Graphical abstract
Keywords: Human cytomegalovirus, Chemokine receptors, Signaling, Post-endocytic sorting, Heteromerization, G-protein coupled receptor
Abstract
Human cytomegalovirus (HCMV) is a widespread pathogen that infects up to 80% of the human population and causes severe complications in immunocompromised patients. HCMV expresses four seven transmembrane (7TM) spanning/G protein-coupled receptors (GPCRs) – US28, US27, UL33 and UL78 – that show close homology to human chemokine receptors. While US28 was shown to bind several chemokines and to constitutively activate multiple signaling cascades, the function(s) of US27, UL33 and UL78 in the viral life cycle have not yet been identified. Here we investigated the possible interaction/heteromerization of US27, UL33 and UL78 with US28 and the functional consequences thereof. We provide evidence that these receptors not only co-localize, but also heteromerize with US28 in vitro. While the constitutive activation of the US28-mediated Gαq/phospholipase C pathway was not affected by receptor heteromerization, UL33 and UL78 were able to silence US28-mediated activation of the transcription factor NF-κB. Summarized, we provide evidence that these orphan viral receptors have an important regulatory capacity on the function of US28 and as a consequence, may ultimately impact on the viral life cycle of HCMV.
1. Introduction
Human cytomegalovirus (HCMV), also known as human herpesvirus 5 (HHV-5), is a large and highly species-specific virus that belongs to the family of beta-herpesviruses. Infection with HCMV is asymptomatic and rarely causes complications in immunocompetent hosts. In immunosuppressed hosts, however, HCMV infection causes severe complications, including graft rejection or systemic infections [1–4]. HCMV expresses four viral seven transmembrane (7TM) spanning/G protein-coupled receptors (GPCRs), i.e. UL33 (unique long 33), UL78, US27 (unique short 27) and US28, which show high homology to human chemokine receptors [5,6].
US28 is the best characterized HCMV encoded 7TM/GPCR and was suggested to (i) be important for the viral life cycle by enhancing cell–cell fusion, thus promoting viral spread [7–10] and (ii) to activate the immediate early HCMV promoter, which generally leads to transactivation of other viral genes [11]. US28 is constitutively active—i.e. transduces signals in a ligand-independent way. The receptor activates the Gαq/phospholipase C (PLC) pathway and induces several transcription factors, such as NF-κB (nuclear factor kappa B), CREB (cyclic AMP responsive element binding) [12,13], NFAT (nuclear factor of activated T cells) [14] or SRF (serum response factor) [15]. Moreover, US28 is constitutively endocytosed, which results in the localization of the receptor in the membranes of intracellular organelles, especially late endosomes/lysosomes and multi-vesicular bodies (MVBs) [13,16,17], where it has been suggested that the virions of HCMV may be assembled [7,8].
US27, UL33 and UL78 are still “orphan” receptors, since no endogenous ligands have been identified to date. Of these, UL33 was reported to constitutively induce inositol phosphate (IP) accumulation via coupling to Gαq and Gαi/o-proteins [13,18] and to activate CREB via Gαq, Gαs, Gβγ and p38 kinase [13,18], as well as to co-localize with endosomes in HCMV infected cells [7,8]. US27 was shown to be expressed during late HCMV infection states and found to be present on HCMV virions [7,19,20]. UL78 shares only very limited homology with endogenous chemokine receptors [21] and was shown to be dispensable for viral replication [22].
Orphan 7TM/GPCRs have recently been shown to influence the signal activity of non-orphan receptors through heteromerization. One prominent example is the orphan receptor GPR50, which was reported to antagonize the functions of the melatonin MT1 receptor. Heteromerization of GPR50 with the MT1 receptor prevented the agonist binding and G protein coupling [23].
Here we set out to test whether the orphan viral receptors US27, UL33 and UL78 possibly interact/heteromerize with US28 and whether this had functional consequences on the signaling and/or endocytic properties of US28.
2. Materials and methods
2.1. Reagents
Mouse anti-Flag M1 and M2 monoclonal antibody, gelatine from bovine skin Type B, Triton X-100, 3,3′,5,5′-tetramethylbenzidine liquid substrate system were purchased from Sigma–Aldrich (Austria). Cell culture media (DMEM), fetal bovine serum (FBS), Dulbecco's phosphate buffered saline (DPBS), Lipofectamine 2000, Alexa Fluor 594 nm conjugated IgG1a, 488 nm conjugated IgG2b and 594 nm conjugated donkey anti rabbit IgG were purchased from Invitrogen (Austria). HRP-conjugated anti-mouse antibody was obtained from Jackson Immuno Research (Dianova, Germany). Vectashield mounting medium and Vectastain ABC Kit were purchased from Vector Laboratories (Szabo-Scandic, Austria). The white 96-well culture plates and the Steadylite Plus Assay Kit, high sensitivity luminescence reporter gene assay system, [3H]myo-inositol and EasyTag 35S-methionine were purchased from PerkinElmer (Austria). Yttrium silicate (YSi) SPA beads were from GE Healthcare (Austria). NaOH, KCl and CaCl2·2H2O were obtained from Merck (Austria), NaCl, Tris and formaldehyde were from Roth (Lactan, Austria). Coelenterazine-H powder (1 mg) was purchased from Interchim (France). Monoclonal rat anti-HA and monoclonal mouse anti-GFP antibodies were purchased from Roche Diagnostics (France). Monoclonal rabbit anti-HA antibody was purchased from Cell Signaling (France). Polyclonal rabbit anti-Flag antibody, protein G sepharose beads and poly-l-lysine were obtained from Sigma–Aldrich (France). Phusion High-Fidelity DNA Polymerase was purchased from Finnzymes (France). IRdye 800-conjugated anti-rat, IRdye 680-conjugated anti-rabbit, IRdye680-conjugated anti-mouse antibodies were purchased from Thermo Scientific (France). JetPEI transfection reagent was obtained from Polyplus Transfection (France).
2.2. DNA constructs
A Flag-tagged version of US28 (Flag-US28) was cloned by using the BamHI and XbaI restriction sites in the SS-Flag pcDNA3.1 zeo (+) vector (16). The cDNA encoding UL78 was kindly provided by Dr. Detlef Michel (University of Ulm, Germany) and was cloned in the SS-Flag pcDNA3.1 zeo (+) and SS-HA pcDNA3.1 zeo (+) vectors using BamHI and EcoRV restriction sites. UL33 (exon 1 + 2) was cloned in the SS-HA pcDNA3.1 zeo (+) vector using BamHI and XhoI restriction sites. A HA-tagged version of US27 (exon 2) was cloned by using the BamHI and EcoRV restriction sites in SS-HA pcDNA3.1 zeo (+) vector. Flag-US28-YFP, HA-US28-Rluc, HA-UL33, HA-UL33-Rluc, Flag-UL78 and HA-UL78-Rluc constructs were obtained using the latter cDNAs as templates and the Phusion High-Fidelity DNA Polymerase. The Flag or HA tags were inserted after the ATG in frame of the US28, UL33 or UL78 sequences. Flag-US28, HA-US28, HA-UL33, Flag-UL78 and HA-UL78 were amplified by PCR. The amplified products were subcloned directly into a pCR2.1 vector (Invitrogen, CA). After sequencing, the fragments were digested with the restriction enzymes HindIII and BamHI and subcloned into pcDNA3.1 vector or pcDNA3.1 vector containing either YFP or Rluc sequences. The vasopressin V2-YFP fusion protein has been described previously [24]. All DNA constructs were verified by sequencing.
