Abstract
Langerhans cells (LC) are distinct dendritic cells (DC) that populate stratified squamous epithelia. Despite extensive studies, our understanding of LC development is incomplete. TGFβ1 is required for LC development, but other epidermis-derived influences may also be important. Recently, EpCAM (CD326) has been identified as cell surface protein discriminating LC from Langerin+ dermal and other DC in skin. EpCAM is a known transcriptional target of the Wnt signaling pathway. We hypothesized that intraepidermal Wnt signaling might influence LC development. Addition of Wnt3A into cultures of bone marrow-derived cells in combination with TGFβ1, GM-CSF, M-CSF resulted in increased (33%; p<0.05) accumulation of EpCAM+ DC. In contrast, addition of the Wnt antagonist Dkk1 decreased numbers of EpCAM+ DC (21%; p<0.05). We used K14-KRM1; K5-rtTA; tetO-Dkk1 triple transgenic and K5-rtTA; tetO-Dkk1 double transgenic mice to test the in vivo relevance of our in vitro findings. Feeding doxycycline to nursing mothers induced expression of Dkk1 in skin of transgenic pups causing an obvious hair phenotype. Expression of Dkk1 reduced LC proliferation (40%; p<0.01) on P7, decreased LC densities (26%; p<0.05) on P14, and decreased EpCAM expression intensities on LC as well (33%). In aggregate, these data suggest that Wnt signaling in skin influences LC development.
INTRODUCTION
Epidermal LC represent a unique subset of dendritic cells (DC) that populate stratified squamous epithelia. They have long been thought to play pivotal roles in initiating immunity by acquiring antigens that are encountered in skin, migrating to draining lymph nodes after activation, and stimulating antigen-specific T cells (Merad et al., 2008). However, recent studies suggest that LC do not function as essential antigen-presenting cells for anti-viral immune responses (Aebischer et al., 2005; Allan et al., 2003) or for contact hypersensitivity reactions (Bennett et al., 2007; Bennett et al., 2005; Bursch et al., 2007; Kaplan et al., 2005; Kissenpfennig et al., 2005) in established murine models. Thus, despite extensive study, important aspects of LC physiology remain to be elucidated. Previous studies with TGFβ1 (Borkowski et al., 1996a) and M-CSF receptor (Ginhoux et al., 2006) knockout mice demonstrated that TGFβ1 and M-CSF are essential for LC development. These cytokines likely act on local precursors (Bogunovic et al., 2006) that proliferate in situ rather than circulating precursors (Merad et al., 2008). However, additional epidermal-derived molecules that act only over short distances may also be relevant for LC development.
We previously determined that EpCAM (CD326) is expressed at high levels by murine LC (Borkowski et al., 1996b) and the ability of EpCAM expression to discriminate LC from Langerin+ dermal DC and other DC has been reported (Bursch et al., 2007). EpCAM is a direct transcriptional target of the canonical Wnt-β-catenin signaling pathway (Yamashita et al., 2007), and Wnt signaling is well known to be involved in epidermal development and homeostasis, and to participate in the development of hematopoietic cells (Clevers, 2006; Fleming et al., 2008; Korinek et al., 1998; Saitoh et al., 1998; Scheller et al., 2006; Wodarz and Nusse, 1998). Thus, we hypothesized that epidermis-derived Wnt proteins might regulate the development and/or homeostasis of EpCAM expressing LC in epidermis.
Binding of Wnt proteins to their receptors (Frizzled proteins), and to members of the low-density lipoprotein receptor-related protein family that serve as essential coreceptors (LRP5 or LRP6) activates the canonical Wnt/β-catening signaling pathway. Pathway activation causes accumulation of β-catenin in the cytoplasm, translocation of β-catenin to the nucleus, formation of active transcription complexes of β-catenin and members of the LEF/TCF family of DNA binding proteins (Bejsovec, 2000; Wodarz and Nusse, 1998) and, subsequently, transcription of genes that are regulated by Wnt-responsive elements. Wnt signaling is regulated via antagonists, including secreted Dickkopf-related protein 1 (Dkk1) (Glinka et al., 1998; Niehrs, 1999, 2001). Dkk1 functions as an inhibitor of Wnt signaling by binding to kremen protein 1 (KRM1) (Mao et al., 2002), a transmembrane high affinity receptor, in conjunction with LPR5/6 (Bafico et al., 2001; Semenov et al., 2001) thereby promoting internalization of LRP5/6 and reduced responsiveness to Wnt (Mao et al., 2002; Wu et al., 2000).
