Abstract
Most spiking neurons are divided into functional compartments: a dendritic input region, a soma, a site of action potential initiation, an axon trunk and its collaterals for propagation of action potentials, and distal arborizations and terminals carrying the output synapses. The axon trunk and lower order branches are probably the most neglected and are often assumed to do nothing more than faithfully conducting action potentials. Nevertheless, there are numerous reports of complex membrane properties in non-synaptic axonal regions, owing to the presence of a multitude of different ion channels. Many different types of sodium and potassium channels have been described in axons, as well as calcium transients and hyperpolarization-activated inward currents. The complex time- and voltage-dependence resulting from the properties of ion channels can lead to activity-dependent changes in spike shape and resting potential, affecting the temporal fidelity of spike conduction. Neural coding can be altered by activity-dependent changes in conduction velocity, spike failures, and ectopic spike initiation. This is true under normal physiological conditions, and relevant for a number of neuropathies that lead to abnormal excitability. In addition, a growing number of studies show that the axon trunk can express receptors to glutamate, GABA, acetylcholine or biogenic amines, changing the relative contribution of some channels to axonal excitability and therefore rendering the contribution of this compartment to neural coding conditional on the presence of neuromodulators. Long-term regulatory processes, both during development and in the context of activity-dependent plasticity may also affect axonal properties to an underappreciated extent.
1. Introduction
Most axons perform three tasks that are crucial for neuronal communication over distances that are prohibitive for passive electrotonic spread of electrical potentials: action potential initiation, propagation, and action potential-mediated transmitter release (Fig. 1A). At the proximal axon, either the hillock or the axon initial segment (AIS), analog signals are converted into digital ones. The mostly graded responses to synaptic input are shaped to different degrees by complex passive and active dendrite properties (Gulledge et al., 2005; London and Hausser, 2005; Magee, 2000; Sidiropoulou et al., 2006; Spruston, 2008), and translated into a temporal pattern of all-or-none regenerative action potentials (“spikes”, Fig. 1B). Spikes initiated at proximal sites are then propagated along the axon trunk and branches towards distal presynaptic sites, where depolarization results in transmitter release. The process of impulse propagation adds considerable delay to the information flow, depending on axon length and conduction velocity (Fig. 1C).
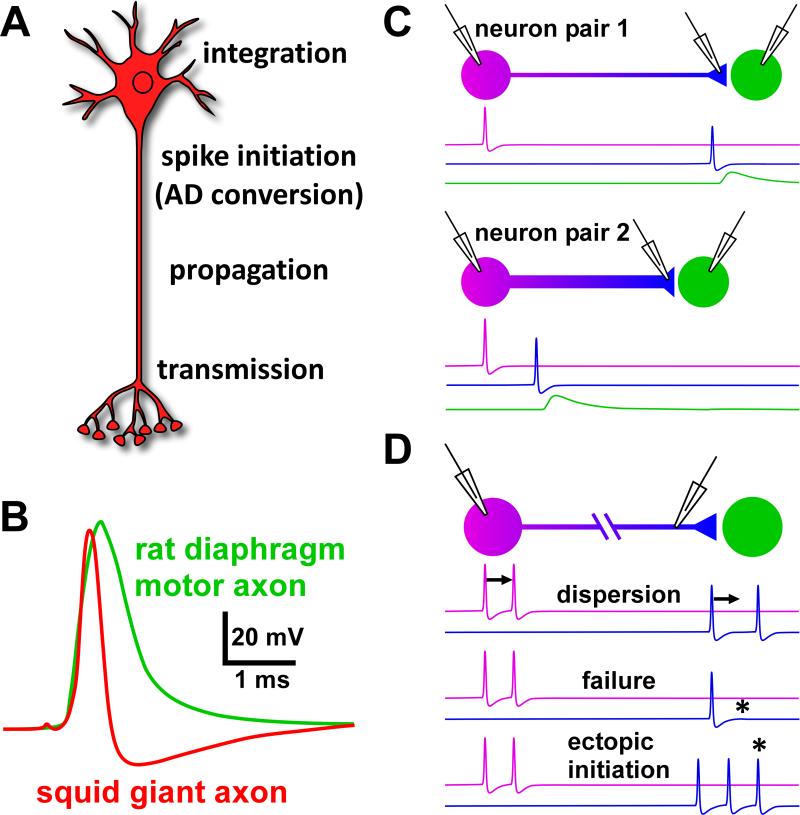
Fig. 1.
Action potential conduction in axons of spiking neurons. A: Schematic representation of a neuron with proximal integration of synaptic input and spike initiation. Spikes are propagated along the axon into distal terminals where depolarization results in transmitter release. B: Typical spike waveforms obtained from intracellular recordings. C: Schematic of conduction delay. The propagation time introduces a latency between spike initiation and postsynaptic responses potentially much larger than the synaptic delay, and very different across different pairs of neurons. D: Changes of temporal patterns between proximal and distal recording sites introduced by the process of propagation, including temporal dispersion, spike failures, and ectopic spike initiation. B is modified from Hodgkin and Huxley, 1939, and David et al., 1995.
A large body of work on axons has focused on the contribution of different voltage-gated ion channels to the initiation of spikes (Baranauskas, 2007; Bean, 2007; Clark et al., 2009), and recent studies have analyzed in detail the functional basis and morphological site of this process in different neuron types (Khaliq and Raman, 2006; Kole et al., 2008; Kress et al., 2008; Lorincz and Nusser, 2008; Meeks and Mennerick, 2007; Shu et al., 2007a). There also has been considerable interest in the dynamics of spike-mediated presynaptic depolarization. For example, activity-dependent changes in spike shape potentially have a significant effect on presynaptic calcium influx and therefore transmitter release (Brody and Yue, 2000; Brown and Randall, 2009; Geiger and Jonas, 2000; Juusola et al., 2007; Ma and Koester, 1995; Sasaki et al., 2011). Such activity-dependent dynamics and presynaptic modulation essentially mean that spikes arriving at the presynaptic terminal are not pure “all-or-none” signals anymore, and that coding is a mix of analog and digital components.
Importantly, activity-dependent changes in excitability are not restricted to distal or proximal axonal sites but also occur along the axonal path, i.e. in the “axon trunk” or “axon proper”. Before John Eccles pioneered intracellular recordings from central neurons and the study of synapses (Brock et al., 1952; Burke, 2006; Eccles, 1964) and for some time after, electrophysiology was dominated by the study of peripheral axons. Because of the experimental accessibility of large axons in peripheral nerve, particularly in invertebrates and lower vertebrates, the basic understanding of neuronal excitability was derived from axons. Interestingly, the study of spike conduction during repetitive activation for a long time represented the only way to gauge the dynamics of neural communication. Based on these studies, it has long been known that repetitive activity can change conduction velocities and alter spike patterns (Bullock, 1951; Swadlow et al., 1980; Swadlow and Waxman, 1976), and can even lead to spike failures (Barron and Matthews, 1935; Krnjevic and Miledi, 1959) or ectopic spike initiation (Standaert, 1963, 1964; Toennies, 1938) (Fig. 1D). Therefore, the process of spike propagation itself can significantly contribute to the patterning of neuronal communication. Whereas for a long time this was either ignored or considered a biological limit of temporal fidelity (Swadlow et al., 1980), non-synaptic axonal membrane has recently become a site of renewed interest (Debanne, 2004; Debanne et al., 2011; Kress and Mennerick, 2009; Segev and Schneidman, 1999; Sidiropoulou et al., 2006). Advances in electrophysiological and molecular techniques have allowed greater insight into the properties and function of axons, even small central ones, and have resulted in the changed view that the axon trunk and branches potentially contribute to the short-term dynamics of neuronal communication and increase the computational capabilities of the neuron.
Here we discuss the possible functional consequences of changes in spike propagation from a physiological perspective. Rather than giving an exhaustive account of different types of ion channels and membrane properties across different axons, and of the molecular underpinnings of axonal specializations, we focus on different aspects of temporal fidelity and activity-dependent changes. Apart from the short-term dynamics of spike propagation that arise mostly from complex complements of ion channels with different time- and voltage-dependences, we also discuss larger time-scale effects. First, there is good evidence from a range of different systems showing that non-synaptic axonal membrane can be endowed with receptors to GABA, acetylcholine, or monoamines. Therefore, axonal properties may be affected by neuromodulators in similar ways to somato-dendritic cell compartments and the proximal axon, and thus render spike propagation conditional on neuromodulatory state. Second, long-term regulatory mechanisms affecting axon morphology and intrinsic membrane properties, as in the context of growth and activity-dependent intrinsic plasticity, may play an important role in determining the dynamics of spike propagation. We discuss experimental evidence for these different aspects of spike propagation from the perspective of morphological diversity and differences in coding strategy that different neurons utilize.
2. Diversity of axons
The vast diversity of neurons within and across species is also reflected in axonal morphology, mode of signal propagation, dynamic range of activity, and complement of ion channels. The delay in communication between neurons that is introduced by spike propagation, and the dynamic changes of this delay, depend on the intricate interplay between axon structure and excitability, and on the type of activity that is propagated. In this section, we will first describe the structural and functional diversity of axons, and then review the diversity of ion channels found in axonal membrane. Readers of the literature on axons, and of published work touching upon axons in passing, will notice that there is a lack of consistent terminology. When the presence of a physiological property or an ion channel or receptor is described, it is not always clear at first glance if the term axon refers to the proximal axon in the context of spike initiation, the “axon proper” or trunk, or to terminal branches or even presynaptic sites alone. Here we will use the term axon for trunk and branches in the context of propagation, unless explicitly stated otherwise.
2.1. Structural and functional diversity of axons
Before considering the dynamics of spike propagation, one has to consider the absolute delay that is introduced by the process of propagation. Delay in itself may be an important part of network computational capacity (Carr and Konishi, 1988; Izhikevich, 2006; Karino et al., 2011), and is carefully regulated in some cases to ensure consistent latencies and synchrony across projections over different distances (Budd et al., 2010; Govind and Lang, 1976a; Salami et al., 2003; Stanford, 1987; Sugihara et al., 1993). The extent to which activity-dependent changes in propagation can alter the temporal structure of spike patterns depends to some degree on the mean total delay, so ultimately on conduction velocity and axonal length.
2.1.1. Axon diameters and conduction velocity
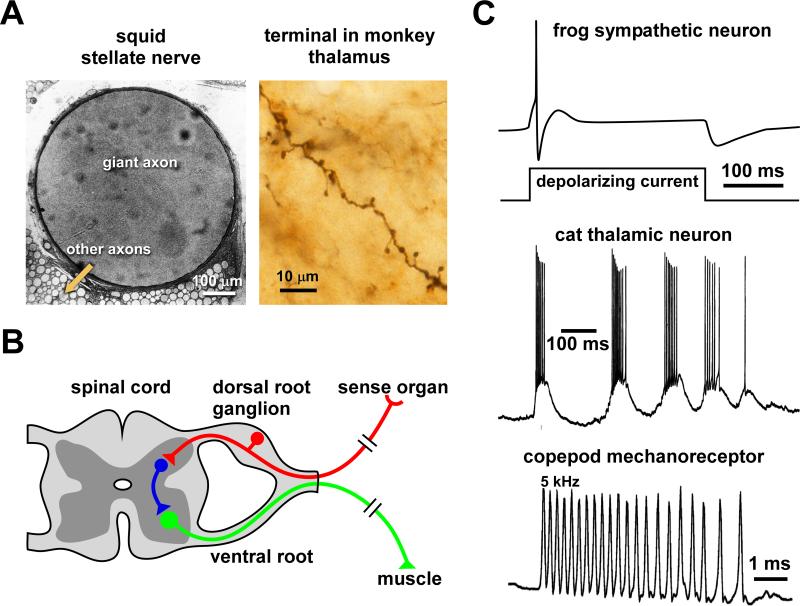
Conduction velocity depends on biophysical parameters like membrane capacitance and resistance, axial resistance, density of voltage-gated ion channels, and on diameter (Colquhoun and Ritchie, 1972; Del Castillo and Moore, 1959; Hodgkin, 1939, 1954; Katz, 1947; Renganathan et al., 2001; Waxman, 1975). The passive spread of current ahead of the active site depolarizes adjacent axon regions, and this process is governed by the length constant of the axon and the time constant of its membrane (Hodgkin and Rushton, 1946; Rall, 1969). Over how large a distance this passive spread can depolarize the membrane to threshold is determined by the length constant, which in turn depends on diameter, specific membrane resistance, and axial resistance. The time constant describes how much the capacitance delays full depolarization. According to these relationships, axons with large length constants and small time constants are propagating impulses rapidly. The specific membrane properties can be quite different across axons of different neurons, and they can be radically different between unmyelinated and myelinated axons. However, within each group they are consistent enough to result in a strong correlation between conduction velocity and diameter. For given specific membrane properties in unmyelinated axons, velocity is proportional to the square root of the axon diameter (Hodgkin, 1954), meaning that large diameter axons propagate impulses rapidly. Diameters of unmyelinated axons vary from just below a millimeter to significantly less than a micrometer. The squid giant axon can be hundreds of micrometers in diameter (Young, 1936)(Fig. 2A, left panel) and has for this reason been the first experimental preparation that allowed intracellular recording (Hodgkin and Huxley, 1939). At the other end of the spectrum, many mammalian central axons have diameters of around 100 nm, with even thinner collaterals and terminals (Westrum and Blackstad, 1962) (Fig. 2A, right panel). The squid giant axon displays conduction velocities between 10 and 25 m/s over the temperature range that the animals are exposed to seasonally (Rosenthal and Bezanilla, 2000). Peripheral crustacean axons of ~1-10 μm diameter display conduction velocities between 1 and 10 m/s over the same temperature range (Young et al., 2006). At the other end of the spectrum, mammalian mossy fiber axons show conduction velocities of less than 0.3 m/s (Kress et al., 2008), and conduction velocities of slow cutaneous C-fibers are also below 1 m/s (Weidner et al., 1999).
Fig. 2.
Diversity of axon morphology and neuronal firing patterns. A: Different axon diameters. The left panel shows a cross section of a squid (Sepioteuthis lessoniana) stellar nerve with vastly different axon sizes. The right panel shows a small-bouton spinous axon terminal of a cortico-thalamic neuron from a macaque monkey, filled with a retrograde tracer. B: Schematic of spinal and dorsal root ganglion neurons. Local interneurons (blue) have fairly short axons, whereas the axons of sensory (red) and motor (green) neurons can be > 1 m long. C: Different firing behavior. The upper panel shows a fast-adapting sympathetic neuron. These neurons often only fire single spikes in response to sustained depolarization, due to substantial M-type potassium currents and slow after-hyperpolarization. The middle panel shows a thalamic neuron during spindle oscillation. The lower panel shows the extreme high-frequency response of a copepod antennal mechanoreceptor to a water jet. A is modified from Lee et al., 1994, and Miyashita et al., 2007; C is modified from Jones, 1985, Contreras and Steriade, 1996, and Fields and Weissburg, 2004.
Whereas impulse conduction in unmyelinated axons is continuous, myelinated axons show saltatory conduction (Bostock and Sears, 1978; Huxley and Stampfli, 1949; Stampfli, 1954). The myelin sheath increases resistance and lowers capacitance, and therefore increases the length constant and decreases the time constant. The sheath is periodically interrupted by the Nodes of Ranvier, regions with a high density of voltage-gated ion channels, particularly sodium channels which regenerate the spike. Channel density in the internodal membrane is low, which also increases resistance. As passive voltage spread between nodes is almost instantaneous (only slightly slowed by capacitance), impulses jump from node to node, effectively increasing conduction velocity. Conduction velocity increases linearly with diameter in myelinated axons, which above a certain diameter are substantially faster than unmyelinated axons of the same size (Arbuthnott et al., 1980; Ritchie, 1982; Rushton, 1951; Waxman, 1980). While conduction velocity in small myelinated fibers is moderate, axons of α- motor neurons and type 1a and 1b sensory neurons with diameters of up to 20 μm can propagate spikes at up to 120 m/s (Gasser and Erlanger, 1927; Manzano et al., 2008), and spikes in some central cortico-spinal axons can reach similar speeds (Evarts, 1965; Takahashi, 1965). Myelination is often considered a hallmark of the vertebrate nervous system, but in fact has convergently evolved in annelids and arthropods (Hartline and Colman, 2007; Roots, 2008). In Penaeus shrimps, saltatory conduction in myelinated axons is almost twice as fast as in the fastest vertebrate axons, with velocities around 200 m/s (Kusano, 1966; Xu and Terakawa, 1999).
2.1.2. Axon length and conduction delay
Many mammalian cortical neurons have axons extending only a few hundred micrometers, and axons of local interneurons in small invertebrates can be even smaller. In contrast, the axons of descending neurons in the spinal cord and of motor and sensory neurons innervating muscles and skin in the distal extremities can be more than a meter long (Fig. 2B). The variability of axon diameters, conduction velocities, and axon lengths results in vastly different total conduction delays between initiation site and presynaptic regions. Local neurons and fast projection neurons with myelinated axons can have axonal conduction delays in the sub-millisecond range (Bartos et al., 2002; Evarts, 1965; Takahashi, 1965), whereas even some central projections with axons several centimeters long can show delays of tens of milliseconds or more (Aston-Jones et al., 1985a; Faiers and Mogenson, 1976). Across mammalian species with vastly different brain sizes, and therefore different axonal projection distances, the degree of myelination and the statistical distribution of axon diameters point toward evolutionary trade-offs in construction costs, metabolic costs, spatial constraints, conduction delay, and temporal precision (Wang, 2008; Wang et al., 2008). Small unmyelinated axons are costly in terms of metabolic rate because they require a continuous distribution of channel and pump molecules. They conduct slowly and show less temporal precision during repetitive firing. On the other hand, they do not take up much space and do not require costly production of myelin in surrounding glial cells. Large myelinated axons and their associated glial cells are costly in construction and take up limited available space. On the other hand, their metabolic cost is low, and they conduct rapidly and therefore with more precision. Mammalian species with large brains have a disproportionally larger number of large myelinated axons, presumably to keep total conduction delays short and spike timing precise.
2.1.3. Axonal branching and diameter changes
Neurons also differ vastly in the complexity of axonal trees, and in the detailed structure of axonal compartments. Geometrical inhomogeneities, above all branch points, have a significant effect on signal propagation. Impedance mismatch between axon sections with different diameters can cause changes in conduction velocity, spike failures, and spike reflection (Debanne, 2004; Manor et al., 1991; Segev and Schneidman, 1999; Swadlow et al., 1980) (discussed in section 3.2.). Some neurons have relatively simple axonal morphology, whereas others can arborize extensively, showing hundreds of branch points (Antonini et al., 1998; Ishizuka et al., 1990; Li et al., 1994; Major et al., 1994). Many cortical axons form en passant boutons (Shepherd et al., 2002), varicosities along the axonal path which from the perspective of spike conduction represent abrupt changes in diameter. Some central axons also have terminaux boutons, spine-like structures that may serve to minimize axonal length and avoid zigzagging of axonal paths (Anderson and Martin, 2001).
2.1.4. Patterns of activity
Perhaps most significant for propagation fidelity, the temporal patterns of spikes can differ substantially across different neurons. Some neurons are fast-adapting and normally only fire single spikes (Fig. 2C, upper panel). Obviously, any short-term activity-dependent dynamics in the axon membrane of such a neuron is irrelevant as long as the interval between successive events is longer than the recovery time of membrane excitability. However, most neurons fire repetitively, producing trains of spikes or regular bursts (Fig. 2C, middle panel). Many neurons even show different modes of activity at different times, probably related to different network states or modes of activation (Grace and Bunney, 1984a, b; Kao et al., 2008; Le Franc and Le Masson, 2010; Neiman et al., 2007). The instantaneous frequency during repetitive activity is usually not higher than a few hundred Hz, limited by the refractory period of the axon, as sodium channel inactivation and elevated potassium conductance following each spike usually prevent spiking for several milliseconds. However, axons in the auditory pathway of gerbils (Scott et al., 2007) and axons in the pacemaker nucleus of weakly electric fish (Moortgat et al., 1998) can sustain firing frequencies of more than 1 kHz. Some specialized mechanoreceptors in copepod crustaceans can even fire at frequencies greater than 5 kHz (Fields and Weissburg, 2004)(Fig. 2C, lower panel).
2.1.5. Deviations from canonical neuron compartmentalization
The schematic representation of neuron compartmentalization into dendrites, soma, and axon, and information flow through these compartments in this order, does of course not do justice to the diversity of neuron morphology. Good examples for neurons not following this pattern are the pseudo-unipolar sensory neurons of the dorsal root ganglia (Fig. 2B) with their spike-conducting distal and proximal processes (Devor, 1999). In addition, not all neurons have a clear morphological division into dendrites and axon. In arthropods and other invertebrates, most central neurons are unipolar, i.e. both dendrites and axons stem from a single primary neurite that leaves the cell body. In some cases, pre- and postsynaptic sites are intermingled, even when a longer axon is present (Watson and Burrows, 1983). In other neurons, there is some separation into input and output branches (Lohr et al., 2002). There also are numerous examples of neurons with multiple spike initiation zones in different segments of the nervous system. These features make it clear that many neurons do not always operate as a single unit that integrates, computes and transmits information, but may be more accurately described as consisting of multiple functional signaling units (Clarac and Cattaert, 1999).
Finally, it should be noted that not all neurons use all-or-none impulse conduction as their only mode of signal transmission. In fact, some neurons do not utilize impulse conduction at all, as they are electrotonically compact enough to release transmitter only as a graded function of membrane potential (Roberts and Bush, 1981). Most of these are small local neurons, like the retinal bipolar cells (Werblin and Dowling, 1969), but even some crustacean proprioceptors with relatively long peripheral axons are non-spiking (DiCaprio, 2003; Ripley et al., 1968). Other neurons, like those in the crustacean stomatogastric ganglion, use both graded and impulse-mediated forms of synaptic communication (Graubard et al., 1980, 1983; Raper, 1979). In cortical axons, it was recently discovered that graded potentials can propagate over long distances and influence spike-mediated synaptic transmission, effectively adding an analog component to digital signaling (Alle and Geiger, 2008; Kress and Mennerick, 2009).
2.2. Diversity of axonal voltage-gated ion channels
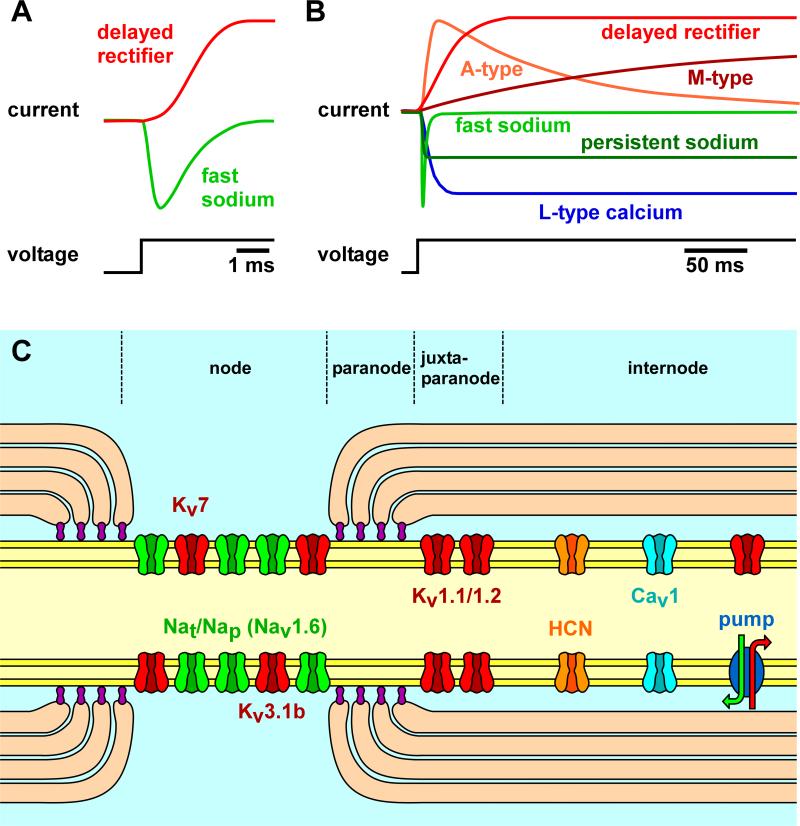
The classic literature describes spike initiation and propagation in unmyelinated invertebrate axons on the basis of minimal complements of voltage-gated ion channels, giving rise to a fast sodium and a delayed rectifier potassium current in the squid giant axon (Hodgkin and Huxley, 1952) (Fig. 3A), and an additional transient potassium current (“A-current”) in crab walking leg axons (Connor, 1975; Connor et al., 1977). It is now clear that many axons, including vertebrate and invertebrate, central and peripheral, myelinated and unmyelinated, posses a significantly more complex complement of ion channels, with diverse time- and voltage-dependences of their gating properties (Fig. 3B). Animals as phylogenetically distant from us as jellyfish can have axonal ion channel complements giving rise to calcium, sodium, and multiple potassium currents (Meech and Mackie, 1993). Still, the consequences for spike propagation are poorly understood in most cases. We do not intend to give an exhaustive account of which types of ion channels have been found in which axon. Rather, we will give some examples to illustrate the diversity and set the stage for a discussion in the following sections of the roles that some of them play in the context of activity-dependent changes in spike conduction.
Fig. 3.
Diversity of ionic currents in axons. A: Fast sodium and delayed rectifier potassium currents in the squid giant axon, as elicited in response to a depolarizing voltage step. B: Cartoon of current responses to depolarizing voltage steps in an axon with a more complex complement of channels which activate and inactivate with very different time constants. C: A range of different ion channels found in either central or peripheral myelinated axons, distributed differentially between node, juxtaparanode, and internode. A is modified from Hodgkin and Huxley, 1952.
Where does our knowledge of axonal ion channels come from? Many axons are small and not easily accessible for physiological studies, and detailed immunohistochemical studies of subcellular ion channel distributions are still rare (Lujan, 2010). However, large peripheral myelinated axons have relatively early proven to be amenable to direct electrophysiological analysis (Baker et al., 1987; Kocsis and Waxman, 1987; Vogel and Schwarz, 1995; Waxman et al., 1995; Waxman and Ritchie, 1993). A multitude of different ion channels or currents with characteristic spatial distributions in relation to the Nodes of Ranvier have been found (Fig. 3C), and their contribution to spike conduction continues to be of great interest because of the role they play in a number of peripheral neuropathies (Krishnan et al., 2009). The same is true for ion channels in spinal axons in the context of demyelination and injury (Nashmi and Fehlings, 2001). Much less is known for small peripheral unmyelinated axons and axons in the brain. In peripheral unmyelinated axons, evidence for the presence of many ion channels is often indirectly derived from the effect that pharmacological substances have on extracellularly recorded spike conduction and excitability (Moalem-Taylor et al., 2007). In cortical neurons and elsewhere in the brain, the spatial distribution of ion channels is very cell type-specific. Despite the fact that there have been some advances in the localization and targeting of channels with immunohistochemical methods (Lujan, 2010; Vacher et al., 2008), there still is a dearth of information about many ion channels, including their presence or absence in axons (Lorincz and Nusser, 2008; Nusser, 2009), and the effect that they have on the dynamics of spike propagation. Direct patch-clamp recordings from small central axons and terminals are a relatively recent achievement (Bischofberger et al., 2006; Geiger and Jonas, 2000; Shu et al., 2006).
Overall, it is not clear to which degree any of the findings regarding the presence and subcellular localization of ion channels can be generalized. Nusser (2009) pointed out, with respect to central neurons, that experimental results about the subcellular distribution of ion channels in one “model” cell are often erroneously thought to hold for all or many cell types. This caveat is particularly important as it is now evident that the diversity of cell types in the brain far exceeds classical categorizations (Diaz, 2009; Sugino et al., 2006). To which degree such diversity is also present in axonal ion channel complements is unknown. In the peripheral nervous system, axons are often only categorized into myelinated and unmyelinated, sensory and motor. There are some accounts of differences in electrophysiological properties between amphibian, human, and other mammalian myelinated axons (Bowe et al., 1987; Reid et al., 1999), and some explicit statements about differences in motor versus sensory axons (Kiernan et al., 2004), or even about differences across different axons of the same general classification (Obreja et al., 2010; Weidner et al., 1999). However, how idiosyncratic findings may be for the particular nerve, axon, or animal species that served as an experimental model, is often undetermined.
Apart from the difficulty in recording from axons, in some neurons studying the impact of specific ionic currents on spike conduction is complicated by another problem. Particularly in neurons with shorter axons, functional compartmentalization is not always easy to assess. If the neuron is electrotonically compact, it can be difficult to determine if the functional role of a channel or current (or other axonal membrane property) lies predominantly in spike initiation, spike propagation, or regulation of presynaptic depolarization, and it may influence the electrical behavior across different compartments (Alle and Geiger, 2008; Paradiso and Wu, 2009; Shah et al., 2008; Shu et al., 2007a). In addition, the presence of en passant synapses in many neurons means that propagation and presynaptic depolarization are not fully anatomically separated.
The question why many axons possess a complement of ionic conductances that may exceed the minimal set necessary to conduct spikes is discussed in the following sections. However, it is important to note that the presence of specific ion channels in specific cell compartments is highly regulated. If a neuron expresses a particular ion channel, this channel is not just distributed throughout the whole cell but follows an exquisitely regulated molecular compartmentalization. This is particularly evident in myelinated axons, where specific ion channel clustering at node, juxtaparanode and internode is achieved through multiple targeting and barrier mechanisms (Poliak and Peles, 2003; Rosenbluth, 2009), but is also true for other neuronal compartments (Lai and Jan, 2006; Lasiecka et al., 2009; Rasband, 2010). Therefore, the presence of specific ion channels in axons should be interpreted as precise targeting, as opposed to a “sloppy” expression pattern resulting in the presence of channels in compartments where their functional role is secondary. This view is consistent with the differential subcellular distribution of currents within one cell type and across different cell types (Vacher et al., 2008). Furthermore, similar ionic currents are actually often produced by different channel isoforms or splice variants in different cell compartments. For example, hippocampal axons from different neuron types show predominantly the Nav1.2 isoform of voltage-gated sodium channels in their axons, while the Nav1.1 isoform is restricted to soma and dendrites (Gong et al., 1999). In cortical pyramidal cells, Nav1.2 and Nav1.6 are differentially distributed along the axon initial segment (Hu et al., 2009). Different splice variants of Kv3.1 potassium channels can be differentially targeted to either somatodendritic or axonal cell compartments (Ozaita et al., 2002).
2.2.1. Voltage-gated sodium channels
Obviously, fast and inactivating sodium currents are present in almost all axons, as their properties are responsible for the regenerative nature of spikes. In mammals (and other vertebrates), these channels are formed by different α-subunits in different cell compartments and cell types (Krishnan et al., 2009; Vacher et al., 2008). Each α-subunit consists of four homologous domains (pseudo-subunits) that form the pore, so there is no heteromerization as in HCN and potassium channels (see sections 2.2.2 and 2.2.3). All voltage-gated sodium channels cloned so far belong to a single subfamily of genes giving rise to 9 or 10 different isoforms (Catterall et al., 2005a). In mammalian central neurons, unmyelinated axons often express Nav1.2 channels along their path, whereas the nodal sodium currents in myelinated axons are typically due to Nav1.6 channels (Vacher et al., 2008). Different isoforms account for different gating properties (Smith and Goldin, 1998). The inactivation and recovery from inactivation of sodium channels critically determine axonal excitability during high frequency repetitive firing. For example, recovery from inactivation is faster in Nav1.6 compared to Nav1.7 channels. Across mouse dorsal root ganglion neurons, large myelinated fibers display much higher firing frequencies than small unmyelinated ones. This has been linked to differential expression of these channel isoforms (Herzog et al., 2003). However, physiological properties depend on the interaction of potentially many different voltage-gated ion channels. Therefore, the functional consequences of differential distribution of isoforms for propagation are not well understood.
In many axons, a small percentage (1-2%) of axonal sodium current is gated differently in that it activates at more hyperpolarized membrane potentials and inactivates slowly (Baker and Bostock, 1997; Stys et al., 1993; Tokuno et al., 2003). This persistent sodium current is important for subthreshold excitability and repetitive firing (Bostock and Rothwell, 1997; French et al., 1990; McIntyre et al., 2002). In some neurons, both transient and persistent currents stem from the same gene (e.g., Nav1.6) and are both blocked by tetrodotoxin, but it is not clear if they represent distinct channel populations or a uniform population in which individual channels can switch between gating modes (Alzheimer et al., 1993; Kiernan et al., 2003; Magistretti et al., 1999; Taddese and Bean, 2002). Tetrodotoxin-resistant Nav1.8 and Nav1.9 channels also produce slowly inactivating currents and are found for example in peripheral and trigeminal axons (Black and Waxman, 2002; Coggeshall et al., 2004; Jeftinija, 1994; Quasthoff et al., 1995). In addition, some sodium channels can produce small “resurgent” currents during the time of recovery from inactivation (Bean, 2005). This behavior is associated with accelerated recovery from inactivation and thought to play a role in allowing rapid repetitive firing, but their functional significance for axonal spike propagation is unknown.
In contrast to mammals, invertebrates usually only have one or a few genes which encode voltage-gated sodium channels (Goldin, 2002; Loughney et al., 1989). Diversity of currents with different gating properties, including persistent ones, seems to predominantly stem from extensive alternative splicing (Dai et al., 2010; Lin et al., 2009).
2.2.2. Voltage-gated potassium channels
Axonal voltage-gated potassium channels are critical for excitability. To which aspect of spike propagation they contribute depends on voltage-dependence and kinetics, and how these properties are matched to the inactivation and de-inactivation properties of sodium channels (Alle et al., 2009; Baranauskas, 2007; Sengupta et al., 2010). Fast activation properties are important for the repolarization of spikes, which in turn influences the excitability during repetitive firing. Slow activation and deactivation properties determine after-potentials and therefore are also important in activity-dependent excitability changes. In addition, how much a potassium channel contributes to activity-dependent changes in spike shape and conduction velocity depends on if and how fast it inactivates. Apart from the classical sustained delayed rectifier currents (Hodgkin and Huxley, 1952) and transient (fast activating and inactivating) A-currents (Connor, 1975; Connor et al., 1977), a large number of intermediate types exist, and their pharmacology is inconsistent. Furthermore, there are axonal potassium currents significantly slower than the classical delayed rectifier (Baker et al., 1987; Kocsis et al., 1987). This diversity of potassium channel properties is partly due to the fact that they are members of a diverse gene family (Coetzee et al., 1999; Lujan, 2010). In addition, their pore is formed by four real subunits that can assemble into heteromeric channels. Molecular identification of the four prototypical voltage-gated potassium channels was first achieved in Drosophila. The vertebrate nomenclature for gene families is according to the sequence homology with the fly single gene orthologues: Kv1 (Shaker), Kv2 (Shab), Kv3 (Shaw), and Kv4 (Shal) (Gutman et al., 2005). In addition, Kv7 codes for channels that give rise to slow sustained (M-type) currents.
Kv1 channels can produce either sustained (Kv1.1, Kv1.2, Kv1.3, Kv1.5, Kv1.6) or transient (Kv1.4) currents, and heteromerization within the family as well as assembly with different auxiliary subunits can produce a wide variety of gating properties, including delayed rectifier and A-type currents (Rettig et al., 1994; Trimmer and Rhodes, 2004). Kv1.1, Kv1.2 and Kv1.4 are widely expressed in mammalian axons, where they often are co-localized and form heteromeric complexes (Rhodes et al., 1997; Trimmer and Rhodes, 2004; Wang et al., 1993), the combinations of which can be very cell-type specific (Veh et al., 1995). The juxtaparanodal fast potassium currents in myelinated axons, important for limiting re-excitation (Benoit and Dubois, 1986; Brau et al., 1990; Corrette et al., 1991; Dubois, 1982; Kocsis et al., 1987; Kocsis et al., 1982b), are mostly due to Kv1.1 and Kv1.2 subunits (Nashmi et al., 2000; Rasband and Trimmer, 2001; Utsunomiya et al., 2008; Wang et al., 1993). Transient Kv1 currents are usually not as rapidly inactivating as the classical A-currents, but in cortical axons even relatively slow inactivation properties of Kv1 channels can cause changes in spike shapes during repetitive activation (Shu et al., 2007b).
Kv2 channels are usually restricted to proximal cell compartments (Du et al., 1998; Vacher et al., 2008), but Kv3 and Kv4 channels are found in axons. The fast nodal delayed rectifier type current in central myelinated axons is usually due to Kv3.1 channels, more specifically the splice variant Kv3.1b (Devaux et al., 2003), and Kv3.4 is often associated with Kv1 channels in unmyelinated central axons, possibly forming heteromers (Laube et al., 1996). Rapidly inactivating Kv4.3 channels can be found in unmyelinated axons (Buniel et al., 2008). Kv7 channels are found both in unmyelinated axons (Buniel et al., 2008; Vervaeke et al., 2006), and at the node in myelinated axons (Devaux et al., 2004), where they produce slow (M-type) currents (Baker et al., 1987; Dubois, 1981; Eng et al., 1988; Schwarz et al., 2006).
