Abstract
Aims
More than two billion people worldwide are deficient in key micronutrients. Single micronutrients have been used at high doses to prevent and treat dietary insufficiencies. Yet the impact of combinations of micronutrients in small doses aiming to improve lipid disorders and the corresponding metabolic pathways remains incompletely understood. Thus, we investigated whether a combination of micronutrients would reduce fat accumulation and atherosclerosis in mice.
Methods and results
Lipoprotein receptor-null mice fed with an original combination of micronutrients incorporated into the daily chow showed reduced weight gain, body fat, plasma triglycerides, and increased oxygen consumption. These effects were achieved through enhanced lipid utilization and reduced lipid accumulation in metabolic organs and were mediated, in part, by the nuclear receptor PPARα. Moreover, the micronutrients partially prevented atherogenesis when administered early in life to apolipoprotein E-null mice. When the micronutrient treatment was started before conception, the anti-atherosclerotic effect was stronger in the progeny. This finding correlated with decreased post-prandial triglyceridaemia and vascular inflammation, two major atherogenic factors.
Conclusion
Our data indicate beneficial effects of a combination of micronutritients on body weight gain, hypertriglyceridaemia, liver steatosis, and atherosclerosis in mice, and thus our findings suggest a novel cost-effective combinatorial micronutrient-based strategy worthy of being tested in humans.
Keywords: Atherosclerosis, Lipids, PPARα, Nutrition, Prevention
1. Introduction
Disorders of lipid metabolism generally result in excessive fat storage, not only in adipose tissue but also in other organs such as liver and arteries. Fat accumulation in hepatocytes results in fatty liver; in arteries, it may result in atherosclerotic plaques. Fatty liver is usually asymptomatic, but 35% of obese individuals with fatty liver will progress to cirrhosis compared with 10% in the general population.1 Ultimately, cirrhosis may lead to liver cancer.2 Obesity is also known to be associated with a systemic inflammatory state that induces endothelial dysfunction, thereby favouring atherogenesis.3 In fact, endothelial cell activation enhances recruitment of macrophages into the subendothelial space where they promote atherosclerotic plaque formation.4 Rupture of an atherosclerotic plaque may cause myocardial infarction, the leading cause of mortality in Western countries.
The high morbidity associated with obesity warrants preventive measures. Strategies for weight gain prevention address socio-environmental factors by promoting healthy nutrition and regular exercise. Unfortunately, there is little evidence for lasting effects of these interventions.5 Plasma lipid-lowering drugs are most commonly used to reduce obesity-related complications,6 but so far, no effective approach is available for prevention of both obesity and its cardiovascular complications.
We hypothesized that combinations of micronutrients may meet this challenge. The most recent definition of a micronutrient from Mosby's Medical Dictionary (2009) describes them as ‘any dietary element essential only in minute amounts for the normal physiologic processes of the body, including vitamins and minerals or chemical elements, such as zinc or iodine'. Most studies have evaluated the beneficial effects of one micronutrient on a single parameter of lipid metabolism. We still do not know all dietary elements that are essential for its fine-tuning, but different micronutrients must cooperate to allow optimal cell activity.7
In the present study, we test an original combination of specific micronutrients, which comprises several phyto-ingredients and oils with beneficial effects on lipid metabolism (Supplementary material online, Table S1). In fact, bioactive phytochemicals have been proposed for the prevention and treatment of diabetic complications and natural product libraries are a rich source of PPAR modulators in the treatment of cardiometabolic syndrome.8,9 We anticipated that when assembled in well-defined proportions, such ingredients combined to classic micronutrients would cooperate to produce effects at low concentrations. We show herein that the combination called lipistase10 meets this expectation and reduces body weight gain, plasma lipids, hepatic steatosis, and atherosclerosis in mice deficient in low-density lipoprotein receptor (LDLrKO) or in apolipoprotein E (APOEKO), two common models for investigating adiposity or atherosclerosis, respectively.
2. Methods
2.1. Ethics statement
All experiments involving animals described herein were approved by the Veterinary Office of the Canton Vaud (Switzerland) (authorizations 1868.1, 1868.2, and 1868.3) in accordance with the Federal Swiss Veterinary Office Guidelines and conform to the European Commission Directive 86/609/EEC. Blood collection and cervical dislocation were carried out after anaesthesia with 1.45% isoflurane. Depth of anaesthesia was monitored by toe pinch reflex test.
2.2. Animals
The mouse strains used are established models for lipid disorder studies and are either deficient in the receptor of low-density lipoprotein (LDLrKO mice, four 9-week-old males per group) or apolipoprotein E (APOEKO mice, ten 3-week-old males per group; ten 10-week-old males per group, and fifteen males per group for the antenatal study). LDLrKO-PPARαKO mice (five 9-week-old males per group) were used to assess the importance of PPARα in the effects of lipistase. All mice had free access to food and water.
2.3. Diet and micronutrition
Mice were fed a standard chow (3436, Provimi Kliba, Switzerland) for 3 months (antenatal treated mice) or 10 months (long-term treated mice). The micronutrient combination lipistase10 was added to this diet before pelleting, using soy oil with or without lipistase given at a concentration of 17 µg/L for a final concentration in food of 350 ng/kg. Littermate control mice were fed with the same standard chow without lipistase. Trace elements, vitamins, and minerals are already present in chow diet and are only marginally increased in the lipistase diet (in total, 174.9 ng more per kg of diet). The ingredients that are exclusively contained in the lipistase diet are the oils and the plant extracts, as detailed in Supplementary material online, Table S1.
