The clinical progestin MPA has no effect on brain mitochondrial function by itself but attenuates estrogen-driven up-regulation of aerobic glycolysis and bioenergetics.
Abstract
The impact of clinical progestins used in contraception and hormone therapies on the metabolic capacity of the brain has long-term implications for neurological health in pre- and postmenopausal women. Previous analyses indicated that progesterone and 17β-estradiol (E2) sustain and enhance brain mitochondrial energy-transducing capacity. Herein we determined the impact of the clinical progestin, medroxyprogesterone acetate (MPA), on glycolysis, oxidative stress, and mitochondrial function in brain. Ovariectomized female rats were treated with MPA, E2, E2+MPA, or vehicle with ovary-intact rats serving as a positive control. MPA alone and MPA plus E2 resulted in diminished mitochondrial protein levels for pyruvate dehydrogenase, cytochrome oxidase, ATP synthase, manganese-superoxide dismutase, and peroxiredoxin V. MPA alone did not rescue the ovariectomy-induced decrease in mitochondrial bioenergetic function, whereas the coadministration of E2 and MPA exhibited moderate efficacy. However, the coadministration of MPA was detrimental to antioxidant defense, including manganese-superoxide dismutase activity/expression and peroxiredoxin V expression. Accumulated lipid peroxides were cleared by E2 treatment alone but not in combination with MPA. Furthermore, MPA abolished E2-induced enhancement of mitochondrial respiration in primary cultures of the hippocampal neurons and glia. Collectively these findings indicate that the effects of MPA differ significantly from the bioenergetic profile induced by progesterone and that, overall, MPA induced a decline in glycolytic and oxidative phosphorylation protein and activity. These preclinical findings on the basis of acute exposure to MPA raise concerns regarding neurological health after chronic use of MPA in contraceptive and hormone therapy.
A debate continues as to whether perimenopausal women should use steroid hormone replacement therapy. The choice of hormone therapy not only affects transient menopausal symptoms but also long-term health outcomes that include cognitive function. The Women's Health Initiative, a large, double-blind, placebo-controlled, randomized clinical trial concluded that estrogens-plus-progestin therapy increased the risk of dementia, cardiovascular disease, stroke, and invasive breast cancer, outweighing any benefits (1–3). Due to limitations in clinical study design including age of participants and degree of hormone responsiveness, further research must be conducted to substantiate causality (4). From a basic neuroscience standpoint, it is evident that ovarian hormones are integral regulators of metabolic functions enabling dynamic responses in neurogenesis, morphogenesis, and synaptic transmission (5). Mitochondrial dysfunction is detectable in the early pathogenesis of neurodegenerative disorders, including Alzheimer's disease (6, 7). Disrupted calcium homeostasis and increased oxidative stress result in apoptotic subpopulations of neurons, proposed to underlie neurodegenerative pathology and associated cognitive decline (8). Furthermore, we have shown that mitochondrial dysfunction precedes Alzheimer's disease pathology and is exacerbated during female reproductive senescence (9). Previously we identified changes in the brain mitochondrial proteome associated with 17β-estradiol (E2) replacement (10). We demonstrated that progesterone (P4) and E2 mechanisms of action sustain and enhance brain mitochondrial energy-transducing capacity (10–12). Whereas E2 and P4 increase mitochondrial function alone, in combination P4 and E2 negate benefits to mitochondrial function (12).
The acetylated pregnane, 17α-acetoxy-6α-methylpregn-4-ene-3,20-dione [medroxyprogesterone acetate (MPA)], is used extensively in hormone replacement therapy (Prempro™, Pfizer Inc, Pearl River, NY: Premarin plus Provera) and contraception (Depo-Provera™, Pfizer Inc). Within specific brain regions and hippocampal subregions, progestins bind to progesterone receptors (PRs) to elicit biological responses that vary from antiproliferative, neuroprotective, and neurogenic effects (13–17). Compared with P4, MPA possesses a 3-fold higher relative binding affinity for PR (18).
At variance with the well-documented protective effects of E2 and P4, MPA alone does not share the same neuroprotective capacity as endogenous P4. E2 promotes mitochondrial calcium-buffering capacity, thereby preventing apoptosis in primary cultured hippocampal neurons and isolated rat brain mitochondria (4, 19, 20). Moreover, E2 activation of protein kinase B (Akt) and MAPK has been demonstrated within single neurons via a phosphatidylinositol-3 kinase-dependent mechanism (21). Activated MAPK subsequently phosphorylates cAMP response element-binding protein, up-regulating gene transcription, including spinophilin (22) and Bcl-2 (19). Phosphorylated ERK is translocated to the nucleus only by E2 and P4, not by MPA (23). P4 alone and in combination with E2 proved to be neuroprotective, whereas MPA was not protective against excitotoxicity and blocked E2's influence (23, 24). Further indication of divergent signaling comes from recent work by the group of Singh and colleagues (25), who demonstrated that P4 elicited increases in cortical brain-derived neurotrophic factor levels, whereas MPA negatively regulated this neurotrophic factor. Ovarian hormones regulate synaptic plasticity in part by modulating N-methyl-d-aspartate (NMDA) receptor function and spine density in the hippocampus. Excitatory synapse formation is induced by E2 and down-regulated by P4 via N-methyl-d-aspartate receptors (26). Zadran et al. (27, 28) showed that E2, brain-derived neurotrophic factor, and epidermal growth factor activate the calcium-dependent protease, m-calpain to influence long-term potentiation.
We propose that in combination with E2, MPA would disrupt mitochondrial function and efficient electron coupling, resulting in increased oxidative damage. To test this hypothesis, we used the classic ovariectomized (OVX) rat model and replaced ovarian hormones with MPA in either the absence or presence of E2. Our results reveal that MPA antagonizes E2 up-regulation of mitochondrial function in whole-brain mitochondria and in hippocampus.
Materials and Methods
Chemicals
All chemicals were from MP Biomed (Irvine, CA) unless otherwise noted. E2 and medroxyprogesterone acetate were obtained from Steraloids (Newport, RI). Steroids were dissolved in ethanol and diluted in tocopherol-stripped corn oil (MP Biomed) with final ethanol concentration less than 0.001%.
Animals
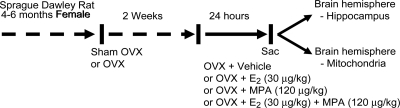
The use of animals for the study was approved by the Institutional Animal Care and Use Committee at the University of Southern California (Protocol 10911). Young adult (4–6 months old) female OVX Sprague Dawley rats purchased from Harlan (Indianapolis, IN) were housed under controlled conditions of temperature (22 C), humidity, and light (14 h light, 10 h dark) with water and food available ad libitum. After 2 wk of habituation to the facilities and surgery recovery (following ovariectomy), rats were injected sc with E2 (30 μg/kg), MPA (120 μg/kg), or corn oil vehicle control 24 h before the animals were killed and forebrain dissection (Fig. 1). The dose of E2 (30 μg/kg body weight) was chosen as representative of a standard systemic E2 therapy used clinically and in previous studies, which ranges from 10 to 100 μg/kg. Based on the E2 (30 μg/kg) concentration, we chose the MPA (120 μg/kg) concentration to be 4-fold higher. This dose relates to the clinically used ratio (1:4) of 0.625 mg conjugated equine estrogens per 2.5 mg MPA in the commonly prescribed hormone therapy (Prempro).
