Abstract
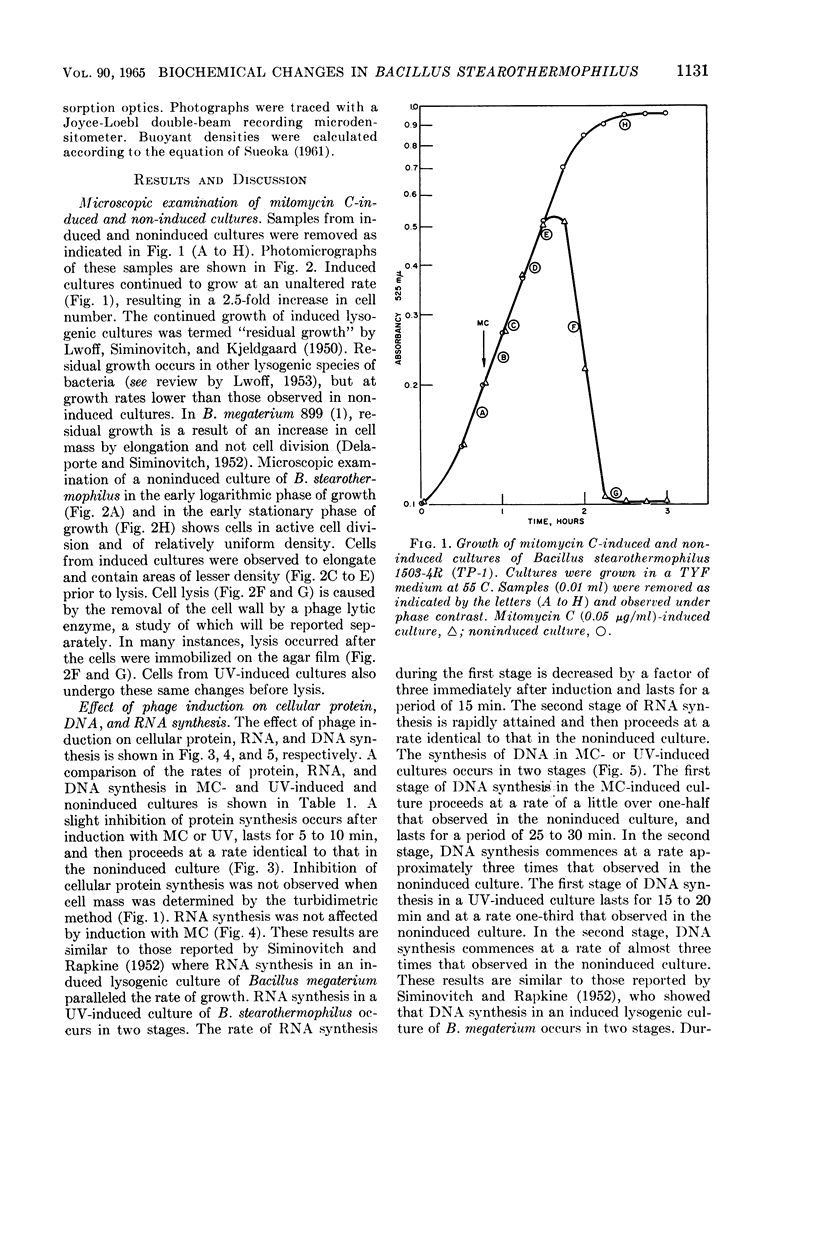
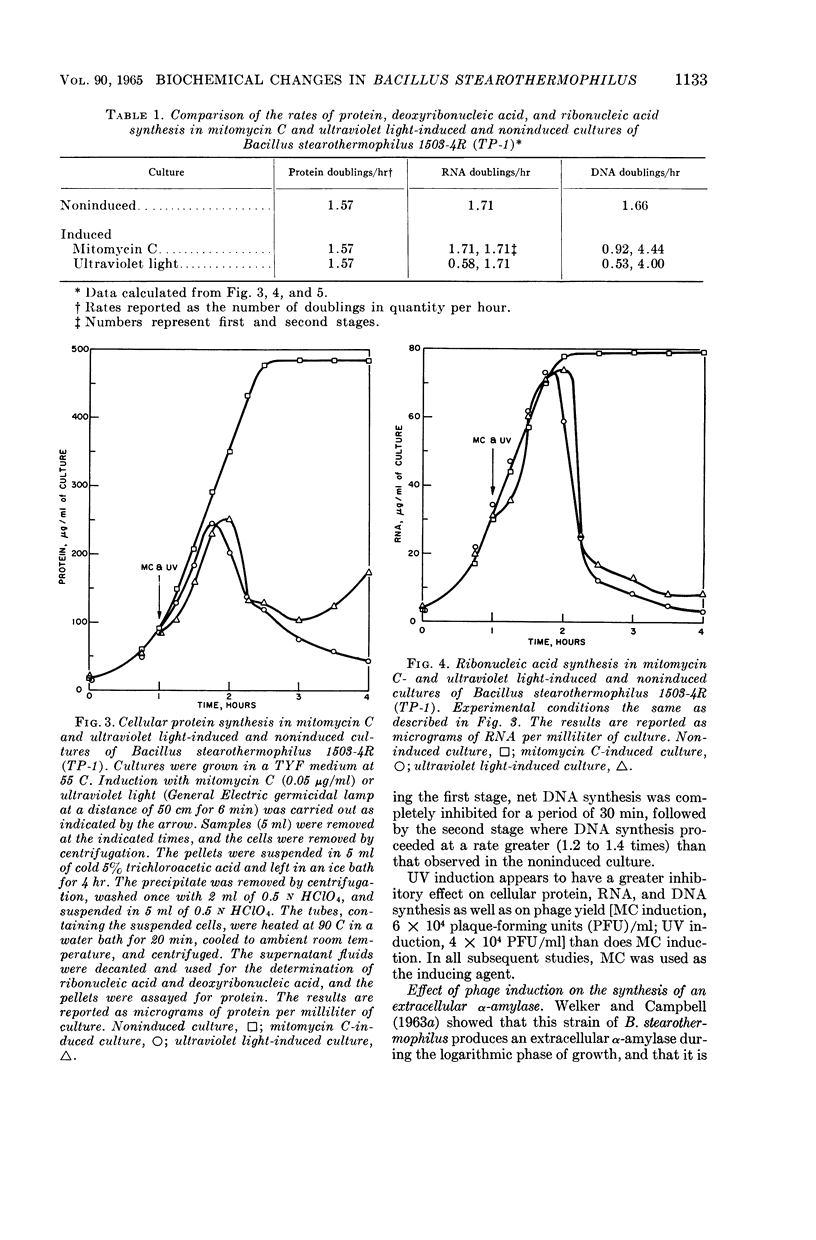
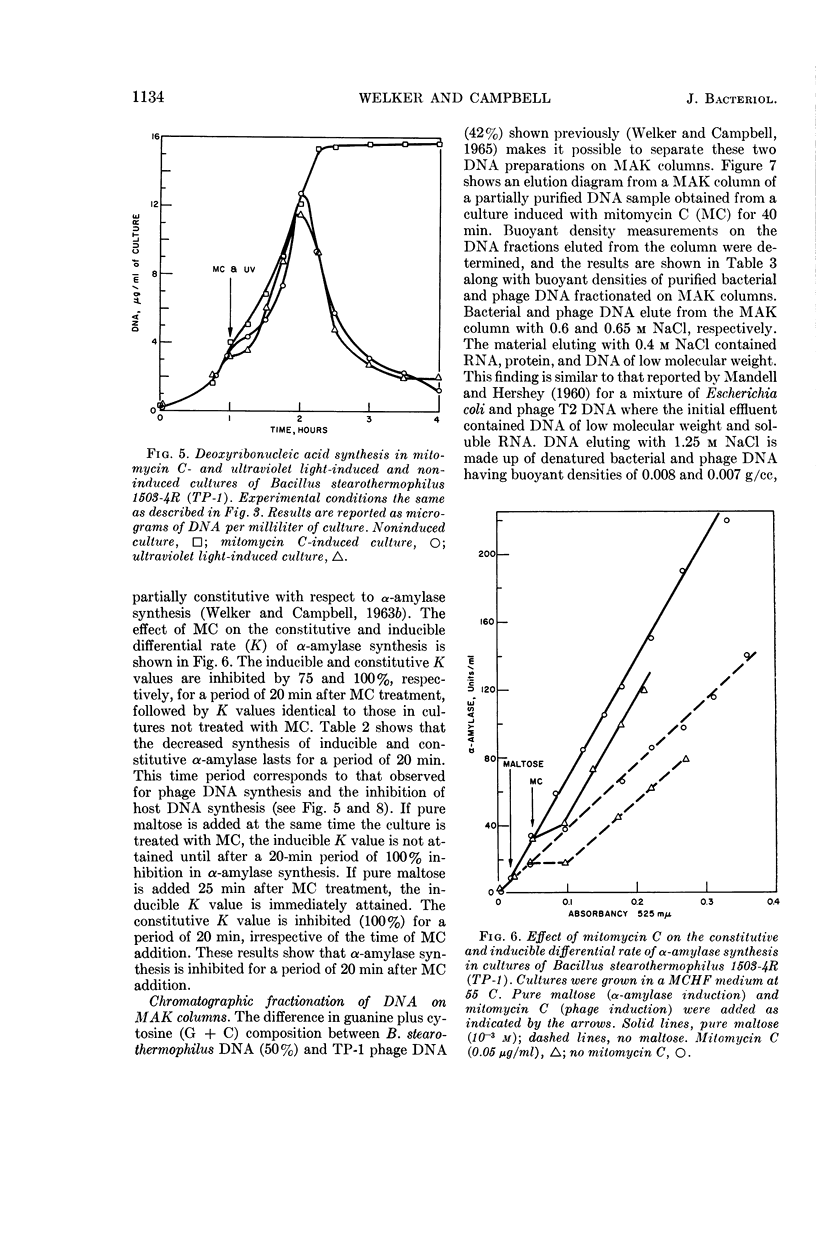
Welker, N. E. (University of Illinois, Urbana), and L. Leon Campbell. Biochemical changes in lysogenic Bacillus stearothermophilus after bacteriophage induction. J. Bacteriol. 90:1129–1137. 1965.—Cultures of Bacillus stearothermophilus 1503-4R (TP-1) continued to grow at an unaltered rate after induction with mitomycin C (MC). MC-induced cultures exhibited a 2.5-fold increase in cell number before lysis occurred. Prior to lysis, cells were observed to elongate and to contain areas of lesser density. Protein synthesis was slightly inhibited in MC- or ultraviolet light (UV)-induced cultures for a period of 5 to 10 min, and then proceeded at a rate identical to that in the