beneficial effects on clinical outcomes or manifested adverse effects [77, 92-96].
In a randomized, placebo-controlled study of 70 patients with cardiovascular risk factors, Shargorodsky et al. [97] demonstrated beneficial effects of combined antioxidant supplementation for 6 months with vitamin C (1000 mg/d), vitamin E (400 IU/d), coenzyme Q10 (120 mg/d) and selenium (200 μg/d) on glucose and lipid metabolism, blood pressure, and arterial elasticity. The authors observed significant effects of antioxidant treatment on systolic blood pressure (p = 0.001), diastolic blood pressure (p = 0.034), large and small arterial elasticity (p = 0.006 and 0.0001), HbA1C (p = 0.022), and HDL (p = 0.022). The improvement of cardiovascular function could not be attributed to changes in blood pressure modulators, because the treatment and placebo groups did not differ with respect to plasma renin, aldosterone, and urinary catecholamines. The authors stated that they have no mechanistic explanations for the observed effects [97]. The study did not include markers of acute or chronic oxidative stress nor did it include measurements of plasma levels of the antioxidants, which makes it difficult to assess the efficacy of the treatment in reducing oxidative stress and LPO.
Vitamin C and nitric oxide bioactivity
Another mediator of vascular function to consider is nitric oxide (NO). Its bioactivity as a vasodilating factor depends on its eNOS-mediated production from arginine and its deactivation by superoxide. The activity of NADPH oxidase, a major source of superoxide in vascular smooth muscle cells and cardiac myocytes [98], therefore inversely affects NO bioactivity. Consumption of low-salt diets indirectly affects NO bioactivity because low-salt levels activate the renin-angiotensin-aldosterone system and the resultant increase in angiotensin II activates NADPH oxidase through agonism of the angiotensin II type 1 (AT1) receptor [98]. Suematsu et al. [99] studied the effects of low-salt diet on NO bioactivity, cardiac function, and mortality in adult male mongrel dogs. They observed that NO-mediated coronary vasodilation (induced by bolus injection of 5 μg/kg veratrine) was inhibited by 44% (p < 0.05) in dogs on a low-salt diet, which was attributed to increased NADPH oxidase activity and superoxide production. This inhibitory effect was completely reversed by intravenous infusion of vitamin C (2000 mg bolus, followed by constant infusion at 25 mg/min for 120 min), or by apocynin (10 mg/kg for 120 min), an inhibitor of NADPH oxidase [100].
NO bioactivity is also impaired in hypertensive patients with a nondipper circadian pattern (i.e., blood pressure remains high at nighttime), which presents a risk factor for cerebrovascular and cardiovascular events. Maio et al. [101] studied the effect of intra-arterial infusion of vitamin C on forearm blood flow response to acetylcholine, an NO-dependent vasodilator. They found that forearm blood flow following acetylcholine administration improved to a greater extent in nondipper patients that were co-treated with vitamin C, supporting the hypothesis that vitamin C improves endothelial function in hypertensive patients with impaired NO physiology [101].
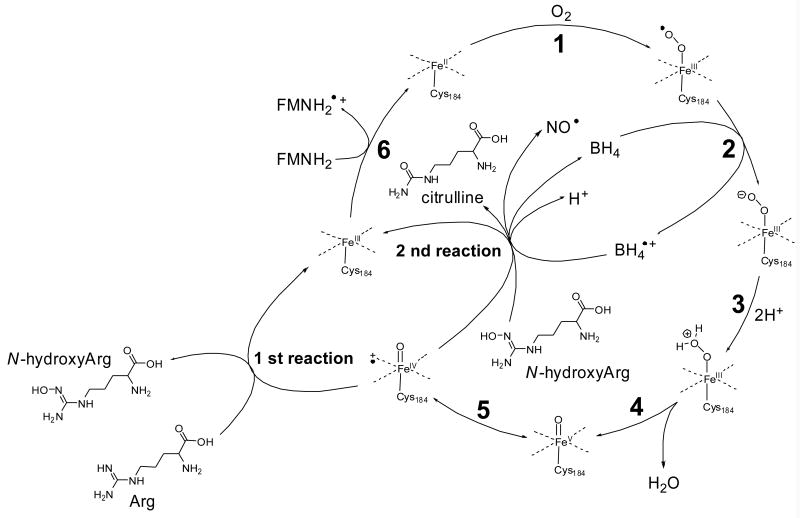
There are several possible mechanistic explanations for the observed beneficial effects of systemically administered vitamin C on endothelial function [77, 102, 103]. As stated above, one explanation is that vitamin C eliminates superoxide intracellularly [12, 103, 104] that would otherwise deactivate NO by forming peroxynitrite [105]. NADPH oxidase is a prominent source of superoxide in vascular cells, but eNOS itself can produce superoxide by the oxygenase component of the enzyme when eNOS becomes uncoupled in the absence of the substrates, arginine and N-hydroxyarginine, and its redox cofactor tetrahydrobiopterin (BH4) [106-108]. In coupled reaction cycles, BH4 donates an electron to the heme-bound FeIII-oxo complex thereby forming protonated BH3• (BH4•+) in the pathway to the formation of the reactive heme-FeIV-oxo species. BH4•+ may recycle back to BH4 during the conversion of the intermediate reaction product, N-hydroxyarginine, into citrulline and NO [109, 110] (Figure 3). In solution, BH4 is unstable and readily auto-oxidizes to form quinonoid dihydrobiopterin (qBH2) and ROS [111]. Stoll et al. [112] demonstrated that NOS stabilizes the protonated BH4•+ radical (to prevent it from autoxidation) and maintains proper one-electron redox cycling of BH4 in coupled NOS-mediated reaction cycles. In uncoupled reactions, NOS generates superoxide and other ROS that decrease NOS activity [113].
Fig. 3.
Proposed mechanism of NO biosynthesis by NOS, a heme-containing flavo-enzyme (adapted from [109]). In the first reaction, arginine is hydroxylated to form Nω-hydroxyarginine, which is oxidatively converted in the second reaction into NO and citrulline. The oxygen in both reactions originates from a heme FeIV=O species, which is formed by oxygenation of heme-FeII, acceptance of an electron from tetrahydrobiopterin (BH4), protonation and loss of a water molecule (steps 1-4; in step 5, the FeIV-oxo complex arises from the FeV-oxo complex by electron transfer from a ligand nitrogen atom to iron. NO is released in the second reaction via an intermediate FeIII-NO complex. The resulting heme-FeIII species is reduced back to heme-FeII by the flavoprotein domain (step 6). In endothelial NOS, the heme-iron is bound to Cys184 of the enzyme [220].
The effect of vitamin C on NO bioavailability has been attributed to its ability to prevent eNOS-related superoxide production [103, 114]. The mechanism of the interaction of vitamin C with the enzyme complex or co-factors is not entirely known and perhaps there are multiple mechanisms. Vitamin C may prevent BH4 from being oxidized by ROS (or even molecular oxygen [111]) by acting as a scavenger of ROS in cells, or vitamin C may directly reduce oxidized intermediates such as BH3• [104, 115]. Unlike thiols such as glutathione, vitamin C is unable to reduce qBH2 to BH4 but it can convert BH3• radical into BH4 with formation of ascorbyl radical [104, 115, 116]. It is also conceivable that ascorbate re-activates uncoupled NOS by reduction of heme iron-oxo species to the resting heme-FeII state, similar to the function of ascorbate in uncoupled prolyl hydroxylase (Figure 1).
BH4 and also vitamin C have been shown by Garry et al. [117] to enhance acetylcholine-induced relaxation of rabbit artery rings, in a way that is endothelium-dependent but NO-independent. Furthermore, the effect disappeared in the presence of catalase and could be mimicked by exogenous H2O2, from which the investigators concluded that the effect was due to the formation of H2O2 produced from molecular oxygen and BH4 and vitamin C, perhaps in the presence of catalytic metal ions [118]. It had previously been shown that H2O2 potentiates (rather than inhibits as expected for an NO-dependent mechanism) vascular relaxation induced by acetylcholine through enhanced Ca2+ mobilization and opening of hyperpolarizing endothelial KCa channels, a phenomenon known as the ‘endothelium-derived hyperpolarizing factor (EDHF)-type’ relaxation [119, 120]. Concentrations of H2O2 following systemic administration of vitamin C or BH4 may reach pharmacologically relevant levels in the interstitial fluid where H2O2 is not degraded by glutathione peroxidase and catalase. Garry et al. [117] hypothesized that H2O2-induced relaxation of the EDHF type may represent a compensatory mechanism to compensate for reduced NO bioavailability.
Vitamin C participates in addition reactions
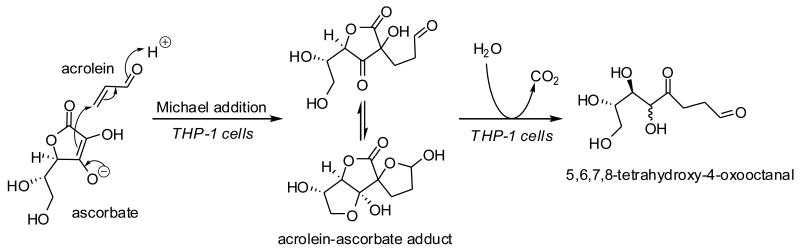
The ascorbate anion form of vitamin C is an enolate capable of forming Michael adducts in aqueous solutions at physiological pH [121]. There are many examples of covalent adducts of vitamin C, including Michael adducts, with natural products in the plant kingdom, of which ascorbigen is most well known [122]. The reaction of ascorbate with acrolein (2-propenal), a ubiquitous electrophile originating from LPO and combustion of organic matter [123], and with other α,β-unsaturated aldehydes/ketones has been reported in the organic chemistry literature [121, 124]. The participation of ascorbate in 2-electron reactions in vitro and in plants raises the question whether vitamin C plays a role in the detoxification of 2-alkenals in the animal kingdom. Our laboratory has studied the covalent interaction between acrolein and ascorbic acid in cultured human THP-1 monocytes [125]. We found that intracellularly ascorbate forms a Michael adduct with acrolein, which is rapidly metabolized via hydrolysis of the ascorbyl lactone and decarboxylation (Figure 4). This metabolic degradation pathway is similar to degradation of ascorbigen at pH > 7 [126]. We further found that paraoxonases 1 and 2, which are known to possess lactonase activity [127, 128], catalyze hydrolysis of the ascorbyl lactone moiety of the ascorbate–acrolein adduct [125]. The extent and biological relevance of 2-electron reactions involving ascorbate remains to be demonstrated.
Fig. 4.
Michael adduction of acrolein with ascorbate and subsequent metabolism of the acrolein– ascorbate adduct in cultured human THP-1 monocytes.
Is there a health benefit associated with vitamin C supplements?
Many authors, including the discoverer of vitamin C, Albert Szent-Györgyi (see [129, 130]), and Linus Pauling [131], have pointed out that there is a large difference between vitamin C status causing scurvy symptoms and vitamin C status required for maintaining optimum health. The large body of research on vitamin C suggests that vitamin C supplementation may provide health benefits beyond prevention of scurvy [75, 130]. The current recommended dietary allowance (RDA) for vitamin C for adult men and women, set at 75 mg/day for women and 90 mg/day for men [132], is sufficient to prevent scurvy and was based on the amount needed to protect leukocytes from oxidative burst. Carr and Frei [75] proposed in 1999 that the RDA be adjusted to 120 mg/day to reduce the risk of cardiovascular disease and cancer. They based their conclusions on data from four dozen prospective cohort studies, case-control studies or cross-sectional studies on the relationship between vitamin C intake, plasma concentrations of vitamin C and clinical endpoints related to cardiovascular disease and cancer. Higher doses of vitamin C (500 mg/day) may be required to achieve vasodilation and decrease of blood pressure [79, 133]. Kris-Etherton et al. [92] stated their opinion that the evidence from human studies is not sufficiently compelling to advise individuals, healthy or at risk (e.g., dialysis patients), to take antioxidant supplements to reduce the risk of cardiovascular disease.