2.4. Body weight, fat mass, PPARα transactivation assay, and mRNA levels
Mice were weighed before treatment and monthly during the study. In parallel, food intake was monitored. The body fat mass was measured with an EchoMRI apparatus (Colombus Instruments, Ohio, USA). PPARα transactivation assay was performed using the GAL4/UAS system. NIH3T3 cells were transfected with either GAL4 alone or GAL4 fused to the PPARα ligand-binding domain (LBD), in addition to a luciferase reporter plasmid. Renilla reporter plasmid was used as a transfection control. The four plasmids used for this transfection, GAL4, GAL4-LBD, 5xUAS-TK-LUC, and RENILLA, were from our in-house stocks. SuperFect Transfection Reagent (QIAGEN, Switzerland) and the Luciferase Assay System (Promega, Switzerland) were used according to the manufacturers’ recommendations. After transfection, cells were incubated with different compounds in a starved medium for 24 h before harvesting. The extraction of RNA and its analysis by RT–qPCR were according to standard procedures. The values were normalized to those obtained for a housekeeping mRNA. Sequences of the oligonucleotides used are given in the Supplementary material online, Table S2.
2.5. Lipid utilization
Blood was collected via retro-orbital puncture and plasma was recovered by centrifugation. Triglyceride plasma levels were measured using a Roche Diagnostics/Hitachi 902 analyzer (Mannheim, Germany). Paraffin embedded LDLrKO mice gastrocnemius muscle samples were sectioned (4 µm) and incubated 2 h at 37°C with 1/200 diluted rabbit polyclonal anti-CD36 antibody (Novus Biologicals, Inc. Littleton, CO, USA). Immunostaining was via a fluorescent secondary antibody, goat anti-rabbit Alexa 568 (Invitrogen, Basel, Switzerland). For CD36 quantification by immunoblotting, 4 µg and 6 µg of cytosolic and membrane extracts, respectively, were loaded and probed with 1/1000 rabbit polyclonal anti-CD36 antibody (Novus Biologicals, Inc. Littleton, CO, USA). An indirect calorimetric analysis, measuring respiratory gases, was performed at the end of the study. Mice were acclimatized in the calorimeter cages of Oxymax/Comprehensive Lab Animal Monitoring System (CLAMS, Columbus Instruments, Columbus, OH, USA) for 24 h and then oxygen consumption was measured for an additional 24 h.
2.6. Adipogenesis
After 10 months, the lipistase-treated LDLrKO mice and the untreated controls were anaesthetized with 1.45% isoflurane and sacrificed. Adipose tissue and liver samples were paraffin embedded. Sections of 4 µm thickness were stained with haematoxylin–eosin and histology was studied under an optic microscope. Rat anti-F4/80 antibody (Abcam, Cambridge, UK) was used to stain adipose tissue macrophages and the immunostaining was revealed by an anti-rat HRP (Invitrogen, Basel, Switzerland).
For the adipogenesis assay, we used the pre-adipocytes 3T3L1 cells (in-house stock) at 2 days post-confluence (day 0). We first incubated the cells for 2 days with 16 µL/mL of the cocktail IBMX (10 mM)/dexamethasone (50 µM)/insulin (10 mg/mL) (Sigma, Buchs, Switzerland) in order to induce their differentiation into adipocytes able to accumulate lipids. During the induction phase, the cells were also treated with either rosiglitazone (1 μM) alone (Alexis Biochemicals, Lausen, Switzerland) or with rosiglitazone (1 μM) and lipistase (160 pg/mL). At day 3, the cells were incubated with these treatments without the cocktail for 5 days. The medium was changed every 2 days during the whole assay. At day 8, cells were fixed with PFA 4% for 10 min and stained with Oil-red O for 10 min. After washing, the optic density of each plate was measured with a spectrophotometer at a wavelength of 520 nm.
2.7. Hepatic neutral lipids content
Hepatic cholesterol, cholesterol esters, and triglycerides were determined by gas chromatography analysis as described elsewhere11 from snap frozen liver biopsies.
2.8. Atherosclerosis
APOEKO mice treated from the age of 3 or 10 weeks were sacrificed after 3 or 10 months of treatment, respectively, and the aortic roots were dissected. In the antenatal study, the pups of lipistase-treated APOEKO mice were again treated for 3 months after weaning and were then sacrificed (the parents were treated 1 month before mating, and the mothers during pregnancy and lactation). Atherosclerotic plaques were quantified in cryo-cross-sections (8 µm thickness) of aortic roots stained with Oil-red O using digital morphometry (Igor6, Wavemetrics Inc., Lake Oswego, USA). Rat monoclonal anti-vascular cell adhesion molecule 1 (VCAM1) and anti-CD68 antibodies, and rabbit polyclonal anti-α smooth muscle actin (αSMA) antibody (Abcam, Cambridge, UK) were used on frozen sections of antenatal-treated aortic roots. The immunostaining was revealed by a fluorescent secondary antibody, goat anti-rat/rabbit Alexa 568 (Invitrogen, Basel, Switzerland). For the postprandial plasma triglyceride test, APOEKO mice after 3 months treatment with lipistase or vehicle from the antenatal study were fasted for 12 h. After fasting, they received 300 µL of soy oil containing or not lipistase (17 µL/L) by oral gavages and blood samples were collected before gavages (time 0) and every hour during 5 h.
2.9. Statistics
Analysis of variance for repeated measurements, paired or unpaired t-test, was used where appropriate. Normal distribution was tested using the Shapiro Wilk's test (SPSS13, SPSS Inc. and Prizm, GraphPad Inc.). Values are expressed as mean ± SEM.
