Abstract
Consuming protein following exercise has been shown to stimulate protein synthesis acutely in skeletal muscle and has been recommended to prevent sarcopenia. It is not known, however, whether acute stimulation persists long term or includes muscle cell division. We asked here whether consuming protein following exercise during aerobic training increases long-term protein and DNA synthesis rates in skeletal muscle of adult humans. Sixteen previously untrained participants (50±8 yr) consumed either a carbohydrate or carbohydrate and protein drink following each session during 6 wk of treadmill training. A younger untrained group provided a nonexercising comparison. Participants were administered heavy water (2H2O; deuterium oxide) continuously for 6 wk to isotopically label newly synthesized skeletal muscle proteins and DNA. Muscle biopsies were performed after 6 wk of training. Contrary to acute studies, consuming protein after exercise did not increase skeletal muscle protein synthesis rates. In contrast, muscle protein synthesis, DNA, and phospholipid synthesis were significantly higher in the older exercise groups than the younger sedentary group. The higher DNA replication rate could not be attributed to mitochondrial DNA and may be due to satellite cell activation. We conclude that postexercise protein supplementation does not increase rates of mixed protein synthesis over 6 wk and that aerobic exercise may stimulate long-term cell division (DNA synthesis) in skeletal muscle of humans. Measurements of long-term synthesis rates provide important insights into aging and exercise adaptations.—Robinson, M. M., Turner, S. M., Hellerstein, M. K., Hamilton, K. L., Miller, B. F. Long-term synthesis rates of skeletal muscle DNA and protein are higher during aerobic training in older humans than in sedentary young subjects but are not altered by protein supplementation.
Keywords: stable isotope tracer, kinetics, deuterium oxide, heavy water, endurance, satellite cell
Aging is a major public health concern due to high social and economic costs associated with impaired mobility and physical function (1). These costs will continue to rise as the world's population >60 yr old increases to an estimated 2 billion by 2050 (2). The loss of skeletal muscle mass and function with age (sarcopenia) contributes to increased morbidity. Understanding the progression of sarcopenia may help the development of lifestyle recommendations to promote healthy aging.
There is a ∼1–2%/yr loss of skeletal muscle mass in people >50 yr (3, 4). The mass of skeletal muscle tissue is determined by the net contribution of the synthesis of new proteins and degradation of existing proteins, collectively called protein turnover. Exercise and protein consumption have repeatedly been shown to increase acute muscle protein synthesis in younger people (5, 6), while older people showed blunted responses to feeding (7), insulin (8), and exercise (9). The blunted response to anabolic signals may lead to lower average rates of protein synthesis over prolonged time frames and contribute to sarcopenia.
Although aerobic exercise is recommended to aging populations, little is known about the potential anabolic responses to aerobic exercise. Further, mitochondria dysfunction is implicated in muscle wasting (10), and potential increases in mitochondrial biogenesis from aerobic training may be beneficial for aging populations (11). Recent studies show that aerobic exercise can stimulate skeletal muscle protein synthesis (12) and myofiber size and function (13). In addition, protein consumption after cycling can increase whole body protein turnover (14) and nitrogen balance (15) in older people. Consuming protein after aerobic exercise is a simple lifestyle modification for aging populations, but it is currently not known whether short-term increases in skeletal muscle protein synthesis will persist over longer duration.
Although previous studies of sarcopenia have focused on mixed or myofibrillar proteins, depletion of satellite cells (16) and loss of mitochondria (17) could also play a role in the loss of muscle function. Satellite cells are resident myogenic precursors that promote muscle growth and repair following exercise, and a decrease in the content (16, 18) or activation (19) of satellite cells with age has been suggested to contribute to sarcopenia. Activation of satellite cells to grow, divide, and fuse to an existing myofibril requires DNA synthesis, protein synthesis, and membrane remodeling. Simultaneously analyzing these pathways has been technically challenging in short-term intravenous tracer infusions.
Deuterium oxide (D2O, 2H2O, or heavy water) can be used to determine kinetics of multiple synthetic processes over several weeks to months (20–23). Deuterium from the body water pool is incorporated into a wide variety of metabolic precursors that produce deuterium-labeled products, including proteins (24), DNA (25), and lipids (22). To our knowledge, there are no reports characterizing multiple long-term synthetic processes in skeletal muscle during exercise training in humans.
The purpose of our study was to assess long-term synthetic processes in skeletal muscle in older individuals undergoing an aerobic exercise-training program with postexercise nutrient ingestion. We hypothesized that protein consumption following exercise would increase synthesis rates of skeletal muscle protein and DNA. Contrary to our hypothesis, postexercise protein nutrition did not enhance anabolic responses to a greater extent than postexercise carbohydrate ingestion. A novel finding was that aerobic exercise-trained older individuals exhibited higher protein synthesis and DNA replication in skeletal muscle than in younger, sedentary subjects, the latter finding potentially attributable to increased satellite cell recruitment.
MATERIALS AND METHODS
Ethical approval
The Institutional Review Board at Colorado State University approved this study (07-199H and 09-1363H). Each volunteer was informed of the potential risks and benefits and provided written consent before participating. The study followed the guidelines set forth by the Declaration of Helsinki.
Subject characteristics
Sixteen sedentary males and females (mean±sd; age 50±8; range 37–64 yr) volunteered for the study (Table 1). Because of novel findings regarding DNA turnover, a second group of participants (3 female and 1 male; age 21±2; range 19–23 yr) who did not perform exercise training was added to provide a young sedentary comparison group (Table 1). Participants in the exercise group were matched for age, sex, body mass index (BMI), and maximal aerobic capacity (Vo2max) and then randomized to consume either a carbohydrate (CHO) or protein + carbohydrate (PRO) drink following each exercise session. There were no baseline differences (P>0.05) between PRO and CHO in age, BMI, or Vo2max (Table 1). The participants were healthy based on medical history questionnaire and results from a Bruce protocol graded exercise test with 12-lead electrocardiogram. Body composition was determined using dual-energy X-ray absorptiometery (DEXA; GE Medical Systems, Madison, WI, USA). All subjects were free of any medications that could impair mitochondrial adaptations, including nonselective β-adrenergic antagonists, HMG-CoA reductase inhibitors (statins), and nonsteroidal anti-inflammatory drugs (26). Medication classes that were allowed included selective serotonin reuptake inhibitors, proton pump inhibitors, and multivitamins.
