Abstract
The timing of events can be implicit or without awareness yet critical for task performance. However, the neural correlates of implicit timing are unknown. One system that has long been implicated in event timing is the olivocerebellar system, which originates exclusively from the inferior olive. By using event-related functional MRI in human subjects and a specially designed behavioral task, we examined the effect of the subjects’ awareness of changes in stimulus timing on the olivocerebellar system response. Subjects were scanned while observing changes in stimulus timing that were presented near each subject's detection threshold such that subjects were aware of such changes in only approximately half the trials. The inferior olive and multiple areas within the cerebellar cortex showed a robust response to time changes regardless of whether the subjects were aware of these changes. Our findings provide support to the proposed role of the olivocerebellar system in encoding temporal information and further suggest that this system can operate independently of awareness and mediate implicit timing in a multitude of perceptual and motor operations, including classical conditioning and implicit learning.
Keywords: cerebellum, visual
Encoding the timing of events is an inherent component of perceptual and motor tasks. Such timing can be implicit or without the subject's awareness yet important for performance regardless of the goal of the task. For example, when throwing a ball at a target, the spatial accuracy relies, at least partially, on precise motor timing; however, subjects may not be explicitly aware of the timing of individual components of this complex multijoint movement (1, 2). Similarly, subjects may improve the speed and accuracy of performing a perceptual task by implicitly using temporal information to predict the timing of sensory stimuli (i.e., temporal expectancy) (3, 4). More directly, implicit timing can be conceptualized as a critical component of classical conditioning and other stimulus/response association processes such as implicit learning and automatic behavior. However, implicit timing or timing without awareness has been difficult to characterize in experimental settings, especially in animal studies, and its neural correlates remain poorly understood (5). One system that has long been implicated in event timing is the olivocerebellar system, which originates exclusively in the inferior olive (6–12). Whether the olivocerebellar system mediates timing without awareness has not been demonstrated directly to our knowledge. However, the capacity of the inferior olive and the climbing fiber system to encode temporal information independently of awareness is supported by indirect evidence from classical conditioning and single cell recording literature (10-11, 13-16). In the few imaging and lesion studies in humans that specifically addressed the cerebellar contribution to implicit timing, the term “implicit timing” has not been strictly defined as timing without awareness (5, 17–20). These studies produced conflicting results and did not dissociate the role of the inferior olive and climbing fibers from that of mossy fibers (5, 17–20).
By using event-related functional MRI (fMRI) and a perceptual task that dissociates the temporal from nontemporal attributes of sensory input, we have shown inferior olive activation when subjects perceived unexpected changes in the timing but not the spatial orientation or color of visual stimuli (21). The results were consistent with the enhanced response of the inferior olive and climbing fiber system to unexpected sensory input consistently shown in electrophysiological studies, and further indicated that the inferior olive response to unexpected stimuli is specific to timing (22, 23). In the present study, we used a similar event-related fMRI paradigm to determine the effect of the subject's awareness on the transient neural responses of the inferior olive and cerebellar cortex to changes in stimulus timing.
Results
We used a behavioral task that relied on the subjects’ own response to determine whether they were aware of the change in timing of single visual stimuli. This task was based on the assumption that, when observing a sequence of stimuli separated by equal interstimulus intervals (ISIs) except for one deviant stimulus (preceded by an ISI of a different duration), subjects will incorrectly perceive the sequence as isochronous only if they are unaware of the change in the timing of deviant stimulus. The term “awareness” is used here to describe the subjects’ conscious perception of the stimulus timing and not the occurrence of the stimulus itself.
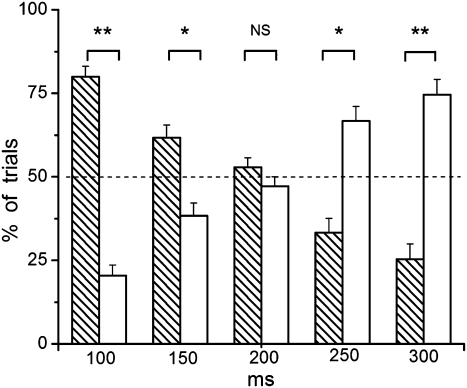
We first performed a prescanning behavioral experiment to test the validity of the task and to determine the threshold for each subject at which a change in stimulus timing was detected (i.e., the threshold at which the subject was aware of the deviant stimulus timing in approximately 50% of the trials). The results show a near-perfect correlation (r2 > 0.99) between the magnitude of change in the timing of deviant stimuli and the percentage of detected deviant stimuli (Fig. 1). This finding confirmed the validity of the behavioral task and indicated that the subjects responded based on what they actually perceived, i.e., they consistently judged the sequence as anisochronous when they were aware of the change in deviant stimulus timing and as isochronous when they were unaware of such a change, even though the deviant stimuli were physically identical in their temporal and nontemporal properties. The subjects’ performance was within the range of anisochrony detection threshold for visual stimuli reported in the literature (24).