Surprisingly little is known about potassium current diversity in invertebrate axons. It is noteworthy though that different channel types may be targeted to different cell compartments with similar specificity as in vertebrates. The A-current found in some crustacean motor axon trunks (Ballo and Bucher, 2009; Connor, 1975) may be of different origin than transient potassium currents in proximal cell compartments and in terminals. In a stomatogastric neuron, antibody staining against Shal and Shaker channels revealed that Shal is targeted to proximal compartments and axon terminals, while Shaker was exclusively found along the path of the axon in the motor nerve (Baro et al., 2000). The same axons also express both Shab and Shaw channels (French et al., 2004). A similar preferred distribution of Shaker over Shal in axons may be present in many insect neurons (Wicher et al., 2001).
There also is some evidence for axonal calcium-activated potassium currents of the large conductance BK type. These channels are gated by both calcium elevations and depolarization and contribute to repolarization and after-hyperpolarization (Lee and Cui, 2010; Sah and Faber, 2002). They were directly characterized with patch-clamp recordings in myelinated peripheral axons of frog (Jonas et al., 1991; Koh et al., 1994b) and rat (Safronov et al., 1993). There also is evidence from electrophysiological and calcium imaging experiments for their presence in some myelinated and unmyelinated central axons (Bielefeldt and Jackson, 1993; Callewaert et al., 1996; Lev-Ram and Grinvald, 1987; Muschol et al., 2003), and immunohistochemistry shows some staining in non-synaptic axonal membrane (Knaus et al., 1996; Misonou et al., 2006). Their functional impact should critically depend on the presence of appreciable calcium transients, but it is not clear how widespread those are among different types of axons (see section 2.2.4.).
In addition, there is evidence for sodium-dependent potassium channels in axons. The current was first described in the nodal region of frog peripheral myelinated axons, where it significantly contributes to after-hyperpolarizing potentials following moderate numbers of spikes (Koh et al., 1994a; Poulter et al., 1995). Later, two genes were identified in mammals (Bhattacharjee and Kaczmarek, 2005), one of which encodes a channel found in many central axons, particularly in brainstem and vestibular nucleus neurons (Bhattacharjee et al., 2002). Both calcium- and sodium-activated potassium currents are present in invertebrates, and their contribution to spike shape has been studied in insect neuron somata (Wicher et al., 2006), but little is known about a possible presence and function in axons.
2.2.3. HCN channels
Many axons display inward rectification because they express hyperpolarization-activated cyclic nucleotide-gated channels. In mammals, there are four genes, called HCN1-4, which encode subunits that can form homo- or heterotetrameric channels with distinct gating properties (Biel et al., 2009; Robinson and Siegelbaum, 2003; Wahl-Schott and Biel, 2009). In invertebrates, extensive splicing of a single gene gives rise to multiple potential transcripts of HCN homologs (Dai et al., 2010; Gisselmann et al., 2005; Marx et al., 1999; Ouyang et al., 2007). HCN channels and their invertebrate homologs are permeable to both sodium and potassium and have fairly unique gating properties. The current they give rise to (Ih) shows a reverse voltage-dependence of activation, being partly activated at resting membrane potential and increasing activation upon hyperpolarization. Therefore, it confers inwardly rectifying properties which play important role in balancing axonal hyperpolarization caused by potassium conductances and sodium/potassium-ATPase activity (Baginskas et al., 2009; Baker et al., 1987; Ballo and Bucher, 2009; Ballo et al., 2010; Grafe et al., 1997; Kiernan et al., 2004; Soleng et al., 2003; Tomlinson et al., 2010).
In the mammalian brain, HCN channel distribution is very cell-type specific. Immunohistochemistry revealed the presence in axons of some neurons, whereas in other axons HCN channels are missing or restricted to distal (albeit often pre-terminal) compartments (Notomi and Shigemoto, 2004; Nusser, 2009). However, Ih is prominent in the “axon proper” in myelinated optic nerve fibers (Eng et al., 1990), in myelinated and unmyelinated peripheral fibers (Baker et al., 1987; Birch et al., 1991; Grafe et al., 1997; Poulter et al., 1993; Takigawa et al., 1998; Tomlinson et al., 2010), and has also been found in crustacean axons (Ballo and Bucher, 2009; Ballo et al., 2010).
2.2.4. Voltage-gated calcium channels
There is some evidence for voltage-gated calcium channels in axons. Obviously, they are present at presynaptic sites where they control calcium influx and subsequent calcium-dependent transmitter release. In non-synaptic axonal membrane, the role they play for excitability and spike propagation is less clear. Like in sodium channels, the pore-forming part of calcium channels consists of single subunits (α1). These are encoded by 3 families of genes in mammals (Catterall, 2000; Catterall et al., 2005b; Dolphin, 2009) which match the original classification of calcium currents by gating properties and pharmacology. Cav1 and Cav2 channels are high voltage-activated (Cav1 = L-type; Cav2 = P/Q-, N-, and R-type), whereas Cav3 channels are low voltage-activated (T-type). In invertebrates, pharmacology, gating properties, and sequence information do not perfectly match mammalian channels, but allow at least the classification in L-type, and non-L-type (Jeziorski et al., 2000).
Information about the role calcium channels may play for axonal excitability is ambiguous for two reasons. First, they can be expressed postnatally but early enough that their role may be predominantly in regulating intracellular calcium concentrations critical for growth. For example, calcium channels are expressed in some axons with incomplete myelination: P-type channels in Purkinje cell axons (Callewaert et al., 1996), and N-type channels in optic nerve axons (Sun and Chiu, 1999). Interestingly, N-type channels in optic nerve axons also appear in demyelination disease models (Gadjanski et al., 2009). Second, even in the adult, axonal calcium channels may be predominantly involved in intracellular calcium signaling in some cases and not play a large role in directly determining excitability. Activity-dependent calcium influx in adult myelinated rat optic nerve axons was first detected by calcium imaging (Lev-Ram and Grinvald, 1987), and it was later shown that L-type calcium channels are present (Brown et al., 2001; Fern et al., 1995a). Calcium influx is not restricted to nodes but homogeneous along the axon (Zhang et al., 2006). Channels do not appear to be of sufficient density to contribute more than subtly to spiking or normal excitability, so their role may be mostly regulatory (Brown, 2003).
However, in other cases calcium channels appear to contribute significantly to excitability. In some unmyelinated peripheral axons, propagated calcium spikes can be observed when sodium currents are blocked and potassium currents are reduced (Elliott et al., 1989; Jackson et al., 2001; Mayer et al., 1999; Quasthoff et al., 1995; Wächtler et al., 1998). In a crustacean stomatogastric motor axon, L-type calcium channels convey bistable membrane behavior in the motor nerve, as a brief electrical stimulation can lead to self-sustained tonic firing for tens of seconds (Le et al., 2006). Antibody staining in the stomatogastric nervous system showed both L-type and P/Q-type channels in peripheral nerve axons (French et al., 2002). Calcium channels may also play an important role in determining excitability in en passant synapses or varicosities during repetitive activation. In distal axons of hypothalamic peptidergic neurons in the neurohypophysis, calcium channel-dependent activation of calcium-dependent potassium channels and subsequent partial membrane inactivation in secretory varicosities has been suggested to cause spike failures and slow spike conduction in an activity-dependent manner (Bielefeldt and Jackson, 1993; Muschol et al., 2003).
There also is evidence for L-type channels in non-synaptic axonal membrane in spinal dorsal column white matter (Ouardouz et al., 2003), hippocampal neurons (Tippens et al., 2008), and Leech Retzius and Leydig neurons (Beck et al., 2001; Lohr et al., 2001). Therefore, voltage-gated calcium channels may be fairly common in non-synaptic axonal membrane, but little is known about their functional roles.
2.2.5. Other ion channels, pumps, and exchangers
As stated above, we did not intend to give a complete account of ion channels in axons. However, we want to point out that it is by no means clear that axonal expression of ion channels is usually limited to the canonical types described here. For example, transient receptor potential (TRPC) channels are expressed in axons of cultured hippocampal neurons (Strubing et al., 2001). In some myelinated axons, both ATP-sensitive potassium channels (Jonas et al., 1991), and voltage-independent potassium channels with unique (flickering) opening properties (Koh et al., 1992; Reid et al., 1999; Safronov et al., 1993) have been found. There also is accumulating evidence that voltage-gated chloride channels play a role in controlling neuronal excitability (Rinke et al., 2010), and such channels can be present in peripheral axons (Strupp and Grafe, 1991; Wu and Shrager, 1994).
Apart from ionic conductances, ion pumps and exchangers can also play a significant part in activity-dependent changes in axonal excitability. Particularly important is the sodium/potassium-ATPase (Na+/K+-pump) which was first isolated from crustacean leg nerves (Skou, 1957). It uses hydrolysis of ATP to pump three sodium ions to the extracellular side for every two potassium ions pumped into the axon, and therefore causes a net deficit of positive charge (Baker et al., 1969; Skou, 1988). In addition to the role of its electrogenic properties for maintaining the resting potential, its sodium-dependence makes it an important contributor to transient changes in membrane potential. The massive influx of sodium during repetitive spiking activates the pump which in turn causes prolonged after-hyperpolarization and an increase in activation threshold (Bostock and Grafe, 1985; Gordon et al., 1990).
3. Short-term dynamics of spike propagation
The gating properties of ion channels endow non-synaptic axonal membrane with a voltage- and time-dependence that renders spike propagation dependent on the history of activity. In addition, geometrical factors can slow down or speed up spike propagation. At the extremes of changes in excitability and conduction velocity, spike propagation can fail in the axon or single propagated spikes can elicit repetitive activity. In this section, we will first describe how the properties of voltage-gated ion channels and other ionic mechanisms can change axonal excitability and spike propagation. We will then review how non-uniform axonal structure and excitability affect spike propagation. Finally, we will discuss what these phenomena may mean with respect to neural coding.
3.1. Activity-dependent changes in axon excitability and conduction velocity
In a hypothetical axon with uniform geometry and ion channel distribution or, in the case of myelinated axons, uniform repetitions of the same spatial pattern of membrane properties, changes in conduction velocity during repetitive activity are solely due to spikes traveling through regions of altered membrane excitability left in the wake of preceding spikes. As described in section 2.2., the complement of voltage-gated ion channels in axonal membrane can be quite complex, including channels with substantially different voltage-dependences and time constants associated with activation and inactivation (Fig. 3B). Therefore, conduction velocity can be a highly nonlinear function of spike frequency and history, not well correlated with membrane potential or spike shape. In addition, spike velocity changes with propagation distance as a function of changing spike intervals.
3.1.1. The recovery cycle of axonal excitability
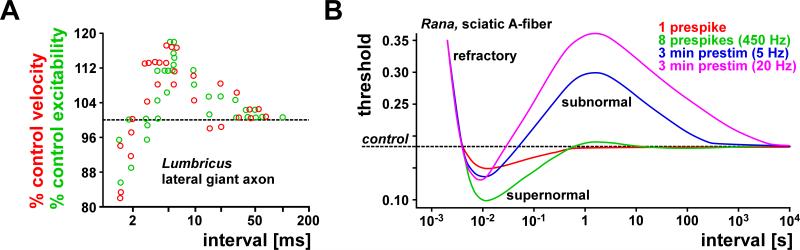
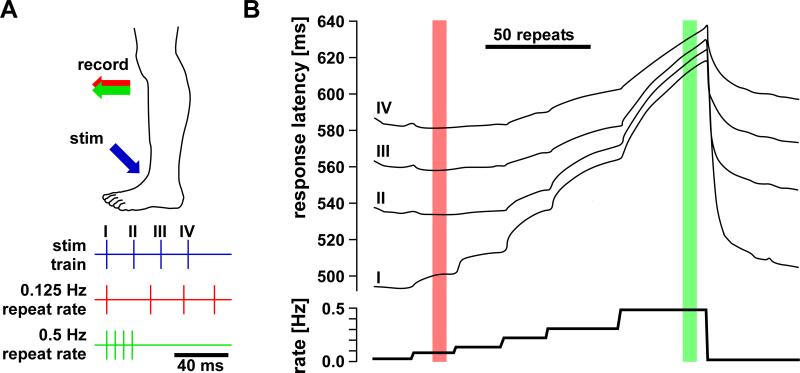
It was recognized early that during repetitive activation nerves undergo absolute and relative refractory periods of decreased excitability and conduction velocity. Detailed studies of excitability changes also showed that under some conditions there can be periods of supernormal excitability (Adrian, 1920), but these were for some time erroneously thought to represent experimental artifacts (Graham and Lorente de No, 1938). Finally, there can be a period of prolonged decrease in excitability, termed the subnormal period. Collectively, this oscillatory sequence of excitability changes is termed the recovery cycle. One of the first systematic and carefully controlled investigations of activity-dependent changes in spike conduction was performed by Bullock (1951). He measured the change in excitability and conduction velocity in earthworm lateral giant axons and frog sciatic A-fibers with repeated stimuli: a conditioning pulse followed by a test pulse at different intervals (Fig. 4A). He found that at intervals of a few milliseconds, excitability and conduction velocity of the spike in response to the test pulse were reduced, but with larger intervals excitability and conduction velocity were increased. This supernormal period extended to intervals of more than 100 milliseconds, and was even greater when more than one conditioning stimulus was used. Importantly, the time courses of activity-dependent changes in excitability and conduction velocity were similar. In frog myelinated sciatic fibers, excitability was later tested for longer time intervals and with a number of different conditioning stimulus regimes (Raymond, 1979). When axons were conditioned with short high-frequency stimulation or lower-frequency stimulation sustained over several minutes, the refractory period and subsequent supernormal period was followed by a long-lasting subnormal period of increased threshold (Fig. 4B).
Fig. 4.
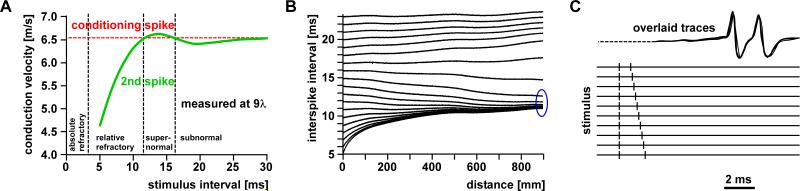
Activity-dependent changes in axonal excitability and conduction delay in earthworm and frog axons. A: Concomitant relative changes of conduction velocity and excitability as a function of interval between paired pulses in an earthworm axon. Excitability was measured as the threshold for spike initiation at the stimulus site. At small intervals, conduction velocity and excitability was reduced. At larger intervals, velocity and excitability was increased and returned to control values only at intervals greater than 100 ms. B: Triphasic changes in excitability and their dependence on conditioning regime in frog sciatic axons. With a single conditioning spike (red), the threshold for the initiation of a second spike was increased at small intervals (relative refractory period), and decreased at larger intervals (supernormal period). When the axon was conditioned with 8 impulses at 450 Hz (green), the supernormal period was increased and followed by a period of slightly increased threshold (subnormal). The subnormal period was dramatically increased when the axon was conditioned with 5 Hz (blue) or 20 Hz (magenta) stimulation sustained over 3 minutes. A is modified from Bullock, 1951; B is modified from Raymond, 1979.
In rabbit corpus callosum neurons, changes in stimulation threshold and conduction velocity have been tested with soma recordings and antidromic axon stimulations (Swadlow, 1974; Swadlow et al., 1980; Swadlow and Waxman, 1975, 1976). Similar sequences of refractory, supernormal, and subnormal periods were observed. As in Bullock's experiments with earthworm axons, changes in excitability and conduction delay closely followed the same time course. It was also found that the duration of the supernormal period depended on the control conduction velocity. Fast conducting axons recovered more rapidly from supernormality. Interestingly, the duration of the supernormal period appeared to be a continuous function of the control conduction velocity when data from both myelinated and unmyelinated axons were plotted concomitantly, suggesting that there was no systematic difference in excitability changes between axons with continuous or saltatory conduction.
Activity-dependent changes in excitability, including supernormal and late subnormal conduction, were also studied in other central axons, like the efferent fibers of basal ganglion neurons (Kocsis and VanderMaelen, 1979), Schaffer collaterals (Soleng et al., 2004; Wigstrom and Gustafsson, 1981), cerebellar parallel fibers (Gardner-Medwin, 1972; Kocsis et al., 1983), and optical nerve fibers (George et al., 1984; Oozeer et al., 2006). Today, the dynamics of excitability and spike conduction are extensively used in the study of peripheral nerves. The good agreement between changes in stimulation thresholds and conduction delays imply that the same ionic mechanisms underlie changes in excitability and conduction velocity. In the literature, both terms are often used interchangeably or at least under the assumption that changes in one parameter are similar to changes in the other. In studies of human peripheral nerves, threshold measurements are now common because they are more sensitive to certain aspects of pathological changes (Bostock et al., 1998). A systematic analysis of changes in human axon excitability was first performed on the median nerve, showing refractory and supernormal phases similar to those in animal models, with some differences between different fiber types (Gilliatt and Willison, 1963; Stohr, 1981). Such excitability studies still play an important role in the study of the human peripheral nervous system and its pathological states. Although non-invasive by nature, and therefore with no cellular electrophysiological access, gauging nerve excitability with measurements of stimulation threshold and conduction velocity allows many inferences about ionic mechanisms, dysregulation of ion channels, and demyelination (Bostock et al., 1998; Bostock and Rothwell, 1997; Burke et al., 2001; Krishnan et al., 2009). In animal model systems, where at least in some cases direct access for the measurement of axonal membrane properties is possible, the relationships between the properties of specific ionic conductances, excitability, and membrane potential have been studied in some detail.
3.1.2. Ionic mechanisms underlying the recovery cycle
The mechanisms underlying the different phases of the recovery cycle have been reviewed extensively, most recently by Krishnan et al. (2009) in the context of neurological disorders. They are best understood in peripheral myelinated axons, but also studied in detail in unmyelinated peripheral axons (Weidner et al., 2002). There are some quantitative differences between motor and sensory fibers (e.g. Kiernan, 2004), C-fibers of different sensory modalities (Bostock et al., 2003; Campero et al., 2004; Obreja et al., 2010; Serra et al., 1999; Weidner et al., 2000), and peripheral versus optic nerve axons (Oozeer et al., 2006). The diversity of ion channels suggests that ionic mechanisms may differ across different axon types, but in general there is good agreement about at least some of the activity-dependent changes in excitability and conduction velocity.
The period of reduced excitability and conduction velocity immediately following a spike is relatively straightforward to explain. According to the ionic theory of spike initiation and propagation by Hodgkin and Huxley (1952), which includes only fast sodium and delayed rectifier potassium conductances, the absolute refractory period following a spike peak is due to the inactivated state of sodium channels and the elevated potassium conductance. No spikes can be elicited during this period. This phenomenon is important because it ensures the unidirectional propagation of spikes under normal circumstances. During the relative refractory period, axonal excitability is still decreased while sodium channels are recovering from inactivation and potassium channels are deactivating. The threshold for spike initiation is higher and the conduction velocity therefore slowed.
Supernormal and subsequent subnormal conduction are less straightforward to explain. Even relatively simple axon models including only Hodgkin-Huxley conductances and cable equations generate this oscillatory sequence of supernormal-subnormal swings to some degree (Miller and Rinzel, 1981; Moradmand and Goldfinger, 1995), likely associated with passive capacitive effects. However, in most axons changes in excitability are due to distinct ionic mechanisms that are not part of the Hodgkin-Huxley model. In frog sciatic nerve, it was noticed early that the supernormal period may be associated with a depolarizing after-potential (Gasser and Erlanger, 1930), later described in detail for myelinated frog and rat axons (Bowe et al., 1987). Originally, it was thought that the main mechanism is an increase in extracellular potassium concentration (Bowe et al., 1987; Gilliatt and Willison, 1963; Kocsis and VanderMaelen, 1979; Malenka et al., 1983; Swadlow and Waxman, 1976). However, a substantial contribution to depolarization comes from passive capacitive charging of the internodal membrane of myelinated axons (Barrett and Barrett, 1982; Blight, 1985; Blight and Someya, 1985; David et al., 1995). A similar passive mechanism has been proposed for unmyelinated peripheral and central axons (Bostock et al., 2003; Soleng et al., 2004; Weidner et al., 2002). In addition, persistent (or potentially “resurgent”) sodium currents can play an important role (Bostock and Rothwell, 1997; Burke et al., 1998; McIntyre et al., 2002; Stys et al., 1993). Peripheral nerves can also show a “paradoxical” hyperexcitability following long high-frequency activation in peripheral nerve that can lead to ectopic spike initiation (Bostock and Bergmans, 1994; Bostock et al., 1998; Kiernan et al., 1997). This phenomenon is thought to arise from a depolarization caused by accumulation of extracellular potassium.
Subnormal excitability can also be due to several underlying mechanisms. The subnormal period following a single spike can arise from a hyperpolarization caused by slow potassium currents (Baker et al., 1987; Lin et al., 2000; Schwarz et al., 2006; Stys and Waxman, 1994; Taylor et al., 1992), and repetitive activation can lead to accumulation of this effect (Burke et al., 2001; Burke et al., 1995; Miller et al., 1995). The sequence of depolarizing and hyperpolarizing after-potentials in a myelinated axon can be seen in Fig. 5A and B, and the associated changes in excitability in a model of the myelinated axon are depicted in Fig. 5C. The after-hyperpolarization in this model (McIntyre et al., 2002) was due only to potassium conductances such as described above. However, higher frequency activation often leads to a more prolonged after-hyperpolarization of different origin. It is due to the sodium-dependence of the Na+/K+-pump and can last many minutes (Fig. 4B). Particularly in smaller axons, repetitive activity leads to a substantial change in intracellular sodium concentration, and subsequent activation of the pump. Because the pump exchanges three sodium ions for two potassium ions, strong activation leads to hyperpolarization. There is ample evidence for this type of hyperpolarization for many different types of axons, including invertebrate and vertebrate, myelinated and unmyelinated (Baker, 2000; Barrett and Barrett, 1982; Bostock and Bergmans, 1994; Gordon et al., 1990; Kiernan et al., 2004; Moldovan and Krarup, 2006; Robert and Jirounek, 1998; Scuri et al., 2007; Vagg et al., 1998; Van Essen, 1973).
Fig. 5.
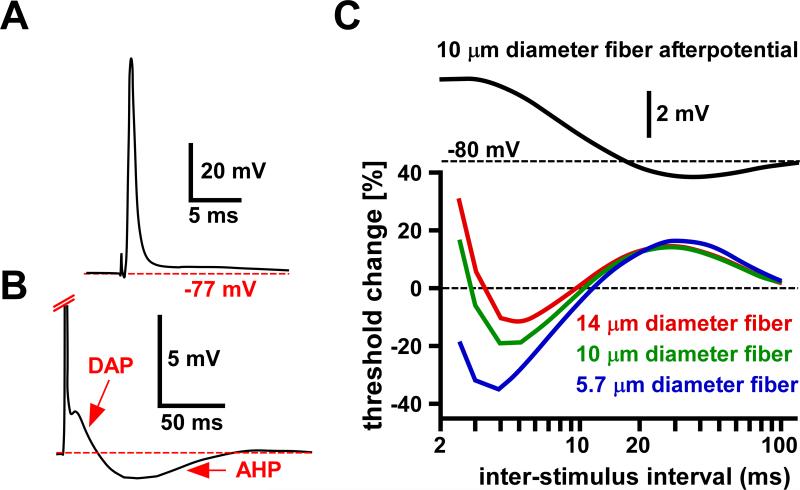
Afterpotentials and activity-dependent changes in axonal excitability. A: Spike recorded from a myelinated motor axon innervating the diaphragm in the rat. B: Enlarged view of the voltage range around resting membrane potential and expanded time base of the same trace shown in A. A depolarizing afterpotential (DAP) is followed by an after-hyperpolarization (AHP). C: The relationship between afterpotentials and excitability changes in a computational model of a myelinated axon, calculated for three different axon diameters. Note that the changes associated with the relative refractory period at short intervals (~2-4 ms) are not reflected in the membrane potential. However, later changes in threshold follow the DAP and AHP quite well. The difference in DAP between axons of different sizes was due to the fact that passive capacitive charging depends on diameter. A and B are modified from David et al., 1995; C is modified from McIntyre et al., 2002.
3.1.3. After-potentials and activity-dependent changes in spike shape
Despite the fact that changes in excitability and conduction velocity are often well correlated with changes in membrane potential, this relationship is complex in many cases. In general, the presence of multiple conductances with diverse time- and voltage-dependences of activation and inactivation leads to substantial nonlinearities that can make it very difficult and non-intuitive to map conductances to membrane behavior (Taylor et al., 2009). In the context of spike propagation, every aspect of changes in membrane potential, excitability, and conduction velocity is shaped by the contribution of multiple, often functionally opposing conductances. There are two types of changes in voltage trajectory that can be observed. The existence of depolarizing and hyperpolarizing after-potentials means that consecutive spikes are fired from different membrane potentials. In addition, the spike shape itself, i.e. its amplitude and duration, can change.
The inactivation of sodium channels on its own has an effect on spike shape during repetitive activity, in that spikes propagating in the relative refractory period have reduced amplitudes. Spike amplitude reduction due to sodium channel inactivation has been observed in a number of systems, but has mostly been discussed in the context of spike failures at branch points (Brody and Yue, 2000; Grossman et al., 1979a, b; see section 3.2.), or with respect to synaptic depression caused by a reduction of presynaptic depolarization (He et al., 2002). In Hodgkin-Huxley type model axons, conduction velocity is positively correlated with spike amplitude, and spike amplitude decreases with decreasing spike intervals (Moradmand and Goldfinger, 1995; Fig. 6A and B). It should be noted that two related mechanisms underlie the slowing of conduction by sodium channel inactivation: spike threshold is increased, and the reduced spike amplitude limits the distance over which adjacent regions can be depolarized to threshold.
Fig. 6.
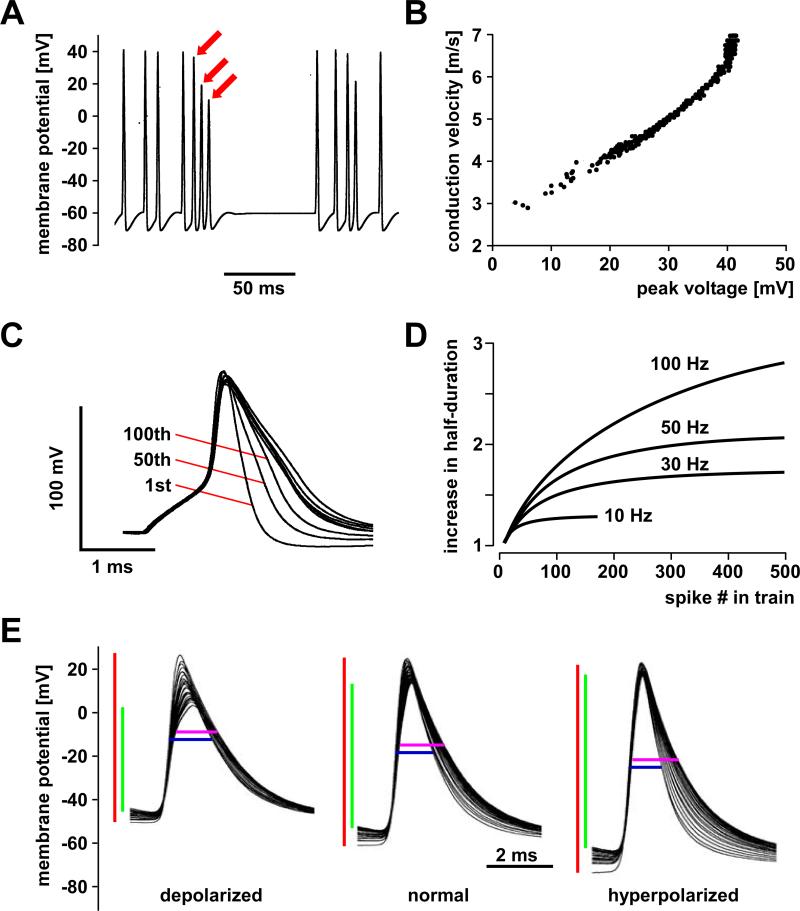
Changes in spike shape during repetitive activity and changes in baseline membrane potential A: In a Hodgkin-Huxley type axon model with only fast sodium and delayed rectifier potassium currents, sodium channel inactivation leads to a reduction in spike amplitude (red arrows). B: Conduction velocity in the same model is well correlated with the peak voltage of spikes, in that spikes with reduced amplitude are slower. C: In hippocampal mossy fibers, inactivation of A-type potassium channels leads to activity-dependent spike broadening. The overlaid traces shown are from a 50 Hz train. D: The magnitude of the increase in spike duration in mossy fibers is dependent on stimulation frequency. E: In a lobster stomatogastric axon, depolarizing and hyperpolarizing current injections during ongoing burst activity change spike amplitudes, spike durations, and the dynamics of both. Shown are overlaid spikes from single bursts under each condition. Depolarizing current injection (left) decreases initial spike amplitude (red bar) and increases the frequency dependent change in amplitude (green bar), compared to control (middle panel). Initial spike duration (blue bar) is prolonged compared to control, but increases very little over the course of the burst (magenta bar). Hyperpolarizing current injection (right) increases spike amplitude and decreases frequency-dependent reduction. Initial spike duration is reduced compared to control but increases substantially over the course of the burst. A and B are modified from Moradmand and Goldfinger, 1995. C and D are modified from Geiger and Jonas, 2000. E is modified from Ballo and Bucher, 2009.
The voltage-dependence of sodium channel inactivation and de-inactivation illustrates the ambiguous effects that membrane potential can have on axonal excitability. A brief subthreshold depolarization brings the membrane closer to the activation threshold of sodium channels and therefore closer to spike threshold. In consequence, depolarization by impulses can spread farther and conduction velocity is increased, as seen in the supernormal conduction associated with after-depolarization. On the other hand, a more sustained subthreshold depolarization will inactivate sodium channels. In consequence, fewer sodium channels are available for activation and conduction velocity will be decreased. In unmyelinated axons innervating the rat cranial meninges, sodium channel inactivation slowed spike conduction during sustained low frequency stimulations (De Col et al., 2008). Interestingly, block of the Na+/K+-pump increased this effect. The pump is often held responsible for the pronounced slowing of conduction in unmyelinated axons, but blocking it can also lead to depolarization and subsequent sodium channel inactivation. Slow depolarization during repetitive activity also leads to sodium channel inactivation and reduced spike amplitudes in crustacean axons (Grossman et al., 1979a) and hippocampal axons (Brody and Yue, 2000; He et al., 2002; Meeks and Mennerick, 2004).
Hyperpolarization moves the membrane potential further away from the activation threshold of sodium channels and slows impulse conduction, as seen in the subnormal conduction associated with after-hyperpolarization. On the other hand, hyperpolarization causes removal of inactivation and renders more sodium channels available for activation, therefore increasing conduction velocity. Graded presynaptic hyperpolarization leads to increased spike amplitudes at the squid giant synapse (Hagiwara and Tasaki, 1958) and at autaptic contacts in cultured hippocampal neurons (Thio and Yamada, 2004). In a crustacean stomatogastric axon, depolarizing current injection during ongoing bursting activity reduces spike amplitude and increases the frequency-dependent reduction of amplitude, whereas hyperpolarizing current injection increases spike amplitude and reduces the frequency-dependent reduction of amplitude (Ballo and Bucher, 2009; Fig. 6E). The inactivation properties of sodium channels are also thought to explain changes in the recovery cycle of peripheral unmyelinated axons in rats and humans in response to sustained depolarization and hyperpolarization by chemical mediators (Moalem-Taylor et al., 2007). Here, depolarization leads to increased conduction velocity for a conditioning compound spike, but to a loss of supernormal conduction in subsequent test spikes. Hyperpolarization leads to overall slowing but to an extension of the supernormal period.
The role that potassium currents play for conduction velocity and excitability can also be ambiguous. Slow potassium currents, like those produced by Kv7 channels, mainly play a role during repetitive activity. They cause hyperpolarization and limit the duration of the supernormal phase. Again, the effect of this hyperpolarization can be ambiguous. While hyperpolarization leads to subnormal conduction, it promotes removal of inactivation from sodium channels. Consequently, block of Kv7 channels can lead to increased sodium channel inactivation in rat hippocampal axons and reduce spike amplitudes (Vervaeke et al., 2006). The functional contribution of fast potassium channels to excitability depends largely on how their activation and de-activation properties are matched to the inactivation and de-inactivation of sodium channels (Alle et al., 2009; Baranauskas, 2007; Sengupta et al., 2010). In the classical Hodgkin-Huxley model, they have a number of effects. Their opening upon depolarization speeds up spike repolarization and therefore limits inactivation of sodium channels. Their relatively slow deactivation produces an after-hyperpolarization that accelerates the removal of inactivation from sodium channels. These two processes reduce the refractory period and therefore limit slowing of spike conduction. On the other hand, delayed deactivation and the fact that a portion of channels are open at resting membrane potential decreases excitability and slows spike conduction. In myelinated axons, the role of fast potassium currents is controversial. They are small at the node, and the juxtaparanodal Kv1 channels are sometimes thought to contribute little to repolarization, but to have their main function in limiting re-excitation (Chiu et al., 1979; Mert, 2006; Oozeer et al., 2006). For example, they limit after-depolarization in rat sciatic nerve (Baker et al., 1987). However, fast potassium channels contribute to spike repolarization in peripheral rat and lizard axons, as specific blockers increase spike duration (Baker et al., 1987; David et al., 1995; Eng et al., 1988; Kocsis et al., 1987). Optical nerve axons and other central axons also show a distinct increase in spike duration when Kv1 channels are blocked (Geiger and Jonas, 2000; Gordon et al., 1988; Shu et al., 2007b). Because some of these channels produce inactivating A-type or D-type currents, their contribution to repolarization and re-excitation changes during repetitive activity. For example, A-current inactivation during repetitive firing is thought to underlie supernormal conduction in crustacean axons (Stockbridge and Yamoah, 1990). Potassium channel inactivation progressively increases spike duration, for example in layer 5 pyramidal axons (Shu et al., 2007b), crustacean stomatogastric motor axons (Ballo and Bucher, 2009), and mossy fibers (Geiger and Jonas, 2000; Fig. 6C and D). Again, the effect on excitability is ambiguous. On the one hand, potassium channel inactivation increases excitability as diminished outward currents reduce the amount of current necessary to reach spike threshold. On the other hand, prolonged spike duration increases sodium channel inactivation during repetitive activity. Because of the voltage-dependence of A-current inactivation, the magnitude of changes in spike duration during repetitive activation is also dependent on membrane potential. During sustained depolarization, inactivation rapidly leads to prolonged spike duration with little subsequent change, while sustained hyperpolarization accelerates initial spike repolarization which then slows down substantially during successive spikes (Ballo and Bucher, 2009; Fig. 6E). During higher frequency spiking, prolonged repolarization times can also contribute to summation, with the consequence that successive spikes are fired from more depolarized membrane potentials (Ballo and Bucher, 2009; Fig. 8).
Fig. 8.
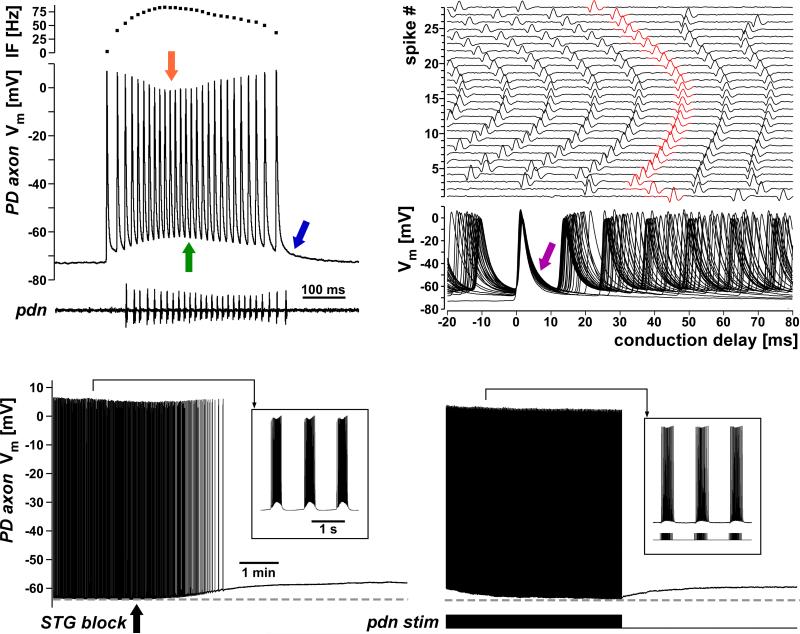
Membrane potential, spike shape, and conduction delay in a crustacean stomatogastric axon. A: Single burst recorded from the pyloric dilator (PD) neuron axon in the motor nerves during ongoing rhythmic pyloric activity. The intracellular recording is from a relatively proximal site, ~1 cm from the stomatogastric ganglion; the extracellular recording (lower panel) is from a distal site close to the motor terminals, ~5 cm from the ganglion. Note the longer delay to the first spike. The upper panel shows the parabolic instantaneous frequency over the course of the burst. The intracellular recording shows that spike amplitude decreases with frequency (orange arrow). In addition, the membrane potential from which each spike is fired increases with frequency (green arrow). This is due to summation caused by very slow repolarization times (blue arrow). B: Multiple sweep view triggered from the intracellularly recorded spikes of the single burst shown in A. Intracellular spikes are shown superimposed in the bottom panel. Note that spike duration increases over the course of the burst (purple arrow), contributing to the summation shown in A. Corresponding extracellular spikes are colored red in the staggered sweeps in the upper panel. The delay from intra- to extracellular recording site changes dramatically. Note that there is an initial decrease in conduction delay that is not correlated with the changes in spike shape. C: Apart from the fast changes in spike shape and membrane potential over the course of a burst, the baseline membrane potential also changes at a slow time scale in an activity-dependent manner. When centrally generated activity in the stomatogastric ganglion is blocked, the axon slowly depolarizes (left panel). When subsequently the motor nerve is stimulated with a realistic spike pattern, it repolarizes with a similar time course (right panel). A, B, and C are adapted from Ballo and Bucher, 2009.