Tissue-selective expression of Dkk1 in transgenic mice can be achieved with lineage-specific promoters and this approach has been used to modulate Wnt signaling in vivo (Andl et al., 2002; Chu et al., 2004; Huelsken et al., 2001; Ito et al., 2007; Liu et al., 2007; Osada et al., 2010; Shu et al., 2005). In situations where constitutive inhibition of Wnt signaling is deleterious, mice with temporal as well as spatial regulation of Dkk1 expression can be utilized. K5-rtTA; tetO-Dkk1 mice are double transgenic animals (DT) that express a tetracycline reverse transactivator (rtTA) protein under control of the keratin 5 promoter. The rtTA protein binds to tetracycline operator elements (tetO) in the presence of doxycycline, resulting in Dkk1 production in the skin of mice that are ingesting the antibiotic. These mice have been used previously to assess the involvement of Wnt signaling in mammary gland development (Chu et al., 2004), wound healing in skin (Ito et al., 2007), and thymus development (Osada et al., 2010).
In this study, we also made use of triple transgenic K14-KRM1; K5-rtTA; tetO-Dkk1 mice that additionally include a Keratin14 promotor-driven KRM1 transgene, since KRM1 is a high-affinity Dkk1 receptor known to functionally cooperate with Dkk1 to inhibit Wnt signaling (Mao et al., 2002). The combination of Dkk1 and KRM1 transgenes potentiates the inhibition of Wnt signaling in keratinocytes (Rothbacher and Lemaire, 2002; Semenov et al., 2001). Although KRM1 single transgenic mice do not display gross alterations in skin architecture or hair cycling, doxycycline-mediated Dkk1-induction in triple transgenic mice reveals an even more severe skin phenotype than that seen in double transgenic K5-rtTA; tetO-Dkk1 mice (Y. S. Choi and S. E. Millar unpublished observations).
Studies of LC function have been constrained by the inability to routinely propagate LC-like cells in vitro. Although we previously described methodology that allowed the generation of LC-like cells from fetal mouse skin (Jakob et al., 1997), this primary culture system no longer supports expansion of cells of interest. Herein, we describe new conditions that allowed us to routinely propagate LC-like cells (CD11c+ MHC class II+ EpCAM+ DC) from murine bone marrow. In the present studies, we assessed the ability of recombinant Wnt protein to promote the development of LC-like DC in vitro, and the ability of the Wnt antagonist Dkk1 to inhibit LC development in vivo in K5-rtTA; tetO-Dkk1 and K14-KRM1; K5-rtTA; tetO-Dkk1 mice. Our results do not conclusively identify an essential role for Wnt signaling in LC development, but do suggest that Wnt signaling can influence LC proliferation, number and phenotype.
RESULTS AND DISCUSSION
Generation of LC-like cells in vitro
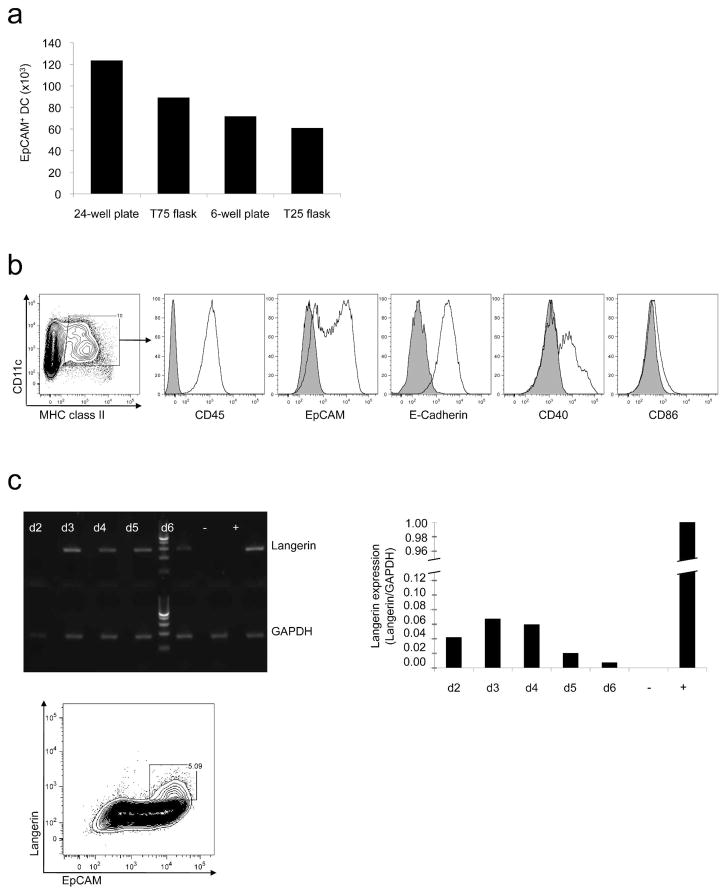
In a series of preliminary experiments, we identified conditions that allowed optimal propagation of LC-like cells in vitro. The shape and size of the culture dishes used had a major impact on the development of CD11c+ MHC class II+ E-Cadherin+ EpCAM+ LC-like cells (Figure 1a). The largest numbers of total leukocytes and LC-like cells were obtained in 24-well plates. The time period after initiation of culture also influenced expression of various markers. After 72 hours, 10% of all cells expressed CD45, CD11c, MHC class II, E-Cadherin, EpCAM, and CD40. As expected, adding TGFβ1 into cultures prevented maturation of the LC-like cells as manifested by expression of low levels of MHC class II and CD86 (Figure 1b). However, stimulation of LC-like cells with 100 ng/ml LPS for 22 hours in subcultures without TGFβ1 increased CD86 and MHC class II expression, indicating that these DC were capable of maturation (data not shown). Langerin expression by cultured EpCAM+ cells was low as compared to freshly isolated epidermal LC. QRT-PCR revealed an increase in Langerin mRNA expression by cultured LC-like cells over the first 72 hours. Accordingly, flow cytometry revealed a peak in intracellular Langerin protein expression after 96 hours (Figure 1c). The number of LC-like cells per well decreased after 120 hours.