Vitamin E
α-Tocopherol is a required nutrient for humans because it is necessary for the prevention of vitamin E deficiency symptoms, including peripheral neuropathy and hemolytic anemia. Vitamin E is a potent lipid-soluble, chain breaking antioxidant.
Vitamin E form and function
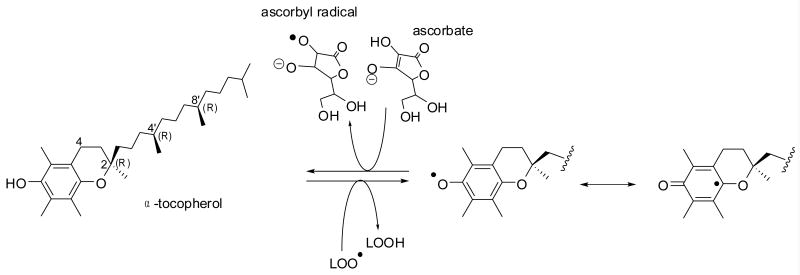
The naturally occurring form of α-tocopherol is RRR-α-tocopherol [132] (Figure 5). This nomenclature means that the chiral carbons are in the R-configuration at positions 2, 4′ and 8′. The 2 position of α-tocopherol determines α-tocopherol biologic activity. Only 2R-α-tocopherol forms meet human vitamin E requirements [132]. Synthetic α–tocopherol is called all-rac-α-tocopherol (all racemic, or dl) and contains an equal mixture of eight different stereoisomers (RRR, RSR, RRS, RSS, SRR, SSR, SRS, SSS), which differ in the configuration of the side chain. All of the stereoisomers have equal antioxidant activities, but only those in the 2R-configuration have high biologic activity.
Fig. 5.
Antioxidant effect of vitamin E. α-Tocopherol reacts with a lipid hydroperoxyl (LOO•) radical. The resultant tocopheryl radical is resonance stabilized, does not react with oxygen (unlike L• radicals) and it can be converted back to α-tocopherol by ascorbate.
Vitamin E, a potent peroxyl radical scavenger, is a chain-breaking antioxidant that prevents the propagation of free radicals in membranes and in plasma lipoproteins [134]. When peroxyl radicals (ROO•) are formed, these react 1000-times faster with vitamin E (Vit E-OH) than with polyunsaturated fatty acids (PUFA) [135]. The hydroxyl group of tocopherol reacts with the peroxyl radical to form the corresponding lipid hydroperoxide and the tocopheryl radical (Vit E-O•). The tocopheryl radical (Vit E-O•) reacts with vitamin C (or other hydrogen donors, AH), thereby oxidizing the latter and returning vitamin E to its reduced state (Figs. 2 and 5) [136]. The function of tocopherol and other tocochromanols, as potent antioxidants in Arabidopsis seedlings, was recently demonstrated by Mène-Saffrané et al. [137], who found that Arabidopsis mutants incapable of biosynthesizing tocochromanols exhibited a severe seedling developmental phenotype associated with massive LPO.
The interaction of vitamins E and C has led to the idea of “vitamin E recycling”, where the antioxidant function of oxidized vitamin E is continuously restored by other antioxidants. This “antioxidant network” depends upon the supply of aqueous antioxidants and the metabolic activity of cells. It should be noted that free metals, such as iron or copper, can re-initiate LPO by reaction with ROOH to form an alkoxy radical. Additionally, if other antioxidants are not present Vit E-O• can re-initiate LPO [138].
Since the tocopheroxyl radical can be reduced back to tocopherol by ascorbate or other reducing agents, oxidized tocopherols are usually not found in vivo. Biologically relevant oxidation products formed from α–tocopherol include 4a,5-epoxy- and 7,8-epoxy-8a(hydroperoxy)tocopherones and their respective hydrolysis products, 2,3-epoxy-tocopherol quinone and 5,6-epoxy-α–tocopherol quinone [139]. These products have been demonstrated during in vitro oxidation; their importance in vivo is unknown [140].
Vitamin E Deficiency Symptoms
Symptoms of vitamin E deficiency suggest that its antioxidant properties play a major role in protecting membranes and nervous tissues from oxidative stress. Clinical deficiency of vitamin E in humans causes varying degrees of hemolysis depending on the subject's age and status of other antioxidants, oxidative stress and PUFAs. For example, pediatric patients with cystic fibrosis and vitamin E deficiency caused by fat malabsorption also develop hemolytic anemia [141, 142].
The primary, and most serious, clinical manifestation of human vitamin E deficiency is a peripheral neuropathy with degeneration of large-caliber axons in sensory neurons [143-146]. The development of a progressive ataxia appears more rapidly in infants and young children with malabsorption from birth than in adults, suggesting that the developing nervous system is dependent on adequate vitamin E for normal development [147].
Vitamin E also appears to be essential in preserving immunologic function in elderly individuals [148], especially concerning the immune synapse among CD4+ T-lymphocytes that is important for T-cell signaling and immune function [149]. Both animal [150, 151] and human studies [148] suggest that relative vitamin E deficiency enhances and vitamin E supplementation prevents aging associated reductions in immune function. However, some studies suggest that vitamin E supplementation in the elderly may not be as protective against lower respiratory tract infectious illness as previously postulated [152].
Vitamin E Antioxidant Effects in Humans
The evidence in humans that α-tocopherol functions as a fat-soluble antioxidant in response to oxidative stress is limited. Oxidative stress caused by ultramarathon running has been shown to increase rates of plasma vitamin E disappearance in humans [153]. Moreover, prior vitamin C and E supplementation decreased markers of LPO in runners [154]. Because cigarette smoking generates free radicals [155], α-tocopherol kinetics in smokers was compared with non-smokers. Cigarette smokers had greater LPO (F2-isoprostane concentrations) and plasma α-tocopherol disappearance rates [156]. Importantly, the rates were inversely correlated with vitamin C status; vitamin E disappearance was faster in smokers with low plasma ascorbic acid concentrations [156]. When smokers were supplemented with vitamin C (500 mg twice daily) for two weeks, α-tocopherol disappearance rates were normalized to rates observed in non-smokers (with or without vitamin C supplements) [157]. Thus, in smokers with greater oxidative stress, additional vitamin C is needed to restore the α-tocopheroxyl radical to its reduced form and thus protect vitamin E concentrations. Importantly, there were no significant effects of vitamin C supplementation on F2-isoprostane concentrations [157, 158]. Thus, vitamin E does not prevent radical formation, or the initial oxidation of fatty acids, but vitamin E stops the LPO chain reaction. The studies in smokers suggest that vitamin E requirements are dependent upon both oxidative stress status and vitamin C status. It is unclear whether the oxidative stress caused by cigarette smoking is equivalent to other forms of oxidative stress.
Obesity is an inflammatory disease associated with increased F2-isoprostanes [159]. Obese children also have been reported to have increased circulating F2-isoprostanes [160]. Obese subjects with diabetes have an even greater degree of oxidative stress, since type II diabetics have higher levels of circulating F2-isoprostanes than do normal subjects [161] and these LPO biomarkers further increase in during bouts of hyperglycemia [162]. Consistent with observations in smokers [157, 158], vitamin C supplementation (1.5 g daily) in Type II diabetics for 3 weeks did not improve LPO biomarkers [163]. Thus, vitamin E is needed to prevent LPO, while one of the roles of vitamin C is to regenerate vitamin E.
α-Tocopherol Pharmacokinetics and Bioavailability
Vitamin E is fat soluble, transported by lipoproteins, and is dependent upon lipid and lipoprotein metabolism for tissue vitamin E delivery [164]. Its tissue depletion takes decades rather than weeks [164]. Importantly, no tissue serves as a vitamin E store, releasing α-tocopherol on demand. However, plasma α-tocopherol concentrations are regulated by the liver, specifically by the α-tocopherol transfer protein (α-TTP), as well as by metabolism and excretion [164].
Some information is known about vitamin E pharmacokinetics using orally administered deuterium-labeled vitamin E(s) [153, 165-184]. Labeled vitamin E absorption and disposition has been characterized in short-term studies (from 3 to 72 h) and in longer protocols to assess delivery to peripheral tissues by administering large oral doses (e.g. 400 IU) [171]. Various tocopherols are absorbed, transported in chylomicrons but only α–tocopherol is maintained in plasma and tissues [166, 185]. The plasma half-life of RRR-α-tocopherol is 48 to 60 h [183, 186], while that of SRR-α-tocopherol (synthetic form) is 15 h [186]. α-Tocopherol recirculation from the liver to the plasma is critical for this long half-life [186], and is dependent upon hepatic α-TTP [167, 187]. The rapid recirculation between the liver and plasma results in the daily replacement of nearly the entire circulating α-tocopherol pool [186].
There are no definitive studies quantitating α-tocopherol absorption in humans. Studies from 1960-70 using radioactive α-tocopherol in humans reported fractional vitamin E absorption in normal subjects ranging from 55 to 79% [188, 189]. These were balance studies and depended upon the complete collection of fecal material; any losses of labeled material result in increased apparent absorption. In contrast, more recent studies using deuterium labeled α-tocopherol, but based on observed plasma labeled α-tocopherol concentrations, absorption in humans was only 33% [184]. These data emphasize our lack of knowledge concerning vitamin E absorption. This measure is important in that the amount of vitamin E that must be consumed will vary depending on absorption efficiency.
Is There A Health Benefit Associated With Optimal α-Tocopherol Intakes?
The 2000 recommended dietary allowance (RDA) for vitamin E (15 mg (22 IU RRR-) α-tocopherol) was defined in the Dietary Reference Intakes (DRIs) by the Food and Nutrition Board, Institute of Medicine [132]. However, 96% of American women and 93% of men do not meet the current vitamin E recommendations [190]; mean dietary intakes in the US are only ∼6 mg α-tocopherol [191]. Are recommendations too high or is dietary vitamin E consumption by most people too low? In general, to set an estimated dietary requirement (EAR), the amount of a nutrient needed to fulfill a specific biochemical function is estimated. Then, the amount needed from the diet is estimated based on the fractional absorption. Given that the biochemical function of vitamin E is its antioxidant activity, and the fractional absorption is not known, the 2000 vitamin E RDAs had to be set by correlation of antioxidant activity with dietary intakes. The only data available were studies conducted more than 50 years ago [132]. The RDA was based on these measures because these were the only available data despite the general agreement that the hemolysis assay is highly dependent on assay conditions, and that no LPO measures have been described that are specific solely for vitamin E [132].
The Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) study tested in Finnish smokers whether supplementation for 5 y with vitamin E (50 IU dl-α-tocopheryl acetate) and β-carotene (20 mg) would decrease cancer incidence [192]. Although supplementation had no effect on mortality, a follow-up report [193] described baseline vitamin E status of the 29,092 Finnish men that were followed for 19 y during which time 13,380 deaths ensued. The men at baseline in the highest compared with the lowest quintiles of serum α-tocopherol had significantly lower incidences of total mortality {relative risk (RR)= 0.82 (95% CI: 0.78, 0.86)} and cause-specific mortality {cancer RR= 0.79 (0.72, 0.86), cardiovascular disease RR=0.81 (0.75, 0.88), and other causes RR=0.70 (0.63, 0.79); P for trend for each <0.0001}. A reduction in mortality occurred at serum α-tocopherol concentrations (30 μmol/L) associated with dietary intakes of about13 mg α-tocopherol [193], consistent with the vitamin E RDA in the 2000 DRIs [132]. Thus, a generous dietary intake of vitamin E over a lifetime apparently can decrease chronic disease incidence.