noninduced culture. Ribonucleic acid (RNA) synthesis was not affected by MC induction. UV induction caused RNA synthesis to occur in two stages: in the first stage, the rate of RNA synthesis was one-third that observed in the noninduced culture and lasted for a period of 15 min; the second stage of RNA synthesis then proceeded at a rate identical to that in the noninduced culture. The synthesis of deoxyribonucleic acid (DNA) in an MC- or UV-induced culture occurred in two stages. In the first stage, DNA synthesis in induced cultures occurred at a rate of one-half (MC) and one-third (UV) of that observed in the noninduced culture. The first stage of DNA synthesis in MC- or UV-induced cultures lasted for 25 to 30 min and 15 to 20 min, respectively. In the second stage, the rate of DNA synthesis in MC- or UV-induced cultures occurred at a rate three times that of the noninduced culture. UV induction appeared to have a greater inhibitory effect than MC induction on protein, RNA, and DNA synthesis as well as phage yield. The differential rate (K) of inducible and constitutive α-amylase synthesis was inhibited by 75 and 100%, respectively, for a period of 20 min after MC induction. After 20 min, the K values for α-amylase synthesis were identical to those obtained in the absence of MC induction. The synthesis of TP-1 phage DNA occurred rapidly and was complete 25 min after MC induction, whereas bacterial DNA was degraded or its rate of synthesis was decreased. During the second stage of DNA synthesis, only bacterial DNA was synthesized, but at a rate greater than that found in the noninduced culture.
