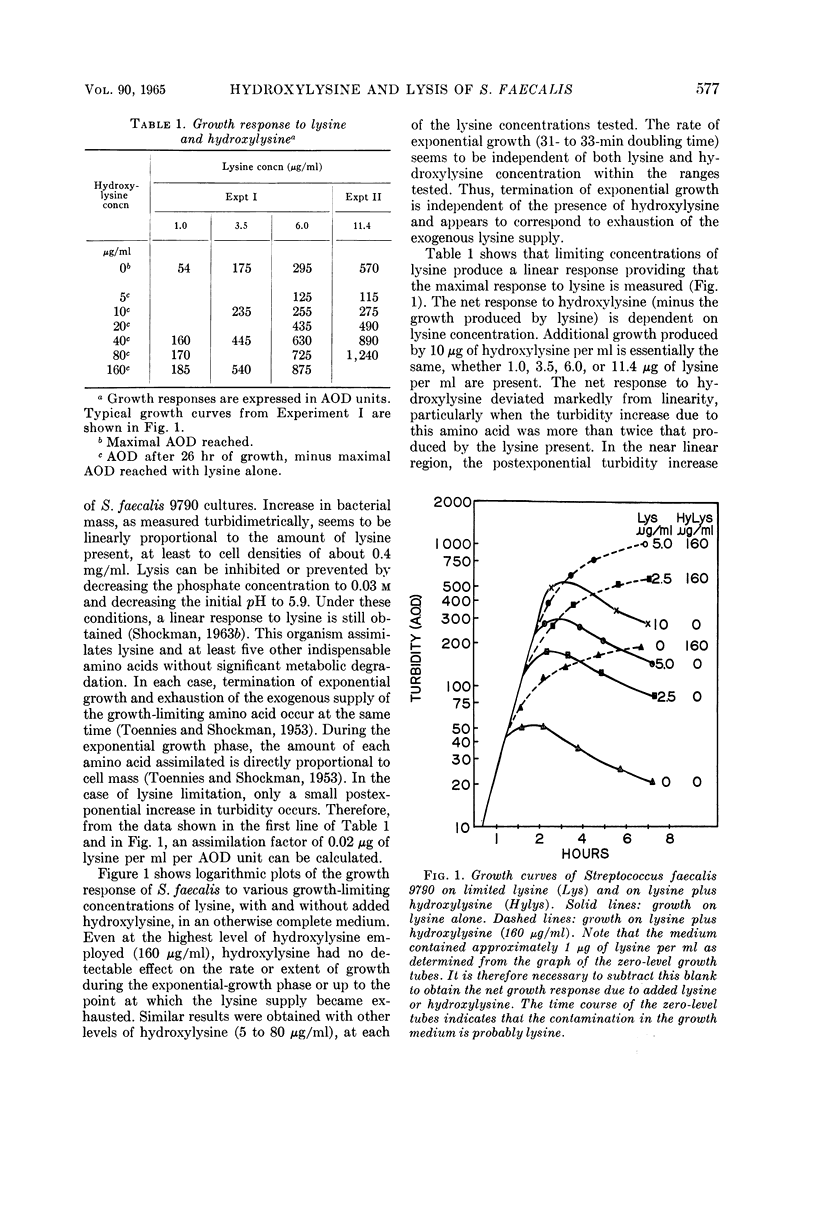
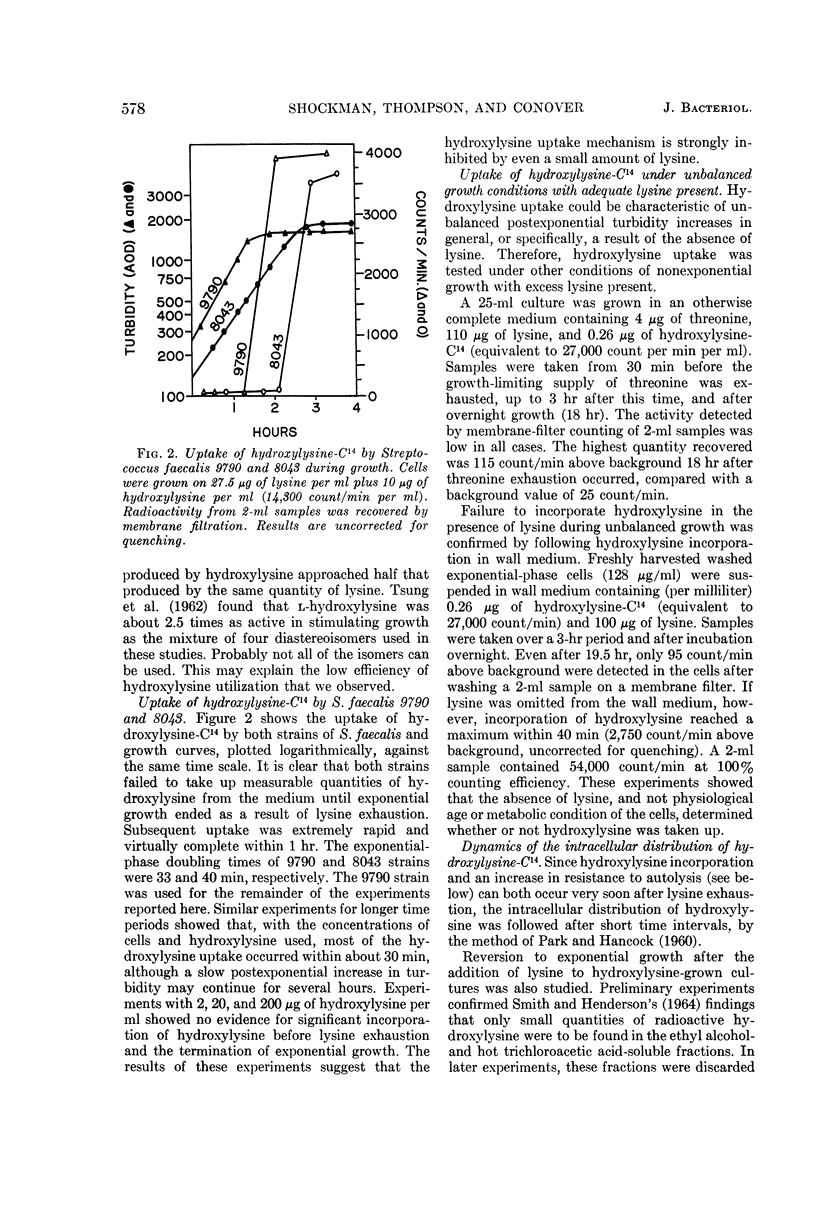
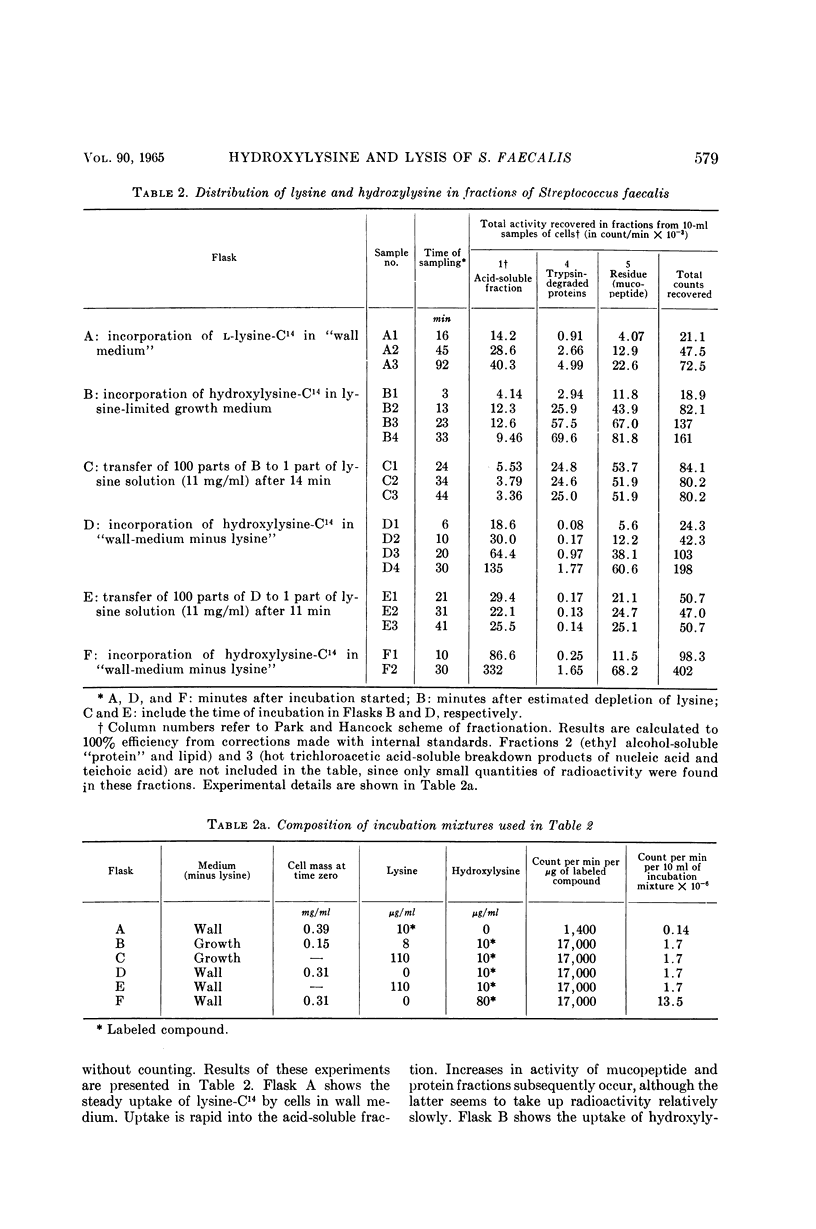
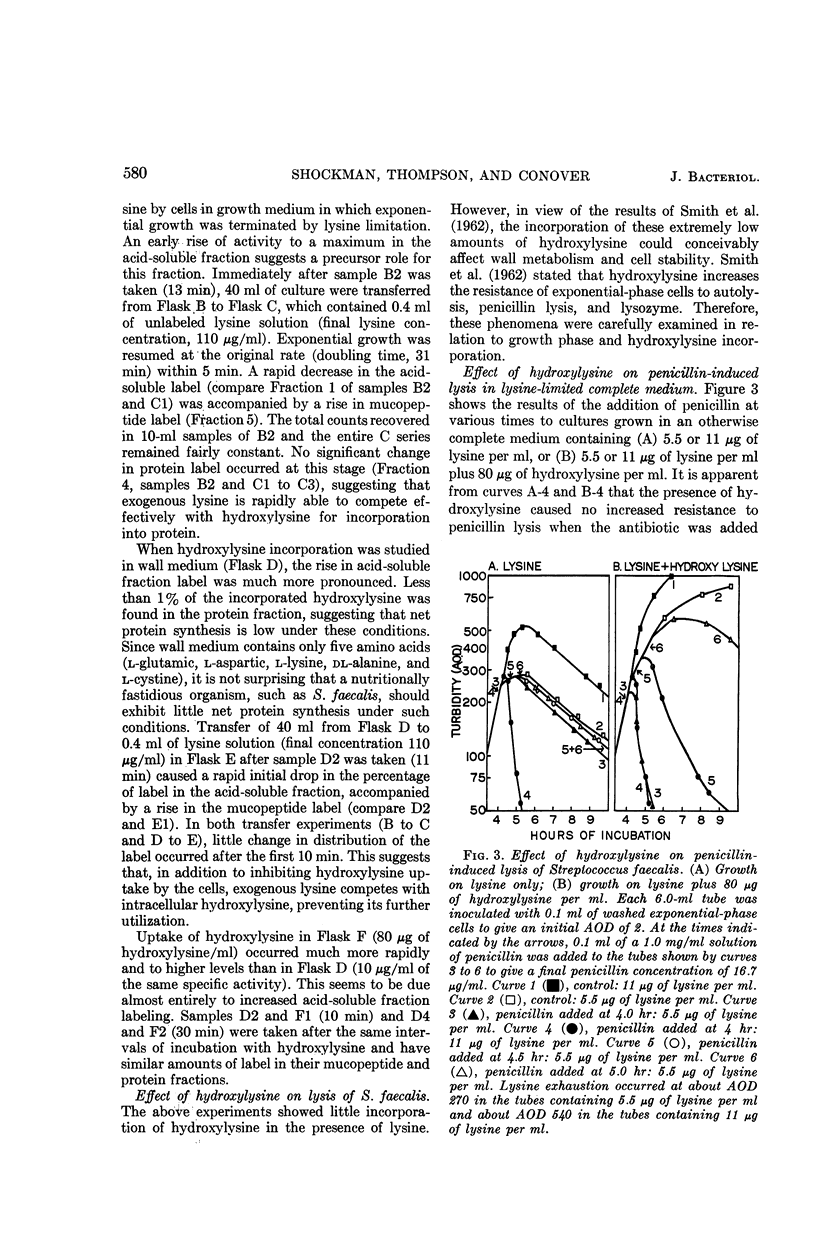
Abstract
Shockman, Gerald D. (Temple University, Philadelphia, Pa.), J. Stuart Thompson, and Margaret J. Conover. Replacement of lysine by hydroxylysine and its effects on cell lysis in Streptococcus faecalis. J. Bacteriol. 90:575–588. 1965.—Hydroxylysine was not significantly incorporated by Streptococcus faecalis ATCC 9790 or 8043 until exponential growth ceased as a result of lysine exhaustion. Uptake was then rapid and virtually complete within 1 hr. Lysine absence, rather than physiological age, seemed to be the governing factor. Hydroxylysine uptake rapidly reached a peak in the acid-soluble fraction, suggesting a precursor role for substances in this fraction. Substitution of hydroxylysine for lysine was much more efficient in mucopeptide synthesis than in protein synthesis. In wall medium, less than 1% of the incorporated hydroxylysine was found in the protein fraction. Addition of lysine to both growth and wall media inhibited both further hydroxylysine uptake and transfer of hydroxylysine from acid-soluble to mucopeptide or protein fractions. Hydroxylysine resulted in decreased penicillin susceptibility only after it was postexponentially incorporated. This effect was physiologically similar to that seen after threonine deprivation or chloramphenicol treatment. Hydroxylysine incorporation increased resistance