In the context of repetitive activation, the function of fast potassium currents in limiting re-excitation usually dominates over the function in limiting sodium channel inactivation. Therefore, blocking potassium currents usually leads to hyperexcitability. For example, block of Kv1 channels can lead to distal axonal spike initiation in a number of axons (Baker et al., 1987; Kocsis et al., 1987; Palani et al., 2010; Shu et al., 2007b). An interesting case in this respect is the presence of fast Kv3 channels at the node of myelinated axons. The contribution of such channels to repetitive activity has been studied with somatic recordings in fast spiking central neurons in the context of spike initiation (Baranauskas, 2007; Bean, 2007; Erisir et al., 1999). They behave differently than the classical delayed rectifier currents in a number of ways. Most importantly, they have a steep voltage-dependence, activate at relatively depolarized potentials, and their deactivation is very fast. Consequently, they do not significantly contribute to the excitability after the spike, but mainly help the rapid repolarization of the spike, which limits the refractory period. Therefore, blocking Kv3 channels has the somewhat counter-intuitive effect of reducing re-excitability.
In general, spike shape critically depends on which types of potassium channels a neuron expresses, and neurons with short spike durations are usually able to sustain higher spike frequencies (Bean, 2007). On the other hand, recent studies have shown that the waveform of a single spike can be quite robust to differences in temporal overlap between sodium and fast potassium currents, as long as appropriate single conductance levels are maintained (Alle et al., 2009; Sengupta et al., 2010). These studies focused on energy expenditure of single spikes, under the premise that large temporal overlap between opposing sodium and fast potassium conductances results in the need for larger sodium currents to generate a spike, and subsequently to increased ATP hydrolysis by the Na+/K+-pump. The consequences for repetitive activation have not been studied but the results demonstrate that in the presence of currents with opposing signs, voltage trajectory is a poor predictor of conductance. This is important in the context of spike conduction velocity because the axonal length constant is dependent on total conductance (the inverse of input resistance). In the context of repetitive activity, the presence of balancing or opposing ionic mechanisms can also mean that total conductance changes are not well reflected in the voltage trajectory. A good example for this may be the balance between after-hyperpolarization and inward rectification through Ih. As hyperpolarization increases, inwardly rectifying channels open and limit further hyperpolarization. This is associated with a decrease in late subnormal excitability in peripheral axons (Baker et al., 1987; Grafe et al., 1997; Moalem-Taylor et al., 2007; Robert and Jirounek, 1998; Takigawa et al., 1998). Ih also reduces spike slowing and conduction failures in unmyelinated central axons (Baginskas et al., 2009; Soleng et al., 2003), and balances activity-dependent hyperpolarization in crustacean axons (Ballo and Bucher, 2009; Ballo et al., 2010; Beaumont et al., 2002; Beaumont and Zucker, 2000). The more pronounced late subexcitability in human motor axons compared to cutaneous afferents is thought to arise from a smaller inward rectification through Ih (Kiernan et al., 2004). The function of Ih in balancing hyperpolarization suggests that inward rectification improves the temporal fidelity of spike conduction. However, the opposing action of after-hyperpolarization and inward rectification means that the change in total conductance is disproportionate to the actual change in membrane potential. In other words, very different underlying membrane resistance can underlie similar membrane potentials, and therefore result in very different conduction velocities. We are not aware of a case where such a relationship has been explicitly investigated, but it is intriguing that Ih is important in determining the steady-state interburst membrane potential in rhythmically active crustacean axons (Ballo and Bucher, 2009; Ballo et al., 2010), and shifts the entire recovery cycle in human c-fibers (Moalem-Taylor et al., 2007).
The changes in excitability and membrane potentials discussed so far result from axonal spiking activity and therefore only play a role during repetitive activity. However, recent work has shown that in some axons fluctuations in membrane potential are due to synaptic or modulatory input. Direct effects from axo-axonal synapses or paracrine release of modulators are discussed in section 4, but there can also be passive spread of sub-threshold potentials from proximal sites over relatively long distances. Recent work on mammalian central neurons shows that even in small diameter axons passive spread of synaptic potentials from dendritic and somatic inputs bridge hundreds of micrometers or more and influence the efficacy of spike-mediated transmission at presynaptic sites (Alle and Geiger, 2006, 2008; Kress and Mennerick, 2009). In addition, fluctuations of membrane potential generated in terminals can spread antidromically to influence spike initiation (Paradiso and Wu, 2009). These results are of course most interesting in the context of presynaptic depolarization and the integration of analog and digital signaling components. However, they also illustrate that in some axons, sub-threshold membrane potential fluctuations generated by barrages of proximal synaptic inputs can spread through a substantial portion of the axon. Therefore, they may also influence conduction velocities and delays.
3.1.4. Spatial variation of activity-dependent changes in excitability
It is important to note that activity-dependent changes in conduction velocity are a function of local excitability changes at any given site that is traversed by propagating spikes. Therefore, there is a spatial component to temporal precision. If conduction velocity is different between consecutive spikes, the interval between the spikes changes along the axon and the excitability of the axon at the time of the second spike is different at different locations. The temporal precision of spike conduction for given local excitability changes depends on the mean absolute velocity and the length of the axon. To some degree, the same percent change in velocity will have a bigger effect in longer or more slowly conducting axons. However, conduction delay does not change linearly with distance. If the second spike travels more slowly than the first, the interval between both will increase along the axon, potentially until it is large enough for both spikes to be conducted at the same speed. If the second spike is faster than the first, it eventually approaches the refractory region left in the wake of the first and starts slowing down, so the interval at which both travel stabilizes. In a long enough axon, this means that smaller variations in spike initiation can be compensated during propagation, and pairs of spikes can be locked into a specific interval. A theoretical demonstration of this principle can be seen in Figure 7A and B, obtained from a Hodgkin Huxley-type long axon model (Moradmand and Goldfinger, 1995). This model shows the usual triphasic change in conduction velocity when pairs of spikes are elicited at different intervals and delay is measured at a fixed distance (Fig. 7A). Figure 7B shows the change in interval over the length of the axon for different stimulus intervals. When the second spike is elicited during the relative refractory period at the stimulation site, conduction velocity is reduced and the interval increases asymptotically to a maximum with axonal distance. When the second spike is elicited during the earlier part of the supernormal period, conduction velocity is increased and interval decreases with axonal distance, converging on the same value as the ones from stimulations during the relative refractory period. This means that for a small range of intervals generated at a spike initiation site, intervals of spikes arriving at presynaptic sites can be equalized. Such equalization has been observed in antidromic stimulations of efferent visual cortical neurons in the rabbit and termed “impulse entrainment” (Kocsis et al., 1979). Figure 7C shows that in this system varying stimulus intervals had no effect on the spike intervals recorded over the distance from thalamus to cortex. Similar entrainment towards intervals that are close to the duration of the relative refractory period has been demonstrated in human C-fibers (Weidner et al., 2002).
Fig. 7.
Spatial variation and entrainment of intervals between spikes propagated at different velocities. A: Conduction velocity as a function of stimulus interval in a Hodgkin-Huxley axon model stimulated with paired pulses. Velocities were calculated from measurements at a distance of 9 length constants from the stimulation site. The conditioning spike traveled at a velocity of 6.54 m/s (dashed red line). The second spike was slowed when elicited during the relative refractory period. With increasing intervals, conduction became first supernormal and then subnormal (green line). B: When the interstimulus interval was varied between 5 and 23 ms in the same model axon, the interspike interval changed as a function of distance from the stimulation site. Over distance, spikes elicited during the relative refractory period and in the early supernormal period converge on an interval that marks the transition between refractory and supernormal periods, where the conduction velocities of conditioning and second spike are identical (blue ellipse). C: Impulse entrainment in a rabbit efferent visual cortex neuron. Thalamic antidromic stimulation at a distance of 14.9 mm was used to evoke spikes in cortical extracellular recordings. Recorded spike intervals were a constant 1.7 ms, despite the fact that stimulus intervals were varied between 0.9 and 2.0 ms (10 pairs of stimuli as shown in the lower scheme). A and B are modified from Moradmand and Goldfinger, 1995; C is modified from Kocsis et al.,1979.
Another aspect of the spatial component of excitability changes is that in neurons with more than one integrative site, spike-mediated interactions occur across compartments. In the lobster stomatogastric nervous system, a motor neuron controlling esophageal muscles has three spike initiation zones, one in the unpaired ganglion that the soma resides in, and one in each of a bilateral pair of ganglia that the axon traverses before it exits through motor nerves. Synaptic inputs in the bilateral pair of ganglia serve two functions. They elicit rhythmic motor activity and rhythmically inhibit conduction of tonic spiking generated in the unpaired ganglion (Nagy et al., 1981). This inhibitory effect may be exerted by excitability changes following each rhythmic burst instead of resulting from direct inhibitory synaptic inputs. Similarly, the spike initiation zones in multi-segmental leech heart interneurons are usually suppressed by bursts generated in the “dominant” ganglion (Calabrese, 1980). Spike initiation and propagation can also be suppressed by antidromic propagation of spikes. Different terminals of the same C-fiber that integrate sensory input can influence each other. Spikes initiated in one terminal arbor can be propagated both orthodromically towards the central nervous system, and antidromically into adjacent arbors where they may block spike initiation or conduction (Weidner et al., 2003). Over longer distances, presynaptic inputs at axon terminals can elicit spikes that back-propagate and inhibit activity, a phenomenon very common in both vertebrate and invertebrate neurons (Cattaert and Bevengut, 2002; Pinault, 1995).
3.1.5. Frequency- and history-dependence of conduction velocity during complex spike patterns
Most of the excitability changes discussed so far have been studied in the conceptual framework of how a “conditioning” spike or train of spikes affects the initiation and conduction of a subsequent “test” spike. However, this framework is insufficient to understand the fluctuations of conduction velocity that occur when a neuron produces spike trains of more or less complex temporal structure. Obviously, if spike intervals are sufficiently large for the axonal membrane to return to the initial state, consecutive spikes will have identical conduction delays. Therefore, changes in spike delay depend on neuronal activity that is sufficiently repetitive, and on the time constants of membrane properties underlying these dynamics. Relatively fast processes at a time scale similar to spike intervals will render conduction velocity dependent on spike frequency. Relatively slow processes at a time scale of multiple spike intervals will in addition render conduction velocity dependent on the history of spiking, i.e. on the preceding pattern of spikes, further back in time than just the last interval.
Potentially, even when only the dynamics arising from the interactions of voltage-gated currents are considered, history-dependence may involve rather large time scales. Such larger time scale processes are not just resulting from slow activation or inactivation properties of some voltage-gated ion channels, which are usually limited to hundreds of milliseconds or a few seconds. Rather, during repetitive spiking they depend on the changed initial conditions at the time of each spike. Repeated activation at intervals smaller than the recovery time leads to cumulative effects. For example, consider the original description of sodium channel gating during spikes in the squid giant axon (Hodgkin and Huxley, 1952). Sodium channels activate and inactivate, and then recover from inactivation within some 4 ms after the onset of the spike. If another spike occurs within the relative refractory period, say after an interval of 3 ms, fewer sodium channels are available for activation because more channels still have closed inactivation gates. The second spike therefore starts under changed initial conditions, which has consequences for the following refractory period. A third spike with the same interspike interval will now start at conditions different from both the first and the second, as even fewer sodium channels are available (see Fig. 6A). With repeated activation, changes in propagation therefore occur over time scales significantly larger than the largest time constant of any channel gating process. Furthermore, the changes in voltage trajectory (e.g., reduced spike amplitude) mean that activation, inactivation and removal of inactivation are affected differently by each spike, leading to non-monotonic changes in conduction velocity (Elphick et al., 1990; Miller and Rinzel, 1981; Moradmand and Goldfinger, 1995). Intrinsic properties of neurons in general arise from multiple interacting time- and voltage-dependent processes. Complex combinations of such processes can make neuronal firing very dependent on the history of activity, potentially at a time scale exceeding the largest time constant present in any separate underlying process (Drew and Abbott, 2006; Gilboa et al., 2005; Tal et al., 2001). Considering that spike propagation in probably all cases involves interactions between different ionic conductances displaying gating properties with several different time constants, and in addition may be influenced by other processes such as summating pump currents or intracellular calcium dynamics, it is likely that many axons show substantial history-dependence of conduction velocity.
The history-dependence of conduction velocity means that in a given axon with given membrane dynamics the types of changes imposed by propagation depend critically on the pattern of activity. A simple aspect of this has already been discussed, as it is apparent from many examples that the relative strength and duration of each part of the recovery cycle is dependent on the conditioning stimulus, i.e. different between a single conditioning stimulus and trains of stimuli of different duration and frequency (e.g., Raymond, 1979; Kiernan et al., 1997; see Fig. 4B). However, in order to understand the contribution of activity-dependent changes in conduction velocity to the shaping of temporal patterns, one has to consider to which degree the conduction delay of each of the spikes in a pattern is affected. In other words, the more interesting question is how the temporal structure, i.e. the pattern of intervals in a train of impulses, is altered by the process of propagation. The problem in many cases is that the dynamic range of naturally occurring spike patterns is not known. In the absence of a good approximation of “natural” temporal patterns, attempts have been made to theoretically explore the extent and sign of temporal dispersion in response to different sustained spike frequencies (Miller and Rinzel, 1981), or to trains with a randomized (Poisson-like) frequency distribution (Moradmand and Goldfinger, 1995). In these models based only on cable theory and Hodgkin-Huxley type ionic conductances, it was found that intervals and temporal structure changes can be substantial and are dependent on mean frequency.
One of the few cases where the extent of changes in delay has been shown for realistic “natural” activity in a biological axon comes from the lobster stomatogastric nervous system (Ballo and Bucher, 2009). This model system reliably produces regular rhythmic patterns in vitro that closely resemble in vivo activity (Marder and Bucher, 2007). The pyloric dilator (PD) neurons in the lobster continuously burst with a duty cycle of ~35% at periods around 1s, and each burst consists of ~20 spikes (Ballo and Bucher, 2009). The interspike interval structure is parabolic, i.e. it shows increasing instantaneous spike frequencies towards the middle of the burst, peaking at around 70 Hz, and decreasing frequencies towards the end. Intracellular recordings of the axon in the motor nerve show a number of changes in voltage trajectory over the course of a single burst (Fig. 8A and B). Spike amplitude is diminished as a function of frequency, and spike duration increases over the course of a single burst (see also Fig. 6E). In addition, the slow spike repolarization causes summation which results in each spike being fired from a different membrane potential. Conduction delays recorded between proximal and distal recording sites at several centimeters distance show a complex pattern of changes that are not clearly correlated with instantaneous frequencies or any of the changes in voltage trajectory (Fig. 8B). These nonlinearities are apparent in single bursts, but are also due to changes occurring at slower time scales changes which are not apparent during steady-state ongoing activity. When centrally generated activity is blocked, the baseline membrane potential slowly depolarizes with a time constant of several hundred seconds (Fig. 8C), an effect that can be reversed when the distal axon is stimulated with a realistic pattern. Therefore, the extent to which conduction delay changes over the course of a single burst probably also depends on the frequency and strength of ongoing bursting activity.
In the lobster axon described, the functional consequences of the substantial changes in the interval structure of spikes within each burst are unknown. One of the few cases where activity-dependent changes in conduction delays have explicitly been considered part of the coding strategy is the signaling of human C-fibers (Weidner et al., 2002). During repetitive activation, C-fibers hyperpolarize and conduction velocity progressively slows. However, slowing of conduction does not mean that all intervals increase during propagation. C-fibers often fire in bursts, and the overall activity level determines how changes in excitability and conduction affect the smaller scale temporal structure. Weidner et al. (2002) stimulated fibers with trains of 4 stimuli at high frequency (20 or 50 Hz) and varied the repetition rate of these trains, i.e. they elicited patterns with varying burst frequencies but constant burst duration and intraburst spike frequency (Fig.9). Compared to initial conditions, overall conduction velocity was always slowed. However, the relative change in conduction velocity between spikes within one burst changed dramatically depending on the overall activity level, i.e. on burst frequency. At low repetition rates, intervals increased, i.e. intraburst frequency decreased. At higher repetition rates, intervals decreased, as consecutive spikes within each burst slowed less than initial ones. This “relative supernormality” led to a several-fold increase in intraburst spike frequency, apparently approaching the point where impulse locking would occur over the propagation distance. The process of propagation therefore enhances the contrast between different levels of overall activity. This effect may be important for coding, as overall activity levels in C-fibers can change dramatically, for example in response to inflammatory mediators.
Fig. 9.
“Contrast enhancement” in human C-fibers. A: Peroneal nerve C-fibers were stimulated at their receptive fields with trains of 4 pulses (intervals: 20 ms) at varying repetition rates and recorded at knee level. At low repetition rates (red), intervals between spikes increased during propagation, and at higher repetition rates (green), intervals decreased. B: Plot of the response latencies at the recording site as a function of successive changes in repetition rate of the 4-spike trains. Graphs labeled I, II, III, and IV correspond to the first to fourth spikes within the train shown in A. All latencies were measured with respect to the first stimulus in the train. Overall, repetitive stimulation slowed conduction compared to the previously quiescent axon, as can be seen in the progressive increase of latency with repetition rate for spike I. However, intervals between spikes I, II, III, and IV were first increased and then decreased with increasing repetition rate. This relative supernormal conduction increased until spikes became locked into a minimum interval that corresponded to the duration of the relative refractory period. Red and green bars correspond to the patterns shown in A. Modified from Weidner et al., 2002.
3.2. The effects of non-uniform membrane properties on spike propagation
So far, we have only considered phenomena that result from activity-dependent changes in ionic currents which would be relevant even in hypothetical axons with uniform morphology and channel distribution. Real axons often have properties far from uniform, as diameters can change along the path and at branch points, and ion channel complements can be different between proximal and distal sites. In theory, excitability changes during propagation of repetitive firing activity in a uniform axon only affect the distribution of intervals in a spike pattern. Once elicited, a spike may travel at changing velocity through regions of altered excitability left in the wake of preceding spikes, but it will neither fail nor elicit additional spikes. This results from the fact that both speeding and slowing can maximally alter spike intervals over the propagation distance to the point in the recovery cycle where velocity becomes equal to the preceding spike, as described in section 3.1.4. In contrast, non-uniform membrane properties can lead to activity-independent slowing and speeding, and can produce both spike failures and ectopic spike initiation. These activity-independent properties can interact with activity-dependent changes in excitability to produce complex filtering of spike patterns.
3.2.1. Non-uniform channel distribution and noise
One way the excitability of an axon can change along its path is if the complement and densities of ion channels are different in different sections. Ion channel complements clearly can be very different between the spike-initiating proximal axon, the “axon proper”, and terminal arborizations or presynaptic membrane (Lujan, 2010; Nusser, 2009; Vacher et al., 2008). However, the extent to which ion channel densities can change along non-synaptic axonal membrane is generally not well known. There are some examples of spike failures in the proximal axon that must be due to differences in excitability between initiation site and propagating part. For example, during Purkinje cell bursting elicited by climbing fiber input, generally only the first and last spike are propagated (Khaliq and Raman, 2005; Monsivais et al., 2005). Bursting activity elicited in the proximal part of climbing fibers can also lead to occasional spike failures within the primary axon (Mathy et al., 2009). Particularly interesting examples of non-uniform ion channel distribution are the en passant varicosities found on many mammalian central axons (Fig. 10A). Apart from the effects of diameter changes that are discussed below, these presynaptic specializations are likely to have a different complement of ionic conductances than the adjacent non-synaptic axonal membrane because they are sites of calcium-mediated transmitter release. For axons of hypothalamic neurons with varicosities in the neurohypophysis, Muschol et al. (2003) proposed a model of “stuttering conduction” (Fig. 10B). Calcium influx and subsequent activation of calcium-dependent potassium currents lead to an activity-dependent loss of excitability that is confined to varicosities (Bielefeldt and Jackson, 1993). During repetitive activation, spikes may invade the varicosity passively but can be actively propagated after they passed it. Therefore, the delay imposed by propagation through varicosities is dependent on spike history and can lead to substantial changes in delay and consequently to temporal dispersion.
Fig. 10.
The effects of non-uniform excitability and geometry. A: three-dimensional confocal reconstruction of a layer 5 pyramidal cell axon and dendrite in rat somatosensory cortex. B: Model of activity-dependent inactivation at varicosities in peptidergic neurons of the neurohypophysis, mediated by calcium-dependent potassium channels. C: The effect of diameter changes at branch points on spike propagation and delay. A is modified from Kalisman et al., 2005; B is modified from Muschol et al., 2003; C is modified from Manor et al., 1991.
Even axons with uniform geometry and ion channel distribution can show non-uniform electrical properties while a spike is propagated. This is due to thermal and channel noise that can produce fluctuations of excitability both in time and in space, which has been explored theoretically in different model axons (Faisal and Laughlin, 2007; Kuriscak et al., 2002). Noise has only a minor impact in larger axons, but Faisal and Laughlin (2007) found distinct mechanisms degrading the reliability of spike propagation in models of thin axons. First, noise-induced variability in channel openings during the spike can gradually affect velocity. Second, noise-induced spontaneous openings of whole clusters of channels ahead of the spike can make the spike leap ahead in discrete jumps, a phenomenon termed “stochastic micro-saltatory propagation”. A small number of spike failures and ectopic spike initiation have also been observed in these models.
3.2.2. Impedance mismatch
Spike conduction can also be affected by morphological inhomogeneities. The theoretical effects of non-uniform geometry such as branch points or diameter changes on spike propagation have been first explored according to cable theory by Rall and Goldstein (Goldstein and Rall, 1974; Rall, 1959), and later in the context of spike conduction through complex axonal trees (Manor et al., 1991). This work has been reviewed in detail elsewhere (Debanne, 2004; Segev and Schneidman, 1999; Swadlow et al., 1980). When a spike approaches an axon region of different diameter, conduction velocity can be increased or decreased depending on the sign of the associated impedance change. An increase in diameter (i.e. a decrease in impedance) means that the current ahead of the spike causes a smaller depolarization and therefore increases the time to reach threshold. This “current sink” slows propagation towards the point where the diameter changes. Once this point is traversed, of course, the current generated by each spike in the axon with increased diameter is larger and therefore velocity is increased. Conversely, a decrease in diameter speeds up conduction towards the point of impedance increase, and subsequently propagation is slowed in the smaller diameter section. Gradual changes in diameter also change conduction velocity. For example, a continuously increasing diameter leads to a gradual increase in conduction velocity, but velocity at any given point is smaller than it would be in a uniform axon with an equivalent diameter.
Special cases of impedance change are branch points. Propagation through a branch point depends on the “3/2 power rule” (Rall, 1959) which describes the geometric ratio (GR) between the diameters of the daughter branches (d1 and d2) and the diameter of the parent branch (dp). This ratio is defined as: GR = (d13/2 + d23/2) / dp3/2. If GR = 1, for example if the diameter of each daughter branch is 63% of the parent branch diameter, impedance is matched and propagation is unperturbed. Conduction velocity is decreased if GR > 1, and increased if GR < 1. Figure 10C shows the difference in spike propagation through a branch point for models with uniform specific membrane properties but different geometric ratios (Manor et al., 1991). With GR = 1, the spike travels with uniform shape and velocity from parent to daughter branches. With GR = 8, spike shape is distorted at the branch point. Spikes resume the initial shape in the daughter branches, but are delayed compared to branch points with GR = 1.
Changes in conduction speed imposed by single changes in diameter or branch points are usually only on the order of tenths of milliseconds, but the interaction of multiple geometric inhomogeneities, as found in neurons with complex axonal branching structure and varicosities in higher order branches, can lead to substantial changes in delay (Manor et al., 1991). Two effects in this context are noteworthy. First, the lag imposed by an increase in diameter or a GR > 1 is larger than the lead imposed by the equivalent decrease in diameter or the reciprocal GR value. This is important because it means that varicosities, i.e. rapid successive increases and decreases in diameter, slow propagation. Second, if two sites of geometrical change are electrotonically close, the change in spike velocity is not the linear sum of the changes that would be imposed by each individual site in isolation. If GR > 1 at two sites close to each other, the imposed delay is larger than the sum of the individual delays each site would impose in isolation. If GR < 1 at two sites close to each other, the increase in velocity is smaller than the sum of the individual increases in velocity. Taken together, these phenomena mean that the absolute delay imposed by the process of spike propagation does not just depend on the distance and average diameter, but also on the number and spatial arrangement of morphological inhomogeneities that a spike has to traverse on its way to a presynaptic site.
3.2.3. Non-uniform excitability and spike failures
Equally important as imposed delays, impedance mismatch can lead to a decreased safety factor for propagation, i.e. spikes can fail at branch points and varicosities (Debanne, 2004; Swadlow et al., 1980; Wall, 1995). Obviously, it would not be advantageous for any neuron if spike failures would occur indiscriminately, solely on the basis of geometry. However, non-uniform geometry in conjunction with activity-dependent changes in excitability can cause frequency-dependent failures or relief of failures. Intermittent spike failures during repetitive activity were first observed in spinal axons (Barron and Matthews, 1935), and later suggested to occur at branch points (Krnjevic and Miledi, 1959). Figure 11A shows a model of a branched myelinated axon in which high-frequency spiking in the parent branch led to intermittent failures in the daughter branches (Zhou and Chiu, 2001). In this model, spike slowing and failures were dependent on a number of biophysical parameters. With identical geometry and ion channel distribution, spike failures were dependent on temperature and frequency and therefore clearly on excitability changes. In addition, the fidelity of propagation changed when the length of internodal sections around the branch point or the size of the periaxonal space between axon membrane and myelin sheath were altered. Importantly, in this model the reliability of spike propagation was very sensitive to the dynamics of periaxonal potassium accumulation and clearing.
Fig. 11.
Branch point propagation failures. A: Intermittent spike failures at a branch point in a computational model of a myelinated axon at two different temperatures. B: Selective spike failure in one of the daughter branches of a spiny lobster motor axon. Traces are from initial stimulation of the parent axon (control) and after 4.5 s of stimulation at 50 Hz. The top two traces are intracellular recordings (sites indicated by triangles in the schematic), and the bottom two traces are extracellular recordings (sites indicated by double lines). Note that during repetitive activity, the spike has a diminished amplitude in the intracellular recording of the medial branch, and fails to propagate to the extracellular recording site (black arrow). C: The role of A-type potassium currents in branch point spike failures in hippocampal pyramidal axons. Hyperpolarizing presteps in the presynaptic cell eliminated postsynaptic responses to single spikes elicited at the soma of the presynaptic cell (left traces). When cells were held at potentials more hyperpolarized than the resting membrane potential (RMP) and then spikes elicited with sustained depolarizing pulses, only initial spikes failed to propagate to presynaptic sites. Subsequent spikes elicited postsynaptic responses. A is modified from Zhou and Chiu, 2001; B is modified from Grossman et al., 1979a; C is modified from Debanne et al., 1997.
Experiments conducted on crustacean motor axons suggested early that extracellular potassium accumulation can play an important role in branch point spike failures. In these axons, repetitive activation leads to spike failures and subsequent synaptic depression at the neuromuscular junction (Grossman et al., 1979a, b; Hatt and Smith, 1976; Parnas, 1972; Smith, 1980). Grossman et al. (1979a) recorded from spiny lobster motor axons close to the innervated muscles. At this site, the parent axon splits into a larger diameter medial, and a smaller diameter lateral daughter branch (Fig. 11B). Multiple simultaneous intra- and extracellular recordings showed that during repetitive stimulation spike amplitude decreased in the medial branch and active propagation eventually failed. Failures were also associated with a slight depolarization of the resting membrane potential. These observations are consistent with extracellular potassium accumulation and subsequent increased sodium channel inactivation, suggesting that failures are due to depolarization. Consistent with this model, hyperpolarization relieved conduction failures in crayfish motor axons (Smith, 1980). In the spiny lobster motor axon, experimentally increasing extracellular potassium concentration also produced conduction failures (Grossman et al., 1979b). However, in this case failures occurred in both daughter branches, and not selectively in the larger branch. According to cable theory, spike failures due to impedance mismatch at an axon branch point with uniform specific membrane properties always occur in both daughter branches, independent of their relative sizes (Goldstein and Rall, 1974). Selective spike failures at branch points therefore cannot be explained by impedance mismatch alone, but require non-uniform excitability. In the spiny lobster axon, it was suggested that extracellular potassium accumulation has a smaller effect on the smaller daughter branch (Grossman et al., 1979b). Because of the larger surface-to-volume ratio in the smaller branch, spiking causes a larger increase in intracellular sodium concentration, and subsequently a stronger activation of the Na+/K+ pump. Therefore, potassium clearance from the periaxonal space is faster around the smaller branch, reducing the probability of spike failures. Consistent with this, block of the pump led to simultaneous failures in both daughter branches. Potassium accumulation is also held partly responsible for spike failures in hippocampal axons (Meeks and Mennerick, 2004).
In section 3.1.3., we described the ambiguous effects that changing membrane potential can have on axon excitability. This ambiguity is also apparent in the context of activity-dependent spike failures at branch points. While the failures in crustacean axons described above are due to depolarization, spike failures in other axons are associated with activity-dependent hyperpolarization. In different types of leech mechanosensory neurons, failures occur at central branch points due to activity-dependent activation of the Na+/K+ pump (Baccus et al., 2000; Cataldo et al., 2005; Gu, 1991; Macagno et al., 1987; Scuri et al., 2007; Van Essen, 1973; Yau, 1976). Spike failures apparently associated with membrane hyperpolarization have also been found in the neurohypophysis (Bielefeldt and Jackson, 1993), C-fibers in rabbit nodose ganglion (Ducreux et al., 1993), and at bifurcations of locust neuromodulatory neurons (Heitler and Goodman, 1978). Activity-dependent hyperpolarization in axons is often balanced by inward rectification through Ih, as discussed in section 3.1.3. Consistent with this function, block of Ih increased the occurrence of spike failures in hippocampal pyramidal cell axons (Soleng et al., 2003) and cerebellar parallel fibers (Baginskas et al., 2009).
The presence of inactivating potassium currents in many axons may also have interesting consequences for spike failures at branch points. In CA3 pyramidal neurons, hyperpolarization can lead to spike failures on the basis of removal of potassium channel inactivation (Debanne et al., 1997; Debanne et al., 1999; Kopysova and Debanne, 1998). Figure 11C (left set of traces) shows that the synaptic response in postsynaptic CA1 or other CA3 neurons could fail when a hyperpolarizing pre-step was applied to the soma of the presynaptic neuron shortly before a spike was elicited by depolarizing current injection. Because these synapses are known to not show transmission failures, block of synaptic responses must have resulted from propagation failure. Furthermore, such failures were only seen in about a third of connections, suggesting that spikes failed in some branches, but not in others. Failures also occurred when spikes followed a somatic IPSP and were abolished when A-type potassium channels were blocked. Therefore, failures most likely occurred due to removal of inactivation from A-type potassium channels by hyperpolarization. Consistent with this model, during repetitive activation and sustained depolarization only the initial spike failed to elicit a postsynaptic response, while subsequent spikes resulted in EPSPs (Fig. 11C, right set of traces). These results suggest that in some neurons repetitive activity may be propagated more reliably than isolated spikes. For example, inactivation of A-type potassium currents by repeated bursting activity may relieve spike failures in some cortical neurons projecting into the nucleus accumbens, resulting in a potentiation of synaptic connections (Casassus et al., 2005). It should also be noted that in the experiments on CA3 neurons (Debanne et al., 1997), the latency between a hyperpolarizing potential and a subsequent spike had a large effect on total delay, suggesting that in these neurons the background of synaptically-induced membrane potential fluctuations significantly affects the temporal patterns of spikes even in the absence of failures (see section 3.1.3.).
Spike failures can also occur as a result of direct synaptic or modulatory input to axons. These phenomena are discussed in section 4.
3.2.4. Ectopic spike initiation and spike reflection
The term “ectopic” signifies that spikes can be initiated at sites other than the axon initial segment or hillock. It is most commonly used to describe cases in which pathological conditions lead to hyperexcitability of the axon, but may also play a role during normal neuronal signaling. There are different modes of ectopic spike initiation. First, ectopic spike initiation may follow a normally propagated spike, increasing the spike rate or prolonging repetitive activity. Second, ectopic spike initiation may spontaneously occur in the axon, even in the absence of propagation of proximally elicited spikes. Third, spikes may be initiated in the axon in response to synaptic or modulatory input, making the axon a site of additional signal integration.
One of the first systematic studies of ectopic spike initiation was performed by Toennies, who reported “reflex discharge” after stimulating the cat saphenous nerve, which contains mostly axons from dorsal root ganglion sensory neurons (Toennies, 1938). An afferent volley generated in response to distal nerve stimulation was followed by potentials traveling antidromically back to the recording site. In motor axons, ectopic spike initiation was observed in cat soleus nerve (Standaert, 1963, 1964). Here, post-tetanic potentiation of muscle responses was found to be due to repetitive spiking initiated in the motor axon terminals in response to a single stimulus. Similar post-tetanic hyperexcitability has been found in human motor axons (Bostock and Bergmans, 1994).
There are only a few cases in which spontaneous ectopic spike initiation can be assigned a clear physiological function. In some cases, axonal membrane properties may allow spontaneous ectopic spike initiation, but this activity is usually suppressed by proximally generated activity. In the leech, heartbeat is controlled mostly neurogenically by motor neurons that receive rhythmic drive from a multisegmental central pattern generating network (Kristan et al., 2005). When centrally generated activity in motor neurons is suppressed, the axons in the peripheral nerves can generate bursting activity (Maranto and Calabrese, 1983). In the lobster stomatogastric nervous system, a motor axon can spontaneously generate tonic spiking or bursting activity in the absence of centrally generated activity (Ballo and Bucher, 2009; Bucher et al., 2003). This activity results from a spike threshold close to the resting membrane potential and slow ionic mechanisms. Presumably, the depolarization is induced by Ih and bursting is generated by a functional antagonism between Ih and a slow after-hyperpolarization. Spike initiation in both examples from leech and lobster only occurs when centrally generated activity is experimentally suppressed and therefore may only reflect the intrinsic membrane properties of the axon rather than representing a biologically relevant form of neural activity. In this respect, it is interesting to ask which mechanisms are normally responsible for limiting excitability to prevent ectopic spike initiation. Both pathological dysregulation of ion channels and their pharmacological block can give insight into this problem. In the long unmyelinated Schaffer collateral axons in hippocampus CA3, block of Kv1 potassium channels with 4-AP results in the conversion of single spikes into bursts (Palani et al., 2010) (Fig. 12A and B). Similarly, the dysregulation and altered spatial organization of potassium channels in particular can be responsible for ectopic spike initiation, e.g. in demyelination diseases or as a result of injury (Poliak and Peles, 2003; Stephanova, 1990). However, many mechanisms can contribute to pathological hyperexcitability, including up-regulation of Ih or persistent sodium currents (Baker, 2000; Jiang et al., 2008; Krishnan et al., 2009; Luo et al., 2007; Vucic and Kiernan, 2006).
Fig. 12.
Ectopic spike initiation. A: When rat hippocampal CA3 pyramidal cell somata were removed by a cut, blocking Kv1 channels with 4-AP induced burst firing in Schaffer collateral axons in response to a single stimulus. Stimulation and recording sites are indicated in the schematic of the hippocampus. Traces are from extracellular potentials recorded from the distal axon. Overlays of 10 repeated stimulations are shown. B: Bursting only occurred at the branch exposed to 4-AP. In CA3 neurons in “intact” hippocampal slices, somatic recordings showed single spikes in response to single stimulation of site 1 (S1) and two spikes when 4-AP was delivered at the stimulation site. Stimulating a different axon collateral (S2) distant to the site of 4-AP application did not evoke repetitive spiking. Traces are overlays of 3 repeated stimulations. C: In a subset of mouse hippocampal interneurons, repeated activation by current injection into the soma eventually leads to long-lasting repetitive spiking generated in the axon. Shown is the response to the 11th 1s soma stimulation. This effect requires hundreds of evoked spikes but is not dependent on somatic depolarization. D: Repeated axonal stimulation in the same preparation leads to long-lasting axonal spike initiation even when the soma is hyperpolarized to the point where antidromic spikes fail to invade it (insert, stimulus artifacts only). A and B are modified from Palani et al., 2010. C and D are modified from Sheffield et al., 2011.