Figure 1. Generation of bone marrow derived LC-like cells.
(a) Shape and size of culture dishes had a major impact on the total number of EpCAM+ DC recovered. Data expressed as total cell numbers of EpCAM+ cells per culture dish per 106 input cells. (b) Phenotypes of LC-like cells after 72 hours as determined by flow cytometry. (c) Expression of Langerin by LC-like cells from day 2 until day 6 of culture as determined by RT-PCR (RNase free water served as negative control (−), freshly isolated epidermal Langerhans cells served as positive control (+), qRT-PCR data expressed as fold expression in relation to GAPDH and flow cytometry on day 4 of culture (gated on CD11c+ MHC class II+ cells).
in vitro effects of Wnt signaling modulators on LC-like cells
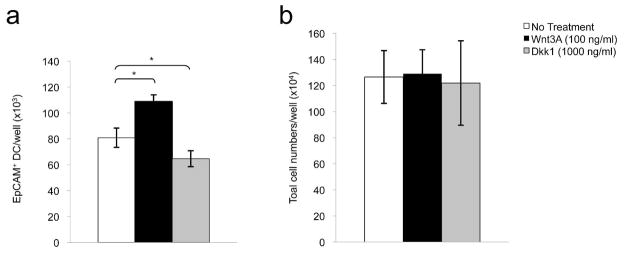
To investigate the involvement of Wnt signaling in LC development, we initially characterized effects of Wnt protein and the Wnt antagonist Dkk1 on the development of murine LC-like DC in C57BL/6 bone marrow cultures. Initial dose response studies revealed maximal effects of Wnt3A and Dkk1 at 100 ng/ml and 1000 ng/ml respectively (data not shown). Addition of Wnt3A (100 ng/ml), which is known to activate the Wnt/β-catenin signaling pathway (Kishida et al., 1999), into bone marrow cultures resulted in modest increases in the numbers of LC-like DC that were recovered after 72 hours (~33%; p<0.05, Figure 2a). In contrast, the potent Wnt inhibitor Dkk1 (1000 ng/ml) decreased the number of LC-like cells accumulating in cultures that were not supplemented with Wnt3A protein (~21%, p<0.05, Figure 2a). Total leukocyte numbers, determined at 72 hours, did not change significantly in the presence of Wnt3A or Dkk1 (Figure 2b). These results indicate that Wnt3A has a modest selective effect on the development of LC-like cells in vitro, and suggest that small amounts of endogenous Wnt proteins may be present and active in bone marrow cultures.
Figure 2. Effects of Wnt protein and a Wnt antagonist on the development of LC-like DC in vitro.
(a) Aggregate results of effects of Wnt3A (100 ng/ml) and and Wnt inhibitor Dkk1 (1000 ng/ml) on EpCAM+ DC accumulation after 72 hours, untreated cells (No Treatment) served as controls (n=4, *p<0.05). (b) Lack of effects of Wnt3A and Dkk1 on total leukocyte numbers in bone marrow cultures after 72 hours (n=4 experiments). Error bars represent the mean ± SEM.
Influence of intraepidermal Wnt signaling on LC in vivo
To assess possible effects of Wnt signaling on LC development in situ, we initially characterized LC in the epidermis of K5-rtTA; tetO-Dkk1 DT mice (Supplemental Figure 1). Keratinocytes in these mice produce the Wnt inhibitor Dkk1 after exposure to doxycycline (Chu et al., 2004). Dkk1 was induced in the skin of young mice by feeding doxycycline to nursing mothers beginning on postnatal day 0 (P0). This approach avoids the limb and dental defects that would result from earlier exposure of developing mice to Dkk1 (Chu et al., 2004). Due to a lack of availability of the DT mice, we performed subsequent studies with K14-KRM1; K5-rtTA; tetO-Dkk1 TT mice. In TT mice, the Wnt inhibiting effect of Dkk1 is potentiated in keratinocytes by the additional expression of KRM1 in K14 expressing cells. Direct effects of Dkk1 on LC or LC precursors are expected to be identical in DT and TT mice.