2.3. Antibody feeding experiments
HEK293 cells were grown on gelatin coated coverslips and transiently transfected with Flag-US28 (1.5 μg/coverslip) in combination with HA-UL33 (1.5 μg/coverslip), HA-UL78 (1.5 μg/coverslip) or HA-US27 (1.5 μg/coverslip) using Lipofectamine 2000. 24 h post-transfection, antibody feeding experiments were conducted essentially as previously described [25]. In brief, living cells were incubated with anti-Flag M1 (1:500) and anti-HA HA11 (1:500) antibodies for 45 min, fixed with 3.7% formaldehyde in PBS and permeabilized in blotto (50 mM Tris–HCl, pH 7.5, 1 mM CaCl2, 0.2% Triton X-100 and 3% milk). Subsequently, the cells were stained with the subtype-selective antibodies Alexa Fluor 488-conjugated IgG2b against the Flag-tag (1:1000, 20 min) and Alexa Fluor 594-conjugated IgG1a against the HA-tag (1:1000, 20 min). Finally, cells were mounted with Vectashield mounting medium and analyzed using an Olympus IX70 fluorescence microscope.
2.4. Immunoprecipitation
For co-immunoprecipitation assays, HEK293T cells were seeded in 10 cm dishes and co-transfected with 8 μg of the indicated plasmids. 48 h after transfection, cells were washed twice in PBS, and lysed in 1 ml cold lysis buffer (25 mM Tris (pH 7.4), 2 mM EDTA, 10 mM MgCl2, protease inhibitor cocktail and 1% Triton). After solubilization for 2 h at 4 °C, lysates were centrifuged at 12,000 × g during 1 h at 4 °C and the supernatant was collected for the immunoprecipitation assay. Immunoprecipitations were performed using 2 μg of the indicated antibodies pre-adsorbed on protein G sepharose beads for 2 h at 4 °C. Immunoprecipitated proteins were eluted with Laemmli buffer 2× (Tris–HCl pH 6.8, 125 mM, SDS 10%, Glycerol 10%, bromophenol blue 1% and DTT 100 mM) and subjected to 10% or 7.5% SDS–PAGE and immunoblotting. Immunoblotting was performed using the indicated antibodies and immunoreactivity was revealed using secondary antibodies coupled to 680 or 800 nm fluorophores using the Odyssey LI-COR infrared fluorescent scanner (ScienceTec).
2.5. BRET (bioluminescence resonance energy transfer) measurement
HEK293T cells were grown in complete medium (Dulbecco's modified Eagle's medium supplemented with 10% (v/v) fetal bovine serum, 4.5 g/L glucose, 100 U/ml penicillin, 0.1 mg/ml streptomycin and 1 mM glutamine) (Invitrogen, CA). Transient transfections were performed using JetPEI transfection reagent according to manufacturer's instructions.
For BRET donor saturation curves, HEK293T cells were seeded in 12-well plates and transiently transfected with 200 ng of HA-UL33-Rluc or 100 ng of HA-UL78-Rluc and 100–1800 ng of YFP plasmids. For BRET competition assays, HEK293T cells were seeded in 6-well plates. Cells were transiently transfected with (i) 200 ng HA-UL33-Rluc and 1000 ng Flag-US28-YFP in the presence or absence of 1800 ng Flag-UL78 or (ii) 100 ng HA-UL78-Rluc and 1000 ng Flag-US28-YFP in the presence or absence of 1900 ng HA-UL33 plasmids. 24 h after transfection, cells were transferred into a 96-well white Optiplate pre-coated with 10 mg/ml poly-l-lysine. Following 24 h incubation, BRET measurements were conducted. Luminescence and fluorescence were measured simultaneously using the lumino/fluorometer Mithras™ (Berthold) that allows the sequential integration of luminescence signals detected with two filter settings (Rluc filter, 485 ± 10 nm; YFP filter, 530 ± 12.5 nm). Emission signals at 530 nm were divided by emission signals at 485 nm and the difference between this emission ratio (obtained with donor and acceptor fused or co-expressed and that obtained with the donor protein expressed alone) was defined as the BRET ratio. The results were expressed in milliBRET units (mBU) corresponding to the BRET ratio values multiplied by 1000. Total fluorescence was measured with the fluorometer Fusion™ (Packard Instrument Company).
2.6. Reporter gene assays
Transcription factor luciferase assays were performed essentially as described in Ref. [13]. In brief, HEK293 cells (30,000 cells/well) were seeded on gelatin coated 96-well plates and transiently transfected with 75 ng/well Flag-US28, the cis-reporter plasmid for NF-κB (50 ng/well) and either pcDNA3.1 (75 ng/well) or the respective receptors (HA-UL33, HA-UL78 or HA-US27; 75 ng/well). Controls were transfected with 150 ng/well of either pcDNA3.1 alone, or pcDNA3.1 (75 ng/well) in combination with UL33, UL78 or US27 (75 ng/well), but in the absence of US28. In parallel to each assay, an additional plate was prepared for ELISA (see below) to control for receptor expression levels. The luciferase reporter gene assays were conducted 24 h post-transfection according to the manufacturer's guidelines. In brief, cells were washed twice with PBS and the cell number was determined by optical density in a FlexStation II Device (Molecular Probes, Endpoint Measurement). Then, 100 μl/well Steadylight luciferase assay reagent were added to 100 μl/well PBS. Following a 10 min incubation period, luminescence was measured using a TopCounter Device (TopCount NXT, PerkinElmer).
2.7. Inositol phosphate (IP) accumulation assay
HEK293 cells were seeded on gelatin coated 96-well plates (30,000 cells/well) and transfected with 75 ng/well Flag-US28 and either pcDNA3.1 (75 ng/well) or the respective orphan receptor (HA-UL33, HA-UL78 or HA-US27; 75 ng/well). Controls were transfected with 150 ng/well of either pcDNA3.1 alone, or pcDNA3.1 (75 ng/well) in combination with UL33, UL78 or US27 (75 ng/well), but in the absence of US28. In parallel to each assay, an additional plate was prepared for ELISA (see below) to control for receptor expression levels. 24 h post-transfection the cells were loaded with 1 μCi/well [3H]myo-inositol in 100 μl/well Optimem and incubated overnight at 37 °C and 5% CO2. The next day, the labeling medium was aspirated, 100 μl/well HBSS buffer (including CaCl2 and MgCl2) containing 10 mM LiCl were added and the cells were incubated for 45 min at 37 °C and 5% CO2. The reaction was terminated by aspiration and the cell number was determined by optical density in a FlexStation II Device. Subsequently, cells were lysed with 50 μl/well 10 mM formic acid for 90 min on ice. 40 μl of the resulting cell extract were transferred to 160 μl of YSi-SPA beads (12.5 mg/ml) and shaken for 90 min at 4 °C. The plates were then stored at 4 °C and the accumulation of inositol phosphate was counted in a TopCount microplate scintillation counter (TopCount NXT, PerkinElmer) 24 h later.