Table 1.
Subject characteristics of treatment and control groups
| Characteristic | CHO | PRO | Young sedentary |
|---|---|---|---|
| Sex | 5 F, 3 M | 5 F, 3 M | 3 F, 1 M |
| Age (yr) | 52 ± 10 | 48 ± 7 | 21 ± 2 |
| Height (m) | 1.67 ± 0.11 | 1.68 ± 0.14 | 1.6 ± 0.1 |
| Weight (kg) | 72.2 ± 24.2 | 71.0 ± 21.0 | 58.5 ± 11.4 |
| BMI (kg/m2) | 25.3 ± 4.8 | 24.7 ± 3.3 | 22.6 ± 1.9 |
| Fat (%) | 29.7 ± 5.6 | 30.7 ± 8.2 | 24.6 ± 5.7 |
| Vo2max (ml/kg/min) | 31.1 ± 7.1 | 25.5 ± 4.2 | 38.4 ± 3.5 |
| Vo2max (L/min) | 2.2 ± 0.77 | 1.83 ± 0.62 | 2.25 ± 0.5 |
Subjects were matched for sex (F, female; M, male), age, BMI, and Vo2max and then randomized to consume either CHO or PRO drinks following each exercise session. A third group did not undergo any training or dietary intervention and provided a young sedentary comparison group.
Study overview
Participants completed a progressive aerobic exercise protocol (3 sessions/wk for 6 wk) and consumed a postexercise drink of either CHO or PRO. The drinks were isocaloric (300 kcal), and PRO included 20 g of mixed milk proteins (drinks were kindly provided by the Gatorade Sports Science Institute, Barrington IL, USA). D2O was consumed daily to isotopically label newly synthesized skeletal muscle proteins, DNA, and membrane phospholipid glycerol. Vo2max, DEXA, muscle biopsy, and blood sampling were performed before and after the 6-wk training protocol (Fig. 1). The participants were instructed to maintain their normal dietary habits. Dietary habits were not changed throughout the study, as shown from 3-d records collected every 2 wk (data not shown).
Figure 1.
Sixteen participants completed 6 wk of progressive aerobic training while consuming either carbohydrates or carbohydrates with 20 g of protein following each exercise session. D2O was consumed 3 times daily (tid) or 2 times daily (bid) to isotopically label newly synthesized products obtained in muscle biopsy samples. Body composition and maximal aerobic fitness (Vo2max) were determined before and after training. Saliva and blood samples were used to determine steady-state D2O content in body water and precursor enrichments for synthesis rate calculations.
Vo2max
Vo2max was measured using a cycle ergometer (Lode Excalibur; Medical Graphics Corp., St. Paul, MN, USA) and indirect calorimeter (ParvoMedics, Sandy, UT, USA), as described previously (15). Heart rate was continuously recorded during the test and used to determine the intensity of exercise sessions. The heart rates recorded at various percentages of Vo2max (60, 65, 75, and 85%) were used to determine exercise intensity during the 6-wk exercise protocol.
Exercise protocol
The 6-wk training program was chosen because this period has been identified as a the period necessary to induce a new steady state of mitochondrial biosynthesis in humans (27). Exercise was performed on a treadmill and included warm-up (15 min at 60%) and workout stages (30 min, progressing from 65 to 85% by wk 5). The participants wore heart rate monitors (Polar Electro, Lake Success, NY, USA), and the speed or grade of the treadmill was changed to maintain heart rate at the specified percentage of Vo2max. A skilled technician supervised each exercise session and monitored all exercise intensities and times. The drinks were consumed immediately after exercise under supervision. We had 100% compliance with all exercise sessions and drink consumption.
Deuterium labeling
Deuterium labeling of newly synthesized products was achieved using oral consumption of D2O (70%; Cambridge Isotope Laboratories, Andover MA, USA) throughout the entire 6-wk exercise protocol (or 4 wk for sedentary group). A target of 1–2% enrichment was achieved during a 1-wk priming stage (50 ml 3×/d = 150 ml/d) and maintained for 5 wk (50 ml 2×d = 100 ml/d). Body water enrichment was determined from saliva swabs collected at wk 2, 4, and 6 as described previously (22, 28). Participants were instructed to not eat or drink anything for 30 min before saliva sampling. Saliva swabs were stored at −80°C until analysis.
Tissue sampling
Participants arrived at the laboratory following an overnight fast for blood and muscle sampling before and after the 6 wk exercise protocol. Venous blood was collected; 1 ml of whole blood was removed and stored at −80°C for peripheral blood mononuclear cell (PMBC) isolation as described below. The remaining whole blood was centrifuged (1200 g, 4°C, 15 min) to separate plasma and buffy coat layers that were removed separately and stored at −80°C. Muscle biopsy samples (∼100–150 mg) of the vastus lateralis were removed while the subjects were under local anesthesia (1% lidocaine) using a 5-mm Bergstrom needle with manual suction and then immediately frozen in liquid nitrogen and stored at −80°C.
Mixed muscle protein synthesis rate
Mixed muscle protein synthesis (MPS) rate was determined from ∼20 mg of the postmuscle using gas-chromatography mass-spectrometry (GC-MS) analysis of deuterium-labeled alanine, as described previously (20). Approximately 20 mg of mixed skeletal muscle was hydrolyzed by incubation in 6 N HCl at 120°C for 24 h. The hydrolysates were ion exchanged, dried under vacuum, and then suspended in 1 ml of 50% acetonitrile and 50 mM K2HPO4, pH 11. Pentafluorobenzyl bromide (20 μl; Pierce Scientific, Rockford, IL, USA) was added, and the sealed mixture was incubated at 100°C for 1 h. Derivatives were extracted into ethyl acetate, and the top, organic layer was removed and dried by addition of solid Na2SO4 followed by vacuum centrifugation. With the use of negative chemical ionization, derivatized amino acids were analyzed on a DB225 gas chromatograph column. The starting temperature was 100°C, increasing 10°C/min to 220°C. The mass spectrometry used negative chemical ionization with helium as the carrier gas and methane as the reagent gas. The mass-to-charge ratios of 448, 449, and 450 were monitored for the pentafluorobenzyl-N,N-di(pentafluorobenzyl)alaninate derivative. In all cases, these mass-to-charge ratios represented the primary daughter ions that included all of the original hydrocarbon bonds from the given amino acid. The newly synthesized fraction (f) of muscle proteins was calculated from the true precursor enrichment (p) using mass isotopomer distribution analysis (MIDA; refs. 20, 29). MPS was calculated as the change in enrichment of deuterium-labeled alanine (25) bound in muscle proteins over the entire labeling period.