LC precursors enter murine skin soon after epidermal differentiation is completed and undergo a massive burst of proliferation between postnatal days 2 (P2) and 7 (P7), reaching “adult” numbers within the first two weeks after birth. (Chang-Rodriguez et al., 2004; Chang-Rodriguez et al., 2005; Chorro et al., 2009; Elbe-Burger and Schuster, 2010; Kobayashi et al., 1987; Tripp et al., 2004). Thus, it was anticipated that an effect of Wnt inhibition by Dkk1 would be evident prior to P14 if Wnt proteins were involved in LC development.
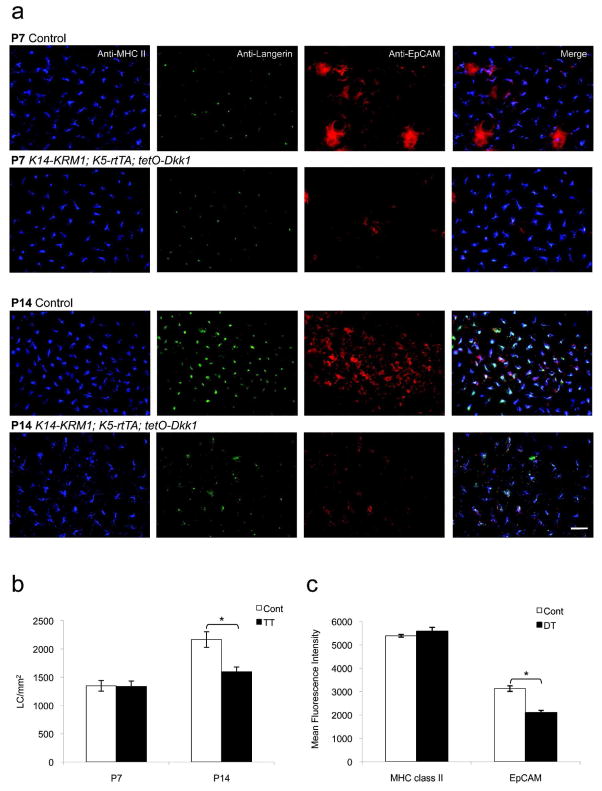
Dkk1 induction resulted in an obvious body size and hair phenotype. DT and TT mice were smaller and had less terminal hair than their littermate controls. This confirms that administration of doxycycline to nursing mothers induced Dkk1 expression in the skin of pups with the appropriate genotype. Immunofluorescence staining of P7 TT epidermal sheets demonstrated that LC in Dkk1-expressing epidermis were present and expressed MHC class II, Langerin, and EpCAM (Figure 3a). Enumeration of LC in TT epidermal sheets on P7 did not reveal significant differences between TT mice and control animals. However, on P14 LC densities were ~26% lower in Dkk1-producing TT mice (p<0.05, Figure 3b), consistent with the lower LC densities (~21%, p<0.05) that had been previously observed in the P14 epidermis of DT mice (Supplemental Figure 1). Careful inspection revealed that LC morphology was also somewhat abnormal and anti-EpCAM immunofluorescence staining intensity was decreased in Dkk1-expressing mice. This impression was confirmed when laser scanning cytometry measurements revealed that EpCAM staining intensities in the epidermis of Dkk1-expressing DT animals were decreased by ~33% as compared to controls, whereas MHC class II staining intensities were equivalent (Figure 3c).
Figure 3. LC densities and characteristics in mice that express a Wnt antagonist in epidermis.
(a) Epidermal sheets on postnatal days 7 and 14 (P7, P14) from doxycycline-exposed littermate control (Cont) and K14-KRM1; K5-rtTA; tetO-Dkk1 (TT) mice, stained with anti-EpCAM, anti-Langerin, anti-MHC class II mAb and visualized via immunofluorescence microscopy (bar = 25 μm). (b) LC densities from TT and Cont mice on P7 and P14 (3 random fields per mouse, n=5 mice per group, *p<0.05). (c) Mean fluorescence intensities of MHC class II and EpCAM expression in epidermal sheets from K5-rtTA; tetO-Dkk1 (DT) and Cont mice as determined with a Laser Scanning Cytometer (see Materials and Methods, 3 random fields per mouse, n=2 mice per group, *p<0.05). Error bars represent the mean ± SEM.