Fig. 1.
Experimental treatment paradigm and OVX rat model. Ovaries were surgically removed from young adult female rats 2 wk before sc injection with E2, MPA, E2+MPA, or oil vehicle control. Sham OVX rats, determined to be in the morning of the estrous phase of the cycle served as a positive control. Twenty-four hours after the treatment, whole-brain mitochondria from the left hemisphere of the forebrain were isolated and subjected to a series of functional assessment experiments. The hippocampus was dissected from the right hemisphere of the forebrain and used to determine the expression of the mitochondrial proteins.
To confirm administration of steroid, plasma, and brain E2 levels were analyzed by commercial ELISA (IBL-Hamburg, Hamburg Germany) after hexane-ethyl acetate extraction. The 30-μg/kg dose produced E2 levels in OVX rats of 42 pg/g in brain tissue and 44 pg/ml in serum (12). Sham OVX rats were cytologically tested for at least two to three cycles and selected at the appropriate day of their natural estrous cycle by vaginal cytology. For sham OVX rats on the day of estrous, cytological smears consisted primarily of anucleated cornified cells. OVX rats all displayed leukocytic smears. Sham OVX rats (and OVX rats) were killed at 1100 h on the day of estrous, 24 h after peak levels of E2 (29). At the time the animals were killed, uteri were removed and weighed (sham OVX, 0.450 ± 0.040 g; OVX, 0.149 ± 0.010 g; E2, 0.288 ± 0.001 g; MPA, 0.182 ± 0.017 g; E2+MPA, 0.242 ± 0.012 g wet weight; n = 5). A well-reported (30–32) increase in body weight was confirmed 2 wk after OVX (sham OVX, 277.9 ± 5.43 g, n = 5; OVX, 290.8 ± 2.34 g, n = 20; P < 0.05). All experiments and procedures were approved by the Institutional Animal Care and Use Committee.
Brain mitochondrial isolation and lipid fractionation
Brain mitochondria were isolated from rats as previously described (33). Rats were decapitated, and the whole forebrain minus the cerebellum and brain stem was rapidly removed, minced, and homogenized at 4 C in mitochondrial isolation buffer [MIB; pH 7.4, containing sucrose (320 mm), EDTA (1 mm), Tris-HCl (10 mm), and Protease Inhibitor Cocktail Set I (Calbiochem, La Jolla, CA; AEBSF-HCl, 500 μm; aprotonin, 150 nm; E-64, 1 μm, EDTA disodium, 500 μm; leupeptin hemisulfate, 1 μm)]. Single forebrain homogenates were then centrifuged at 1500 × g for 5 min. The pellet was resuspended in MIB, rehomogenized, and centrifuged again at 1500 × g for 5 min. The postnuclear supernatants from both centrifugations were combined and crude mitochondria were pelleted by centrifugation at 21,000 × g for 10 min. The resulting mitochondrial pellet and lipid layer, mostly as a result of myelin, were resuspended in 15% Percoll made in MIB, layered over a preformed 23/40% Percoll discontinuous gradient, and centrifuged at 31,000 × g for 10 min. After centrifugation, the least dense layer of lipids was collected at the top of the 15% Percoll and stored at −80 C for lipid peroxide measurements. The purified mitochondria were collected at the 23/40% interface and washed with 10 ml MIB by centrifugation at 16,700 × g for 13 min. The loose pellet was collected and transferred to a microcentrifuge tube and washed in MIB by centrifugation at 9000 × g for 8 min. The resulting mitochondrial pellet was resuspended in MIB to an approximate concentration of 1 mg/ml. The resulting mitochondrial samples were used immediately for respiratory activity measurements or stored at −80 C for protein, enzymatic, and lipid assays. In previous experiments with purified mitochondria, we confirmed the purity and integrity of isolated mitochondria by Western blot analysis as previously described (10).
Respiratory measurements
Mitochondrial oxygen consumption was measured using MitoExpress A65N phosphorescent oxygen-sensitive probe (Luxcel Biosciences, Cork, Ireland). Fifty micrograms of isolated rat brain mitochondria were placed in the respiration chamber at 37 C in respiratory buffer (130 mm KCl, 2 mm KH2PO4, 3 mm HEPES, 2 mm MgCl2, 1 mm EGTA) to yield a final concentration of 250 μg/ml. Following the manufacturer's protocol, mitochondria were energized by the addition of glutamate (5 mm) and malate (5 mm) as substrates. State 3 respiration was stimulated by the addition of ADP (410 μm). In separate wells, state 2 respiration was measured by excluding ADP (a surrogate for state 4). The rate of oxygen consumption was calculated based on the slope of the response. The respiratory control ratio (RCR) was determined by dividing the rate of oxygen consumption per minute for state 3 (presence of ADP) by the rate of oxygen consumption per minute for state 2 respiration (absence of ADP).
Pyruvate dehydrogenase (PDH) activity
PDH activity was measured by monitoring the conversion of oxidation of nicotinamide adenine dinucleotide to nicotinamide adenine dinucleotide hydroxide by following the change in absorption at 340 nm as previously described (9). Isolated brain mitochondria were dissolved in 2% [(3-cholamidopropyl) dimethyl-ammonio]-1-propane-sulfonate buffer to yield a final concentration of 10 μg/μl and incubated at 37 C in PDH assay buffer [35 mm KH2PO4, 2 mm KCN, 0.5 mm EDTA, 5 mm MgCl2 (adjusted to pH 7.25 with KOH), 200 mm sodium pyruvate, 2.5 mm rotenone, 4 mm sodium CoA, 40 mm thiamine pyrophosphate chloride]. The reaction was initiated by the addition of 15 mm of the oxidation of nicotinamide adenine dinucleotide.
Complex IV/cytochrome c oxidase (COX) activity
Complex IV activity was determined colormetrically by monitoring change in absorbance (550 nm) of reduced cytochrome c to oxidized form in permeabilized immunocaptured mitochondrial. COX activity of isolated mitochondria (20 μg) was measured using the Rapid Microplate Assay kit for Rat complex IV activity (Mitosciences, Eugene, OR) following the manufacturer's instructions.
Manganese superoxide dismutase (MnSOD) activity
Enzyme activity of MnSOD was measured with 10 μg isolated brain mitochondria according to the manufacturer's instructions (Cayman Chemical, Ann Arbor, MI). MnSOD activity was determined in the presence of KCN, which inhibits Cu-ZnSOD. A proprietary solution containing a tetrazolium salt, supplied in the kit, was used to quantify dismutation of superoxide radicals.
Western blot analysis
Equal amounts of mitochondrial protein (20 μg/well) were loaded into each well of 12% SDS-PAGE gels (Bio-Rad Laboratories, Hercules, CA), electrophoresed with a Tris/glycine running buffer, and transferred to a polyvinylidine difluoride membrane. The blots were probed with anti-PDH subunit E1α (1:2000; Mitosciences), antiphosphorylated PDH subunit E1α (1:1000; Calbiochem), COX complex IV (COXIV; 1:1000; Cell Signaling Technology, Danvers, MA), anti-complex V, subunit-α (1:1000; Mitosciences), anti-MnSOD (1:500; BD Biosciences, San Jose, CA), anti-heat shock protein 60 (1:500; BD Biosciences) or anti-peroxiredoxin V (1:500; BD Biosciences) and the corresponding horseradish peroxidase-conjugated horse antimouse or antirabbit secondary antibody (Vector, Burlingame, CA) at a concentration of 1:10,000. Anti-β-tubulin (1:3000; AbCam, Cambridge, MA) was used as a loading control for hippocampal lysates. Antigen-antibody complexes were visualized with Pierce SuperSignal chemiluminescent substrates (Thermo Scientific, Waltham, MA) and captured by Molecular Imager ChemiDoc XRS system (Bio-Rad Laboratories). All band intensities were quantified using Un-Scan-it software (Silk Scientific, Salt Lake City, UT).
