Abstract
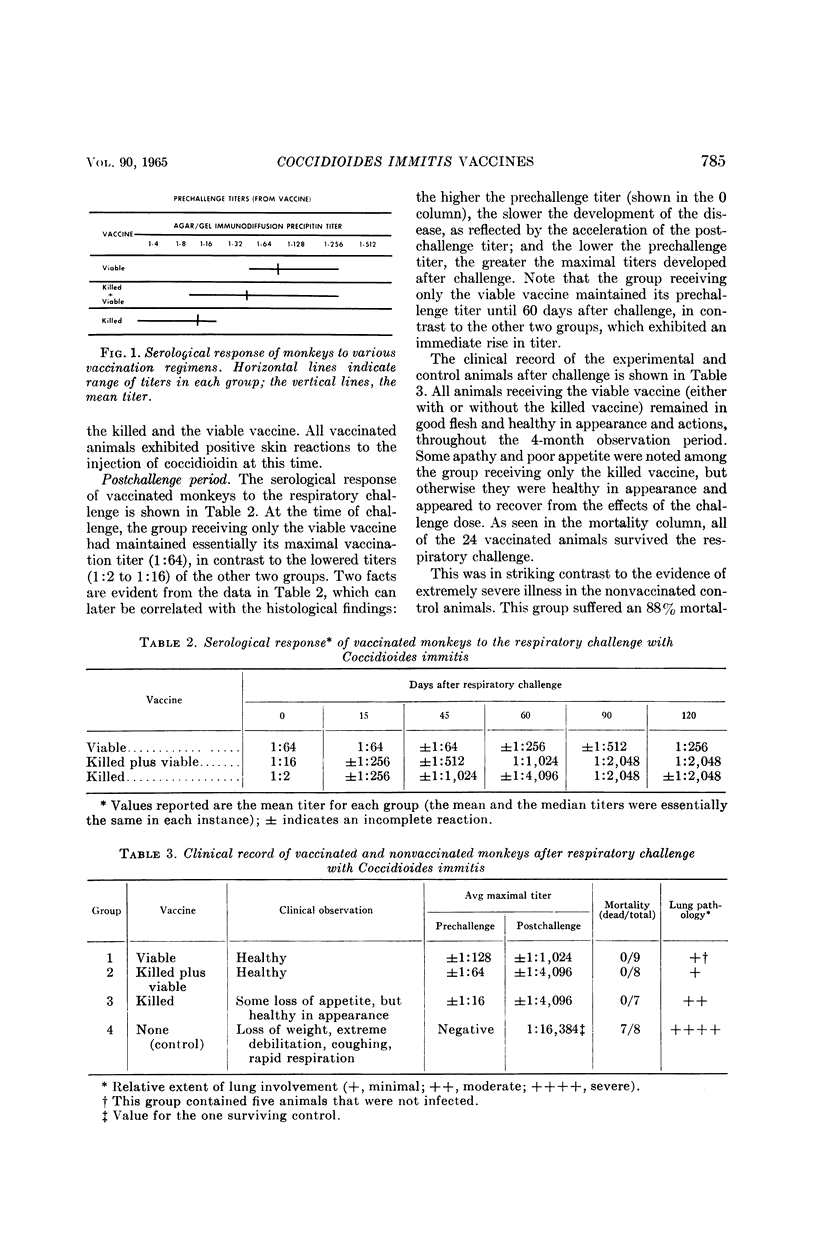
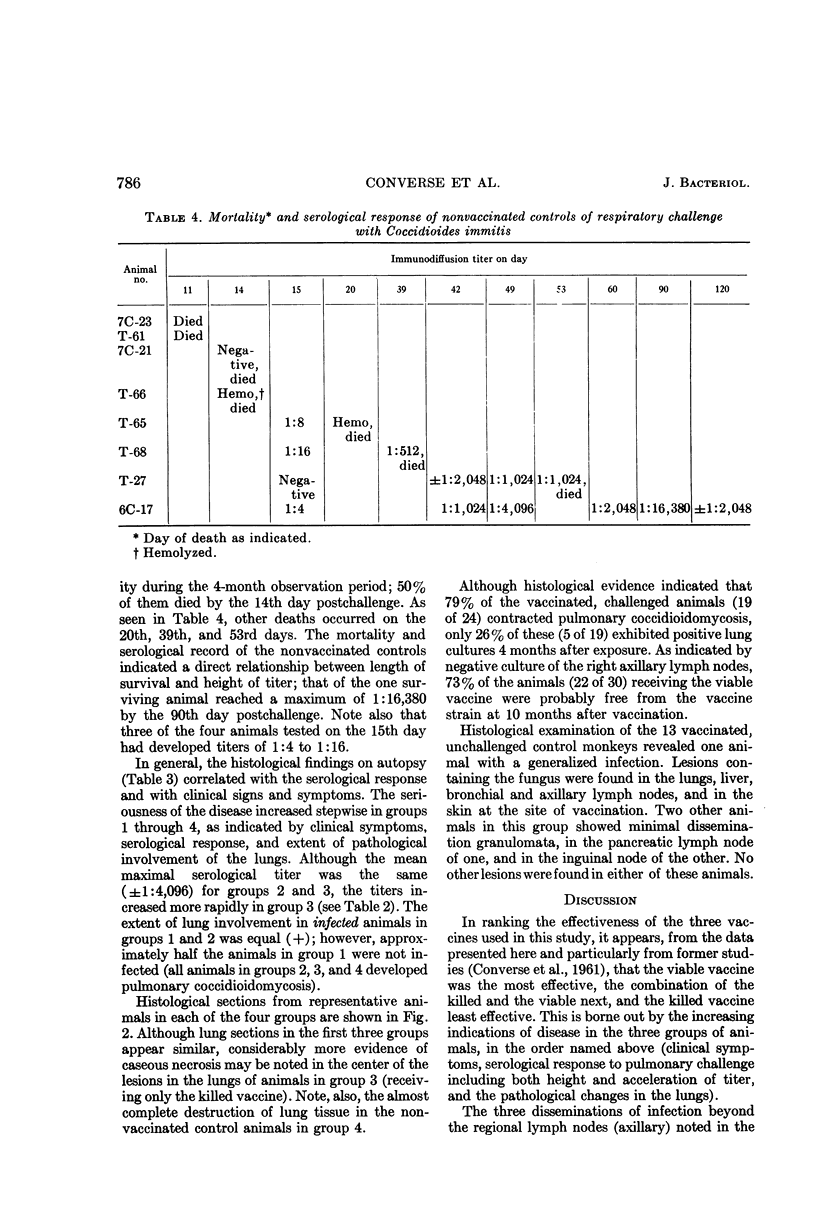
Converse, J. L. (U.S. Army Biological Laboratories, Fort Detrick, Frederick, Md.), G. A. Deauville, E. M. Snyder, J. G. Ray, and M. E. Seaquist. Control of tissue reactions in monkeys vaccinated with viable Coccidioides immitis by prevaccination with killed Coccidioides immities. J. Bacteriol. 90:783–788. 1965.—Control of undesirable tissue reactions resulting from the subcutaneous injection of 150 viable arthrospores of Coccidioides immitis (strain D-76) was obtained by four injections of formalin-killed arthrospores 14, 12, 8, and 4 weeks (total dose, 36 mg) before injection of the viable arthrospores. Only 6 and 12% of these vaccinated animals exhibited ulceration and lymphadenopathy, respectively, as compared with 100 and 83% of the animals receiving only the viable vaccine. Agar-gel immunodiffusion precipitin titers of approximately 1:64 were evident 3 months after vaccination in animals receiving both vaccines, as compared with 1:128 in those injected with the viable vaccine alone. The above data indicated that somatic reactions to injection of a viable vaccine could be eliminated by preinjection of a killed vaccine. However, 6 months after vaccination, respiratory challenge (7,500 strain Cash arthrospores) indicated that this treatment also impaired the protective effect of the viable vaccine. All animals receiving both vaccines developed mild pulmonary coccidioidomycosis, whereas only 50% of the animals receiving only the viable vaccine were infected. In addition, the group receiving both vaccines demonstrated a more rapid and higher postchallenge precipitin titer. All vaccinated animals (those receiving the killed, the viable, or a combination of the two vaccines) survived for 4 months after challenge, as compared with 88% mortality (50% within 14 days) in the nonvaccinated controls.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CONVERSE J. L., CASTLEBERRY M. W., BESEMER A. R., SNYDER E. M. Immunization of mice against coccidioidomycosis. J Bacteriol. 