Fig. 1.
Percentage of trials in which subjects were aware (blank columns) or unaware (hatched columns) of the change in stimulus timing. In each trial, subjects observed a sequence of visual stimuli occurring at 1 Hz except for one deviant stimulus that occurred sooner than expected by 100, 150, 200, 250, or 300 ms. Subjects performed 18 randomly presented trials in each category, judging whether the sequence was anisochronous or isochronous (which indicated whether they were aware or unaware of the change of deviant stimulus timing, respectively). As expected, the larger the deviation from isochronicity, the easier deviant stimuli were detected and vice versa. A deviation of 200 ms in stimulus timing corresponded to an approximate 50% detection rate for the whole group (N = 17). Error bars indicate SEM (**P ≤ 0.0001 and *P ≤ 0.001; NS, not significant).
Subjects were then scanned while performing the same task. The scanning paradigm was designed to measure the event-related hemodynamic response that is time-locked to the onset of single stimuli. In each trial, the subjects observed a sequence of visual stimuli and, after a delay, they were prompted by a visual cue to indicate whether the sequence was anisochronous or isochronous by pushing one of two buttons in a forced-choice fashion. Each anisochronous sequence consisted of seven stimuli occurring at 1 Hz with the exception of one deviant stimulus that was presented 150 to 250 ms earlier than expected based on each subject's threshold as determined by the prescanning experiment. To prevent subjects from judging the isochronicity of the sequence by attending to one particular stimulus, the deviant stimulus was presented pseudorandomly as the fifth, sixth, or seventh stimulus. Thus, subjects observed sequences in which at least four stimuli (and three ISIs) occurring at 1 Hz preceded the deviant stimulus that established isochronicity, allowing the subjects to recognize the change in timing of the deviant stimulus. Failure to detect such a change (and therefore judge anisochronous sequence as isochronous) indicated that the subjects were unaware of the change in timing of one particular stimulus even though they were “aware” that they were performing a timing task and were paying attention to the timing of the whole sequence of stimuli. To monitor the subjects’ performance during scanning, we mixed trials with anisochronous sequences with randomly presented “catch” trials with true isochronous sequences (with no deviant stimuli).
Deviant stimuli were classified post hoc as detected or undetected based on each subject's responses during scanning. By using a general linear model and a canonical hemodynamic response function as a covariate, we performed voxel-based event-related analysis of fMRI data modeling detected deviant stimuli (AWARE), undetected deviant stimuli (UNAWARE), regular stimuli (REGULAR), visual cue, and motor response as separate event types. From each subject, an equal number of events with detected and undetected deviant stimuli was used for statistical comparison of the AWARE and UNAWARE conditions.
We focused on the activations of the cerebellum and brainstem; cerebral cortical and subcortical activations data are presented in Table S1.
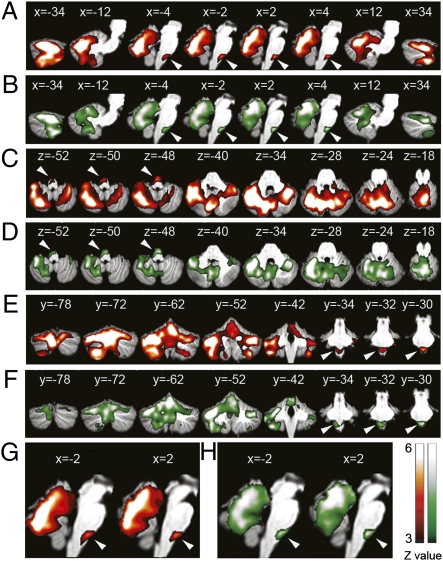
Both AWARE and UNAWARE conditions activated the inferior olive bilaterally as well as multiple areas in the cerebellar cortex (Table 1 and Fig. 2). The bilateral inferior olives also showed a greater response to AWARE (2 −28 −48; z = 3.22; and −2 −30 −49; z = 3.54) and UNWARE (2 −30 −49; z = 3.15; and −2 −29 −50; z = 3.14) compared with regular stimuli using the contrasts [AWARE minus REGULAR] and [UNAWARE minus REGULAR], respectively. The contrast [AWARE minus REGULAR] also showed activations in left lobule V (−26 −44 −27; z = 3.26), left lobule VI (−24 −68 −27; z = 3.24), bilateral crus I (38 −50 −35; z = 4.47; and −24 −68 −33; z = 4.91), left lobule VIIb (−26 −70 −51; z = 4.79), and bilateral lobule VIIIa (36 −48 −53; z = 3.97; and −38 −42 −53; z = 4.13). By using the contrast [UNAWARE minus REGULAR], we found activations in left lobule V (−24 −44 −27; z = 4.52), bilateral lobule VI (34 −48 −35; z = 3.19; and −28 −56 −23; z = 3.20), left crus I (−46 −44 −41; z = 3.50), left lobule VIIIa (−32 −42 −53; z = 3.33), and left lobule VIIIb (−24 −48 −57; z = 3.16).