There are other examples where a biological function of ectopic axonal spike initiation is plausible. Some animals can autotomize (voluntarily self-amputate) limbs or tails to escape the grip of a predator. In some cases, the severed appendage generates rhythmic movements, presumably to distract the predator. In stick insects, this rhythmic movement is generated by the axon of a leg flexor motor neuron. Experimental axotomy at the normal site of autotomy in the leg generates long-lasting and regular rhythmic bursting, suggesting a complement of ionic conductances similar to those found in central compartments of rhythmically active neurons (Schmidt and Grund, 2003). A subset of CA1 hippocampal and neocortical inhibitory interneurons has recently been shown to generate persistent spiking initiated in the distal axons in response to long lasting somatodendritic activation (Sheffield et al., 2011). When these neurons were stimulated either with realistic activation patterns or repeated tonic current injection into the soma, eventually the distal axons generated minute-long ectopic spiking. The axon here appears to act as a slow integrator of neural activity, as hundreds of proximally evoked spikes were required to elicit ectopic spiking. Figure 12C shows the persistent unstimulated spiking following repeated 1 second long depolarizing somatic current injections. Figure 12 D shows that it was not dependent on somatic depolarization. When the soma was hyperpolarized during antidromic axon stimulation, spikes failed to actively invade the soma but persistent spiking after stimulation was still elicited. The ionic mechanism underlying this slow axonal integration has yet to be described, but spiking persisted in the presence of GABA and glutamate receptor blockers and was therefore not dependent on chemical synaptic interactions. However, there is some evidence that it may depend on distal axonal electrical coupling. This kind of distally generated activity uncouples neurons from their usual circuit function and may either be protective during seizure development, or have important implications for the generation of cortical oscillations and memory storage (Connors and Ahmed, 2011).
Autonomous axonal spiking in mammalian central axons has been reported for a range of pathological conditions (reviewed in Pinault, 1995). It is common in cortical axons or thalamocortical projections during seizure activity. Distal or presynaptic axonal spike initiation appears to result mostly from synaptic interactions of axon terminals. Orthodromic and antidromic activity influence each other in a complex manner, as propagated spikes induce local excitability changes in distant compartments. In some cases, ectopic axonal spike initiation may occur to some degree under non-pathological conditions in thalamocortical axons and CA1 pyramidal cell axons (Papatheodoropoulos, 2008; Pinault, 1990, 1995; Pinault and Pumain, 1989), but the functional significance of such activity is not well understood.
Another way that additional spikes can be produced in the axon is through reverse propagation (reflection). Reflection can occur at branch points when a spike is close to failure (Goldstein and Rall, 1974). If the delay imposed by the branch point exceeds the refractory period, a second spike can be elicited that travels antidromically. It is unknown how common this phenomenon is in vertebrate neurons, but it has been studied in detail in leech mechanosensory neurons, particularly in pressure-sensitive (P-) neurons (Baccus, 1998; Baccus et al., 2000). These cells have their soma in the central nervous system and send out a thick primary axon to their major receptive field in the skin. A thinner collateral branches from the main axon in the central nervous system and projects to a minor receptive field in the skin. Spikes elicited at the terminals of the minor receptive field reach different presynaptic sites before and after invading the larger primary axon and evoke EPSPs in different postsynaptic neurons. Repetitive activity leads to slight hyperpolarization and can cause the spike to fail to invade the larger diameter branch. In this case only the postsynaptic neurons contacted by the thin collateral branch propagating the spike before it reaches the larger branch show a postsynaptic response. However, with slightly less hyperpolarization, spikes can be reflected. In this case, presynaptic sites on the side before the thin branch connects with the larger branch get depolarized from the initial spike and with very little delay by a second spike that was reflected from the central branch point and reaches the presynaptic site antidromically. In consequence, the postsynaptic cells contacted at this site show a much larger EPSP.
3.3. Synchronization by ephaptic interactions and electrical coupling
Communication between neurons is not necessarily only achieved by chemical synaptic transmission. More rapidly, activity in one or a group of neurons can influence neighboring neurons through electrical coupling, changes in extracellular ion concentrations, and very local changes in extracellular potentials as well as fairly widespread changes in extracellular field potentials (Jefferys, 1995). Particularly the effects arising from changes in extracellular potentials are not well understood, and their functional implications have been studied mostly theoretically (Anastassiou et al., 2010; Holt and Koch, 1999), or in the context of massive pathological firing, e.g. during epileptiform activity (Jefferys, 1995). In axons, activity-dependent changes in excitability can be due to impulse propagation in neighboring axons. In the classic experiments on crab leg axons by Katz and Schmitt, a propagated impulse in one axon caused a biphasic change in excitability in an adjacent axon, first a reduction and then an increase (Katz and Schmitt, 1940, 1942). This effect was due to a subthreshold depolarization induced by a small extracellular potential. Such interactions were briefly thereafter named “ephaptic” (Arvanitaki, 1942), from the Greek for “to touch on”, as opposed to “synaptic”, from the Greek for “to clasp together”. They also occur in vertebrate nerves (Kocsis et al., 1982a). Neighboring axons can to some degree be synchronized by ephaptic interactions. In Katz and Schmitt's experiments, a spike that followed briefly after a spike in the adjacent axon was accelerated, and synchronous spikes in both axons were slowed.
Interestingly, synchronization may also be achieved by neuron-glia interactions (Fields, 2008a; Yamazaki et al., 2007). Oligodendrocytes in the hippocampus depolarize in response to axonal activity, and this depolarization in turn increases axonal conduction velocity by an unknown mechanism. Because single oligodendrocytes can myelinate ~20 axons, glial depolarization may represent a powerful way to synchronize activity across bundles of myelinated axons.
Finally, synchronization across axons may be achieved by electrical coupling. Fast “ripple” oscillations in the hippocampus are due to synchronous firing of principal cells. This synchrony is not dependent on chemical synaptic transmission but due to gap-junctional coupling of proximal axons (Schmitz et al., 2001; Traub et al., 2002). Recordings from these neurons actually show small amplitude “spikelets” which are sensitive to tetrodotoxin and gap-junctional blockers, and therefore likely to represent spikes in adjacent axons. Similarly, fast cerebellar oscillations are mediated by gap-junctional coupling between proximal Purkinje cell axons (Middleton et al., 2008; Traub et al., 2008). The obvious advantage of this kind of synchronization is that the frequency of oscillations (up to 200 Hz in hippocampus, and ~75 Hz in cerebellum) may be too high to achieve synchronization by chemical synaptic interactions. It is not clear if electrical coupling also helps to synchronize activity at more distal sites, but there is some evidence from hippocampal interneurons that electrical coupling may underlie coordinated ectopic axonal spike initiation at higher order axon branches (Sheffield et al., 2011).
3.4. Potential consequences of activity-dependent changes in propagation for neural coding
The functional impact of changes in spike propagation has only been investigated in a few instances, for obvious reasons. Ideally, such an assessment requires that axons are accessible to the study of membrane properties, that they allow simultaneous recordings from different sites to measure conduction delays, and that the physiological range of activity is known. The latter is particularly important as the theoretical consequences that different axonal properties can have for neural coding depend on spike patterns, coding strategy, and overall conduction delay. Information may be coded in the rate of spikes, in the temporal pattern of interspike intervals, or in bursts, sometimes in parallel within the same activity pattern, or multiplexing information transfer at different time scales (Friedrich et al., 2004; Oswald et al., 2004; Panzeri et al., 2010).
3.4.1. Temporal coding
Temporal coding means that information is contained in the interspike intervals, spike latencies, or phase of firing (Cessac et al., 2010; Lestienne, 2001; Panzeri et al., 2010; Theunissen and Miller, 1995). Temporal coding may be affected by the changes in interspike intervals during propagation from spike initiation site to presynaptic sites that occur when conduction velocity changes in an activity-dependent manner during repetitive firing. In the absence of more extreme consequences of changes in axonal excitability, like spike failures or ectopic spike initiation, the overall rate of firing is not affected, but the precise temporal structure of activity is altered. The impact that changes in axonal propagation can have therefore depends on the absolute change in conduction delay and the temporal precision of the code, i.e. the coarsest temporal resolution sufficient for decoding. In sensory systems, this precision can vary between the sub-millisecond range and tens of milliseconds (Panzeri et al., 2010).
To which degree temporal dispersion of spike patterns during axonal conduction impacts temporal coding schemes is generally not known and largely ignored, but the theoretical implications have been recognized early (George, 1977; Miller and Rinzel, 1981). The diversity of axonal properties described above suggests that in some cases changes in conduction delay may not matter because they remain within the temporal precision of the code, whereas in others changes in conduction delay may exceed the temporal precision and therefore contribute to the code.
3.4.2. Rate coding
In systems with an encoding time window larger than the interspike intervals present, information transfer depends on the rate of spikes (Panzeri et al., 2010; Theunissen and Miller, 1995). In this case, changes during propagation that affect the precise temporal structure of spike intervals may be unimportant, but changes in overall spike rate due to spike failures or ectopic spike initiation, or changes in spike rate at intermediate time windows induced by supernormal or subnormal propagation (Fig. 9), still matter. There are several contexts in which such phenomena may play an important role. The critical frequencies that an axon allows to be conducted in a 1:1 manner limit the total rate with which a neuron can communicate, independently of the spike initiation process. If repetitive activity leads to spike failures, for example because of sodium channel inactivation, the axon acts as a low-pass filter, limiting signal transmission at higher frequencies. If repetitive activation decreases the probability of spike failure, for example because of potassium channel inactivation, the axon acts as a band pass filter, additionally limiting signal transmission at low frequencies. Ectopic spike initiation can increase spike rate independent of synaptic integration. In this case, the axon can act as an amplifier, either increasing spike frequency or prolonging spiking. However, if ectopic spike initiation is suppressed by activity initiated in the proximal axon (so that it predominantly plays a role during phases of weak synaptic activation), the axon basically acts as an equalizing filter that can compensate changes in spike rate to some degree and ensure minimal tonic activity.
3.4.3. Synaptic dynamics
Presynaptic calcium dynamics, postsynaptic receptor activation, and signal summation, and therefore short-term synaptic dynamics, are critically dependent on the temporal relationship between consecutive spikes. In fact, the dynamics of these processes are probably determining the different integration time windows underlying temporal and rate coding strategies. However, another aspect of synaptic integration could be affected by changes in axonal conduction delay, namely the temporal relationship of convergent synaptic input from different presynaptic neurons. In some cases, differential axonal propagation delay is important in coincidence detection, for example in the processing of interaural time difference during sound localization (Carr and Konishi, 1988; Joris and Yin, 2007; Karino et al., 2011). In others, the temporal relationship between pre- and postsynaptic firing determines the sign of synaptic plasticity (Bi and Poo, 1998). It is not well understood how such spike timing-dependent forms of plasticity operate in the context of repetitive activity and across different synapses. It is notable that computational principles relying on the synchrony or other temporal relationships between spikes from different neurons converging on the same postsynaptic cell have to be viewed in the context of potentially very different conduction delays. The grouping of synchronous inputs can rely on these delays in a computationally significant manner (Izhikevich, 2006). Therefore, activity-dependent changes in propagation delay that alter the temporal relationship of parallel inputs from different presynaptic neurons potentially affect long-term synaptic plasticity (Bakkum et al., 2008).
Synaptic efficacy and dynamics may also be affected by spike failures at a shorter time scale (Debanne et al., 1997; Debanne et al., 1999). If spike failures occur not in the main trunk of the axons but selectively at branch points, they do not necessarily affect the temporal code or rate. Instead, if the functional synaptic connection to a postsynaptic cell is distributed over contact sites located in different axon branches, spike failures at branch points may cause consecutive spikes to activate different numbers of presynaptic sites. Alternatively, selective spike failures at branch points may differentially affect different postsynaptic neurons if there is a spatial segregation of specific contact sites in the axonal tree (Debanne et al., 1997; Grossman et al., 1979a).
4. Synaptic and neuromodulatory effects on axons
So far, we have mostly discussed changes in spike propagation arising from morphological inhomogeneities and the dynamics of voltage-gated ion channels. In this section, we are describing evidence from a range of different systems for synaptic and neuromodulatory effects on axonal properties. Presynaptic integration of inputs, i.e. distinct excitatory and inhibitory synaptic responses in axon terminals close to or at presynaptic sites, is a common phenomenon (Dudel and Kuffler, 1961; Pinault, 1995). Such inputs can modulate spike-mediated presynaptic depolarization or gate the arrival of presynaptic spikes at terminals. In addition, it has long been known that neurotransmitter receptors are expressed in myelinated and unmyelinated peripheral and central axons at some distance from terminal arborizations (Kocsis and Sakatani, 1995).
Early evidence of axonal modulation was found mainly for ionotropic receptors like GABAA and nicotinic acetylcholine receptors (nAChRs). Activation of ionotropic receptors leads to a direct increase in conductance which can result in shunting or in changes in axonal membrane potential that in turn affect the contribution of different voltage-gated ion channels to excitability. There is also increasing evidence of such interactions for axonal glutamate receptors in the cortex. Both AMPA and kainate receptors are found on presynaptic axons (Kamiya, 2002; Schenk and Matteoli, 2004), and glutamate-mediated depolarization affects spike shape, calcium influx and subsequent transmitter release (Kamiya, 2002). Recently, the role of AMPA receptor-mediated depolarization has been studied in detail in hippocampal CA3 pyramidal neurons (Sasaki et al., 2011). Here, astrocytes release glutamate and the axonal depolarization leads to inactivation of A-type potassium currents, which in turn leads to prolonged spike duration. The broadened spike triggers larger calcium elevations in presynaptic boutons and strengthens synaptic transmission to postsynaptic neurons. However, the astrocyte-mediated signaling affects fairly long stretches of the axon, including several en passant boutons, and is therefore likely to also affect spike propagation.
In addition, axons can be modulated by metabotropic pathways. Neuromodulators acting through metabotropic receptors can alter intrinsic properties of neurons by changing the gating properties of voltage-gated ion channels (Harris-Warrick et al., 1998; Levitan, 1994; Marder and Thirumalai, 2002). This includes phosphorylation-dependent changes in gating properties of voltage-gated sodium channels which have long been thought not to be modulated (Bevan and Storey, 2002; Cantrell and Catterall, 2001; Carr et al., 2003).
The presence of neuromodulatory effects on axonal excitability means that the short-term dynamics of spike propagation itself may be conditional on modulatory state. The fidelity of spike conduction may therefore not only be dependent on spike frequency and history, but also be modified by signaling from other cells. Neuromodulators can stem from hormonal, synaptic, or paracrine release by other neurons or glial cells.
Among the earliest evidence for axonal targeting by chemical transmission was the presynaptic inhibition and shunting by presynaptic afferent depolarizations that are well described in a range of systems (Cattaert et al., 1999; Dudel and Kuffler, 1961; Eccles et al., 1962; Thompson et al., 1993). In some cases, axo-axonal input occurs close to synaptic release sites and exerts graded control over the shape of the presynaptic action potentials. In others, shunting, i.e. the decrease of input resistance associated with opening of ion channels, prevents active invasion of axon terminals by spikes (Jackson and Zhang, 1995; Segev, 1990; Zhang and Jackson, 1993). However, in both cases axo-axonal input occurs close to the presynaptic sites. In Aplysia, conduction of afferent activity can also be gated by synaptic input to sites at a considerable distance from output synapses. In the mechanoafferent bipolar neuron B21, the incoming sensory process splits into a medial process that connects it to the cell body and a process connecting it to the contralateral hemisphere of the ganglion. Both processes reliably propagate spikes (Evans et al., 2003; Evans et al., 2008). However, a third process leaving the soma laterally fails to propagate spikes at normal resting potentials. Depolarization relieves propagation failures as it allows spikes to be propagated through the relatively inexcitable soma (Evans et al., 2007). Interestingly, synaptic output from B21 to motor neurons is also dependent on subthreshold membrane potential oscillation during motor activity, which changes intracellular calcium concentration in a graded manner (Ludwar et al., 2009). Together, these mechanisms make signal transmission dependent on the phase of rhythmic activity.
Whereas in B21 rhythmic gating is achieved by relief of conduction failure, in other cases rhythmic inhibition of conduction has been observed. A phase-dependent inhibition of spike propagation during rhythmic activity has been found in mammalian brainstem spindle afferents. In this case, different axon compartments can show different activity patterns as only one part of the axon is rhythmically gated during motor activity (Verdier et al., 2003).
Synaptic or modulatory control of axon excitability can also be functionally excitatory. Depolarizing input can provide a shunt at presynaptic terminals but also elicit spikes (Cattaert et al., 1999; Pinault, 1995). Some of the examples of ectopic spike initiation discussed in section 3.2.4 depend on synaptic interactions between different axon terminals. In addition, neuromodulation of the axon proper can be sufficient to lead to ectopic spike initiation.
4.1. Receptors on axons and release from endogenous sources
It is far from clear how widespread the phenomenon of axonal modulation is, and how diverse the modulators involved are. The discussion of axonal neuromodulation below is restricted to GABA, acetylcholine, and biogenic amines. These represent some of the best described examples, but this is not to say that other common neuromodulators, e.g. the vast array of neuropeptides (Merighi, 2002; Nusbaum et al., 2001; Salio et al., 2006), are not potential modulators of axonal properties. It is notable that information about spatial distribution of receptors and presence in axons in particular, is scarce. Receptor distribution is often described at the level of brain regions and cell types, but information about subcellular distribution is lacking or not explicit (Nusser, 2009). Receptor immunoreactivity in tracts and fibers is quite common, but often it is undetermined whether immunoreactivity corresponds to proteins in axonal membrane or in the cytosol. The latter may mostly represent transport through the axon. There are also cases in which axons clearly respond to exogenous application of modulators, but an endogenous source is not known. It is a common phenomenon in the brain that receptor and transmitter expression are mismatched, raising the question whether receptor expression is always of functional significance (Herkenham, 1987). However, it is now widely recognized, particularly for monoamines, opioids, and peptides, that “volume transmission” through extracellular and cerebrospinal fluid, i.e. over substantial distances between release sites and receptors, is quite common (Fuxe et al., 2010). Finally, there are some cases that show clear physiological and pharmacological effects but are lacking immunohistochemical evidence for the presence of receptors. This could be mostly due to the limits of antibody detection of low abundance proteins. However, axonal neuromodulation may not just be a matter of local receptor expression. In long axons, one would expect that neuromodulatory action requires the presence of receptors to a neuromodulator, but in shorter axons this may not necessarily be true. Although changes in concentrations of second messengers like cAMP or calcium can be very locally restricted (Zaccolo et al., 2002), they can also spread globally through different cell compartments, or show mixtures of local and global signaling (Nikolaev et al., 2006; Rich et al., 2001). It is therefore possible that axonal properties may be modulated by activation of receptors at some distance to the target site. Such cross-compartmental signaling may be of relevance particularly in small central neurons, where it is hard to study.
Most evidence of axonal neuromodulation comes from peripheral nerves where selective drug application to axons is straightforward. There are a number of examples from large unmyelinated axons in invertebrates, and some from peripheral nerves in mammals. Special cases here are the non-myelinated C-fibers. These neurons respond to a variety of noxious and inflammatory signals, both physical and chemical. Some of the transduction mechanisms for chemical mediators (including neuropeptides) can be transferred to axonal membrane after injury and lead to ectopic spike initiation (Michaelis et al., 1997). However, recent evidence suggests that both some of the physical stimuli (e.g., heat) and a subset of chemical mediators can activate axons under normal conditions, suggesting that “ectopic” spike initiation is part of regular sensory transduction (Hoffmann et al., 2008; Moalem et al., 2005). These include responses to GABA (Brown and Marsh, 1978), acetylcholine (Armett and Ritchie, 1960; Lang et al., 2003a), serotonin (5-HT) (Lang et al., 2006), and adenosine/ATP (Lang et al., 2003b; Lang et al., 2002).
4.2. GABA
Axonal responses to GABA have first been described in the context of presynaptic inhibition (Dudel and Kuffler, 1961). Apart from phasic presynaptic inputs, GABA also plays an important role in tonic modulation of axons through high affinity GABAA receptors (Belelli et al., 2009; Farrant and Nusser, 2005; Trigo et al., 2008). It has long been known that GABAA receptors are present and can modulate excitability in peripheral nerve trunks and spinal axons. In peripheral vagus nerve trunks of adult rats, GABA reduces the amplitude of compound spikes, associated with a depolarization of the nerve (Brown, 1979; Brown and Marsh, 1978). In the central nervous system, spinal dorsal column tracts from young rats with incomplete myelination respond similarly and also show a pronounced slowing of conduction (Sakatani et al., 1991a). Experiments with re-uptake inhibitors suggest that there is an endogenous source of GABA (Sakatani et al., 1993). GABA sensitivity is still present in adult rats, albeit far less pronounced (Sakatani et al., 1991b), suggesting a predominantly developmental role. It remains unclear, at least in the adult, what the source of GABA may be, and if it plays a functional role in the adult animal (Kocsis and Sakatani, 1995). Transient developmental GABA modulation has also been described in the optic nerve. Here, GABAA-mediated reduction of spike amplitude and slowing of conduction is caused by GABA release from glial cells (Lim and Ho, 1998; Sakatani et al., 1992; Sakatani et al., 1994; Sakatani et al., 1991c). In central neurons, activation of perisynaptic and even axonal receptors at moderate distances to synapses may be activated by “spill-over” from synaptic release (Kullmann et al., 2005). Some of these receptors may be expressed along most of the axon and have a significant effect on spike propagation, for example in hippocampal mossy fibers (Ruiz et al., 2003), where the synapses that mossy fibers form with CA3 neurons may be modulated by GABA spill-over from interneurons (Alle and Geiger, 2007).
Whereas the GABAA-mediated chloride conductance at postsynaptic sites most often results in a hyperpolarizing potential, the intracellular chloride concentration appears to be high in many axons, resulting in a depolarizing response. For example, GABA depolarizes peripheral myelinated sensory axons, whereas motor axons do not respond (Bhisitkul et al., 1987; Morris et al., 1983). In primary sensory neurons, it is well established that the depolarizing response is due to distinct expression patterns of different chloride transporters (Rocha-Gonzalez et al., 2008). Independent of the sign of the voltage response, presynaptically the increase in conductance can lead to shunting of spikes and therefore be functionally inhibitory (Cattaert et al., 1992; Eccles et al., 1962). GABA-mediated spike failures in terminal branches are for example found in sensory axons in the spinal cord (Wall, 1995). A GABAA-mediated shunting mechanism has also been proposed for axonal segments of rat jaw-closer muscle spindle afferents. The rostral and caudal parts of the axons can show different firing patterns during fictive rhythmic motor activity, with only the caudal compartment of the central axon showing phasic modulation in time with the motor pattern (Westberg et al., 2000). This appears to result from GABAA - mediated axo-axonal synaptic input from interneurons (Verdier et al., 2003).
On the other hand, as mentioned above, presynaptic GABA-mediated depolarization can lead to ectopic spike initiation. This can have important consequences for the excitability of proximal cell compartments that receive the antidromically propagated spike (Cattaert et al., 1999; Pinault, 1995), but may also play a role for orthodromic signaling. Slight depolarization can increase excitability, depending on the stimulus paradigm used. This is for example true for GABAA-mediated responses in a subset of unmyelinated peripheral axons in humans (Carr et al., 2010). Some hippocampal pyramidal cells fire ectopic axonal spikes during some forms of oscillatory network activity during the phase when proximal compartments are inhibited (Papatheodoropoulos, 2008). These ectopic spikes are presumably due to GABA-mediated synaptic interactions. In addition to shunting and spike initiation, GABA-mediated depolarization can also be sufficient to affect intrinsic voltage-gated ion channels. At nerve terminals of the pituitary, GABA-mediated depolarization can cause spike failures (Jackson and Zhang, 1995; Zhang and Jackson, 1993, 1995). Here, the shunting mechanism appears to be of only minor importance. Figure 13A shows recordings obtained with a chloride concentration in the pipette that kept the chloride equilibrium potential close to the resting potential. In consequence, GABA application caused an increase in conductance but no change in membrane potential. This increase in conductance led to an increased current threshold for spike initiation. However, a more dramatic effect was seen when the chloride equilibrium potential was smaller (more depolarized) than the resting potential (Fig. 13B). The depolarization resulting from GABA application led to a decrease in spike amplitude and failures of active responses. This was likely due to sodium channel inactivation and not to the increase in chloride conductance, as depolarization by current injection had similar effects.
Fig. 13.
Modulation of axon excitability by ionotropic receptors. A and B: GABAA receptor activation in rat posterior pituitary axon terminals. The chloride equilibrium potential was manipulated by changing chloride concentration in the patch pipette use for recordings. B: When the chloride equilibrium potential was close to the resting membrane potential, GABA did not cause a change in membrane potential, but the increase in chloride conductance caused a shunting effect. In consequence, the spike threshold was increased. Shown are overlaid and time-shifted responses to the same current injections of increasing amplitudes in control and GABA. Spike threshold increased by ~15% in the presence of GABA and only the two largest current injections elicited a spike. B: When the chloride equilibrium potential was more depolarized than the resting membrane potential, GABA caused a sustained depolarization. Due to sodium channel inactivation, this depolarization led to spike failures. The same effect was seen when terminals were depolarized not by GABA application but current injection through the pipette. C and D: Activation of 5-HT(3) receptors reduces temporal dispersion in rat C-fibers. C: Extracelluar recordings of stimulated compound spikes in rat sural nerve. In control, repeated paired stimulations lead to supernormal conduction of the 2nd spike, which is observed as a reduced latency between stimulus and peak. Supernormality is reduced in the presence of m-chlorophenylbiguanide (mCPBG), a 5-HT(3) receptor agonist. D: Calculated effect of 5-HT(3) receptor activation on the change in interspike interval during conduction along the nerve. Intervals are less reduced in the presence of the agonist. A and B are modified from Zhang and Jackson, 1995; C and D are modified from Lang et al., 2006.
Optic nerve axons in the neonatal rat also express GABAB receptors. While GABAA receptors seem to be mostly responsible for the reduction of spike amplitude, GABAB receptors regulate calcium transients through N-type channels (Sun and Chiu, 1999), and may play an important protective role in anoxia-induced injury (Fern et al., 1995b).
4.3. Acetylcholine
The presence of nAChRs on axon trunks has been recognized early in the peripheral nervous system and optic nerves. In rabbit vagus nerve, ACh depolarizes C-fibers, reduces spike amplitude and substantially slows conduction velocity (Armett and Ritchie, 1960). The underlying ionic mechanism and pharmacology suggest that effects are due to nAChRs (Armett and Ritchie, 1961, 1963). In human sural nerve, C-fiber excitability is increased by ACh during repetitive activation, mediated by specific nAChR subunits (Lang et al., 2003a). In early postnatal mouse and rat optic nerves, nicotine induces axonal calcium elevation dependent on extracellular calcium (Zhang et al., 2004). This effect decreases substantially with increasing age and myelination. Nicotine also reduces compound spike amplitude. Interestingly, repetitive stimulation leads to a decrease in compound spike amplitude that can be prevented by blockers of nAChRs, suggesting that axon excitability is modulated by endogenous ACh in an activity-dependent manner.
In other axons of the central nervous system, the role of nAChRs is less clear. Despite the fact that they are ligand-gated ion channels and therefore predisposed for fast synaptic transmission, they seem to predominantly act in a non-synaptic fashion (Lendvai and Vizi, 2008). Cholinergic modulation of cortical circuits often involves targeting of pre- and perisynaptic sites (Mansvelder et al., 2009). It should be noted that even if receptors are mostly presynaptic, those synapses are en passant in many cases. Conceivably, spike propagation through these synaptic sites may also be affected. There is also evidence for “pre-terminal” receptors (Engelman and MacDermott, 2004; Lena et al., 1993; McGehee and Role, 1996; McMahon et al., 1994; Zhu et al., 2005). These receptors are on higher order axon arborizations, but at some distance from presynaptic sites. Activation of these receptors enhances transmitter release from the terminals but this effect is blocked by TTX. Therefore, it is likely that ACh controls spike shape and/or active propagation into terminals. In the rat midbrain, nicotine dramatically increases spontaneous IPSCs mediated by GABAergic axon terminals in interpeduncular nucleus neurons (Lena et al., 1993). As this effect is blocked by TTX, cholinergic activation of the terminal axon arborizations appears to be sufficient for distal ectopic spike initiation. Similarly, high affinity nAChRs on thalamocortical terminals in prefrontal cortex can elicit ectopic spikes that increase glutamatergic transmission to postsynaptic neurons (Lambe et al., 2003).
Surprisingly, nAChRs are also found in myelinated axon trunks. Positron emission tomography with radioactive ligands in humans showed receptors in myelinated axons of thalamocortical pathways (Ding et al., 2004). Subsequently, the role of nAChRs in modulating thalamocortical signaling has been studied in mice, mainly focusing on spike initiation (Kawai et al., 2007). ACh lowers spike threshold and enhances synchrony of spike initiation across different neurons. However, ACh responses occur in nodes of the extended proximal portion of the tract. Therefore, spike propagation may also be affected.
4.4. Amines
Spike propagation in mammalian unmyelinated peripheral fibers can be modulated by serotonin (5-HT). 5-HT signaling in the periphery is part of inflammatory responses to degenerative neuropathies, viral infections and ischemia, in which long sections of peripheral axons can be exposed to such mediators. C-fibers in isolated rat sural nerve sections respond to 5-HT(3) receptor agonists, and show distinct changes in the dynamics of post-spike excitability and activity-dependent changes of conduction delay (Lang et al., 2006) (Fig. 13C). The main effect appears to be a reduction of supernormality, so that spike intervals change less over distance (Fig. 13D). Frequency of repetitive spiking is a crucial determinant of pain perception. Interestingly, 5-HT improves temporal fidelity, but as temporal dispersion may be part of the normal coding strategy and serve to enhance stimulus contrast (Weidner et al., 2002; Fig. 9), this potentially impairs function. It should be noted that 5-HT(3) receptors are ionotropic, whereas probably most other examples of amine modulation involve second messenger signaling and modulation of intrinsic axonal properties. For example, α-adrenoreceptors appear to reduce voltage-gated calcium currents in unmyelinated fibers of rat sympathetic nerve trunks (Elliott et al., 1989).
Amine modulation of axons has been described in several neurons in the crustacean stomatogastric nervous system (STNS). In this system, an anatomically separate ganglion, the stomatogastric ganglion (STG), contains the central pattern generating circuits (CPGs) which control rhythmic movements of the posterior foregut (Harris-Warrick et al., 1992; Marder and Bucher, 2007). The neurons comprising these circuits are modulated by a multitude of substances released either directly into the neuropil of the STG by descending projections from more anterior ganglia, or through the circulatory system as neurohormones (Marder and Bucher, 2007; Nusbaum and Beenhakker, 2002; Stein, 2009). The monoamines dopamine (DA), 5-HT, and octopamine (OA) all have multiple network targets in the STG, acting on the gating properties of intrinsic voltage-gated ion channels as well as on synaptic properties (Ayali and Harris-Warrick, 1999; Harris-Warrick et al., 1998; Marder and Bucher, 2007; Marder and Thirumalai, 2002). It is now clear that they also directly target the axons of sensory neurons and modulatory projection neurons, which play an important role in activating the CPGs, and the motor axons leaving the STG. Consequently, the motor output is shaped through changes in the firing patterns of individual neurons that occur at some distance to classical integration sites.
The sites of axon modulation by amines in the crab STNS are shown schematically in Figure 14A. OA activates a central spike initiation zone in a mechanosensory neuron (Daur et al., 2009). This bipolar neuron, called anterior gastric receptor (AGR), has its cell body close to the STG and a peripheral projection to a specific set of muscles at which it initiates spikes in response to contraction. AGR does not make direct connections to neurons in the STG, but its ascending axon projects to ganglia anterior to the STG where it affects the firing pattern of descending modulatory neurons. These modulatory neurons in turn shape the motor patterns generated in the STG. Propagation of spikes elicited in AGR peripherally in response to muscle contraction suppress spontaneous firing, but in the absence of strong peripheral activation, a second spike initiation zone situated in the ascending axon is active. OA increases the rate of spikes generated at this second initiation zone, and this change has a significant effect on the phasing of the motor patterns generated in the STG.
Fig. 14.
“Ectopic” spike initiation elicited by biogenic amines in the stomatogastric nervous system. A: Schematic of the stomatogastric nervous system of the crab, Cancer borealis. The axons of an ascending sensory neuron (anterior gastric receptor, AGR), and a descending modulatory neuron (modulatory commissural neuron 5, MCN5) pass through a nerve region that contains synaptic release sites. Octopamine application to this site elicits additional spikes in both axons. 5-HT application to a motor nerve elicits sustained firing in response to centrally generated bursts in the axon of a motor neuron (lateral gastric neuron, LG). CoG: commissural ganglion; STG: stomatogastric ganglion; ion: inferior esophageal nerve; son: superior esophageal nerve; stn: stomatogastric nerve, dgn: dorsal gastric nerve. B: Synaptic release sites at the stn-son junction (the site of octopamine modulation shown in A). The upper panels are confocal images of this nerve regions stained for synaptotagmin (red) to visualize release sites. Neurobiotin-filled axons are shown in green. The lower panel is an electron micrograph of the same region showing synaptic release sites of large terminals containing both clear and dense core vesicles. C: Schematic of the stomatogastric nervous system of the lobster, Homarus americanus. The peripheral axons of a motor neuron in the stomatogastric ganglion (pyoric dilator, PD) express receptors to dopamine. D: Intracellular recording from the PD neuron axon in the motor nerve show that in the absence of centrally generated activity, dopamine depolarizes the membrane and leads to peripheral spike initiation. E: Steady-state activation curves of Ih from voltage-clamp experiments in the PD axon. The dopamine effect is due to direct cAMP-mediated modulation of Ih. Dopamine reduces the maximal conductance, but changes the voltage-dependence so that conductance is increased at biologically relevant membrane potentials. A summarizes findings from Meyrand et al., 1992, Goaillard et al., 2004, and Daur et al., 2009. B is modified from Goaillard et al., 2004. C summarizes findings from Bucher et al., 2003. D is modified from Ballo and Bucher, 2009. E is modified from Ballo et al., 2010.
The descending axons of a bilateral pair of identified descending modulatory neurons to the STG, called modulatory commissural neurons 5 (MCN5), also respond to OA (Goaillard et al., 2004). MCN5 has its cell body in a ganglion anterior to the STG and a long projection that runs for most of its way through the single input nerve into the STG. This nerve contains a small (a few hundred micrometers long) anatomical specialization which contains synaptic structures, at centimeter distance to the STG (Fig. 14A&B). When OA is applied to this site, additional spikes are initiated in the axon of MCN5. This axonal increase in MCN5 spike frequency in turn increases the cycle frequency of rhythmic activity in the STG. The effect is functionally distinct from direct actions of OA on the central pattern generating neurons in the STG. Whereas OA effects on MCN5 firing only change cycle frequency in the STG, direct modulation of STG neuron properties also affects the relative timing between STG neurons. Interestingly, the anatomical specialization in the nerve with synaptic neuropil shows release sites from cells with both clear and dense core vesicles (Fig. 14B) and contains varicosities that are immunoreactive to a number of neuropeptides (Skiebe and Ganeshina, 2000), suggesting that axons passing through this site may also be subject to peptidergic modulation.
Finally, some of the motor axons leaving the STG are modulated by 5-HT or DA. In the crab STNS, 5-HT modulates a peripheral spike initiation site in the lateral gastric (LG) neuron (Meyrand et al., 1992). This spike initiation site is situated in the peripheral motor nerve, 0.5-2 cm distal to the STG (Fig. 14A). Central depolarization activates it in the presence of 5-HT, so that after a centrally generated burst the axon continues to fire, albeit at a lower frequency. Peripherally initiated spikes travel antidromically to the STG but fail to activate LG output synapses. However, they also travel orthodromically towards different muscles innervated by LG. All muscles show electrical responses to the lower frequency input by peripherally generated spikes, but only one of the three muscles tested showed contraction responses. Interestingly, the source of 5-HT may be sensory neurons signaling contraction of LG target muscles.
In the lobster STNS, the peripheral axons of a pair of motor neurons, the pyloric dilator (PD) neurons, are sensitive to DA (Fig. 14C). In the absence of centrally generated activity, low concentrations (<nM) can elicit peripheral spike initiation (Bucher et al., 2003). This usually occurs at a specific site, 1-2 cm distal to the STG. However, focal application shows that almost the entire 4-5 cm of peripheral axons can be activated. There is no specific DA release targeted to the peripheral nerves but the low concentrations sufficient for activation match hemolymph concentrations of amines. Therefore, modulation here appears to be hormonal. The PD axons represent one of the first systems where neuromodulation of axonal excitability has been investigated directly by measuring the changes in activation properties of a single current. The depolarizing effect of DA on the axon resting membrane potential (Fig. 14D) is sensitive to blockers of Ih (Ballo and Bucher, 2009). Both the signaling pathway and the effect of DA on Ih activation has been studied (Ballo et al., 2010). Pharmacological experiments showed that DA acts through D1-type receptors and leads to an increase in cAMP concentration. Voltage-clamp measurements showed an increase in Ih activation around resting membrane potential, consistent with the cAMP sensitivity of the channels underlying this current (Fig. 14E). In the light of these findings, it is interesting to note that Ih in this axon appears to be crucial in balancing slow after-hyperpolarization. This may have a significant effect on temporal fidelity of spike conduction. In fact, during normal centrally generated bursting, after-hyperpolarization suppresses DA-activated peripheral spike initiation, so the DA effect may be more important in controlling conduction delay than in eliciting additional spikes.