to autolysis, but failed to decrease lysozyme susceptibility when measured after heat inactivation of autolysis. Electron microscopy of negatively stained cells showed increased thickness of cell walls containing hydroxylysine. Thus, most of the effects of replacement of lysine by hydroxylysine resemble those seen after deprivation of a nonwall amino acid (e.g., threonine or valine) or after chloramphenicol treatment. Each of these conditions results in inhibition of protein synthesis while permitting cell-wall synthesis to continue, resulting in autolysis-resistant, thick-walled cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brock T. D. CHLORAMPHENICOL. Bacteriol Rev. 1961 Mar;25(1):32–48. doi: 10.1128/br.25.1.32-48.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUC-NGUYEN H., WEED L. L. D-ORNITHINE AS A CONSTITUENT OF A BACTERIAL CELL WALL. J Biol Chem. 1964 Oct;239:3372–3376. [PubMed] [Google Scholar]
- Hancock R., Fitz-James P. C. Some differences in the action of penicillin, bacitracin, and vancomycin on Bacillus megaterium. J Bacteriol. 1964 May;87(5):1044–1050. doi: 10.1128/jb.87.5.1044-1050.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ E. Influence of valine, isoleucine, and related compounds on actinomycin synthesis. J Biol Chem. 1960 Apr;235:1090–1094. [PubMed] [Google Scholar]
- LARK C., LARK K. G. Studies on the mechanism by which D-amino acids block cell wall synthesis. Biochim Biophys Acta. 1961 May 13;49:308–322. doi: 10.1016/0006-3002(61)90130-5. [DOI] [PubMed] [Google Scholar]