Lipid peroxidation
Accumulated lipid peroxides in purified brain mitochondria and whole-brain lipid fraction were measured using the leucomethylene blue assay. t-Butyl hydroperoxide was used as a standard. Leucomethylene blue was measured by monitoring absorbance at 650 nm after 1 h incubation at room temperature. Data values were plotted as picomoles per microgram of protein.
Seahorse XF-24 metabolic flux analysis
Primary hippocampal neurons or mixed glia from embryonic day 18 embryos of timed-pregnant Sprague Dawley rats were cultured on Seahorse XF-24 (Seahorse Bioscience, Billerica, MA) plates at a density of 75,000 cells/well. Neurons were grown in neurobasal medium and B27 supplement for 10 d before the experiment. Mixed glial cells were grown in phenol-red-free DMEM with 10% fetal bovine serum and plated 1 d before treatment. Cells were serum starved 4 h before treatment. On the day of metabolic flux analysis, both cell cultures were changed to unbuffered DMEM [DMEM base medium supplemented with 25 mm glucose; 1 mm sodium pyruvate; 31 mm NaCl; 2 mm GlutaMAX (Invitrogen, Carlsbad, CA), pH 7.4] and incubated at 37 C in a CO2-free incubator for 1 h. All media and injection reagents were adjusted to pH 7.4 on the day of the assay. Four baseline measurements of oxygen consumption rate (OCR) in picomoles per minute were taken before the sequential injection of mitochondrial inhibitors. Four readings were taken after each addition of the mitochondrial inhibitor and before the automated injection of the subsequent inhibitor. The mitochondrial inhibitors were added sequentially: oligomycin (1 μm) to inhibit ATP synthase, p-trifluoromethoxycarbonylcyanide phenylhydrazone (1 μm) to uncouple mitochondria, and rotenone (1 μm) to inhibit complex I of the electron transport chain. OCR was automatically calculated and recorded by the Seahorse XF-24 software.
Statistics
Data are presented as means ± sem and, where indicated, as percent change relative to OVX. Statistically significant differences were determined by one-way ANOVA followed by Student-Newman Keuls post hoc analysis when appropriate. P < 0.05 was considered statistically significant.
Results
Medroxyprogesterone acetate has no effect on E2 potentiation of brain mitochondrial respiratory activity
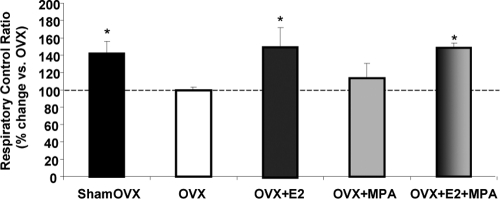
We measured the respiratory rate of the isolated whole-brain mitochondria using glutamate (5 mm) and malate (5 mm) as basal (state 2) respiratory substrates. The ADP addition to the mitochondrial suspensions initiated state 3 respirations. Compared with OVX vehicle control, there was a significant increase in the RCR in the E2 and E2+MPA treatment groups but not in the MPA alone group (Fig. 2). In vivo treatment with E2 resulted in a 50% increase in the brain mitochondria RCR, restoring it to the level of sham OVX (Fig. 2). In vivo treatment with E2+MPA resulted in a 48% increase in the RCR of isolated brain mitochondria (Fig. 2). These data indicate that under ex vivo conditions, in which energizing substrates were provided directly to isolated mitochondria, the E2-induced benefit to mitochondrial respiration was essentially unaffected by the addition of MPA.
Fig. 2.
Medroxyprogesterone acetate has no effect on brain mitochondrial respiratory activity alone or in combination with E2. Mitochondrial oxygen consumption ± in vivo E2 (30 μg/kg), MPA (120 μg/kg), E2+MPA (30 μg/kg + 120 μg/kg), or corn oil vehicle control (OVX). Ovary-intact (shamOVX) rats served as a positive control and were selected on the morning after estrus by vaginal cytology. Oxygen consumption measurements of isolated rat brain mitochondria were performed using MitoExpress A65N phosphorescent oxygen sensitive probe (Luxcel Biosciences, Cork). Isolated mitochondria were energized by addition of l-malate (5 mm) and l-glutamate (5 mm) as substrates. ADP (410 μm) was added to stimulate state 3 respiration, and rates were compared with unstimulated ADP-null, representing basal state 2 respiration. The traces are representative of five separate experiments. Data are expressed as a percent change in mitochondrial RCR (state 3/state 2) relative to OVX. The data represents mean ± sem of five separate experiments. *, P < 0.05 compared with OVX (n = 5).
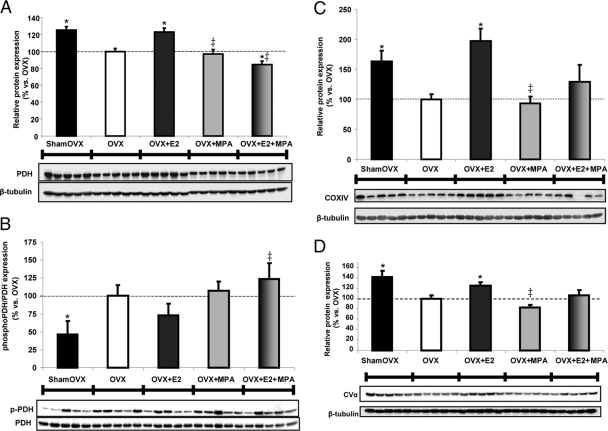
Medroxyprogesterone acetate inhibits E2 potentiation of mitochondrial bioenergetics panel including protein expression of PDH, phosphorylated PDH (pPDH), complex IV, and complex V
PDH plays a central role in brain mitochondria: the enzyme complex links anaerobic glycolysis (cytosol) to the tricarboxylic acid cycle (TCA) by catalyzing oxidative decarboxylation of pyruvate to acetyl-CoA. To determine the expression pattern of PDH E1α, an immunoblot of this critical regulatory subunit was performed on hippocampal lysate relative to β-tubulin loading control. Results indicate that PDH E1α levels were decreased by 25% when OVX is compared with sham OVX. E2 restored this decline in expression by 25% after 24 h. MPA did not restore PDH levels relative to OVX. Furthermore, coadministration of MPA abolished E2-induced benefits below OVX levels (Fig. 3A). One known posttranslational modification is the phosphorylation of PDH by PDH kinase, which inactivates the enzyme (34). Results indicate that pPDH (inactive) levels after OVX are increased by 55% when compared with those of sham OVX. Compared with OVX, E2 decreased the ratio of pPDH to PDH by 28%, but this did not reach significance. MPA alone had no effect on the pPDH to PDH ratio compared with OVX. Interestingly, E2+MPA increased pPDH/PDH (PDH inhibition) by 50% compared with E2 alone. In combination, MPA not only blocked E2, but it also exacerbated the inhibition of PDH. Phosphorylation of proteins is rapid and transient, possibly explaining the variability observed 24 h after treatment (Fig. 3B).