3. Results
3.1. Body weight, fat mass, and circulating triglycerides
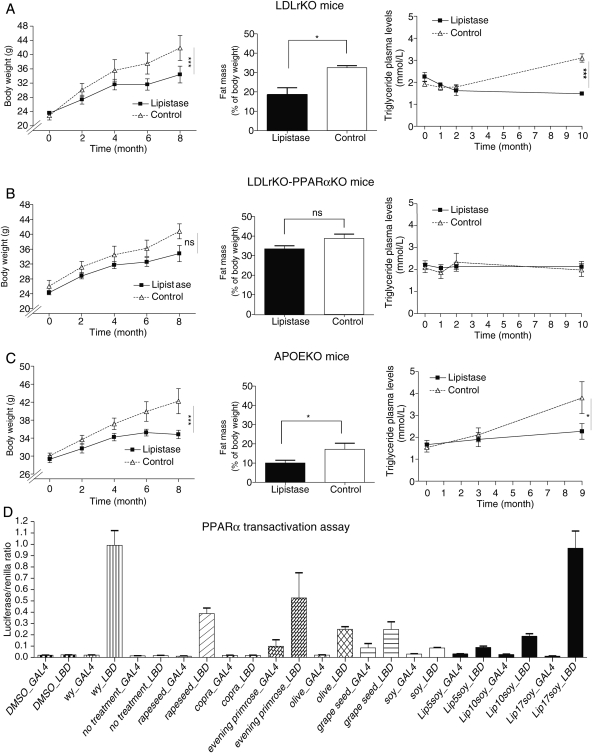
Lipistase comprises 26 ingredients, including trace metals, minerals, vitamins, phyto-ingredients, and oils designed to impact lipid metabolism.10 To assess the effects of long-term lipistase treatment on body weight, three mouse strains (LDLrKO, LDLrKO-PPARαKO, and APOEKO) received the combinatorial micronutrient preparation at low doses in the daily chow for up to 10 months. Control littermates received the same diet without micronutrients. Despite a similar food intake (Supplementary material online, Figure S1), the treated LDLrKO and APOEKO mice gained less weight, developed less fat mass, and showed reduced plasma triglyceride levels when compared with untreated mice (Figure 1A and C). Interestingly, these differences were reduced in micronutrient-treated LDLrKO-PPARαKO mice compared with their control littermates, suggesting a role of the nuclear receptor PPARα in mediating the effects of lipistase (Figure 1B). This was further supported by the almost two-fold increase of fat accumulation in lipistase-treated LDLrKO-PPARαKO compared with the lipistase-treated LDLrKO mice. The untreated LDLrKO-PPARαKO mice also accumulated more fat than the LDLrKO mice, but to a lesser extent. In addition, the LDLrKO-PPARαKO animals accumulated three–four-fold more neutral lipids in their liver (see below), which together with their increased adiposity may explain their unchanged triglyceride levels during the course of the experiment (Figure 1B). These observations prompted us to investigate whether lipistase activates PPARα, a major regulator of lipid homeostasis including blood triglyceride levels.12,13 Using NIH3T3 cells and the GAL4/UAS system as transactivation assay for the PPARα LBD (Figure 1D), we observed a dose-dependent PPARα activation by lipistase, reaching levels obtained with the synthetic PPARα ligand, WY-14 643, and exceeding the level of activation by several lipistase phyto-ingredients tested individually. Moreover, this assay showed that evening primrose oil is a powerful PPARα activator. Taken together, these results indicate that lipistase reduces weight gain in different mouse models by decreasing fat mass expansion. PPARα may participate in these beneficial effects.
Figure 1.
Lipistase reduces body weight gain and body fat mass via PPARα activation. (A–C) Body weight gain, body fat mass, and plasma triglycerides in lipistase-treated LDLrKO, LDLrKO-PPARαKO, and APOEKO mice vs. controls. (D) PPARα transactivation assay using the GAL4/UAS system. NIH3T3 cells were transfected with either GAL4 (GAL4) alone or GAL4 linked to the PPARα LBD. The activators of PPARα bind to LBD and hence trigger the transcription of a Luciferase reporter gene via binding of the GAL4 DNA-binding domain to the upstream activation sequence (UAS) in the regulatory region of this gene. A Renilla reporter gene is used as a transfection control. LIP5SOY, LIP10SOY, LIP17SOY: lipistase at 5, 10, and 17 µg/L of soy oil used at 1 µL/mL (final concentrations of lipistase: 5, 10, and 17 pg/mL of medium); Soy: soy oil as control of the effect of the lipistase vehicle used at 1 µL/mL; WY-14 643 (wy): synthetic PPARα ligand used as positive control (1 µM as final concentration). Rapeseed oil, copra oil, evening primrose oil, olive oil, and grape seed oil were all used at 1 µL/mL of medium [ns, not significant; *P<0.05; ***P < 0.001; n(NIH3T3 cells, D) = 2 × 3 wells].
3.2. Lipid utilization in muscle
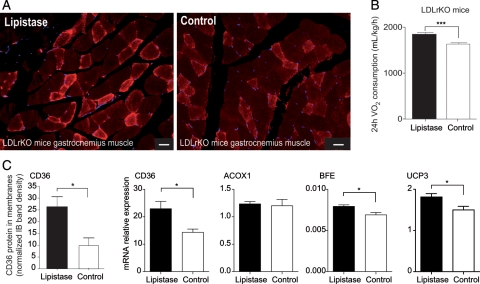
Skeletal muscle is the primary site of lipid utilization.14 Thus, we investigated the effects of lipistase on the expression of CD36, a key factor involved in lipid uptake and utilization.15 LDLrKO mice treated with lipistase for 10 months expressed higher levels of CD36 mRNA and protein in muscle (Figure 2A and C). Consistent with enhanced lipid oxidation, the mRNA levels of the peroxisomal bifunctional enzyme (BFE) and mitochondrial uncoupling protein 3 (UCP3) as well as oxygen consumption were significantly higher in lipistase-treated LDLrKO mice compared with controls (Figure 2B and C). These results suggest a lipistase-dependent stimulation of muscle oxidative activity.
Figure 2.
Lipistase enhances muscle lipid utilization in LDLrKO mice. (A) Representative images of anti-CD36 immunostaining on paraffin-embedded sections of oxidative muscle (gastrocnemius) from mice after 10-month lipistase treatment vs. controls (bars = 50 μm). (B) Oxygen consumption measured after the 10-month treatment (***P<0.001). (C) Immunoblot quantification of the membrane-bound CD36 protein in muscle and mRNA expression of marker genes for fatty acid transport and oxidation measured in gastrocnemius 10 months after lipistase treatment (*P<0.05).