Table 1.
Cerebellar and brainstem areas showing event-related activation when observing deviant stimuli that were detected (AWARE) and undetected (UNAWARE)
| Deviant stimuli |
||||
| AWARE |
UNAWARE |
|||
| Brain area | xyz | z Score | xyz | z Score |
| Right lobule IV | 4 −43 −11 | 4.51 | 4 −42 −11 | 4.59 |
| Left lobule IV/III | −2 −43 −11 | 3.93 | −2 −42 −9 | 4.64 |
| Left lobule V | −26–42 −29 | 4.23 | −24 −44 −27 | 5.81 |
| Right lobule VI | 38 −64 −23 | 3.77 | 32 −60 −29 | 3.38 |
| Left lobule VI | −24 −68 −27 | 6.02 | −32 −64 −27 | 5.53 |
| Right crus I | 42 −54 −33 | 6.91 | 42 −54 −33 | 5.06 |
| Left crus I | −40 −56 −33 | 7.45 | −36 −54 −31 | 6.38 |
| Left lobule VIIb | −24 −70 −51 | 6.71 | −36 −52 −49 | 5.06 |
| Right lobule VIIIa | 36 −50 −55 | 6.05 | 36 −48 −53 | 4.26 |
| Left lobule VIIIa | −38 −42 −53 | 6.05 | −34 −42 −53 | 5.28 |
| Left lobule VIIIb/ IX | −10 −58 −59 | 4.23 | −13 −58 −47 | 4.38 |
| Right inferior olive | 2 28 −48 | 4.94 | 2 −30 −50 | 4.82 |
| Left inferior olive | −2 −30 −49 | 5.01 | −2 −30 −48 | 4.42 |
Direct subtraction using the contrast [ AWARE minus UNAWARE] showed higher response in left crus II (−34 −72 −51; z = 3.90). [UNAWARE minus AWARE] contrast showed no significant activations. There was no significant difference between inferior olive response to AWARE and its response to UNAWARE conditions using either contrast [(AWARE minus UNAWARE) or (UNAWARE minus AWARE)].
Fig. 2.
Statistical parametric maps of event-related activations time-locked to detected (red) and undetected (green) deviant stimuli. Activations are shown on sagittal (A, B, G, and H), axial (C and D), and coronal (E and F) templates of the cerebellum and brainstem. Shown are stereotaxic coordinates (in millimeters relative to anterior commissure): X, +, right; −, left; Y, +, anterior; −, posterior; Z, +, superior; −, inferior. Arrowheads indicate inferior olive.
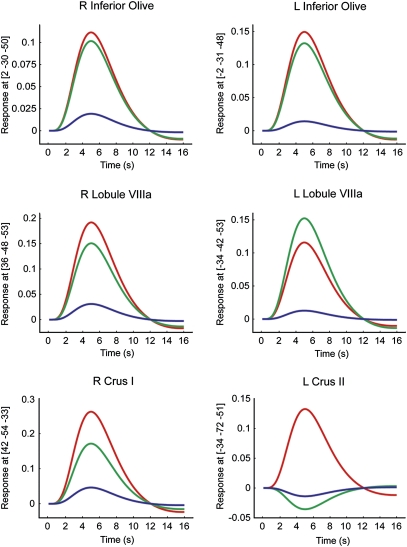
Next, we defined areas that respond to deviant stimuli regardless of awareness as areas that were activated in common in AWARE and UNAWARE conditions without significant difference between these conditions. These areas were identified by using conjunction analysis (23) and were in right lobule IV (4 −42 −11; z = 4.51), left lobule III/IV(−2 −42 −11; z = 3.93), left lobule V (−26 −44 −27; z = 4.17), lobule VI bilaterally (38 −68 −27; z = 3.53; and −32 −64 −27; z = 5.53), crus I bilaterally (36 −82 −27; z = 3.13; and −36 −54 −31; z = 6.38), left lobule VIIb (−36 −52 −49; z = 5.06), lobule VIIIa bilaterally (36 −48 −53; z = 4.26; and −34 −44 −53; z = 5.28), and left lobule VIIIb/IX (−12 −54 −55; z = 3.43). As expected, conjunction of AWARE and UNAWARE conditions also showed bilateral inferior olive activation (2 −31 −48; z = 4.82; and −2 −30 −50; z = 4.55; Fig. 3).
Fig. 3.