Fig. 3.
Medroxyprogesterone acetate inhibits the E2 potentiation of the protein profile of the bioenergetics-related panel. Total protein was isolated from hippocampus after 24 h exposure to (30 μg/kg), MPA (120 μg/kg), E2+MPA (30 μg/kg + 120 μg/kg), or corn oil vehicle control (OVX). Ovary-intact (shamOVX) rats served as a positive control and were selected on the morning after estrus by vaginal cytology. All immunoblot data were normalized to β-tubulin loading control and compared relative to OVX as 100% (except for the pPDH to PDH ratio, Fig. 4B). A, Expression of PDH, subunit E1α, was determined by Western blot analysis. Bars represent mean relative expression ± sem from five animals per group. *, P < 0.05 vs. OVX; ‡, P < 0.05 vs. E2 (n = 5). B, Expression of phosphorylated PDH, subunit E1α, was determined by Western blot analysis (phosphorylation inhibits PDH activity). Bars represent the mean ratio of pPDH to PDH expression ± sem from five animals per group. *, P < 0.05 vs. OVX; ‡, P < 0.05 vs. E2 (n = 5). C, Expression of COXIV was determined by Western blot analysis. Note that one band did not develop in the E2+MPA group and was excluded from the analysis. Bars represent mean relative expression ± sem from four to five animals per group. *, P < 0.05 vs. OVX; ‡, P < 0.05 vs. E2 (n = 4–5). D, Expression of complex V, subunit α, was determined by Western blot analysis. Bars represent mean relative expression ± sem from five animals per group. *, P < 0.05 vs. OVX; ‡, P < 0.05 vs. E2 (n = 5).
Electrons flow down the electron transport chain to the terminal complex, COX. Results indicate that OVX decreased COXIV levels 65% when compared with sham OVX. E2 significantly restored this OVX-induced decline in expression after 24 h treatment. There was nearly a 100% increase in COXIV expression after 24 h E2 treatment compared with OVX. When E2 was compared with E2+MPA, there was approximately a 70% reduction in expression indicating an inhibitory effect by MPA. In accordance, when compared with OVX, both MPA and E2+MPA treatment failed to alter COXIV expression (Fig. 3C).
We reported previously that both E2 and P4 increased CVα expression (10, 12). In this study, E2 increased CVα by about 25 and 40%, respectively vs. the OVX and MPA treatment groups. E2+MPA demonstrated that MPA inhibits E2-induced CVα expression (Fig. 3D).
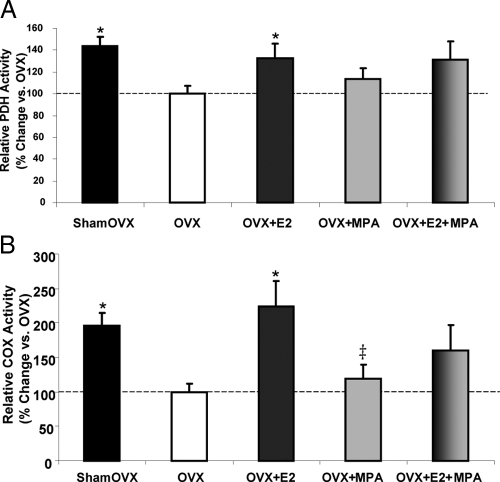
Medroxyprogesterone acetate inhibits E2 potentiation of PDH activity, the link between glycolysis and the TCA cycle, and COX activity, the terminal complex of the electron transport chain
Brain mitochondria isolated from sham OVX rats displayed a 44% difference in PDH activity vs. OVX (Fig. 4A). Likewise, E2 resulted in a 33% increase in brain mitochondrial PDH activity vs. OVX. MPA and E2+MPA failed to elicit a significant increase in PDH activity (Fig. 4A). Increased PDH activity could be accounted for by either an increased protein expression (Fig. 3A) or modulation (Fig. 3B) of activity.
Fig. 4.
Medroxyprogesterone acetate inhibits the E2 potentiation of PDH activity and COX activity. A, Relative rate of PDH activity from isolated whole-brain mitochondria ± in vivo E2 (30 μg/kg), MPA (120 μg/kg), E2+MPA (30 μg/kg + 120 μg/kg), or corn oil vehicle control (OVX). Ovary-intact (shamOVX) rats served as a positive control and were selected on the morning of estrus by vaginal cytology consisting primarily of anucleated cornified cells. The bars represent mean ± sem from five separate experiments with one animal per group for each experiment. *, P < 0.05 vs. OVX; (n = 5). B, Relative rate of COX activity of isolated whole-brain mitochondria. The bars represent mean ± sem from five separate experiments with one animal per group for each experiment. *, P < 0.05 vs. OVX; ‡, P < 0.05 vs. E2 (n = 5).
Brain mitochondrial COX activity in MPA-treated rats did not show a statistically significant difference from the OVX control (Fig. 4B). E2 treatment resulted in a 125% increase in brain mitochondrial COX activity, consistent with previous results (Fig. 4B) (12). E2+MPA failed to alter brain mitochondrial COX activity compared with OVX. Although the average COX activity was 60% above OVX, the data suggest that MPA diminished E2 modulation of COX activity.
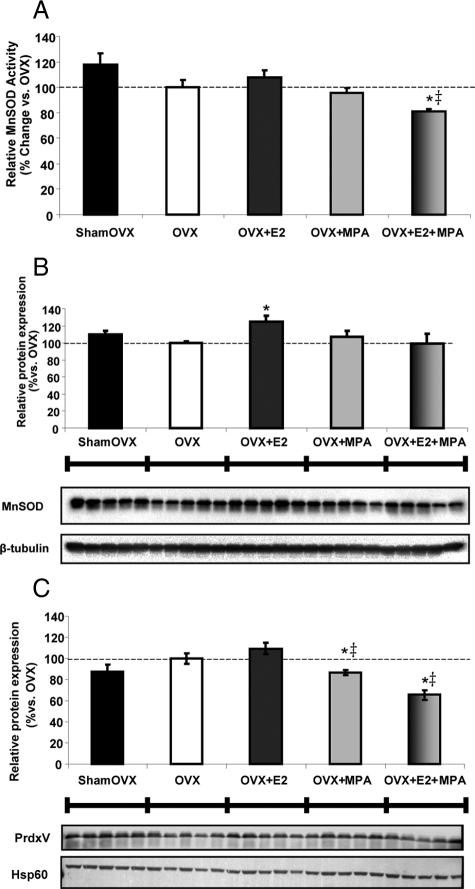
Medroxyprogesterone acetate combined with E2 altered the antioxidant profile of brain mitochondria
We determined the impact of MPA and E2+MPA on the enzyme activity and protein expression of the mitochondrial matrix protein MnSOD and peroxiredoxin V (PrdxV) (Fig. 5, A–C). As a positive control, there was an expected 25% increase in MnSOD expression with E2-treatment, whereas the MPA and E2+MPA groups were not significantly different from the OVX vehicle control (Fig. 5B). Interestingly, MPA directly decreased PrdxV expression vs. OVX. E2+MPA decreased expression of PrdxV by 35% vs. OVX and 45% vs. E2 alone (Fig. 5C). E2+MPA decreased MnSOD activity by 20% vs. OVX and 30% vs. E2 (Fig. 5A). These results fit with the lipid peroxidation outcome measures (Fig 6, A and B) because MnSOD generates H2O2 from O2.− disproportionation and PrdxV is involved in the reduction of H2O2 and the prevention of peroxidative damage. The antioxidant capabilities of E2 are blocked by the addition of MPA, which suggests that mitochondrial peroxiredoxin may be critical as the brain's defense mechanism for maintaining low levels of hydrogen peroxide.