Muscle DNA extraction
Muscle DNA synthesis rate (DNA %F) was determined from DNA extracted from muscle biopsy samples. Total muscle DNA (∼8 μg) was extracted from 50 mg tissue using the MiniDNA kit (Qiagen, Valencia, CA, USA), following manufacturer's instructions, and eluted in 200 μl TE buffer (10 mM Tris and 1 mM EDTA, pH 8.0). A 20-μl aliquot of total DNA was used for PCR procedures (below). The remaining DNA was precipitated with cold ethanol, suspended into 200 μl nuclease-free H2O, and hydrolyzed to free deoxyribonucleic acids. Briefly, isolated DNA was hydrolyzed overnight at 37°C with nuclease S1 and potato acid phosphatase. Hydrolyzates were reacted with pentafluorobenzyl hydroxylamine and acetic acid and then acetylated with acetic anhydride and 1-methylimidazole. Dichloromethane extracts were dried, resuspended in ethyl acetate, and analyzed by GC-MS on a DB-17 column with negative chemical ionization, using He as carrier and CH4 as reagent gas. The fractional molar isotope abundances at m/z 435 (M0 mass isotopomer) and 436 (M1) of the pentafluorobenzyl triacetyl derivative of purine dR were quantified using ChemStation software (Agilent Technologies, Santa Clara, CA, USA). The deoxyadenosine fraction was separated and analyzed for deuterium content by GC-MS, as described previously (25).
Blood processing
PMBCs were purified from frozen whole-blood samples using magnetic beads (Miltenyi Biotech, Auburn, CA, USA) following the manufacturer's protocol. The buffy coat layer was used for one participant because the whole-blood sample yielded insufficient DNA. Briefly, anti-CD14+ beads were added to whole blood (1 ml) or buffy coat (∼500 μl) and collected using whole-blood columns and MiniMacs separator (Miltenyi Biotech). The PMBC fraction was suspended in 200 μl PBS (3.2 mM Na2HPO4, 0.5 mM KH2PO4, 1.3 mM KCl, and 135 mM NaCl, pH 7.5). DNA was extracted from PBMCs using a DNA Mini kit (Qiagen), eluted using 200 μl nuclease-free H2O, and processed for GC-MS analysis as described for DNA %F (25).
Calculation of DNA %F
Deuterium labeling of the deoxyribose moiety of DNA occurs exclusively through de novo nucleotide synthesis and allows calculation of the rate of newly synthesized DNA over extended periods (21, 25). The enrichment of a synthesized product cannot exceed the enrichment of the true precursor; therefore, the enrichment of DNA in a cell that is fully replaced during the labeling period (e.g., PMBC) can be used as an estimate of the enrichment of the precursor in the individual (EM1*). We determined EM1* using 2 methods: PMBC DNA enrichment, and the relationship between measured body water deuterium enrichment and MIDA calculations of EM1* at different body water D2O enrichments, as described previously (25, 29). A generalized form of the relationship between EM1* and body water D2O is EM1* = 3.193 * BW + 0.0013, where BW is determined from the body water D2O enrichment measured in saliva samples. There was no difference between the DNA %F calculated from body water and PMBCs, and the reported values are from body water.
Muscle membrane phospholipid synthesis
Total phospholipids were extracted from muscle biopsy samples, and the glycerol fraction was separated by thin-layer chromatography and analyzed by mass spectrometry (22). Briefly, a lipid-containing fraction was collected during the DNA isolation as described above. The digested muscle sample was applied to a spin column and centrifuged, and then a lipid-enriched fraction was collected from the flowthrough. Lipids were extracted using chloroform, and then fatty acid methyl esters were separated from glycerol by a modified Folch technique. The aqueous phase containing glycerol was lyophilized, derivatized to glycerol triacetate, and resolved by thin-layer chromatography. The labeling of phospholipid glycerol represents the formation of new phospholipid molecules in the same manner as for triglyceride molecules (22); glycerol hydrogens equilibrate rapidly with body water during the formation of triosephosphate, and these hydrogens are then permanently incorporated into the glycerol backbone of monoacylglycerides, which in turn form phospholipids. The synthesis rate of membrane phospholipids (PL %F) is an index of membrane lipid synthesis.
Real-time PCR
The ratio of mitochondrial DNA (mtDNA) to nuclear DNA (nDNA) copy number was determined using real-time PCR from an aliquot of total muscle DNA. Briefly, 10 ng of DNA was amplified in a 20-μl reaction with a premade master mix (Thermo Fisher, Rockford, IL, USA) and TaqMan primers and probes (Applied Biosystems, Carlsbad, CA, USA). Samples were amplified in triplicate for each primer/probe sequence (singleplex), and all plates included blank and internal controls. PCR conditions were a hot start (15 min at 95°C) followed by 40 cycles of denaturing (15 s at 95°C) and annealing (60 s at 60°C) in 96-well clear reaction plates using a 7300 Real-Time PCR System (Applied Biosystems). One nuclear and one mitochondrial primer/probe set was custom designed using Primer Express 3.0 software (Applied Biosystems), and the other primers and probes were inventoried TaqMan gene expression assays for genomic DNA (Applied Biosystems; Table 2). The inventoried assays include proprietary sequences that are not specified by Applied Biosystems.
Table 2.
Sequence information of PCR gene targets
| Name | Gene | Chr | Sequence |
|---|---|---|---|
| Displacement loop | MT-Dloop | mt | F: AGCACATTACAGTCAAATCCCTTCTC |
| R: CACGGAGGATGGTGGTCAAG | |||
| P: CCCCATGGATGACCCC | |||
| β-2-Microglobulin | B2M | 15 | F: GTGCCTGATATAGCTTGACACCAA |
| R: TCGGGAAAAGACACATTAATATTGCCA | |||
| P: CCCCAAGTGAAATACC | |||
| Mitochondrial encoded 16S RNA | MT-RNR2 | mt | Proprietary sequence assay Hs02596860_s1 |
| TATA box binding protein | TAF-1L | X | Proprietary sequence assay Hs00542346_s1 |
Primers and probes for MT-Dloop and B2M were custom designed and are reported. MT-RNR2 and TAF-1L were purchased as proprietary sequences from Applied Biosystems. F, forward; R, reverse; P, probe.
mtDNA content
The relative copy (RC) number of mtDNA to nDNA (30) adjusted for qPCR efficiency (31) was calculated as RC = EΔCt where
The slope of the linear regression line of diluted DNA samples covering the expected threshold cycle Ct for gene targets revealed equal efficiency E between gene targets (E=75%). Ct values were determined automatically using the SDS 1.4 software (Applied Biosystems), and then it was manually verified that the threshold line was located early in the linear amplification phase. Ct values were consistent between the control samples run on separate plates.
