Abstract
Peptide–MHC (pMHC) multimers, in addition to being tools for tracking and quantifying antigen-specific T cells, can mediate downstream signaling after T-cell receptor engagement. In the absence of costimulation, this can lead to anergy or apoptosis of cognate T cells, a property that could be exploited in the setting of autoimmune disease. Most studies with class I pMHC multimers used noncovalently linked peptides, which can allow unwanted CD8+ T-cell activation as a result of peptide transfer to cellular MHC molecules. To circumvent this problem, and given the role of self-reactive CD8+ T cells in the development of type 1 diabetes, we designed a single-chain pMHC complex (scKd.IGRP) by using the class I MHC molecule H-2Kd and a covalently linked peptide derived from islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP206–214), a well established autoantigen in NOD mice. X-ray diffraction studies revealed that the peptide is presented in the groove of the MHC molecule in canonical fashion, and it was also demonstrated that scKd.IGRP tetramers bound specifically to cognate CD8+ T cells. Tetramer binding induced death of naive T cells and in vitro- and in vivo-differentiated cytotoxic T lymphocytes, and tetramer-treated cytotoxic T lymphocytes showed a diminished IFN-γ response to antigen stimulation. Tetramer accessibility to disease-relevant T cells in vivo was also demonstrated. Our study suggests the potential of single-chain pMHC tetramers as possible therapeutic agents in autoimmune disease. Their ability to affect the fate of naive and activated CD8+ T cells makes them a potential intervention strategy in early and late stages of disease.
CD8+ cytotoxic T lymphocytes (CTLs) use their T-cell receptors (TCRs) to recognize peptides presented by class I MHC molecules, and this recognition can lead to the demise of the cell displaying the cognate peptide–MHC (pMHC) complex. As a result, CD8+ T cells are important pathogenic effectors in a number of autoimmune diseases, including type 1 diabetes (1). The development of strategies to interfere with their function offers new therapeutic opportunities. Treatment of CTLs with multimers of pMHC complexes has shown promise in inhibiting CTL-mediated cytotoxicity (2–5). For example, pMHC multimers constructed with short flexible linkers cause rapid death of peptide-specific CTLs (3), whereas those with long rigid linkers inhibit CTL-mediated cytotoxicity by interfering with integrin-mediated CTL adhesion (2). In addition, dimeric Ig fusions of pMHC complexes have been shown to inhibit lysis of target cells by alloreactive CTLs (4, 5).
We reasoned that, in addition to their inhibition of already differentiated CTLs (2–5), pMHC multimers should also be effective against naive T cells, as they would present antigen in the absence of a second costimulatory signal and would be predicted to drive the T cells to apoptosis or anergy (6–9). This is a profoundly unexplored area, perhaps because of the early unexpected finding that pMHC tetramers could instead activate naive CD8+ T cells (10). This behavior was subsequently found to result from the release of the peptide from the tetramers and its transfer to MHC molecules on T cells, which then acted as antigen-presenting cells capable of activating their naive counterparts (11, 12). Thus, the activity of pMHC multimers against CD8+ T cells, both naive and antigen-experienced, requires reevaluation with the use of pMHC complexes in which the peptide is rendered nonexchangeable by virtue of covalent linkage to the complex (13, 14).
To this end, we used a disease-relevant model system consisting of autoreactive CD8+ 8.3 T cells. The 8.3 T-cell clone was originally isolated from the pancreatic islets of a nonobese diabetic (NOD) mouse (15), a model system for type 1 diabetes in which CD8+ T cells have an important pathogenic role (16). The 8.3 T-cell clone is specific for the peptide composed of residues 206 to 214 of islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP206–214) presented by H-2Kd (17), and its pathogenicity has been demonstrated by adoptive transfer studies (15) and the accelerated disease that occurs in NOD mice that transgenically express the 8.3 TCR (18). T cells specific for IGRP206–214 represent a prevalent population in the islets of NOD mice (17, 19, 20), and the monitoring of their numbers in the blood can be used to predict disease (20). IGRP epitopes have also been found to be targeted by CD8+ T cells in human type 1 diabetes (21–23).
