Abstract
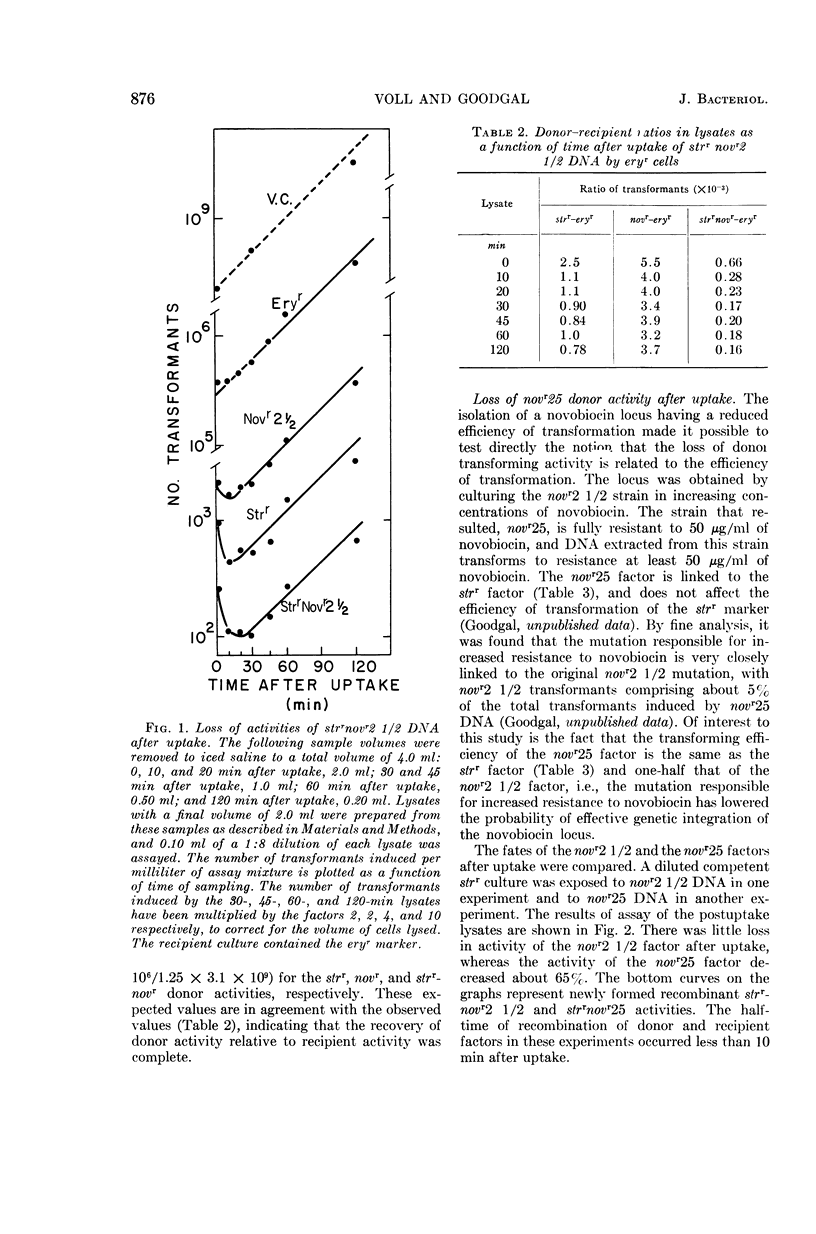
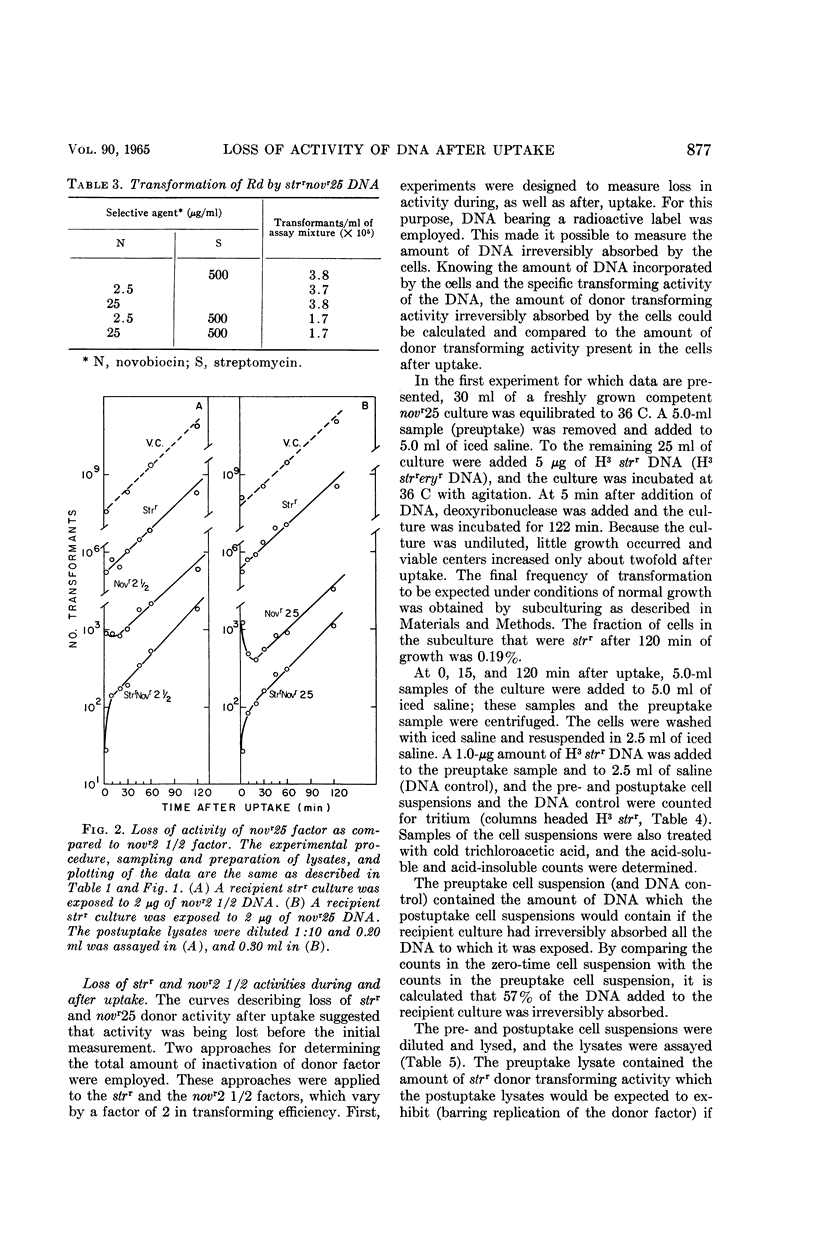
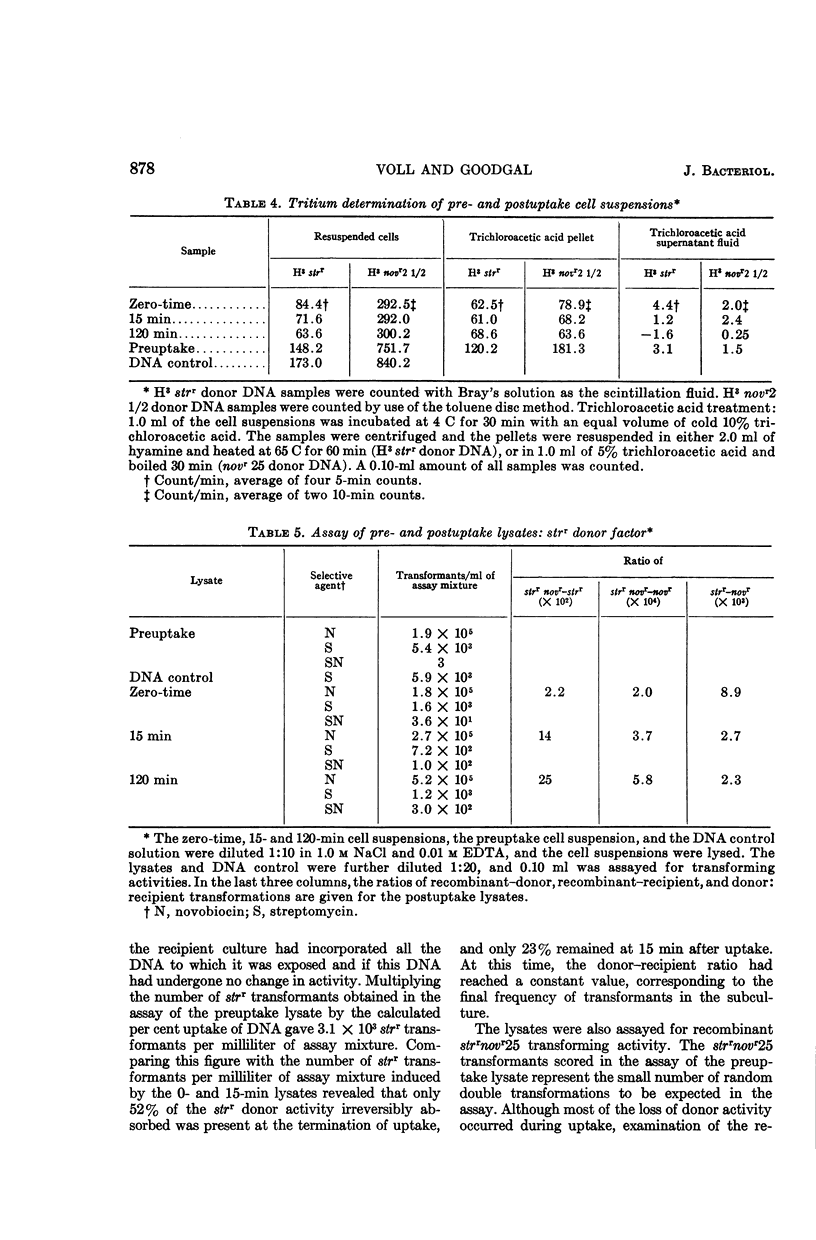
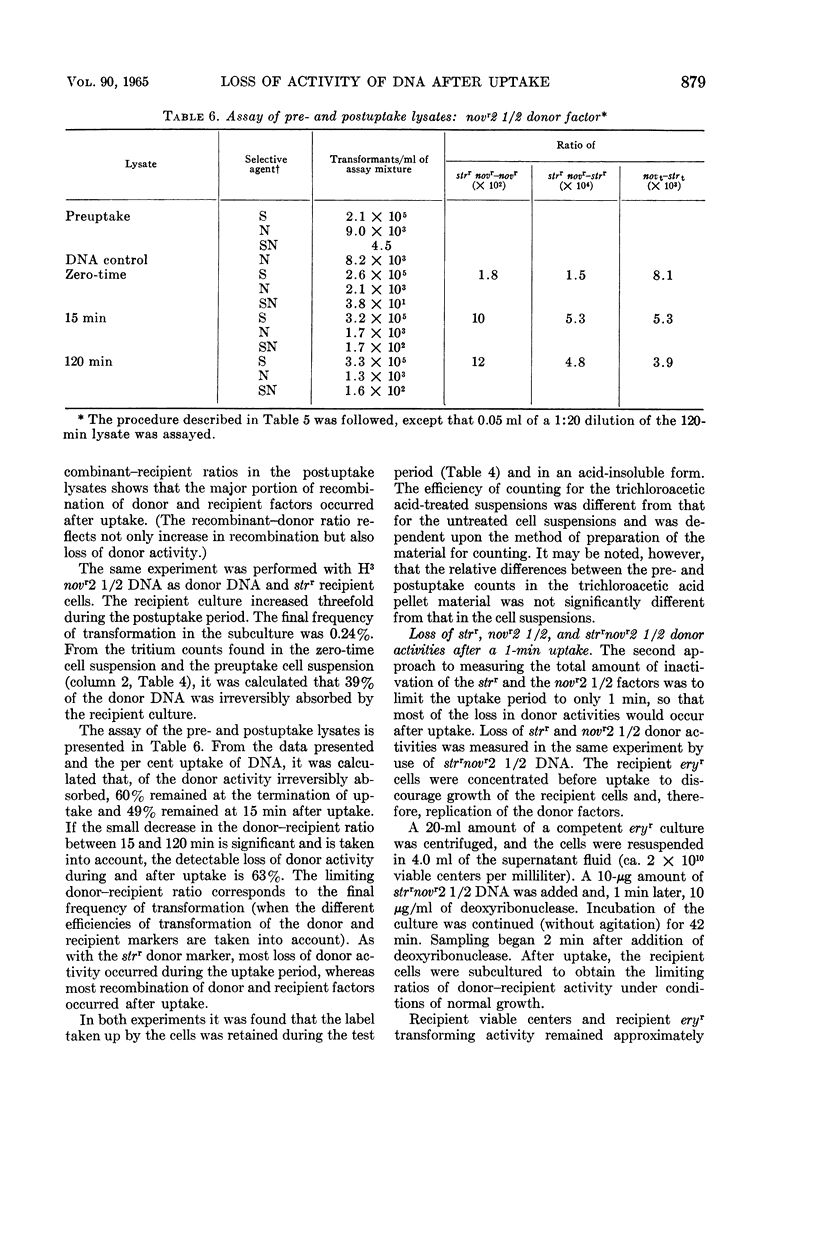
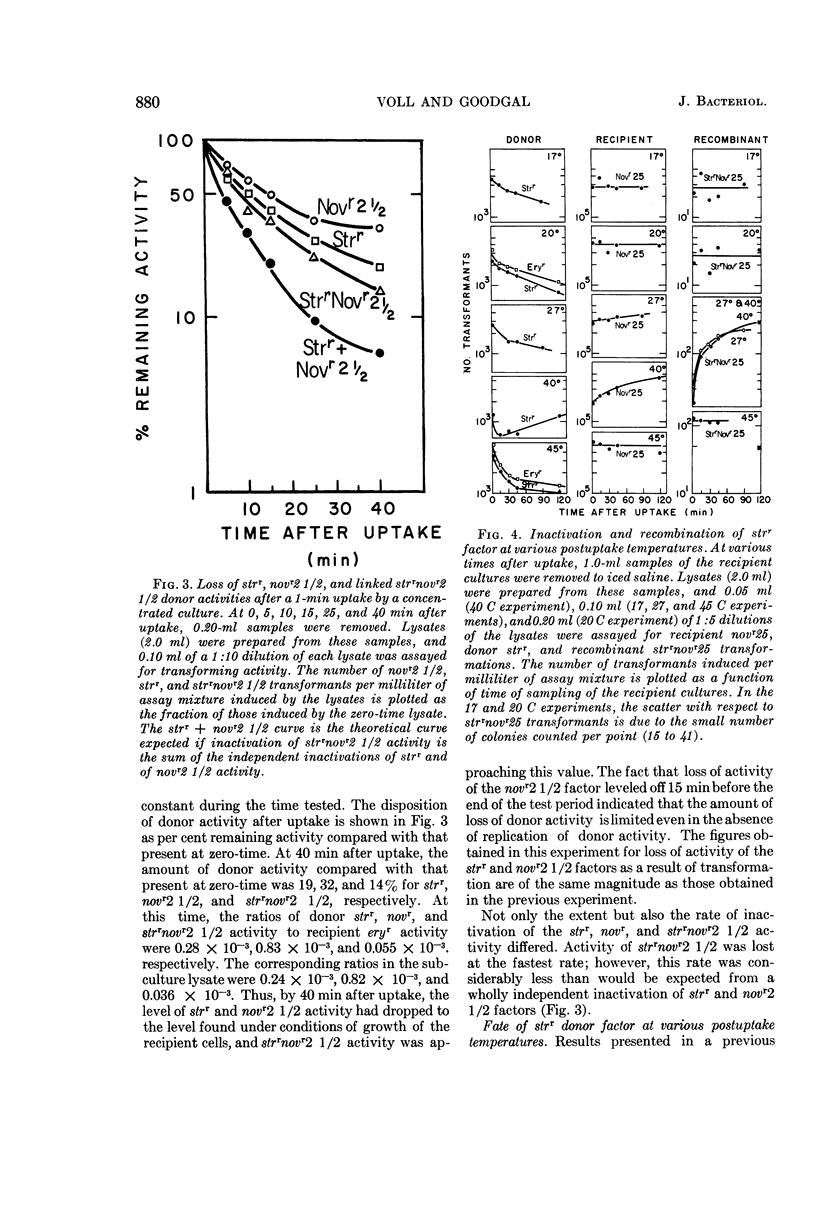
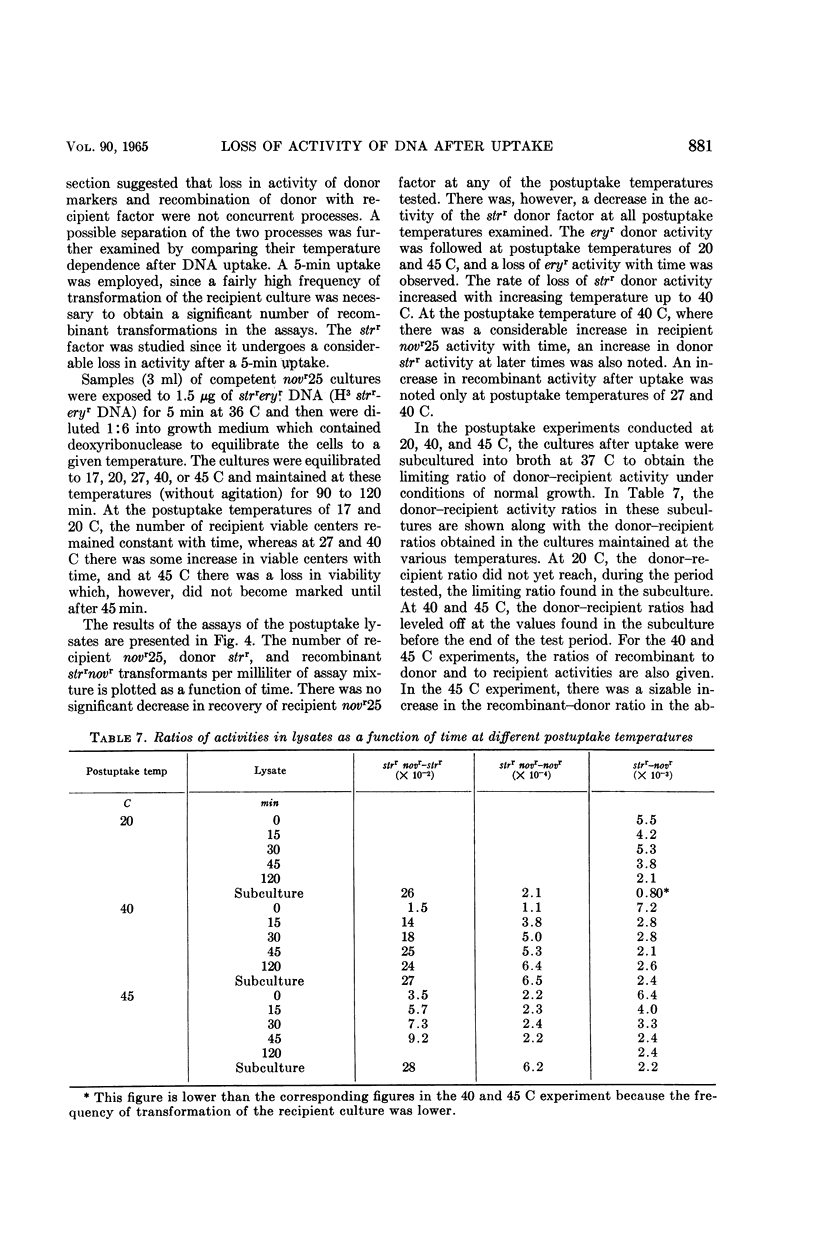
Voll, Mary Jane (University of Pennsylvania, Philadelphia), and Sol H. Goodgal. Loss of activity of transforming deoxyribonucleic acid after uptake by Haemophilus influenzae. J. Bacteriol. 90:873–883. 1965.—Transforming deoxyribonucleic acid (DNA) which has been irreversibly removed from solution by competent cells undergoes a progressive loss in marker activity when tested by lysis of the