2.8. ELISA (enzyme linked immunosorbent assay)
The ELISAs were performed on the same day as the corresponding signaling experiments (see above). Cells were fixed with 3.7% formaldehyde in PBS and permeabilized in blotto (50 mM Tris–HCl, pH7.5, 1 mM CaCl2, 0.2% Triton X-100 and 3% milk) for 1 h. Next, the cells were incubated with either anti-Flag M1 (1:500) or anti-HA HA11 (1:1000) antibody overnight at 4 °C. Then cells were washed with TBS (25 mM Tris base, 135 mM NaCl, 2.5 mM KCl, 1 mM CaCl2·2H2O, pH 7.4) and incubated with HRP-conjugated anti-mouse antibody (1:2500) in blotto (50 mM Tris–HCl, pH 7.5, 1 mM CaCl2 and 1.5% milk) for 2 h at room temperature. After three washes with TBS, the cell number was determined by optical density in a FlexStation II Device. Then, 75 μl/well TMB (3,3′,5,5′-tetramethylbenzidine) substrate was added and the coloring reaction was stopped by the addition of 50 μl/well 0.5 M sulfuric acid after 2 min at room temperature. Receptor levels were measured at the optical density of 450 nm in a BioRad xMark Microplate Spectrophotometer.
2.9. Statistical analysis
Statistical analyses were performed using ANOVA analysis of variance for comparisons between multiple groups, followed by a Bonferroni's post-hoc analysis using GraphPad Prism software.
3. Results
3.1. Co-localization of HCMV encoded chemokine receptors
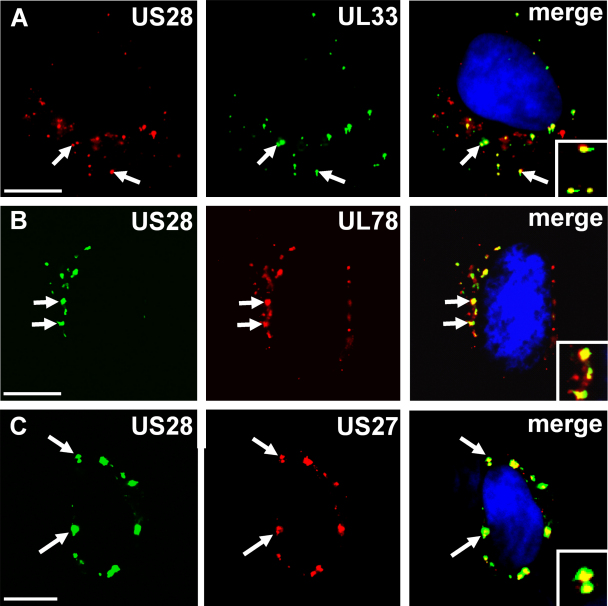
US28, UL33 and US27 have previously been shown to constitutively internalize and localize in intracellular compartments [7,13,16]. Here we set out to investigate a possible co-localization of US28 with the three orphan HCMV encoded receptors US27, UL33 and UL78. Therefore, the respective receptors were transiently co-expressed with US28 in HEK293 cells at a 1:1 ratio and antibody feeding experiments were conducted (see Section 2). These experiments revealed that US28 co-internalizes and co-localizes with UL33 (Fig. 1A) and UL78 (Fig. 1B) in HEK293 cells. As described before [26], US28 was also found to co-localize with US27 (Fig. 1C).
Fig. 1.
US28 co-localizes with UL33, UL78 and US27. HEK293 cells transiently transfected with Flag-US28 and either HA-UL33 (A), HA-UL78 (B) or HA-US27 (C) were ‘fed’ antibody to the extracellular Flag-tag and HA-tag for 60 min. The cells were then fixed and stained with the secondary antibodies under permeabilizing conditions. Finally, cells were mounted with Vectashield (+DAPI, blue) and analyzed by fluorescence microscopy. Merge: yellow. Scale bars = 10 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
3.2. Dimerization of US28 with US27, UL33 and UL78
We were able to demonstrate that after constitutive internalization US28 co-localizes with all three orphan receptors in endosomal compartments (Fig. 1). Next, we conducted co-immunoprecipitation (co-IP) experiments to test whether the receptors are not only co-localizing, but furthermore able to physically interact with each other. Therefore, HEK293T cells were transiently co-transfected with Flag-US28-YFP and HA-US28 (A), HA-UL33-Rluc (B), HA-UL78-Rluc (C) or HA-US27 (D). These experiments revealed that US28 not only forms homomers (Fig. 2A), but moreover interacts with all three orphan receptors (Fig. 2B–D).
Fig. 2.
(A) Detection of US28/US28 homomers using co-immunoprecipitation. HEK293T cells were transfected with HA-US28 in the presence or absence of Flag-US28-YFP. Lysates were immunoblotted with monoclonal rabbit anti-HA (bottom blot) and polyclonal rabbit anti-Flag antibodies (middle blot). Lysates were immunoprecipitated with monoclonal rat anti-HA, resolved by SDS–PAGE and immunoblotted with polyclonal rabbit anti-Flag antibodies (top blot). (B) Detection of US28/UL33 heteromers using co-immunoprecipitation. HEK293T cells were transfected with HA-UL33-Rluc in the presence or absence of Flag-US28-YFP. The expression of HA-UL33-Rluc was monitored by measuring Renilla luciferase activity following coelenterazine H addition (bottom graph). Lysates were immunoblotted with monoclonal mouse anti-GFP antibodies (middle blot). Lysates were immunoprecipitated with monoclonal rat anti-HA, resolved by SDS–PAGE and immunoblotted with monoclonal mouse anti-GFP antibodies (top blot). (C) Detection of US28/UL78 heteromers using co-immunoprecipitation. HEK293T cells were transfected with HA-UL78-Rluc in the presence or absence of Flag-US28-YFP. The expression of HA-UL78-Rluc was monitored by measuring Renilla luciferase activity following coelenterazine H addition (bottom graph). Lysates were immunoblotted with polyclonal rabbit anti-Flag antibodies (middle blot). Lysates were immunoprecipitated with monoclonal rat anti-HA, resolved by SDS–PAGE and immunoblotted with polyclonal rabbit anti-Flag antibodies (top blot). All immunoblots shown are representative of three independent experiments. (D) Detection of US28/US27 heteromers using co-immunoprecipitation. HEK293T cells were transfected with HA-US27 in the presence or absence of Flag-US28-YFP to assess US27/US28 heteromer formation. Lysates were immunoblotted with monoclonal rat anti-HA (bottom blot) and polyclonal rabbit anti-Flag antibodies (middle blot). Lysates were immunoprecipitated with monoclonal rat anti-HA, resolved by SDS–PAGE and immunoblotted with polyclonal rabbit anti-Flag antibodies (top blot).