Is There A Health Benefit Associated With Vitamin E Supplements?
Studies of vitamin E effects on heart attack risk have resulted in conflicting outcomes: beneficial effects [194-196], limited effects [197], no benefit [198], and possible harm [199-201]. Meta-analysis of antioxidant-intervention trials in humans suggests that the doses of vitamin E supplements (400 or 800 IU) given in many clinical trials are not associated with adverse effects [202, 203] or are associated with increased risk of death [204, 205]. However, there have been no studies documenting the mechanism for adverse effects of vitamin E, other than its tendency to increase bleeding.
The Women's Health Study [206] tested the efficacy of vitamin E supplements to prevent heart disease or cancer in normal healthy women. They evaluated 600 IU vitamin E or placebo taken every other day for ten years by 40,000 healthy women aged 45 years and older. Overall, vitamin E supplements had no effect on the incidence of cancer, cardiovascular events or on total mortality. However, deaths from cardiovascular disease were reduced 24% {RR=0.76; 95% CI, 0.59-0.98; P=0.03}. “In subgroup analyses, women aged at least 65 years comprised 10% of study participants but contributed 31% of end points. A significant 26% reduction in major cardiovascular events was observed among women aged at least 65 years assigned to vitamin E {RR=0.74; 95%CI, 0.59-0.93; P=0.009} and 49% reduction in cardiovascular death {RR=0.51; 95% CI, 0.33-0.77; P<0.001} rates.” The decrease in cardiovascular death was attributed to a decrease in sudden death [206]. Given that the incidence of cardiovascular disease is quite low in women until they are over 65 y and that women lag behind men by 20 years with respect to sudden death (http://www.americanheart.org), these findings suggest that vitamin E supplements are effective in decreasing death from cardiovascular disease. It is not clear if supplements just insure that women consume “optimal amounts” of vitamin E (15 mg, as discussed above) or if other mechanisms are involved.
In contrast, studies in healthy physicians have shown no benefit of vitamin E supplements with regard to heart disease [77] or cancer, specifically prostate cancer [2, 207]. Apparently, α-tocopherol supplements are beneficial only if chronic diseases result, at least in part, from suboptimal protection by antioxidants. Importantly, the amounts of vitamin E that exerted beneficial effects in intervention studies are not achievable by dietary means, but it should be noted that dietary levels of vitamin E did have benefit in this regard when they were followed for a lifetime [193]. These latter findings also suggest that subjects in the placebo group who routinely consume dietary levels of 15 mg α-tocopherol are not likely to benefit from supplements and that the “healthy volunteer effect” [208] likely precludes finding a benefit for vitamin E in chronic disease prevention if the subjects are already well nourished with respect to α- tocopherol.
Boaz et al. [195] suggested that in the clinical trials where vitamin E has had benefit, the subjects consuming the placebo had higher incidences of cardiovascular disease and perhaps greater oxidative stress; examples include end-stage renal disease and heart transplant patients. Although the HOPE trial was the first of many randomized controlled intervention studies to show that vitamin E supplements given to high-risk patients did not decrease heart disease incidence [198], it is also the first example of vitamin E supplements having benefit in subjects with increased oxidative stress. Specifically, vitamin E supplementation decreased heart attack risk in subjects with demonstrable inadequate antioxidant protection due to impaired haptoglobin function. Sub-group analysis of diabetic patients in the HOPE trial who have the haptoglobin 2-2 (Hp 2-2) genotype had a “statistically significant reduction in the risk of CV death (0.45 [0.23–0.90]) and nonfatal myocardial infarction (0.57 [0.33–0.97])”, when they were given vitamin E supplements [209]. Importantly, Milman et al. [210], in a separate placebo-controlled study carried out only in Hp 2-2 diabetics, found that daily vitamin E supplementation (400 IU) reduced cardiovascular events.
It may be that the benefit arising from vitamin E supplementation in heart disease is not its antioxidant function, but as a result of the ability of vitamin E to interfere with clot formation by interfering with vitamin K status. This is an important function in the prevention of thrombosis, which can lead to heart attacks or stroke. Findings from the Women's Health Study showed that vitamin E supplementation significantly decreased venous thromboembolism by 21% [211]. This beneficial vitamin E effect may be due to interactions between vitamin E and vitamin K. Plant-derived, dietary vitamin K is phylloquinone (vitamin K1), which has a 20 carbon phytyl side chain, while menaquinones (MK), have multiple prenyl units, as indicated by their suffix number (i.e., MK-n) [212]. The liver is thought to convert phylloquinone to menadione, which is then used by extra-hepatic tissues for MK-4 synthesis [213]. Importantly, it appears that vitamin E interferes with this process because extrahepatic tissue vitamin K concentrations were lower in rats fed a high vitamin E diet [214]. Additionally, high-dose vitamin E supplementation (1000 IU) in humans decreased the degree of γ-carboxylation of prothrombin (proteins induced by vitamin K absence-factor II, PIVKA-II) [215]. Potentially, high dose vitamin E supplements increase liver α-tocopherol concentrations to the point that α-tocopherol competes with phylloquinone preventing the latter's hepatic transformation to menadione, and thus limiting MK-4 synthesis. Although vitamin E may limit the active form of vitamin K, this vitamin E action may be beneficial. Indeed, Glynn et al. [211] proposed that vitamin E supplements would be a safer alternative to warfarin in humans with increased risk of thrombosis. However, this vitamin E effect also brings up the potential risks. Supplemental α-tocopherol may increase bleeding tendencies. Additionally, vitamin E may potentiate the effects of aspirin with respect to blood clotting [216]. Certainly, the upper level set by the FNB of 1000 mg α-tocopherol (1100 IU dl- or 1500 IU d-α-tocopherol) should not be exceeded by supplement users [132].
Conclusions
Vitamin C exerts its biological effects primarily by acting as an enzyme cofactor and as an antioxidant. Other functions of vitamin C are under active investigation such as its role in brain development and cell signaling through production of hydrogen peroxide, and its participation in Michael addition reactions to form conjugates of electrophilic intermediates. As an essential nutrient, vitamin E functions as an anti-oxidant by scavenging lipid hydroperoxyl radicals. At high levels, vitamin E may compromise blood coagulation by interfering with vitamin K synthesis and/or redox cycling, which can be beneficial in some individuals but pose a health risk in others. Many vitamin C and E supplementation trials, conducted with the aim to reduce oxidative stress and LPO, have failed to show beneficial effects on clinical endpoints in medical disorders associated with increased levels of oxidative stress. Explanations for the apparent lack of effect include 1) no data on vitamin levels at baseline, 2) no data on biomarkers of oxidative stress and LPO, and 3) suboptimal doses and route of administration. Alternatively, the subjects may have consumed sufficient dietary antioxidants to counteract any potential benefits of supplements, or are relatively healthy with low levels of oxidative stress.
If disease progression proceeds faster at low vitamin status, then little effect is expected in patients with adequate vitamin levels at baseline. Measurement of vitamin levels would be most important in patients with lower baseline vitamin levels, notably elderly and hospitalized patients. Another important factor determining the success of a clinical trial is the choice of endpoints, especially those that are related to the targets of vitamin supplementation. Many of the larger antioxidant trials we reviewed did not include measurements of vitamin levels or measurements of biomarkers of oxidative stress. Such measurements would provide information on dose-effect relationships and prediction of effective dose regimens for definitive efficacy trials using biomarker and clinical endpoints.
Acknowledgments
We thank Dr. G.R. Buettner for suggestions for improvement of the manuscript and especially for help with Figure 1. MGT was supported in part by National Institutes of Health (NIH) Office of Dietary Supplements (ODS), DK 067930, DK081761 and by US Department of Agriculture (USDA) 2008-01875; JFS was supported in part by NIH HL081721.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gann PH. Randomized trials of antioxidant supplementation for cancer prevention: first bias, now chance--next, cause. Jama. 2009;301:102–103. doi: 10.1001/jama.2008.863. [DOI] [PubMed] [Google Scholar]
- 2.Gaziano JM, Glynn RJ, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Sesso HD, Buring JE. Vitamins E and C in the prevention of prostate and total cancer in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2009;301:52–62. doi: 10.1001/jama.2008.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Padayatty SJ, Sun AY, Chen Q, Espey MG, Drisko J, Levine M. Vitamin C: intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS One. 2010;5:e11414. doi: 10.1371/journal.pone.0011414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson JX. Mechanism of action of vitamin C in sepsis: ascorbate modulates redox signaling in endothelium. Biofactors. 2009;35:5–13. doi: 10.1002/biof.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butterfield DA, Bader Lange ML, Sultana R. Involvements of the lipid peroxidation product, HNE, in the pathogenesis and progression of Alzheimer's disease. Biochim Biophys Acta. 1801:924–929. doi: 10.1016/j.bbalip.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowman GL, Dodge H, Frei B, Calabrese C, Oken BS, Kaye JA, Quinn JF. Ascorbic acid and rates of cognitive decline in Alzheimer's disease. J Alzheimers Dis. 2009;16:93–98. doi: 10.3233/JAD-2009-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majamaa K, Gunzler V, Hanauske-Abel HM, Myllyla R, Kivirikko KI. Partial identity of the 2-oxoglutarate and ascorbate binding sites of prolyl 4-hydroxylase. J Biol Chem. 1986;261:7819–7823. [PubMed] [Google Scholar]