Full text
PDF

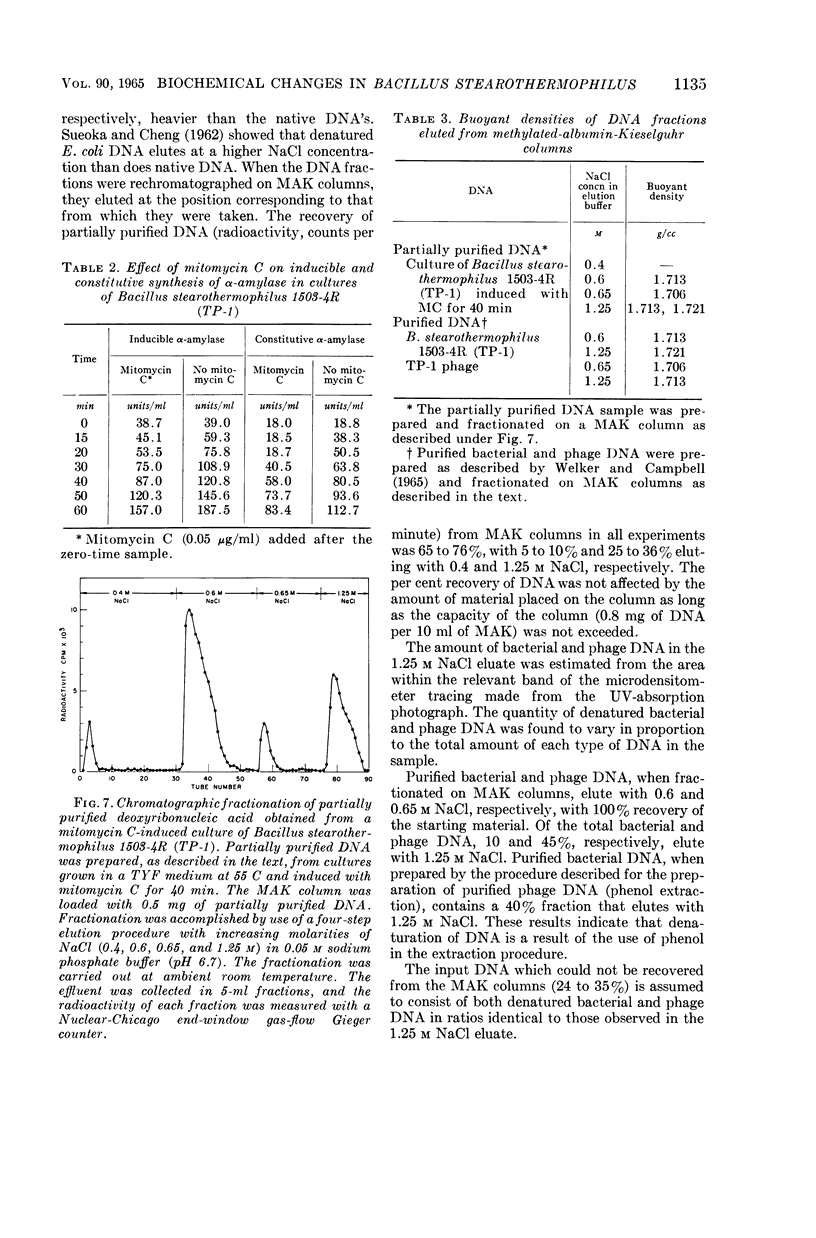
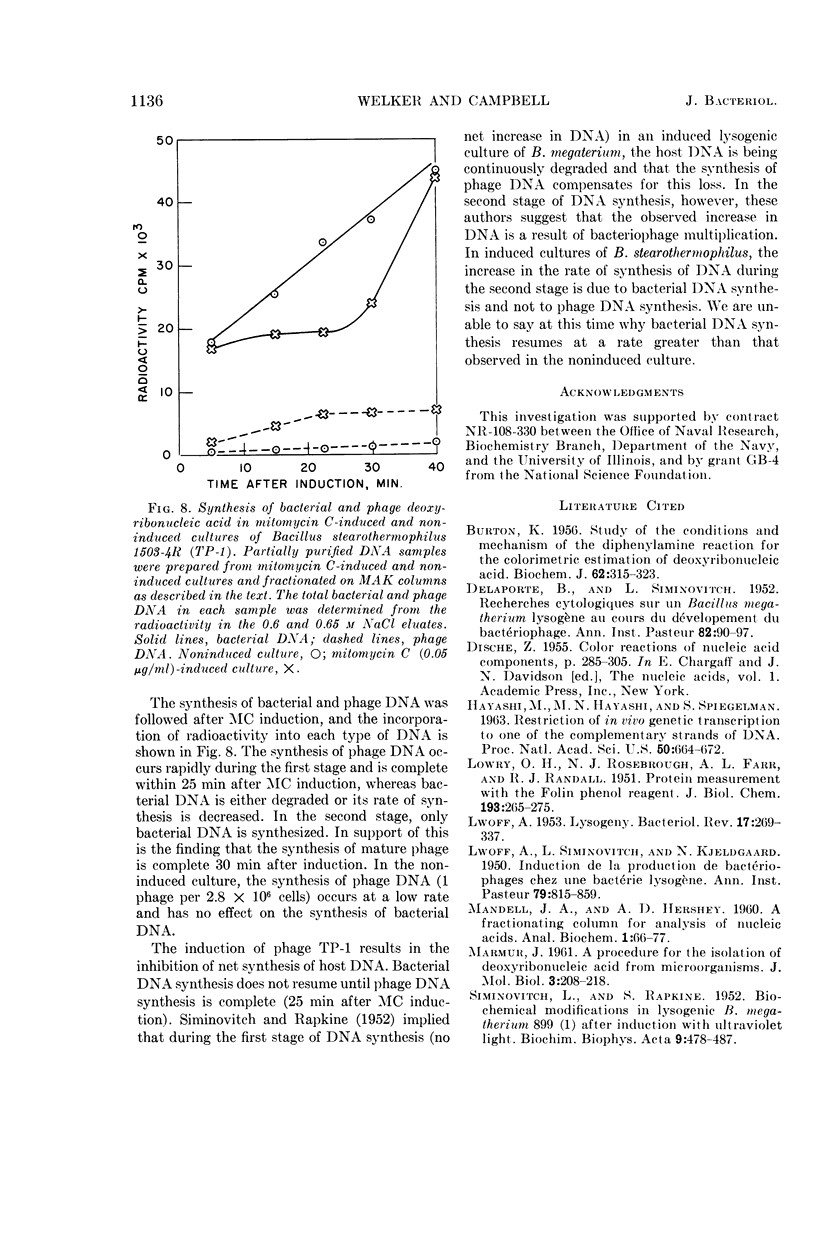

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELAPORTE B., SIMINOVITCH L. Recherches cytologiques sur un Bacillus megatherium lysogène au cours du développement du bactériophage. Ann Inst Pasteur (Paris) 1952 Jan;82(1):90–97. [PubMed] [Google Scholar]
- HAYASHI M., HAYASHI M. N., SPIEGELMAN S. RESTRICTION OF IN VIVO GENETIC TRANSCRIPTION TO ONE OF THE COMPLEMENTARY STRANDS OF DNA. Proc Natl Acad Sci U S A. 1963 Oct;50:664–672. doi: 10.1073/pnas.50.4.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LWOFF A. Lysogeny. Bacteriol Rev. 1953 Dec;17(4):269–337. doi: 10.1128/br.17.4.269-337.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LWOFF A., SIMINOVITCH L., KJELDGAARD N. Induction de la production de bacteriophages chez une bactérie lysogène. Ann Inst Pasteur (Paris) 1950 Dec;79(6):815–859. [PubMed] [Google Scholar]
