Abstract
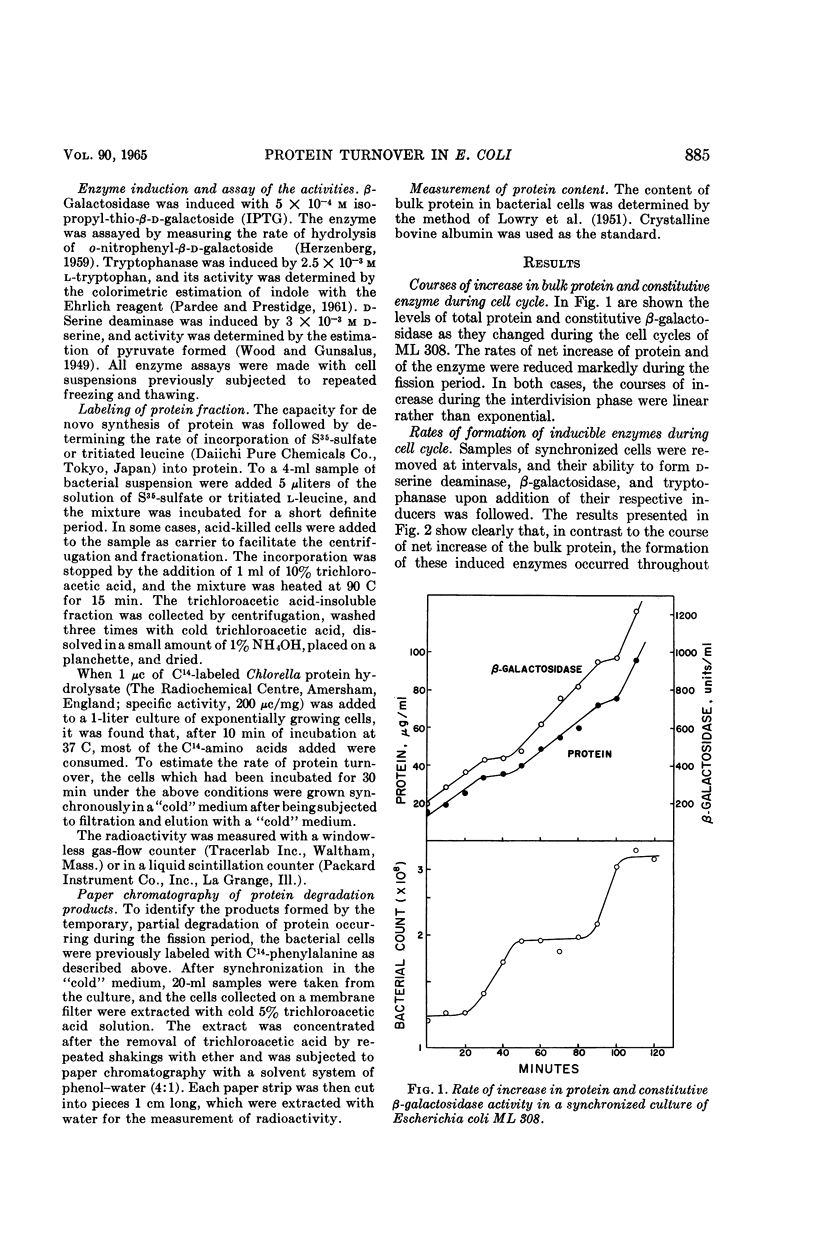
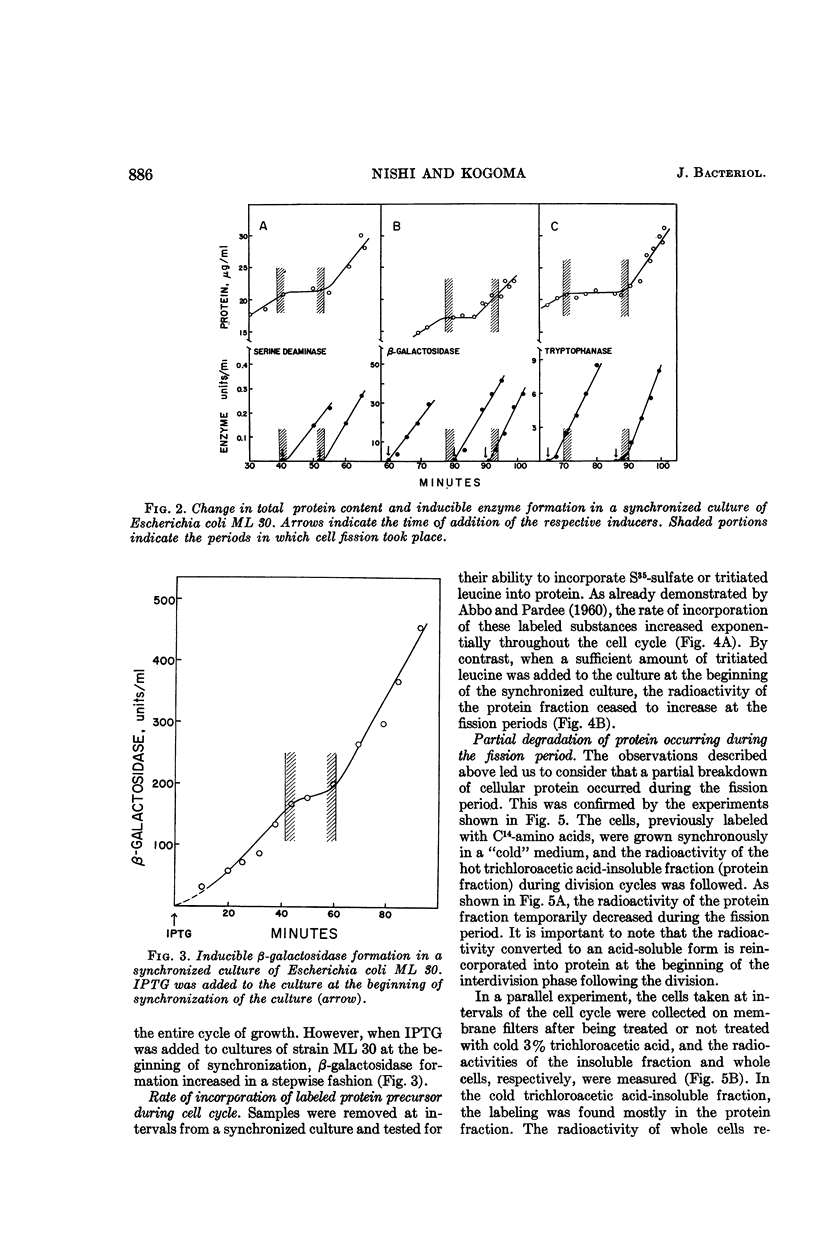
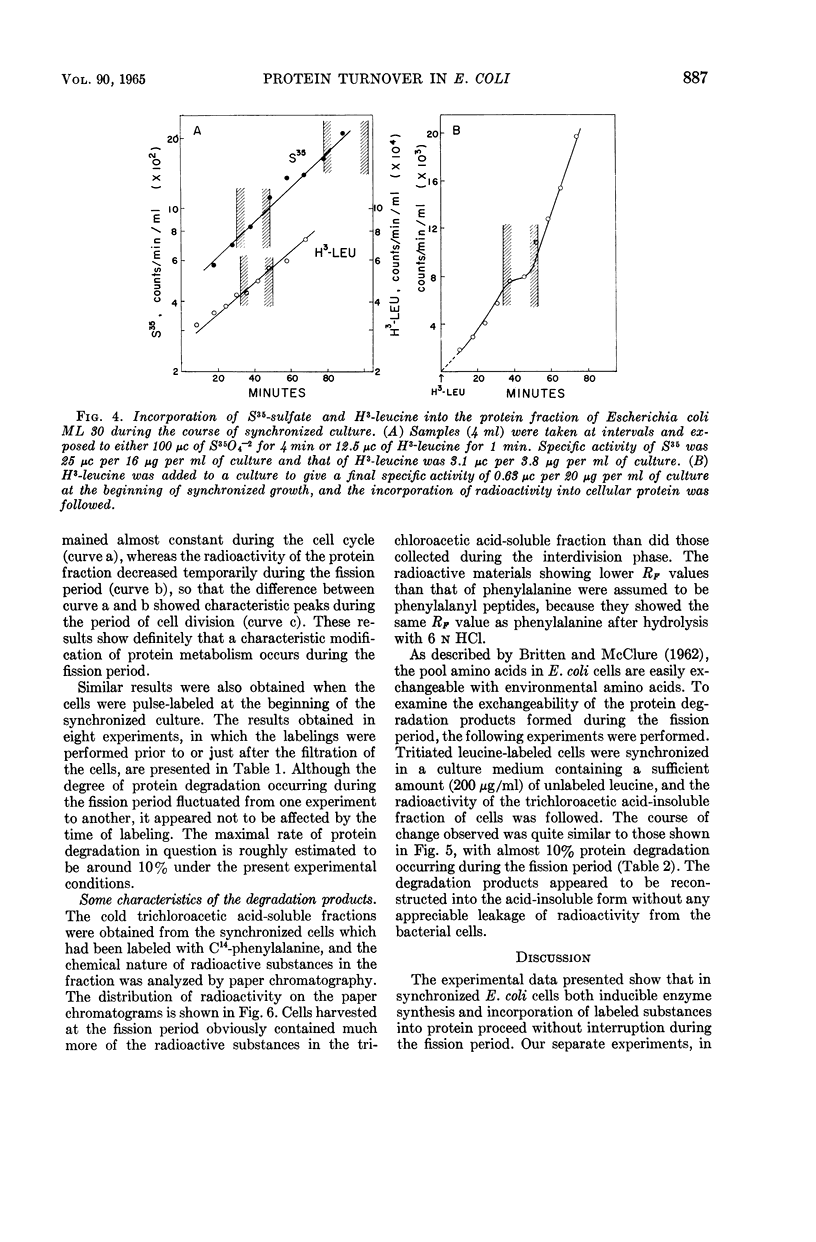
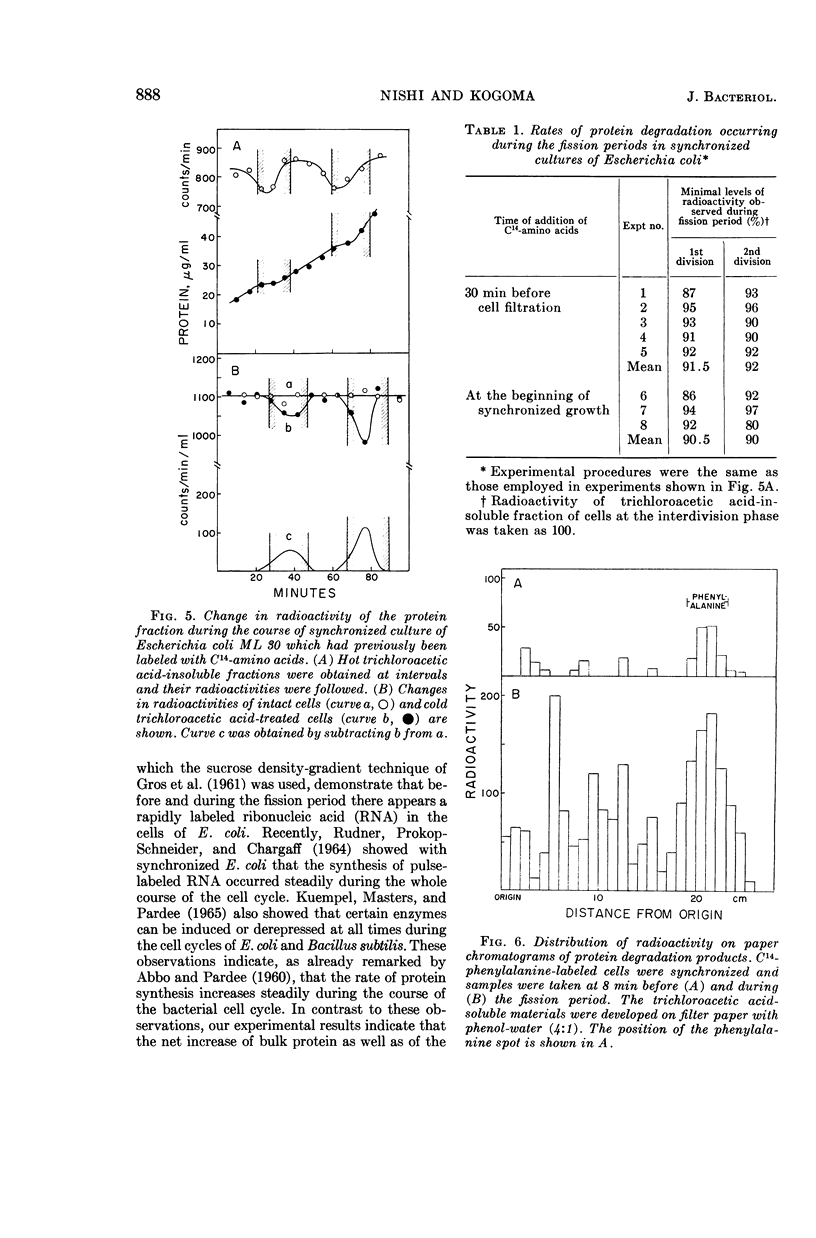
Nishi, Arasuke (University of Tokyo, Tokyo, Japan), and Tokio Kogoma. Protein turnover in the cell cycle of Escherichia coli. J. Bacteriol. 90:884–890. 1965.—Protein metabolism and enzyme formation throughout the cell cycle were investigated in synchronized cultures of Escherichia coli. The cells showed a temporary cessation of the net increase of bulk protein and of constitutive β-galactosidase activity during the division period. By contrast, when tested by short-term experiments performed with cells at different growth stages, the bacteria displayed a constant incorporation of labeled protein precursors into the protein fraction, even during the fission period. Similar results were obtained with respect to the capacities for induced enzyme formation. On the other hand, when the cells were previously labeled and then subjected