- 8.Siegel B. Bioorganic Chemistry. 1979;8:219. [Google Scholar]
- 9.Silverman RB. The Organic Chemistry of Enzyme-Catalyzed Reactions. San Diego: Academic Press; 2002. [Google Scholar]
- 10.Myllyharju J, Kivirikko KI. Characterization of the iron- and 2-oxoglutarate-binding sites of human prolyl 4-hydroxylase. Embo J. 1997;16:1173–1180. doi: 10.1093/emboj/16.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myllyla R, Majamaa K, Gunzler V, Hanauske-Abel HM, Kivirikko KI. Ascorbate is consumed stoichiometrically in the uncoupled reactions catalyzed by prolyl 4-hydroxylase and lysyl hydroxylase. J Biol Chem. 1984;259:5403–5405. [PubMed] [Google Scholar]
- 12.Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine. New York: Oxford University Press; 1999. [Google Scholar]
- 13.Telang S, Clem AL, Eaton JW, Chesney J. Depletion of ascorbic acid restricts angiogenesis and retards tumor growth in a mouse model. Neoplasia. 2007;9:47–56. doi: 10.1593/neo.06664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coucke PJ, Willaert A, Wessels MW, Callewaert B, Zoppi N, De Backer J, Fox JE, Mancini GM, Kambouris M, Gardella R, Facchetti F, Willems PJ, Forsyth R, Dietz HC, Barlati S, Colombi M, Loeys B, De Paepe A. Mutations in the facilitative glucose transporter GLUT10 alter angiogenesis and cause arterial tortuosity syndrome. Nat Genet. 2006;38:452–457. doi: 10.1038/ng1764. [DOI] [PubMed] [Google Scholar]
- 15.Segade F. Glucose transporter 10 and arterial tortuosity syndrome: the vitamin C connection. FEBS Lett. 2010;584:2990–2994. doi: 10.1016/j.febslet.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Nardai G, Braun L, Csala M, Mile V, Csermely P, Benedetti A, Mandl J, Banhegyi G. Protein-disulfide isomerase- and protein thiol-dependent dehydroascorbate reduction and ascorbate accumulation in the lumen of the endoplasmic reticulum. J Biol Chem. 2001;276:8825–8828. doi: 10.1074/jbc.M010563200. [DOI] [PubMed] [Google Scholar]
- 17.Mercer BM. Preterm premature rupture of the membranes. Obstet Gynecol. 2003;101:178–193. doi: 10.1016/s0029-7844(02)02366-9. [DOI] [PubMed] [Google Scholar]
- 18.Mercer BM, Abdelrahim A, Moore RM, Novak J, Kumar D, Mansour JM, Perez-Fournier M, Milluzzi CJ, Moore JJ. The impact of vitamin C supplementation in pregnancy and in vitro upon fetal membrane strength and remodeling. Reprod Sci. 2010;17:685–695. doi: 10.1177/1933719110368870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLaren J, Taylor DJ, Bell SC. Prostaglandin E(2)-dependent production of latent matrix metalloproteinase-9 in cultures of human fetal membranes. Mol Hum Reprod. 2000;6:1033–1040. doi: 10.1093/molehr/6.11.1033. [DOI] [PubMed] [Google Scholar]
- 20.Boulahbel H, Duran RV, Gottlieb E. Prolyl hydroxylases as regulators of cell metabolism. Biochem Soc Trans. 2009;37:291–294. doi: 10.1042/BST0370291. [DOI] [PubMed] [Google Scholar]
- 21.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 22.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 23.Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. Embo J. 2003;22:4082–4090. doi: 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flashman E, Davies SL, Yeoh KK, Schofield CJ. Investigating the dependence of the hypoxia-inducible factor hydroxylases (factor inhibiting HIF and prolyl hydroxylase domain 2) on ascorbate and other reducing agents. Biochem J. 2010;427:135–142. doi: 10.1042/BJ20091609. [DOI] [PubMed] [Google Scholar]
- 25.Kuiper C, Molenaar IG, Dachs GU, Currie MJ, Sykes PH, Vissers MC. Low ascorbate levels are associated with increased hypoxia-inducible factor-1 activity and an aggressive tumor phenotype in endometrial cancer. Cancer Res. 2010;70:5749–5758. doi: 10.1158/0008-5472.CAN-10-0263. [DOI] [PubMed] [Google Scholar]
- 26.Maxwell P, Salnikow K. HIF-1: an oxygen and metal responsive transcription factor. Cancer Biol Ther. 2004;3:29–35. doi: 10.4161/cbt.3.1.547. [DOI] [PubMed] [Google Scholar]
- 27.Kaczmarek M, Timofeeva OA, Karaczyn A, Malyguine A, Kasprzak KS, Salnikow K. The role of ascorbate in the modulation of HIF-1alpha protein and HIF-dependent transcription by chromium(VI) and nickel(II) Free Radic Biol Med. 2007;42:1246–1257. doi: 10.1016/j.freeradbiomed.2007.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Page EL, Chan DA, Giaccia AJ, Levine M, Richard DE. Hypoxia-inducible factor-1alpha stabilization in nonhypoxic conditions: role of oxidation and intracellular ascorbate depletion. Mol Biol Cell. 2008;19:86–94. doi: 10.1091/mbc.E07-06-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerald D, Berra E, Frapart YM, Chan DA, Giaccia AJ, Mansuy D, Pouyssegur J, Yaniv M, Mechta-Grigoriou F. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell. 2004;118:781–794. doi: 10.1016/j.cell.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Russo TA, Kwon O, Chanock S, Rumsey SC, Levine M. Ascorbate recycling in human neutrophils: induction by bacteria. Proc Natl Acad Sci U S A. 1997;94:13816–13819. doi: 10.1073/pnas.94.25.13816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miranda CL, Reed RL, Kuiper HC, Alber S, Stevens JF. Ascorbic acid promotes detoxification and elimination of 4-hydroxy-2(E)-nonenal in human monocytic THP-1 cells. Chem Res Toxicol. 2009;22:863–874. doi: 10.1021/tx900042u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chavez J, Chung WG, Miranda CL, Singhal M, Stevens JF, Maier CS. Site-specific protein adducts of 4-hydroxy-2(E)-nonenal in human THP-1 monocytic cells: protein carbonylation is diminished by ascorbic acid. Chem Res Toxicol. 2010;23:37–47. doi: 10.1021/tx9002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma P, Raghavan SA, Dikshit M. Role of ascorbate in the regulation of nitric oxide generation by polymorphonuclear leukocytes. Biochem Biophys Res Commun. 2003;309:12–17. doi: 10.1016/s0006-291x(03)01523-7. [DOI] [PubMed] [Google Scholar]
- 34.Chatterjee M, Saluja R, Kumar V, Jyoti A, Kumar Jain G, Kumar Barthwal M, Dikshit M. Ascorbate sustains neutrophil NOS expression, catalysis, and oxidative burst. Free Radic Biol Med. 2008;45:1084–1093. doi: 10.1016/j.freeradbiomed.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 35.Mateo J, Garcia-Lecea M, Cadenas S, Hernandez C, Moncada S. Regulation of hypoxia-inducible factor-1alpha by nitric oxide through mitochondria-dependent and -independent pathways. Biochem J. 2003;376:537–544. doi: 10.1042/BJ20031155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas DD, Espey MG, Ridnour LA, Hofseth LJ, Mancardi D, Harris CC, Wink DA. Hypoxic inducible factor 1alpha, extracellular signal-regulated kinase, and p53 are regulated by distinct threshold concentrations of nitric oxide. Proc Natl Acad Sci U S A. 2004;101:8894–8899. doi: 10.1073/pnas.0400453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vissers MC, Wilkie RP. Ascorbate deficiency results in impaired neutrophil apoptosis and clearance and is associated with up-regulation of hypoxia-inducible factor 1alpha. J Leukoc Biol. 2007;81:1236–1244. doi: 10.1189/jlb.0806541. [DOI] [PubMed] [Google Scholar]
- 38.Roberts JM, Redman CW. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet. 1993;341:1447–1451. doi: 10.1016/0140-6736(93)90889-o. [DOI] [PubMed] [Google Scholar]
- 39.Hubel CA, Roberts JM, Taylor RN, Musci TJ, Rogers GM, McLaughlin MK. Lipid peroxidation in pregnancy: new perspectives on preeclampsia. Am J Obstet Gynecol. 1989;161:1025–1034. doi: 10.1016/0002-9378(89)90778-3. [DOI] [PubMed] [Google Scholar]
- 40.Roberts JM, Hubel CA. Is oxidative stress the link in the two-stage model of pre-eclampsia? Lancet. 1999;354:788–789. doi: 10.1016/S0140-6736(99)80002-6. [DOI] [PubMed] [Google Scholar]
- 41.Gupta S, Aziz N, Sekhon L, Agarwal R, Mansour G, Li J, Agarwal A. Lipid peroxidation and antioxidant status in preeclampsia: a systematic review. Obstet Gynecol Surv. 2009;64:750–759. doi: 10.1097/OGX.0b013e3181bea0ac. [DOI] [PubMed] [Google Scholar]
- 42.Chappell LC, Seed PT, Briley AL, Kelly FJ, Lee R, Hunt BJ, Parmar K, Bewley SJ, Shennan AH, Steer PJ, Poston L. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial. Lancet. 1999;354:810–816. doi: 10.1016/S0140-6736(99)80010-5. [DOI] [PubMed] [Google Scholar]
- 43.Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367:1145–1154. doi: 10.1016/S0140-6736(06)68433-X. [DOI] [PubMed] [Google Scholar]
- 44.Villar J, Purwar M, Merialdi M, Zavaleta N, Thi Nhu Ngoc N, Anthony J, De Greeff A, Poston L, Shennan A. World Health Organisation multicentre randomised trial of supplementation with vitamins C and E among pregnant women at high risk for pre-eclampsia in populations of low nutritional status from developing countries. Bjog. 2009;116:780–788. doi: 10.1111/j.1471-0528.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- 45.Holmes VA, Young IS, Maresh MJ, Pearson DW, Walker JD, McCance DR. The Diabetes and Pre-eclampsia Intervention Trial. Int J Gynaecol Obstet. 2004;87:66–71. doi: 10.1016/j.ijgo.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 46.Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, Pearson GD, Wapner RJ, Varner MW, Thorp JM, Jr, Mercer BM, Peaceman AM, Ramin SM, Carpenter MW, Samuels P, Sciscione A, Harper M, Smith WJ, Saade G, Sorokin Y, Anderson GB. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362:1282–1291. doi: 10.1056/NEJMoa0908056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS. Vitamins C and E and the risks of preeclampsia and perinatal complications. N Engl J Med. 2006;354:1796–1806. doi: 10.1056/NEJMoa054186. [DOI] [PubMed] [Google Scholar]
- 48.Spinnato JA, 2nd, Freire S, Pinto ESJL, Cunha Rudge MV, Martins-Costa S, Koch MA, Goco N, Santos Cde B, Cecatti JG, Costa R, Ramos JG, Moss N, Sibai BM. Antioxidant therapy to prevent preeclampsia: a randomized controlled trial. Obstet Gynecol. 2007;110:1311–1318. doi: 10.1097/01.AOG.0000289576.43441.1f. [DOI] [PubMed] [Google Scholar]