cells and exposure to new recipient cells. The loss of activity is limited and marker-specific, with greater inactivation of those markers with lower efficiencies of transformation. Recipient factors or donor factors which have undergone recombination, as measured by the appearance of linked markers, do not undergo inactivation. The efficiency of transformation can be correlated with the sensitivity of a marker to inactivation after DNA uptake. A mutation which affects the efficiency of transformation is found to increase sensitivity to postuptake inactivation. The rate of inactivation is temperature-dependent. At temperatures of 20 and 45 C, marker inactivation can occur without concomitant recombination. During the uptake process, DNA is retained in an acid-insoluble form, indicating that the fate of Haemophilus influenzae DNA differs from the fate of transforming DNA in pneumococcus.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER H. E., LEIDY G. Induction of streptomycin resistance in sensitive Hemophilus influenzae by extracts containing desoxyribonucleic acid from resistant Hemophilus influenzae. J Exp Med. 1953 Jan;97(1):17–31. doi: 10.1084/jem.97.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNHART B. J., HERRIOTT R. M. PENETRATION OF DEOXYRIBONUCLEIC ACID INTO HEMOPHILUS INFLUENZAE. Biochim Biophys Acta. 1963 Sep 17;76:25–39. [PubMed] [Google Scholar]
- FOX M. S., HOTCHKISS R. D. Fate of transforming deoxyribonucleate following fixation by transformable bacteria. Nature. 1960 Sep 17;187:1002–1006. doi: 10.1038/1871002a0. [DOI] [PubMed] [Google Scholar]
- GOODGAL S. H., HERRIOTT R. M. Studies on transformations of Hemophilus influenzae. I. Competence. J Gen Physiol. 1961 Jul;44:1201–1227. doi: 10.1085/jgp.44.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN D. M. A host-specific variation affecting relative frequency of transformation of two markers in pneumococcus. Exp Cell Res. 1959 Nov;18:466–480. doi: 10.1016/0014-4827(59)90312-x. [DOI] [PubMed] [Google Scholar]
- HOTCHKISS R. D. Size limitations governing the incorporation of genetic material in the bacterial transformations and other non-reciprocal recombinations. Symp Soc Exp Biol. 1958;12:49–59. [PubMed] [Google Scholar]
- LACKS S., HOTCHKISS R. D. A study of the genetic material determining an enzyme in Pneumococcus. Biochim Biophys Acta. 1960 Apr 22;39:508–518. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- LACKS S. Molecular fate of DNA in genetic transformation of Pneumococcus. J Mol Biol. 1962 Jul;5:119–131. doi: 10.1016/s0022-2836(62)80067-9. [DOI] [PubMed] [Google Scholar]
- RAVIN A. W., IYER V. N. Genetic mapping of DNA: influence of the mutated configuration on the frequency of recombination along the length of the molecule. Genetics. 1962 Oct;47:1369–1384. doi: 10.1093/genetics/47.10.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAEFFER P. Interspecific reactions in bacterial transformation. Symp Soc Exp Biol. 1958;12:60–74. [PubMed] [Google Scholar]
- VOLL M. J., GOODGAL S. H. Recombination during transformation in Hemophilus influenzae. Proc Natl Acad Sci U S A. 1961 Apr 15;47:505–512. doi: 10.1073/pnas.47.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]