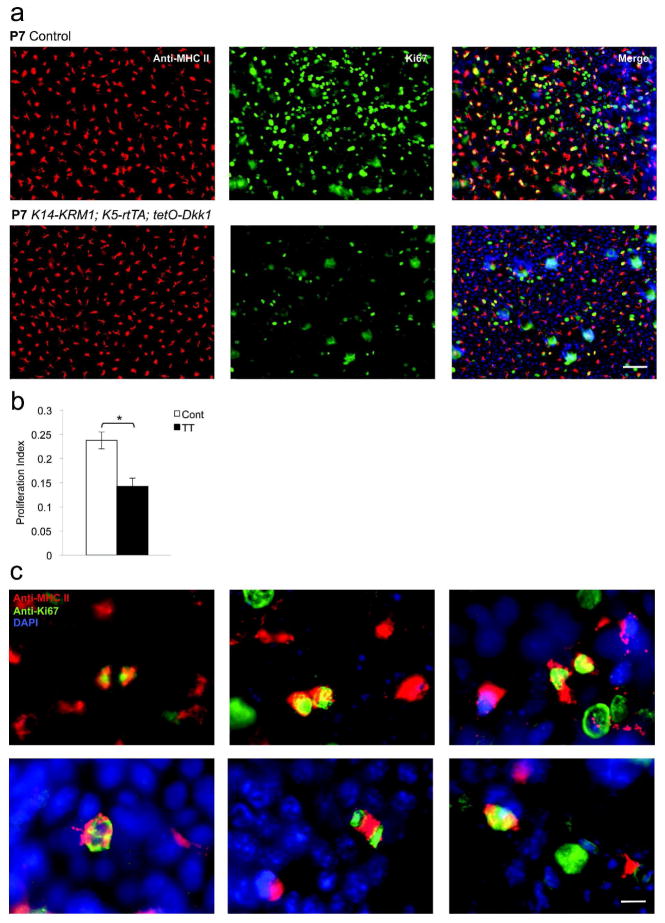
Consistent with the decreased LC densities that were observed, Dkk1-producing TT mice also displayed a 40% decrease in LC proliferation on P7 as determined by quantifying Ki67 proliferation indices (p<0.01, Figure 4a and b). Interestingly, analysis of epidermal sheets revealed numerous MHC class II+ cells at various stages of the mitosis (Figure 4c).
Figure 4. LC proliferation in mice that express a Wnt antagonist in epidermis.
(a) Epidermal sheets were prepared on postnatal day 7 (P7) from doxycycline-exposed littermate control (Cont) and K14-KRM1; K5-rtTA; tetO-Dkk1 (TT) mice, stained with anti-MHC class II mAb and anti-Ki67 pAb and visualized via immunofluorescence microscopy (bar = 50 μm). (b) LC proliferation indices as determined by dividing MHC class II+ Ki67+ cells by MHC class II+ cells (3 random fields per mouse, n=5, *p<0.01). Error bars represent the mean ± SEM. (c) Various stages of mitosis in MHC class II+ cells as depicted by Ki67 staining (bar = 15 μm).
Multiple secreted proteins and differentiation signaling pathways may regulate proliferation of LC precursors in fetal/neonatal skin (Elbe-Burger and Schuster, 2010). Our in vivo experiments suggest that Wnt signaling regulates LC proliferation, but that it is not absolutely required for LC development in mice post-weaning. Our data also suggest that Wnt signaling influences murine LC phenotype and regulates EpCAM expression by LC, as has been reported for other cells (Munz et al., 2009; Yamashita et al., 2007). It remains possible that Wnt signaling is essential for LC development at earlier stages of postnatal life than examined in the present study. Since LC survive for months to years in unperturbed epidermis, Wnt dependency might be very difficult to demonstrate after LC differentiation has been completed or even initiated. Additional studies regarding factors and signaling pathways that regulate LC precursors in fetal mouse epidermis, and identification of culture conditions that allow routine propagation of LC in vitro will be important for further characterization of this incompletely understood developmental process.
MATERIALS AND METHODS
Mice
Adult female C57BL/6 mice were obtained from the NCI-Fredrick Animal Production Program, Frederick, MD. K5-rtTA; tetO-Dkk1 mice have been described previously (Chu et al., 2004). FvB female K5rtTA Tg mice were mated to FvB male tetO-Dkk1 Tg to generate K5-rtTA; tetO-Dkk1 DT animals for study. These mice were additionally crossed to K14-KRM1 mice to obtain TT animals. Littermates without the doxycycline responsive transgene were used as controls. Doxycycline was fed to nursing mothers beginning on postnatal day 0 (P0) to induce Dkk1 production in the epidermis of DT and TT animals. Mice were studied on P7 and P14, as indicated. All mice were bred and housed in a pathogen-free environment and used in experiments in accordance with institutional guidelines. Mice were genotyped using tail clip DNA isolated via the Qiagen DNeasy Blood and Tissue Kit (Quiagen, Valencia, California) used according to the manufacturer’s protocol and PCR. PCR primers for rtTA (F: 5′ AGC TGC TTA ATG AGG TCG GA -3; R: 5′ GCT TGT CGT AAT AAT GGC GG -3), Dkk1 (F: 5′-CCC GGA TCC GCG TCC TTC GGA GAT GAT GG-3′; R: 5′-AAT GGA TCC TTT AGA CTG TCG GTT TAG TGT CTC-3′) and KRM1 (F: 5′-CCG AGT GCA ATA GTG TCT GC-3′; R: 5′-GGC TTG CTC GGT GAT CAC CTC CTC-3′) were used in conjunction with the following incubation conditions: 95°C for 2 minutes, 75°C for 95 seconds and 35 repeats of a cycle at 95°C for 30 seconds, 55°C for 40 seconds and 72°C for 2 minutes.