- 49.Xu H, Perez-Cuevas R, Xiong X, Reyes H, Roy C, Julien P, Smith G, von Dadelszen P, Leduc L, Audibert F, Moutquin JM, Piedboeuf B, Shatenstein B, Parra-Cabrera S, Choquette P, Winsor S, Wood S, Benjamin A, Walker M, Helewa M, Dube J, Tawagi G, Seaward G, Ohlsson A, Magee LA, Olatunbosun F, Gratton R, Shear R, Demianczuk N, Collet JP, Wei S, Fraser WD. An international trial of antioxidants in the prevention of preeclampsia (INTAPP) Am J Obstet Gynecol. 2010;202:239 e231–239 e210. doi: 10.1016/j.ajog.2010.01.050. [DOI] [PubMed] [Google Scholar]
- 50.McCance DR, Holmes VA, Maresh MJ, Patterson CC, Walker JD, Pearson DW, Young IS. Vitamins C and E for prevention of pre-eclampsia in women with type 1 diabetes (DAPIT): a randomised placebo-controlled trial. Lancet. 2010;376:259–266. doi: 10.1016/S0140-6736(10)60630-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Talaulikar V. Vitamin C and E to prevent pre-eclampsia in diabetic women. Lancet. 2010;376:1642–1643. doi: 10.1016/S0140-6736(10)62092-2. author reply 1643. [DOI] [PubMed] [Google Scholar]
- 52.Hauth JC, Clifton RG, Roberts JM, Spong CY, Myatt L, Leveno KJ, Pearson GD, Varner MW, Thorp JM, Jr, Mercer BM, Peaceman AM, Ramin SM, Sciscione A, Harper M, Tolosa JE, Saade G, Sorokin Y, Anderson GB. Vitamin C and E supplementation to prevent spontaneous preterm birth: a randomized controlled trial. Obstet Gynecol. 2010;116:653–658. doi: 10.1097/AOG.0b013e3181ed721d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joshi SR, Mehendale SS, Dangat KD, Kilari AS, Yadav HR, Taralekar VS. High maternal plasma antioxidant concentrations associated with preterm delivery. Ann Nutr Metab. 2008;53:276–282. doi: 10.1159/000189789. [DOI] [PubMed] [Google Scholar]
- 54.Hung TH, Chen SF, Li MJ, Yeh YL, Hsieh TT. Differential effects of concomitant use of vitamins C and E on trophoblast apoptosis and autophagy between normoxia and hypoxia-reoxygenation. PLoS One. 2010;5:e12202. doi: 10.1371/journal.pone.0012202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Talaulikar V, Manyonda I. The myth of vitamins C and E for the prevention of preeclampsia: just when will the penny drop? Am J Obstet Gynecol. 2010;203:e7–8. doi: 10.1016/j.ajog.2010.06.063. author reply e8. [DOI] [PubMed] [Google Scholar]
- 56.Gropper SS, Smith JL, Groff JL. Advanced Nutrition and Human Metabolism. Belmont CA: Wadsworth; 2009. [Google Scholar]
- 57.Talwar D, McConnachie A, Welsh P, Upton M, O'Reilly D, Davey Smith G, Watt G, Sattar N. Which circulating antioxidant vitamins are confounded by socioeconomic deprivation? The MIDSPAN family study. PLoS One. 2010;5:e11312. doi: 10.1371/journal.pone.0011312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Michiels M, Mellema M, Peters FP. Haemorrhages due to vitamin C deficiency. Scurvy in the 21st century. Ned Tijdschr Geneeskd. 2010;154:A1638. [PubMed] [Google Scholar]
- 59.Singer RF. Vitamin C supplementation in kidney failure: effect on uraemic symptoms. Nephrol Dial Transplant. 2010 doi: 10.1093/ndt/gfq412. [DOI] [PubMed] [Google Scholar]
- 60.Finkelstein FO, Juergenson P, Wang S, Santacroce S, Levine M, Kotanko P, Levin NW, Handelman GJ. Hemoglobin and Plasma Vitamin C Levels in Patients on Peritoneal Dialysis. Perit Dial Int. 2010 doi: 10.3747/pdi.2009.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fain O, Paries J, Jacquart B, Le Moel G, Kettaneh A, Stirnemann J, Heron C, Sitbon M, Taleb C, Letellier E, Betari B, Gattegno L, Thomas M. Hypovitaminosis C in hospitalized patients. Eur J Intern Med. 2003;14:419–425. doi: 10.1016/j.ejim.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 62.Reed RM. Captain Ignose to the rescue. Am J Med. 2010;123:704–706. doi: 10.1016/j.amjmed.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 63.Cole JA, Warthan MM, Hirano SA, Gowen CW, Jr, Williams JV. Scurvy in a 10-Year-Old Boy. Pediatr Dermatol. 2010 doi: 10.1111/j.1525-1470.2010.01095.x. [DOI] [PubMed] [Google Scholar]
- 64.Raynaud-Simon A, Cohen-Bittan J, Gouronnec A, Pautas E, Senet P, Verny M, Boddaert J. Scurvy in hospitalized elderly patients. J Nutr Health Aging. 2010;14:407–410. doi: 10.1007/s12603-010-0032-y. [DOI] [PubMed] [Google Scholar]
- 65.Riess KP, Farnen JP, Lambert PJ, Mathiason MA, Kothari SN. Ascorbic acid deficiency in bariatric surgical population. Surg Obes Relat Dis. 2009;5:81–86. doi: 10.1016/j.soard.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 66.Christen S, Finckh B, Lykkesfeldt J, Gessler P, Frese-Schaper M, Nielsen P, Schmid ER, Schmitt B. Oxidative stress precedes peak systemic inflammatory response in pediatric patients undergoing cardiopulmonary bypass operation. Free Radic Biol Med. 2005;38:1323–1332. doi: 10.1016/j.freeradbiomed.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 67.Alexandrescu DT, Dasanu CA, Kauffman CL. Acute scurvy during treatment with interleukin-2. Clin Exp Dermatol. 2009;34:811–814. doi: 10.1111/j.1365-2230.2008.03052.x. [DOI] [PubMed] [Google Scholar]
- 68.Marcus SL, Dutcher JP, Paietta E, Ciobanu N, Strauman J, Wiernik PH, Hutner SH, Frank O, Baker H. Severe hypovitaminosis C occurring as the result of adoptive immunotherapy with high-dose interleukin 2 and lymphokine-activated killer cells. Cancer Res. 1987;47:4208–4212. [PubMed] [Google Scholar]
- 69.Marcus SL, Petrylak DP, Dutcher JP, Paietta E, Ciobanu N, Strauman J, Wiernik PH, Hutner SH, Frank O, Baker H. Hypovitaminosis C in patients treated with high-dose interleukin 2 and lymphokine-activated killer cells. Am J Clin Nutr. 1991;54:1292S–1297S. doi: 10.1093/ajcn/54.6.1292s. [DOI] [PubMed] [Google Scholar]
- 70.Lee SH, Oe T, Blair IA. Vitamin C-induced decomposition of lipid hydroperoxides to endogenous genotoxins. Science. 2001;292:2083–2086. doi: 10.1126/science.1059501. [DOI] [PubMed] [Google Scholar]
- 71.Suh J, Zhu BZ, Frei B. Ascorbate does not act as a pro-oxidant towards lipids and proteins in human plasma exposed to redox-active transition metal ions and hydrogen peroxide. Free Radic Biol Med. 2003;34:1306–1314. doi: 10.1016/s0891-5849(03)00147-3. [DOI] [PubMed] [Google Scholar]
- 72.Mattson MP. Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders. Exp Gerontol. 2009;44:625–633. doi: 10.1016/j.exger.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Butterfield DA, Yatin SM, Varadarajan S, Koppal T. Amyloid beta-peptide-associated free radical oxidative stress, neurotoxicity, and Alzheimer's disease. Methods Enzymol. 1999;309:746–768. doi: 10.1016/s0076-6879(99)09050-3. [DOI] [PubMed] [Google Scholar]
- 74.Traverso N, Menini S, Cosso L, Odetti P, Albano E, Pronzato MA, Marinari UM. Immunological evidence for increased oxidative stress in diabetic rats. Diabetologia. 1998;41:265–270. doi: 10.1007/s001250050902. [DOI] [PubMed] [Google Scholar]
- 75.Carr AC, Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr. 1999;69:1086–1107. doi: 10.1093/ajcn/69.6.1086. [DOI] [PubMed] [Google Scholar]
- 76.Retana-Ugalde R, Casanueva E, Altamirano-Lozano M, Gonzalez-Torres C, Mendoza-Nunez VM. High dosage of ascorbic acid and alpha-tocopherol is not useful for diminishing oxidative stress and DNA damage in healthy elderly adults. Ann Nutr Metab. 2008;52:167–173. doi: 10.1159/000129652. [DOI] [PubMed] [Google Scholar]
- 77.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. Jama. 2008;300:2123–2133. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lykkesfeldt J, Poulsen HE. Is vitamin C supplementation beneficial? Lessons learned from randomised controlled trials. Br J Nutr. 2010;103:1251–1259. doi: 10.1017/S0007114509993229. [DOI] [PubMed] [Google Scholar]
- 79.Duffy SJ, Gokce N, Holbrook M, Huang A, Frei B, Keaney JF, Jr, Vita JA. Treatment of hypertension with ascorbic acid. Lancet. 1999;354:2048–2049. doi: 10.1016/s0140-6736(99)04410-4. [DOI] [PubMed] [Google Scholar]
- 80.Loria CM. Vitamin C Status and Cardiovascular Disease: A Review of Prospective Studies. In: Packer L, Traber MG, Kraemer K, Frei B, editors. The Antioxidant Vitamins C and E. Champaign, Illinois: AOCS Press; 2002. pp. 105–115. [Google Scholar]
- 81.Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kuiper HC, Miranda CL, Sowell JD, Stevens JF. Mercapturic acid conjugates of 4-hydroxy-2-nonenal and 4-oxo-2-nonenal metabolites are in vivo markers of oxidative stress. J Biol Chem. 2008;283:17131–17138. doi: 10.1074/jbc.M802797200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kuiper HC, Langsdorf BL, Miranda CL, Joss J, Jubert C, Mata JE, Stevens JF. Quantitation of mercapturic acid conjugates of 4-hydroxy-2-nonenal and 4-oxo-2-nonenal metabolites in a smoking cessation study. Free Radic Biol Med. 2010;48:65–72. doi: 10.1016/j.freeradbiomed.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuiper HC, Bruno RS, Traber MG, Stevens JF. Vitamin C supplementation lowers urinary levels of 4-hydroperoxy-2-nonenal metabolites in humans. Free Radic Biol Med. 2011 doi: 10.1016/j.freeradbiomed.2011.01.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harrison FE, May JM. Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic Biol Med. 2009;46:719–730. doi: 10.1016/j.freeradbiomed.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tveden-Nyborg P, Lykkesfeldt J. Does vitamin C deficiency result in impaired brain development in infants? Redox Rep. 2009;14:2–6. doi: 10.1179/135100009X392412. [DOI] [PubMed] [Google Scholar]
- 87.Harrison FE, Meredith ME, Dawes SM, Saskowski JL, May JM. Low ascorbic acid and increased oxidative stress in gulo(-/-) mice during development. Brain Res. 2010;1349:143–152. doi: 10.1016/j.brainres.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem. 1997;272:20963–20966. doi: 10.1074/jbc.272.34.20963. [DOI] [PubMed] [Google Scholar]
- 89.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 90.Diaz MN, Frei B, Vita JA, Keaney JF., Jr Antioxidants and atherosclerotic heart disease. N Engl J Med. 1997;337:408–416. doi: 10.1056/NEJM199708073370607. [DOI] [PubMed] [Google Scholar]
- 91.Navab M, Ananthramaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fonarow GC, Vahabzadeh K, Hama S, Hough G, Kamranpour N, Berliner JA, Lusis AJ, Fogelman AM. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J Lipid Res. 2004;45:993–1007. doi: 10.1194/jlr.R400001-JLR200. [DOI] [PubMed] [Google Scholar]
- 92.Kris-Etherton PM, Lichtenstein AH, Howard BV, Steinberg D, Witztum JL. Antioxidant vitamin supplements and cardiovascular disease. Circulation. 2004;110:637–641. doi: 10.1161/01.CIR.0000137822.39831.F1. [DOI] [PubMed] [Google Scholar]