Fig. 5.
Medroxyprogesterone acetate inhibits the E2 potentiation of the antioxidant profile of brain mitochondria. A, Relative rate of MnSOD activity of isolated whole-brain mitochondria ± in vivo E2 (30 μg/kg), MPA (120 μg/kg), E2+MPA (30 μg/kg + 120 μg/kg), or corn oil vehicle control (OVX). Ovary-intact (shamOVX) rats served as a positive control and were selected on the morning after the estrous cycle by vaginal cytology. The bars represent mean ± sem from five separate experiments with one animal per group for each experiment. *, P < 0.05 vs. OVX; ‡, P < 0.05 vs. E2 (n = 5). Immunoblot data were normalized to loading control and compared relative to OVX. Expression of the mitochondrial antioxidant proteins MnSOD in hippocampus (B) and PrdxV in whole-brain mitochondria (C) was measured using Western blot analysis. The bars represent mean ± sem from five animals per group. *, P < 0.05 vs. OVX; ‡, P < 0.05 vs. E2 (n = 5).
Fig. 6.
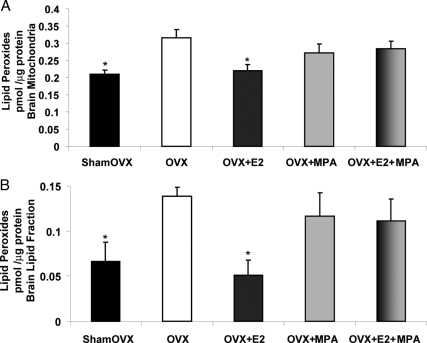
Medroxyprogesterone acetate inhibits E2 protection against ovariectomy-induced lipid peroxidation of brain mitochondria and whole-brain lipid fraction. Whole-brain mitochondria and whole-brain lipid fraction were isolated 24 h after in vivo exposure to E2 (30 μg/kg), MPA (120 μg/kg), E2+MPA (30 μg/kg + 120 μg/kg), or corn oil vehicle control (OVX). Ovary-intact (shamOVX) rats served as a positive control and were selected on the morning after the estrous cycle by vaginal cytology. Lipid peroxides in purified brain mitochondria (A) and brain lipid fraction (B) were measured using the leucomethylene blue assay. The bars represent mean ± sem from four animals per group. *, P < 0.05 compared with OVX (n = 4).
Medroxyprogesterone acetate inhibits E2 protection against ovariectomy-induced lipid peroxidation of purified brain mitochondria and total brain lipid fractions
Electron leakage, associated with free radical production, is increased in mitochondria deprived of ovarian hormones (12). We reasoned that the accumulated oxidative damage to mitochondrial membranes should also be increased. Conversely, replacement of ovarian hormones is expected to reduce oxidative damage. Results of these analyses indicated that within 2 wk, brain lipid peroxidation was significantly increased in ovarian hormone-deprived OVX rats compared with sham OVX (Fig. 6, A and B). When assessed as lipid peroxides, there was a reduction in purified brain mitochondrial accumulated lipid peroxides of 30% in E2 group (Fig. 6A). Similarly, there was a reduction in whole-brain lipid peroxidation of approximately 60% for the E2 group vs. OVX vehicle (Fig. 6B). The antioxidant defense system afforded by E2 was diminished when in combination with MPA.
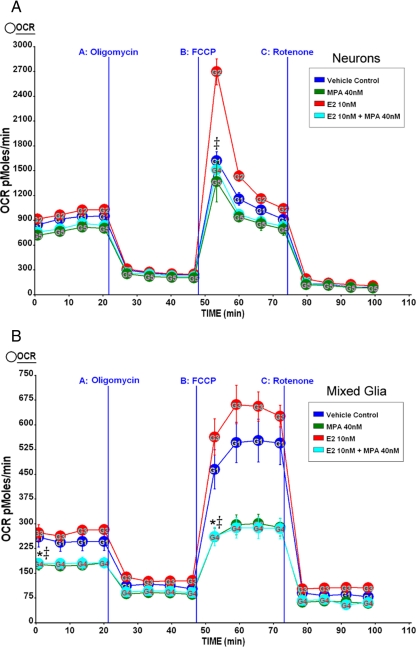
Medroxyprogesterone acetate inhibits E2 potentiation of hippocampal neuron and glia mitochondrial respiratory reserve capacity in vitro
Primary rat embryonic (E18) neurons from hippocampus were cultured for 10 d in neurobasal media+B27 and were treated with E2 (10 nm), MPA (40 nm), or E2+MPA [E2 (10 nm) + MPA (40 nm)] for 24 h before the experiment. OCR was determined using Seahorse XF-24 metabolic flux analyzer as described in Materials and Methods. In primary neurons, E2 (10 nm) elicited higher maximal mitochondrial respiratory capacity than all other conditions (vehicle; MPA, 40 nm; E2, 10 nm + MPA, 40 nm) (Fig. 7A). Importantly, in primary hippocampal neurons, the E2-induced maximal respiratory reserve capacity was inhibited by MPA coadministration. This differs from the results obtained from whole-brain mitochondria (Fig. 2), wherein E2+MPA was similar to the sham OVX and E2-alone groups. The discrepancy between ex vivo respiration, in which glutamate and malate are provided thus able to bypass PDH, and in vitro whole-cell oxygen consumption could possibly be explained by the inactivation of PDH by E2+MPA (Fig. 3B). Primary cultured mixed glia from hippocampus were cultured for 14 d in DMEM + 10% serum and were serum starved for 4 h and then treated with E2 (10 nm), MPA (40 nm), or E2+MPA [E2 (10 nm) + MPA (40 nm)] for 24 h before the experiment. As expected, basal respiration of mixed glia was much lower than pure neuronal culture. After the addition of the uncoupler FCCP, MPA alone and in combination with E2 significantly inhibited maximal mitochondrial respiratory capacity compared with vehicle or E2 conditions (Fig. 7B).
Fig. 7.