Next, bioluminescence resonance energy transfer (BRET) assays were performed to confirm the co-IP results. Therefore, UL33 or UL78 were fused at their C-terminus to Renilla luciferase (HA-UL33-Rluc, HA-UL78-Rluc) and used as energy donors. US28 was fused at its C-terminus to YFP (Flag-US28-YFP) and used as the energy acceptor. A possible heteromerization between US28 and US27 could not be validated using the BRET approach, since the co-expression of US27-Rluc led to a significant decrease in US28-YFP expression levels (data not shown).
Donor saturation curves were performed by co-transfecting a fixed amount of HA-UL33-Rluc or HA-UL78-Rluc in the presence of increasing amounts of Flag-US28-YFP in HEK293T cells. The vasopressin V2 receptor YFP (V2-YFP) fusion protein was used as a negative control. A specific interaction between BRET donor and acceptor pairs is reflected by a hyperbolic donor saturation curve, which reaches an asymptote with increasing YFP/Rluc ratios. This is shown for the US28/UL33 couple (Fig. 3A) and the US28/UL78 couple (Fig. 3B), respectively. In contrast, a non-specific interaction, due to random collision, was observed with V2-YFP where a quasi-linear increase in BRET was observed with increasing YFP/Rluc ratios (Fig. 3B). BRET competition assays were then performed to assess, if UL78 and UL33 can disrupt US28/UL33 and US28/UL78 heteromerization, respectively. The YFP/Rluc ratio in the ascending portion of the saturation curve before the plateau was selected and the corresponding amounts of donor and acceptor plasmids were transfected in the presence of saturating amounts of the competitor receptor (Flag-UL78 in the case of HA-UL33-Rluc/Flag-US28-YFP and HA-UL33 in the case of HA-UL78-Rluc/Flag-US28-YFP). The BRET signal was significantly decreased in the presence of the competitor receptor indicating that UL78 (Fig. 3C) and UL33 (Fig. 3D) interfere with US28/UL33 and US28/UL78 heteromerization, respectively.
Fig. 3.
Heteromerization of US28 with UL33 and UL78. (A) Donor saturation curves were performed by co-transfecting a fixed amount of HA-UL33-Rluc in the presence of increasing amounts of Flag-US28-YFP in HEK 293T cells. The saturation curves are obtained from three independent experiments. (B) Donor saturation curves were performed by co-transfecting a fixed amount of HA-UL78-Rluc in the presence of increasing amounts of Flag-US28-YFP (●) in HEK 293T cells. V2-YFP (■) was used as a negative control. The saturation curves are obtained from three independent experiments. (C) Bret competition assays were performed by co-transfecting a fixed amount of HA-UL33-Rluc and Flag-US28-YFP corresponding to a YFP/Rluc ratio in the ascending portion of the saturation curve before the plateau in the presence of saturating amounts of the competitor receptor (Flag-UL78). Lysates were immunoblotted with polyclonal rabbit anti-Flag antibody (bottom blot) to assess the expression levels of the competitor receptor. (D) Bret competition assays were performed by co-transfecting a fixed amount of HA-UL78-Rluc and Flag-US28-YFP corresponding to a YFP/Rluc ratio in the ascending portion of the saturation curve before the plateau in the presence of saturating amounts of the competitor receptor (HA-UL33). Lysates were immunoblotted with monoclonal rabbit anti-HA antibody (bottom blot) to assess the expression levels of the competitor receptor. Statistical differences were assessed using the student t-test (***p < 0.001).
Summarized, the co-IP and BRET experiments show that US28 forms homomers and is furthermore able to heteromerize with the three viral HCMV encoded receptors US27, UL33 and UL78.
3.3. US28-mediated transcription factor activation, but not G protein-mediated inositol phosphate accumulation, is altered by receptor heteromerization
It has previously been demonstrated that receptor heteromerization alters the trafficking and signaling properties of 7TM/GPCRs [27–29]. Importantly, orphan receptors – such as GPR50 – have been demonstrated to modulate the activity of non-orphan receptors through heteromerization [30].
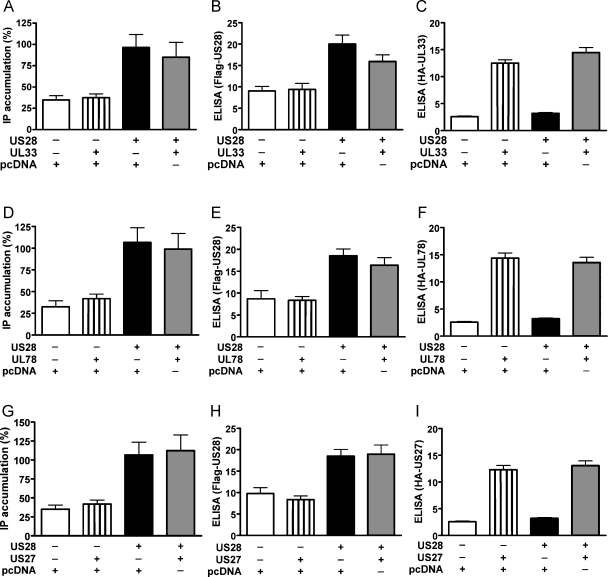
Hence, we next tested the functional consequences of the heteromerization of US28 with US27, UL33 and UL78. First, the ability of US28 to mediate the Gαq/phospholipase C (PLC) dependent accumulation of inositol phosphate (IP) was assessed in cells co-expressing the orphan HCMV encoded receptors. US28 constitutively induced IP turnover in the absence (Fig. 4A, D and G, black bars) and presence (Fig. 4A, D and G, grey bars) of the other viral receptors. The expression levels of Flag-tagged US28 (Fig. 4B, E and H) as well as the HA-tagged orphan receptors (Fig. 4C, F and I) were monitored by ELISA. These results suggest that the orphan receptors had no direct influence on the G protein mediated signaling capacity of US28.
Fig. 4.
US28-mediated inositol phosphate (IP) accumulation is not altered by heteromerization with UL33, UL78 or US27. (A, D and G) IP accumulation in HEK293 cells co-expressing receptor heteromers. HEK293 cells were transiently transfected with Flag-US28 (75 ng/well) and either pcDNA3.1 (black bars, 75 ng/well) or 75 ng/well of HA-UL33 (A), HA-UL78 (D) or HA-US27 (G), respectively (grey bars). Controls were transfected with either pcDNA3.1 alone (150 ng/well, white bars) or pcDNA3.1 (75 ng/well) in combination with 75 ng/well of HA-UL33 (A), HA-UL78 (D) or HA-US27 (G) (striped bars) in the absence of Flag-US28. IP production mediated by US28 was allowed to accumulate for 45 min. Values were normalized to the cell number, and 100% corresponds to the basal activity of US28 (black bars). Data are means of four experiments ± SEM carried out in quadruplicates. (B, C, E, F, H and I) Receptor expression levels evaluated by ELISA. HEK293 cells were transfected as described for the respective IP accumulation assay. The expression levels of US28 were assayed by ELISA against the Flag epitope tag of US28 (B, E and H). The levels of UL33 (C), UL78 (F) and US27 (I) were assessed by ELISA against the HA epitope tag of the respective receptors. Values were normalized to the cell number. Data are means ± SEM of four independent experiments carried out in quadruplicates.