We used 8.3 T cells to investigate whether a single multimeric pMHC reagent could be developed that would inactivate or eradicate both CTLs and naive CD8+ T cells. We designed a single-chain pMHC complex in which IGRP206–214 is covalently attached to β2-microglobulin (β2m), which itself is covalently linked to the heavy chain of H-2Kd. X-ray diffraction analysis of the single-chain H-2Kd/IGRP206–214 (scKd.IGRP) demonstrated that the covalently linked peptide is presented in the canonical binding groove of the MHC molecule in a fashion that would support productive TCR engagement. Tetramers of scKd.IGRP exhibit high-specificity binding for the cognate 8.3 TCR. Most importantly, scKd.IGRP tetramers specifically induce apoptosis of naive CD8+ 8.3 T cells, as well as of in vitro-generated CTLs and islet-infiltrating CTLs naturally differentiated in vivo. The tetramers also gain access to splenic and pancreatic T cells when administered in vivo. These characteristics support further exploration of the therapeutic potential of single-chain pMHC tetramers for type 1 diabetes and other conditions in which CD8+ T cells contribute to the pathogenic process.
Results
Design and Biochemical Characterization of scKd.IGRP.
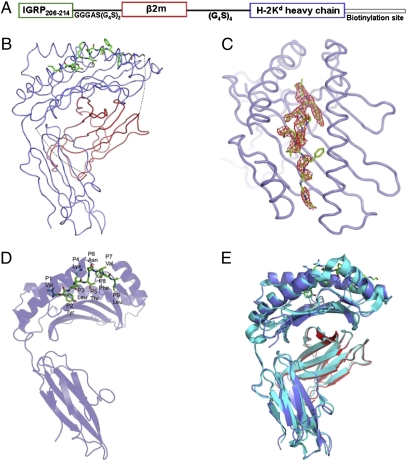
To circumvent the complications associated with peptide transfer to cellular MHC molecules (11, 12), we designed the single-chain construct scKd.IGRP, which contained the heavy chain of H-2Kd, β2m, and the target peptide, following a strategy similar to that reported for a single-chain construct of H-2Kb presenting a peptide derived from ovalbumin (13, 14). Specifically, the C terminus of IGRP206–214 (VYLKTNVFL) was fused to the N terminus of β2m with a GGGAS(G4S)2 linker and the C terminus of β2m was fused to the N terminus of the H-2Kd heavy chain with a (G4S)4 linker (Fig. 1A).
Fig. 1.
Single-chain pMHC design and structural features. (A) Schematic representation of the scKd.IGRP construct is shown. The IGRP peptide, β2m, and the heavy chain are depicted in green, red, and blue, respectively. The same color scheme is maintained in B. At the C terminus of the heavy chain, the biotinylation site is represented as a thin white box. (B) Ribbon diagram of scKd.IGRP. The IGRP peptide is rendered as a ball-and-stick model and the atoms are colored as follows: carbon, green; nitrogen, blue; and oxygen, red. The complex is oriented with the IGRP N terminus on the top left and the membrane proximal α3 domain at the bottom. The linkers between the IGRP peptide and β2m and between β2m and the heavy chain are represented as dotted lines. The two linker glycines immediately C-terminal to the peptide are shown in black. (C) Experimental electron density and overall conformation of the IGRP peptide. The Fo-Fc map contoured at the 2σ level is shown around the peptide. The membrane distal peptide-binding platform of H-2Kd is depicted as a blue ribbon and the IGRP peptide is displayed as a ball-and-stick model. The N terminus of the peptide is at the top of the figure. (D) Ribbon representation of the heavy chain and ball-and-stick representation of the IGRP peptide emphasizes the orientation of the side chains of the IGRP peptide (labeled). (E) Superposition of scKd.IGRP onto the structure of H-2Kd/FLU (2FWO), which reflects a very high degree of similarity between these two complexes. The Cα RMSD calculated over all of the residues of β2m and the heavy chain is 1.08 Å. Both structures are shown as ribbon representations, with the H-2Kd/FLU complex in cyan.
The scKd.IGRP construct was expressed in Escherichia coli as inclusion bodies that were refolded and purified in milligram quantities (Fig. S1A). The refolded material exhibited excellent solution properties and was monodisperse as demonstrated by analytical size-exclusion chromatography (Fig. S1C), and exhibited a well resolved single tight band on a native polyacrylamide gel (Fig. S1B). A control single-chain H-2Kd molecule presenting the tumor-derived peptide KYQAVTTTL (24), which is not recognized by 8.3 T cells, was prepared in an identical fashion and designated scKd.TUM (Fig. S2).
Anchoring of IGRP206–214 in H-2Kd Groove and Implications for TCR Recognition.