Statistics
Statistical analysis was performed using Prism 4.0c (GraphPad Software, La Jolla, CA, USA). Differences in subject characteristics were compared using 2-way ANOVA (group×time) with repeated measures. Changes in Vo2max were calculated as the percentage increase over baseline and compared using an unpaired t test. MPS and DNA %F were compared with unpaired t test. Correlations were calculated using Pearson's r. Significance was set at P = 0.05; data are presented as means ± sd.
RESULTS
Changes in subject characteristics
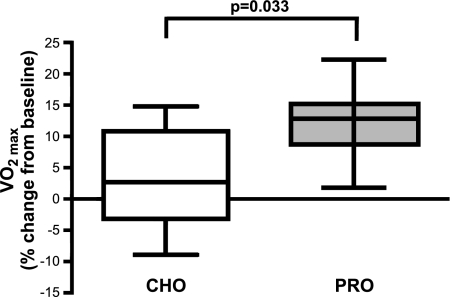
Absolute Vo2max increased in the PRO but not the CHO group following 6 wk of aerobic training (change from baseline: PRO, 12.2±6.2%; CHO, 3.3±8.7%; P=0.033; Fig. 2). Average total body weight, fat free mass, and percentage body fat did not change for either group (data not shown) and indicate the subjects were in net energy balance during training.
Figure 2.
Absolute Vo2max increased in PRO group but not CHO group following 6 wk of aerobic training. Box plots indicate mean, interquartile range, and sample range of the percentage increase from baseline.
Muscle protein and DNA synthesis
Body water enrichment reached ∼1.5–2.5% by wk 2 and was maintained through wk 6 (Fig. 3). The increased body water enrichments had no adverse effects, and only one participant, with the lowest body weight, reported a slight and transient dizziness during the initial loading phase of heavy water consumption. Raw enrichment values from skeletal muscle fractions are displayed in Table 3. Average MPS (Fig. 4A) over 6 wk of endurance exercise did not differ between PRO and CHO groups (PRO, 39.6±5.9; CHO, 43.5±5.9% new in 6 wk; P = 0.22). Because MPS did not differ between CHO and PRO groups, we combined the two groups into a single older exercise group and compared it with the younger sedentary group. The older exercise group had a higher MPS than the younger sedentary group (7.4±1.0 vs. 5.54±1.0%/wk; P = 0.0043; Fig. 4B).
Figure 3.
Body water D2O enrichment measured from saliva swabs was raised to 1.5–2.5% during the 6-wk labeling period.
Table 3.
Precursor and product enrichment of skeletal muscle protein, DNA, and membrane phospholipids following 2H2O labeling
| Fraction | Older exercise |
Young sedentary | |
|---|---|---|---|
| CHO | PRO | ||
| DNA | |||
| Precursor (%) | 6.74 ± 2.50 | 5.69 ± 1.85 | 3.03 ± 1.10 |
| Product (%) | 0.42 ± 0.22 | 0.28 ± 0.14 | 0.05 ± 0.04 |
| Protein | |||
| Precursor (%) | 4.34 ± 1.56 | 3.97 ± 2.75 | 2.83 ± 0.81 |
| Product (%) | 1.91 ± 0.60 | 1.58 ± 1.00 | 1.20 ± 0.33 |
| Phospholipids | |||
| Precursor (%) | 6.96 ± 2.42 | 6.39 ± 1.81 | 7.53 ± 2.85 |
| Product (%) | 2.79 ± 0.82 | 2.36 ± 0.82 | 0.41 ± 0.08 |
Data are means ± sd. Older exercise groups were labeled for 6 wk; young sedentary group was labeled for 4 wk.
Figure 4.
A) There were no differences in MPS between CHO or PRO nutrition with aerobic exercise training. B) Younger sedentary group (Yg-SED) had lower MPS than the older exercise group (Older-EX). *P < 0.005.
The average DNA %F (Fig. 5A) over 6 wk of endurance exercise did not differ between the PRO and CHO groups. Combining the two groups resulted in an average DNA %F of 4.2 ± 2.8% (95% CI: 2.7–5.8% newly synthesized DNA) in 6 wk or ∼0.5–1%/wk. In contrast, the calculated DNA %F was nearly 0 in the young sedentary group; even considering the shorter labeling time, there was significantly less 2H incorporation into DNA compared with the older exercise group (Fig. 5B). The final enrichment of the young sedentary group was similar to background samples and resulted in DNA %F that did not differ from 0 (P>0.05). DNA %F was weakly correlated with MPS in the older exercise group (Pearson r2=0.25; P=0.054; Fig. 5C).
Figure 5.
A)There were no differences in DNA %F between CHO or PRO nutrition with aerobic training. B) DNA %F was lower in the younger sedentary group (Yg-SED) than in the older exercise group (Older-EX). Low enrichment of Yg-SED resulted in a DNA synthesis rate that did not differ from 0. Data are expressed as percentage new per week because the Yg-SED group was labeled for 4 wk and the Older-EX group for 6 wk. C) MPS and DNA %F were correlated over 6 wk of aerobic training but not for the Yg-SED (not shown). *P = 0.013.
The average PL %F over 6 wk of endurance exercise did not differ between the PRO and CHO groups (37.6±11.9 vs. 42.2±11.2% new in 6 wk, respectively; Fig. 6A). The combined CHO and PRO groups (older exercise group) had higher average PL %F than the young sedentary group (7.13±2.1 vs. 1.36±0.55%/wk, respectively; P<0.0001; Fig 6B). PL %F was not correlated to MPS or DNA %F (data not shown).
Figure 6.