1962 Jul;84:46–52. doi: 10.1128/jb.84.1.46-52.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castleberry M. W., Converse J. L., Soto P. J., Jr Antibiotic control of tissue reactions in dogs vaccinated with viable cells of Coccidioides immitis. J Bacteriol. 1964 May;87(5):1216–1220. doi: 10.1128/jb.87.5.1216-1220.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUCETTE J., TRIMBLE J. R. Primary cutaneous coccidioidomycosis; report of a case of a laboratory infection. AMA Arch Derm. 1956 Oct;74(4):405–410. [PubMed] [Google Scholar]
- GOLDSCHMIDT E. P., TAYLOR G. W. Nutritional requirements for the growth and arthrospore formation of Coccidioides immitis. J Bacteriol. 1958 Mar;75(3):265–271. doi: 10.1128/jb.75.3.265-271.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODMAN D. H., SCHABARUM B. PRIMARY CUTANEOUS COCCIDIOIDOMYCOSIS. VISIBLE CLASSIC DEMONSTRATION OF DELAYED HYPERSENSITIVITY. Ann Intern Med. 1963 Jul;59:84–90. doi: 10.7326/0003-4819-59-1-84. [DOI] [PubMed] [Google Scholar]
- HARRELL E. R., HONEYCUTT W. M. Coccidioidomycosis: a traveling fungus disease. Arch Dermatol. 1963 Feb;87:188–196. doi: 10.1001/archderm.1963.01590140050009. [DOI] [PubMed] [Google Scholar]
- PAPPAGIANIS D., MILLER R. L., SMITH C. E., KOBAYASHI G. S. Response of monkeys to respiratory challenge following subcutaneous inoculation with Coccidioides immitis. Am Rev Respir Dis. 1960 Aug;82:244–250. doi: 10.1164/arrd.1960.82.2.244. [DOI] [PubMed] [Google Scholar]
- PAPPAGIANIS D., SMITH C. E., BERMAN R. J., KOBAYASHI G. S. Experimental subcutaneous coccidioidal infection in the mouse. J Invest Dermatol. 1959 May;32(5):589–598. doi: 10.1038/jid.1959.98. [DOI] [PubMed] [Google Scholar]
- RAY J. G., Jr, KADULL P. J. AGAR-GEL PRECIPITIN TECHNIQUE IN ANTHRAX ANTIBODY DETERMINATIONS. Appl Microbiol. 1964 Jul;12:349–354. doi: 10.1128/am.12.4.349-354.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON J. W., SMITH C. E., PLUNKETT O. A. Primary cutaneous coccidioidomycosis; the criteria for diagnosis and a report of a case. Calif Med. 1953 Sep;79(3):233–239. [PMC free article] [PubMed] [Google Scholar]