to synchronization in a nonradioactive medium, the radioactivity of the protein fraction decreased temporarily by nearly 10% during the fission period and then regained its previous level at the beginning of the ensuing phase of growth. This indicates that the products of partial degradation of protein were again utilized for protein synthesis in the next cell cycle. It was concluded that the temporary lagging of net increase of bulk protein may be due to the partial breakdown of protein occurring during the fission period.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRITTEN R. J., McCLURE F. T. The amino acid pool in Escherichia coli. Bacteriol Rev. 1962 Sep;26:292–335. doi: 10.1128/br.26.3.292-335.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROS F., HIATT H., GILBERT W., KURLAND C. G., RISEBROUGH R. W., WATSON J. D. Unstable ribonucleic acid revealed by pulse labelling of Escherichia coli. Nature. 1961 May 13;190:581–585. doi: 10.1038/190581a0. [DOI] [PubMed] [Google Scholar]
- HAMBURGER K., ZEUTHEN E. Some characteristics of growth in normal and synchronized populations of Tetrahymena pyriformis. C R Trav Lab Carlsberg. 1960;32:1–18. [PubMed] [Google Scholar]
- HERZENBERG L. A. Studies on the induction of beta-galactosidase in a cryptic strain of Escherichia coli. Biochim Biophys Acta. 1959 Feb;31(2):525–538. doi: 10.1016/0006-3002(59)90029-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANDELSTAM J. The intracellular turnover of protein and nucleic acids and its role in biochemical differentiation. Bacteriol Rev. 1960 Sep;24(3):289–308. doi: 10.1128/br.24.3.289-308.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARUYAMA Y. Biochemical aspects of the cell growth of Escherichia coli as studied by the method of synchronous culture. J Bacteriol. 1956 Dec;72(6):821–826. doi: 10.1128/jb.72.6.821-826.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARUYAMA Y., YANAGITA T. Physical methods for obtaining synchronous culture of Escherichia coli. J Bacteriol. 