The bacterially expressed and refolded scKd.IGRP protein crystallized in the monoclinic space group P21 with one molecule per asymmetric unit. Initial phase estimates were obtained by molecular replacement using a conventional H-2Kd complex (Protein Data Bank ID 2FWO) (25) incorporating the influenza virus-derived peptide TYQRTRALV (FLU). After initial refinement, interpretable electron density was observed for the IGRP206–214 peptide and the two linker glycine residues immediately C-terminal to the peptide (Fig. 1B). The final electron density map was of excellent quality, with no ambiguities observed for the main-chain or side-chain atoms of IGRP206–214, except for the phenylalanine present at position 8 of the peptide (Fig. 1C and Fig. S3). Although the electron density of that phenylalanine side chain was weak, electrospray ionization Fourier transform MS revealed a mass of 49,463.6 Da, which is consistent with the predicted molecular weight of 49,463.1 Da, including the phenylalanine at position 8 (Fig. S4). The phenylalanine side chain was modeled on the basis of weak density and occupies one of the most favored rotamer positions. Diffraction data to 2.7 Å resolution were used for refinement, resulting in a final atomic model with Rwork and Rfree of 20.8% and 28.4%, respectively, and good stereochemistry (Table S1).
Despite the importance of H-2Kd/IGRP206–214 as a target of pathogenic CD8+ T cells in type 1 diabetes in NOD mice (17, 19, 20), the structure of this pMHC complex has not been reported previously to our knowledge. We found that IGRP206–214 (VYLKTNVFL) is presented in the H-2Kd groove between the α1 and α2 helices and on top of the β-sheet platform in canonical fashion (Fig. 1B and C) (26). The overall conformation of the IGRP peptide is shown in Fig. 1D and Fig. S5. The binding of the IGRP peptide is associated with complete or partial burial of TyrP2, ThrP5, and LeuP9 in the H-2Kd groove (Fig. 1D and Table S2). Tyrosine is found at the P2 position of nearly all naturally processed H-2Kd–binding nonameric peptides (25). Amino acids present at the C-terminal P9 position are also highly conserved, with a preference for Ile, Leu, and Val (25). Our structural data suggest that TyrP2 and LeuP9 are indeed important residues for anchoring IGRP206–214 in the H-2Kd groove. Our structural data also suggest that LysP4, AsnP6, ValP7, and PheP8 are the most likely to be involved in contacting residues of the cognate TCRs, as the side chains of these residues protrude from the groove and are accessible for TCR recognition (Fig. 1D and Table S2). This is consistent with the identification of LysP4 and PheP8 as major 8.3 TCR contact residues (27), and the finding that alteration of P7 in the IGRP206–214 mimotope peptide NRP (KYNKANWFL) to Ala or Val endows the resulting NRP-A7 and NRP-V7 peptides with superagonist activity (28).
Structural alignment of scKd.IGRP with the native (i.e., noncovalently linked) H-2Kd/FLU (25) highlights a high degree of similarity between these two complexes (Fig. 1E), with an rmsd of 1.06 Å calculated over all Cα atoms from the β2m domain, the heavy chain, and the peptide (0.47 Å between the β2m domains and 1.08 Å between the heavy chains). Overall, the structural organization of the complex and the canonical mode of peptide binding are conserved in the covalently linked pMHC complex. These results further suggest that the single-chain pMHC complex presents peptide and contacts its cognate TCR in a canonical fashion.
Tetramerization of scKd.IGRP.
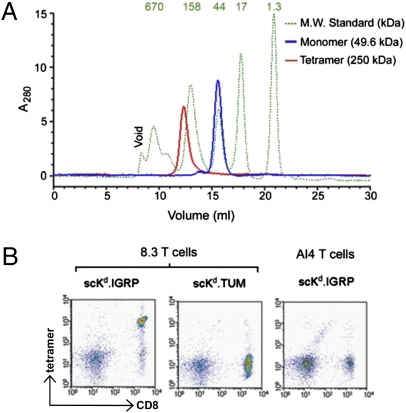
Because of the relatively weak binding affinity between pMHC complexes and their cognate TCRs, we generated scKd.IGRP tetramers with enhanced avidity to assess the functional activity of the single-chain pMHC complex. The tetramers were constructed by using standard procedures based on the high-affinity biotin–streptavidin interaction. The scKd.IGRP tetramers and the control tetramers (scKd.TUM) behaved as single highly homogeneous peaks on size-exclusion chromatography (Fig. 2A and Fig. S2B). A potential challenge associated with the production of such tetramers is the generation of heterogeneous mixtures containing ill-defined multimers, which complicates mechanistic interpretations (29). Our preparations consisted nearly exclusively of tetrameric complexes (Fig. 2A and Fig. S2B).