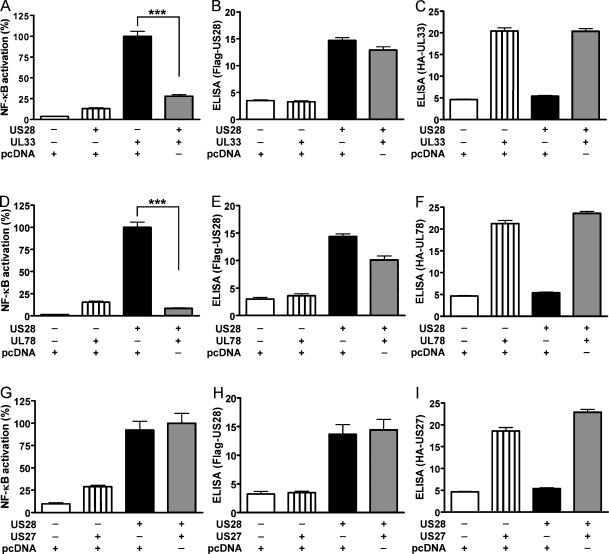
US28 has been reported to activate downstream transcription factors such as NF-κB, CREB [12,13], NFAT [14] or SRF [15]. We next tested whether the heteromerization of US28 with the orphan HCMV encoded receptors alters the downstream signal transduction profile of US28. Hence, US28 was co-expressed with the respective orphan receptors and reporter gene assays were performed. Interestingly, the co-expression of UL33 and UL78 was found to significantly decrease the activation of the transcription factor NF-κB by US28 (Fig. 5A and D, compare black bars vs. grey bars). The reduction of US28 signaling capacity in the presence of UL33 and UL78 was unlikely to be due to altered expression levels of the receptors, since they remained relatively stable under the different experimental conditions (see Fig. 5B, C, E and F). Co-expression of US27 on the other hand, did not alter the signaling capacity of US28 (Fig. 5G compare black bar vs. grey bar).
Fig. 5.
UL33 and UL78 alter US28 mediated NF-κB activation. (A, D and G) NF-κB activation in HEK293 cells co-expressing receptor heteromers. HEK293 cells were transiently transfected with Flag-US28 (75 ng/well), the cis-reporter luciferase plasmid for NF-κB (50 ng/well) and either pcDNA3.1 (black bars, 75 ng/well) or 75 ng/well of HA-UL33 (A), HA-UL78 (D) or HA-US27 (G), respectively (grey bars). Controls were transfected with either pcDNA3.1 alone (150 ng/well, white bars) or pcDNA3.1 (75 ng/well) in combination with 75 ng/well of HA-UL33 (A), HA-UL78 (D) or HA-US27 (G) (striped bars) in the absence of Flag-US28. A luciferase reporter assay was conducted 24 h post-transfection. Values were normalized to the cell number, whereby 100% corresponds to the basal activity of US28 (black bars). Data are means of three independent experiments ± SEM carried out in quadruplicates. ***p < 0.001. (B, C, E, F, H and I) Receptor expression levels evaluated by ELISA. HEK293 cells were transfected as described for the respective NF-κB assay. The expression levels of US28 were assayed by ELISA against the Flag epitope tag of US28 (B, E and H). The levels of UL33 (C), UL78 (F) and US27 (I) were assessed by ELISA against the HA epitope tag of the respective receptors. Values were normalized to the cell number. Data are means ± SEM of four independent experiments carried out in quadruplicates.
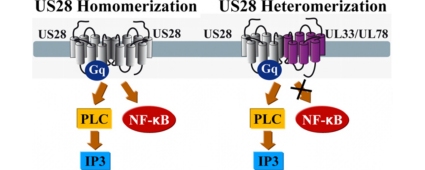
Summarized, these data suggest that the constitutive signaling activity of US28 is selectively modulated via heteromerization with the orphan HCMV-encoded UL33 and UL78. The activation of the Gαq/PLC/IP3 pathway via US28 was not altered, whereas the activation of the transcription factor NF-κB by US28 was almost completely blocked by UL33 or UL78, respectively.
4. Discussion
Out of the four HCMV encoded 7TM/GPCRs, US28 is the only receptor that has been shown to bind endogenous chemokines [13,31,32]. The other three receptors – US27, UL33 and UL78 – are yet orphan receptors with mainly unknown function [5,6]. Here we tested whether these orphan receptors – similar to recent reports on other orphan 7TM/GPCRs [23] – may have ligand-independent functions by modulating the signaling and trafficking capacities of US28.
Antibody feeding experiments revealed that all four viral 7TM/GPCRs are predominantly found in intracellular compartments. This is in accordance with previous data on US27, US28 and UL33 [7,33], which have all been described to constitutively internalize. Here we show for the first time that US28 co-localizes with all three orphan receptors in HEK293 cells (Fig. 1).
Heteromerization and thus a direct protein–protein interaction between these HCMV encoded receptors were confirmed by co-IP and BRET experiments (Figs. 2 and 3). To date it is widely accepted that most, if not all, 7TM/GPCRs are organized in dimeric/oligomeric complexes. The importance of 7TM/GPCR oligomerization is most convincingly illustrated by the functional alterations induced when two different 7TM/GPCRs associate to form heteromeric complexes. Modifications in pharmacological, signaling and trafficking properties have been observed for many 7TM/GPCRs when engaged into heteromers [27–29,34]. For instance, the chemokine receptor CXCR4 was shown to heteromerize with CCR5 [35,36], whereby the CCR5/CXCR4 heteromer was causally involved in the modulation of T lymphocyte responses [37]. Moreover, homomerization of CCR5 was shown to prevent infection with HIV-1 [38].
In addition, orphan receptors were shown to be capable of influencing the activity of non-orphan receptors through heteromerization. The orphan receptor GPR50, for example, was reported to antagonize the functions of the melatonin MT1 receptor via heteromerization. Heteromerization of GPR50 with the MT1 receptor prevented the agonist binding and G protein coupling [23]. Moreover, it was found that the carboxy-terminus of GPR50 is involved in these inhibitory effects. As opposed to the wild type receptor, heteromerization with a carboxy-terminally deleted GPR50 did not alter MT1 related functions [23].
To evaluate the functional consequences of viral HCMV receptor heteromerization, the Gαq/PLC/IP pathway and the activation of the downstream transcription factor NF-κB were monitored. Surprisingly, the IP accumulation was not altered upon co-expression of US28 with the orphan receptors (Fig. 4). On the other hand, the constitutive activation of NF-κB was almost completely blocked by the co-expression of UL33 (Fig. 5A) and UL78 (Fig. 5D). US27, the HCMV encoded receptor that shares the highest sequence homology with US28, did neither alter the IP accumulation (Fig. 4G), nor the activation of the transcription factor (Fig. 5G).