A shift in the balance between slow after-hyperpolarization and Ih has also been observed in crayfish axon terminals in response to 5-HT (Beaumont et al., 2002; Beaumont and Zucker, 2000). In this example, the amine modulation plays a role in the context of synaptic transmission and not in spike propagation. However, amine action on the control of activity-dependent hyperpolarization may be a common theme. In leech mechanosensory neurons, 5-HT decreases the occurrence of spike propagation failures, apparently by decreasing hyperpolarization due to activity of the sodium/potassium-ATPase (Mar and Drapeau, 1996; Scuri et al., 2007).
5. Long-term regulation of axonal properties
The dynamics of spike conduction described in the preceding sections arise from given sets of axonal physical properties, and from specific ionic mechanisms and their modulation. At longer time scales, axonal properties may also be subject to more persistent regulatory changes that affect axon morphology and the properties or expression levels of membrane proteins. We abstain from an in-depth discussion of the vast literature on pathological changes of axonal morphology and excitability in the context of channelopathies, demyelination, ischemia, and injury. Such changes have recently been reviewed in detail elsewhere (Krarup and Moldovan, 2009; Krishnan et al., 2009; Mert, 2007; Waxman, 2006). Instead, we will focus on findings suggesting that axonal properties can be modified concomitantly with, or in response to, changes in cellular and network function, generally referred to as neural plasticity.
5.1. Stability and plasticity of conduction velocity
It is now well established for other neuronal compartments that neural plasticity encompasses diverse phenomena that can occur in response to changes in the environment sensu lato (Nelson and Turrigiano, 2008). They include activity-dependent as well as activity-independent mechanisms that change or stabilize morphological parameters, synaptic properties, and intrinsic excitability of neurons. In theory, there are two aspects of spike propagation that could be affected. First, the dynamics of spike propagation during repetitive activity, i.e. the temporal fidelity of axonal conduction, could be changed. In this scenario, the potential consequences of delay changes, spike failures, and ectopic spike initiation for neural coding could be altered in a persistent manner. Second, the mean delay from spike initiation to presynaptic depolarization could be adjusted. This would affect coding strategies depending on absolute delay or synchrony across different axons. There is very little data available on the potential long-term regulation of the dynamics of spike propagation, but plenty of evidence for regulation of mean delay.
Because absolute delay and synchrony across different presynaptic neurons may be an important aspect of neural coding strategies (Carr and Konishi, 1988; Izhikevich, 2006; Karino et al., 2011), one would expect delays to be stable and well-regulated in many cases. Short of actually observing stability of conduction delay over time, a convincing argument for stability comes from the observation that in some systems different conduction delays compensate for different axonal path lengths. In a lobster motor neuron, axon branches that innervate more distal muscle fibers conduct spikes faster to ensure synchronous depolarization of all fibers (Govind and Lang, 1976a). In other cases, such correlation is also found across different axons. Variations in conduction velocity compensate for different axonal path lengths across different retinal ganglion cell axons (Stanford, 1987), across different Purkinje cell axons (Sugihara et al., 1993), and across different thalamocortical axons (Salami et al., 2003).
However, there is at least some evidence for plasticity of axonal conduction delay. A few studies directly investigated long-term changes in spike propagation. In rabbit visual corticotectal and callosal axons, conduction velocity as well as refractory period and supernormal conduction were measured over several months with implanted recording and stimulation electrodes (Swadlow, 1982, 1985) (Fig. 15A). While the majority of axons recorded had stable conduction delays, in others delay either continuously increased or decreased (Fig. 15B). Changes in excitability, measured as stimulation threshold, closely matched the changes in conduction delays. However, re-excitability (measured as minimum spike interval and supernormal latency) appeared to be a lot more stable than mean conduction delay, or at least were not correlated with changes in mean delay (Fig. 15C&D). Recordings of the same axon at two different sites demonstrated that the changes in conduction velocity were similar in the proximal and distal regions of the axon, suggesting uniform changes in the intrinsic properties of the axon trunk.
Fig. 15.
Long-term changes in axonal excitability. A: Stimulation and recording of rabbit callosal axons Shown on the left are superimposed recording traces from electrodes implanted into superficial cortical layers near the border of visual areas I and II. Stimulation electrodes were implanted into the corpus callosum. Conduction delay was measured as the latency from axon stimulation (blue arrowhead) to the antidromic spike (red circle). Excitability changes were measured with paired stimulations. At intervals of several milliseconds, conduction was supernormal (note that the spike interval is smaller than the stimulus interval). In addition, the minimum stimulus interval was determined that allowed initiation and propagation of the second spike. B: Over several months, antidromic latency in some axons remained stable (cell 1), in others either decreased (cell 2) or increased (cell 3). C&D: Supernormal latency and minimum interval remained relatively stable, or at least did not appear to change systematically with antidromic latency. Modified from Swadlow, 1982.
In networks of cultured cortical neurons, patterned stimulation induced changes in conduction velocity of extracellularly measured spikes (Bakkum et al., 2008). As in the experiments with callosal axons described above, conduction velocity increased in some axons and decreased in others. These changes required activation of the post-synaptic targets. Spike amplitudes also changed, albeit not monotonically with conduction speed. The absence of a strict correlation between changes in conduction velocity and spike amplitude suggests that these modifications of axon function do not only involve changes in channel densities, but may also be due to changes in morphology.
5.2. Activity-dependent plasticity of intrinsic axonal excitability
The mechanisms potentially underlying changes in conduction velocity could include modifications of axonal morphology, which we will discuss later, and changes in intrinsic excitability. Due to the limited experimental accessibility of axons, so far little information is available on the molecular basis of changes in excitability, but results from other cell compartments shed some light on the potential regulation of voltage-gated ion channels in the axon. At least two types of activity-dependent plasticity are usually distinguished. The terms Hebbian or Hebbian-like plasticity refer to long-term changes underlying the acquisition and storage of new information at the cellular level. The term homeostatic plasticity refers to the mechanisms regulating synaptic and intrinsic properties to ensure long-term stability of neural function.
For a long time, studies of synaptic plasticity were focused on presynaptic changes in transmitter release, but it is now well established that changes in pre- and postsynaptic intrinsic excitability play an important role in Hebbian plasticity (Campanac and Debanne, 2007; Debanne et al., 2003; Debanne and Poo, 2010; Zhang and Linden, 2003). In fact, even in the early descriptions of long-term potentiation of hippocampal synapses, a contribution of changes in postsynaptic excitability was suggested (Bliss and Lomo, 1973). Although most studies focused on changes in dendritic voltage-gated ion channels, there are some examples of axonal contributions. Experimentally induced long-term potentiation in hippocampal CA1 pyramidal cells can involve both changes in synaptic transmission and changes in postsynaptic excitability at the level of the axon (Xu et al., 2005). In this case, the activation and inactivation of the voltage-dependent sodium currents were both shifted to more hyperpolarized potentials, leading to a decrease in the threshold of the action potential synergistic with the potentiation of the pure synaptic component. Because currents were recorded from the soma, it was not clear whether these changes in sodium channel properties were restricted to the AIS or also expressed in the axon trunk. In cultured hippocampal neurons, long-term potentiation induced by correlated firing of the pre- and postsynaptic neurons was associated with a change in excitability of the presynaptic neuron (Ganguly et al., 2000). This change was mediated by shifts in the voltage-dependence of sodium currents leading to a decrease in the threshold for spiking and a reduction of interspike interval variability. The location of the changes in intrinsic excitability was not precisely defined. However, they appeared to require retrograde signaling from the postsynaptic neuron. This suggests that the sodium channels involved are located near the synaptic terminal involved in Hebbian plasticity. In vivo, where the same neuron is sending collaterals to many distinct postsynaptic targets, such a mechanism may affect only the collateral carrying the potentiated synapse. This could favor the propagation of action potentials into specific branches and could for example have a significant influence on spike failures at branch points, selectively directing action potential traffic to stronger synapses. Changes involving both spike initiation and spike propagation were investigated following operant conditioning of spinal reflexes. Rats and monkeys were conditioned to increase or decrease their muscle response to the stimulation of afferent sensory fibers (H-reflex) (Carp et al., 2001; Carp and Wolpaw, 1994; Wolpaw and Tennissen, 2001). The physiological changes underlying the conditioned responses, while involving supraspinal influences, were found to mainly occur at the level of the intrinsic excitability of motor neurons. The spike threshold recorded at the soma was increased when the animal learned to decrease its response to the sensory stimulus. In addition, the conduction velocity was decreased. Therefore, classical learning paradigms can induce changes in the excitability of the axon, not only at the spike initiation site, but also in the axon trunk. In Aplysia, the long-term effects of local depolarization were investigated in the axons of sensory and motor neurons (Weragoda et al., 2004). Transient local application of high potassium saline centimeters away from the soma induced long-term hyperexcitability in the axon, similar to changes in excitability after nerve crush. The action potential threshold was significantly lower in the treated region 24 hours after the 2-minute treatment, whereas conduction and spike initiation were unchanged in untreated regions. The change in spike threshold was shown to be dependent on local protein synthesis, and may involve changes in the expression level of ion channels. This suggests that the machinery necessary for local regulation of protein expression is present in the axon trunk, and that long-term changes in axon excitability can occur in response to relatively brief changes in activity.
Homeostatic plasticity also involves changes in both synaptic properties and in intrinsic excitability (Davis, 2006; Marder and Goaillard, 2006; Turrigiano, 2011). Imposed perturbations of activity in many cases lead to a regulation of intrinsic excitability that restores activity levels. In the study by Swadlow (1985) described in section 5.1, the dependence of conduction velocity on activity was tested in one animal by enucleation of one eye and subsequent recordings of contralateral corticotectal axonal activity. In the two cells monitored after ablation, conduction velocity started increasing 5 days post-ablation, and reached a new steady-state value after 25-30 days at 110% of the control values (Swadlow, 1985). This change could be interpreted as homeostatic, i.e. representing an upregulation of excitability to compensate for loss of visual activation. An interesting case in this respect is the recent finding of activity-dependent regulation of spike initiation. In cultured hippocampal neurons, chronic depolarization with high potassium saline induced a shift of the AIS away from the cell body, which should increase the spike threshold (Grubb and Burrone, 2010). The shift of the AIS involved multiple molecular components, including voltage-gated sodium channels, and was reversible when neurons returned to normal membrane potentials. In neurons involved in sound localization in birds, auditory input deprivation led to a redistribution of voltage-gated sodium channels that lengthened the AIS, associated with an increase in excitability and spontaneous activity (Kuba et al., 2010).
In two studies investigating the changes in excitability associated with homeostatic plasticity, sodium currents were found to be strongly regulated by changes in activity levels (Desai et al., 1999; Turrigiano et al., 1994). However, physiological measurements were made at the soma and therefore could not distinguish between changes affecting only spike initiation and changes also affecting spike propagation. Other evidence for compensatory regulation of ion channels in the axons comes from mutant mice. In optic nerves and elsewhere in the brain, Nav1.6 channels are found in high expression levels at the axon initial segment and in the Nodes of Ranvier in mature myelinated axons. During development, these compartments initially express predominantly Nav1.2 channels, most of which subsequently get replaced by Nav1.6 channels (Van Wart and Matthews, 2006). Mutant mice with reduced expression of Nav1.6 channels partially compensate for axonal dysfunctions by retaining elevated Nav1.2 expression (Vega et al., 2008). However, it is not known if this compensatory expression can only happen during a critical developmental period, and if it is dependent on activity. Taken together, these findings suggest that the expression and properties of sodium channels are strongly regulated in various types of plasticity. Because of the fundamental importance of sodium currents for spike generation and propagation, such regulatory processes have the potential to significantly influence the temporal fidelity of axonal spike conduction, as well as the overall conduction delay.
Studies of the activity-dependent regulation of potassium channels have also mostly been focused on proximal cell compartments, but at least one study shows a clear effect of activity on the distribution of channels in the axon trunk (Grosse et al., 2000). In hippocampal neurons, Kv1 channels at appreciable expression levels appear postnatally, i.e. relatively late in development. This late expression is regulated by activity. Block of activity in cultured neurons prevented the axonal expression of some types of Kv1 α-subunits. While this was shown with immunocytochemical methods, a recent electrophysiological study in rat hippocampal slices showed that in response to a decrease in network activity, pyramidal neuron D-type (Kv1.1) current, which is known to be prominently expressed along the axon in these cells, is downregulated (Cudmore et al., 2010).
Altogether, these studies suggest that conduction velocity can be regulated in an activity-dependent manner in different behavioral contexts. It should be noted, however, that such plasticity obviously has some limits. Presumably deleterious changes in conduction velocity during normal aging imply that activity-dependent plasticity fails to compensate for changes in axonal excitability. For example, axonal conduction delays in cholinergic projections to the cortex are increased by 50% in aged squirrel monkeys (Aston-Jones et al., 1985b). Likewise, the functional impairments occurring during demyelinating diseases shed some light on the limits of axon plasticity (Krishnan et al., 2009).
5.3. Plasticity of axon morphology
Because of the dependence of axonal conduction velocity on morphological parameters like diameter, myelin thickness, and internodal length, it is not surprising that changes in these parameters are utilized to regulate conduction delay. The morphological plasticity of axonal arborizations has been known for more than 30 years from the pioneering studies on the effect of monocular deprivation on the connectivity of thalamocortical afferents (Hubel et al., 1977; Shatz and Stryker, 1978). This manipulation results in a significant decrease of the total length and number of branching points of thalamocortical axons coming from the deprived eye (Antonini and Stryker, 1993). The activity-dependence of axon growth has since been studied extensively at the molecular level (Ibarretxe et al., 2007). However, monocular deprivation during critical developmental periods has such dramatic consequences for gross axon morphology and synaptic connectivity that from the point of view of spike propagation, changes may be rather unselective. It is now also well established that synaptic plasticity, above all LTP and LTD, is associated with morphological rearrangements of both the presynaptic and postsynaptic site (Holtmaat and Svoboda, 2009). Higher order axon branches and terminals can stabilize and grow, or disappear, concomitantly with strengthening and weakening of synaptic connections (Becker et al., 2008; De Paola et al., 2006; Gogolla et al., 2007; Stettler et al., 2006). Because such changes could affect the electrotonic structure of axons, including impedance matching or mismatching at branch points, consequences for spike propagation are easily conceivable. However, to our knowledge no studies have addressed this question yet.
An interesting perspective on the regulation of axon morphology is provided by the problems posed by growth and development. Depending on species and circuit in question, adult-like neural function may either be established early, mature slowly, or even be absent because of an operating mode that is age-specific. However, in most cases development is associated with dramatic changes in the size of the body, and hence of the nervous system. The lengths, diameters and intrinsic membrane properties of neural processes in general have to be increased in a carefully controlled way and at relative rates that either preserve their electrotonic structure or allow appropriate functional adjustment (Hill et al., 1994; Olsen et al., 1996). An obvious challenge for axons that comes with an increase in size is the adjustment of conduction velocity that is necessary if a stable delay of neuronal response is needed. In other words, if the total conduction delay has to remain stable, an increase in conduction velocity can compensate for an increase in axon length. In species that grow continuously, such as lobsters, the body size can increase by a factor of 20 or more between juvenile and adult stages, although juvenile and adult animals share common behaviors and produce similar neural activity. In some cases, total conduction delay is not maintained. In lobster stomatogastric motor axons between juvenile animals and adults, conduction velocity remains either stable or increases by less than 20% while axon lengths increase by a factor of four (Bucher et al., 2005). Consequently, conduction delay almost quadruples. In contrast, lobster giant axons increase substantially in diameter and roughly quadruple their conduction velocity, presumably to compensate for changes in axon length (Govind and Lang, 1976b). Similarly, in postnatally growing rat triceps surae motor neurons changes in conduction velocity compensate well for changes in axon length to achieve stable conduction delays (Chen et al., 1992). It should be noted, however, that in the absence of specific information about the importance of absolute delay values for coding, interpretation of such data can be difficult. In goldfish, Mauthner cell axons of larger animals actually have smaller diameters and conduction delays (Funch et al., 1981).
In addition to the relationship between axon diameter and conduction velocity, delay can be regulated by a coordinated increase in diameter and myelination (Buckley et al., 2010; Franklin and Hinks, 1999). In mammals, where growth is finite, some peripheral nerves still increase in length by more than four times postnatally. Peripheral sensory axons are unmyelinated at birth and become myelinated during the first three postnatal weeks (Peters and Muir, 1959). The stages at which myelination starts and ends largely depend on the behavioral maturity of the species considered at birth (Szalay, 2001; Tessitore and Brunjes, 1988; Yakovlev and Lecours, 1967). In precocial animals, which are able to feed and walk at birth, myelination is almost complete at birth. In altricial animals, which are immature at birth and depend on their mothers to feed, myelination extends late into postnatal life. In humans, central motor and sensory axons achieve adult conduction delays by the age of two, while body size keeps substantially increasing afterwards (Eyre et al., 1991). Adjustments necessary even after the initial myelinating process is completed can be achieved through changes in myelin thickness and internode spacing that help maintain conduction time (Friede et al., 1985). This is also evident in adult animals. For example, thalamocortical projections make use of regional differences in axon myelination to compensate for different path lengths and ensure similar conduction delays (Salami et al., 2003).
Importantly, a number of studies suggest that myelination is modulated by activity, and that this coupling likely relies on the sensitivity of glial cells to different signaling molecules produced by axons (Emery, 2010; Fields, 2008b; Markham and Greenough, 2004). In postnatal developmental stages, manipulations of visual input, including deprivation, premature eye-opening, enrichment or impoverishment of visual environment, and length of day, affect the degree and onset of axon myelination (Gyllensten and Malmfors, 1963; Juraska and Kopcik, 1988; Sirevaag and Greenough, 1987; Spears et al., 1990; Szeligo and Leblond, 1977; Tauber et al., 1980). Recent work also suggests that activity-dependent modifications of myelination are present in adults and may be involved in learning processes (Fields, 2010; Zalc and Fields, 2000). Plasticity of myelination can be revealed when changes in axonal activity are imposed or fiber tracts are lesioned. For example, imposed hind limb load changes induce changes in myelin thickness and internodal length in different peripheral nerves, partly associated with changes in conduction velocity (Canu et al., 2009). Axonal sprouting observed in the hippocampus following lesion of the entorhino-hippocampal perforant pathway is associated with the recruitment of newly formed myelinating cells, suggesting that myelination is still plastic in adult animals (Drojdahl et al., 2010). Whether this plasticity is associated with a recovery of axon function has yet to be determined.
In humans, functional imaging has been used to directly investigate the relationship between white matter structure and cognitive function at different developmental stages (Fields, 2008b). These studies have shown that myelination of specific brain regions correlates with cognitive development in children (Kraft et al., 1980; Mabbott et al., 2006; Nagy et al., 2004; Pujol et al., 2006), and that differences in white matter structure are correlated with reading abilities (Klingberg et al., 2000; Niogi and McCandliss, 2006). Several recent studies used diffusion tensor imaging to analyze the microstructure of white matter in humans. Changes in the microstructure are mostly interpreted as changes in the degree of myelination of axons. Manual dexterity, for example in professional pianists, appears to be correlated with increased myelination in cortical, subcortical, and spinal axons (Bengtsson et al., 2005; Imfeld et al., 2009; Lindberg et al., 2010). One study even directly assessed the effect of task learning on white matter microstructure. Changes were found in subjects learning to juggle after 6 weeks of training and persisted 4 weeks after cessation of training (Scholz et al., 2009). Such complex sensory-motor tasks depend on precise timing, and temporal precision of spike propagation may be a critical parameter in this context. These studies suggest that the cellular mechanisms of learning and memory, including complex behaviors in the adult, may not just involve changes in synaptic efficacy, but also the tuning of axonal conduction properties.
6. Concluding remarks
The findings discussed here clearly show that many axons do not faithfully propagate spikes. Nonlinearities resulting from the gating properties of ion channels and geometric inhomogeneities, as well as neuromodulation, may alter activity patterns between initiation and arrival at presynaptic sites. Curiously, reports of activity-dependent changes in conduction velocity and impulse failures have appeared over the better part of the 20th century, and some of the potential functional implications have been insightfully discussed more than 30 years ago (George, 1977; Swadlow et al., 1980). Despite this long-standing knowledge, the understanding that axons act as conditional rather than faithful conduits of information is only now entering the common consciousness of cellular neuroscientists. This may have to do with the fact that the relevance for neural coding and processing is far from understood. In most cases, we do not even know the magnitude of changes in spike conduction in the context of biologically relevant patterns of activity. It is likely that the extent to which the dynamics of axonal spike propagation are truly utilized as part of the coding strategy is not consistent across different neurons and model systems.
There are different viewpoints on this issue that one might adopt. From one perspective, activity-dependent changes in conduction velocity can be viewed as a limit of temporal fidelity. If there is a complex frequency- and history-dependence of spike propagation as seen in some of the examples discussed above, it may impose constraints on the coding strategy that the neuron can use. In other words, some neurons may have limited temporal fidelity of axonal conduction but there is no adaptive disadvantage as long as this is not functionally relevant in the computational tasks the neuron is involved in. Why then would such a neuron express a complex and specific complement of voltage-gated ion channels in its axonal membrane instead of relying on the minimal set sufficient for spike propagation? The biophysical properties of an axon are the result of the evolutionary history of the cell type or lineage, and not necessarily optimized for all aspects of temporal fidelity of spike propagation. The presence of ion channels that exceed our notion of what the minimal complement necessary for spike conduction is may have evolved in a different functional context, or may contribute to different aspects of the same functional context. For example, the tuning of the axonal complement of different voltage-gated ion channels did not just evolve in the context of excitability and re-excitability, but was likely also driven towards minimizing energy expenditure (Alle et al., 2009; Sengupta et al., 2010). In addition, the ambiguous effects of voltage-gated ionic conductances on excitability mean that their presence and regulation can be a compromise between beneficial and deleterious effects in specific functional contexts. For example, Ih can balance excessive hyperpolarization caused by the Na+/K+-pump and therefore be crucial in preventing spike failures during repetitive activity (Baginskas et al., 2009; Grafe et al., 1997; Kiernan et al., 2004; Soleng et al., 2003). On the other hand, it may contribute to a depolarized resting potential in the absence of proximally initiated spikes and lead to ectopic spike initiation (Ballo and Bucher, 2009). Accordingly, one might interpret the fact that some axons have excellent temporal fidelity of propagation as an adaptive specialization. The biophysical properties of some axons may have specifically evolved in the context of maintaining spike timing precision. For example, some axons of crustacean proprioceptive afferents, which clearly use temporal coding schemes, show almost no changes in conduction delay even at fairly high spike frequencies (DiCaprio et al., 2007). The apparent excess in ion channel types in many axons may also play a role in the robustness of spike conduction. In a system relying on a minimal number of components, the loss or dysfunction of any of these components likely impairs performance dramatically (Barbaric et al., 2007; Kitano, 2004; Marder and Goaillard, 2006). The presence of functionally overlapping and therefore conceivably partially redundant ion channels in axons may ensure the preservation of propagation in the case of loss or dysfunction of a single ion channel type. However, because distinct ion channels rarely are identical in their gating properties, this may add nonlinearities to the spike propagation process.
From a different perspective, the dynamics of spike propagation can be viewed as an integral part of signal transmission. In fact, the usual view of membrane properties in other neuronal compartments than axons is somewhat “teleological”: The presence of a specific set of passive properties and ionic conductances determines the electrical behavior and therefore all of these properties and every type of ion channel are thought to be present for a specific reason in the context of excitability. Accordingly, changes in spike patterns during propagation must be interpreted as part of the coding strategy, as long as they occur within the range of patterns that are generated in vivo (which are often not well known). For example, if the inactivation of a potassium current leads to decrease in spike failures at branch points during repetitive activity, postsynaptic activation is enhanced at higher firing frequencies and this represents a biologically relevant form of non-synaptic short-term plasticity (Debanne et al., 1999).
We do not hold these two viewpoints as mutually exclusive, but they illustrate that the interpretation of axonal contribution to neural processing in most cases is difficult in the absence of a better understanding of realistic activity patterns and general coding strategy. It is to be expected that the substantial advances in electrophysiological approaches, voltage-sensitive dye imaging, and molecular mapping of ion channels and receptors will allow a better understanding of the dynamics of the axonal membrane in many systems in the nearer future. Together with advances in techniques allowing the recording of realistic activity patterns in vivo and over longer time spans, such approaches will facilitate our understanding of axons as computing devices.
Particularly interesting will be to explore to which degree axons are subject to neuromodulation and long-term regulatory processes, and therefore involved in neural plasticity. Taken together, the observations described in section 5 suggest that virtually all parameters involved in the definition of conduction properties, like the density of ion channels, myelination, axon diameters and branching patterns, can be modified by activity. Changes in conduction properties are very rarely taken into account in the theoretical framework of learning processes and information coding in the brain. However, a significant part of the computations performed by neuronal networks relies on exquisitely precise timing that could not be achieved without optimization of conduction properties (Bakkum et al., 2008; Izhikevich, 2006; Karino et al., 2011). Therefore, learning of complex fine-tuned behaviors cannot be understood if considering only plasticity of synaptic and somatodendritic properties of neurons.
Highlights.
Across phyla and cell types, axonal complements of voltage-gated ion channels by far exceed the classic description of spike initiation and propagation in the squid axon.
Due to the complexity of ion channel complements and morphological inhomogeneities of axons, temporal patterns of neuronal activity can change substantially during propagation from proximal to distal axonal sites.
Neuromodulators can alter axonal excitability and affect spike propagation.
Short-term dynamics, neuromodulation, and long-term regulation of axonal spike conduction may play important roles in neural coding and plasticity in the brain.
7. Acknowledgements
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS058825 to DB, and Avenir (Inserm), CG13, and Fondation Fyssen grants to JMG. We thank Drs Farzan Nadim and Dominique Debanne for helpful discussions.
Abbreviations
- 5-HT
serotonin
- ACh
acetylcholine
- AGR
anterior gastric receptor neuron
- AIS
axon initial segment
- AMPA receptor
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- ATP
adenosine-5'-triphosphate
- CA
cornu ammonis
- Cav
voltage-gated calcium channel
- CPG
central pattern generator
- DA
dopamine
- DG
dentate gyrus
- EPSP
excitatory postsynaptic potential
- GABA
γ-Aminobutyric acid
- GR
geometric ratio
- HCN
hyperpolarization-activated cyclic nucleotide-gated channel
- IA
A-type potassium current
- Ih
hyperpolarization-activated inward current
- IPSP
inhibitory postsynaptic potential
- Kv
voltage-gated potassium channel
- LG
lateral gastric neuron
- MCN5
modulatory commissural neuron 5
- nAChR
nicotinic acetylcholine receptor
- Nap
persistent sodium current
- Nat
transient sodium current
- Nav
voltage-gated sodium channel
- OA
octopamine
- PD
pyloric dilator neuron
- pdn
pyloric dilator nerve
- STG
stomatogastric ganglion
- STNS
stomatogastric nervous system
- TRPC
classical transient receptor potential channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8. References
- Adrian ED. The recovery process of excitable tissues: Part I. J Physiol. 1920;54:1–31. doi: 10.1113/jphysiol.1920.sp001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alle H, Geiger JR. Combined analog and action potential coding in hippocampal mossy fibers. Science. 2006;311:1290–1293. doi: 10.1126/science.1119055. [DOI] [PubMed] [Google Scholar]
- Alle H, Geiger JR. GABAergic spill-over transmission onto hippocampal mossy fiber boutons. J Neurosci. 2007;27:942–950. doi: 10.1523/JNEUROSCI.4996-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alle H, Geiger JR. Analog signalling in mammalian cortical axons. Curr Opin Neurobiol. 2008;18:314–320. doi: 10.1016/j.conb.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Alle H, Roth A, Geiger JR. Energy-efficient action potentials in hippocampal mossy fibers. Science. 2009;325:1405–1408. doi: 10.1126/science.1174331. [DOI] [PubMed] [Google Scholar]
- Alzheimer C, Schwindt PC, Crill WE. Modal gating of Na+ channels as a mechanism of persistent Na+ current in pyramidal neurons from rat and cat sensorimotor cortex. J Neurosci. 1993;13:660–673. doi: 10.1523/JNEUROSCI.13-02-00660.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastassiou CA, Montgomery SM, Barahona M, Buzsaki G, Koch C. The effect of spatially inhomogeneous extracellular electric fields on neurons. J Neurosci. 2010;30:1925–1936. doi: 10.1523/JNEUROSCI.3635-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JC, Martin KA. Does bouton morphology optimize axon length? Nat Neurosci. 2001;4:1166–1167. doi: 10.1038/nn772. [DOI] [PubMed] [Google Scholar]
- Antonini A, Stryker MP. Rapid remodeling of axonal arbors in the visual cortex. Science. 1993;260:1819–1821. doi: 10.1126/science.8511592. [DOI] [PubMed] [Google Scholar]
- Antonini A, Gillespie DC, Crair MC, Stryker MP. Morphology of single geniculocortical afferents and functional recovery of the visual cortex after reverse monocular deprivation in the kitten. J Neurosci. 1998;18:9896–9909. doi: 10.1523/JNEUROSCI.18-23-09896.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuthnott ER, Boyd IA, Kalu KU. Ultrastructural dimensions of myelinated peripheral nerve fibres in the cat and their relation to conduction velocity. J Physiol. 1980;308:125–157. doi: 10.1113/jphysiol.1980.sp013465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armett CJ, Ritchie JM. The action of acetylcholine on conduction in mammalian non-myelinated fibres and its prevention by an anticholinesterase. J Physiol. 1960;152:141–158. doi: 10.1113/jphysiol.1960.sp006476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armett CJ, Ritchie JM. The action of acetylcholine and some related substances on conduction in mammalian non-myelinated nerve fibres. J Physiol. 1961;155:372–384. doi: 10.1113/jphysiol.1961.sp006634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armett CJ, Ritchie JM. The ionic requirements for the action of acetylcholine on mammalian non-myelinated fibres. J Physiol. 1963;165:141–159. doi: 10.1113/jphysiol.1963.sp007048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitaki A. Effects evoked in an axon by the activity of a contiguous one. Journal of Neurophysiology. 1942;5:89–108. [Google Scholar]