Event-related BOLD response time-locked to detected deviant stimuli (red), undetected deviant stimuli (green), and stimuli with regular timing (blue) at peak voxels of areas activated in common between aware and unaware conditions without significant difference between the two conditions (bilateral inferior olive, left crus II, right crus I, and bilateral lobule VIIIa) and at the peak voxel in an area with higher response in aware than unaware conditions (left crus II). There were no areas with significantly higher response in unaware than aware conditions. Shown are plots of the fitted hemodynamic response (in arbitrary units) and coordinates of peak voxels. Time (S), peristimulus time in seconds.
Finally, we used direct subtraction to compare AWARE and UNAWARE conditions. One area in the cerebellar cortex (left crus II, −34 −72 −51; z = 3.90) showed higher response using the contrast [AWARE minus UNAWARE] (Fig. 3). There were no areas with significantly higher response in unaware than aware conditions using the contrast [UNAWARE minus AWARE]. The inferior olive showed no activation using the contrasts [AWARE minus UNAWARE] or [UNAWARE minus AWARE] even at a low threshold (P < 0.05, uncorrected).We obtained the same result when the ISI change was fixed among subjects. The inferior olive showed no activation using the contrasts [AWARE minus UNAWARE] or [UNAWARE minus AWARE], even at a low threshold (P < 0.05, uncorrected), when analysis was limited to the 12 subjects with the same threshold (200 ms for all 12 subjects).
Discussion
The present study significantly extends the findings of previous studies on the importance of the olivocerebellar system in the timing of sensory events (21, 25). Our data demonstrate that the response of the inferior olive and multiple areas within the cerebellar cortex to changes in stimulus timing occur regardless of whether the subjects were aware of such changes. This finding has not been directly demonstrated in previous studies to our knowledge. However, evidence from studies of trace conditioning (in which an empty interval intervenes between the neutral stimulus and the aversive stimulus) and delay conditioning (in which the neutral stimulus overlaps and coterminates with the aversive stimulus) indirectly supports a role for the inferior olive and climbing fiber system in implicit timing (26, 27). In both trace and delay conditioning, intact inferior olive was shown to be critical for encoding ISIs (13-14, 16). However, unlike trace conditioning, which was shown to require awareness and intact forebrain structures, delay conditioning was shown to be independent of the subject's awareness (26, 27). Indeed, delay conditioning paradigms were used in most studies that demonstrated the importance of the inferior olive in classical conditioning, including studies that specifically identified the role of electrotonic coupling between olivary neurons in learning-dependent timing (16).
In humans, both trace and delay eye-blink conditioning were shown to be impaired in patients with cerebellar cortex lesions, thus supporting the view that the cerebellum mediates timing regardless of awareness (28–30). In contrast to this view, however, the consensus from motor timing studies in patients with cerebellar lesions is that the cerebellum is critical for explicit representation of time but not implicit timing (18, 20). In these studies, however, the subjects’ awareness was not determined, and the term “implicit” was used to describe the timing that occurs as an emergent property of the motor behavior itself (18, 20). Furthermore, studies of implicit timing in humans were mostly limited to cerebellar cortex and did not address the specific role of the inferior olive and climbing fiber system (17, 18, 20, 31). The present results show a robust neural response of the inferior olive and multiple areas within the cerebellar cortex to implicit timing defined here as timing without awareness. Although the contribution of the climbing fibers to cerebellar cortex activations shown in the present study cannot be dissociated from that of mossy fibers, the activation of the inferior olive does represent activation of the climbing fiber system (32, 33).Therefore, our data provide evidence specifically linking the climbing fiber system to timing without the subject's awareness.
The response of the inferior olive to deviant stimuli shown in the present study is consistent with early electrophysiological studies demonstrating that the olivary neurons are highly sensitive to unexpected external sensory stimuli (22, 34–37). This finding was interpreted from the perspective of motor control to indicate that the inferior olive signals unexpected events and therefore errors or mismatches between intended and achieved movement (38–41). However, increases in inferior olive response were also shown during passive limb displacement in the absence of active movement, and indeed certain phases of movement were shown to inhibit the inferior olive responsiveness to sensory input (22, 36, 42). An alternative to the long-held error detection hypothesis is that the inferior olive is sensitive to the timing of sensory input. The intrinsic capacity of the olivocerebellar system to encode temporal information is supported by evidence from more recent electrophysiological studies demonstrating that stimulus timing is encoded relative to the phase of oscillations of the olivary neurons (10, 11, 14, 15). In vitro experiments that used intracellular recording and voltage-sensitive dye imaging of inferior olive slices have shown that an extracellular stimulus “resets” the olivary neuronal oscillations and leads to synchronized firing of a large group of neurons in phase with the external stimulus (43, 44). This self-referential phase resetting of subthreshold intrinsic oscillations of the olivary neurons and their synchronized firing in response to external stimuli have been proposed as the underlying mechanism of the enhanced inferior olive and climbing fibers response to unexpected stimuli (11, 22, 23, 38, 39, 45). A similar mechanism likely explains the inferior olive response to the modulation of stimulus timing with or without awareness shown by fMRI methods. Therefore, temporal encoding by the olivocerebellar system can be viewed as a low-level information-processing mechanism that is dependent on the intrinsic oscillatory properties of the inferior olive but largely independent of attentional, top-down, or cognitive control mechanisms. The present fMRI data in human subjects support this view and show that the inferior olive response to changes in stimulus timing is not significantly modulated by whether these changes were consciously perceived.