The NF-κB pathway is known to be an important regulator of the innate and the adaptive immune response, inflammation and cell survival [39]. The major immediate early promoter (MIEP) region of HCMV, which controls the expression of immediate early (IE) genes, contains four binding sites for the transcription factor NF-κB. The role of these binding sites is not completely established. In some studies, inhibition of NF-κB signaling yielded an impaired viral replication [40,41], whereas other reports show that the deletion of the binding sites within the MIEP region had no effects [42,43]. The mechanisms employed by HCMV for the establishment of viral latency and reactivation are still an important source of speculation. In general, HCMV infection and reactivation were shown to yield the enhancement of cellular responses, including the activation of transcription factors, accumulation of cAMP, inositol phosphate hydrolysis and the metabolism of arachidonic acid [44,45]. For γ-herpesviruses, such as KSHV (Kaposi's sarcoma-associated herpesvirus), EBV (Epstein-Barr virus) or γ-herpesvirus 68 (γ-HV68), the NF-κB pathway was reported to be involved in cell growth as well as in the establishment of viral latency [46–50]. Hence, it is tempting to speculate that the selective modulation of the NF-κB activation via heteromerization with US28 might be important in viral reactivation or latency. Indeed, heteromerization of US28 and UL78, which is co-expressed during the early phases of HCMV infection, could provide a way to downregulate the NF-κB activation of US28. In addition, during late stages of HCMV infection, the constitutive activity of US28 could be modulated via heteromerization with UL33. After the initial re-programming of newly infected cells, the downregulation of US28 via heteromerization with the orphan receptors UL33 and UL78 might thus represent a mechanism to negatively modulate the NF-κB pathway.
Recent reports suggest that US28 might play a causative role in multiple diseases associated with HCMV infection. Human cytomegalovirus has been shown to be present in various malignancies, including breast cancer [51], colon cancer [52] or malignant glioblastoma [53]. Indeed, US28 seems to be involved in tumorigenesis via the up-regulation of COX-2 [54,55]. Moreover, Bongers et al. showed that the receptor also promotes intestinal neoplasia and cancer in transgenic mice [56]. In addition, US28 might also represent the causative link between HCMV infection and the accelerated progression of vascular diseases, since it is able to mediate the migration of vascular smooth muscle cells [57].
Hence, the finding that US28 is engaged in hetero-/oligomeric complexes provides important information for future drug design. Furthermore, the manipulation of the NF-κB pathway via heteromerization with the other HCMV encoded receptors might contribute to understanding the mechanisms underlying viral latency and reactivation in vivo.
Acknowledgements
This work was supported by grants from the Austrian Science Fund (P18723 to MW), the Jubiläumsfonds of the Austrian National Bank (to MW), the Lanyar Stiftung Graz, Austria (to MW and PT), the ‘Molecular Medicine Ph.D. program’ of the Medical University of Graz, Austria (to PT), grants from the Fondation Recherche Médicale (“Equipe FRM”), the Association pour la Recherche sur le Cancer (ARC, n° 5051), the Institut National de la Santé et de la Recherche Médicale (INSERM) and the Centre National de la Recherche Scientifique (CNRS). PT and KT were supported by research fellowships of the Medical University of Graz (Austria) and the Université Paris Descartes (France), respectively.
Contributor Information
Pia Tschische, Email: ptschische@gallo.ucsf.edu.
Kenjiro Tadagaki, Email: kenjiro.tadagaki@inserm.fr.
Maud Kamal, Email: maud.kamal@inserm.fr.
Ralf Jockers, Email: ralf.jockers@inserm.fr.
Maria Waldhoer, Email: maria.waldhoer@medunigraz.at.
References
- 1.Humar A., Michaels M. American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant. 2006;6(2):262–274. doi: 10.1111/j.1600-6143.2005.01207.x. [DOI] [PubMed] [Google Scholar]
- 2.Gandhi M.K., Khanna R. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect Dis. 2004;4(12):725–738. doi: 10.1016/S1473-3099(04)01202-2. [DOI] [PubMed] [Google Scholar]
- 3.Ross S.A., Boppana S.B. Congenital cytomegalovirus infection: outcome and diagnosis. Semin Pediatr Infect Dis. 2005;16(1):44–49. doi: 10.1053/j.spid.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths P.D. CMV as a cofactor enhancing progression of AIDS. J Clin Virol. 2006;35(4):489–492. doi: 10.1016/j.jcv.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 5.Rosenkilde M.M., Waldhoer M., Luttichau H.R., Schwartz T.W. Virally encoded 7TM receptors. Oncogene. 2001;20(13):1582–1593. doi: 10.1038/sj.onc.1204191. [DOI] [PubMed] [Google Scholar]
- 6.Rosenkilde M.M., Smit M.J., Waldhoer M. Structure, function and physiological consequences of virally encoded chemokine seven transmembrane receptors. Br J Pharmacol. 2008;153(Suppl. 1):S154–S166. doi: 10.1038/sj.bjp.0707660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraile-Ramos A., Pelchen-Matthews A., Kledal T.N., Browne H., Schwartz T.W., Marsh M. Localization of HCMV UL33 and US27 in endocytic compartments and viral membranes. Traffic. 2002;3(3):218–232. doi: 10.1034/j.1600-0854.2002.030307.x. [DOI] [PubMed] [Google Scholar]
- 8.Cepeda V, Esteban M, Fraile-Ramos A. Human cytomegalovirus final envelopment on membranes containing both trans-Golgi network and endosomal markers. Cell Microbiol 2010; 12(3):386–404. [DOI] [PubMed]
- 9.Casarosa P., Waldhoer M., LiWang P.J., Vischer H.F., Kledal T., Timmerman H. CC and CX3C chemokines differentially interact with the N terminus of the human cytomegalovirus-encoded US28 receptor. J Biol Chem. 2005;280(5):3275–3285. doi: 10.1074/jbc.M407536200. [DOI] [PubMed] [Google Scholar]
- 10.Pleskoff O., Treboute C., Alizon M. The cytomegalovirus-encoded chemokine receptor US28 can enhance cell–cell fusion mediated by different viral proteins. J Virol. 1998;72(8):6389–6397. doi: 10.1128/jvi.72.8.6389-6397.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boomker J.M., The T.H., de Leij L.F., Harmsen M.C. The human cytomegalovirus-encoded receptor US28 increases the activity of the major immediate-early promoter/enhancer. Virus Res. 2006;118(1-2):196–200. doi: 10.1016/j.virusres.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Casarosa P., Bakker R.A., Verzijl D., Navis M., Timmerman H., Leurs R. Constitutive signaling of the human cytomegalovirus-encoded chemokine receptor US28. J Biol Chem. 2001;276(2):1133–1137. doi: 10.1074/jbc.M008965200. [DOI] [PubMed] [Google Scholar]