3.3. Lipid storage and macrophage recruitment in white adipose tissue
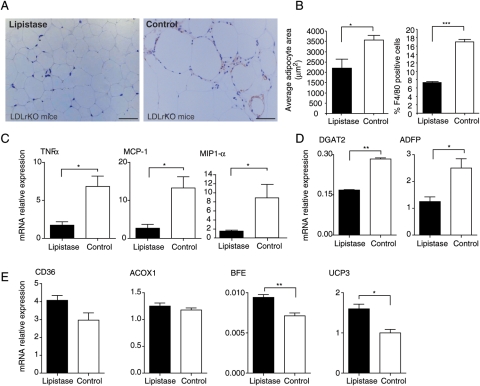
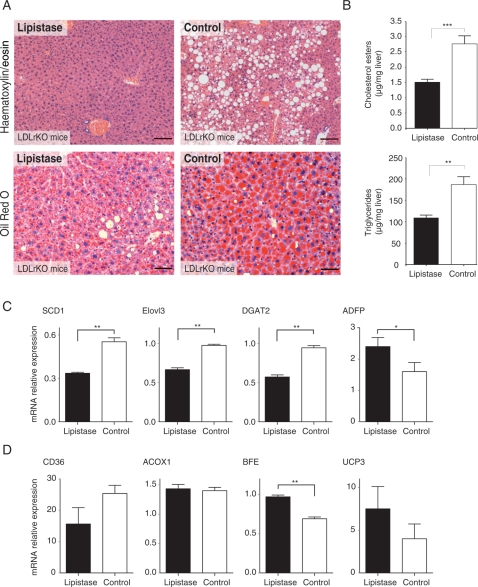
By increasing lipid catabolism, lipistase was anticipated to decrease body fat accumulation. In fact, lipistase treatment resulted in smaller adipocytes and reduced body fat mass (Figures 1A and 3A and B). Furthermore, macrophage infiltration of adipose tissue and associated inflammatory markers, which are hallmarks of obesity,16 were much reduced in the adipose tissue of lipistase-treated LDLrKO mice (Figure 3A–C). Consistent with these observations, the mRNA levels of diacylglycerol O-acyltransferase 2 (DGAT2) and of the major constituent of the lipid droplet surface, the adipocyte differentiation-related protein (ADFP), were decreased by lipistase treatment (Figure 3D). In parallel, BFE and UCP3 were increased, most likely reflecting an enhanced oxidative fatty acid catabolism (Figure 3E). These results indicate that lipistase prevents adipose tissue expansion and inflammation and increases its oxidative capacity.
Figure 3.
Lipistase reduces inflammation and impacts lipogenic and oxidative pathways in adipocytes of LDLrKO mice. (A) Anti-F4/80 immunostaining of macrophages (appearing in brown) of epididymal white adipose tissue paraffin-embedded sections counterstained with haematoxylin and eosin from animals after 10-month lipistase treatment vs. controls (bars = 50 μm). (B) Adipocyte surface area (left) and F4/80 positive cells (right) were quantified from representative sections. (C) mRNA expression levels of inflammatory markers and (D) of lipogenic markers. (E) Expression levels of fatty acid transport and oxidation marker genes measured on white adipose tissue (*P<0.05; **P<0.01).
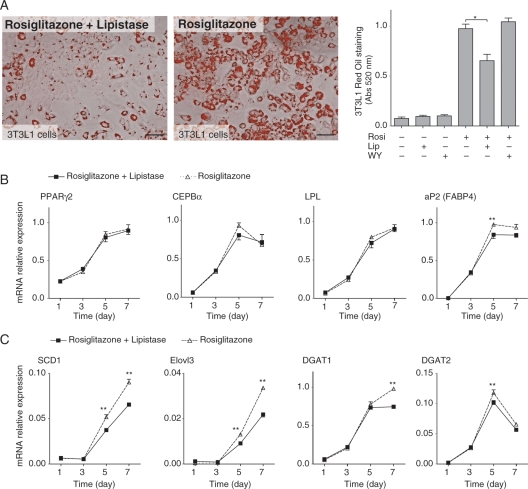
To investigate a possible direct effect of lipistase on adipogenesis, cultured 3T3L1 cells were induced to accumulate lipids with the standard IBMX cocktail and then treated with either rosiglitazone, a stimulator of adipogenesis, or rosiglitazone and lipistase. Lipistase prevented triglyceride and neutral lipid (Oil-red O staining) accumulation compared with cells treated with rosiglitazone alone (Figure 4A). Interestingly, lipid loading was not significantly impacted by the PPARα ligand WY-14 643, indicating that in this experimental cell culture set-up, the lipistase effects do not depend on PPARα (Supplementary material online, Figure S2). Lipistase did not inhibit genes involved in adipocyte differentiation (PPARγ2; CCAAT/enhancer binding protein α, CEBPα; lipoprotein lipase, LPL), with the exception of adipocyte protein 2 (aP2/FABP4), which was reduced (Figure 4B). Genes encoding secreted adipokines, such as leptin, adiponectin, resistin, and adipsin, were not affected either (Supplementary material online, Figure S2B). However, the expression of four lipogenic genes (steaoryl-CoA desaturase, SCD1; elongation of very long chain fatty acids 3, Elovl3; diacylglycerol acyltransferase 1, DGTA1; DGAT2) among the eight analysed was significantly reduced by lipistase (Figure 4C, Supplementary material online, Figure S2C). Together, these results indicate that lipistase downregulated the lipogenic pathway both in vivo and in 3T3L1 cells, a likely cause for reduced adipocyte lipid accumulation.
Figure 4.
Lipistase reduces lipogenesis in 3T3L1 differentiating cells. (A) Oil-red O staining (left panels; bars = 50 μm) and quantification (right panel) for 3T3L1 differentiated cells (day 8), treated with the PPARγ ligand rosiglitazone (Rosi, 1 μM), the PPARα ligand WY-14643 (WY, 1 μM), and lipistase (Lip, 160 pg/mL). *P = 0.015. Lipistase effect on mRNA expression of (B) adipogenic transcription factors (PPARγ2, CEBPα), adipose differentiation markers (LPL, aP2), and (C) lipogenic enzymes (SCD1, Elovl3, DGAT1, and DGAT2) during differentiation of 3T3L1 cells at the indicated days [**P<0.01; n(3T3L1) = 3 × 6 wells].