Medroxyprogesterone acetate inhibits mitochondrial respiration in vitro. A, Primary rat embryonic (E18) neurons from hippocampus were cultured for 10 d in neurobasal media+B27 and were treated with E2 (10 nm), MPA (40 nm), E2+MPA (10 nm + 40 nm), or vehicle for 24 h before the experiment. The OCR was determined using a Seahorse XF-24 metabolic flux analyzer. Vertical lines indicate time of addition of mitochondrial inhibitors A [oligomycin (1 μm)], B [FCCP (1 μm)], or C [rotenone (1 μm)]. The OCR in primary neurons treated with E2 (10 nm) had higher maximal mitochondrial respiratory capacity than all other conditions (vehicle; MPA, 40 nm; E2, 10 nm + MPA, 40 nm). *, P < 0.05 vs. vehicle; ‡, P < 0.05 vs. E2 (n = 3–4). B, Primary rat embryonic (E18) mixed glia from hippocampus were cultured for 14 d in DMEM supplemented with 10% serum. The glia were serum starved 4 h and then treated with E2 (10 nm), MPA (40 nm), E2+MPA (10 nm + 40 nm), or vehicle for 24 h before the experiment. The OCR was determined using a Seahorse XF-24 metabolic flux analyzer. Vertical lines indicate the time of addition of mitochondrial inhibitors A [oligomycin (1 μm)], B [FCCP (1 μm)], or C [rotenone (1 μm)]. The OCR in primary glia treated with MPA (40 nm) alone or in combination (E2, 10 nm + MPA, 40 nm) had lower maximal mitochondrial respiratory capacity relative to vehicle or E2 conditions (E2, 10 nm). *, P < 0.05 vs. vehicle; ‡, P < 0.05 vs. E2; (n = 4–5).
Discussion
We investigated the impact of MPA and E2+MPA on key mitochondrial enzymes, energy-transducing capacity, and oxidative stress. To the best of our knowledge, this is the first study to assess brain mitochondrial function and hippocampal mitochondrial protein expression after MPA and E2+MPA in vivo pretreatment. Results of these analyses indicate that pretreatment with MPA alone did not enhance mitochondrial respiration compared with OVX when energizing substrates were provided to isolated mitochondria ex vivo. However, E2+MPA significantly increased mitochondrial respiration relative to vehicle-treated OVX 24 h after a single in vivo exposure. After measuring mitochondrial respiration, we examined other critical components of mitochondrial bioenergetics. Our hypothesis led us to discover a hypometabolic state in OVX rat brain mitochondria that can be rescued by in vivo ovarian hormone replacement but not by the synthetic progestin MPA. This metabolic requirement is also lacking in diseases associated with mitochondrial dysfunction and diminished respiratory capacity (6, 9, 35–37). Many of the molecular events that lead to ATP production in normal and aging brain occur at the level of gene and protein expression. In addition, posttranslational modifications modulate enzymatic activity. By Western blot analysis of pPDH and therefore PDH inactivation, MPA significantly attenuated E2-induced dephosphorylation. Reduction in energy-transducing capacity also leads to secondary dysregulation of metabolic processes required for synaptic function and generation of action potentials.
In the current study, we demonstrated that complex IV activity is decreased in response to ovarian hormone deprivation. Consistent with this finding, only E2 rescued COX enzyme activity back to sham OVX levels. Other groups have reported increased COX activity with E2 treatment in various tissues including brain in which E2 treatment prevented ethanol withdrawal-induced declines in COX activity (38). Enhanced COX activity is largely due to increased expression of COX subunits. Corresponding to the responses in COX activity, in our previous studies, we found robust changes in COX subunit mRNA expression after E2- and P4-alone groups, with more modest increases in the E2+P4 coadministration group under the same 24-h treatment paradigm as the current study (Table 1) (12). To our knowledge, this is the first study to examine COX activity in the central nervous system in response to MPA alone and in combination with E2.
Table 1.
Consensus of estrogen and progestin effects on brain mitochondrial parameters
| Results compared with vehicle | Sham OVX | OVX +vehicle | OVX +E2 | OVX +P4a | OVX +E2+P4a | OVX +MPA | OVX +E2+MPA |
|---|---|---|---|---|---|---|---|
| RCR | ↑ | ⇆ | ↑ | ↑ | ∼ | ∼ | ↑ |
| PDH expression | ↑ | ⇆ | ↑ | X | X | ∼ | ↓ |
| PDH activity | ↑ | ⇆ | ↑ | X | X | ∼ | ∼ |
| pPDH | ↓ | ⇆ | ∼ | X | X | ∼ | ∼ |
| COXIV expression | ↑ | ⇆ | ↑ | ↑ | ∼ | ∼ | ∼ |
| COX activity | ↑ | ⇆ | ↑ | ↑ | ↑ | ∼ | ∼ |
| CVα | ↑ | ⇆ | ↑ | ↑ | ↑ | ∼ | ∼ |
| MnSOD expression | ∼ | ⇆ | ↑ | ↑ | ↑ | ∼ | ∼ |
| MnSOD activity | ∼ | ⇆ | ∼ | X | X | ∼ | ↓ |
| PrdxV | ∼ | ⇆ | ↑ | ∼ | ∼ | ↓ | ↓ |
| Mitochondrial lipid peroxides | ↓ | ⇆ | ↓ | ↓ | ↓ | ∼ | ∼ |
| OCR neurons | N/A | ⇆ | ↑ | ↑ | ∼ | ∼ | ∼ |
| OCR glia | N/A | ⇆ | ∼ | X | X | ↓ | ↓ |
Tabular comparison of mitochondrial function summarizes 24-h steroid hormone-replaced OVX rats. E2 (30 μg/kg), P4 (30 μg/kg), E2+P4 (30 μg/kg + 30 μg/kg), MPA (120 μg/kg), E2+MPA (30 μg/kg + 120 μg/kg), or corn oil vehicle control (OVX). Ovary-intact (sham OVX) rats served as a positive control and were selected on the morning after estrus by vaginal cytology. For cultured rat neuron and glial experiments: vehicle; MPA, 40 nm; E2, 10 nm; P4, 40 nm; E2, 10 nm + P4, 40 nm; E2, 10 nm + MPA, 40 nm. Significant changes relative to vehicle control (⇆) are represented as ↑ (increased), ↓ (decreased), ∼ (unchanged), X (not measured), or N/A (not applicable).
P4 and E2+P4 data referenced from Irwin et al. (12).
Previously we determined that E2, P4, or coadministration reduced electron leak, indicating greater efficiency of electron transport (12). Consistent with reduced generation of free radicals, E2 induced a significant reduction back to sham OVX levels in mitochondrial lipid peroxidation, whereas MPA and E2+MPA combination were not efficacious. The data further indicate that MPA does not directly regulate mitochondrial function but rather attenuates antioxidant defense capabilities associated with E2 treatment. Mechanisms underlying the MPA disruption of ovarian hormone actions involve signaling mechanisms such as PR signaling to MAPKs, critical for neuroprotection (23, 24). In the current study, when administered alone, MPA generally displayed neither positive nor negative effects on brain mitochondrial function when compared with OVX. MPA altered E2-induced restoration of mitochondrial protein expression related to energy transduction and oxidative stress levels in vivo, corroborated by in vitro data. These findings suggest that MPA is a mixed regulator of E2's effect on mitochondrial function. Functionally, although not significantly interfering with the magnitude of whole-brain mitochondria respiratory capacity, PDH, and COX activity, MPA did interfere with the antioxidant capacity measured by MnSOD activity. Although we did not observe direct action of MPA in most mitochondrial end points, the E2-afforded benefits of hormone therapy on mitochondrial health are diminished when MPA is administered in combination.