Dendritic Cell Cultures
LC-like DC were propagated as described previously (Inaba et al., 2009), with some modifications. Briefly, femurs and tibias from C57BL/6 mice were flushed, and recovered cells were counted and resuspended at 106 cells/ml in MEM α medium (Gibco Invitrogen, Carlsbad, California), containing 10% heat-inactivated FBS (HyClone Thermo Scientific, Waltham, Massachusetts), 2 mM Glutamine, 0.1 mM NEAA, 10 mM HEPES, 1% PenStrep (all Gibco Invitrogen, Carlsbad, California), 50 μM 2-mercaptoethanol (Sigma, St. Louis, Missouri), and the recombinant cytokines human TGFβ1, murine GM-CSF, murine M-CSF (all PeproTech, Rocky Hill, New Jersey) at concentrations of 10 ng/ml each. Recombinant murine Wnt3A and Dkk1 were purchased from R&D Systems (Minneapolis, Minnesota). Wnt3A was provided in lyophilized form from PBS, 01 mM EDTA and 0.5% (w/v) CHAPS, pH 6.8 with BSA as a carrier protein. Diluent controls were utilized as indicated.
Antibodies
Purified rat IgG2aκ anti-mouse Langerin mAb (clone L31) and the corresponding isotype control were purchased from eBioscience (San Diego, California) and labeled with Alexa Fluor 488 or 647 using mAb labeling kits (Invitrogen). Poloyclonal rabbit anti-Ki67 (Abcam, Cambridge, Massachusetts) was used in combination with an donkey anti-rabbit Alexa 488-labeled secondary Ab (Invitrogen). Additional directly-labeled mAb and their isotype controls (BD Biosciences, San Jose, California unless otherwise indicated) were used for immunofluorescence microscopy and flow cytometry to detect the following: EpCAM (Alexa Fluor 488 or 647-G8.8, BioLegend, San Diego, California), CD11c (APC-HL3), and MHC class II (FITC-M5/114.15.2). Rat anti-mouse CD16/32 (2.4G2) and rat IgG2aκ were routinely used for blocking (2.5 μg/ml) before staining, for Ki67 staining 5% donkey serum (Jackson ImmunoResearch Laboratories, West Grove, Pennsylvania) was added into the blocking buffer.
Flow Cytometry
Data was collected with a FACSCalibur flow cytometer (BD) and analyzed with FlowJo software (Treestar, Ashland, Oregon). Nonviable cells were excluded after 7-AAD (BD Biosciences) staining, unless cells had been fixed and permeabilized (Cytofix/Cytoperm Kit, BD Biosciences) before analysis.
Assessment of Langerin mRNA Expression by LC-like Cells and LC
Cultured LC-like cells were enriched for EpCAM+ cells by incubation with Alexa Fluor 647 labeled anti-mouse EpCAM mAb (G8.8 clone) and positive selection using anti-Alexa Fluor 647 magnetic beads and the MACS Separation Unit (Miltenyi, Biotec, Bergisch Gladbach, Germany). Total RNA from the EpCAM+ cells was extracted using TRIzol Reagent (Gibco Invitrogen), purified via the RNeasy Mini Kit (Qiagen) and used to prepare cDNA with SuperScript III First-Strand Synthesis SuperMix (Invitrogen) in accordance with the manufacturers’ protocols. As a positive control, freshly isolated Langerhans cells were prepared from epidermal cell suspensions (Tang et al., 1993) using Lympholyte M (Cedarlane Laboratories Limited, Burlington, North Carolina) density gradients. Interphase cells were further enriched for EpCAM+ cells using magnetic beads as mentioned above. Flow cytometry of the positive selected cell fraction ensured an enrichment of 95% EpCAM+ cells (data not shown). RNase free water served as the negative control.
Semi-quantitative PCR was performed using Platinum PCR SuperMix (Invitrogen) as well as primers for Langerin (5′-ACGCACCCCAAAGACCTGGTACAG-3′, 5′-AGACACCC TGATATTGGCACAGTG-3′) and GAPDH, and cycling conditions of 95°C for 5 minutes, 30 repeats of cycles at 95°C for 30 seconds, 60°C for 30 seconds and 72°C for 1 minute, and a final extension at 72°C for 7 minutes.
Quantitative PCR was performed using Maxima SYBR Green qPCR Master Mix (Fermentas Thermo Scientific), primers for Langerin and GAPDH, and cycling conditions of 95°C for 10 minutes, 40 repeats of cycles at 95°C for 30 seconds, 60°C for 30 seconds and 72°C for 1 minute, and a final extension at 65°C for 7 minutes.
Preparation of Epidermal Sheets
Ears were split into dorsal and ventral halves, cartilage and subcutaneous tissue were removed, and skin was floated on 3.8% ammonium thiocyanate (Sigma) in PBS for 20 minutes at 37° C. Epidermis was separated from dermis and fixed in acetone at 20°C for 15 minutes prior to rehydration in PBS.