A) PL %F, an indication of membrane turnover, did not differ between CHO or PRO nutrition groups. B) PL %F was lower in the younger sedentary (Yg-SED) than older exercise (Older-EX) group. *P < 0.0001.
mtDNA content
The ratio of mtDNA to nDNA did not differ between groups at baseline or following 6 wk of endurance exercise (Fig. 7). The internal controls were consistent across different plates [Ct (CV) for B2M=24.2±0.41 (0.017%); TAF1-L=23.8±0.42 (0.018%); Dloop=16.0±0.46 (0.029%); mtRNR=16.4±0.46 (0.028%)].
Figure 7.
There were no differences at baseline or following 6 wk of aerobic training for RC number of mtDNA:nDNA using primers/probes for Dloop and B2M.
DISCUSSION
We used heavy water to simultaneously measure the synthesis rates of multiple processes in response to 6 wk of endurance exercise training combined with either carbohydrate or carbohydrate plus protein consumption after exercise. Our results showed that maximal aerobic fitness increased in the protein but not carbohydrate group. Counter to our initial hypothesis, the synthesis rates of mixed muscle proteins, DNA, and membrane phospholipids did not differ between protein and carbohydrate groups during the 6-wk endurance-training protocol. We anticipated that the DNA synthesis rate would be near zero since skeletal muscle fibers do not undergo regular cell divisions unless stimulated by a strong anabolic stimulus, such as growth during childhood or resistance training. However, we measured ∼4% newly synthesized DNA in adults during aerobic training, a significantly higher value than we observed in sedentary young subjects (in whom values did not differ from zero). These results can be interpreted to indicate that the DNA synthesis is due to satellite cell division and add to a growing body of evidence that aerobic exercise can stimulate skeletal muscle adaptations that previously were considered only in response to resistance exercise.
DNA synthesis rate
The D2O technique labels DNA through de novo nucleoside synthesis pathways and represents almost exclusively S-phase DNA synthesis rather than DNA repair (21). In principle, 3 sources could contribute to the measured DNA synthesis: mitochondrial biogenesis, cells that are continuously replicating in muscle tissue, or satellite cell recruitment.
The measured DNA synthesis can not be accounted for by mtDNA turnover. First, the lack of mtDNA:nDNA change with training suggests that even if mtDNA changed, then nDNA would have changed in the same direction with equal magnitude. Second, the small size of mtDNA (∼16.5 kb) and mtDNA copy number (∼2500 per nuclear genome) is <1% of total DNA (∼3 Gb). Therefore, even a fully turned over mtDNA pool could not account for the ∼4% newly synthesized DNA reported in our study.
Second, it is not likely that cells other than myocytes are contributing to our measured DNA synthesis rate. A cell type or collection of multiple types that contribute ∼4% of total DNA could be fully turned over and could theoretically account for the newly synthesized DNA over 6 wk. We recruited the sedentary group to determine the rate of DNA synthesis under sedentary and free-living conditions to account for basal turnover of other cell types. DNA in the sedentary group had enrichments that did not differ from background following 4 wk of labeling, indicating that the cells represented in the biopsy samples have basal DNA synthesis rates near zero. Thus, any contribution of cells other than myofibrils is small and does not adequately account for our measured DNA synthesis rate during aerobic training.
The third potential source of DNA synthesis is satellite cell replication. Satellite cells are resident stem cells within skeletal muscle that are usually quiescent but can be activated to divide into 2 daughter cells. A portion of activated satellite cells is used to maintain the population of satellite cells, and the others fuse to existing myofibrils or form nascent myofibrils (32, 33). Early work has shown that isotopic labeling of DNA in skeletal muscle can be attributed to satellite cell replication (34). In addition, ∼80% of satellite cells can be labeled within 5 d in growing rats (33). Satellite cells make up ∼2–5% of total nuclei in muscle samples from adult humans (35, 36); therefore, it is possible that replication of satellite cells could account for ∼4% newly synthesized DNA.
Few studies have considered satellite cell activation with aerobic exercise; however, a recent report in humans showed a single bout of aerobic exercise increased markers of satellite cell activation (37). If satellite cells are activated and differentiated, then an increase in MPS would be stimulated to maintain the posited myonuclear domain (38), which is maintained with aging (39). Consistent with this hypothesis, a positive correlation (r2∼25%) was documented between muscle protein and DNA synthesis over the 6 wk of aerobic training.
Muscle protein synthesis
Average muscle protein synthesis did not differ between protein and carbohydrate groups, in contrast to short-term studies. Postexercise protein consumption has been repeatedly shown to increase muscle protein synthesis in the several hours following aerobic (40, 41) and resistance exercise (6, 42). In addition, the importance of timed protein consumption after exercise is supported by studies over several days and weeks that reported increased nitrogen retention (15) and strength gains (43). Our results suggest that any short-term increases in protein synthesis following exercise and protein consumption may not augment cumulative protein synthesis over several weeks compared with carbohydrates only. Instead, they are consistent with results showing that the timing of protein intake can vary around exercise and still allow increased amino acid uptake over several hours (44, 45) or muscle cross-sectional area with training (46).
There are some important considerations when comparing our results to previous findings. First, acute tracer studies are conducted in inpatient settings and commonly have strict dietary controls. Our long-term measures were made on free-living people with fluctuations in daily energy balance, which can alter protein balance (47). Second, we used heavy water to directly measure skeletal muscle protein synthesis, while others have used nonspecific methods that measure net changes, such as nitrogen balance (15) or muscle cross-sectional area (43). Perhaps the timing of protein intake is less critical in people who are consuming adequate calories and protein outside of a tightly controlled inpatient setting.
Muscle protein synthesis was higher in our older exercise group compared with the younger sedentary group and provides strong, though indirect, support for the hypothesis that aerobic training increases protein synthesis in older subjects. The younger untrained group represents an upper bound on the rate of long-term basal synthesis because previous reports show either similar (48) or lower skeletal muscle protein synthesis with aging (10, 49). Therefore, the higher protein synthesis during aerobic training over resting may be greater if compared with an older nonexercising group.