- Aston-Jones G, Foote SL, Segal M. Impulse conduction properties of noradrenergic locus coeruleus axons projecting to monkey cerebrocortex. Neuroscience. 1985a;15:765–777. doi: 10.1016/0306-4522(85)90077-6. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rogers J, Shaver RD, Dinan TG, Moss DE. Age-impaired impulse flow from nucleus basalis to cortex. Nature. 1985b;318:462–464. doi: 10.1038/318462a0. [DOI] [PubMed] [Google Scholar]
- Ayali A, Harris-Warrick RM. Monoamine control of the pacemaker kernel and cycle frequency in the lobster pyloric network. J Neurosci. 1999;19:6712–6722. doi: 10.1523/JNEUROSCI.19-15-06712.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccus SA. Synaptic facilitation by reflected action potentials: enhancement of transmission when nerve impulses reverse direction at axon branch points. Proc Natl Acad Sci U S A. 1998;95:8345–8350. doi: 10.1073/pnas.95.14.8345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccus SA, Burrell BD, Sahley CL, Muller KJ. Action potential reflection and failure at axon branch points cause stepwise changes in EPSPs in a neuron essential for learning. J Neurophysiol. 2000;83:1693–1700. doi: 10.1152/jn.2000.83.3.1693. [DOI] [PubMed] [Google Scholar]
- Baginskas A, Palani D, Chiu K, Raastad M. The H-current secures action potential transmission at high frequencies in rat cerebellar parallel fibers. Eur J Neurosci. 2009;29:87–96. doi: 10.1111/j.1460-9568.2008.06566.x. [DOI] [PubMed] [Google Scholar]
- Baker MD. Axonal flip-flops and oscillators. Trends Neurosci. 2000;23:514–519. doi: 10.1016/s0166-2236(00)01645-3. [DOI] [PubMed] [Google Scholar]
- Baker MD, Bostock H. Low-threshold, persistent sodium current in rat large dorsal root ganglion neurons in culture. J Neurophysiol. 1997;77:1503–1513. doi: 10.1152/jn.1997.77.3.1503. [DOI] [PubMed] [Google Scholar]
- Baker M, Bostock H, Grafe P, Martius P. Function and distribution of three types of rectifying channel in rat spinal root myelinated axons. J Physiol. 1987;383:45–67. doi: 10.1113/jphysiol.1987.sp016395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker PF, Blaustein MP, Keynes RD, Manil J, Shaw TI, Steinhardt RA. The ouabain-sensitive fluxes of sodium and potassium in squid giant axons. J Physiol. 1969;200:459–496. doi: 10.1113/jphysiol.1969.sp008703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkum DJ, Chao ZC, Potter SM. Long-term activity-dependent plasticity of action potential propagation delay and amplitude in cortical networks. PLoS ONE. 2008;3:e2088. doi: 10.1371/journal.pone.0002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballo AW, Bucher D. Complex intrinsic membrane properties and dopamine shape spiking activity in a motor axon. J Neurosci. 2009;29:5062–5074. doi: 10.1523/JNEUROSCI.0716-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballo AW, Keene JC, Troy PJ, Goeritz ML, Nadim F, Bucher D. Dopamine modulates Ih in a motor axon. J Neurosci. 2010;30:8425–8434. doi: 10.1523/JNEUROSCI.0405-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranauskas G. Ionic channel function in action potential generation: current perspective. Mol Neurobiol. 2007;35:129–150. doi: 10.1007/s12035-007-8001-0. [DOI] [PubMed] [Google Scholar]
- Barbaric I, Miller G, Dear TN. Appearances can be deceiving: phenotypes of knockout mice. Brief Funct Genomic Proteomic. 2007;6:91–103. doi: 10.1093/bfgp/elm008. [DOI] [PubMed] [Google Scholar]
- Baro DJ, Ayali A, French L, Scholz NL, Labenia J, Lanning CC, Graubard K, Harris-Warrick RM. Molecular underpinnings of motor pattern generation: differential targeting of shal and shaker in the pyloric motor system. J Neurosci. 2000;20:6619–6630. doi: 10.1523/JNEUROSCI.20-17-06619.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett EF, Barrett JN. Intracellular recording from vertebrate myelinated axons: mechanism of the depolarizing afterpotential. J Physiol. 1982;323:117–144. doi: 10.1113/jphysiol.1982.sp014064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron DH, Matthews BH. Intermittent conduction in the spinal cord. J Physiol. 1935;85:73–103. doi: 10.1113/jphysiol.1935.sp003303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Frotscher M, Meyer A, Monyer H, Geiger JR, Jonas P. Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc Natl Acad Sci U S A. 2002;99:13222–13227. doi: 10.1073/pnas.192233099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. The molecular machinery of resurgent sodium current revealed. Neuron. 2005;45:185–187. doi: 10.1016/j.neuron.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- Beaumont V, Zucker RS. Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic Ih channels. Nat Neurosci. 2000;3:133–141. doi: 10.1038/72072. [DOI] [PubMed] [Google Scholar]
- Beaumont V, Zhong N, Froemke RC, Ball RW, Zucker RS. Temporal synaptic tagging by I(h) activation and actin: involvement in long-term facilitation and cAMP-induced synaptic enhancement. Neuron. 2002;33:601–613. doi: 10.1016/s0896-6273(02)00581-0. [DOI] [PubMed] [Google Scholar]
- Beck A, Lohr C, Deitmer JW. Calcium transients in subcompartments of the leech Retzius neuron as induced by single action potentials. J Neurobiol. 2001;48:1–18. doi: 10.1002/neu.1039. [DOI] [PubMed] [Google Scholar]
- Becker N, Wierenga CJ, Fonseca R, Bonhoeffer T, Nagerl UV. LTD induction causes morphological changes of presynaptic boutons and reduces their contacts with spines. Neuron. 2008;60:590–597. doi: 10.1016/j.neuron.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- Benoit E, Dubois JM. Toxin I from the snake Dendroaspis polylepis polylepis: a highly specific blocker of one type of potassium channel in myelinated nerve fiber. Brain Res. 1986;377:374–377. doi: 10.1016/0006-8993(86)90884-x. [DOI] [PubMed] [Google Scholar]
- Bevan S, Storey N. Modulation of sodium channels in primary afferent neurons. Novartis Found Symp. 2002;241:144–153. discussion 153-148, 226-132. [PubMed] [Google Scholar]
- Bhattacharjee A, Kaczmarek LK. For K+ channels, Na+ is the new Ca2+. Trends Neurosci. 2005;28:422–428. doi: 10.1016/j.tins.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A, Gan L, Kaczmarek LK. Localization of the Slack potassium channel in the rat central nervous system. J Comp Neurol. 2002;454:241–254. doi: 10.1002/cne.10439. [DOI] [PubMed] [Google Scholar]
- Bhisitkul RB, Villa JE, Kocsis JD. Axonal GABA receptors are selectively present on normal and regenerated sensory fibers in rat peripheral nerve. Exp Brain Res. 1987;66:659–663. doi: 10.1007/BF00270698. [DOI] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel M, Wahl-Schott C, Michalakis S, Zong X. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev. 2009;89:847–885. doi: 10.1152/physrev.00029.2008. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K, Jackson MB. A calcium-activated potassium channel causes frequency-dependent action-potential failures in a mammalian nerve terminal. J Neurophysiol. 1993;70:284–298. doi: 10.1152/jn.1993.70.1.284. [DOI] [PubMed] [Google Scholar]
- Birch BD, Kocsis JD, Di Gregorio F, Bhisitkul RB, Waxman SG. A voltage- and time-dependent rectification in rat dorsal spinal root axons. J Neurophysiol. 1991;66:719–728. doi: 10.1152/jn.1991.66.3.719. [DOI] [PubMed] [Google Scholar]
- Bischofberger J, Engel D, Li L, Geiger JR, Jonas P. Patch-clamp recording from mossy fiber terminals in hippocampal slices. Nat Protoc. 2006;1:2075–2081. doi: 10.1038/nprot.2006.312. [DOI] [PubMed] [Google Scholar]
- Black JA, Waxman SG. Molecular identities of two tetrodotoxin-resistant sodium channels in corneal axons. Exp Eye Res. 2002;75:193–199. doi: 10.1006/exer.2002.2014. [DOI] [PubMed] [Google Scholar]
- Blight AR. Computer simulation of action potentials and afterpotentials in mammalian myelinated axons: the case for a lower resistance myelin sheath. Neuroscience. 1985;15:13–31. doi: 10.1016/0306-4522(85)90119-8. [DOI] [PubMed] [Google Scholar]
- Blight AR, Someya S. Depolarizing afterpotentials in myelinated axons of mammalian spinal cord. Neuroscience. 1985;15:1–12. doi: 10.1016/0306-4522(85)90118-6. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H, Bergmans J. Post-tetanic excitability changes and ectopic discharges in a human motor axon. Brain. 1994;117(Pt 5):913–928. doi: 10.1093/brain/117.5.913. [DOI] [PubMed] [Google Scholar]
- Bostock H, Grafe P. Activity-dependent excitability changes in normal and demyelinated rat spinal root axons. J Physiol. 1985;365:239–257. doi: 10.1113/jphysiol.1985.sp015769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H, Rothwell JC. Latent addition in motor and sensory fibres of human peripheral nerve. J Physiol. 1997;498(Pt 1):277–294. doi: 10.1113/jphysiol.1997.sp021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H, Sears TA. The internodal axon membrane: electrical excitability and continuous conduction in segmental demyelination. J Physiol. 1978;280:273–301. doi: 10.1113/jphysiol.1978.sp012384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H, Campero M, Serra J, Ochoa J. Velocity recovery cycles of C fibres innervating human skin. J Physiol. 2003;553:649–663. doi: 10.1113/jphysiol.2003.046342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H, Cikurel K, Burke D. Threshold tracking techniques in the study of human peripheral nerve. Muscle Nerve. 1998;21:137–158. doi: 10.1002/(sici)1097-4598(199802)21:2<137::aid-mus1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Bowe CM, Kocsis JD, Waxman SG. The association of the supernormal period and the depolarizing afterpotential in myelinated frog and rat sciatic nerve. Neuroscience. 1987;21:585–593. doi: 10.1016/0306-4522(87)90144-8. [DOI] [PubMed] [Google Scholar]
- Brau ME, Dreyer F, Jonas P, Repp H, Vogel W. A K+ channel in Xenopus nerve fibres selectively blocked by bee and snake toxins: binding and voltage-clamp experiments. J Physiol. 1990;420:365–385. doi: 10.1113/jphysiol.1990.sp017918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock LG, Coombs JS, Eccles JC. The recording of potentials from motoneurones with an intracellular electrode. J Physiol. 1952;117:431–460. doi: 10.1113/jphysiol.1952.sp004759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody DL, Yue DT. Release-independent short-term synaptic depression in cultured hippocampal neurons. J Neurosci. 2000;20:2480–2494. doi: 10.1523/JNEUROSCI.20-07-02480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM. A modeling study predicts the presence of voltage gated Ca2+ channels on myelinated central axons. Comput Methods Programs Biomed. 2003;71:25–31. doi: 10.1016/s0169-2607(02)00031-7. [DOI] [PubMed] [Google Scholar]
- Brown AM, Westenbroek RE, Catterall WA, Ransom BR. Axonal L-type Ca2+ channels and anoxic injury in rat CNS white matter. J Neurophysiol. 2001;85:900–911. doi: 10.1152/jn.2001.85.2.900. [DOI] [PubMed] [Google Scholar]
- Brown DA, Marsh S. Axonal GABA-receptors in mammalian peripheral nerve trunks. Brain Res. 1978;156:187–191. doi: 10.1016/0006-8993(78)90098-7. [DOI] [PubMed] [Google Scholar]
- Brown D,A. Extrasynaptic GABA systems. Trends Neurosci. 1979;2:271–273. [Google Scholar]
- Brown JT, Randall AD. Activity-dependent depression of the spike after-depolarization generates long-lasting intrinsic plasticity in hippocampal CA3 pyramidal neurons. J Physiol. 2009;587:1265–1281. doi: 10.1113/jphysiol.2008.167007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher D, Prinz AA, Marder E. Animal-to-animal variability in motor pattern production in adults and during growth. J Neurosci. 2005;25:1611–1619. doi: 10.1523/JNEUROSCI.3679-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher D, Thirumalai V, Marder E. Axonal dopamine receptors activate peripheral spike initiation in a stomatogastric motor neuron. J Neurosci. 2003;23:6866–6875. doi: 10.1523/JNEUROSCI.23-17-06866.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley CE, Marguerie A, Alderton WK, Franklin RJ. Temporal dynamics of myelination in the zebrafish spinal cord. Glia. 2010;58:802–812. doi: 10.1002/glia.20964. [DOI] [PubMed] [Google Scholar]
- Budd JM, Kovacs K, Ferecsko AS, Buzas P, Eysel UT, Kisvarday ZF. Neocortical axon arbors trade-off material and conduction delay conservation. PLoS Comput Biol. 2010;6:e1000711. doi: 10.1371/journal.pcbi.1000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock TH. Facilitation of conduction rate in nerve fibers. J Physiol. 1951;114:89–97. doi: 10.1113/jphysiol.1951.sp004605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buniel M, Glazebrook PA, Ramirez-Navarro A, Kunze DL. Distribution of voltage-gated potassium and hyperpolarization-activated channels in sensory afferent fibers in the rat carotid body. J Comp Neurol. 2008;510:367–377. doi: 10.1002/cne.21796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Kiernan MC, Bostock H. Excitability of human axons. Clin Neurophysiol. 2001;112:1575–1585. doi: 10.1016/s1388-2457(01)00595-8. [DOI] [PubMed] [Google Scholar]
- Burke D, Miller TA, Kiernan MC, Mogyoros I. Activity-dependent modulation of excitability. Muscle Nerve. 1995;18:675–677. [PubMed] [Google Scholar]
- Burke D, Mogyoros I, Vagg R, Kiernan MC. Quantitative description of the voltage dependence of axonal excitability in human cutaneous afferents. Brain. 1998;121(Pt 10):1975–1983. doi: 10.1093/brain/121.10.1975. [DOI] [PubMed] [Google Scholar]
- Burke RE. John Eccles' pioneering role in understanding central synaptic transmission. Prog Neurobiol. 2006;78:173–188. doi: 10.1016/j.pneurobio.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Calabrese RL. Control of multiple impulse-initiation sites in a leech interneuron. J Neurophysiol. 1980;44:878–896. doi: 10.1152/jn.1980.44.5.878. [DOI] [PubMed] [Google Scholar]
- Callewaert G, Eilers J, Konnerth A. Axonal calcium entry during fast ‘sodium’ action potentials in rat cerebellar Purkinje neurones. J Physiol. 1996;495(Pt 3):641–647. doi: 10.1113/jphysiol.1996.sp021622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanac E, Debanne D. Plasticity of neuronal excitability: Hebbian rules beyond the synapse. Arch Ital Biol. 2007;145:277–287. [PubMed] [Google Scholar]
- Campero M, Serra J, Bostock H, Ochoa JL. Partial reversal of conduction slowing during repetitive stimulation of single sympathetic efferents in human skin. Acta Physiol Scand. 2004;182:305–311. doi: 10.1111/j.1365-201X.2004.01357.x. [DOI] [PubMed] [Google Scholar]
- Cantrell AR, Catterall WA. Neuromodulation of Na+ channels: an unexpected form of cellular plasticity. Nat Rev Neurosci. 2001;2:397–407. doi: 10.1038/35077553. [DOI] [PubMed] [Google Scholar]
- Canu MH, Carnaud M, Picquet F, Goutebroze L. Activity-dependent regulation of myelin maintenance in the adult rat. Brain Res. 2009;1252:45–51. doi: 10.1016/j.brainres.2008.10.079. [DOI] [PubMed] [Google Scholar]
- Carp JS, Wolpaw JR. Motoneuron plasticity underlying operantly conditioned decrease in primate H-reflex. J Neurophysiol. 1994;72:431–442. doi: 10.1152/jn.1994.72.1.431. [DOI] [PubMed] [Google Scholar]
- Carp JS, Chen XY, Sheikh H, Wolpaw JR. Operant conditioning of rat H-reflex affects motoneuron axonal conduction velocity. Exp Brain Res. 2001;136:269–273. doi: 10.1007/s002210000608. [DOI] [PubMed] [Google Scholar]
- Carr CE, Konishi M. Axonal delay lines for time measurement in the owl's brainstem. Proc Natl Acad Sci U S A. 1988;85:8311–8315. doi: 10.1073/pnas.85.21.8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Day M, Cantrell AR, Held J, Scheuer T, Catterall WA, Surmeier DJ. Transmitter modulation of slow, activity-dependent alterations in sodium channel availability endows neurons with a novel form of cellular plasticity. Neuron. 2003;39:793–806. doi: 10.1016/s0896-6273(03)00531-2. [DOI] [PubMed] [Google Scholar]
- Carr RW, Sittl R, Fleckenstein J, Grafe P. GABA increases electrical excitability in a subset of human unmyelinated peripheral axons. PLoS ONE. 2010;5:e8780. doi: 10.1371/journal.pone.0008780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casassus G, Blanchet C, Mulle C. Short-term regulation of information processing at the corticoaccumbens synapse. J Neurosci. 2005;25:11504–11512. doi: 10.1523/JNEUROSCI.2466-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo E, Brunelli M, Byrne JH, Av-Ron E, Cai Y, Baxter DA. Computational model of touch sensory cells (T Cells) of the leech: role of the afterhyperpolarization (AHP) in activity-dependent conduction failure. J Comput Neurosci. 2005;18:5–24. doi: 10.1007/s10827-005-5477-3. [DOI] [PubMed] [Google Scholar]
- Cattaert D, Bevengut M. Effects of antidromic discharges in crayfish primary afferents. J Neurophysiol. 2002;88:1753–1765. doi: 10.1152/jn.2002.88.4.1753. [DOI] [PubMed] [Google Scholar]
- Cattaert D, El Manira A, Bevengut M. Presynaptic inhibition and antidromic discharges in crayfish primary afferents. J Physiol Paris. 1999;93:349–358. doi: 10.1016/s0928-4257(00)80062-5. [DOI] [PubMed] [Google Scholar]
- Cattaert D, el Manira A, Clarac F. Direct evidence for presynaptic inhibitory mechanisms in crayfish sensory afferents. J Neurophysiol. 1992;67:610–624. doi: 10.1152/jn.1992.67.3.610. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005a;57:397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev. 2005b;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- Cessac B, Paugam-Moisy H, Vieville T. Overview of facts and issues about neural coding by spikes. J Physiol Paris. 2010;104:5–18. doi: 10.1016/j.jphysparis.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Chen XY, Carp JS, Wolpaw JR. Constancy of motor axon conduction time during growth in rats. Exp Brain Res. 1992;90:343–345. doi: 10.1007/BF00227247. [DOI] [PubMed] [Google Scholar]
- Chiu SY, Ritchie JM, Rogart RB, Stagg D. A quantitative description of membrane currents in rabbit myelinated nerve. J Physiol. 1979;292:149–166. doi: 10.1113/jphysiol.1979.sp012843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarac F, Cattaert D. Functional multimodality of axonal tree in invertebrate neurons. J Physiol Paris. 1999;93:319–327. doi: 10.1016/s0928-4257(00)80060-1. [DOI] [PubMed] [Google Scholar]
- Clark BD, Goldberg EM, Rudy B. Electrogenic tuning of the axon initial segment. Neuroscientist. 2009;15:651–668. doi: 10.1177/1073858409341973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Tate S, Carlton SM. Differential expression of tetrodotoxin-resistant sodium channels Nav1.8 and Nav1.9 in normal and inflamed rats. Neurosci Lett. 2004;355:45–48. doi: 10.1016/j.neulet.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Colquhoun D, Ritchie JM. The interaction at equilibrium between tetrodotoxin and mammalian non-myelinated nerve fibres. J Physiol. 1972;221:533–553. doi: 10.1113/jphysiol.1972.sp009766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JA. Neural repetitive firing: a comparative study of membrane properties of crustacean walking leg axons. J Neurophysiol. 1975;38:922–932. doi: 10.1152/jn.1975.38.4.922. [DOI] [PubMed] [Google Scholar]
- Connor JA, Walter D, McKown R. Neural repetitive firing: modifications of the Hodgkin-Huxley axon suggested by experimental results from crustacean axons. Biophys J. 1977;18:81–102. doi: 10.1016/S0006-3495(77)85598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors BW, Ahmed OJ. Integration and autonomy in axons. Nat Neurosci. 2011;14:128–130. doi: 10.1038/nn0211-128. [DOI] [PubMed] [Google Scholar]
- Contreras D, Steriade M. Spindle oscillation in cats: the role of corticothalamic feedback in a thalamically generated rhythm. J Physiol. 1996;490(Pt 1):159–179. doi: 10.1113/jphysiol.1996.sp021133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrette BJ, Repp H, Dreyer F, Schwarz JR. Two types of fast K+ channels in rat myelinated nerve fibres and their sensitivity to dendrotoxin. Pflugers Arch. 1991;418:408–416. doi: 10.1007/BF00550879. [DOI] [PubMed] [Google Scholar]
- Cudmore RH, Fronzaroli-Molinieres L, Giraud P, Debanne D. Spike-time precision and network synchrony are controlled by the homeostatic regulation of the D-type potassium current. J Neurosci. 2010;30:12885–12895. doi: 10.1523/JNEUROSCI.0740-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai A, Temporal S, Schulz DJ. Cell-specific patterns of alternative splicing of voltage-gated ion channels in single identified neurons. Neuroscience. 2010;168:118–129. doi: 10.1016/j.neuroscience.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Daur N, Nadim F, Stein W. Regulation of motor patterns by the central spike-initiation zone of a sensory neuron. Eur J Neurosci. 2009 doi: 10.1111/j.1460-9568.2009.06866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Modney B, Scappaticci KA, Barrett JN, Barrett EF. Electrical and morphological factors influencing the depolarizing after-potential in rat and lizard myelinated axons. J Physiol. 1995;489(Pt 1):141–157. doi: 10.1113/jphysiol.1995.sp021037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW. Homeostatic Control of Neural Activity: From Phenomenology to Molecular Design. Annu Rev Neurosci. 2006 doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- De Col R, Messlinger K, Carr RW. Conduction velocity is regulated by sodium channel inactivation in unmyelinated axons innervating the rat cranial meninges. J Physiol. 2008;586:1089–1103. doi: 10.1113/jphysiol.2007.145383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paola V, Holtmaat A, Knott G, Song S, Wilbrecht L, Caroni P, Svoboda K. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron. 2006;49:861–875. doi: 10.1016/j.neuron.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Debanne D. Information processing in the axon. Nat Rev Neurosci. 2004;5:304–316. doi: 10.1038/nrn1397. [DOI] [PubMed] [Google Scholar]
- Debanne D, Poo MM. Spike-timing dependent plasticity beyond synapse - pre- and post-synaptic plasticity of intrinsic neuronal excitability. Front Synaptic Neurosci. 2010;2:21. doi: 10.3389/fnsyn.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Campanac E, Bialowas A, Carlier E, Alcaraz G. Axon physiology. Physiol Rev. 2011;91:555–602. doi: 10.1152/physrev.00048.2009. [DOI] [PubMed] [Google Scholar]
- Debanne D, Daoudal G, Sourdet V, Russier M. Brain plasticity and ion channels. J Physiol Paris. 2003;97:403–414. doi: 10.1016/j.jphysparis.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Debanne D, Guerineau NC, Gahwiler BH, Thompson SM. Action-potential propagation gated by an axonal I(A)-like K+ conductance in hippocampus. Nature. 1997;389:286–289. doi: 10.1038/38502. [DOI] [PubMed] [Google Scholar]
- Debanne D, Kopysova IL, Bras H, Ferrand N. Gating of action potential propagation by an axonal A-like potassium conductance in the hippocampus: a new type of non-synaptic plasticity. J Physiol Paris. 1999;93:285–296. doi: 10.1016/s0928-4257(00)80057-1. [DOI] [PubMed] [Google Scholar]
- Del Castillo J, Moore JW. On increasing the velocity of a nerve impulse. J Physiol. 1959;148:665–670. doi: 10.1113/jphysiol.1959.sp006315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci. 1999;2:515–520. doi: 10.1038/9165. [DOI] [PubMed] [Google Scholar]
- Devaux JJ, Kleopa KA, Cooper EC, Scherer SS. KCNQ2 is a nodal K+ channel. J Neurosci. 2004;24:1236–1244. doi: 10.1523/JNEUROSCI.4512-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux J, Alcaraz G, Grinspan J, Bennett V, Joho R, Crest M, Scherer SS. Kv3.1b is a novel component of CNS nodes. J Neurosci. 2003;23:4509–4518. doi: 10.1523/JNEUROSCI.23-11-04509.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor M. Unexplained peculiarities of the dorsal root ganglion. Pain. 1999;(Suppl 6):S27–35. doi: 10.1016/S0304-3959(99)00135-9. [DOI] [PubMed] [Google Scholar]
- Diaz E. One Decade Later: What has Gene Expression Profiling Told us About Neuronal Cell Types, Brain Function and Disease? Curr Genomics. 2009;10:318–325. doi: 10.2174/138920209788921029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCaprio RA. Nonspiking and spiking proprioceptors in the crab: nonlinear analysis of nonspiking TCMRO afferents. J Neurophysiol. 2003;89:1826–1836. doi: 10.1152/jn.00978.2002. [DOI] [PubMed] [Google Scholar]
- DiCaprio RA, Billimoria CP, Ludwar B. Information rate and spike-timing precision of proprioceptive afferents. J Neurophysiol. 2007;98:1706–1717. doi: 10.1152/jn.00176.2007. [DOI] [PubMed] [Google Scholar]
- Ding YS, Fowler JS, Logan J, Wang GJ, Telang F, Garza V, Biegon A, Pareto D, Rooney W, Shea C, Alexoff D, Volkow ND, Vocci F. 6-[18F]Fluoro-A-85380, a new PET tracer for the nicotinic acetylcholine receptor: studies in the human brain and in vivo demonstration of specific binding in white matter. Synapse. 2004;53:184–189. doi: 10.1002/syn.20051. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. Calcium channel diversity: multiple roles of calcium channel subunits. Curr Opin Neurobiol. 2009;19:237–244. doi: 10.1016/j.conb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Drew PJ, Abbott LF. Models and properties of power-law adaptation in neural systems. J Neurophysiol. 2006;96:826–833. doi: 10.1152/jn.00134.2006. [DOI] [PubMed] [Google Scholar]
- Drojdahl N, Nielsen HH, Gardi JE, Wree A, Peterson AC, Nyengaard JR, Eyer J, Finsen B. Axonal plasticity elicits long-term changes in oligodendroglia and myelinated fibers. Glia. 2010;58:29–42. doi: 10.1002/glia.20897. [DOI] [PubMed] [Google Scholar]
- Du J, Tao-Cheng JH, Zerfas P, McBain CJ. The K+ channel, Kv2.1, is apposed to astrocytic processes and is associated with inhibitory postsynaptic membranes in hippocampal and cortical principal neurons and inhibitory interneurons. Neuroscience. 1998;84:37–48. doi: 10.1016/s0306-4522(97)00519-8. [DOI] [PubMed] [Google Scholar]
- Dubois JM. Evidence for the existence of three types of potassium channels in the frog Ranvier node membrane. J Physiol. 1981;318:297–316. doi: 10.1113/jphysiol.1981.sp013865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois JM. Capsaicin blocks one class of K+ channels in the frog node of Ranvier. Brain Res. 1982;245:372–375. doi: 10.1016/0006-8993(82)90820-4. [DOI] [PubMed] [Google Scholar]
- Ducreux C, Reynaud JC, Puizillout JJ. Spike conduction properties of T-shaped C neurons in the rabbit nodose ganglion. Pflugers Arch. 1993;424:238–244. doi: 10.1007/BF00384348. [DOI] [PubMed] [Google Scholar]
- Dudel J, Kuffler SW. Presynaptic inhibition at the crayfish neuromuscular junction. J Physiol. 1961;155:543–562. doi: 10.1113/jphysiol.1961.sp006646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC. The Physiology of Synapses. Academic Press; New York: 1964. [Google Scholar]
- Eccles JC, Magni F, Willis WD. Depolarizations of central terminals of group I afferent fibres from muscle. J Physiol (Lond) 1962;160:62–93. doi: 10.1113/jphysiol.1962.sp006835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott P, Marsh SJ, Brown DA. Inhibition of Ca-spikes in rat preganglionic cervical sympathetic nerves by sympathomimetic amines. Br J Pharmacol. 1989;96:65–76. doi: 10.1111/j.1476-5381.1989.tb11785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elphick C, Meron E, Rinzel J, Spiegel EA. Impulse patterning and relaxational propagation in excitable media. J Theor Biol. 1990;146:249–268. doi: 10.1016/s0022-5193(05)80138-9. [DOI] [PubMed] [Google Scholar]
- Emery B. Regulation of oligodendrocyte differentiation and myelination. Science. 2010;330:779–782. doi: 10.1126/science.1190927. [DOI] [PubMed] [Google Scholar]
- Eng DL, Gordon TR, Kocsis JD, Waxman SG. Development of 4-AP and TEA sensitivities in mammalian myelinated nerve fibers. J Neurophysiol. 1988;60:2168–2179. doi: 10.1152/jn.1988.60.6.2168. [DOI] [PubMed] [Google Scholar]
- Eng DL, Gordon TR, Kocsis JD, Waxman SG. Current-clamp analysis of a time-dependent rectification in rat optic nerve. J Physiol. 1990;421:185–202. doi: 10.1113/jphysiol.1990.sp017940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman HS, MacDermott AB. Presynaptic ionotropic receptors and control of transmitter release. Nat Rev Neurosci. 2004;5:135–145. doi: 10.1038/nrn1297. [DOI] [PubMed] [Google Scholar]
- Erisir A, Lau D, Rudy B, Leonard CS. Function of specific K(+) channels in sustained high-frequency firing of fast-spiking neocortical interneurons. J Neurophysiol. 1999;82:2476–2489. doi: 10.1152/jn.1999.82.5.2476. [DOI] [PubMed] [Google Scholar]
- Evans CG, Jing J, Rosen SC, Cropper EC. Regulation of spike initiation and propagation in an Aplysia sensory neuron: gating-in via central depolarization. J Neurosci. 2003;23:2920–2931. doi: 10.1523/JNEUROSCI.23-07-02920.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CG, Kang T, Cropper EC. Selective spike propagation in the central processes of an invertebrate neuron. J Neurophysiol. 2008;100:2940–2947. doi: 10.1152/jn.90807.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CG, Ludwar B, Cropper EC. Mechanoafferent neuron with an inexcitable somatic region: consequences for the regulation of spike propagation and afferent transmission. J Neurophysiol. 2007;97:3126–3130. doi: 10.1152/jn.01341.2006. [DOI] [PubMed] [Google Scholar]
- Evarts EV. Relation of Discharge Frequency to Conduction Velocity in Pyramidal Tract Neurons. J Neurophysiol. 1965;28:216–228. doi: 10.1152/jn.1965.28.2.216. [DOI] [PubMed] [Google Scholar]
- Eyre JA, Miller S, Ramesh V. Constancy of central conduction delays during development in man: investigation of motor and somatosensory pathways. J Physiol. 1991;434:441–452. doi: 10.1113/jphysiol.1991.sp018479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiers AA, Mogenson GJ. Electrophysiological identification of neurons in locus coeruleus. Exp Neurol. 1976;53:254–266. doi: 10.1016/0014-4886(76)90295-8. [DOI] [PubMed] [Google Scholar]
- Faisal AA, Laughlin SB. Stochastic simulations on the reliability of action potential propagation in thin axons. PLoS Comput Biol. 2007;3:e79. doi: 10.1371/journal.pcbi.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fern R, Ransom BR, Waxman SG. Voltage-gated calcium channels in CNS white matter: role in anoxic injury. J Neurophysiol. 1995a;74:369–377. doi: 10.1152/jn.1995.74.1.369. [DOI] [PubMed] [Google Scholar]
- Fern R, Waxman SG, Ransom BR. Endogenous GABA attenuates CNS white matter dysfunction following anoxia. J Neurosci. 1995b;15:699–708. doi: 10.1523/JNEUROSCI.15-01-00699.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields DM, Weissburg MJ. Rapid firing rates from mechanosensory neurons in copepod antennules. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2004;190:877–882. doi: 10.1007/s00359-004-0543-2. [DOI] [PubMed] [Google Scholar]
- Fields RD. Oligodendrocytes changing the rules: action potentials in glia and oligodendrocytes controlling action potentials. Neuroscientist. 2008a;14:540–543. doi: 10.1177/1073858408320294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008b;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. Neuroscience. Change in the brain's white matter. Science. 2010;330:768–769. doi: 10.1126/science.1199139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ, Hinks GL. Understanding CNS remyelination: clues from developmental and regeneration biology. J Neurosci Res. 1999;58:207–213. [PubMed] [Google Scholar]
- French CR, Sah P, Buckett KJ, Gage PW. A voltage-dependent persistent sodium current in mammalian hippocampal neurons. J Gen Physiol. 1990;95:1139–1157. doi: 10.1085/jgp.95.6.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French LB, Lanning CC, Harris-Warrick RM. The localization of two voltage-gated calcium channels in the pyloric network of the lobster stomatogastric ganglion. Neuroscience. 2002;112:217–232. doi: 10.1016/s0306-4522(01)00621-2. [DOI] [PubMed] [Google Scholar]
- French LB, Lanning CC, Matly M, Harris-Warrick RM. Cellular localization of Shab and Shaw potassium channels in the lobster stomatogastric ganglion. Neuroscience. 2004;123:919–930. doi: 10.1016/j.neuroscience.2003.08.036. [DOI] [PubMed] [Google Scholar]
- Friede RL, Brzoska J, Hartmann U. Changes in myelin sheath thickness and internode geometry in the rabbit phrenic nerve during growth. J Anat. 1985;143:103–113. [PMC free article] [PubMed] [Google Scholar]
- Friedrich RW, Habermann CJ, Laurent G. Multiplexing using synchrony in the zebrafish olfactory bulb. Nat Neurosci. 2004;7:862–871. doi: 10.1038/nn1292. [DOI] [PubMed] [Google Scholar]
- Funch PG, Kinsman SL, Faber DS, Koenig E, Zottoli SJ. Mauthner axon diameter and impulse conduction velocity decrease with growth of goldfish. Neurosci Lett. 1981;27:159–164. doi: 10.1016/0304-3940(81)90261-5. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Dahlstrom AB, Jonsson G, Marcellino D, Guescini M, Dam M, Manger P, Agnati L. The discovery of central monoamine neurons gave volume transmission to the wired brain. Prog Neurobiol. 2010;90:82–100. doi: 10.1016/j.pneurobio.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Gadjanski I, Boretius S, Williams SK, Lingor P, Knoferle J, Sattler MB, Fairless R, Hochmeister S, Suhs KW, Michaelis T, Frahm J, Storch MK, Bahr M, Diem R. Role of n-type voltage-dependent calcium channels in autoimmune optic neuritis. Ann Neurol. 2009;66:81–93. doi: 10.1002/ana.21668. [DOI] [PubMed] [Google Scholar]
- Ganguly K, Kiss L, Poo M. Enhancement of presynaptic neuronal excitability by correlated presynaptic and postsynaptic spiking. Nat Neurosci. 2000;3:1018–1026. doi: 10.1038/79838. [DOI] [PubMed] [Google Scholar]
- Gardner-Medwin AR. An extreme supernormal period in cerebellar parallel fibres. J Physiol. 1972;222:357–371. doi: 10.1113/jphysiol.1972.sp009802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser HS, Erlanger J. The role played by the sizes of the constituent fibers of a nerve-trunk in determining the form of its action potential wave. Am J Physiol. 1927;80:522–547. [Google Scholar]
- Gasser HS, Erlanger J. The ending of the axon action potential and its relation to other events in nerve acivity. Am J Physiol. 1930;94:247–277. [Google Scholar]
- Geiger JR, Jonas P. Dynamic control of presynaptic Ca(2+) inflow by fast-inactivating K(+) channels in hippocampal mossy fiber boutons. Neuron. 2000;28:927–939. doi: 10.1016/s0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]
- George SA. Changes in interspike interval during propagation: quantitative description. Biol Cybern. 1977;26:209–213. doi: 10.1007/BF00366592. [DOI] [PubMed] [Google Scholar]
- George SA, Mastronarde DN, Dubin MW. Prior activity influences the velocity of impulses in frog and cat optic nerve fibers. Brain Res. 1984;304:121–126. doi: 10.1016/0006-8993(84)90867-9. [DOI] [PubMed] [Google Scholar]
- Gilboa G, Chen R, Brenner N. History-dependent multiple-time-scale dynamics in a single-neuron model. J Neurosci. 2005;25:6479–6489. doi: 10.1523/JNEUROSCI.0763-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliatt RW, Willison RG. The refractory and supernormal periods of the human median nerve. J Neurol Neurosurg Psychiatry. 1963;26:136–147. doi: 10.1136/jnnp.26.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisselmann G, Marx T, Bobkov Y, Wetzel CH, Neuhaus EM, Ache BW, Hatt H. Molecular and functional characterization of an I(h)-channel from lobster olfactory receptor neurons. Eur J Neurosci. 2005;21:1635–1647. doi: 10.1111/j.1460-9568.2005.03992.x. [DOI] [PubMed] [Google Scholar]
- Goaillard JM, Schulz DJ, Kilman VL, Marder E. Octopamine modulates the axons of modulatory projection neurons. J Neurosci. 2004;24:7063–7073. doi: 10.1523/JNEUROSCI.2078-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N, Galimberti I, Caroni P. Structural plasticity of axon terminals in the adult. Curr Opin Neurobiol. 2007;17:516–524. doi: 10.1016/j.conb.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Evolution of voltage-gated Na(+) channels. J Exp Biol. 2002;205:575–584. doi: 10.1242/jeb.205.5.575. [DOI] [PubMed] [Google Scholar]
- Goldstein SS, Rall W. Changes of action potential shape and velocity for changing core conductor geometry. Biophys J. 1974;14:731–757. doi: 10.1016/S0006-3495(74)85947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B, Rhodes KJ, Bekele-Arcuri Z, Trimmer JS. Type I and type II Na(+) channel alpha-subunit polypeptides exhibit distinct spatial and temporal patterning, and association with auxiliary subunits in rat brain. J Comp Neurol. 1999;412:342–352. [PubMed] [Google Scholar]
- Gordon TR, Kocsis JD, Waxman SG. Evidence for the presence of two types of potassium channels in the rat optic nerve. Brain Res. 1988;447:1–9. doi: 10.1016/0006-8993(88)90959-6. [DOI] [PubMed] [Google Scholar]
- Gordon TR, Kocsis JD, Waxman SG. Electrogenic pump (Na+/K(+)-ATPase) activity in rat optic nerve. Neuroscience. 1990;37:829–837. doi: 10.1016/0306-4522(90)90112-h. [DOI] [PubMed] [Google Scholar]
- Govind CK, Lang F. Coordinated activation of muscle fibres by different conduction velocities in branches of a crustacean motor axon. Experientia. 1976a;32:1170–1171. doi: 10.1007/BF01927607. [DOI] [PubMed] [Google Scholar]
- Govind CK, Lang F. Growth of lobster giant axons: correlation between conduction velocity and axon diameter. J Comp Neurol. 1976b;170:421–433. doi: 10.1002/cne.901700403. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984a;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci. 1984b;4:2866–2876. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe P, Quasthoff S, Grosskreutz J, Alzheimer C. Function of the hyperpolarization-activated inward rectification in nonmyelinated peripheral rat and human axons. J Neurophysiol. 1997;77:421–426. doi: 10.1152/jn.1997.77.1.421. [DOI] [PubMed] [Google Scholar]
- Graham HT, Lorente de No R. Recovery of blood-perfused mamalian nerves. Am J Physiol. 1938;123:326–340. [Google Scholar]
- Graubard K, Raper JA, Hartline DK. Graded synaptic transmission between spiking neurons. Proc Natl Acad Sci U S A. 1980;77:3733–3735. doi: 10.1073/pnas.77.6.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graubard K, Raper JA, Hartline DK. Graded synaptic transmission between identified spiking neurons. J Neurophysiol. 1983;50:508–521. doi: 10.1152/jn.1983.50.2.508. [DOI] [PubMed] [Google Scholar]