- LOFTFIELD R. B. THE FREQUENCY OF ERRORS IN PROTEIN BIOSYNTHESIS. Biochem J. 1963 Oct;89:82–92. doi: 10.1042/bj0890082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACH B., TATUM E. L. ENVIRONMENTAL CONTROL OF AMINO ACID SUBSTITUTIONS IN THE BIOSYNTHESIS OF THE ANTIBIOTIC POLYPEPTIDE TYROCIDINE. Proc Natl Acad Sci U S A. 1964 Oct;52:876–884. doi: 10.1073/pnas.52.4.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy B. J., Britten R. J., Roberts R. B. The Synthesis of Ribosomes in E. coli: III. Synthesis of Ribosomal RNA. Biophys J. 1962 Jan;2(1):57–82. doi: 10.1016/s0006-3495(62)86841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., HANCOCK R. A fractionation procedure for studies of the synthesis of cell-wall mucopeptide and of other polymers in cells of Staphylococcus aureus. J Gen Microbiol. 1960 Feb;22:249–258. doi: 10.1099/00221287-22-1-249. [DOI] [PubMed] [Google Scholar]
- PERKINS H. R., CUMMINS C. S. CHEMICAL STRUCTURE OF BACTERIAL CELL WALLS. ORNITHINE AND 2,4-DIAMINOBUTYRIC ACID AS COMPONENTS OF THE CELL WALLS OF PLANT PATHOGENIC CORYNEBACTERIA. Nature. 1964 Mar 14;201:1105–1107. doi: 10.1038/2011105a0. [DOI] [PubMed] [Google Scholar]
- PETERSEN C. S., CARROLL R. W. Biological effect of hydroxylysine. Science. 1956 Mar 30;123(3196):546–547. doi: 10.1126/science.123.3196.546. [DOI] [PubMed] [Google Scholar]