Fig. 2.
scKd.IGRP tetramers are homogeneous and bind specifically to cognate 8.3 T cells. (A) scKd.IGRP monomers (blue line), tetramers (red line), and molecular weight standards (green dotted line) were analyzed by size-exclusion chromatography on a Superdex 200 column. The standard peaks are labeled with their molecular weights (in kDa) at the top of the figure. (B) Splenocytes from 8.3 or AI4 TCR-transgenic NOD mice were stained with anti-CD8 and the indicated PE-labeled tetramers and analyzed by flow cytometry.
Specific Binding of scKd.IGRP Tetramers to CD8+ T Cells Bearing a Cognate TCR.
To assess the binding capacity of the scKd.IGRP for the cognate 8.3 TCR, scKd.IGRP was tetramerized by using phycoerythrin (PE)-labeled streptavidin, and its binding to splenocytes from 8.3 TCR-transgenic NOD mice was assessed by flow cytometry. Tetramers of scKd.IGRP bound to nearly all the CD8+ T cells from these mice, whereas PE-conjugated tetramers of scKd.TUM did not bind (Fig. 2B). This behavior indicates that the scKd.IGRP complex adopts a conformation in which the covalently linked antigenic peptide is properly presented for specific recognition by the cognate 8.3 T cells. The specificity of the binding was further substantiated by the lack of interaction between scKd.IGRP tetramers and AI4 T cells (Fig. 2B), which recognize an autoantigenic peptide in the context of H-2Db (19).
scKd.IGRP Tetramers Do Not Activate Cognate Naive CD8+ T Cells and Instead Drive Them to Apoptosis.
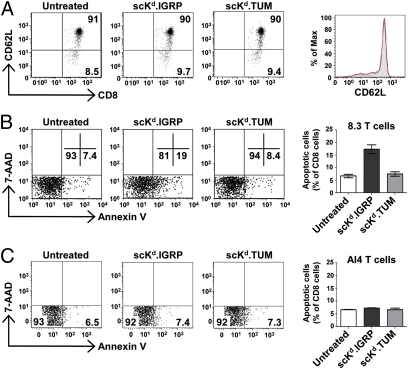
To examine the biological activity of scKd.IGRP tetramers on naive T cells, splenocytes isolated from nondiabetic 8.3 TCR-transgenic NOD mice were treated with scKd.IGRP or scKd.TUM tetramers for 3 h. Splenic CD8+ T cells from these mice were previously demonstrated to be largely naive (18). We confirmed this observation on the basis of high CD62L expression, which was insensitive to tetramer treatment (Fig. 3A), showing that the tetramer binding did not activate the CD8+ T cells. However, staining with Annexin V–FITC demonstrated that the scKd.IGRP tetramers induced phosphatidylserine externalization, a marker of apoptosis, in nearly 20% of cognate CD8+ 8.3 T cells, whereas the irrelevant scKd.TUM tetramers did not have this effect (Fig. 3B). In contrast, the scKd.IGRP tetramers were unable to induce apoptosis of noncognate CD8+ AI4 T cells (Fig. 3C).
Fig. 3.
Induction of apoptosis in naive CD8+ T cells by scKd.IGRP tetramers. (A) Splenocytes from 6- to 8-wk-old 8.3 TCR-transgenic NOD mice were incubated at 37 °C for 3 h with 25 nM scKd.IGRP tetramers (red histogram) or 25 nM scKd.TUM tetramers (blue) or left untreated (filled gray), and then stained with anti-CD8 and anti-CD62L and analyzed by flow cytometry. Samples were gated on CD8+ cells. (B) As in A, except that treated and untreated splenocytes were stained with anti-CD8, Annexin V, and 7-AAD and analyzed by flow cytometry. Samples were gated on CD8+ 7-AAD− cells. (C) As in B, except that splenocytes from 6- to 8-wk-old AI4 TCR-transgenic NOD mice were used. In A–C, numbers denote the percentage of cells present in the indicated quadrants of the dot plots.
Death of in Vitro- and in Vivo-Generated CTLs upon Treatment with scKd.IGRP Tetramers.