- Grosse G, Draguhn A, Hohne L, Tapp R, Veh RW, Ahnert-Hilger G. Expression of Kv1 potassium channels in mouse hippocampal primary cultures: development and activity-dependent regulation. J Neurosci. 2000;20:1869–1882. doi: 10.1523/JNEUROSCI.20-05-01869.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman Y, Parnas I, Spira ME. Differential conduction block in branches of a bifurcating axon. J Physiol. 1979a;295:283–305. doi: 10.1113/jphysiol.1979.sp012969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman Y, Parnas I, Spira ME. Mechanisms involved in differential conduction of potentials at high frequency in a branching axon. J Physiol. 1979b;295:307–322. doi: 10.1113/jphysiol.1979.sp012970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MS, Burrone J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature. 2010;465:1070–1074. doi: 10.1038/nature09160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu XN. Effect of conduction block at axon bifurcations on synaptic transmission to different postsynaptic neurones in the leech. J Physiol. 1991;441:755–778. doi: 10.1113/jphysiol.1991.sp018777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Kampa BM, Stuart GJ. Synaptic integration in dendritic trees. J Neurobiol. 2005;64:75–90. doi: 10.1002/neu.20144. [DOI] [PubMed] [Google Scholar]
- Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, Robertson GA, Rudy B, Sanguinetti MC, Stuhmer W, Wang X. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev. 2005;57:473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- Gyllensten L, Malmfors T. Myelinization of the optic nerve and its dependence on visual function--a quantitative investigation in mice. J Embryol Exp Morphol. 1963;11:255–266. [PubMed] [Google Scholar]
- Hagiwara S, Tasaki I. A study on the mechanism of impulse transmission across the giant synapse of the squid. J Physiol. 1958;143:114–137. doi: 10.1113/jphysiol.1958.sp006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick RM, Johnson BR, Peck JH, Kloppenburg P, Ayali A, Skarbinski J. Distributed effects of dopamine modulation in the crustacean pyloric network. Ann N Y Acad Sci. 1998;860:155–167. doi: 10.1111/j.1749-6632.1998.tb09046.x. [DOI] [PubMed] [Google Scholar]
- Harris-Warrick R, Marder E, Selverston AI, Moulins M. Dynamic Biological Networks: The Stomatogastric Nervous System. MIT Press; Cambridge, Massachusetts; London, England: 1992. [Google Scholar]
- Hartline DK, Colman DR. Rapid conduction and the evolution of giant axons and myelinated fibers. Curr Biol. 2007;17:R29–35. doi: 10.1016/j.cub.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Hatt H, Smith DO. Synaptic depression related to presynaptic axon conduction block. J Physiol. 1976;259:367–393. doi: 10.1113/jphysiol.1976.sp011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zorumski CF, Mennerick S. Contribution of presynaptic Na(+) channel inactivation to paired-pulse synaptic depression in cultured hippocampal neurons. J Neurophysiol. 2002;87:925–936. doi: 10.1152/jn.00225.2001. [DOI] [PubMed] [Google Scholar]
- Heitler WJ, Goodman CS. Multiple sites of spike initiation in a bifurcating locust neurone. J Exp Biol. 1978;76:63–84. [Google Scholar]
- Herkenham M. Mismatches between neurotransmitter and receptor localizations in brain: observations and implications. Neuroscience. 1987;23:1–38. doi: 10.1016/0306-4522(87)90268-5. [DOI] [PubMed] [Google Scholar]
- Herzog RI, Cummins TR, Ghassemi F, Dib-Hajj SD, Waxman SG. Distinct repriming and closed-state inactivation kinetics of Nav1.6 and Nav1.7 sodium channels in mouse spinal sensory neurons. J Physiol. 2003;551:741–750. doi: 10.1113/jphysiol.2003.047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AA, Edwards DH, Murphey RK. The effect of neuronal growth on synaptic integration. J Comput Neurosci. 1994;1:239–254. doi: 10.1007/BF00961736. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL. The relation between conduction velocity and the electrical resistance outside a nerve fibre. J Physiol. 1939;94:560–570. doi: 10.1113/jphysiol.1939.sp003702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL. A note on conduction velocity. J Physiol. 1954;125:221–224. doi: 10.1113/jphysiol.1954.sp005152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. Action potentials recorded from inside nerve fiber. Nature. 1939;144:710–711. [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Rushton WA. The electrical constants of a crustacean nerve fibre. Proc R Soc Med. 1946;134:444–479. doi: 10.1098/rspb.1946.0024. [DOI] [PubMed] [Google Scholar]
- Hoffmann T, Sauer SK, Horch RE, Reeh PW. Sensory transduction in peripheral nerve axons elicits ectopic action potentials. J Neurosci. 2008;28:6281–6284. doi: 10.1523/JNEUROSCI.1627-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt GR, Koch C. Electrical interactions via the extracellular potential near cell bodies. J Comput Neurosci. 1999;6:169–184. doi: 10.1023/a:1008832702585. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Hu W, Tian C, Li T, Yang M, Hou H, Shu Y. Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat Neurosci. 2009;12:996–1002. doi: 10.1038/nn.2359. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977;278:377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Huxley AF, Stampfli R. Evidence for saltatory conduction in peripheral myelinated nerve fibres. J Physiol. 1949;108:315–339. [PubMed] [Google Scholar]
- Ibarretxe G, Perrais D, Jaskolski F, Vimeney A, Mulle C. Fast regulation of axonal growth cone motility by electrical activity. J Neurosci. 2007;27:7684–7695. doi: 10.1523/JNEUROSCI.1070-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imfeld A, Oechslin MS, Meyer M, Loenneker T, Jancke L. White matter plasticity in the corticospinal tract of musicians: a diffusion tensor imaging study. Neuroimage. 2009;46:600–607. doi: 10.1016/j.neuroimage.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Izhikevich EM. Polychronization: computation with spikes. Neural Comput. 2006;18:245–282. doi: 10.1162/089976606775093882. [DOI] [PubMed] [Google Scholar]
- Jackson MB, Zhang SJ. Action potential propagation and propagation block by GABA in rat posterior pituitary nerve terminals. J Physiol. 1995;483(Pt 3):597–611. doi: 10.1113/jphysiol.1995.sp020609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson VM, Trout SJ, Brain KL, Cunnane TC. Characterization of action potential-evoked calcium transients in mouse postganglionic sympathetic axon bundles. J Physiol. 2001;537:3–16. doi: 10.1111/j.1469-7793.2001.0003k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys JG. Nonsynaptic modulation of neuronal activity in the brain: electric currents and extracellular ions. Physiol Rev. 1995;75:689–723. doi: 10.1152/physrev.1995.75.4.689. [DOI] [PubMed] [Google Scholar]
- Jeftinija S. The role of tetrodotoxin-resistant sodium channels of small primary afferent fibers. Brain Res. 1994;639:125–134. doi: 10.1016/0006-8993(94)91772-8. [DOI] [PubMed] [Google Scholar]
- Jeziorski MC, Greenberg RM, Anderson PA. The molecular biology of invertebrate voltage-gated Ca(2+) channels. J Exp Biol. 2000;203:841–856. doi: 10.1242/jeb.203.5.841. [DOI] [PubMed] [Google Scholar]
- Jiang YQ, Xing GG, Wang SL, Tu HY, Chi YN, Li J, Liu FY, Han JS, Wan Y. Axonal accumulation of hyperpolarization-activated cyclic nucleotide-gated cation channels contributes to mechanical allodynia after peripheral nerve injury in rat. Pain. 2008;137:495–506. doi: 10.1016/j.pain.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Jonas P, Koh DS, Kampe K, Hermsteiner M, Vogel W. ATP-sensitive and Ca-activated K channels in vertebrate axons: novel links between metabolism and excitability. Pflugers Arch. 1991;418:68–73. doi: 10.1007/BF00370453. [DOI] [PubMed] [Google Scholar]
- Jones SW. Muscarinic and peptidergic excitation of bull-frog sympathetic neurones. J Physiol. 1985;366:63–87. doi: 10.1113/jphysiol.1985.sp015785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris P, Yin TC. A matter of time: internal delays in binaural processing. Trends Neurosci. 2007;30:70–78. doi: 10.1016/j.tins.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Juraska JM, Kopcik JR. Sex and environmental influences on the size and ultrastructure of the rat corpus callosum. Brain Res. 1988;450:1–8. doi: 10.1016/0006-8993(88)91538-7. [DOI] [PubMed] [Google Scholar]
- Juusola M, Robinson HP, de Polavieja GG. Coding with spike shapes and graded potentials in cortical networks. Bioessays. 2007;29:178–187. doi: 10.1002/bies.20532. [DOI] [PubMed] [Google Scholar]
- Kalisman N, Silberberg G, Markram H. The neocortical microcircuit as a tabula rasa. Proc Natl Acad Sci U S A. 2005;102:880–885. doi: 10.1073/pnas.0407088102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H. Kainate receptor-dependent presynaptic modulation and plasticity. Neurosci Res. 2002;42:1–6. doi: 10.1016/s0168-0102(01)00303-0. [DOI] [PubMed] [Google Scholar]
- Kao MH, Wright BD, Doupe AJ. Neurons in a forebrain nucleus required for vocal plasticity rapidly switch between precise firing and variable bursting depending on social context. J Neurosci. 2008;28:13232–13247. doi: 10.1523/JNEUROSCI.2250-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karino S, Smith PH, Yin TCT, Joris PX. Axonal Branching Patterns as Sources of Delay in the Mammalian Auditory Brainstem: A Re-Examination. The Journal of Neuroscience. 2011;31:3016–3031. doi: 10.1523/JNEUROSCI.5175-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. The effect of electrolyte deficiency on the rate of conduction in a single nerve fibre. J Physiol. 1947;106:411–417. doi: 10.1113/jphysiol.1947.sp004221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Schmitt OH. Electric interaction between two adjacent nerve fibres. J Physiol. 1940;97:471–488. doi: 10.1113/jphysiol.1940.sp003823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Schmitt OH. A note on interaction between nerve fibres. J Physiol. 1942;100:369–371. doi: 10.1113/jphysiol.1942.sp003948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai H, Lazar R, Metherate R. Nicotinic control of axon excitability regulates thalamocortical transmission. Nat Neurosci. 2007;10:1168–1175. doi: 10.1038/nn1956. [DOI] [PubMed] [Google Scholar]
- Khaliq ZM, Raman IM. Axonal propagation of simple and complex spikes in cerebellar Purkinje neurons. J Neurosci. 2005;25:454–463. doi: 10.1523/JNEUROSCI.3045-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan MC, Baker MD, Bostock H. Characteristics of late Na(+) current in adult rat small sensory neurons. Neuroscience. 2003;119:653–660. doi: 10.1016/s0306-4522(03)00194-5. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Lin CS, Burke D. Differences in activity-dependent hyperpolarization in human sensory and motor axons. J Physiol. 2004;558:341–349. doi: 10.1113/jphysiol.2004.063966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan MC, Mogyoros I, Hales JP, Gracies JM, Burke D. Excitability changes in human cutaneous afferents induced by prolonged repetitive axonal activity. J Physiol. 1997;500(Pt 1):255–264. doi: 10.1113/jphysiol.1997.sp022015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano H. Biological robustness. Nat Rev Genet. 2004;5:826–837. doi: 10.1038/nrg1471. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, Poldrack RA. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Knaus HG, Schwarzer C, Koch RO, Eberhart A, Kaczorowski GJ, Glossmann H, Wunder F, Pongs O, Garcia ML, Sperk G. Distribution of high-conductance Ca(2+)-activated K+ channels in rat brain: targeting to axons and nerve terminals. J Neurosci. 1996;16:955–963. doi: 10.1523/JNEUROSCI.16-03-00955.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis JD, Sakatani K. Modulation of axonal excitability by neurotransmitter receptors. In: Waxman SG, Kocsis JD, Stys PK, editors. The Axon. Structure, Function and Pathophysiology. Oxford University Press; New York: 1995. [Google Scholar]
- Kocsis JD, VanderMaelen CP. A supernormal period in central axons following single cell stimulation. Exp Brain Res. 1979;36:381–386. doi: 10.1007/BF00238919. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Waxman SG. Ionic channel organization of normal and regenerating mammalian axons. In: Seil FJ, Herbert E, Karlson BM, editors. Progress in Brain Research. Elsevier; Amsterdam: 1987. pp. 89–101. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Eng DL, Gordon TR, Waxman SG. Functional differences between 4-aminopyridine and tetraethylammonium-sensitive potassium channels in myelinated axons. Neurosci Lett. 1987;75:193–198. doi: 10.1016/0304-3940(87)90296-5. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Malenka RC, Waxman SG. Effects of extracellular potassium concentration on the excitability of the parallel fibres of the rat cerebellum. J Physiol. 1983;334:225–244. doi: 10.1113/jphysiol.1983.sp014491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis JD, Ruiz JA, Cummins KL. Modulation of axonal excitability mediated by surround electric activity: an intra-axonal study. Exp Brain Res. 1982a;47:151–153. doi: 10.1007/BF00235898. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Swadlow HA, Waxman SG, Brill MH. Variation in conduction velocity during the relative refractory and supernormal periods: a mechanism for impulse entrainment in central axons. Exp Neurol. 1979;65:230–236. doi: 10.1016/0014-4886(79)90263-2. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Waxman SG, Hildebrand C, Ruiz JA. Regenerating mammalian nerve fibres: changes in action potential waveform and firing characteristics following blockage of potassium conductance. Proc R Soc Lond B Biol Sci. 1982b;217:77–87. doi: 10.1098/rspb.1982.0095. [DOI] [PubMed] [Google Scholar]
- Koh DS, Jonas P, Vogel W. Na(+)-activated K+ channels localized in the nodal region of myelinated axons of Xenopus. J Physiol. 1994a;479(Pt 2):183–197. doi: 10.1113/jphysiol.1994.sp020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh DS, Jonas P, Brau ME, Vogel W. A TEA-insensitive flickering potassium channel active around the resting potential in myelinated nerve. J Membr Biol. 1992;130:149–162. doi: 10.1007/BF00231893. [DOI] [PubMed] [Google Scholar]
- Koh DS, Reid G, Vogel W. Activating effect of the flavonoid phloretin on Ca(2+)-activated K+ channels in myelinated nerve fibers of Xenopus laevis [corrected]. Neurosci Lett. 1994b;165:167–170. doi: 10.1016/0304-3940(94)90736-6. [DOI] [PubMed] [Google Scholar]
- Kole MH, Ilschner SU, Kampa BM, Williams SR, Ruben PC, Stuart GJ. Action potential generation requires a high sodium channel density in the axon initial segment. Nat Neurosci. 2008;11:178–186. doi: 10.1038/nn2040. [DOI] [PubMed] [Google Scholar]
- Kopysova IL, Debanne D. Critical role of axonal A-type K+ channels and axonal geometry in the gating of action potential propagation along CA3 pyramidal cell axons: a simulation study. J Neurosci. 1998;18:7436–7451. doi: 10.1523/JNEUROSCI.18-18-07436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft RH, Mitchell OR, Languis ML, Wheatley GH. Hemispheric asymmetries during six- to eight-year-olds performance of Piagetian conservation and reading tasks. Neuropsychologia. 1980;18:637–643. doi: 10.1016/0028-3932(80)90103-7. [DOI] [PubMed] [Google Scholar]
- Krarup C, Moldovan M. Nerve conduction and excitability studies in peripheral nerve disorders. Curr Opin Neurol. 2009 doi: 10.1097/WCO.0b013e3283304c9d. [DOI] [PubMed] [Google Scholar]
- Kress GJ, Mennerick S. Action potential initiation and propagation: upstream influences on neurotransmission. Neuroscience. 2009;158:211–222. doi: 10.1016/j.neuroscience.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress GJ, Dowling MJ, Meeks JP, Mennerick S. High threshold, proximal initiation, and slow conduction velocity of action potentials in dentate granule neuron mossy fibers. J Neurophysiol. 2008;100:281–291. doi: 10.1152/jn.90295.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan AV, Lin CS, Park SB, Kiernan MC. Axonal ion channels from bench to bedside: a translational neuroscience perspective. Prog Neurobiol. 2009;89:288–313. doi: 10.1016/j.pneurobio.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Kristan WB, Jr., Calabrese RL, Friesen WO. Neuronal control of leech behavior. Prog Neurobiol. 2005;76:279–327. doi: 10.1016/j.pneurobio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Krnjevic K, Miledi R. Presynaptic failure of neuromuscular propagation in rats. J Physiol. 1959;149:1–22. doi: 10.1113/jphysiol.1959.sp006321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba H, Oichi Y, Ohmori H. Presynaptic activity regulates Na(+) channel distribution at the axon initial segment. Nature. 2010;465:1075–1078. doi: 10.1038/nature09087. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Ruiz A, Rusakov DM, Scott R, Semyanov A, Walker MC. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog Biophys Mol Biol. 2005;87:33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriscak E, Trojan S, Wunsch Z. Model of spike propagation reliability along the myelinated axon corrupted by axonal intrinsic noise sources. Physiol Res. 2002;51:205–215. [PubMed] [Google Scholar]
- Kusano K. Electrical activity and structural correlates of giant nerve fibers in kuruma shrimp (Penaeus japonicus). J Cell Physiol. 1966;68:361–384. [Google Scholar]
- Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7:548–562. doi: 10.1038/nrn1938. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28:216–225. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- Lang PM, Burgstahler R, Sippel W, Irnich D, Schlotter-Weigel B, Grafe P. Characterization of neuronal nicotinic acetylcholine receptors in the membrane of unmyelinated human C-fiber axons by in vitro studies. J Neurophysiol. 2003a;90:3295–3303. doi: 10.1152/jn.00512.2003. [DOI] [PubMed] [Google Scholar]
- Lang PM, Moalem-Taylor G, Tracey D, Bostock H, Grafe P. Activity-dependent modulation of axonal excitability in unmyelinated peripheral rat nerve fibers by the 5-HT(3) serotonin receptor. J Neurophysiol. 2006 doi: 10.1152/jn.00716.2006. [DOI] [PubMed] [Google Scholar]
- Lang PM, Sippel W, Schmidbauer S, Irnich D, Grafe P. Functional evidence for P2X receptors in isolated human vagus nerve. Anesthesiology. 2003b;99:232–235. doi: 10.1097/00000542-200307000-00038. [DOI] [PubMed] [Google Scholar]
- Lang PM, Tracey DJ, Irnich D, Sippel W, Grafe P. Activation of adenosine and P2Y receptors by ATP in human peripheral nerve. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:449–457. doi: 10.1007/s00210-002-0624-0. [DOI] [PubMed] [Google Scholar]
- Lasiecka ZM, Yap CC, Vakulenko M, Winckler B. Compartmentalizing the neuronal plasma membrane from axon initial segments to synapses. Int Rev Cell Mol Biol. 2009;272:303–389. doi: 10.1016/S1937-6448(08)01607-9. [DOI] [PubMed] [Google Scholar]
- Laube G, Roper J, Pitt JC, Sewing S, Kistner U, Garner CC, Pongs O, Veh RW. Ultrastructural localization of Shaker-related potassium channel subunits and synapse-associated protein 90 to septate-like junctions in rat cerebellar Pinceaux. Brain Res Mol Brain Res. 1996;42:51–61. doi: 10.1016/s0169-328x(96)00120-9. [DOI] [PubMed] [Google Scholar]
- Le Franc Y, Le Masson G. Multiple firing patterns in deep Dorsal Horn Neurons of the spinal cord: computational analysis of mechanisms and functional implications. J Neurophysiol. 2010 doi: 10.1152/jn.00919.2009. [DOI] [PubMed] [Google Scholar]
- Le T, Verley DR, Goaillard JM, Messinger DI, Christie AE, Birmingham JT. Bistable behavior originating in the axon of a crustacean motor neuron. J Neurophysiol. 2006;95:1356–1368. doi: 10.1152/jn.00893.2005. [DOI] [PubMed] [Google Scholar]
- Lee PG, Turk PE, Yang WT, Hanlon RT. Biological characteristics and biomedical applications of the squid Sepioteuthis lessoniana cultured through multiple generations. Biol Bull. 1994;186:328–341. doi: 10.2307/1542279. [DOI] [PubMed] [Google Scholar]
- Lee US, Cui J. BK channel activation: structural and functional insights. Trends Neurosci. 2010 doi: 10.1016/j.tins.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lena C, Changeux JP, Mulle C. Evidence for “preterminal” nicotinic receptors on GABAergic axons in the rat interpeduncular nucleus. J Neurosci. 1993;13:2680–2688. doi: 10.1523/JNEUROSCI.13-06-02680.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendvai B, Vizi ES. Nonsynaptic chemical transmission through nicotinic acetylcholine receptors. Physiol Rev. 2008;88:333–349. doi: 10.1152/physrev.00040.2006. [DOI] [PubMed] [Google Scholar]
- Lestienne R. Spike timing, synchronization and information processing on the sensory side of the central nervous system. Prog Neurobiol. 2001;65:545–591. doi: 10.1016/s0301-0082(01)00019-3. [DOI] [PubMed] [Google Scholar]
- Levitan IB. Modulation of ion channels by protein phosphorylation and dephosphorylation. Annu Rev Physiol. 1994;56:193–212. doi: 10.1146/annurev.ph.56.030194.001205. [DOI] [PubMed] [Google Scholar]
- Lev-Ram V, Grinvald A. Activity-dependent calcium transients in central nervous system myelinated axons revealed by the calcium indicator Fura-2. Biophys J. 1987;52:571–576. doi: 10.1016/S0006-3495(87)83246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XG, Somogyi P, Ylinen A, Buzsaki G. The hippocampal CA3 network: an in vivo intracellular labeling study. J Comp Neurol. 1994;339:181–208. doi: 10.1002/cne.903390204. [DOI] [PubMed] [Google Scholar]
- Lim CH, Ho SM. GABAergic modulation of axonal conduction in the developing rat retinotectal pathway. Brain Res Dev Brain Res. 1998;108:299–302. doi: 10.1016/s0165-3806(98)00052-2. [DOI] [PubMed] [Google Scholar]
- Lin CS, Mogyoros I, Burke D. Recovery of excitability of cutaneous afferents in the median and sural nerves following activity. Muscle Nerve. 2000;23:763–770. doi: 10.1002/(sici)1097-4598(200005)23:5<763::aid-mus14>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Lin WH, Wright DE, Muraro NI, Baines RA. Alternative splicing in the voltage-gated sodium channel DmNav regulates activation, inactivation, and persistent current. J Neurophysiol. 2009;102:1994–2006. doi: 10.1152/jn.00613.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg PG, Feydy A, Maier MA. White matter organization in cervical spinal cord relates differently to age and control of grip force in healthy subjects. J Neurosci. 2010;30:4102–4109. doi: 10.1523/JNEUROSCI.5529-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr C, Beck A, Deitmer JW. Activity-dependent accumulation of Ca2+ in axon and dendrites of the leech Leydig neuron. Neuroreport. 2001;12:3649–3653. doi: 10.1097/00001756-200112040-00009. [DOI] [PubMed] [Google Scholar]
- Lohr R, Godenschwege T, Buchner E, Prokop A. Compartmentalization of central neurons in Drosophila: a new strategy of mosaic analysis reveals localization of presynaptic sites to specific segments of neurites. J Neurosci. 2002;22:10357–10367. doi: 10.1523/JNEUROSCI.22-23-10357.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London M, Hausser M. Dendritic computation. Annu Rev Neurosci. 2005;28:503–532. doi: 10.1146/annurev.neuro.28.061604.135703. [DOI] [PubMed] [Google Scholar]
- Lorincz A, Nusser Z. Cell-type-dependent molecular composition of the axon initial segment. J Neurosci. 2008;28:14329–14340. doi: 10.1523/JNEUROSCI.4833-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughney K, Kreber R, Ganetzky B. Molecular analysis of the para locus, a sodium channel gene in Drosophila. Cell. 1989;58:1143–1154. doi: 10.1016/0092-8674(89)90512-6. [DOI] [PubMed] [Google Scholar]
- Ludwar BC, Evans CG, Jing J, Cropper EC. Two Distinct Mechanisms Mediate Potentiating Effects of Depolarization on Synaptic Transmission. J Neurophysiol. 2009 doi: 10.1152/jn.00418.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan R. Organisation of potassium channels on the neuronal surface. J Chem Neuroanat. 2010;40:1–20. doi: 10.1016/j.jchemneu.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Luo L, Chang L, Brown SM, Ao H, Lee DH, Higuera ES, Dubin AE, Chaplan SR. Role of peripheral hyperpolarization-activated cyclic nucleotide-modulated channel pacemaker channels in acute and chronic pain models in the rat. Neuroscience. 2007;144:1477–1485. doi: 10.1016/j.neuroscience.2006.10.048. [DOI] [PubMed] [Google Scholar]
- Ma M, Koester J. Consequences and mechanisms of spike broadening of R20 cells in Aplysia californica. J Neurosci. 1995;15:6720–6734. doi: 10.1523/JNEUROSCI.15-10-06720.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabbott DJ, Noseworthy M, Bouffet E, Laughlin S, Rockel C. White matter growth as a mechanism of cognitive development in children. Neuroimage. 2006;33:936–946. doi: 10.1016/j.neuroimage.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Macagno ER, Muller KJ, Pitman RM. Conduction block silences parts of a chemical synapse in the leech central nervous system. J Physiol. 1987;387:649–664. doi: 10.1113/jphysiol.1987.sp016593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic integration of excitatory synaptic input. Nat Rev Neurosci. 2000;1:181–190. doi: 10.1038/35044552. [DOI] [PubMed] [Google Scholar]
- Magistretti J, Ragsdale DS, Alonso A. High conductance sustained single-channel activity responsible for the low-threshold persistent Na(+) current in entorhinal cortex neurons. J Neurosci. 1999;19:7334–7341. doi: 10.1523/JNEUROSCI.19-17-07334.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major G, Larkman AU, Jonas P, Sakmann B, Jack JJ. Detailed passive cable models of whole-cell recorded CA3 pyramidal neurons in rat hippocampal slices. J Neurosci. 1994;14:4613–4638. doi: 10.1523/JNEUROSCI.14-08-04613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Kocsis JD, Waxman SG. The supernormal period of the cerebellar parallel fibers effects of [Ca2+]o and [K+]o. Pflugers Arch. 1983;397:176–183. doi: 10.1007/BF00584354. [DOI] [PubMed] [Google Scholar]
- Manor Y, Koch C, Segev I. Effect of geometrical irregularities on propagation delay in axonal trees. Biophys J. 1991;60:1424–1437. doi: 10.1016/S0006-3495(91)82179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, Mertz M, Role LW. Nicotinic modulation of synaptic transmission and plasticity in cortico-limbic circuits. Semin Cell Dev Biol. 2009;20:432–440. doi: 10.1016/j.semcdb.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzano GM, Giuliano LM, Nobrega JA. A brief historical note on the classification of nerve fibers. Arq Neuropsiquiatr. 2008;66:117–119. doi: 10.1590/s0004-282x2008000100033. [DOI] [PubMed] [Google Scholar]
- Mar A, Drapeau P. Modulation of conduction block in leech mechanosensory neurons. J Neurosci. 1996;16:4335–4343. doi: 10.1523/JNEUROSCI.16-14-04335.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranto AR, Calabrese RL. Neural control of the hearts in the leech, Hirudo medicinales. II. Myogenic activity and its control by heart motoneurons. J Comp Physiol A. 1983;154:381–391. [Google Scholar]
- Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- Marder E, Thirumalai V. Cellular, synaptic and network effects of neuromodulation. Neural Netw. 2002;15:479–493. doi: 10.1016/s0893-6080(02)00043-6. [DOI] [PubMed] [Google Scholar]
- Markham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biol. 2004;1:351–363. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx T, Gisselmann G, Stortkuhl KF, Hovemann BT, Hatt H. Molecular cloning of a putative voltage- and cyclic nucleotide-gated ion channel present in the antennae and eyes of Drosophila melanogaster. Invert Neurosci. 1999;4:55–63. doi: 10.1007/pl00022368. [DOI] [PubMed] [Google Scholar]
- Mathy A, Ho SS, Davie JT, Duguid IC, Clark BA, Hausser M. Encoding of oscillations by axonal bursts in inferior olive neurons. Neuron. 2009;62:388–399. doi: 10.1016/j.neuron.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer C, Quasthoff S, Grafe P. Confocal imaging reveals activity-dependent intracellular Ca2+ transients in nociceptive human C fibres. Pain. 1999;81:317–322. doi: 10.1016/S0304-3959(99)00015-9. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW. Presynaptic ionotropic receptors. Curr Opin Neurobiol. 1996;6:342–349. doi: 10.1016/s0959-4388(96)80118-8. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Richardson AG, Grill WM. Modeling the excitability of mammalian nerve fibers: influence of afterpotentials on the recovery cycle. J Neurophysiol. 2002;87:995–1006. doi: 10.1152/jn.00353.2001. [DOI] [PubMed] [Google Scholar]
- McMahon LL, Yoon KW, Chiappinelli VA. Nicotinic receptor activation facilitates GABAergic neurotransmission in the avian lateral spiriform nucleus. Neuroscience. 1994;59:689–698. doi: 10.1016/0306-4522(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Meech RW, Mackie GO. Ionic currents in giant motor axons of the jellyfish, Aglantha digitale. J Neurophysiol. 1993;69:884–893. doi: 10.1152/jn.1993.69.3.884. [DOI] [PubMed] [Google Scholar]
- Meeks JP, Mennerick S. Selective effects of potassium elevations on glutamate signaling and action potential conduction in hippocampus. J Neurosci. 2004;24:197–206. doi: 10.1523/JNEUROSCI.4845-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks JP, Mennerick S. Action potential initiation and propagation in CA3 pyramidal axons. J Neurophysiol. 2007;97:3460–3472. doi: 10.1152/jn.01288.2006. [DOI] [PubMed] [Google Scholar]
- Merighi A. Costorage and coexistence of neuropeptides in the mammalian CNS. Prog Neurobiol. 2002;66:161–190. doi: 10.1016/s0301-0082(01)00031-4. [DOI] [PubMed] [Google Scholar]
- Mert T. Kv1 channels in signal conduction of myelinated nerve fibers. Rev Neurosci. 2006;17:369–374. doi: 10.1515/revneuro.2006.17.3.369. [DOI] [PubMed] [Google Scholar]
- Mert T. Roles of axonal voltage-dependent ion channels in damaged peripheral nerves. Eur J Pharmacol. 2007;568:25–30. doi: 10.1016/j.ejphar.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Meyrand P, Weimann JM, Marder E. Multiple axonal spike initiation zones in a motor neuron: serotonin activation. J Neurosci. 1992;12:2803–2812. doi: 10.1523/JNEUROSCI.12-07-02803.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis M, Vogel C, Blenk KH, Janig W. Algesics excite axotomised afferent nerve fibres within the first hours following nerve transection in rats. Pain. 1997;72:347–354. doi: 10.1016/s0304-3959(97)00050-x. [DOI] [PubMed] [Google Scholar]
- Middleton SJ, Racca C, Cunningham MO, Traub RD, Monyer H, Knopfel T, Schofield IS, Jenkins A, Whittington MA. High-frequency network oscillations in cerebellar cortex. Neuron. 2008;58:763–774. doi: 10.1016/j.neuron.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RN, Rinzel J. The dependence of impulse propagation speed on firing frequency, dispersion, for the Hodgkin-Huxley model. Biophys J. 1981;34:227–259. doi: 10.1016/S0006-3495(81)84847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TA, Kiernan MC, Mogyoros I, Burke D. Activity-dependent changes in impulse conduction in normal human cutaneous axons. Brain. 1995;118(Pt 5):1217–1224. doi: 10.1093/brain/118.5.1217. [DOI] [PubMed] [Google Scholar]
- Misonou H, Menegola M, Buchwalder L, Park EW, Meredith A, Rhodes KJ, Aldrich RW, Trimmer JS. Immunolocalization of the Ca2+-activated K+ channel Slo1 in axons and nerve terminals of mammalian brain and cultured neurons. J Comp Neurol. 2006;496:289–302. doi: 10.1002/cne.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Ichinohe N, Rockland KS. Differential modes of termination of amygdalothalamic and amygdalocortical projections in the monkey. J Comp Neurol. 2007;502:309–324. doi: 10.1002/cne.21304. [DOI] [PubMed] [Google Scholar]
- Moalem G, Grafe P, Tracey DJ. Chemical mediators enhance the excitability of unmyelinated sensory axons in normal and injured peripheral nerve of the rat. Neuroscience. 2005;134:1399–1411. doi: 10.1016/j.neuroscience.2005.05.046. [DOI] [PubMed] [Google Scholar]
- Moalem-Taylor G, Lang PM, Tracey DJ, Grafe P. Post-spike excitability indicates changes in membrane potential of isolated C-fibers. Muscle Nerve. 2007;36:172–182. doi: 10.1002/mus.20793. [DOI] [PubMed] [Google Scholar]
- Moldovan M, Krarup C. Evaluation of Na+/K+ pump function following repetitive activity in mouse peripheral nerve. J Neurosci Methods. 2006;155:161–171. doi: 10.1016/j.jneumeth.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Monsivais P, Clark BA, Roth A, Hausser M. Determinants of action potential propagation in cerebellar Purkinje cell axons. J Neurosci. 2005;25:464–472. doi: 10.1523/JNEUROSCI.3871-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moortgat KT, Keller CH, Bullock TH, Sejnowski TJ. Submicrosecond pacemaker precision is behaviorally modulated: the gymnotiform electromotor pathway. Proc Natl Acad Sci U S A. 1998;95:4684–4689. doi: 10.1073/pnas.95.8.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradmand K, Goldfinger MD. Computation of long-distance propagation of impulses elicited by Poisson-process stimulation. J Neurophysiol. 1995;74:2415–2426. doi: 10.1152/jn.1995.74.6.2415. [DOI] [PubMed] [Google Scholar]
- Morris ME, Di Costanzo GA, Fox S, Werman R. Depolarizing action of GABA (gamma-aminobutyric acid) on myelinated fibers of peripheral nerves. Brain Res. 1983;278:117–126. doi: 10.1016/0006-8993(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Muschol M, Kosterin P, Ichikawa M, Salzberg BM. Activity-dependent depression of excitability and calcium transients in the neurohypophysis suggests a model of “stuttering conduction”. J Neurosci. 2003;23:11352–11362. doi: 10.1523/JNEUROSCI.23-36-11352.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy F, Dickinson PS, Moulins M. Rhythmical synaptic control of axonal conduction in a lobster motor neuron. J Neurophysiol. 1981;45:1109–1124. doi: 10.1152/jn.1981.45.6.1109. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci. 2004;16:1227–1233. doi: 10.1162/0898929041920441. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Fehlings MG. Mechanisms of axonal dysfunction after spinal cord injury: with an emphasis on the role of voltage-gated potassium channels. Brain Res Brain Res Rev. 2001;38:165–191. doi: 10.1016/s0165-0173(01)00134-5. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Jones OT, Fehlings MG. Abnormal axonal physiology is associated with altered expression and distribution of Kv1.1 and Kv1.2 K+ channels after chronic spinal cord injury. Eur J Neurosci. 2000;12:491–506. doi: 10.1046/j.1460-9568.2000.00926.x. [DOI] [PubMed] [Google Scholar]
- Neiman AB, Yakusheva TA, Russell DF. Noise-induced transition to bursting in responses of paddlefish electroreceptor afferents. J Neurophysiol. 2007;98:2795–2806. doi: 10.1152/jn.01289.2006. [DOI] [PubMed] [Google Scholar]
- Nelson SB, Turrigiano GG. Strength through diversity. Neuron. 2008;60:477–482. doi: 10.1016/j.neuron.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev VO, Bunemann M, Schmitteckert E, Lohse MJ, Engelhardt S. Cyclic AMP imaging in adult cardiac myocytes reveals far-reaching beta1-adrenergic but locally confined beta2-adrenergic receptor-mediated signaling. Circ Res. 2006;99:1084–1091. doi: 10.1161/01.RES.0000250046.69918.d5. [DOI] [PubMed] [Google Scholar]
- Niogi SN, McCandliss BD. Left lateralized white matter microstructure accounts for individual differences in reading ability and disability. Neuropsychologia. 2006;44:2178–2188. doi: 10.1016/j.neuropsychologia.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J Comp Neurol. 2004;471:241–276. doi: 10.1002/cne.11039. [DOI] [PubMed] [Google Scholar]
- Nusbaum MP, Beenhakker MP. A small-systems approach to motor pattern generation. Nature. 2002;417:343–350. doi: 10.1038/417343a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E. The roles of co-transmission in neural network modulation. Trends Neurosci. 2001;24:146–154. doi: 10.1016/s0166-2236(00)01723-9. [DOI] [PubMed] [Google Scholar]
- Nusser Z. Variability in the subcellular distribution of ion channels increases neuronal diversity. Trends Neurosci. 2009;32:267–274. doi: 10.1016/j.tins.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Obreja O, Ringkamp M, Namer B, Forsch E, Klusch A, Rukwied R, Petersen M, Schmelz M. Patterns of activity-dependent conduction velocity changes differentiate classes of unmyelinated mechano-insensitive afferents including cold nociceptors, in pig and in human. Pain. 2010;148:59–69. doi: 10.1016/j.pain.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Olsen O, Nadim F, Hill AA, Edwards DH. Uniform growth and neuronal integration. J Neurophysiol. 1996;76:1850–1857. doi: 10.1152/jn.1996.76.3.1850. [DOI] [PubMed] [Google Scholar]
- Oozeer M, Veraart C, Legat V, Delbeke J. A model of the mammalian optic nerve fibre based on experimental data. Vision Res. 2006;46:2513–2524. doi: 10.1016/j.visres.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Oswald AM, Chacron MJ, Doiron B, Bastian J, Maler L. Parallel processing of sensory input by bursts and isolated spikes. J Neurosci. 2004;24:4351–4362. doi: 10.1523/JNEUROSCI.0459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouardouz M, Nikolaeva MA, Coderre E, Zamponi GW, McRory JE, Trapp BD, Yin X, Wang W, Woulfe J, Stys PK. Depolarization-induced Ca2+ release in ischemic spinal cord white matter involves L-type Ca2+ channel activation of ryanodine receptors. Neuron. 2003;40:53–63. doi: 10.1016/j.neuron.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Ouyang Q, Goeritz M, Harris-Warrick RM. Panulirus interruptus Ih-channel gene PIIH: modification of channel properties by alternative splicing and role in rhythmic activity. J Neurophysiol. 2007;97:3880–3892. doi: 10.1152/jn.00246.2007. [DOI] [PubMed] [Google Scholar]
- Ozaita A, Martone ME, Ellisman MH, Rudy B. Differential subcellular localization of the two alternatively spliced isoforms of the Kv3.1 potassium channel subunit in brain. J Neurophysiol. 2002;88:394–408. doi: 10.1152/jn.2002.88.1.394. [DOI] [PubMed] [Google Scholar]