Methods
Subjects.
Eighteen subjects (nine women; mean age ± SD, 29 ± 4 y) performed a prescanning experiment to establish the validity of the behavioral task and to determined each subject's threshold. All subjects were right-handed, had normal or corrected-to-normal visual acuity, and gave a written informed consent according to the guidelines approved by the Minneapolis Veterans Affairs Medical Center and the University of Minnesota Human Subjects Committees.
During scanning, one subject reported perceiving “true” isochronous sequences as anisochronous in 40.9% of trials. This subject was excluded from further analysis as a result of poor performance. Behavioral and fMRI data from the remaining 17 subjects (nine women; mean age ± SD, 26.9 ± 4.1 y) are presented.
fMRI.
Task.
The subjects were instructed to indicate whether the observed sequences were anisochronous or isochronous. The ratio of trials with anisochronous sequences to those with isochronous sequences was kept at 3:1 to maximize the number of trials with deviant stimuli; however, subjects were not informed about this ratio. Subjects underwent four scanning runs. The total scanning time for each run was 6 min and 32 s, during which 24 trials were randomly presented (18 trials with anisochronous sequences mixed with six catch trials with isochronous sequences). Each anisochronous sequence contained one deviant stimulus that was presented “prematurely” by 150 ms for three subjects, 200 ms for 12 subjects, and 250 ms for two subjects based on each individual subject's threshold as determined by the prescanning behavioral data. Within the anisochronous sequence, the deviant stimulus was presented pseudorandomly as the fifth stimulus in six trials, sixth stimulus in six trials, and seventh stimulus in six trials.
After a delay of 2 or 4 s, subjects were prompted by a visual cue to indicate whether the sequence was “regular” or “irregular” by pressing one of two buttons with the right or left thumb. Buttons corresponding to regular and irregular sequences were counterbalanced among subjects. The delay between the conclusion of the sequence and the visual cue was introduced to separate the neural response to the visual stimuli in the sequence from that to the visual cue and motor response. The jittered timing of visual cue (and motor response) was used to optimize estimation power of event-related neural activity (46). We used an ISI that was shorter than the regular ISI (of 1,000 ms) to avoid accounting for a neural response to what might be perceived as an “omitted” stimulus if the deviant stimulus was presented later than expected.
The visual display was projected through a backlit screen at the head of the scanner bed and viewed via a mirror attached to the head coil. Subjects were instructed to fixate on a very dark nonflashing disk at the center of the screen. Visual stimuli (150 ms duration) consisted of a white disk (visual angle 1.2°) that flashed on a black background replacing the fixation point. The written visual cue consisted of the words “Regular” and “Irregular” presented for 1 s to the right or left of midline, corresponding to the button to be pressed. Trials in which subjects failed to respond within the 2 s after the onset of a visual cue were regarded as “missed trials” and excluded from further analysis.
Image acquisition and preprocessing.
Blood oxygenation level-dependent (BOLD) contrast functional images were acquired with a 3-T MRI scanner (Magnetom Trio; Siemens) using a gradient echoplanar (T2*) sequence with the following parameters: echo time, 28 ms; repetition time, 2,000 ms; flip angle, 90°; field of view, 200 mm2; in-plane resolution, 3 × 3 mm; and slice thickness, 3 mm with no gap. A total of 35 axial slices were obtained, covering subcortical structures including the brainstem, cerebellum, basal ganglia, and cerebral cortex except for the superior portion of the frontal and parietal lobes. A high-resolution anatomical T1 image was obtained with the following parameters: echo time, 4.7 m; repetition time, 20 ms; flip angle, 22°; field of view, 256 mm2; in-plane resolution, 1 × 1 mm; and slice thickness, 1 mm.
fMRI data were preprocessed and analyzed by using Statistical Parametric Mapping 5 software (Wellcome Department of Cognitive Neurology) implemented in MATLAB (Mathworks).