- RICHMOND M. H. The effect of amino acid analogues on growth and protein synthesis in microorganisms. Bacteriol Rev. 1962 Dec;26:398–420. doi: 10.1128/br.26.4.398-420.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOCKMAN G. D. AMINO-ACID DEPRIVATION AND BACTERIAL CELL-WALL SYNTHESIS. Trans N Y Acad Sci. 1963 Dec;26:182–195. doi: 10.1111/j.2164-0947.1963.tb01241.x. [DOI] [PubMed] [Google Scholar]
- SHOCKMAN G. D. Bacterial cell wall synthesis: the effect of threonine depletion. J Biol Chem. 1959 Sep;234:2340–2342. [PubMed] [Google Scholar]
- SHOCKMAN G. D., KOLB J. J., TOENNIES G. Relations between bacterial cell wall synthesis, growth phase, and autolysis. J Biol Chem. 1958 Feb;230(2):961–977. [PubMed] [Google Scholar]
- SHOCKMAN G. D. Reversal of cycloserine inhibition by D-alanine. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:693–695. doi: 10.3181/00379727-101-25064. [DOI] [PubMed] [Google Scholar]
- SMITH W. G., GILBOE D. P., HENDERSON L. M. INCORPORATION OF HYDROXYLYSINE INTO THE CELL WALL AND A CELL-WALL PRECURSOR IN STAPHYLOCOCCUS AUREUS. J Bacteriol. 1965 Jan;89:136–140. doi: 10.1128/jb.89.1.136-140.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH W. G., HENDERSON L. M. RELATIONSHIPS OF LYSINE AND HYDROXYLYSINE IN STREPTOCOCCUS FAECALIS AND LEUCONOSTOC MESENTEROIDES. J Biol Chem. 1964 Jun;239:1867–1871. [PubMed] [Google Scholar]
- SMITH W. G., NEWMAN M., LEACH F. R., HENDERSON L. M. The effect of hydroxylysine on cell wall synthesis and cell stability in Streptococcus faecalis. J Biol Chem. 1962 Apr;237:1198–1202. [PubMed] [Google Scholar]