- 13.Waldhoer M., Kledal T.N., Farrell H., Schwartz T.W. Murine cytomegalovirus (CMV) M33 and human CMV US28 receptors exhibit similar constitutive signaling activities. J Virol. 2002;76(16):8161–8168. doi: 10.1128/JVI.76.16.8161-8168.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLean K.A., Holst P.J., Martini L., Schwartz T.W., Rosenkilde M.M. Similar activation of signal transduction pathways by the herpesvirus-encoded chemokine receptors US28 and ORF74. Virology. 2004;325(2):241–251. doi: 10.1016/j.virol.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 15.Moepps B., Tulone C., Kern C., Minisini R., Michels G., Vatter P. Constitutive serum response factor activation by the viral chemokine receptor homologue pUS28 is differentially regulated by Galpha(q/11) and Galpha(16) Cell Signal. 2008;20(8):1528–1537. doi: 10.1016/j.cellsig.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Fraile-Ramos A., Kledal T.N., Pelchen-Matthews A., Bowers K., Schwartz T.W., Marsh M. The human cytomegalovirus US28 protein is located in endocytic vesicles and undergoes constitutive endocytosis and recycling. Mol Biol Cell. 2001;12(6):1737–1749. doi: 10.1091/mbc.12.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tschische P, Moser E, Thompson D, Vischer HF, Parzmair GP, Pommer V, et al. The G-protein coupled receptor associated sorting protein GASP-1 regulates the signalling and trafficking of the viral chemokine receptor US28. Traffic 2010; 11(5):660–74. [DOI] [PMC free article] [PubMed]
- 18.Casarosa P., Gruijthuijsen Y.K., Michel D., Beisser P.S., Holl J., Fitzsimons C.P. Constitutive signaling of the human cytomegalovirus-encoded receptor UL33 differs from that of its rat cytomegalovirus homolog R33 by promiscuous activation of G proteins of the Gq, Gi, and Gs classes. J Biol Chem. 2003;278(50):50010–50023. doi: 10.1074/jbc.M306530200. [DOI] [PubMed] [Google Scholar]
- 19.Margulies B.J., Gibson W. The chemokine receptor homologue encoded by US27 of human cytomegalovirus is heavily glycosylated and is present in infected human foreskin fibroblasts and enveloped virus particles. Virus Res. 2007;123(1):57–71. doi: 10.1016/j.virusres.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Connor C.M., Shenk T. Human Cytomegalovirus pUS27 G Protein-coupled Receptor Homologue Is Required for Efficient Spread by the Extracellular Route but Not for Direct Cell-to-Cell Spread. J Virol. 2011;85(8):3700–3707. doi: 10.1128/JVI.02442-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vischer H.F., Leurs R., Smit M.J. HCMV-encoded G-protein-coupled receptors as constitutively active modulators of cellular signaling networks. Trends Pharmacol Sci. 2006;27(1):56–63. doi: 10.1016/j.tips.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Michel D., Milotic I., Wagner M., Vaida B., Holl J., Ansorge R. The human cytomegalovirus UL78 gene is highly conserved among clinical isolates, but is dispensable for replication in fibroblasts and a renal artery organ-culture system. J Gen Virol. 2005;86(Pt 2):297–306. doi: 10.1099/vir.0.80436-0. [DOI] [PubMed] [Google Scholar]
- 23.Levoye A., Dam J., Ayoub M.A., Guillaume J.L., Couturier C., Delagrange P. The orphan GPR50 receptor specifically inhibits MT1 melatonin receptor function through heterodimerization. EMBO J. 2006;25(13):3012–3023. doi: 10.1038/sj.emboj.7601193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terrillon S., Durroux T., Mouillac B., Breit A., Ayoub M.A., Taulan M. Oxytocin and vasopressin V1a and V2 receptors form constitutive homo- and heterodimers during biosynthesis. Mol Endocrinol. 2003;17(4):677–691. doi: 10.1210/me.2002-0222. [DOI] [PubMed] [Google Scholar]
- 25.Martini L., Waldhoer M., Pusch M., Kharazia V., Fong J., Lee J.H. Ligand-induced down-regulation of the cannabinoid 1 receptor is mediated by the G-protein-coupled receptor-associated sorting protein GASP1. Faseb J. 2007;21(3):802–811. doi: 10.1096/fj.06-7132com. [DOI] [PubMed] [Google Scholar]
- 26.Fraile-Ramos A., Kohout T.A., Waldhoer M., Marsh M. Endocytosis of the viral chemokine receptor US28 does not require beta-arrestins but is dependent on the clathrin-mediated pathway. Traffic. 2003;4(4):243–253. doi: 10.1034/j.1600-0854.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 27.Neote K., DiGregorio D., Mak J.Y., Horuk R., Schall T.J. Molecular cloning, functional expression, and signaling characteristics of a C–C chemokine receptor. Cell. 1993;72(3):415–425. doi: 10.1016/0092-8674(93)90118-a. [DOI] [PubMed] [Google Scholar]
- 28.Ferre S., Baler R., Bouvier M., Caron M.G., Devi L.A., Durroux T. Building a new conceptual framework for receptor heteromers. Nat Chem Biol. 2009;5(3):131–134. doi: 10.1038/nchembio0309-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milligan G. G protein-coupled receptor dimerization: function and ligand pharmacology. Mol Pharmacol. 2004;66(1):1–7. doi: 10.1124/mol.104.000497.. [DOI] [PubMed] [Google Scholar]
- 30.Levoye A., Dam J., Ayoub M.A., Guillaume J.L., Jockers R. Do orphan G-protein-coupled receptors have ligand-independent functions? New insights from receptor heterodimers. EMBO Rep. 2006;7(11):1094–1098. doi: 10.1038/sj.embor.7400838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kledal T.N., Rosenkilde M.M., Schwartz T.W. Selective recognition of the membrane-bound CX3C chemokine, fractalkine, by the human cytomegalovirus-encoded broad-spectrum receptor US28. FEBS Lett. 1998;441(2):209–214. doi: 10.1016/s0014-5793(98)01551-8. [DOI] [PubMed] [Google Scholar]
- 32.Kuhn D.E., Beall C.J., Kolattukudy P.E. The cytomegalovirus US28 protein binds multiple CC chemokines with high affinity. Biochem Biophys Res Commun. 1995;211(1):325–330. doi: 10.1006/bbrc.1995.1814. [DOI] [PubMed] [Google Scholar]
- 33.Waldhoer M., Casarosa P., Rosenkilde M.M., Smit M.J., Leurs R., Whistler J.L. The carboxyl terminus of human cytomegalovirus-encoded 7 transmembrane receptor US28 camouflages agonism by mediating constitutive endocytosis. J Biol Chem. 2003;278(21):19473–19482. doi: 10.1074/jbc.M213179200. [DOI] [PubMed] [Google Scholar]
- 34.Prinster S.C., Hague C., Hall R.A. Heterodimerization of g protein-coupled receptors: specificity and functional significance. Pharmacol Rev. 2005;57(3):289–298. doi: 10.1124/pr.57.3.1. [DOI] [PubMed] [Google Scholar]