1956 May;71(5):542–546. doi: 10.1128/jb.71.5.542-546.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. The initial kinetics of enzyme induction. Biochim Biophys Acta. 1961 Apr 29;49:77–88. doi: 10.1016/0006-3002(61)90871-x. [DOI] [PubMed] [Google Scholar]
- RUDNER R., PROKOP-SCHNEIDER B., CHARGAFF E. RHYTHMIC ALTERNATIONS IN THE RATE OF SYNTHESIS AND THE COMPOSITION OF RAPIDLY LABELLED RIBONUCLEIC ACID DURING THE SYNCHRONOUS GROWTH OF BACTERIA. Nature. 1964 Aug 1;203:479–483. doi: 10.1038/203479a0. [DOI] [PubMed] [Google Scholar]
- SYLVEN B., TOBIAS C. A., MALMGREN H., OTTOSON R., THORELL B. Cyclic variations in the peptidase and catheptic activities of yeast cultures synchronized with respect to cell multiplication. Exp Cell Res. 1959 Jan;16(1):75–87. doi: 10.1016/0014-4827(59)90197-1. [DOI] [PubMed] [Google Scholar]
- WOOD W. A., GUNSALUS I. C. Serine and threonine desaminaes of Escherichia coli; activators for a cell-free enzyme. J Biol Chem. 1949 Nov;181(1):171–182. [PubMed] [Google Scholar]