Membrane phospholipid synthesis
The synthesis of membrane phospholipids did not differ between protein and carbohydrate groups and was not correlated to synthesis of skeletal muscle DNA or protein. Interestingly, the synthesis of membrane phospholipids was greater in the aerobic training group compared with sedentary controls and suggests cellular proliferation or remodeling within myofibrils. By area, cellular organelles contain most of the phospholipid content and likely represent the majority of membrane remodeling. In particular, the mitochondrial reticulum is a large portion (20–40%) of intracellular membranes with continuous remodeling (50). Application of this method to isolated mitochondria could facilitate investigation of long-term adaptive changes to the mitochondrial reticulum. Deuterium labeling combined with subfractional analysis can provide unique insight into long-term adaptive changes to cellular membranes.
Mitochondrial adaptations
We did not detect any differences in mtDNA:nDNA between groups at baseline or following training. mtDNA copy number per nDNA is an index of mitochondrial content (11, 51). Interestingly, the increase in Vo2max in the protein group suggests that consuming protein postexercise during endurance training can promote long-term aerobic adaptations independent to changes in mtDNA:nDNA. Each participant in the protein group increased both absolute and relative Vo2max by ∼10%, and the average value for the protein group was greater than for the carbohydrate group. These findings indicate that postexercise protein consumption increases aerobic adaptations to endurance exercise. Mixed muscle protein did not differ between the groups, but that does not rule out that mitochondrial specific protein synthesis increased to a greater degree in the protein group. Previous work has shown that aerobic exercise and feeding increases mitochondrial protein synthesis in the untrained and trained state (12). It is likely that the aerobic exercise increases mRNA of mitochondrial related proteins and the consumption of protein increases the translation of those mRNA (52). The combination of exercise and protein consumption is an easily achievable lifestyle modification that may help promote aerobic fitness during aging.
Limitations of this study
We did not include an age-matched older group that did not undergo training; thus, we were not able to directly compare the effects of exercise on our long-term synthetic measurements. However, we do not expect protein synthesis or DNA synthesis to be increased with aging under sedentary conditions. On the contrary, previous reports showed depletion of satellite cells (16) and decreased protein synthesis with age (49). Thus, our use of a younger sedentary group provides indirect but strong evidence that exercise increases DNA synthesis rates even in an aging population.
We chose treadmill training because brisk walking is a common exercise recommendation, and we intended to study people using a simple lifestyle modification for aging (e.g., exercise followed by protein consumption). It should be noted that our training protocol used treadmills while the Vo2max used cycle ergometry. Cycling ergometry tends to yield lower Vo2max values, and perhaps our differences between pre-and posttesting would be greater if the same modality was used.
Our training intervention of walking and running includes both shortening and lengthening contractions. It could be argued that changes in synthesis observed with our exercise training are due to repair processes associated with higher force lengthening contractions. Although lengthening contractions may stimulate inflammatory responses, it is very difficult to demonstrate skeletal muscle damage with voluntary lengthening contractions in human subjects (53). In addition, it has been clearly demonstrated that skeletal muscle protein synthesis is increased in humans following strenuous voluntary contractions that lack muscle damage (54). Therefore, it is likely that our synthetic measurements reflect a coordination of adaptive as opposed to repair processes in response to aerobic training.
Our techniques are expressed as relative rates of synthesis. It is possible that absolute rates of DNA or protein synthesis were different between younger and older groups. Although we measured fat-free mass, it is not possible to make an absolute quantification of pool size, a necessary component of absolute rates, from this measure. Therefore, any calculation of absolute rates based on fat free mass would be at best a gross estimation. Further studies with an isolated muscle mass and direct measures of protein and DNA content could distinguish absolute from relative rates.
CONCLUSIONS
We conclude that long-term measurements of synthetic processes within skeletal muscle provide important insights into aging, exercise, and nutrition that are missed in acute studies. Our results show that postexercise protein consumption does not alter long-term measures of multiple synthetic processes but can improve maximal aerobic performance. The measured DNA synthesis (∼4% over 6 wk) could be due to satellite cell activation during aerobic exercise training. Further studies should consider the implications of aerobic exercise on satellite cell recruitment and skeletal muscle function with aging.
Acknowledgments
The authors appreciate the enthusiasm of all the study participants during the project and thank everyone who helped with exercise training and heavy water preparation: Jon Land, Kyle Barnes, Karen Warnersdorfer, Rebecca Peterson, Carrie Sousek, Shannon Wells, and the Adult Fitness Program at Colorado State University. The authors also thank Christopher Bell, Rebecca Scalzo, and Garrett Peltonen for providing the sedentary control data and Wyatt Voyles for providing medical oversight. Claire Emson and Kelvin Li (KineMed Inc.) provided technical expertise for deuterium analysis.
Funding for the project was provided to B.F.M. by U.S. National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases pilot grant P30 DK048520 and Colorado State University.