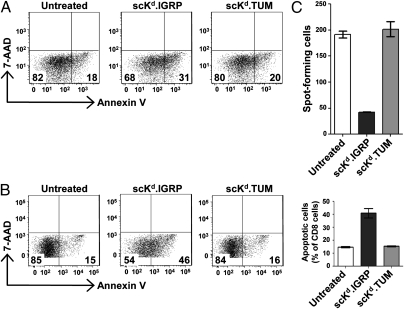
Peptide–MHC class I multimers, which are widely used as cell-surface staining reagents, have been reported to inhibit CTL activity and induce apoptosis of differentiated CD8+ T cells (2–5). However, single-chain pMHC multimers were not examined in these earlier studies. To explore the effect of the scKd.IGRP tetramers on CTLs, we first generated CTLs in vitro by using splenocytes from 8.3 TCR-transgenic NOD mice. CTLs were then treated with the single-chain tetramers for 3 h and stained with Annexin V–FITC to assess apoptosis induction. It was found that, as with naive cells, the CTLs were also driven to death specifically by the scKd.IGRP tetramers, whereas the noncognate scKd.TUM tetramers had no effect (Fig. 4A).
Fig. 4.
Induction of apoptosis in in vitro- and in vivo-generated CTLs and modulation of CTL activity by scKd.IGRP tetramers. (A) In vitro-generated 8.3 CTLs were incubated at 37 °C for 3 h with tetramers of scKd.IGRP or scKd.TUM at 25 nM. Cells were stained with anti-CD8, Annexin V, and 7-AAD and analyzed by flow cytometry. Samples were gated on CD8+ 7-AAD− cells. (B) As in A, except that islet-infiltrating cells from 8.3 TCR-transgenic NOD mice were used. (C) Islet infiltrates from 8.3 TCR-transgenic mice were incubated at 37 °C for 3 h with tetramers of either scKd.IGRP or scKd.TUM at 25 nM. The T-cell response to the superagonist peptide NRP-V7 presented by RMA-S/Kd cells was measured by IFN-γ ELISPOT assay. Spot-forming cells per 103 islet-infiltrating cells are shown.
In NOD mice, diabetes is accompanied by infiltration of the pancreatic islets by autoreactive CTLs. In the 8.3 TCR-transgenic mice, the vast majority of the islet-infiltrating CD8+ T-cell population is specific for H-2Kd/IGRP206–214 (30). These islet-infiltrating cells have previously encountered their antigen during priming in the pancreatic lymph node (31). To investigate the effect of single-chain tetramers on these in vivo-differentiated CTLs, islets from diabetic 8.3 TCR-transgenic mice were isolated and cultured with IL-2 for 6 d. The islet infiltrates that exited the islets were then treated with scKd.IGRP or scKd.TUM tetramers for 3 h and analyzed for apoptosis by Annexin V staining. The scKd.IGRP tetramers induced an approximately threefold increase in apoptosis of these in vivo-differentiated CTLs compared with untreated cells or those treated with the scKd.TUM tetramers (Fig. 4B).
scKd.IGRP Tetramer Treatment Causes Abrogation of Responsiveness in Islet-Infiltrating Cognate CD8+ T Cells.
Our studies demonstrated efficient binding of the scKd.IGRP tetramers to 8.3 T cells (Fig. 2B) and induction of apoptosis in islet-infiltrating CD8+ T cells specific for the IGRP peptide (Fig. 4B). However, there remained a large number of 8.3 T cells that were Annexin V-negative. It is of considerable interest to consider the possible fate of those 8.3 T cells that bound tetramer but did not undergo apoptosis. A second pathway associated with scKd.IGRP tetramer binding (i.e., TCR engagement in the absence of costimulatory interactions) could be the induction of anergy or unresponsiveness (7, 8). For this purpose, islet-infiltrating CD8+ T cells from the 8.3 TCR-transgenic mice were treated with the scKd.IGRP tetramers or the noncognate scKd.TUM tetramers, and used in an IFN-γ enzyme-linked immunosorbent spot (ELISPOT) assay to determine their responsiveness. There was an approximately 80% reduction in the number of spot-forming cells when the CD8+ T cells were treated with the scKd.IGRP tetramers and presented with the superagonist mimotope peptide NRP-V7 (28), whereas those treated with the scKd.TUM tetramers retained their responsiveness to the mimotope (Fig. 4C). Therefore, the binding of the single-chain pMHC tetramers to cells that have already been activated in vivo, in addition to causing cell death (Fig. 4B), may also render them nonresponsive even when presented with a superagonist peptide.
scKd.IGRP Tetramers Can Access Splenic and Islet-Infiltrating T Cells in Vivo.