3.4. Liver steatosis
Lipistase prevented the development of a massive macrovacuolar liver steatosis (Figure 5A). The lipistase-treated animals accumulated much less cholesterol esters and triglycerides, which are hallmarks of the steatotic condition, while the total cholesterol levels were not changed (Figure 5B and Supplementary material online, Figure S3). It is worth to note that the LDLrKO-PPARαKO mice accumulated three to four times more neutral lipids on which lipistase had less—but still significant for triglycerides—impact in the absence of PPARα (Supplementary material online, Figure S3). The PPARα effect in LDLrKO mice was associated with a lipistase-dependent lower expression of lipogenic genes (Figure 5C) and significantly and moderately higher expression of the fatty acid catabolism genes BFE and UCP3, respectively (Figure 5D), which is reminiscent of the role of lipistase in the adipose tissue.
Figure 5.
Lipistase prevents hepatic steatosis. (A) Liver paraffin-embedded sections, stained with haematoxylin and eosin (upper panels) from animals after 10-month lipistase treatment vs. controls (bars = 100 μm); Oil-red O staining of equivalent sections (lower panels) (bars = 50 µm). (B) Levels of cholesterol esters and triglycerides in the liver of the same animals. (C) mRNA expression levels of lipogenic markers and (D) of fatty acid transport and catabolism markers (*P<0.05; **P<0.01).
Collectively, the decrease in adipose tissue mass and liver steatosis and the increase in oxygen consumption provide evidence that lipistase enhances fatty acid oxidation and attenuates lipogenesis through regulation of the corresponding genes.
3.5. Lipistase-dependent PPARα activity
Previous work has attributed hypolipidaemic properties to PPARα.13 The similarity between the effects of lipistase and PPARα ligands, such as fibrates, prompted us to assess whether the action of lipistase on the expression of genes involved in fatty acid metabolism (CD36, BFE, UCP3) and in lipogenesis (SCD1, Elovl3, DGAT2) is PPARα-dependent. Gene expression analyses in lipistase-treated LDLrKO and LDLrKO-PPARαKΟ mice provided evidence that PPARα contributes to the regulation by lipistase of both analysed pathways (Table 1 and Supplementary material online, Figure S4), but the action of lipistase is not solely mediated by PPARα. Thus, our results provide evidence for both independent and interactive roles of lipistase and PPARα in lipid metabolism.
Table 1.
PPARα-dependent regulation by lipistase
| Gene | Muscle | WAT | Liver |
|---|---|---|---|
| FA uptake and catabolism | |||
| CD 36 | + | NE | + |
| BFE | + | + | + |
| UCP3 | + | + | nd |
| Lipogenesis | |||
| SCD 1 | NE | + | + |
| Elovl 3 | − | NE | +R |
| DGAT 2 | − | − | +R |
The effect of lipistase in LDLrKO mice and in LDLrKO-PPARαKO mice was compared to determine the involvement of PPARα in the lipistase-dependent action. The data are derived from results show in Figures 2–4 and in Supplementary material online, Figure S3. NE, no effect of lipistase; +, PPARα-dependent effect of lipistase; − , no PPARα-dependent effect of lipistase; +R, PPARα-dependent effect of lipistase; inversed effect in LDLrKO-PPARαKO; nd, not determined.
3.6. Prevention of atherosclerosis
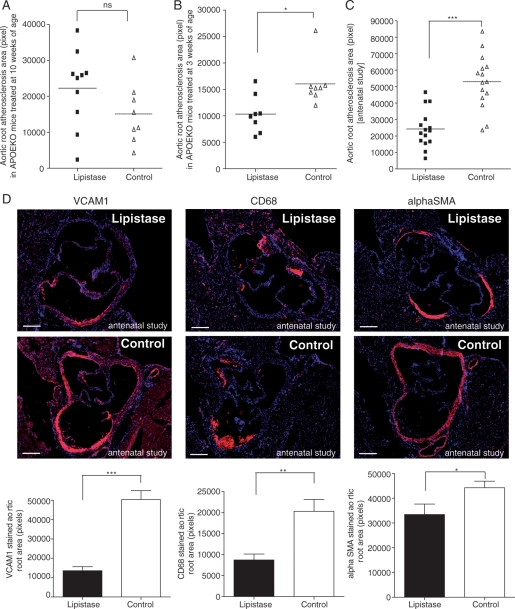
To evaluate the effects of micronutrients on progression of atherosclerosis, we treated APOEKO mice with lipistase for up to 10 months after which an increase in oxygen consumption was observed (Supplementary material online, Figure S5) similarly to LDLrKO mice (Figure 2B). We also evaluated its effects on initiation of atherosclerosis in a third experiment: parent mice, gestating females, lactating females, and post-weaning pups were all fed with lipistase for up to 3 months after weaning, at an age when atherosclerosis has normally developed in untreated animals. Atherosclerotic plaque areas in aortic roots of mice treated with lipistase in adulthood (starting at 10 weeks of age) were not different from controls (Figure 6A). In contrast, mice receiving early (at 3 weeks of age) or antenatal lipistase developed less atherosclerosis at 3 months of age than their control mates (Figure 6B and C; Supplementary material online, Figure S6A and B). Atherosclerotic plaques result from endothelial activation with the expression of adhesion molecules and recruitment of macrophages to the subendothelial space. Upon ingestion of modified lipoproteins, these macrophages become foam cells17 and stimulate proliferation of vascular smooth muscle cells, thereby leading to plaque growth.18 Antenatal lipistase treatment of APOEKO mice decreased VCAM1 and diminished CD68-positive plaque macrophages (Figure 6D). Furthermore, lipistase reduced the number of αSMA-positive smooth muscle cells in micronutrient-treated mice compared with controls (Figure 6D). Since postprandial hypertriglyceridaemia is a main risk factor of atherosclerosis,19 we tested the effect of the lipistase treatment on this parameter. Lipistase reduced postprandial hypertriglyceridaemia in mice with antenatal treatment (Supplementary material online, Figure S7). Importantly, lipistase did not have adverse effects on fertility or pup survival (Supplementary material online, Figure S8).