REFERENCES
- 1. Janssen I., Shepard D. S., Katzmarzyk P. T., Roubenoff R. (2004) The healthcare costs of sarcopenia in the United States. J. Am. Geriatr. Soc. 52, 80–85 [DOI] [PubMed] [Google Scholar]
- 2. Koopman R., van Loon L. J. (2009) Aging, exercise, and muscle protein metabolism. J. Appl. Physiol. 106, 2040–2048 [DOI] [PubMed] [Google Scholar]
- 3. Goodpaster B. H., Park S. W., Harris T. B., Kritchevsky S. B., Nevitt M., Schwartz A. V., Simonsick E. M., Tylavsky F. A., Visser M., Newman A. B. (2006) The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 61, 1059–1064 [DOI] [PubMed] [Google Scholar]
- 4. Hughes V. A., Frontera W. R., Roubenoff R., Evans W. J., Singh M. A. (2002) Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am. J. Clin. Nutr. 76, 473–481 [DOI] [PubMed] [Google Scholar]
- 5. Phillips S. M., Tipton K. D., Aarsland A., Wolf S. E., Wolfe R. R. (1997) Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am. J. Physiol. Endocrinol. Metab. Physiol. 273, E99–E107 [DOI] [PubMed] [Google Scholar]
- 6. Moore D. R., Robinson M. J., Fry J. L., Tang J. E., Glover E. I., Wilkinson S. B., Prior T., Tarnopolsky M. A., Phillips S. M. (2009) Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 89, 161–168 [DOI] [PubMed] [Google Scholar]
- 7. Cuthbertson D., Smith K., Babraj J., Leese G., Waddell T., Atherton P., Wackerhage H., Taylor P. M., Rennie M. J. (2004) Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 19, 422–424 [DOI] [PubMed] [Google Scholar]
- 8. Wilkes E. A., Selby A. L., Atherton P. J., Patel R., Rankin D., Smith K., Rennie M. J. (2009) Blunting of insulin inhibition of proteolysis in legs of older subjects may contribute to age-related sarcopenia. Am. J. Clin. Nutr. 90, 1343–1350 [DOI] [PubMed] [Google Scholar]
- 9. Kumar V., Selby A., Rankin D., Patel R., Atherton P., Hildebrandt W., Williams J., Smith K., Seynnes O., Hiscock N., Rennie M. J. (2009) Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J. Physiol. 587, 211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rooyackers O. E., Adey D. B., Ades P. A., Nair K. S. (1996) Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc. Natl. Acad. Sci. U. S. A. 93, 15364–15369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Menshikova E. V., Ritov V. B., Fairfull L., Ferrell R. E., Kelley D. E., Goodpaster B. H. (2006) Effects of exercise on mitochondrial content and function in aging human skeletal muscle. J. Gerontol. A Biol. Sci. Med. Sci. 61, 534–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilkinson S. B., Phillips S. M., Atherton P. J., Patel R., Yarasheski K. E., Tarnopolsky M. A., Rennie M. J. (2008) Differential effects of resistance and endurance exercise in the fed state on signalling molecule phosphorylation and protein synthesis in human muscle. J. Physiol. 586, 3701–3717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harber M. P., Konopka A. R., Douglass M. D., Minchev K., Kaminsky L. A., Trappe T. A., Trappe S. (2009) Aerobic exercise training improves whole muscle and single myofiber size and function in older women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R1452–R1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murphy C., Miller B. F. (2010) Protein consumption following aerobic exercise increases whole-body protein turnover in older adults. Appl. Physiol. Nutr. Metab. 35, 583–590 [DOI] [PubMed] [Google Scholar]
- 15. Jordan L. Y., Melanson E. L., Melby C. L., Hickey M. S., Miller B. F. (2010) Nitrogen balance in older individuals in energy balance depends on timing of protein intake. J. Gerontol. A Biol. Sci. Med. Sci. 65, 1068–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Renault V., Thornell L. E., Eriksson P. O., Butler-Browne G., Mouly V. (2002) Regenerative potential of human skeletal muscle during aging. Aging Cell 1, 132–139 [DOI] [PubMed] [Google Scholar]
- 17. Short K. R., Bigelow M. L., Kahl J., Singh R., Coenen-Schimke J., Raghavakaimal S., Nair K. S. (2005) Decline in skeletal muscle mitochondrial function with aging in humans. Proc. Natl. Acad. Sci. U. S. A. 102, 5618–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shefer G., Rauner G., Yablonka-Reuveni Z., Benayahu D. (2010) Reduced satellite cell numbers and myogenic capacity in aging can be alleviated by endurance exercise. PLoS One 5, e13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beccafico S., Riuzzi F., Puglielli C., Mancinelli R., Fulle S., Sorci G., Donato R. (2010) Human muscle satellite cells show age-related differential expression of S100B protein and RAGE. [E-pub ahead of print] Age (Dordr.) doi: 10.1007/s11357-010-9197-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Busch R., Kim Y. K., Neese R. A., Schade-Serin V., Collins M., Awada M., Gardner J. L., Beysen C., Marino M. E., Misell L. M., Hellerstein M. K. (2006) Measurement of protein turnover rates by heavy water labeling of nonessential amino acids. Biochim. Biophys. Acta. 1760, 730–744 [DOI] [PubMed] [Google Scholar]
- 21. Neese R. A., Misell L. M., Turner S., Chu A., Kim J., Cesar D., Hoh R., Antelo F., Strawford A., McCune J. M., Christiansen M., Hellerstein M. K. (2002) Measurement in vivo of proliferation rates of slow turnover cells by 2H2O labeling of the deoxyribose moiety of DNA. Proc. Natl. Acad. Sci. U. S. A. 99, 15345–15350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Turner S. M., Murphy E. J., Neese R. A., Antelo F., Thomas T., Agarwal A., Go C., Hellerstein M. K. (2003) Measurement of TG synthesis and turnover in vivo by 2H2O incorporation into the glycerol moiety and application of MIDA. Am. J. Physiol. Endocrinol. Metab. 285, E790–E803 [DOI] [PubMed] [Google Scholar]
- 23. Roohk D. J., Varady K. A., Turner S. M., Emson C. L., Gelling R. W., Shankaran M., Lindwall G., Shipp L. E., Scanlan T. S., Wang J. C., Hellerstein M. K. (2010) Differential in vivo effects on target pathways of a novel arylpyrazole glucocorticoid receptor modulator compared with prednisolone. J. Pharmacol. Exp. Ther. 333, 281–289 [DOI] [PubMed] [Google Scholar]
- 24. Previs S. F., Fatica R., Chandramouli V., Alexander J. C., Brunengraber H., Landau B. R. (2004) Quantifying rates of protein synthesis in humans by use of 2H2O: application to patients with end-stage renal disease. Am. J. Physiol. Endocrinol. Metab. 286, E665–E672 [DOI] [PubMed] [Google Scholar]
- 25. Busch R., Neese R. A., Awada M., Hayes G. M., Hellerstein M. K. (2007) Measurement of cell proliferation by heavy water labeling. Nat. Protoc. 2, 3045–3057 [DOI] [PubMed] [Google Scholar]