For each subject, 196 volumes were corrected for head motion, realigned to the first image and sinc-interpolated over time to correct for phase advance during volume acquisition. Images were then normalized to a Montreal Neurological Institute echoplanar imaging brain template with an enlarged box (inferiorly) to include the whole cerebellum and brainstem (Z values of +20 to −70 mm). Data were resampled to 2 × 2 × 2 mm and spatially smoothed with a Gaussian kernel of 6-mm full-width at half-maximum to decrease spatial noise. The functional data were temporally smoothed to remove slow BOLD signal drifts by using a high-pass filter with a 128-s cutoff. T1-weighted anatomical images were coregistered to the functional scans and transformed into the same normalized Montreal Neurological Institute brain template. Intersubject alignment of cerebellar and brainstem maps was optimized by using nonlinear normalization to a high-resolution atlas template of the human cerebellum and brainstem (spatially unbiased infratentorial template) (47).
Data Analysis.
Behavioral data during scanning show that the subjects accurately recognized isochronous sequences as isochronous in 93.77% ± 3.42 of trials (range, 88–100%). Deviant stimuli were undetected in 49.28% ± 5.42 (range, 40–57%) and were detected in 50.72% ± 5.42 (range, 43–60%) of trials with anisochronous sequences. There was no significant effect of the deviant stimulus order (fifth, sixth, or seventh) within the sequence on the ratio of detected/undetected deviant stimuli [F(2,16) = 2.26; P = 0.15]. There was also no significant effect of the duration of delay after the sequence (2 or 4 s) on the ratio of detected/undetected deviant stimuli (t = 1.09; P = 0.29; Table S2). Voxel-based event-related analysis of fMRI data were performed at two levels of a mixed-effects model. In the first-level analysis (fixed-effects), detected deviant stimuli, undetected deviant stimuli, regular stimuli (stimuli following isochronous ISI), visual cues, and motor responses were modeled as separate event types and were convolved with a canonical hemodynamic response function in a general linear model as implemented in Statistical Parametric Mapping 5 (specifying event duration as zero). Thus, each trial with an anisochronous sequence (total of 72 trials per subject) contained either a detected or an undetected deviant stimulus (one event per trial). From each subject, the number of events with detected deviant stimuli entered into the statistical model was equal to the number of events with undetected deviant stimuli. This number was determined in each subject by whichever number (of detected or undetected deviant stimuli) was smaller. An average of 31.88 ± 2.60 events (range, 26–35) with detected (or undetected) deviant stimuli per subject was used, discarding an average of 6.82 ± 3.97 trials (range, 2–14) per subject from the last run (Table S2).
Linear contrasts of parameter estimates for each event type were obtained from every voxel in each subject. Statistical comparisons between event types (conditions) were limited to: (i) detected deviant stimuli (AWARE), (ii) undetected deviant stimuli (UNAWARE), and (iii) regular stimuli (REGULAR). Single subjects’ maps were then subjected to the second-level group analysis treating intersubject variability as a random effect and thus allowing statistical inference at the population level (48). Statistical thresholds of Z ≥ 3.09 and P ≤ 0.05 (corrected for multiple comparisons) were used for group results. False discovery rate correction was used except for areas of prespecified hypothesis (inferior olive), in which small volume correction (5-mm radius sphere) was applied. Homologous areas below statistical threshold are listed for comparison. Cerebellar lobules were identified based on the Diedrichsen human cerebellum MRI atlas (49) and Schmahmann atlas nomenclature (50). Based on the location of peak voxel within the activation cluster, more than one lobule is listed for one activation cluster when appropriate.
Supplementary Material
Acknowledgments
We thank Dr. Mark Hallett for his comments on the manuscript. This work was supported by a Department of Veterans Affairs Merit Review grant (to K.O.B.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1104096108/-/DCSupplemental.
References
- 1.Chowdhary AG, Challis JH. Timing accuracy in human throwing. J Theor Biol. 1999;201:219–229. doi: 10.1006/jtbi.1999.1024. [DOI] [PubMed] [Google Scholar]
- 2.Timmann D, Watts S, Hore J. Failure of cerebellar patients to time finger opening precisely causes ball high-low inaccuracy in overarm throws. J Neurophysiol. 1999;82:103–114. doi: 10.1152/jn.1999.82.1.103. [DOI] [PubMed] [Google Scholar]