- SIMINOVITCH L., RAPKINE S. Biochemical modifications in lysogenic B. megatherium 899 (1) after induction with ultraviolet light. Biochim Biophys Acta. 1952 Nov;9(5):478–487. doi: 10.1016/0006-3002(52)90196-0. [DOI] [PubMed] [Google Scholar]
- SUEOKA N., CHENG T. Y. Fractionation of nucleic acids with the methylated albumin column. J Mol Biol. 1962 Mar;4:161–172. doi: 10.1016/s0022-2836(62)80048-5. [DOI] [PubMed] [Google Scholar]
- WELKER N. E., CAMPBELL L. L. EFFECT OF CARBON SOURCES ON FORMATION OF ALPHA-AMYLASE BY BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1963 Oct;86:681–686. doi: 10.1128/jb.86.4.681-686.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELKER N. E., CAMPBELL L. L. INDUCTION AND PROPERTIES OF A TEMPERATURE BACTERIOPHAGE FROM BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1965 Jan;89:175–184. doi: 10.1128/jb.89.1.175-184.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELKER N. E., CAMPBELL L. L. INDUCTION OF ALPHA-AMYLASE OF BACILLUS STEAROTHERMOPHILUS BY MALTODEXTRINS. J Bacteriol. 1963 Oct;86:687–691. doi: 10.1128/jb.86.4.687-691.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]