Figure 6.
Lipistase confers atheroprotection in APOEKO mice. (A) Graph represents area of atherosclerotic lesions in aortic roots from mice treated with lipistase (10 mice) or vehicle (Control, 8 mice) starting at 10 weeks of age for 10 months. (B) The graph illustrates atherosclerotic plaque area in aortic roots (lipistase, eight animals; control, eight animals) from mice treated with lipistase or vehicle (Control) starting at 3 weeks of age for 3 months. (C) The graph shows plaque area in aortic roots (15 animals) from offspring of lipistase- or vehicle- (control) treated mice. These offsprings were also treated after weaning for 3 months. (D) Anti-VCAM1, anti-CD68, and anti-αSMA immunostaining (red) of aortic roots from the offspring of lipistase- or vehicle-treated mice (DAPI, blue) (left panels) with corresponding quantification (graphs, right panels). Samples from the same animals as in (C) are shown (bars = 200µm) (ns, not significant; *P<0.05; **P<0.01; ***P<0.001).
4. Discussion
The global escalation of obesity is a major health problem, but so far, preventive measures have received little attention. Our study demonstrates a preventive impact of a fine-tuned micronutrient combination on obesity and atherosclerosis in different mouse strains. Indeed, in LDLrKO and APOEKO mice, but less in LDLrKO-PPARαKO mice, we observed a reduction in body weight gain, reflecting a lower fat mass despite a similar food intake when compared with controls. Lipistase treatment in LDLrKO mice increased lipid utilization by skeletal muscle and reduced fat accumulation in white adipose tissue and liver by stimulating fatty acid uptake and catabolism and by reducing lipogenesis. Lipistase administration in APOEKO mice had preventive effects on atherogenesis, which was strongest in the offspring of parents already treated with lipistase.
PPARα, which is known to reduce fat accumulation and atherosclerosis,13 was found to be activated by lipistase, revealing a role of PPARα in participating in micronutrient actions. Previous work has shown that reduction in body weight gain via PPARα activation is mediated by an up-regulation of fatty acid oxidation and a decreased de novo lipogenesis in liver and adipose tissue, respectively.13,20 Activated PPARα causes a reduction in plasma triglyceride levels by inducing genes that decrease the availability of fatty acids for hepatic triglyceride production, and genes that promote plasma triglyceride clearance through lipoprotein lipase-dependent triglyceride lipolysis.21 Our data demonstrate that lipistase affects most of these pathways.
The primary site for triglyceride clearance and lipid utilization is the skeletal muscle, an organ that plays a major role in the regulation of triglyceridaemia and body weight.14 The fatty acid receptor CD36, which is coded by a PPARα target gene in muscle, is one of the rate-limiting factors for skeletal muscle lipid catabolism.22 Its expression was increased by lipistase treatment in a PPARα-dependent manner, suggesting an enhancement of lipid utilization and fatty acid oxidation.23 Lipistase-treated mice showed increased whole-body oxygen consumption, known to positively correlate with fat catabolism in skeletal muscle.24,25 Stimulation of lipid utilization was reflected in our study by decreased body fat mass, adipocyte size, and hepatic steatosis. Supporting these observations, lipistase counteracted in vitro the effects of rosiglitazone, a stimulator of adipogenesis. This effect was not PPARα-dependent in cell culture showing that lipistase has a broad spectrum of effects and is not merely a PPARα activator.
In our study, the anti-atherosclerotic effects observed upon lipistase treatment are also reminiscent of those described for PPARα.26–30 Lipistase effects were observed when APOEKO mice were treated immediately after weaning and they were more pronounced in animals for which treatment started already in their parents. It remains to be determined whether lipistase unfolds its effects mainly before or during gestation, lactation, or both and whether epigenetic modifications are involved. Interestingly, recent studies indicated that a parental diet may affect lipid metabolism in offspring. Epigenomic profiling of offspring livers revealed reproducible changes in methylation over a likely enhancer for the PPARα gene.31 Metabolic adaptations in utero to nutritional supply have been shown to determine the risk of adult chronic diseases, such as metabolic syndrome and atherosclerosis.32,33 The present study is the first one to show that experimental atherosclerosis in mice can be reduced by an antenatal nutritional intervention and hence offers a prevention strategy to diminish foetal determinants of adult vascular disease.
Further studies are required to address the safety profile of lipistase treatment in terms of fertility, unharmed pregnancy, and breast-feeding. The absence of difference in the litter size between treated and control mice (under observation for 10 months: 14 litters per group) and the normal morphology and behaviour of pups are encouraging. If effective in humans, using lipistase in primary prevention could contribute to fight the world pandemics of obesity and cardiovascular diseases, in support of the World Health Organization recommendation to begin prevention early in life.34
Endothelial dysfunction may be triggered by postprandial hypertriglyceridaemia35 and clearance of postprandial plasma triglycerides is delayed in patients with atherosclerosis.36 In our antenatally treated APOEKO mice, lipistase decreased postprandial plasma triglyceride levels. In addition, lipistase-mediated increase in CD36 expression in skeletal muscle suggests increased lipid uptake in muscle tissue. These findings imply that lipistase reduces atherogenesis at least in part by increasing postprandial plasma triglyceride clearance through an elevated lipid uptake in skeletal muscle, the first tissue destination of dietary lipids during the immediate postprandial period.37 Obviously, several mechanisms by which lipistase may prevent atherogenesis are possible and some of which may implicate PPARα. Reduced intestinal absorption and/or an accelerated plasma clearance of dietary fat are two of them to be studied in the future.