- 3.Barnes R, Jones MR. Expectancy, attention, and time. Cognit Psychol. 2000;41:254–311. doi: 10.1006/cogp.2000.0738. [DOI] [PubMed] [Google Scholar]
- 4.Sohn MH, Carlson RA. Implicit temporal tuning of working memory strategy during cognitive skill acquisition. Am J Psychol. 2003;116:239–256. [PubMed] [Google Scholar]
- 5.Coull J, Nobre A. Dissociating explicit timing from temporal expectation with fMRI. Curr Opin Neurobiol. 2008;18:137–144. doi: 10.1016/j.conb.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Braitenberg V, Atwood RP. Morphological observations on the cerebellar cortex. J Comp Neurol. 1958;109:1–33. doi: 10.1002/cne.901090102. [DOI] [PubMed] [Google Scholar]
- 7.Llinas R, Baker R, Sotelo C. Electrotonic coupling between neurons in cat inferior olive. J Neurophysiol. 1974;37:560–571. doi: 10.1152/jn.1974.37.3.560. [DOI] [PubMed] [Google Scholar]
- 8.Welsh JP, Lang EJ, Suglhara I, Llinás R. Dynamic organization of motor control within the olivocerebellar system. Nature. 1995;374:453–457. doi: 10.1038/374453a0. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson GA, Rokni D, Yarom Y. A model of the olivo-cerebellar system as a temporal pattern generator. Trends Neurosci. 2008;31:617–625. doi: 10.1016/j.tins.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Mathy A, et al. Encoding of oscillations by axonal bursts in inferior olive neurons. Neuron. 2009;62:388–399. doi: 10.1016/j.neuron.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llinás RR. Inferior olive oscillation as the temporal basis for motricity and oscillatory reset as the basis for motor error correction. Neuroscience. 2009;162:797–804. doi: 10.1016/j.neuroscience.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivry R. Cerebellar timing systems. Int Rev Neurobiol. 1997;41:555–573. [PubMed] [Google Scholar]
- 13.Yeo CH, Hardiman MJ, Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. IV. Lesions of the inferior olive. Exp Brain Res. 1986;63:81–92. doi: 10.1007/BF00235649. [DOI] [PubMed] [Google Scholar]
- 14.Welsh JP, Harvey JA. Acute inactivation of the inferior olive blocks associative learning. Eur J Neurosci. 1998;10:3321–3332. doi: 10.1046/j.1460-9568.1998.00400.x. [DOI] [PubMed] [Google Scholar]
- 15.Chorev E, Yarom Y, Lampl I. Rhythmic episodes of subthreshold membrane potential oscillations in the rat inferior olive nuclei in vivo. J Neurosci. 2007;27:5043–5052. doi: 10.1523/JNEUROSCI.5187-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Der Giessen RS, et al. Role of olivary electrical coupling in cerebellar motor learning. Neuron. 2008;58:599–612. doi: 10.1016/j.neuron.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 17.O'Reilly JX, Mesulam MM, Nobre AC. The cerebellum predicts the timing of perceptual events. J Neurosci. 2008;28:2252–2260. doi: 10.1523/JNEUROSCI.2742-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zelaznik HN, Spencer RM, Ivry RB. Dissociation of explicit and implicit timing in repetitive tapping and drawing movements. J Exp Psychol Hum Percept Perform. 2002;28:575–588. doi: 10.1037//0096-1523.28.3.575. [DOI] [PubMed] [Google Scholar]
- 19.Spencer RM, Verstynen T, Brett M, Ivry R. Cerebellar activation during discrete and not continuous timed movements: an fMRI study. Neuroimage. 2007;36:378–387. doi: 10.1016/j.neuroimage.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spencer RM, Zelaznik HN, Diedrichsen J, Ivry RB. Disrupted timing of discontinuous but not continuous movements by cerebellar lesions. Science. 2003;300:1437–1439. doi: 10.1126/science.1083661. [DOI] [PubMed] [Google Scholar]
- 21.Liu T, Xu D, Ashe J, Bushara K. Specificity of inferior olive response to stimulus timing. J Neurophysiol. 2008;100:1557–1561. doi: 10.1152/jn.00961.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gellman R, Gibson AR, Houk JC. Inferior olivary neurons in the awake cat: detection of contact and passive body displacement. J Neurophysiol. 1985;54:40–60. doi: 10.1152/jn.1985.54.1.40. [DOI] [PubMed] [Google Scholar]
- 23.Gellman R, Houk JC, Gibson AR. Somatosensory properties of the inferior olive of the cat. J Comp Neurol. 1983;215:228–243. doi: 10.1002/cne.902150210. [DOI] [PubMed] [Google Scholar]
- 24.Grondin S. Discriminating time intervals presented in sequences marked by visual signals. Percept Psychophys. 2001;63:1214–1228. doi: 10.3758/bf03194535. [DOI] [PubMed] [Google Scholar]