- Palani D, Baginskas A, Raastad M. Bursts and hyperexcitability in non-myelinated axons of the rat hippocampus. Neuroscience. 2010 doi: 10.1016/j.neuroscience.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Panzeri S, Brunel N, Logothetis NK, Kayser C. Sensory neural codes using multiplexed temporal scales. Trends Neurosci. 2010;33:111–120. doi: 10.1016/j.tins.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Papatheodoropoulos C. A possible role of ectopic action potentials in the in vitro hippocampal sharp wave-ripple complexes. Neuroscience. 2008;157:495–501. doi: 10.1016/j.neuroscience.2008.09.040. [DOI] [PubMed] [Google Scholar]
- Paradiso K, Wu LG. Small voltage changes at nerve terminals travel up axons to affect action potential initiation. Nat Neurosci. 2009;12:541–543. doi: 10.1038/nn.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas I. Differential block at high frequency of branches of a single axon innervating two muscles. J Neurophysiol. 1972;35:903–914. doi: 10.1152/jn.1972.35.6.903. [DOI] [PubMed] [Google Scholar]
- Peters A, Muir AR. The relationship between axons and Schwann cells during development of peripheral nerves in the rat. Q J Exp Physiol Cogn Med Sci. 1959;44:117–130. doi: 10.1113/expphysiol.1959.sp001366. [DOI] [PubMed] [Google Scholar]
- Pinault D. Antidromic firing occurs spontaneously on thalamic relay neurons: triggering of ectopic action potentials by somatic intrinsic burst discharges. Neuroscience. 1990;34:281–292. doi: 10.1016/0306-4522(90)90138-t. [DOI] [PubMed] [Google Scholar]
- Pinault D. Backpropagation of action potentials generated at ectopic axonal loci: hypothesis that axon terminals integrate local environmental signals. Brain Res Brain Res Rev. 1995;21:42–92. doi: 10.1016/0165-0173(95)00004-m. [DOI] [PubMed] [Google Scholar]
- Pinault D, Pumain R. Antidromic firing occurs spontaneously on thalamic relay neurons: triggering of somatic intrinsic burst discharges by ectopic action potentials. Neuroscience. 1989;31:625–637. doi: 10.1016/0306-4522(89)90428-4. [DOI] [PubMed] [Google Scholar]
- Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nat Rev Neurosci. 2003;4:968–980. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- Poulter MO, Hashiguchi T, Padjen AL. An examination of frog myelinated axons using intracellular microelectrode recording: the role of voltage-dependent and leak conductances on the steady-state electrical properties. J Neurophysiol. 1993;70:2301–2312. doi: 10.1152/jn.1993.70.6.2301. [DOI] [PubMed] [Google Scholar]
- Poulter MO, Hashiguchi T, Padjen AL. Evidence for a sodium-dependent potassium conductance in frog myelinated axon. Neuroscience. 1995;68:487–495. doi: 10.1016/0306-4522(95)00138-9. [DOI] [PubMed] [Google Scholar]
- Pujol J, Soriano-Mas C, Ortiz H, Sebastian-Galles N, Losilla JM, Deus J. Myelination of language-related areas in the developing brain. Neurology. 2006;66:339–343. doi: 10.1212/01.wnl.0000201049.66073.8d. [DOI] [PubMed] [Google Scholar]
- Quasthoff S, Grosskreutz J, Schroder JM, Schneider U, Grafe P. Calcium potentials and tetrodotoxin-resistant sodium potentials in unmyelinated C fibres of biopsied human sural nerve. Neuroscience. 1995;69:955–965. doi: 10.1016/0306-4522(95)00307-5. [DOI] [PubMed] [Google Scholar]
- Rall W. Branching dendritic trees and motoneuron membrane resistivity. Exp Neurol. 1959;1:491–527. doi: 10.1016/0014-4886(59)90046-9. [DOI] [PubMed] [Google Scholar]
- Rall W. Time constants and electrotonic length of membrane cylinders and neurons. Biophys J. 1969;9:1483–1508. doi: 10.1016/S0006-3495(69)86467-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper JA. Nonimpulse-mediated synaptic transmission during the generation of a cyclic motor program. Science. 1979;205:304–306. doi: 10.1126/science.221982. [DOI] [PubMed] [Google Scholar]
- Rasband MN. The axon initial segment and the maintenance of neuronal polarity. Nat Rev Neurosci. 2010;11:552–562. doi: 10.1038/nrn2852. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Trimmer JS. Developmental clustering of ion channels at and near the node of Ranvier. Dev Biol. 2001;236:5–16. doi: 10.1006/dbio.2001.0326. [DOI] [PubMed] [Google Scholar]
- Raymond SA. Effects of nerve impulses on threshold of frog sciatic nerve fibres. J Physiol. 1979;290:273–303. doi: 10.1113/jphysiol.1979.sp012771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G, Scholz A, Bostock H, Vogel W. Human axons contain at least five types of voltage-dependent potassium channel. J Physiol. 1999;518(Pt 3):681–696. doi: 10.1111/j.1469-7793.1999.0681p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renganathan M, Cummins TR, Waxman SG. Contribution of Na(v)1.8 sodium channels to action potential electrogenesis in DRG neurons. J Neurophysiol. 2001;86:629–640. doi: 10.1152/jn.2001.86.2.629. [DOI] [PubMed] [Google Scholar]
- Rettig J, Heinemann SH, Wunder F, Lorra C, Parcej DN, Dolly JO, Pongs O. Inactivation properties of voltage-gated K+ channels altered by presence of beta-subunit. Nature. 1994;369:289–294. doi: 10.1038/369289a0. [DOI] [PubMed] [Google Scholar]
- Rhodes KJ, Strassle BW, Monaghan MM, Bekele-Arcuri Z, Matos MF, Trimmer JS. Association and colocalization of the Kvbeta1 and Kvbeta2 beta-subunits with Kv1 alpha-subunits in mammalian brain K+ channel complexes. J Neurosci. 1997;17:8246–8258. doi: 10.1523/JNEUROSCI.17-21-08246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich TC, Fagan KA, Tse TE, Schaack J, Cooper DM, Karpen JW. A uniform extracellular stimulus triggers distinct cAMP signals in different compartments of a simple cell. Proc Natl Acad Sci U S A. 2001;98:13049–13054. doi: 10.1073/pnas.221381398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinke I, Artmann J, Stein V. ClC-2 voltage-gated channels constitute part of the background conductance and assist chloride extrusion. J Neurosci. 2010;30:4776–4786. doi: 10.1523/JNEUROSCI.6299-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley SH, Bush BM, Roberts A. Crab muscle receptor which responds without impulses. Nature. 1968;218:1170–1171. doi: 10.1038/2181170a0. [DOI] [PubMed] [Google Scholar]
- Ritchie JM. On the relation between fibre diameter and conduction velocity in myelinated nerve fibres. Proc R Soc Lond B Biol Sci. 1982;217:29–35. doi: 10.1098/rspb.1982.0092. [DOI] [PubMed] [Google Scholar]
- Robert A, Jirounek P. Axonal and glial currents activated during the post-tetanic hyperpolarization in non-myelinated nerve. Pflugers Arch. 1998;436:529–537. doi: 10.1007/s004240050668. [DOI] [PubMed] [Google Scholar]
- Roberts A, Bush BMH. Neurons Without Impulses. Cambridge University Press; Cambridge, UK: 1981. [Google Scholar]
- Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- Rocha-Gonzalez HI, Mao S, Alvarez-Leefmans FJ. Na+,K+,2Cl- cotransport and intracellular chloride regulation in rat primary sensory neurons: thermodynamic and kinetic aspects. J Neurophysiol. 2008;100:169–184. doi: 10.1152/jn.01007.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roots BI. The phylogeny of invertebrates and the evolution of myelin. Neuron Glia Biol. 2008;4:101–109. doi: 10.1017/S1740925X0900012X. [DOI] [PubMed] [Google Scholar]
- Rosenbluth J. Multiple functions of the paranodal junction of myelinated nerve fibers. J Neurosci Res. 2009;87:3250–3258. doi: 10.1002/jnr.22013. [DOI] [PubMed] [Google Scholar]
- Rosenthal JJ, Bezanilla F. Seasonal variation in conduction velocity of action potentials in squid giant axons. Biol Bull. 2000;199:135–143. doi: 10.2307/1542873. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Fabian-Fine R, Scott R, Walker MC, Rusakov DA, Kullmann DM. GABAa receptors at hippocampal mossy fibers. Neuron. 2003;39:961–973. doi: 10.1016/s0896-6273(03)00559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton WA. A theory of the effects of fibre size in medullated nerve. J Physiol. 1951;115:101–122. doi: 10.1113/jphysiol.1951.sp004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safronov BV, Kampe K, Vogel W. Single voltage-dependent potassium channels in rat peripheral nerve membrane. J Physiol. 1993;460:675–691. doi: 10.1113/jphysiol.1993.sp019493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber ES. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol. 2002;66:345–353. doi: 10.1016/s0301-0082(02)00004-7. [DOI] [PubMed] [Google Scholar]
- Sakatani K, Black JA, Kocsis JD. Transient presence and functional interaction of endogenous GABA and GABAa receptors in developing rat optic nerve. Proc R Soc Lond B Biol Sci. 1992;247:155–161. doi: 10.1098/rspb.1992.0022. [DOI] [PubMed] [Google Scholar]
- Sakatani K, Chesler M, Hassan AZ. GABAa receptors modulate axonal conduction in dorsal columns of neonatal rat spinal cord. Brain Res. 1991a;542:273–279. doi: 10.1016/0006-8993(91)91578-o. [DOI] [PubMed] [Google Scholar]
- Sakatani K, Chesler M, Hassan AZ, Lee M, Young W. Non-synaptic modulation of dorsal column conduction by endogenous GABA in neonatal rat spinal cord. Brain Res. 1993;622:43–50. doi: 10.1016/0006-8993(93)90799-s. [DOI] [PubMed] [Google Scholar]
- Sakatani K, Hassan AZ, Chesler M. GABA-sensitivity of dorsal column axons: an in vitro comparison between adult and neonatal rat spinal cords. Brain Res Dev Brain Res. 1991b;61:139–142. doi: 10.1016/0165-3806(91)90123-z. [DOI] [PubMed] [Google Scholar]
- Sakatani K, Hassan AZ, Chesler M. Effects of GABA on axonal conduction and extracellular potassium activity in the neonatal rat optic nerve. Exp Neurol. 1994;127:291–297. doi: 10.1006/exnr.1994.1105. [DOI] [PubMed] [Google Scholar]
- Sakatani K, Hassan AZ, Ching W. Age-dependent extrasynaptic modulation of axonal conduction by exogenous and endogenous GABA in the rat optic nerve. Exp Neurol. 1991c;114:307–314. doi: 10.1016/0014-4886(91)90156-7. [DOI] [PubMed] [Google Scholar]
- Salami M, Itami C, Tsumoto T, Kimura F. Change of conduction velocity by regional myelination yields constant latency irrespective of distance between thalamus and cortex. Proc Natl Acad Sci U S A. 2003;100:6174–6179. doi: 10.1073/pnas.0937380100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salio C, Lossi L, Ferrini F, Merighi A. Neuropeptides as synaptic transmitters. Cell Tissue Res. 2006;326:583–598. doi: 10.1007/s00441-006-0268-3. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Matsuki N, Ikegaya Y. Action-potential modulation during axonal conduction. Science. 2011;331:599–601. doi: 10.1126/science.1197598. [DOI] [PubMed] [Google Scholar]
- Schenk U, Matteoli M. Presynaptic AMPA receptors: more than just ion channels? Biol Cell. 2004;96:257–260. doi: 10.1016/j.biolcel.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Schmidt J, Grund M. Rhythmic activity in a motor axon induced by axotomy. Neuroreport. 2003;14:1267–1271. doi: 10.1097/00001756-200307010-00016. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Schuchmann S, Fisahn A, Draguhn A, Buhl EH, Petrasch-Parwez E, Dermietzel R, Heinemann U, Traub RD. Axo-axonal coupling. a novel mechanism for ultrafast neuronal communication. Neuron. 2001;31:831–840. doi: 10.1016/s0896-6273(01)00410-x. [DOI] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H. Training induces changes in white-matter architecture. Nature Neuroscience. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JR, Glassmeier G, Cooper EC, Kao TC, Nodera H, Tabuena D, Kaji R, Bostock H. KCNQ channels mediate IKs, a slow K+ current regulating excitability in the rat node of Ranvier. J Physiol. 2006;573:17–34. doi: 10.1113/jphysiol.2006.106815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LL, Hage TA, Golding NL. Weak action potential backpropagation is associated with high-frequency axonal firing capability in principal neurons of the gerbil medial superior olive. J Physiol. 2007;583:647–661. doi: 10.1113/jphysiol.2007.136366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuri R, Lombardo P, Cataldo E, Ristori C, Brunelli M. Inhibition of Na+/K+ ATPase potentiates synaptic transmission in tactile sensory neurons of the leech. Eur J Neurosci. 2007;25:159–167. doi: 10.1111/j.1460-9568.2006.05257.x. [DOI] [PubMed] [Google Scholar]
- Segev I. Computer study of presynaptic inhibition controlling the spread of action potentials into axonal terminals. J Neurophysiol. 1990;63:987–998. doi: 10.1152/jn.1990.63.5.987. [DOI] [PubMed] [Google Scholar]
- Segev I, Schneidman E. Axons as computing devices: basic insights gained from models. J Physiol Paris. 1999;93:263–270. doi: 10.1016/s0928-4257(00)80055-8. [DOI] [PubMed] [Google Scholar]
- Sengupta B, Stemmler M, Laughlin SB, Niven JE. Action potential energy efficiency varies among neuron types in vertebrates and invertebrates. PLoS Comput Biol. 2010;6:e1000840. doi: 10.1371/journal.pcbi.1000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra J, Campero M, Ochoa J, Bostock H. Activity-dependent slowing of conduction differentiates functional subtypes of C fibres innervating human skin. J Physiol. 1999;515(Pt 3):799–811. doi: 10.1111/j.1469-7793.1999.799ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MM, Migliore M, Valencia I, Cooper EC, Brown DA. Functional significance of axonal Kv7 channels in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 2008;105:7869–7874. doi: 10.1073/pnas.0802805105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ, Stryker MP. Ocular dominance in layer IV of the cat's visual cortex and the effects of monocular deprivation. J Physiol. 1978;281:267–283. doi: 10.1113/jphysiol.1978.sp012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield ME, Best TK, Mensh BD, Kath WL, Spruston N. Slow integration leads to persistent action potential firing in distal axons of coupled interneurons. Nat Neurosci. 2011;14:200–207. doi: 10.1038/nn.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Raastad M, Andersen P. General and variable features of varicosity spacing along unmyelinated axons in the hippocampus and cerebellum. Proc Natl Acad Sci U S A. 2002;99:6340–6345. doi: 10.1073/pnas.052151299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Duque A, Yu Y, Haider B, McCormick DA. Properties of action-potential initiation in neocortical pyramidal cells: evidence from whole cell axon recordings. J Neurophysiol. 2007a;97:746–760. doi: 10.1152/jn.00922.2006. [DOI] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, Duque A, Yu Y, McCormick DA. Modulation of intracortical synaptic potentials by presynaptic somatic membrane potential. Nature. 2006;441:761–765. doi: 10.1038/nature04720. [DOI] [PubMed] [Google Scholar]
- Shu Y, Yu Y, Yang J, McCormick DA. Selective control of cortical axonal spikes by a slowly inactivating K+ current. Proc Natl Acad Sci U S A. 2007b;104:11453–11458. doi: 10.1073/pnas.0702041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidiropoulou K, Pissadaki EK, Poirazi P. Inside the brain of a neuron. EMBO Rep. 2006;7:886–892. doi: 10.1038/sj.embor.7400789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirevaag AM, Greenough WT. Differential rearing effects on rat visual cortex synapses. III. Neuronal and glial nuclei, boutons, dendrites, and capillaries. Brain Res. 1987;424:320–332. doi: 10.1016/0006-8993(87)91477-6. [DOI] [PubMed] [Google Scholar]
- Skiebe P, Ganeshina O. Synaptic neuropil in nerves of the crustacean stomatogastric nervous system: an immunocytochemical and electron microscopical study. J Comp Neurol. 2000;420:373–397. [PubMed] [Google Scholar]
- Skou JC. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta. 1957;23:394–401. doi: 10.1016/0006-3002(57)90343-8. [DOI] [PubMed] [Google Scholar]
- Skou JC. The Na,K-pump. Methods Enzymol. 1988;156:1–25. doi: 10.1016/0076-6879(88)56004-4. [DOI] [PubMed] [Google Scholar]
- Smith DO. Mechanisms of action potential propagation failure at sites of axon branching in the crayfish. J Physiol. 1980;301:243–259. doi: 10.1113/jphysiol.1980.sp013202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RD, Goldin AL. Functional analysis of the rat I sodium channel in xenopus oocytes. J Neurosci. 1998;18:811–820. doi: 10.1523/JNEUROSCI.18-03-00811.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleng AF, Baginskas A, Andersen P, Raastad M. Activity-dependent excitability changes in hippocampal CA3 cell Schaffer axons. J Physiol. 2004;560:491–503. doi: 10.1113/jphysiol.2004.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleng AF, Chiu K, Raastad M. Unmyelinated axons in the rat hippocampus hyperpolarize and activate an H current when spike frequency exceeds 1 Hz. J Physiol. 2003;552:459–470. doi: 10.1113/jphysiol.2003.048058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears N, Meyer JS, Whaling CS, Wade GN, Zucker I, Dark J. Long day lengths enhance myelination of midbrain and hindbrain regions of developing meadow voles. Brain Res Dev Brain Res. 1990;55:103–108. doi: 10.1016/0165-3806(90)90110-k. [DOI] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci. 2008;9:206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Stampfli R. Saltatory conduction in nerve. Physiol Rev. 1954;34:101–112. doi: 10.1152/physrev.1954.34.1.101. [DOI] [PubMed] [Google Scholar]
- Standaert FG. Post-tetanic repetitive activity in the cat soleus nerve: its origin, course, and mechanism of generation. J Gen Physiol. 1963;47:53–70. doi: 10.1085/jgp.47.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert FG. The mechanisms of post-tetanic potentiation in cat soleus and gastrocnemius muscles. J Gen Physiol. 1964;47:987–1001. doi: 10.1085/jgp.47.5.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford LR. Conduction velocity variations minimize conduction time differences among retinal ganglion cell axons. Science. 1987;238:358–360. doi: 10.1126/science.3659918. [DOI] [PubMed] [Google Scholar]
- Stein W. Modulation of stomatogastric rhythms. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009;195:989–1009. doi: 10.1007/s00359-009-0483-y. [DOI] [PubMed] [Google Scholar]
- Stephanova DI. Conduction along myelinated and demyelinated nerve fibres with a reorganized axonal membrane during the recovery cycle: model investigations. Biol Cybern. 1990;64:129–134. doi: 10.1007/BF02331341. [DOI] [PubMed] [Google Scholar]
- Stettler DD, Yamahachi H, Li W, Denk W, Gilbert CD. Axons and synaptic boutons are highly dynamic in adult visual cortex. Neuron. 2006;49:877–887. doi: 10.1016/j.neuron.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Stockbridge N, Yamoah N. Excitability changes in the crustacean motor axons following activity. J Math Biol. 1990;28:487–499. doi: 10.1007/BF00164160. [DOI] [PubMed] [Google Scholar]
- Stohr M. Activity-dependent variations in threshold and conduction velocity of human sensory fibers. J Neurol Sci. 1981;49:47–54. doi: 10.1016/0022-510x(81)90187-8. [DOI] [PubMed] [Google Scholar]
- Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Strupp M, Grafe P. A chloride channel in rat and human axons. Neurosci Lett. 1991;133:237–240. doi: 10.1016/0304-3940(91)90578-h. [DOI] [PubMed] [Google Scholar]
- Stys PK, Waxman SG. Activity-dependent modulation of excitability: implications for axonal physiology and pathophysiology. Muscle Nerve. 1994;17:969–974. doi: 10.1002/mus.880170902. [DOI] [PubMed] [Google Scholar]
- Stys PK, Sontheimer H, Ransom BR, Waxman SG. Noninactivating, tetrodotoxin-sensitive Na+ conductance in rat optic nerve axons. Proc Natl Acad Sci U S A. 1993;90:6976–6980. doi: 10.1073/pnas.90.15.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara I, Lang EJ, Llinas R. Uniform olivocerebellar conduction time underlies Purkinje cell complex spike synchronicity in the rat cerebellum. J Physiol. 1993;470:243–271. doi: 10.1113/jphysiol.1993.sp019857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino K, Hempel CM, Miller MN, Hattox AM, Shapiro P, Wu C, Huang ZJ, Nelson SB. Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nat Neurosci. 2006;9:99–107. doi: 10.1038/nn1618. [DOI] [PubMed] [Google Scholar]
- Sun BB, Chiu SY. N-type calcium channels and their regulation by GABAB receptors in axons of neonatal rat optic nerve. J Neurosci. 1999;19:5185–5194. doi: 10.1523/JNEUROSCI.19-13-05185.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA. Systematic variations in the conduction velocity of slowly conducting axons in the rabbit corpus callosum. Exp Neurol. 1974;43:445–451. doi: 10.1016/0014-4886(74)90183-6. [DOI] [PubMed] [Google Scholar]
- Swadlow HA. Impulse conduction in the mammalian brain: physiological properties of individual axons monitored for several months. Science. 1982;218:911–913. doi: 10.1126/science.7134984. [DOI] [PubMed] [Google Scholar]
- Swadlow HA. Physiological properties of individual cerebral axons studied in vivo for as long as one year. J Neurophysiol. 1985;54:1346–1362. doi: 10.1152/jn.1985.54.5.1346. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Waxman SG. Observations on impulse conduction along central axons. Proc Natl Acad Sci U S A. 1975;72:5156–5159. doi: 10.1073/pnas.72.12.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA, Waxman SG. Variations in conduction velocity and excitability following single and multiple impulses of visual callosal axons in the rabbit. Exp Neurol. 1976;53:128–150. doi: 10.1016/0014-4886(76)90288-0. [DOI] [PubMed] [Google Scholar]
- Swadlow HA, Kocsis JD, Waxman SG. Modulation of impulse conduction along the axonal tree. Annu Rev Biophys Bioeng. 1980;9:143–179. doi: 10.1146/annurev.bb.09.060180.001043. [DOI] [PubMed] [Google Scholar]
- Szalay F. Development of the equine brain motor system. Neurobiology (Bp) 2001;9:107–135. doi: 10.1556/neurob.9.2001.2.4. [DOI] [PubMed] [Google Scholar]
- Szeligo F, Leblond CP. Response of the three main types of glial cells of cortex and corpus callosum in rats handled during suckling or exposed to enriched, control and impoverished environments following weaning. J Comp Neurol. 1977;172:247–263. doi: 10.1002/cne.901720205. [DOI] [PubMed] [Google Scholar]
- Taddese A, Bean BP. Subthreshold sodium current from rapidly inactivating sodium channels drives spontaneous firing of tuberomammillary neurons. Neuron. 2002;33:587–600. doi: 10.1016/s0896-6273(02)00574-3. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Slow and fast groups of pyramidal tract cells and their respective membrane properties. J Neurophysiol. 1965;28:908–924. doi: 10.1152/jn.1965.28.5.908. [DOI] [PubMed] [Google Scholar]
- Takigawa T, Alzheimer C, Quasthoff S, Grafe P. A specific blocker reveals the presence and function of the hyperpolarization-activated cation current IH in peripheral mammalian nerve fibres. Neuroscience. 1998;82:631–634. doi: 10.1016/s0306-4522(97)00383-7. [DOI] [PubMed] [Google Scholar]
- Tal D, Jacobson E, Lyakhov V, Marom S. Frequency tuning of input-output relation in a rat cortical neuron in-vitro. Neurosci Lett. 2001;300:21–24. doi: 10.1016/s0304-3940(01)01534-8. [DOI] [PubMed] [Google Scholar]
- Tauber H, Waehneldt TV, Neuhoff V. Myelination in rabbit optic nerves is accelerated by artificial eye opening. Neurosci Lett. 1980;16:235–238. doi: 10.1016/0304-3940(80)90003-8. [DOI] [PubMed] [Google Scholar]
- Taylor AL, Goaillard JM, Marder E. How multiple conductances determine electrophysiological properties in a multicompartment model. J Neurosci. 2009;29:5573–5586. doi: 10.1523/JNEUROSCI.4438-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Burke D, Heywood J. Physiological evidence for a slow K+ conductance in human cutaneous afferents. J Physiol. 1992;453:575–589. doi: 10.1113/jphysiol.1992.sp019245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore C, Brunjes PC. A comparative study of myelination in precocial and altricial murid rodents. Brain Res. 1988;471:139–147. doi: 10.1016/0165-3806(88)90159-9. [DOI] [PubMed] [Google Scholar]
- Theunissen F, Miller JP. Temporal encoding in nervous systems: a rigorous definition. J Comput Neurosci. 1995;2:149–162. doi: 10.1007/BF00961885. [DOI] [PubMed] [Google Scholar]
- Thio LL, Yamada KA. Differential presynaptic modulation of excitatory and inhibitory autaptic currents in cultured hippocampal neurons. Brain Res. 2004;1012:22–28. doi: 10.1016/j.brainres.2004.02.077. [DOI] [PubMed] [Google Scholar]
- Thompson SM, Capogna M, Scanziani M. Presynaptic inhibition in the hippocampus. Trends Neurosci. 1993;16:222–227. doi: 10.1016/0166-2236(93)90160-n. [DOI] [PubMed] [Google Scholar]
- Tippens AL, Pare JF, Langwieser N, Moosmang S, Milner TA, Smith Y, Lee A. Ultrastructural evidence for pre- and postsynaptic localization of Cav1.2 L-type Ca2+ channels in the rat hippocampus. J Comp Neurol. 2008;506:569–583. doi: 10.1002/cne.21567. [DOI] [PubMed] [Google Scholar]
- Toennies JF. Reflex discharge from the spinal cord over the dorsal roots. J Neurophysiol. 1938;1:378–390. [Google Scholar]
- Tokuno HA, Kocsis JD, Waxman SG. Noninactivating, tetrodotoxin-sensitive Na+ conductance in peripheral axons. Muscle Nerve. 2003;28:212–217. doi: 10.1002/mus.10421. [DOI] [PubMed] [Google Scholar]
- Tomlinson S, Burke D, Hanna M, Koltzenburg M, Bostock H. In vivo assessment of HCN channel current (I(h)) in human motor axons. Muscle Nerve. 2010;41:247–256. doi: 10.1002/mus.21482. [DOI] [PubMed] [Google Scholar]
- Traub RD, Draguhn A, Whittington MA, Baldeweg T, Bibbig A, Buhl EH, Schmitz D. Axonal gap junctions between principal neurons: a novel source of network oscillations, and perhaps epileptogenesis. Rev Neurosci. 2002;13:1–30. doi: 10.1515/revneuro.2002.13.1.1. [DOI] [PubMed] [Google Scholar]
- Traub RD, Middleton SJ, Knopfel T, Whittington MA. Model of very fast (> 75 Hz) network oscillations generated by electrical coupling between the proximal axons of cerebellar Purkinje cells. Eur J Neurosci. 2008;28:1603–1616. doi: 10.1111/j.1460-9568.2008.06477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigo FF, Marty A, Stell BM. Axonal GABAA receptors. Eur J Neurosci. 2008;28:841–848. doi: 10.1111/j.1460-9568.2008.06404.x. [DOI] [PubMed] [Google Scholar]
- Trimmer JS, Rhodes KJ. Localization of voltage-gated ion channels in mammalian brain. Annu Rev Physiol. 2004;66:477–519. doi: 10.1146/annurev.physiol.66.032102.113328. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Too Many Cooks? Intrinsic and Synaptic Homeostatic Mechanisms in Cortical Circuit Refinement. Annu Rev Neurosci. 2011 doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- Turrigiano G, Abbott LF, Marder E. Activity-dependent changes in the intrinsic properties of cultured neurons. Science. 1994;264:974–977. doi: 10.1126/science.8178157. [DOI] [PubMed] [Google Scholar]
- Utsunomiya I, Yoshihashi E, Tanabe S, Nakatani Y, Ikejima H, Miyatake T, Hoshi K, Taguchi K. Expression and localization of Kv1 potassium channels in rat dorsal and ventral spinal roots. Exp Neurol. 2008;210:51–58. doi: 10.1016/j.expneurol.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Vacher H, Mohapatra DP, Trimmer JS. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol Rev. 2008;88:1407–1447. doi: 10.1152/physrev.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagg R, Mogyoros I, Kiernan MC, Burke D. Activity-dependent hyperpolarization of human motor axons produced by natural activity. J Physiol. 1998;507(Pt 3):919–925. doi: 10.1111/j.1469-7793.1998.919bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. The contribution of membrane hyperpolarization to adaptation and conduction block in sensory neurones of the leech. J Physiol. 1973;230:509–534. doi: 10.1113/jphysiol.1973.sp010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wart A, Matthews G. Expression of sodium channels Nav1.2 and Nav1.6 during postnatal development of the retina. Neurosci Lett. 2006;403:315–317. doi: 10.1016/j.neulet.2006.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega AV, Henry DL, Matthews G. Reduced expression of Na(v)1.6 sodium channels and compensation by Na(v)1.2 channels in mice heterozygous for a null mutation in Scn8a. Neurosci Lett. 2008;442:69–73. doi: 10.1016/j.neulet.2008.06.065. [DOI] [PubMed] [Google Scholar]
- Veh RW, Lichtinghagen R, Sewing S, Wunder F, Grumbach IM, Pongs O. Immunohistochemical localization of five members of the Kv1 channel subunits: contrasting subcellular locations and neuron-specific co-localizations in rat brain. Eur J Neurosci. 1995;7:2189–2205. doi: 10.1111/j.1460-9568.1995.tb00641.x. [DOI] [PubMed] [Google Scholar]
- Verdier D, Lund JP, Kolta A. GABAergic control of action potential propagation along axonal branches of mammalian sensory neurons. J Neurosci. 2003;23:2002–2007. doi: 10.1523/JNEUROSCI.23-06-02002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervaeke K, Gu N, Agdestein C, Hu H, Storm JF. Kv7/KCNQ/M-channels in rat glutamatergic hippocampal axons and their role in regulation of excitability and transmitter release. J Physiol. 2006;576:235–256. doi: 10.1113/jphysiol.2006.111336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel W, Schwarz JR. Voltage-clamp studies in axons: macroscopic and single channel currents. In: Waxman SG, Kocsis JD, Stys PK, editors. The Axon. Structure, Function and Pathophysiology. Oxford University Press; New York: 1995. pp. 257–280. [Google Scholar]
- Vucic S, Kiernan MC. Axonal excitability properties in amyotrophic lateral sclerosis. Clin Neurophysiol. 2006;117:1458–1466. doi: 10.1016/j.clinph.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Wächtler J, Mayer C, Grafe P. Activity-dependent intracellular Ca2+ transients in unmyelinated nerve fibres of the isolated adult rat vagus nerve. Pflugers Arch. 1998;435:678–686. doi: 10.1007/s004240050569. [DOI] [PubMed] [Google Scholar]
- Wahl-Schott C, Biel M. HCN channels: structure, cellular regulation and physiological function. Cell Mol Life Sci. 2009;66:470–494. doi: 10.1007/s00018-008-8525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PD. Do nerve impulses penetrate terminal arborizations? A pre-presynaptic control mechanism. Trends Neurosci. 1995;18:99–103. doi: 10.1016/0166-2236(95)93883-y. [DOI] [PubMed] [Google Scholar]
- Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature. 1993;365:75–79. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- Wang SS. Functional tradeoffs in axonal scaling: implications for brain function. Brain Behav Evol. 2008;72:159–167. doi: 10.1159/000151475. [DOI] [PubMed] [Google Scholar]
- Wang SS, Shultz JR, Burish MJ, Harrison KH, Hof PR, Towns LC, Wagers MW, Wyatt KD. Functional trade-offs in white matter axonal scaling. J Neurosci. 2008;28:4047–4056. doi: 10.1523/JNEUROSCI.5559-05.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AH, Burrows M. The morphology, ultrastructure, and distribution of synapses on an intersegmental interneuron of the locust. J Comp Neurol. 1983;214:154–169. doi: 10.1002/cne.902140205. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Integrative properties and design principles of axons. Int Rev Neurobiol. 1975;18:1–40. doi: 10.1016/s0074-7742(08)60032-x. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve. 1980;3:141–150. doi: 10.1002/mus.880030207. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Axonal conduction and injury in multiple sclerosis: the role of sodium channels. Nat Rev Neurosci. 2006;7:932–941. doi: 10.1038/nrn2023. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Ritchie JM. Molecular dissection of the myelinated axon. Ann Neurol. 1993;33:121–136. doi: 10.1002/ana.410330202. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Kocsis JD, Stys PK, editors. The Axon. Structure, Function and Pathophysiology. Oxford University Press; New York: 1995. [Google Scholar]
- Weidner C, Schmelz M, Schmidt R, Hammarberg B, Orstavik K, Hilliges M, Torebjork HE, Handwerker HO. Neural signal processing: the underestimated contribution of peripheral human C-fibers. J Neurosci. 2002;22:6704–6712. doi: 10.1523/JNEUROSCI.22-15-06704.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner C, Schmelz M, Schmidt R, Hansson B, Handwerker HO, Torebjork HE. Functional attributes discriminating mechano-insensitive and mechano-responsive C nociceptors in human skin. J Neurosci. 1999;19:10184–10190. doi: 10.1523/JNEUROSCI.19-22-10184.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner C, Schmidt R, Schmelz M, Hilliges M, Handwerker HO, Torebjork HE. Time course of post-excitatory effects separates afferent human C fibre classes. J Physiol 527 Pt. 2000;1:185–191. doi: 10.1111/j.1469-7793.2000.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner C, Schmidt R, Schmelz M, Torebjork HE, Handwerker HO. Action potential conduction in the terminal arborisation of nociceptive C-fibre afferents. J Physiol. 2003;547:931–940. doi: 10.1113/jphysiol.2002.028712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weragoda RM, Ferrer E, Walters ET. Memory-like alterations in Aplysia axons after nerve injury or localized depolarization. J Neurosci. 2004;24:10393–10401. doi: 10.1523/JNEUROSCI.2329-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin FS, Dowling JE. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969;32:339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Westberg KG, Kolta A, Clavelou P, Sandstrom G, Lund JP. Evidence for functional compartmentalization of trigeminal muscle spindle afferents during fictive mastication in the rabbit. Eur J Neurosci. 2000;12:1145–1154. doi: 10.1046/j.1460-9568.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- Westrum LE, Blackstad TW. An electron microscopic study of the stratum radiatum of the rat hippocampus (regio superior, CA 1) with particular emphasis on synaptology. J Comp Neurol. 1962;119:281–309. doi: 10.1002/cne.901190303. [DOI] [PubMed] [Google Scholar]
- Wicher D, Berlau J, Walther C, Borst A. Peptidergic counter-regulation of Ca(2+)- and Na(+)-dependent K(+) currents modulates the shape of action potentials in neurosecretory insect neurons. J Neurophysiol. 2006;95:311–322. doi: 10.1152/jn.00904.2005. [DOI] [PubMed] [Google Scholar]
- Wicher D, Walther C, Wicher C. Non-synaptic ion channels in insects--basic properties of currents and their modulation in neurons and skeletal muscles. Prog Neurobiol. 2001;64:431–525. doi: 10.1016/s0301-0082(00)00066-6. [DOI] [PubMed] [Google Scholar]
- Wigstrom H, Gustafsson B. Increased excitability of hippocampal unmyelinated fibres following conditioning stimulation. Brain Res. 1981;229:507–513. doi: 10.1016/0006-8993(81)91013-1. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Tennissen AM. Activity-dependent spinal cord plasticity in health and disease. Annu Rev Neurosci. 2001;24:807–843. doi: 10.1146/annurev.neuro.24.1.807. [DOI] [PubMed] [Google Scholar]
- Wu JV, Shrager P. Resolving three types of chloride channels in demyelinated Xenopus axons. J Neurosci Res. 1994;38:613–620. doi: 10.1002/jnr.490380603. [DOI] [PubMed] [Google Scholar]
- Xu J, Kang N, Jiang L, Nedergaard M, Kang J. Activity-dependent long-term potentiation of intrinsic excitability in hippocampal CA1 pyramidal neurons. J Neurosci. 2005;25:1750–1760. doi: 10.1523/JNEUROSCI.4217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Terakawa S. Fenestration nodes and the wide submyelinic space form the basis for the unusually fast impulse conduction of shrimp myelinated axons. J Exp Biol. 1999;202:1979–1989. doi: 10.1242/jeb.202.15.1979. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR. Regional Development of the Brain in Early Life. Blackwell; 1967. The myelogenetic cycles of regional maturation of the brain. pp. 3–70. [Google Scholar]
- Yamazaki Y, Hozumi Y, Kaneko K, Sugihara T, Fujii S, Goto K, Kato H. Modulatory effects of oligodendrocytes on the conduction velocity of action potentials along axons in the alveus of the rat hippocampal CA1 region. Neuron Glia Biol. 2007;3:325–334. doi: 10.1017/S1740925X08000070. [DOI] [PubMed] [Google Scholar]
- Yau KW. Receptive fields, geometry and conduction block of sensory neurones in the central nervous system of the leech. J Physiol. 1976;263:513–538. doi: 10.1113/jphysiol.1976.sp011643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JS, Peck LS, Matheson T. The effects of temperature on peripheral neuronal function in eurythermal and stenothermal crustaceans. J Exp Biol. 2006;209:1976–1987. doi: 10.1242/jeb.02224. [DOI] [PubMed] [Google Scholar]
- Young JZ. The giant nerve fibres and epistellar body of cephalopods. Q J Microsc Sci. 1936;78:367–386. [Google Scholar]
- Zaccolo M, Magalhaes P, Pozzan T. Compartmentalisation of cAMP and Ca(2+) signals. Curr Opin Cell Biol. 2002;14:160–166. doi: 10.1016/s0955-0674(02)00316-2. [DOI] [PubMed] [Google Scholar]
- Zalc B, Fields RD. Do Action Potentials Regulate Myelination? Neuroscientist. 2000;6:5–13. doi: 10.1177/107385840000600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, Verbny Y, Malek SA, Stys PK, Chiu SY. Nicotinic acetylcholine receptors in mouse and rat optic nerves. J Neurophysiol. 2004;91:1025–1035. doi: 10.1152/jn.00769.2003. [DOI] [PubMed] [Google Scholar]
- Zhang CL, Wilson JA, Williams J, Chiu SY. Action potentials induce uniform calcium influx in mammalian myelinated optic nerves. J Neurophysiol. 2006;96:695–709. doi: 10.1152/jn.00083.2006. [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Jackson MB. GABA-activated chloride channels in secretory nerve endings. Science. 1993;259:531–534. doi: 10.1126/science.8380942. [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Jackson MB. GABAA receptor activation and the excitability of nerve terminals in the rat posterior pituitary. J Physiol. 1995;483(Pt 3):583–595. doi: 10.1113/jphysiol.1995.sp020608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]
- Zhou L, Chiu SY. Computer model for action potential propagation through branch point in myelinated nerves. J Neurophysiol. 2001;85:197–210. doi: 10.1152/jn.2001.85.1.197. [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Stewart RR, McIntosh JM, Weight FF. Activation of nicotinic acetylcholine receptors increases the frequency of spontaneous GABAergic IPSCs in rat basolateral amygdala neurons. J Neurophysiol. 2005;94:3081–3091. doi: 10.1152/jn.00974.2004. [DOI] [PubMed] [Google Scholar]