- SNELL E. E., RADIN N. S., IKAWA M. The nature of D-alanine in lactic acid bacteria. J Biol Chem. 1955 Dec;217(2):803–818. [PubMed] [Google Scholar]
- Shockman G. D., Conover M. J., Kolb J. J., Phillips P. M., Riley L. S., Toennies G. LYSIS OF STREPTOCOCCUS FAECALIS. J Bacteriol. 1961 Jan;81(1):36–43. doi: 10.1128/jb.81.1.36-43.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Conover M. J., Kolb J. J., Riley L. S., Toennies G. NUTRITIONAL REQUIREMENTS FOR BACTERIAL CELL WALL SYNTHESIS. J Bacteriol. 1961 Jan;81(1):44–50. doi: 10.1128/jb.81.1.44-50.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOENNIES G., BAKAY B., SHOCKMAN G. D. Bacterial composition and growth phase. J Biol Chem. 1959 Dec;234:3269–3275. [PubMed] [Google Scholar]
- TOENNIES G., SHOCKMAN G. D. Quantitative amino acid assimilation in homofermentative metabolism. Arch Biochem Biophys. 1953 Aug;45(2):447–458. doi: 10.1016/s0003-9861(53)80021-4. [DOI] [PubMed] [Google Scholar]
- TSUNG C. M., SMITH W. G., LEACH F. R., HENDERSON L. M. Hydroxylysine metabolism in Streptococcus faecalis. J Biol Chem. 1962 Apr;237:1194–1197. [PubMed] [Google Scholar]
- WORK E. AMINO ACIDS OF WALLS OF MICROCOCCUS RADIODURANS. Nature. 1964 Mar 14;201:1107–1109. doi: 10.1038/2011107a0. [DOI] [PubMed] [Google Scholar]
- Whitney J. G., Grula E. A. Incorporation of D-serine into the cell wall mucopeptide of Micrococcus lysodeikticus. Biochem Biophys Res Commun. 1964;14:375–381. doi: 10.1016/s0006-291x(64)80013-9. [DOI] [PubMed] [Google Scholar]