- 25.Xu D, Liu T, Ashe J, Bushara KO. Role of the olivo-cerebellar system in timing. J Neurosci. 2006;26:5990–5995. doi: 10.1523/JNEUROSCI.0038-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark RE, Squire LR. Classical conditioning and brain systems: the role of awareness. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- 27.Clark RE, Manns JR, Squire LR. Classical conditioning, awareness, and brain systems. Trends Cogn Sci. 2002;6:524–531. doi: 10.1016/s1364-6613(02)02041-7. [DOI] [PubMed] [Google Scholar]
- 28.Topka H, Valls-Solé J, Massaquoi SG, Hallett M. Deficit in classical conditioning in patients with cerebellar degeneration. Brain. 1993;116:961–969. doi: 10.1093/brain/116.4.961. [DOI] [PubMed] [Google Scholar]
- 29.Gerwig M, et al. Trace eyeblink conditioning in human subjects with cerebellar lesions. Exp Brain Res. 2006;170:7–21. doi: 10.1007/s00221-005-0171-2. [DOI] [PubMed] [Google Scholar]
- 30.Daum I, et al. Classical conditioning after cerebellar lesions in humans. Behav Neurosci. 1993;107:748–756. doi: 10.1037//0735-7044.107.5.748. [DOI] [PubMed] [Google Scholar]
- 31.Boyd LA, Winstein CJ. Cerebellar stroke impairs temporal but not spatial accuracy during implicit motor learning. Neurorehabil Neural Repair. 2004;18:134–143. doi: 10.1177/0888439004269072. [DOI] [PubMed] [Google Scholar]
- 32.Desclin JC. Histological evidence supporting the inferior olive as the major source of cerebellar climbing fibers in the rat. Brain Res. 1974;77:365–384. doi: 10.1016/0006-8993(74)90628-3. [DOI] [PubMed] [Google Scholar]
- 33.Palay SL, Chan-Palay V. Cerebellar Cortex: Cytology and Organization. Berlin: Springer; 1974. [Google Scholar]
- 34.Eccles JC, Sabah NH, Schmidt RF, Táboríková H. Cutaneous mechanoreceptors influencing impulse discharges in cerebellar cortex. 3. In Purkynĕ cells by climbing fiber input. Exp Brain Res. 1972;15:484–497. doi: 10.1007/BF00236404. [DOI] [PubMed] [Google Scholar]
- 35.Armstrong DM. Functional significance of connections of the inferior olive. Physiol Rev. 1974;54:358–417. doi: 10.1152/physrev.1974.54.2.358. [DOI] [PubMed] [Google Scholar]
- 36.Rushmer DS, Roberts WJ, Augter GK. Climbing fiber responses of cerebellar Purkinje cells to passive movement of the cat forepaw. Brain Res. 1976;106:1–20. doi: 10.1016/0006-8993(76)90069-x. [DOI] [PubMed] [Google Scholar]
- 37.Oscarsson O. Functional organization of olivary projection to the cerebellar anterior lobe. In: Courville J, De Montigny C, Lamarre Y, editors. The Inferior Olivary Nucleus: Anatomy and Physiology. New York: Raven; 1980. [Google Scholar]
- 38.Kim JH, Wang JJ, Ebner TJ. Climbing fiber afferent modulation during treadmill locomotion in the cat. J Neurophysiol. 1987;57:787–802. doi: 10.1152/jn.1987.57.3.787. [DOI] [PubMed] [Google Scholar]
- 39.Andersson G, Armstrong DM. Complex spikes in Purkinje cells in the lateral vermis (b zone) of the cat cerebellum during locomotion. J Physiol. 1987;385:107–134. doi: 10.1113/jphysiol.1987.sp016487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barmack NH, Simpson JI. Effects of microlesions of dorsal cap of inferior olive of rabbits on optokinetic and vestibuloocular reflexes. J Neurophysiol. 1980;43:182–206. doi: 10.1152/jn.1980.43.1.182. [DOI] [PubMed] [Google Scholar]
- 41.Winkelman B, Frens M. Motor coding in floccular climbing fibers. J Neurophysiol. 2006;95:2342–2351. doi: 10.1152/jn.01191.2005. [DOI] [PubMed] [Google Scholar]
- 42.Gibson AR, Horn KM, Pong M. Activation of climbing fibers. Cerebellum. 2004;3:212–221. doi: 10.1080/14734220410018995. [DOI] [PubMed] [Google Scholar]
- 43.Leznik E, Makarenko V, Llinás R. Electrotonically mediated oscillatory patterns in neuronal ensembles: an in vitro voltage-dependent dye-imaging study in the inferior olive. J Neurosci. 2002;22:2804–2815. doi: 10.1523/JNEUROSCI.22-07-02804.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kazantsev VB, Nekorkin VI, Makarenko VI, Llinás R. Self-referential phase reset based on inferior olive oscillator dynamics. Proc Natl Acad Sci USA. 2004;101:18183–18188. doi: 10.1073/pnas.0407900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lidierth M, Apps R. Gating in the spino-olivocerebellar pathways to the c1 zone of the cerebellar cortex during locomotion in the cat. J Physiol. 1990;430:453–469. doi: 10.1113/jphysiol.1990.sp018301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. Neuroimage. 2006;33:127–138. doi: 10.1016/j.neuroimage.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 48.Penny W, Holmes A. Random effect analysis. In: Frackowiak RSJFK, et al., editors. Human Brain Function. New York: Academic; 2003. pp. 843–850. [Google Scholar]
- 49.Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- 50.Schmahmann JD, et al. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage. 1999;10:233–260. doi: 10.1006/nimg.1999.0459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.