The findings indicate that acute effects of MPA differ significantly from the bioenergetic profile induced by P4 and that overall, MPA induced a decline in glycolytic and OXPHOS protein and activity. On all outcome measures except respiration, the combination of MPA and E2 was not synergistic and when administered in combination led to a decreased response relative to E2 alone. In hindsight, our method to measure mitochondrial respiration with isolated mitochondria, by providing the energizing substrates glutamate and malate, bypasses PDH, the link between glycolysis and the TCA cycle. Therefore, we did not test whether the treatment increased substrate utilization by the whole cell. To address this point, in vitro, we measured extracellular flux and determined that pretreatment with E2+MPA abolished E2's ability to increase the maximal respiratory burst in hippocampal neurons. In contrast to cultured neurons, data from mixed glial cultures suggest that pretreatment with MPA has a direct negative effect on glial cell function that overrides E2. The key to explain this discrepancy lies in the deleterious effect of E2+MPA on the antioxidant activity and expression profile of mitochondrial MnSOD, resulting in oxidative stress, which we observed by a marker of oxidant stress, lipid peroxides in both isolated brain mitochondria and total brain lipid extract. Lipid peroxidation is the end product of nonspecific oxidation of polyunsaturated fatty acids, altering the structure and function of cellular membranes. Oxidative stress activates mechanisms that result in glia-mediated inflammation that causes secondary neuronal damage (39).
Because the concentration of MPA was sufficient to inhibit E2 induction of uterine proliferation, the data herein represent the effects of an acute dose, similar to the clinically relevant ratio in the most commonly prescribed hormone therapy. E2+MPA coadministration resulted in a pronounced inhibition of E2's ability to reverse lipid peroxidation. Although the effects of the combined treatment were not entirely consistent across all outcome measures, it is clear that in the brain MPA does not elicit a synergistic response with E2. In some outcomes, as in mitochondrial respiration, there was no inhibitory effect of E2+MPA treatment relative to sham OVX- or E2-alone-positive controls. With other measures, such as PDH, COX, and CVα expression, the combined treatment down-regulated the response relative to OVX control. Disparate mitochondrial outcome profiles between P4 and MPA suggest that the distinct molecular structures of progestins alter steroid receptor-controlled mechanisms of mitochondrial function.
We showed previously that E2 up-regulates PrdxV and MnSOD expression 24 h after treatment in OVX rat brain mitochondria under identical conditions to the current study (10, 12). Although E2+MPA treatment did not change MnSOD expression relative to OVX, MnSOD activity decreased. Our data suggest that MnSOD is posttranslationally modified, thereby decreasing activity in this case, possibly by MPA interfering with signaling mechanisms controlled by E2. Phospholipid hydroperoxide glutathione peroxidase possesses the unique ability to eliminate lipid peroxides from biomembranes (40). E2 has been shown to up-regulate phospholipid hydroperoxide glutathione peroxidase and suppress cytochrome c release from mitochondria that would otherwise trigger apoptosis (41). Up-regulation of phospholipid hydroperoxide glutathione peroxidase prevents the accumulation of the MnSOD-deactivating 4-hydroxynonenal derived from the breakdown of lipid peroxides (42). From the data presented herein, a correlation exists between decreased MnSOD activity with E2+MPA treatment and under the same conditions, decreased mitochondrial PrdxV expression. Purified mitochondria from whole brain were immunoblotted to demonstrate that E2+MPA treatment significantly decreases PrdxV expression. MPA alone significantly decreased PrdxV expression compared with vehicle treated OVX, which potentially allows for inactivation of MnSOD by oxidative mechanisms (43). Thus, MPA negatively affects antioxidant defense capacity within the mitochondrial compartment.
Our studies show that MPA interferes with E2 potentiation of brain mitochondrial function in vitro and in vivo. Previous studies from our laboratory determined that P4 alone potentiated brain mitochondrial function. Here we show that MPA alone does not improve brain mitochondrial function, and therefore, its action is different from P4 (Table 1). The data suggest that the signaling mechanisms induced by P4 are not fully activated by MPA. Moreover, like P4, there is an antagonist effect of MPA on E2 signaling that prevents mitochondrial vigor. Our findings in a rodent model of hormone deprivation and replacement suggest that MPA could be detrimental to women's neurological health. These studies serve as a template for improved testing of progestin efficacy in brain with a focus on mitochondrial energetics and oxidative stress. In this vein, we recently determined the impact of seven clinically relevant progestins on neuroprotection and neurogenesis. The findings from that study indicated that P4 and nestorone were both neurogenic, whereas MPA was not (44). Of the outcomes measured, several progestins (P4, levonorgestrel, nestorone) increased mitochondrial ATP synthase protein expression, whereas MPA did not (44).
In summary, we have demonstrated that MPA diminished the E2-induced increase in oxidative capacity of whole-brain mitochondria, which was confirmed by in vitro cultured neuron and glia experiments. For E2+MPA conditions, increased respiratory activity correlated with increased oxidative stress as a result of antioxidant system inhibition by MPA. As such, using MPA as a progestin replacement may induce mitochondrial alterations in the central nervous system that exacerbate oxidative damage.
We conclude that ovarian hormone deprivation renders the brain susceptible to oxidative changes triggered by H2O2 and resulting in lipid peroxidation. MPA inhibition of antioxidant systems afforded by ovarian hormones exacerbates vulnerability to oxidative stress and neurodegeneration. These findings provide insights into progestin regulation of mitochondrial respiration and the impact of clinical regimens of hormone therapy on mitochondrial function in the brain. A major goal now is to identify a selective progestin that is better suited for hormone therapy and protective against age-related cognitive decline.
Acknowledgments
This work was supported by grants from the National Institute of Aging (5PO1AG026572; Progesterone in Brain Aging and Alzheimer's Disease, Project 1, to E.C. and R.D.B.).
Disclosure Summary: The authors have nothing to declare.
For editorial see page 343
- COX
- Cytochrome c oxidase
- COXIV
- cytochrome c oxidase complex IV
- E2
- 17β-estradiol (estradiol)
- MIB
- mitochondrial isolation buffer
- MnSOD
- manganese superoxide dismutase
- MPA
- 17α-acetoxy-6α-methylpregn-4-ene-3,20-dione (medroxyprogesterone acetate)
- OCR
- oxygen consumption rate
- OVX
- ovariectomized
- P4
- pregn-4-ene-3,20-dione (progesterone)
- PDH
- pyruvate dehydrogenase
- pPDH
- phosphorylated PDH
- PR
- progesterone receptor
- PrdxV
- peroxiredoxin V
- RCR
- respiratory control ratio
- TCA
- tricarboxylic acid cycle.