Immunofluorescence Microscopy
Rehydrated epidermal sheets were incubated in 3% dry milk-PBS (Bio-Rad Laboratories, Hercules, California) including 5 μg/ml rat anti-CD16/32 mAb (BD Biosciences) for 1 hour at RT to minimize nonspecific staining before incubation with fluorochrome-labeled mAb for 1 hour at RT or overnight at 4°C. For Ki67 staining, 5% donkey serum was added into the blocking buffer. Labeled cells were visualized using a Zeiss AxioImager A1 Imunofluorescence Microscope. Intensities of digital images in experimental and control specimens were adjusted within the linear range with Zeiss Axiovison software (all Carl Zeiss, Oberkochen, Germany). LC densities and Ki67 proliferation indices were determined by counting at least 3 random fields per animal at 200x final magnification. The latter was obtained by dividing the number of Ki67/MHC class II double positive cells by MHC class II positive cells in each epidermal sheet. Mean fluorescence intensities corresponding to expression of MHC class II and EpCAM in microscopic fields in epidermal sheets were determined using a Compucyte Laser Scanning Cytometer and the iCYS 3.4 software (CompuCyte Corporation, Westwood, Massachusetts).
Statistics
P values were calculated with Microsoft Excel 2008 for Mac using the Student’s t-test (p<0.05 was considered to be statistically significant). Error bars represent the mean ± SEM, n as indicated.
Supplementary Material
Acknowledgments
We thank Dr. William Telford for his advice and assistance with Flow and Laser Scanning Cytometer related experiments. We also thank Jay Linton, Michael Lu, and Dr. Sei-ichiro Motegi for advice and assistance with other experimental procedures. This work was supported by the Intramural Research Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Abbreviations
- Cont
littermate controls without the doxcycline responsive transgene
- DC
dendritic cells
- Dkk1
dickkopf-related protein 1
- DT
double transgenic animals (K5-rtTA; tetO-Dkk1)
- KRM1
kremen protein 1
- LC
Langerhans cells
- TT
triple transgenic animals (K14-KRM1; K5-rtTA; tetO-Dkk1 mice)
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- Aebischer T, Bennett CL, Pelizzola M, et al. A critical role for lipophosphoglycan in proinflammatory responses of dendritic cells to Leishmania mexicana. Eur J Immunol. 2005;35:476–86. doi: 10.1002/eji.200425674. [DOI] [PubMed] [Google Scholar]
- Allan RS, Smith CM, Belz GT, et al. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–8. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- Andl T, Reddy ST, Gaddapara T, et al. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–53. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Bafico A, Liu G, Yaniv A, et al. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf-1 interaction with LRP6/Arrow. Nat Cell Biol. 2001;3:683–6. doi: 10.1038/35083081. [DOI] [PubMed] [Google Scholar]
- Bejsovec A. Wnt signaling: an embarrassment of receptors. Curr Biol. 2000;10:R919–22. doi: 10.1016/s0960-9822(00)00852-6. [DOI] [PubMed] [Google Scholar]
- Bennett CL, Noordegraaf M, Martina CA, et al. Langerhans cells are required for efficient presentation of topically applied hapten to T cells. J Immunol. 2007;179:6830–5. doi: 10.4049/jimmunol.179.10.6830. [DOI] [PubMed] [Google Scholar]
- Bennett CL, van Rijn E, Jung S, et al. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–76. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogunovic M, Ginhoux F, Wagers A, et al. Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men. J Exp Med. 2006;203:2627–38. doi: 10.1084/jem.20060667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkowski TA, Letterio JJ, Farr AG, et al. A role for endogenous transforming growth factor beta 1 in Langerhans cell biology: the skin of transforming growth factor beta 1 null mice is devoid of epidermal Langerhans cells. J Exp Med. 1996a;184:2417–22. doi: 10.1084/jem.184.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkowski TA, Nelson AJ, Farr AG, et al. Expression of gp40, the murine homologue of human epithelial cell adhesion molecule (Ep-CAM), by murine dendritic cells. Eur J Immunol. 1996b;26:110–4. doi: 10.1002/eji.1830260117. [DOI] [PubMed] [Google Scholar]
- Bursch LS, Wang L, Igyarto B, et al. Identification of a novel population of Langerin+ dendritic cells. J Exp Med. 2007;204:3147–56. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang-Rodriguez S, Ecker R, Stingl G, et al. Autocrine IL-10 partially prevents differentiation of neonatal dendritic epidermal leukocytes into Langerhans cells. J Leukoc Biol. 2004;76:657–66. doi: 10.1189/jlb.0204087. [DOI] [PubMed] [Google Scholar]
- Chang-Rodriguez S, Hoetzenecker W, Schwarzler C, et al. Fetal and neonatal murine skin harbors Langerhans cell precursors. J Leukoc Biol. 2005;77:352–60. doi: 10.1189/jlb.1004584. [DOI] [PubMed] [Google Scholar]
- Chorro L, Sarde A, Li M, et al. Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J Exp Med. 2009;206:3089–100. doi: 10.1084/jem.20091586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu EY, Hens J, Andl T, et al. Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. Development. 2004;131:4819–29. doi: 10.1242/dev.01347. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Elbe-Burger A, Schuster C. Development of the prenatal cutaneous antigen-presenting cell network. Immunol Cell Biol. 2010;88:393–9. doi: 10.1038/icb.2010.13. [DOI] [PubMed] [Google Scholar]