- 93.Zureik M, Galan P, Bertrais S, Mennen L, Czernichow S, Blacher J, Ducimetiere P, Hercberg S. Effects of long-term daily low-dose supplementation with antioxidant vitamins and minerals on structure and function of large arteries. Arterioscler Thromb Vasc Biol. 2004;24:1485–1491. doi: 10.1161/01.ATV.0000136648.62973.c8. [DOI] [PubMed] [Google Scholar]
- 94.Stanner SA, Hughes J, Kelly CN, Buttriss J. A review of the epidemiological evidence for the ‘antioxidant hypothesis’. Public Health Nutr. 2004;7:407–422. doi: 10.1079/phn2003543. [DOI] [PubMed] [Google Scholar]
- 95.Asleh R, Levy AP. Divergent effects of alpha-tocopherol and vitamin C on the generation of dysfunctional HDL associated with diabetes and the Hp 2-2 genotype. Antioxid Redox Signal. 2010;12:209–217. doi: 10.1089/ars.2009.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roberts LJ, 2nd, Traber MG, Frei B. Vitamins E and C in the prevention of cardiovascular disease and cancer in men. Free Radic Biol Med. 2009;46:1558. doi: 10.1016/j.freeradbiomed.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 97.Shargorodsky M, Debby O, Matas Z, Zimlichman R. Effect of long-term treatment with antioxidants (vitamin C, vitamin E, coenzyme Q10 and selenium) on arterial compliance, humoral factors and inflammatory markers in patients with multiple cardiovascular risk factors. Nutr Metab (Lond) 2010;7:55. doi: 10.1186/1743-7075-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 99.Suematsu N, Ojaimi C, Recchia FA, Wang Z, Skayian Y, Xu X, Zhang S, Kaminski PM, Sun D, Wolin MS, Kaley G, Hintze TH. Potential mechanisms of low-sodium diet-induced cardiac disease: superoxide-NO in the heart. Circ Res. 2010;106:593–600. doi: 10.1161/CIRCRESAHA.109.208397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Williams HC, Griendling KK. NADPH oxidase inhibitors: new antihypertensive agents? J Cardiovasc Pharmacol. 2007;50:9–16. doi: 10.1097/FJC.0b013e318063e820. [DOI] [PubMed] [Google Scholar]
- 101.Maio R, Perticone M, Sciacqua A, Tassone EJ, Naccarato P, Bagnato C, Iannopollo G, Sesti G, Perticone F. Oxidative Stress Impairs Endothelial Function in Nondipper Hypertensive Patients. Cardiovasc Ther. 2010 doi: 10.1111/j.1755-5922.2010.00183.x. [DOI] [PubMed] [Google Scholar]
- 102.Frikke-Schmidt H, Lykkesfeldt J. Role of marginal vitamin C deficiency in atherogenesis: in vivo models and clinical studies. Basic Clin Pharmacol Toxicol. 2009;104:419–433. doi: 10.1111/j.1742-7843.2009.00420.x. [DOI] [PubMed] [Google Scholar]
- 103.May JM. How does ascorbic acid prevent endothelial dysfunction? Free Radic Biol Med. 2000;28:1421–1429. doi: 10.1016/s0891-5849(00)00269-0. [DOI] [PubMed] [Google Scholar]
- 104.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 2003;278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 105.Koppenol WH, Moreno JJ, Pryor WA, Ischiropoulos H, Beckman JS. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem Res Toxicol. 1992;5:834–842. doi: 10.1021/tx00030a017. [DOI] [PubMed] [Google Scholar]
- 106.Berka V, Wu G, Yeh HC, Palmer G, Tsai AL. Three different oxygen-induced radical species in endothelial nitric-oxide synthase oxygenase domain under regulation by L-arginine and tetrahydrobiopterin. J Biol Chem. 2004;279:32243–32251. doi: 10.1074/jbc.M404044200. [DOI] [PubMed] [Google Scholar]
- 107.Schmidt TS, Alp NJ. Mechanisms for the role of tetrahydrobiopterin in endothelial function and vascular disease. Clin Sci (Lond) 2007;113:47–63. doi: 10.1042/CS20070108. [DOI] [PubMed] [Google Scholar]
- 108.Pou S, Pou WS, Bredt DS, Snyder SH, Rosen GM. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- 109.Stuehr DJ, Santolini J, Wang ZQ, Wei CC, Adak S. Update on mechanism and catalytic regulation in the NO synthases. J Biol Chem. 2004;279:36167–36170. doi: 10.1074/jbc.R400017200. [DOI] [PubMed] [Google Scholar]
- 110.Wei CC, Wang ZQ, Tejero J, Yang YP, Hemann C, Hille R, Stuehr DJ. Catalytic reduction of a tetrahydrobiopterin radical within nitric-oxide synthase. J Biol Chem. 2008;283:11734–11742. doi: 10.1074/jbc.M709250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Davis MD, Kaufman S, Milstien S. The auto-oxidation of tetrahydrobiopterin. Eur J Biochem. 1988;173:345–351. doi: 10.1111/j.1432-1033.1988.tb14004.x. [DOI] [PubMed] [Google Scholar]
- 112.Stoll S, NejatyJahromy Y, Woodward JJ, Ozarowski A, Marletta MA, Britt RD. Nitric oxide synthase stabilizes the tetrahydrobiopterin cofactor radical by controlling its protonation state. J Am Chem Soc. 2010;132:11812–11823. doi: 10.1021/ja105372s. [DOI] [PubMed] [Google Scholar]
- 113.Sun J, Druhan LJ, Zweier JL. Reactive oxygen and nitrogen species regulate inducible nitric oxide synthase function shifting the balance of nitric oxide and superoxide production. Arch Biochem Biophys. 2010;494:130–137. doi: 10.1016/j.abb.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sherman DL, Keaney JF, Jr, Biegelsen ES, Duffy SJ, Coffman JD, Vita JA. Pharmacological concentrations of ascorbic acid are required for the beneficial effect on endothelial vasomotor function in hypertension. Hypertension. 2000;35:936–941. doi: 10.1161/01.hyp.35.4.936. [DOI] [PubMed] [Google Scholar]
- 115.Valent S, Toth M. Activation energy determinations suggest that thiols reverse autooxidation of tetrahydrobiopterin by a different mechanism than ascorbate. Int J Biochem Cell Biol. 2006;38:1786–1793. doi: 10.1016/j.biocel.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 116.Patel KB, Stratford MR, Wardman P, Everett SA. Oxidation of tetrahydrobiopterin by biological radicals and scavenging of the trihydrobiopterin radical by ascorbate. Free Radic Biol Med. 2002;32:203–211. doi: 10.1016/s0891-5849(01)00777-8. [DOI] [PubMed] [Google Scholar]
- 117.Garry A, Edwards DH, Fallis IF, Jenkins RL, Griffith TM. Ascorbic acid and tetrahydrobiopterin potentiate the EDHF phenomenon by generating hydrogen peroxide. Cardiovasc Res. 2009;84:218–226. doi: 10.1093/cvr/cvp235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Buettner GR. In the absence of catalytic metals ascorbate does not autoxidize at pH 7: ascorbate as a test for catalytic metals. J Biochem Biophys Methods. 1988;16:27–40. doi: 10.1016/0165-022x(88)90100-5. [DOI] [PubMed] [Google Scholar]
- 119.Griffith TM. Endothelium-dependent smooth muscle hyperpolarization: do gap junctions provide a unifying hypothesis? Br J Pharmacol. 2004;141:881–903. doi: 10.1038/sj.bjp.0705698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sandow SL. Factors, fiction and endothelium-derived hyperpolarizing factor. Clin Exp Pharmacol Physiol. 2004;31:563–570. doi: 10.1111/j.1440-1681.2004.04048.x. [DOI] [PubMed] [Google Scholar]
- 121.Fodor G, Arnold R, Mohacsi T, Karle I, Flippen-Anderson J. A new role for l-ascorbic acid: Michael donor to alpha,beta-unsaturated carbonyl compounds. Tetrahedron. 1983;39:2137–2145. [Google Scholar]
- 122.Kesinger NG, Stevens JF. Covalent interaction of ascorbic acid with natural products. Phytochemistry. 2009;70:1930–1939. doi: 10.1016/j.phytochem.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008;52:7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fodor G, Sussangkarn K, Mathelier H, Fang K, Arnold R. Stereospecificity of a new reaction of L-ascorbic acid with cis and trans olefinic 1,4-dicarbonyl compounds. J Org Chem. 1986;51:3148–3150. [Google Scholar]
- 125.Kesinger NG, Langsdorf BL, Yokochi AF, Miranda CL, Stevens JF. Formation of a Vitamin C Conjugate of Acrolein and its Paraoxonase-mediated Conversion into 5,6,7,8-Tetrahydroxy-4-oxooctanal. Chem Res Toxicol. 2010 doi: 10.1021/tx900452j. Epub March, 30 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Preobrazhenskaya M, Lazhko E, Korolev A, Reznikova M, Rozhkov I. Transformation of ascorbigen into 1-deoxy-1-(indol-3-yl)-a-L-sorbopyranose and 1-deoxy-1-(indol-3-yl)-a-L-tagatopyranose. Tetrahedron: Asymmetry. 1996;7:461–467. [Google Scholar]
- 127.Teiber JF, Draganov DI, La Du BN. Lactonase and lactonizing activities of human serum paraoxonase (PON1) and rabbit serum PON3. Biochem Pharmacol. 2003;66:887–896. doi: 10.1016/s0006-2952(03)00401-5. [DOI] [PubMed] [Google Scholar]
- 128.Aviram M, Rosenblat M. Paraoxonases 1, 2, and 3, oxidative stress, and macrophage foam cell formation during atherosclerosis development. Free Radic Biol Med. 2004;37:1304–1316. doi: 10.1016/j.freeradbiomed.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 129.Stone I. The Healing Factor Vitamin C Against Disease. New York: Putnam Publishing Group; 1972. [Google Scholar]
- 130.Aguirre R, May JM. Inflammation in the vascular bed: importance of vitamin C. Pharmacol Ther. 2008;119:96–103. doi: 10.1016/j.pharmthera.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pauling L. How to Live Longer and Feel Better. Corvallis: Oregon State University Press; 2006. [Google Scholar]
- 132.Food and Nutrition Board, Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium and Carotenoids. Washington, D.C.: National Academy Press; 2000. pp. 95–185. [PubMed] [Google Scholar]
- 133.Gokce N, Keaney JF, Jr, Frei B, Holbrook M, Olesiak M, Zachariah BJ, Leeuwenburgh C, Heinecke JW, Vita JA. Long-term ascorbic acid administration reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1999;99:3234–3240. doi: 10.1161/01.cir.99.25.3234. [DOI] [PubMed] [Google Scholar]
- 134.Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic Biol Med. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300:535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 136.Neuzil J, Witting PK, Stocker R. Alpha-tocopheryl hydroquinone is an efficient multifunctional inhibitor of radical-initiated oxidation of low density lipoprotein lipids. Proc Natl Acad Sci U S A. 1997;94:7885–7890. doi: 10.1073/pnas.94.15.7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mene-Saffrane L, Jones AD, Dellapenna D. Plastochromanol-8 and tocopherols are essential lipid-soluble antioxidants during seed desiccation and quiescence in Arabidopsis. Proc Natl Acad Sci U S A. 2010;107:17815–17820. doi: 10.1073/pnas.1006971107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Thomas SR, Stocker R. Molecular action of vitamin E in lipoprotein oxidation: implications for atherosclerosis. Free Radic Biol Med. 2000;28:1795–1805. doi: 10.1016/s0891-5849(00)00236-7. [DOI] [PubMed] [Google Scholar]
- 139.Liebler DC, Burr JA, Philips L, Ham AJ. Gas chromatography-mass spectrometry analysis of vitamin E and its oxidation products. Anal Biochem. 1996;236:27–34. doi: 10.1006/abio.1996.0127. [DOI] [PubMed] [Google Scholar]