Taken together, our results demonstrate beneficial effects of this novel combinatorial micronutrition on obesity, hypertriglyceridaemia, liver steatosis, and atherosclerosis that are mediated in part by PPARα and achieved through enhanced lipid utilization by skeletal muscle, as well as increased oxidative capacity and reduced lipid accumulation in adipose tissue and liver. In contrast to cholesterol lowering agents, which should be avoided in pregnancy and/or lactation,38 the combination of micronutrients analysed in mice seems to be safe. Lipistase represents a nutraceutical that could be taken as tablet or included into human food such as diary products. Thus, double-blind placebo-controlled lipistase studies in small patient cohorts are the next logical step to assess its value with regard to body fat accumulation, plasma lipids, and vascular disease. Moreover, the complex combinatorial mode and the low doses of micronutrients used introduce a novel concept and highlight the attractive potential of micronutrition-based, cost-effective preventive interventions.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by Bioresearch & Partners SA (Monthey, Switzerland) and the Swiss National Science Foundation through grants to W.W. (310030-113404) and C.M.M. (33CM30-124112). Funding to pay the Open Access publication charges for this article was provided by a grant from the Bonizzi–Theler Foundation.
Supplementary Material
Acknowledgements
We thank Catherine Roger, Marianne Carrard, Corinne Tallichet-Blanc (Center for Integrative Genomics), and Justine Bertrand-Michel (Toulouse Lipidomic Core Facility (MetaToul-INSERM U1048)) for expert technical assistance, Annette Cornély (Bioresearch & Partners) for the lipistase solutions, Benoit Lhermitte for pathology analyses, Hervé Guillou for advice and discussions, and Nathalie Constantin for her help in preparing the manuscript.
Conflict of interest: W.W. owns equity in a company developing therapeutic nutrition.
References
- 1.Reddy JK, Rao MS. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am J Physiol Gastrointest Liver Physiol. 2006;290:G852–G858. doi: 10.1152/ajpgi.00521.2005. doi:10.1152/ajpgi.00521.2005. [DOI] [PubMed] [Google Scholar]
- 2.Qian Y, Fan JG. Obesity, fatty liver and liver cancer. Hepatobiliary Pancreat Dis Int. 2005;4:173–177. [PubMed] [Google Scholar]
- 3.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. doi:10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 4.Fantuzzi G, Mazzone T. Adipose tissue and atherosclerosis: exploring the connection. Arterioscler Thromb Vasc Biol. 2007;27:996–1003. doi: 10.1161/ATVBAHA.106.131755. doi:10.1161/ATVBAHA.106.131755. [DOI] [PubMed] [Google Scholar]
- 5.Lemmens VE, Oenema A, Klepp KI, Henriksen HB, Brug J. A systematic review of the evidence regarding efficacy of obesity prevention interventions among adults. Obes Rev. 2008;9:446–455. doi: 10.1111/j.1467-789X.2008.00468.x. doi:10.1111/j.1467-789X.2008.00468.x. [DOI] [PubMed] [Google Scholar]
- 6.Frisinghelli A, Mafrici A. Regression or reduction in progression of atherosclerosis, and avoidance of coronary events, with lovastatin in patients with or at high risk of cardiovascular disease: a review. Clin Drug Investig. 2007;27:591–604. doi: 10.2165/00044011-200727090-00001. doi:10.2165/00044011-200727090-00001. [DOI] [PubMed] [Google Scholar]
- 7.Walden RJ. Micronutrition. Lancet. 1995;346:635. doi: 10.1016/s0140-6736(95)91464-1. doi:10.1016/S0140-6736(95)91464-1. [DOI] [PubMed] [Google Scholar]
- 8.Huang TH, Teoh AW, Lin BL, Lin DS, Roufogalis B. The role of herbal PPAR modulators in the treatment of cardiometabolic syndrome. Pharmacol Res. 2009;60:195–206. doi: 10.1016/j.phrs.2009.03.020. doi:10.1016/j.phrs.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Omar EA, Kam A, Alqahtani A, Li KM, Razmovski-Naumovski V, Nammi S, et al. Herbal medicines and nutraceuticals for diabetic vascular complications: mechanisms of action and bioactive phytochemicals. Curr Pharm Des. 2010;16:3776–3807. doi: 10.2174/138161210794455076. doi:10.2174/138161210794455076. [DOI] [PubMed] [Google Scholar]
- 10.Bourgeois-Lugand MFY, Wahli W, El Kochairi I, Pradervand S. Composition for regulating lipid metabolism. 2008. World Intellectual Property Organization WO 2009/050580: Bioresearch & Partners et al.