References
- 1. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. 2002. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 288:321–333 [DOI] [PubMed] [Google Scholar]
- 2. Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. for the Women's Health Initiative Memory Study 2004. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA 291:2947–2958 [DOI] [PubMed] [Google Scholar]
- 3. Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. 2003. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 289:2651–2662 [DOI] [PubMed] [Google Scholar]
- 4. Brinton RD. 2008. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci 31:529–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brinton RD. 2009. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci 30:212–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beal MF. 2007. Mitochondria and neurodegeneration. Novartis Found Symp 287:183–192; discussion 192–196 [DOI] [PubMed] [Google Scholar]
- 7. Mosconi L, Pupi A, De Leon MJ. 2008. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer's disease. Ann NY Acad Sci 1147:180–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reddy PH, Beal MF. 2008. Amyloid β, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer's disease. Trends Mol Med 14:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. 2009. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci USA 106:14670–14675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nilsen J, Irwin RW, Gallaher TK, Brinton RD. 2007. Estradiol in vivo regulation of brain mitochondrial proteome. J Neurosci 27:14069–14077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brinton RD. 2008. Estrogen regulation of glucose metabolism and mitochondrial function: therapeutic implications for prevention of Alzheimer's disease. Adv Drug Deliv Rev 60:1504–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Irwin RW, Yao J, Hamilton RT, Cadenas E, Brinton RD, Nilsen J. 2008. Progesterone and estrogen regulate oxidative metabolism in brain mitochondria. Endocrinology 149:3167–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. 2008. Progesterone receptors: form and function in brain. Front Neuroendocrinol 29:313–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krebs CJ, Jarvis ED, Chan J, Lydon JP, Ogawa S, Pfaff DW. 2000. A membrane-associated progesterone-binding protein, 25-Dx, is regulated by progesterone in brain regions involved in female reproductive behaviors. Proc Natl Acad Sci USA 97:12816–12821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schumacher M, Guennoun R, Ghoumari A, Massaad C, Robert F, El-Etr M, Akwa Y, Rajkowski K, Baulieu EE. 2007. Novel perspectives for progesterone in hormone replacement therapy, with special reference to the nervous system. Endocr Rev 28:387–439 [DOI] [PubMed] [Google Scholar]
- 16. Zhu Y, Bond J, Thomas P. 2003. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci USA 100:2237–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. 2003. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA 100:2231–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sitruk-Ware R. 2004. Pharmacological profile of progestins. Maturitas 47:277–283 [DOI] [PubMed] [Google Scholar]
- 19. Nilsen J, Diaz Brinton R. 2003. Mechanism of estrogen-mediated neuroprotection: regulation of mitochondrial calcium and Bcl-2 expression. Proc Natl Acad Sci USA 100:2842–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nilsen J, Chen S, Irwin RW, Iwamoto S, Brinton RD. 2006. Estrogen protects neuronal cells from amyloid beta-induced apoptosis via regulation of mitochondrial proteins and function. BMC Neurosci 7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mannella P, Brinton RD. 2006. Estrogen receptor protein interaction with phosphatidylinositol 3-kinase leads to activation of phosphorylated Akt and extracellular signal-regulated kinase 1/2 in the same population of cortical neurons: a unified mechanism of estrogen action. J Neurosci 26:9439–9447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao L, Chen S, Ming Wang J, Brinton RD. 2005. 17β-estradiol induces Ca2+ influx, dendritic and nuclear Ca2+ rise and subsequent cyclic AMP response element-binding protein activation in hippocampal neurons: a potential initiation mechanism for estrogen neurotrophism. Neuroscience 132:299–311 [DOI] [PubMed] [Google Scholar]
- 23. Nilsen J, Brinton RD. 2003. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc Natl Acad Sci USA 100:10506–10511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nilsen J, Brinton RD. 2002. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology 143:205–212 [DOI] [PubMed] [Google Scholar]
- 25. Jodhka PK, Kaur P, Underwood W, Lydon JP, Singh M. 2009. The differences in neuroprotective efficacy of progesterone and medroxyprogesterone acetate correlate with their effects on brain-derived neurotrophic factor expression. Endocrinology 150:3162– 3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McEwen B. 2002. Estrogen actions throughout the brain. Recent Prog Horm Res 57:357–384 [DOI] [PubMed] [Google Scholar]
- 27. Zadran S, Jourdi H, Rostamiani K, Qin Q, Bi X, Baudry M. 2010. Brain-derived neurotrophic factor and epidermal growth factor activate neuronal m-calpain via mitogen-activated protein kinase-dependent phosphorylation. J Neurosci 30:1086–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zadran S, Qin Q, Bi X, Zadran H, Kim Y, Foy MR, Thompson R, Baudry M. 2009. 17β-Estradiol increases neuronal excitability through MAP kinase-induced calpain activation. Proc Natl Acad Sci USA 106:21936–21941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshinaga K. Handbook of physiology endocrinology II, part 1. Gonadotrophin-induced hormone secretion and structural changes in the ovary during the nonpregnant reproductive cycle 1973 [Google Scholar]
- 30. Butera PC. 2010. Estradiol and the control of food intake. Physiol Behav 99:175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roesch DM. 2006. Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiol Behav 87:39–44 [DOI] [PubMed] [Google Scholar]
- 32. Wegorzewska IN, Walters K, Weiser MJ, Cruthirds DF, Ewell E, Larco DO, Handa RJ, Wu TJ. 2008. Postovariectomy weight gain in female rats is reversed by estrogen receptor α agonist, propylpyrazoletriol. Am J Obstet Gynecol 199:67.e1–67.e5 [DOI] [PubMed] [Google Scholar]
- 33. Han D, Williams E, Cadenas E. 2001. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem J 353:411–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martin E, Rosenthal RE, Fiskum G. 2005. Pyruvate dehydrogenase complex: metabolic link to ischemic brain injury and target of oxidative stress. J Neurosci Res 79:240–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Blass JP, Sheu RK, Gibson GE. 2000. Inherent abnormalities in energy metabolism in Alzheimer disease. Interaction with cerebrovascular compromise. Ann NY Acad Sci 903:204–221 [DOI] [PubMed] [Google Scholar]
- 36. Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. 2005. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann Neurol 57:695–703 [DOI] [PubMed] [Google Scholar]
- 37. Ishii K, Sasaki M, Kitagaki H, Yamaji S, Sakamoto S, Matsuda K, Mori E. 1997. Reduction of cerebellar glucose metabolism in advanced Alzheimer's disease. J Nucl Med 38:925–928 [PubMed] [Google Scholar]
- 38. Jung ME, Agarwal R, Simpkins JW. 2007. Ethanol withdrawal posttranslationally decreases the activity of cytochrome c oxidase in an estrogen reversible manner. Neurosci Lett 416:160–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang JY, Wen LL, Huang YN, Chen YT, Ku MC. 2006. Dual effects of antioxidants in neurodegeneration: direct neuroprotection against oxidative stress and indirect protection via suppression of glia-mediated inflammation. Curr Pharm Des 12:3521–3533 [DOI] [PubMed] [Google Scholar]
- 40. Ursini F, Maiorino M, Valente M, Ferri L, Gregolin C. 1982. Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim Biophys Acta 710:197–211 [DOI] [PubMed] [Google Scholar]
- 41. Lapointe J, Kimmins S, Maclaren LA, Bilodeau JF. 2005. Estrogen selectively up-regulates the phospholipid hydroperoxide glutathione peroxidase in the oviducts. Endocrinology 146:2583–2592 [DOI] [PubMed] [Google Scholar]
- 42. Roede JR, Jones DP. 2010. Reactive species and mitochondrial dysfunction: mechanistic significance of 4-hydroxynonenal. Environ Mol Mutagen 51:380–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dubuisson M, Vander Stricht D, Clippe A, Etienne F, Nauser T, Kissner R, Koppenol WH, Rees JF, Knoops B. 2004. Human peroxiredoxin 5 is a peroxynitrite reductase. FEBS Lett 571:161–165 [DOI] [PubMed] [Google Scholar]
- 44. Liu L, Zhao L, She H, Chen S, Wang JM, Wong C, McClure K, Sitruk-Ware R, Brinton RD. 2010. Clinically relevant progestins regulate neurogenic and neuroprotective responses in vitro and in vivo. Endocrinology 151:5782–5794 [DOI] [PMC free article] [PubMed] [Google Scholar]