- 140.Brigelius-Flohé R, Traber MG. Vitamin E: function and metabolism. FASEB J. 1999;13:1145–1155. [PubMed] [Google Scholar]
- 141.Swann IL, Kendra JR. Anaemia, vitamin E deficiency and failure to thrive in an infant. Clin Lab Haematol. 1998;20:61–63. doi: 10.1046/j.1365-2257.1998.00099.x. [DOI] [PubMed] [Google Scholar]
- 142.Benabdeslam H, Abidi H, Garcia I, Bellon G, Gilly R, Revol A. Lipid peroxidation and antioxidant defenses in cystic fibrosis patients. Clin Chem Lab Med. 1999;37:511–516. doi: 10.1515/CCLM.1999.082. [DOI] [PubMed] [Google Scholar]
- 143.Sokol RJ, Heubi JE, Iannaccone S, Bove KE, Balistreri WF. Mechanism causing vitamin E deficiency during chronic childhood cholestasis. Gastroenterology. 1983;85:1172–1182. [PubMed] [Google Scholar]
- 144.Rosenblum JL, Keating JP, Prensky AL, Nelson JS. A progressive neurologic syndrome in children with chronic liver disease. N Engl J Med. 1981;304:503–508. doi: 10.1056/NEJM198102263040902. [DOI] [PubMed] [Google Scholar]
- 145.Sokol RJ. Vitamin E and neurologic deficits. Adv Pediatr. 1990;37:119–148. [PubMed] [Google Scholar]
- 146.Guggenheim MA, Jackson V, Lilly J, Silverman A. Vitamin E deficiency and neurologic disease in children with cholestasis: A prospective study. J Pediatr. 1983;102:577–579. doi: 10.1016/s0022-3476(83)80189-9. [DOI] [PubMed] [Google Scholar]
- 147.Sokol RJ, Guggenheim MA, Heubi JE, Iannaccone ST, Butler-Simon N, Jackson V, Miller C, Cheney M, Balistreri WF, Silverman A. Frequency and clinical progression of the vitamin E deficiency neurologic disorder in children with prolonged neonatal cholestasis. Am J Dis Child. 1985;139:1211–1215. doi: 10.1001/archpedi.1985.02140140045024. [DOI] [PubMed] [Google Scholar]
- 148.Meydani SN, Meydani M, Blumberg JB, Leka LS, Siber G, Loszewski R, Thompson C, Pedrosa MC, Diamond RD, Stollar BD. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial. JAMA. 1997;277:1380–1386. doi: 10.1001/jama.1997.03540410058031. [DOI] [PubMed] [Google Scholar]
- 149.Ahmed T, Marko M, Wu D, Chung H, Huber B, Meydani SN. Vitamin E supplementation reverses the age-associated decrease in effective immune synapse formation in CD4+ T cells. Ann N Y Acad Sci. 2004;1031:412–414. doi: 10.1196/annals.1331.059. [DOI] [PubMed] [Google Scholar]
- 150.Han SN, Adolfsson O, Lee CK, Prolla TA, Ordovas J, Meydani SN. Age and vitamin E-induced changes in gene expression profiles of T cells. J Immunol. 2006;177:6052–6061. doi: 10.4049/jimmunol.177.9.6052. [DOI] [PubMed] [Google Scholar]
- 151.Marko MG, Pang HJ, Ren Z, Azzi A, Huber BT, Bunnell SC, Meydani SN. Vitamin E reverses impaired linker for activation of T cells activation in T cells from aged C57BL/6 mice. J Nutr. 2009;139:1192–1197. doi: 10.3945/jn.108.103416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Meydani SN, Leka LS, Fine BC, Dallal GE, Keusch GT, Singh MF, Hamer DH. Vitamin E and respiratory tract infections in elderly nursing home residents: a randomized controlled trial. JAMA. 2004;292:828–836. doi: 10.1001/jama.292.7.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mastaloudis A, Leonard SW, Traber MG. Oxidative stress in athletes during extreme endurance exercise. Free Radic Biol Med. 2001;31:911–922. doi: 10.1016/s0891-5849(01)00667-0. [DOI] [PubMed] [Google Scholar]
- 154.Mastaloudis A, Morrow JD, Hopkins DW, Deveraj S, Traber MG. Antioxidant supplementation prevents exercise-induced lipid peroxidation, but not inflammation, in ultramarathon runners. Free Radic Biol Med. 2004;36:1329–1341. doi: 10.1016/j.freeradbiomed.2004.02.069. [DOI] [PubMed] [Google Scholar]
- 155.Pryor WA. Biological effects of cigarette smoke, wood smoke, and the smoke from plastics: the use of electron spin resonance. Free Radic Biol Med. 1992;13:659–676. doi: 10.1016/0891-5849(92)90040-n. [DOI] [PubMed] [Google Scholar]
- 156.Bruno RS, Ramakrishnan R, Montine TJ, Bray TM, Traber MG. α-Tocopherol disappearance is faster in cigarette smokers and is inversely related to their ascorbic acid status. Am J Clin Nutr. 2005;81:95–103. doi: 10.1093/ajcn/81.1.95. [DOI] [PubMed] [Google Scholar]
- 157.Bruno RS, Leonard SW, Atkinson JK, Montine TJ, Ramakrishnan R, Bray TM, Traber MG. Faster vitamin E disappearance in smokers is normalized by vitamin C supplementation. Free Radic Biol Med. 2006;40:689–697. doi: 10.1016/j.freeradbiomed.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 158.Taylor AW, Bruno RS, Frei B, Traber MG. Benefits of prolonged gradient separation for high-performance liquid chromatography-tandem mass spectrometry quantitation of plasma total 15-Series F2-isoprostanes. Anal Biochem. 2006;350:41–51. doi: 10.1016/j.ab.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 159.Keaney JF, Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- 160.Oliver SR, Rosa JS, Milne GL, Pontello AM, Borntrager HL, Heydari S, Galassetti PR. Increased oxidative stress and altered substrate metabolism in obese children. Int J Pediatr Obes. 2010;5:436–444. doi: 10.3109/17477160903545163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gopaul NK, Anggard EE, Mallet AI, Betteridge DJ, Wolff SP, Nourooz-Zadeh J. Plasma 8-epi-PGF2 alpha levels are elevated in individuals with non-insulin dependent diabetes mellitus. FEBS Lett. 1995;368:225–229. doi: 10.1016/0014-5793(95)00649-t. [DOI] [PubMed] [Google Scholar]
- 162.Sampson MJ, Gopaul N, Davies IR, Hughes DA, Carrier MJ. Plasma F2 isoprostanes: direct evidence of increased free radical damage during acute hyperglycemia in type 2 diabetes. Diabetes Care. 2002;25:537–541. doi: 10.2337/diacare.25.3.537. [DOI] [PubMed] [Google Scholar]
- 163.Darko D, Dornhorst A, Kelly FJ, Ritter JM, Chowienczyk PJ. Lack of effect of oral vitamin C on blood pressure, oxidative stress and endothelial function in Type II diabetes. Clin Sci (Lond) 2002;103:339–344. doi: 10.1042/cs1030339. [DOI] [PubMed] [Google Scholar]
- 164.Traber MG. Vitamin E regulatory mechanisms. Annu Rev Nutr. 2007;27:347–362. doi: 10.1146/annurev.nutr.27.061406.093819. [DOI] [PubMed] [Google Scholar]
- 165.Traber MG, Ingold KU, Burton GW, Kayden HJ. Absorption and transport of deuterium-substituted 2R,4′R,8′R-α-tocopherol in human lipoproteins. Lipids. 1988;23:791–797. doi: 10.1007/BF02536223. [DOI] [PubMed] [Google Scholar]
- 166.Traber MG, Burton GW, Ingold KU, Kayden HJ. RRR- and SRR-alpha-tocopherols are secreted without discrimination in human chylomicrons, but RRR-alpha-tocopherol is preferentially secreted in very low density lipoproteins. J Lipid Res. 1990;31:675–685. [PubMed] [Google Scholar]
- 167.Traber MG, Sokol RJ, Burton GW, Ingold KU, Papas AM, Huffaker JE, Kayden HJ. Impaired ability of patients with familial isolated vitamin E deficiency to incorporate alpha-tocopherol into lipoproteins secreted by the liver. J Clin Invest. 1990;85:397–407. doi: 10.1172/JCI114452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Traber MG, Rader D, Acuff R, Brewer HB, Kayden HJ. Discrimination between RRR- and all rac-α-tocopherols labeled with deuterium by patients with abetalipoproteinemia. Atherosclerosis. 1994;108:27–37. doi: 10.1016/0021-9150(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 169.Munro LH, Burton G, Kelly FJ. Plasma RRR-alpha-tocopherol concentrations are lower in smokers than in non-smokers after ingestion of a similar oral load of this antioxidant vitamin. Clin Sci (Colch) 1997;92:87–93. doi: 10.1042/cs0920087. [DOI] [PubMed] [Google Scholar]
- 170.Acuff RV, Dunworth RG, Webb LW, Lane JR. Transport of deuterium-labeled tocopherols during pregnancy. Am J Clin Nutr. 1998;67:459–464. doi: 10.1093/ajcn/67.3.459. [DOI] [PubMed] [Google Scholar]
- 171.Burton GW, Traber MG, Acuff RV, Walters DN, Kayden H, Hughes L, Ingold KU. Human plasma and tissue alpha-tocopherol concentrations in response to supplementation with deuterated natural and synthetic vitamin E. Am J Clin Nutr. 1998;67:669–684. doi: 10.1093/ajcn/67.4.669. [DOI] [PubMed] [Google Scholar]
- 172.Traber MG, Elsner A, Brigelius-Flohe R. Synthetic as compared with natural vitamin E is preferentially excreted as alpha-CEHC in human urine: studies using deuterated alpha-tocopheryl acetates. FEBS Lett. 1998;437:145–148. doi: 10.1016/s0014-5793(98)01210-1. [DOI] [PubMed] [Google Scholar]
- 173.Traber MG, Rader D, Acuff R, Ramakrishnan R, Brewer HB, Kayden HJ. Vitamin E dose response studies in humans using deuterated RRR-α–tocopherol. Am J Clin Nutr. 1998;68:847–853. doi: 10.1093/ajcn/68.4.847. [DOI] [PubMed] [Google Scholar]
- 174.Galli F, Lee R, Atkinson J, Floridi A, Kelly FJ. gamma-Tocopherol biokinetics and transformation in humans. Free Radic Res. 2003;37:1225–1233. doi: 10.1080/10715760310001604125. [DOI] [PubMed] [Google Scholar]
- 175.Hall WL, Jeanes YM, Pugh J, Lodge JK. Development of a liquid chromatographic time-of-flight mass spectrometric method for the determination of unlabelled and deuterium-labelled alpha-tocopherol in blood components. Rapid Commun Mass Spectrom. 2003;17:2797–2803. doi: 10.1002/rcm.1263. [DOI] [PubMed] [Google Scholar]
- 176.Traber MG, Paterson E, Atkinson J, Ramakrishnan R, Iacovoni V, Cross C. Studies in humans using deuterium-labeled α- and γ-tocopherols demonstrate rapid plasma γ-tocopherol disappearance. FASEB J. 2003;17:A279. doi: 10.1016/j.freeradbiomed.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 177.Brigelius-Flohe R, Roob JM, Tiran B, Wuga S, Ribalta J, Rock E, Winklhofer-Roob BM. The effect of age on vitamin E status, metabolism, and function: metabolism as assessed by labeled tocopherols. Ann N Y Acad Sci. 2004;1031:40–43. doi: 10.1196/annals.1331.004. [DOI] [PubMed] [Google Scholar]
- 178.Leonard SW, Bruno RS, Ramakrishnan R, Bray T, Traber MG. Cigarette smoking increases human vitamin E requirements as estimated by plasma deuterium-labeled CEHC. Ann NY Acad Sci. 2004;1031:357–360. doi: 10.1196/annals.1331.044. [DOI] [PubMed] [Google Scholar]
- 179.Vaule H, Leonard SW, Traber MG. Vitamin E delivery to human skin; studies using deuterated α-tocopherol measured By APCI LC-MS. Free Radic Biol Med. 2004;36:456–463. doi: 10.1016/j.freeradbiomed.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 180.Bruno RS, Leonard SW, Li J, Bray TM, Traber MG. Lower plasma α-carboxyethyl-hydroxychroman after deuterium labeled α-tocopherol supplementation suggests decreased vitamin E metabolism in smokers. Am J Clin Nutr. 2005;81:1052–1059. doi: 10.1093/ajcn/81.5.1052. [DOI] [PubMed] [Google Scholar]