- 11.Zadravec D, Brolinson A, Fisher RM, Carneheim C, Csikasz RI, Bertrand-Michel J, et al. Ablation of the very-long-chain fatty acid elongase ELOVL3 in mice leads to constrained lipid storage and resistance to diet-induced obesity. FASEB J. 2010;24:4366–4377. doi: 10.1096/fj.09-152298. doi:10.1096/fj.09-152298. [DOI] [PubMed] [Google Scholar]
- 12.van Raalte DH, Li M, Pritchard PH, Wasan KM. Peroxisome proliferator-activated receptor (PPAR)-alpha: a pharmacological target with a promising future. Pharm Res. 2004;21:1531–1538. doi: 10.1023/b:pham.0000041444.06122.8d. doi:10.1023/B:PHAM.0000041444.06122.8d. [DOI] [PubMed] [Google Scholar]
- 13.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–424. doi: 10.1038/35013000. doi:10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 14.Bessesen DH, Rupp CL, Eckel RH. Trafficking of dietary fat in lean rats. Obes Res. 1995;3:191–203. doi: 10.1002/j.1550-8528.1995.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 15.Holloway GP, Luiken JJ, Glatz JF, Spriet LL, Bonen A. Contribution of FAT/CD36 to the regulation of skeletal muscle fatty acid oxidation: an overview. Acta Physiol (Oxf) 2008;194:293–309. doi: 10.1111/j.1748-1716.2008.01878.x. doi:10.1111/j.1748-1716.2008.01878.x. [DOI] [PubMed] [Google Scholar]
- 16.Tordjman J, Guerre-Millo M, Clement K. Adipose tissue inflammation and liver pathology in human obesity. Diabetes Metab. 2008;34:658–663. doi: 10.1016/S1262-3636(08)74601-9. doi:10.1016/S1262-3636(08)74601-9. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan M, Aviram M. Oxidized low density lipoprotein: atherogenic and proinflammatory characteristics during macrophage foam cell formation. An inhibitory role for nutritional antioxidants and serum paraoxonase. Clin Chem Lab Med. 1999;37:777–787. doi: 10.1515/CCLM.1999.118. doi:10.1515/CCLM.1999.118. [DOI] [PubMed] [Google Scholar]
- 18.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr., et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1995;15:1512–1531. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- 19.Fujioka Y, Ishikawa Y. Remnant lipoproteins as strong key particles to atherogenesis. J Atheroscler Thromb. 2009;16:145–154. doi: 10.5551/jat.e598. [DOI] [PubMed] [Google Scholar]
- 20.Mancini FP, Lanni A, Sabatino L, Moreno M, Giannino A, Contaldo F, et al. Fenofibrate prevents and reduces body weight gain and adiposity in diet-induced obese rats. FEBS Lett. 2001;491:154–158. doi: 10.1016/s0014-5793(01)02146-9. doi:10.1016/S0014-5793(01)02146-9. [DOI] [PubMed] [Google Scholar]
- 21.Duval C, Muller M, Kersten S. PPARalpha and dyslipidemia. Biochim Biophys Acta. 2007;1771:961–971. doi: 10.1016/j.bbalip.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Hajri T, Abumrad NA. Fatty acid transport across membranes: relevance to nutrition and metabolic pathology. Annu Rev Nutr. 2002;22:383–415. doi: 10.1146/annurev.nutr.22.020402.130846. doi:10.1146/annurev.nutr.22.020402.130846. [DOI] [PubMed] [Google Scholar]
- 23.Holloway GP, Bonen A, Spriet LL. Regulation of skeletal muscle mitochondrial fatty acid metabolism in lean and obese individuals. Am J Clin Nutr. 2009;89:455S–462S. doi: 10.3945/ajcn.2008.26717B. doi:10.3945/ajcn.2008.26717B. [DOI] [PubMed] [Google Scholar]
- 24.Helge JW, Stallknecht B, Richter EA, Galbo H, Kiens B. Muscle metabolism during graded quadriceps exercise in man. J Physiol. 2007;581:1247–1258. doi: 10.1113/jphysiol.2007.128348. doi:10.1113/jphysiol.2007.128348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coyle EF. Fat oxidation during whole body exercise appears to be a good example of regulation by the interaction of physiological systems. J Physiol. 2007;581:886. doi: 10.1113/jphysiol.2007.134890. doi:10.1113/jphysiol.2007.134890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marx N, Sukhova GK, Collins T, Libby P, Plutzky J. PPARalpha activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation. 1999;99:3125–3131. doi: 10.1161/01.cir.99.24.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nawa T, Nawa MT, Cai Y, Zhang C, Uchimura I, Narumi S, et al. Repression of TNF-alpha-induced E-selectin expression by PPAR activators: involvement of transcriptional repressor LRF-1/ATF3. Biochem Biophys Res Commun. 2000;275:406–411. doi: 10.1006/bbrc.2000.3332. doi:10.1006/bbrc.2000.3332. [DOI] [PubMed] [Google Scholar]
- 28.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. doi:10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 29.Zandbergen F, Plutzky J. PPARalpha in atherosclerosis and inflammation. Biochim Biophys Acta. 2007;1771:972–982. doi: 10.1016/j.bbalip.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dashwood MR, Tsui JC. Endothelin-1 and atherosclerosis: potential complications associated with endothelin-receptor blockade. Atherosclerosis. 2002;160:297–304. doi: 10.1016/s0021-9150(01)00586-x. doi:10.1016/S0021-9150(01)00586-X. [DOI] [PubMed] [Google Scholar]
- 31.Carone BR, Fauquier L, Habib N, Shea JM, Hart CE, Li R, et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell. 2010;143:1084–1096. doi: 10.1016/j.cell.2010.12.008. doi:10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. doi:10.1016/0140-6736(93)91224-A. [DOI] [PubMed] [Google Scholar]
- 33.Lanigan J, Singhal A. Early nutrition and long-term health: a practical approach. Proc Nutr Soc. 2009;68:1–8. doi: 10.1017/S002966510999019X. [DOI] [PubMed] [Google Scholar]
- 34.Lombard CB, Deeks AA, Teede HJ. A systematic review of interventions aimed at the prevention of weight gain in adults. Public Health Nutr. 2009;12:1–11. doi: 10.1017/S1368980009990577. [DOI] [PubMed] [Google Scholar]
- 35.Anderson RA, Evans ML, Ellis GR, Graham J, Morris K, Jackson SK, et al. The relationships between post-prandial lipaemia, endothelial function and oxidative stress in healthy individuals and patients with type 2 diabetes. Atherosclerosis. 2001;154:475–483. doi: 10.1016/s0021-9150(00)00499-8. doi:10.1016/S0021-9150(00)00499-8. [DOI] [PubMed] [Google Scholar]
- 36.Groot PH, van Stiphout WA, Krauss XH, Jansen H, van Tol A, van Ramshorst E, et al. Postprandial lipoprotein metabolism in normolipidemic men with and without coronary artery disease. Arterioscler Thromb. 1991;11:653–662. doi: 10.1161/01.atv.11.3.653. [DOI] [PubMed] [Google Scholar]
- 37.Bessesen DH, Vensor SH, Jackman MR. Trafficking of dietary oleic, linolenic, and stearic acids in fasted or fed lean rats. Am J Physiol Endocrinol Metab. 2000;278:E1124–E1132. doi: 10.1152/ajpendo.2000.278.6.E1124. [DOI] [PubMed] [Google Scholar]
- 38.Qasqas SA, McPherson C, Frishman WH, Elkayam U. Cardiovascular pharmacotherapeutic considerations during pregnancy and lactation. Cardiol Rev. 2004;12:240–261. doi: 10.1097/01.crd.0000102421.89332.43. doi:10.1097/01.crd.0000102421.89332.43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.