- Fleming HE, Janzen V, Lo Celso C, et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–83. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Tacke F, Angeli V, et al. Langerhans cells arise from monocytes in vivo. Nat Immunol. 2006;7:265–73. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, et al. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–62. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, et al. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–45. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Inaba K, Swiggard WJ, Steinman RM, et al. Isolation of dendritic cells. Curr Protoc Immunol. 2009;Chapter 3(Unit 3):7. doi: 10.1002/0471142735.im0307s86. [DOI] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–20. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- Jakob T, Saitoh A, Udey MC. E-cadherin-mediated adhesion involving Langerhans cell-like dendritic cells expanded from murine fetal skin. J Immunol. 1997;159:2693–701. [PubMed] [Google Scholar]
- Kaplan DH, Jenison MC, Saeland S, et al. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–20. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Kishida M, Koyama S, Kishida S, et al. Axin prevents Wnt-3a-induced accumulation of beta-catenin. Oncogene. 1999;18:979–85. doi: 10.1038/sj.onc.1202388. [DOI] [PubMed] [Google Scholar]
- Kissenpfennig A, Henri S, Dubois B, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–54. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Asano H, Fujita Y, et al. Development of ATPase-positive, immature Langerhans cells in the fetal mouse epidermis and their maturation during the early postnatal period. Cell Tissue Res. 1987;248:315–22. doi: 10.1007/BF00218198. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–83. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- Liu F, Thirumangalathu S, Gallant NM, et al. Wnt-beta-catenin signaling initiates taste papilla development. Nat Genet. 2007;39:106–12. doi: 10.1038/ng1932. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Davidson G, et al. Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature. 2002;417:664–7. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–47. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- Munz M, Baeuerle PA, Gires O. The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res. 2009;69:5627–9. doi: 10.1158/0008-5472.CAN-09-0654. [DOI] [PubMed] [Google Scholar]
- Niehrs C. Head in the WNT: the molecular nature of Spemann’s head organizer. Trends Genet. 1999;15:314–9. doi: 10.1016/s0168-9525(99)01767-9. [DOI] [PubMed] [Google Scholar]
- Niehrs C. Developmental biology. Solving a sticky problem. Nature. 2001;413:787–8. doi: 10.1038/35101682. [DOI] [PubMed] [Google Scholar]
- Osada M, Jardine L, Misir R, et al. DKK1 mediated inhibition of Wnt signaling in postnatal mice leads to loss of TEC progenitors and thymic degeneration. PLoS One. 2010;5:e9062. doi: 10.1371/journal.pone.0009062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbacher U, Lemaire P. Creme de la Kremen of Wnt signalling inhibition. Nat Cell Biol. 2002;4:E172–3. doi: 10.1038/ncb0702-e172. [DOI] [PubMed] [Google Scholar]
- Saitoh A, Hansen LA, Vogel JC, et al. Characterization of Wnt gene expression in murine skin: possible involvement of epidermis-derived Wnt-4 in cutaneous epithelial-mesenchymal interactions. Exp Cell Res. 1998;243:150–60. doi: 10.1006/excr.1998.4152. [DOI] [PubMed] [Google Scholar]
- Scheller M, Huelsken J, Rosenbauer F, et al. Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat Immunol. 2006;7:1037–47. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Tamai K, Brott BK, et al. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol. 2001;11:951–61. doi: 10.1016/s0960-9822(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Shu W, Guttentag S, Wang Z, et al. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev Biol. 2005;283:226–39. doi: 10.1016/j.ydbio.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Tang A, Amagai M, Granger LG, et al. Adhesion of epidermal Langerhans cells to keratinocytes mediated by E-cadherin. Nature. 1993;361:82–5. doi: 10.1038/361082a0. [DOI] [PubMed] [Google Scholar]
- Tripp CH, Chang-Rodriguez S, Stoitzner P, et al. Ontogeny of Langerin/CD207 expression in the epidermis of mice. J Invest Dermatol. 2004;122:670–2. doi: 10.1111/j.0022-202X.2004.22337.x. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Wu W, Glinka A, Delius H, et al. Mutual antagonism between dickkopf1 and dickkopf2 regulates Wnt/beta-catenin signalling. Curr Biol. 2000;10:1611–4. doi: 10.1016/s0960-9822(00)00868-x. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Budhu A, Forgues M, et al. Activation of hepatic stem cell marker EpCAM by Wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Res. 2007;67:10831–9. doi: 10.1158/0008-5472.CAN-07-0908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.