- 181.Hall WL, Jeanes YM, Lodge JK. Hyperlipidemic Subjects Have Reduced Uptake of Newly Absorbed Vitamin E into Their Plasma Lipoproteins, Erythrocytes, Platelets, and Lymphocytes, as Studied by Deuterium-Labeled {alpha}-Tocopherol Biokinetics. J Nutr. 2005;135:58–63. doi: 10.1093/jn/135.1.58. [DOI] [PubMed] [Google Scholar]
- 182.Jeanes YM, Hall WL, Lodge JK. Comparative (2)H-labelled alpha-tocopherol biokinetics in plasma, lipoproteins, erythrocytes, platelets and lymphocytes in normolipidaemic males. Br J Nutr. 2005;94:92–99. doi: 10.1079/bjn20051434. [DOI] [PubMed] [Google Scholar]
- 183.Leonard SW, Paterson E, Atkinson JK, Ramakrishnan R, Cross CE, Traber MG. Studies in humans using deuterium-labeled α- and γ-tocopherol demonstrate faster plasma γ-tocopherol disappearance and greater γ-metabolite production. Free Radic Biol Med. 2005;38:857–866. doi: 10.1016/j.freeradbiomed.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 184.Bruno RS, Leonard SW, Park SI, Zhao Y, Traber MG. Human vitamin E requirements assessed with the use of apples fortified with deuterium-labeled α-tocopheryl acetate. Am J Clin Nutr. 2006;83:299–304. doi: 10.1093/ajcn/83.2.299. [DOI] [PubMed] [Google Scholar]
- 185.Traber MG, Rudel LL, Burton GW, Hughes L, Ingold KU, Kayden HJ. Nascent VLDL from liver perfusions of cynomolgus monkeys are preferentially enriched inRRR-compared with SRR-α tocopherol: studies using deuterated tocopherols. J Lipid Res. 1990;31:687–694. [PubMed] [Google Scholar]
- 186.Traber MG, Ramakrishnan R, Kayden HJ. Human plasma vitamin E kinetics demonstrate rapid recycling of plasma RRR-α-tocopherol. Proc Natl Acad Sci USA. 1994;91:10005–10008. doi: 10.1073/pnas.91.21.10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Traber MG, Sokol RJ, Kohlschütter A, Yokota T, Muller DPR, Dufour R, Kayden HJ. Impaired discrimination between stereoisomers of α-tocopherol in patients with familial isolated vitamin E deficiency. J Lipid Res. 1993;34:201–210. [PubMed] [Google Scholar]
- 188.Kelleher J, Losowsky MS. The absorption of vitamin E in man. Biochem J. 1968;110:20P–21P. doi: 10.1042/bj1100020pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.MacMahon MT, Neale G. The absorption of alpha-tocopherol in control subjects and in patients with intestinal malabsorption. Clin Sci. 1970;38:197–210. doi: 10.1042/cs0380197. [DOI] [PubMed] [Google Scholar]
- 190.Moshfegh A, Goldman J, Cleveland L. What we eat in America, NHANES 2001-2002: usual nutrient intakes from food compared to dietary reference intakes. United States Department of Agriculture, Agricultural Research Service; 2005. [Google Scholar]
- 191.Gao X, Martin A, Lin H, Bermudez OI, Tucker KL. alpha-Tocopherol intake and plasma concentration of Hispanic and non-Hispanic white elders is associated with dietary intake pattern. J Nutr. 2006;136:2574–2579. doi: 10.1093/jn/136.10.2574. [DOI] [PubMed] [Google Scholar]
- 192.Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M, Hartman AM, Palmgren J, Freedman LS, Haapakoski J, Barrett MJ, Pietinen P, Malila N, Tala E, Liippo K, Salomaa ER, Tangrea JA, Teppo L, Askin FB, Taskinen E, Erozan Y, Greenwald P, Huttunen JK. Alpha-Tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. J Natl Cancer Inst. 1996;88:1560–1570. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- 193.Wright ME, Lawson KA, Weinstein SJ, Pietinen P, Taylor PR, Virtamo J, Albanes D. Higher baseline serum concentrations of vitamin E are associated with lower total and cause-specific mortality in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Clin Nutr. 2006;84:1200–1207. doi: 10.1093/ajcn/84.5.1200. [DOI] [PubMed] [Google Scholar]
- 194.Stephens NG, Parsons A, Schofield PM, Kelly F, Cheeseman K, Mitchinson MJ. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS) Lancet. 1996;347:781–786. doi: 10.1016/s0140-6736(96)90866-1. [DOI] [PubMed] [Google Scholar]
- 195.Boaz M, Smetana S, Weinstein T, Matas Z, Gafter U, Iaina A, Knecht A, Weissgarten Y, Brunner D, Fainaru M, Green MS. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): randomised placebo-controlled trial. Lancet. 2000;356:1213–1218. doi: 10.1016/s0140-6736(00)02783-5. [DOI] [PubMed] [Google Scholar]
- 196.Salonen RM, Nyyssonen K, Kaikkonen J, Porkkala-Sarataho E, Voutilainen S, Rissanen TH, Tuomainen TP, Valkonen VP, Ristonmaa U, Lakka HM, Vanharanta M, Salonen JT, Poulsen HE. Six-year effect of combined vitamin C and E supplementation on atherosclerotic progression: the Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) Study. Circulation. 2003;107:947–953. doi: 10.1161/01.cir.0000050626.25057.51. [DOI] [PubMed] [Google Scholar]
- 197.Gruppo Italiano per lo Studio della Streptochinasi nell'Infarcto Miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 198.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 199.Cheung MC, Zhao XQ, Chait A, Albers JJ, Brown BG. Antioxidant supplements block the response of HDL to simvastatin-niacin therapy in patients with coronary artery disease and low HDL. Arterioscler Thromb Vasc Biol. 2001;21:1320–1326. doi: 10.1161/hq0801.095151. [DOI] [PubMed] [Google Scholar]
- 200.Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, Frohlich J, Albers JJ. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 201.Waters DD, Alderman EL, Hsia J, Howard BV, Cobb FR, Rogers WJ, Ouyang P, Thompson P, Tardif JC, Higginson L, Bittner V, Steffes M, Gordon DJ, Proschan M, Younes N, Verter JI. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: a randomized controlled trial. JAMA. 2002;288:2432–2440. doi: 10.1001/jama.288.19.2432. [DOI] [PubMed] [Google Scholar]
- 202.Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ. Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials. Lancet. 2003;361:2017–2023. doi: 10.1016/S0140-6736(03)13637-9. [DOI] [PubMed] [Google Scholar]
- 203.Berry D, Wathen JK, Newell M. Bayesian model averaging in meta-analysis: vitamin E supplementation and mortality. Clin Trials. 2009;6:28–41. doi: 10.1177/1740774508101279. [DOI] [PubMed] [Google Scholar]
- 204.Miller ER, 3rd, Paston-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 205.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in Randomized Trials of Antioxidant Supplements for Primary and Secondary Prevention: Systematic Review and Meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 206.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 207.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, 3rd, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL, Jr, Baker LH, Coltman CA., Jr Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Pinsky PF, Miller A, Kramer BS, Church T, Reding D, Prorok P, Gelmann E, Schoen RE, Buys S, Hayes RB, Berg CD. Evidence of a healthy volunteer effect in the prostate, lung, colorectal, and ovarian cancer screening trial. Am J Epidemiol. 2007;165:874–881. doi: 10.1093/aje/kwk075. [DOI] [PubMed] [Google Scholar]
- 209.Levy AP, Gerstein HC, Miller-Lotan R, Ratner R, McQueen M, Lonn E, Pogue J. The effect of vitamin E supplementation on cardiovascular risk in diabetic individuals with different haptoglobin phenotypes. Diabetes Care. 2004;27:2767. doi: 10.2337/diacare.27.11.2767. [DOI] [PubMed] [Google Scholar]
- 210.Milman U, Blum S, Shapira C, Aronson D, Miller-Lotan R, Anbinder Y, Alshiek J, Bennett L, Kostenko M, Landau M, Keidar S, Levy Y, Khemlin A, Radan A, Levy AP. Vitamin E Supplementation Reduces Cardiovascular Events in a Subgroup of Middle-Aged Individuals With Both Type 2 Diabetes Mellitus and the Haptoglobin 2-2 Genotype. A Prospective Double-Blinded Clinical Trial. Arterioscler Thromb Vasc Biol. 2008;28:1–7. doi: 10.1161/ATVBAHA.107.153965. [DOI] [PubMed] [Google Scholar]
- 211.Glynn RJ, Ridker PM, Goldhaber SZ, Zee RY, Buring JE. Effects of random allocation to vitamin E supplementation on the occurrence of venous thromboembolism: report from the Women's Health Study. Circulation. 2007;116:1497–1503. doi: 10.1161/CIRCULATIONAHA.107.716407. [DOI] [PubMed] [Google Scholar]
- 212.Harrington DJ, Soper R, Edwards C, Savidge GF, Hodges SJ, Shearer MJ. Determination of the urinary aglycone metabolites of vitamin K by HPLC with redox-mode electrochemical detection. J Lipid Res. 2005;46:1053–1060. doi: 10.1194/jlr.D400033-JLR200. [DOI] [PubMed] [Google Scholar]
- 213.Thijssen HH, Vervoort LM, Schurgers LJ, Shearer MJ. Menadione is a metabolite of oral vitamin K. Br J Nutr. 2006;95:260–266. doi: 10.1079/bjn20051630. [DOI] [PubMed] [Google Scholar]
- 214.Tovar A, Ameho CK, Blumberg JB, Peterson JW, Smith D, Booth SL. Extrahepatic tissue concentrations of vitamin K are lower in rats fed a high vitamin E diet. Nutr Metab (Lond) 2006;3:29. doi: 10.1186/1743-7075-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Booth SL, Golly I, Sacheck JM, Roubenoff R, Dallal GE, Hamada K, Blumberg JB. Effect of vitamin E supplementation on vitamin K status in adults with normal coagulation status. Am J Clin Nutr. 2004;80:143–148. doi: 10.1093/ajcn/80.1.143. [DOI] [PubMed] [Google Scholar]
- 216.Steiner M, Glantz M, Lekos A. Vitamin E plus aspirin compared with aspirin alone in patients with transient ischemic attacks. Am J Clin Nutr. 1995;62:1381S–1384S. doi: 10.1093/ajcn/62.6.1381S. [DOI] [PubMed] [Google Scholar]
- 217.Purpero V, Moran GR. The diverse and pervasive chemistries of the alpha-keto acid dependent enzymes. J Biol Inorg Chem. 2007;12:587–601. doi: 10.1007/s00775-007-0231-0. [DOI] [PubMed] [Google Scholar]
- 218.Yi C, Jia G, Hou G, Dai Q, Zhang W, Zheng G, Jian X, Yang CG, Cui Q, He C. Iron-catalysed oxidation intermediates captured in a DNA repair dioxygenase. Nature. 2010;468:330–333. doi: 10.1038/nature09497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Hoffart LM, Barr EW, Guyer RB, Bollinger JM, Jr, Krebs C. Direct spectroscopic detection of a C-H-cleaving high-spin Fe(IV) complex in a prolyl-4-hydroxylase. Proc Natl Acad Sci U S A. 2006;103:14738–14743. doi: 10.1073/pnas.0604005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Fischmann TO, Hruza A, Niu XD, Fossetta JD, Lunn CA, Dolphin E, Prongay AJ, Reichert P, Lundell DJ, Narula SK, Weber PC. Structural characterization of nitric oxide synthase isoforms reveals striking active-site conservation. Nat Struct Biol. 1999;6:233–242. doi: 10.1038/6675. [DOI] [PubMed] [Google Scholar]