- 26. Robinson M. M., Hamilton K. L., Miller B. F. (2009) The interactions of some commonly consumed drugs with mitochondrial adaptations to exercise. J. Appl. Physiol. 107, 8–16 [DOI] [PubMed] [Google Scholar]
- 27. Hood D. A. (2001) Invited review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J. Appl. Physiol. 90, 1137–1157 [DOI] [PubMed] [Google Scholar]
- 28. Neese R. A., Siler S. Q., Cesar D., Antelo F., Lee D., Misell L., Patel K., Tehrani S., Shah P., Hellerstein M. K. (2001) Advances in the stable isotope-mass spectrometric measurement of DNA synthesis and cell proliferation. Anal. Biochem. 298, 189–195 [DOI] [PubMed] [Google Scholar]
- 29. Hellerstein M. K., Neese R. A. (1999) Mass isotopomer distribution analysis at eight years: theoretical, analytic, and experimental considerations. Am. J. Physiol. Endocrinol. Metab. Physiol. 276, E1146–E1170 [DOI] [PubMed] [Google Scholar]
- 30. Szuhai K., Ouweland J., Dirks R., Lemaitre M., Truffert J., Janssen G., Tanke H., Holme E., Maassen J., Raap A. (2001) Simultaneous A8344G heteroplasmy and mitochondrial DNA copy number quantification in myoclonus epilepsy and ragged-red fibers (MERRF) syndrome by a multiplex molecular beacon based real-time fluorescence PCR. Nucleic Acids Res. 29, e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pfaffl M. W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuang S., Kuroda K., Le Grand F., Rudnicki M. A. (2007) Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129, 999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schultz E. (1996) Satellite cell proliferative compartments in growing skeletal muscles. Dev. Biol. 175, 84–94 [DOI] [PubMed] [Google Scholar]
- 34. Moss F. P., Leblond C. P. (1971) Satellite cells as the source of nuclei in muscles of growing rats. Anat. Rec. 170, 421–435 [DOI] [PubMed] [Google Scholar]
- 35. Thornell L. E., Lindstrom M., Renault V., Mouly V., Butler-Browne G. S. (2003) Satellite cells and training in the elderly. Scand. J. Med. Sci. Sports 13, 48–55 [DOI] [PubMed] [Google Scholar]
- 36. Hawke T. J., Garry D. J. (2001) Myogenic satellite cells: physiology to molecular biology. J. Appl. Physiol. 91, 534–551 [DOI] [PubMed] [Google Scholar]
- 37. Roberts M. D., Dalbo V. J., Hassell S. E., Brown R., Kerksick C. M. (2010) Effects of preexercise feeding on markers of satellite cell activation. Med. Sci. Sports Ex. 42, 1861–1869 [DOI] [PubMed] [Google Scholar]
- 38. Harber M. P., Konopka A. R., Jemiolo B., Trappe S. W., Trappe T. A., Reidy P. T. (2010) Muscle protein synthesis and gene expression during recovery from aerobic exercise in the fasted and fed states. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R1254–R1262 [DOI] [PubMed] [Google Scholar]
- 39. Cristea A., Qaisar R., Edlund P. K., Lindblad J., Bengtsson E., Larsson L. (2010) Effects of aging and gender on the spatial organization of nuclei in single human skeletal muscle cells. Aging Cell 9, 685–697 [DOI] [PubMed] [Google Scholar]
- 40. Pennings B., Koopman R., Beelen M., Senden J. M., Saris W. H., van Loon L. J. (2011) Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am. J. Clin. Nutr. 93, 322–331 [DOI] [PubMed] [Google Scholar]
- 41. Levenhagen D. K., Gresham J. D., Carlson M. G., Maron D. J., Borel M. J., Flakoll P. J. (2001) Postexercise nutrient intake timing in humans is critical to recovery of leg glucose and protein homeostasis. Am. J. Physiol. Endocrinol. Metab. 280, E982–E993 [DOI] [PubMed] [Google Scholar]
- 42. Koopman R., Wagenmakers A. J., Manders R. J., Zorenc A. H., Senden J. M., Gorselink M., Keizer H. A., van Loon L. J. (2005) Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am. J. Physiol. Endocrinol. Metab. 288, E645–E653 [DOI] [PubMed] [Google Scholar]
- 43. Esmarck B., Andersen J. L., Olsen S., Richter E. A., Mizuno M., Kjaer M. (2001) Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. J. Physiol. 535, 301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tipton K. D., Rasmussen B. B., Miller S. L., Wolf S. E., Owens-Stovall S. K., Petrini B. E., Wolfe R. R. (2001) Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am. J. Physiol. Endocrinol. Metab. 281, E197–E206 [DOI] [PubMed] [Google Scholar]
- 45. Tipton K. D., Elliott T. A., Cree M. G., Aarsland A. A., Sanford A. P., Wolfe R. R. (2007) Stimulation of net muscle protein synthesis by whey protein ingestion before and after exercise. Am. J. Physiol. Endocrinol. Metab. 292, E71–E76 [DOI] [PubMed] [Google Scholar]
- 46. Verdijk L. B., Jonkers R. A., Gleeson B. G., Beelen M., Meijer K., Savelberg H. H., Wodzig W. K., Dendale P., van Loon L. J. (2009) Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am. J. Clin. Nutr. 89, 608–616 [DOI] [PubMed] [Google Scholar]
- 47. Todd K. S., Butterfield G. E., Calloway D. H. (1984) Nitrogen balance in men with adequate and deficient energy intake at three levels of work. J. Nutr. 114, 2107–2118 [DOI] [PubMed] [Google Scholar]
- 48. Volpi E., Sheffield-Moore M., Rasmussen B. B., Wolfe R. R. (2001) Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA 286, 1206–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Henderson G. C., Dhatariya K., Ford G. C., Klaus K. A., Basu R., Rizza R. A., Jensen M. D., Khosla S., O'Brien P., Nair K. S. (2009) Higher muscle protein synthesis in women than men across the lifespan, and failure of androgen administration to amend age-related decrements. FASEB J. 23, 631–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu X., Weaver D., Shirihai O., Hajnoczky G. (2009) Mitochondrial “kiss-and-run”: interplay between mitochondrial motility and fusion-fission dynamics. EMBO J. 28, 3074–3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Menshikova E. V., Ritov V. B., Ferrell R. E., Azuma K., Goodpaster B. H., Kelley D. E. (2007) Characteristics of skeletal muscle mitochondrial biogenesis induced by moderate-intensity exercise and weight loss in obesity. J. Appl. Physiol. 103, 21–27 [DOI] [PubMed] [Google Scholar]
- 52. Miller B. F. (2007) Human muscle protein synthesis after physical activity and feeding. Ex. Sport Sci. Rev. 35, 50–55 [DOI] [PubMed] [Google Scholar]
- 53. Crameri R. M., Aagaard P., Qvortrup K., Langberg H., Olesen J., Kjaer M. (2007) Myofibre damage in human skeletal muscle: effects of electrical stimulation versus voluntary contraction. J. Physiol. 583, 365–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Miller B. F., Olesen J. L., Hansen M., Dossing S., Crameri R. M., Welling R. J., Langberg H., Flyvbjerg A., Kjaer M., Babraj J. A., Smith K., Rennie M. J. (2005) Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J. Physiol. 567, 1021–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]