- 35.Guo W., Shi L., Filizola M., Weinstein H., Javitch J.A. Crosstalk in G protein-coupled receptors: changes at the transmembrane homodimer interface determine activation. Proc Natl Acad Sci U S A. 2005;102(48):17495–17500. doi: 10.1073/pnas.0508950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sohy D., Yano H., de Nadai P., Urizar E., Guillabert A., Javitch J.A. Hetero-oligomerization of CCR2, CCR5, and CXCR4 and the protean effects of “selective” antagonists. J Biol Chem. 2009;284(45):31270–31279. doi: 10.1074/jbc.M109.054809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Contento R.L., Molon B., Boularan C., Pozzan T., Manes S., Marullo S. CXCR4-CCR5: a couple modulating T cell functions. Proc Natl Acad Sci U S A. 2008;105(29):10101–10106. doi: 10.1073/pnas.0804286105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vila-Coro A.J., Mellado M., Martin de Ana A., Lucas P., del Real G., Martinez A.C. HIV-1 infection through the CCR5 receptor is blocked by receptor dimerization. Proc Natl Acad Sci U S A. 2000;97(7):3388–3393. doi: 10.1073/pnas.050457797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ashall L., Horton C.A., Nelson D.E., Paszek P., Harper C.V., Sillitoe K. Pulsatile stimulation determines timing and specificity of NF-kappaB-dependent transcription. Science. 2009;324(5924):242–246. doi: 10.1126/science.1164860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DeMeritt I.B., Milford L.E., Yurochko A.D. Activation of the NF-kappaB pathway in human cytomegalovirus-infected cells is necessary for efficient transactivation of the major immediate-early promoter. J Virol. 2004;78(9):4498–4507. doi: 10.1128/JVI.78.9.4498-4507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caposio P., Musso T., Luganini A., Inoue H., Gariglio M., Landolfo S. Targeting the NF-kappaB pathway through pharmacological inhibition of IKK2 prevents human cytomegalovirus replication and virus-induced inflammatory response in infected endothelial cells. Antiviral Res. 2007;73(3):175–184. doi: 10.1016/j.antiviral.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Benedict C.A., Angulo A., Patterson G., Ha S., Huang H., Messerle M. Neutrality of the canonical NF-kappaB-dependent pathway for human and murine cytomegalovirus transcription and replication in vitro. J Virol. 2004;78(2):741–750. doi: 10.1128/JVI.78.2.741-750.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gustems M., Borst E., Benedict C.A., Perez C., Messerle M., Ghazal P. Regulation of the transcription and replication cycle of human cytomegalovirus is insensitive to genetic elimination of the cognate NF-kappaB binding sites in the enhancer. J Virol. 2006;80(19):9899–9904. doi: 10.1128/JVI.00640-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evers D.L., Wang X., Huang E.S. Cellular stress and signal transduction responses to human cytomegalovirus infection. Microbes Infect. 2004;6(12):1084–1093. doi: 10.1016/j.micinf.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 45.Zhu H., Cong J.P., Mamtora G., Gingeras T., Shenk T. Cellular gene expression altered by human cytomegalovirus: global monitoring with oligonucleotide arrays. Proc Natl Acad Sci U S A. 1998;95(24):14470–14475. doi: 10.1073/pnas.95.24.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaudhary P.M., Jasmin A., Eby M.T., Hood L. Modulation of the NF-kappa B pathway by virally encoded death effector domains-containing proteins. Oncogene. 1999;18(42):5738–5746. doi: 10.1038/sj.onc.1202976. [DOI] [PubMed] [Google Scholar]
- 47.Matta H., Chaudhary P.M. Activation of alternative NF-kappa B pathway by human herpes virus 8-encoded Fas-associated death domain-like IL-1 beta-converting enzyme inhibitory protein (vFLIP) Proc Natl Acad Sci U S A. 2004;101(25):9399–9404. doi: 10.1073/pnas.0308016101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitchell T., Sugden B. Stimulation of NF-kappa B-mediated transcription by mutant derivatives of the latent membrane protein of Epstein-Barr virus. J Virol. 1995;69(5):2968–2976. doi: 10.1128/jvi.69.5.2968-2976.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lei X, Bai Z, Ye F, Xie J, Kim CG, Huang Y, et al. Regulation of NF-kappaB inhibitor IkappaBalpha and viral replication by a KSHV microRNA. Nat Cell Biol 2010; 12(2):193–9. [DOI] [PMC free article] [PubMed]
- 50.Krug L.T., Moser J.M., Dickerson S.M., Speck S.H. Inhibition of NF-kappaB activation in vivo impairs establishment of gammaherpesvirus latency. PLoS Pathog. 2007;3(1):e11. doi: 10.1371/journal.ppat.0030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soderberg-Naucler C. HCMV microinfections in inflammatory diseases and cancer. J Clin Virol. 2008;41(3):218–223. doi: 10.1016/j.jcv.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 52.Harkins L., Volk A.L., Samanta M., Mikolaenko I., Britt W.J., Bland K.I. Specific localisation of human cytomegalovirus nucleic acids and proteins in human colorectal cancer. Lancet. 2002;360(9345):1557–1563. doi: 10.1016/S0140-6736(02)11524-8. [DOI] [PubMed] [Google Scholar]
- 53.Sabatier J., Uro-Coste E., Pommepuy I., Labrousse F., Allart S., Tremoulet M. Detection of human cytomegalovirus genome and gene products in central nervous system tumours. Br J Cancer. 2005;92(4):747–750. doi: 10.1038/sj.bjc.6602339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maussang D., Langemeijer E., Fitzsimons C.P., Stigter-van Walsum M., Dijkman R., Borg M.K. The human cytomegalovirus-encoded chemokine receptor US28 promotes angiogenesis and tumor formation via cyclooxygenase-2. Cancer Res. 2009;69(7):2861–2869. doi: 10.1158/0008-5472.CAN-08-2487. [DOI] [PubMed] [Google Scholar]
- 55.Maussang D., Verzijl D., van Walsum M., Leurs R., Holl J., Pleskoff O. Human cytomegalovirus-encoded chemokine receptor US28 promotes tumorigenesis. Proc Natl Acad Sci U S A. 2006;103(35):13068–13073. doi: 10.1073/pnas.0604433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bongers G, Maussang D, Muniz LR, Noriega VM, Fraile-Ramos A, Barker N, et al. The cytomegalovirus-encoded chemokine receptor US28 promotes intestinal neoplasia in transgenic mice. J Clin Invest 2010; 120(11):3969–78. [DOI] [PMC free article] [PubMed]
- 57.Streblow D.N., Vomaske J., Smith P., Melnychuk R., Hall L., Pancheva D. Human cytomegalovirus chemokine receptor US28-induced smooth muscle cell migration is mediated by focal adhesion kinase and Src. J Biol Chem. 2003;278(50):50456–50465. doi: 10.1074/jbc.M307936200. [DOI] [PubMed] [Google Scholar]