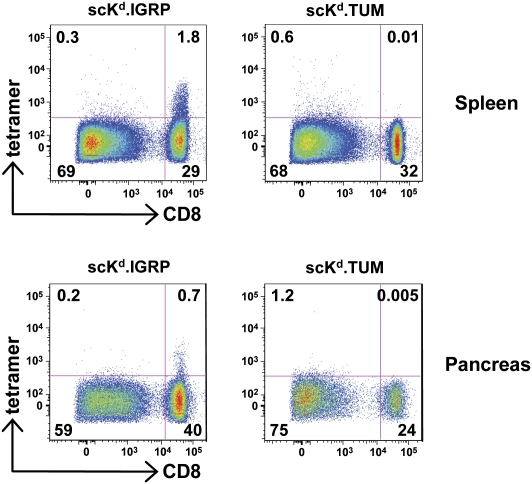
Given the ability of the scKd.IGRP tetramers to induce apoptosis or unresponsiveness of the cognate CD8+ T cells, it was of particular interest to determine whether they could be delivered in vivo. For these studies, we used tetramers prepared with PE-labeled streptavidin for the purpose of visualizing them in the different organs. The 8.3 TCR-transgenic mice were injected i.v. with the scKd.IGRP or the scKd.TUM PE-labeled tetramers. After 4 h of treatment, cells from the spleen and the pancreas were isolated and stained with anti-CD8 and analyzed by flow cytometry for PE-tetramer binding. CD8+ T cells in the spleen and the pancreas showed tetramer binding only in mice treated with the scKd.IGRP tetramers (Fig. 5), thus demonstrating the ability of this reagent to bind to cognate CD8+ T cells in vivo. These results also establish that the tetramers can traffic to the pancreas, the site of autoreactivity in type 1 diabetes, and bind the targeted cognate CD8+ T cells. Based on our in vitro results, this delivery would be expected to ultimately lead to apoptosis and/or the induction of unresponsiveness in this specific T-cell population.
Fig. 5.
scKd.IGRP tetramers access splenic and islet-infiltrating T cells in vivo. 8.3 TCR-transgenic NOD mice were injected i.v. with PE-labeled tetramers of scKd.IGRP or scKd.TUM. After 4 h, cell suspensions of the spleen and pancreas were stained with anti-CD8 and analyzed by flow cytometry for PE-tetramer binding. Numbers denote the percentage of cells present in the indicated quadrants of the dot plots.
Discussion
For naive and antigen-experienced T cells, TCR engagement by pMHC in the absence of the costimulatory signal provided by binding of CD28 to its ligands (CD80 and CD86) can result in T-cell anergy or apoptosis (6–9). This so-called two-signal hypothesis represents the rationale for a variety of therapeutic approaches that are currently being used or explored for the treatment of autoimmune disease (32). Although one such effective strategy is the use of CTLA4-Ig in the treatment of rheumatoid arthritis (33), antigen-specific approaches might be more desirable, as they would reduce the increased risk of infections and cancers that can accompany systemic immunosuppression. Single-chain pMHC class I molecules were originally designed to be used in the context of DNA vaccination to augment immune responses, as covalent linkage of the peptide would result in very stable cell surface expression of defined tumor- or pathogen-derived peptides (13, 14, 34). Their potential to manipulate autoreactive T cells in an antigen-specific manner when multimerized is underexplored. Here we demonstrate that these reagents possess considerable utility for this purpose, without the potential complication of peptide transfer to cells with costimulatory properties and the unintended activation of naive T cells (11, 12). Such reagents should pose advantages over both peptide therapy, which suffers from short peptide half-life in vivo (35) and the risk of anaphylaxis (36), and administration of antigen-coupled fixed syngeneic cells (37, 38), which will require ex vivo manipulation of a patient’s cells.
The apoptosis, as marked by phosphatidylserine externalization, that we observed in naive T cells treated with scKd.IGRP tetramers is consistent with the requirement for both antigen exposure and costimulation to support the survival of naive T cells (9). In contrast, the apoptosis observed upon treatment of CTLs with the cognate tetramers may be more akin to activation-induced cell death, such as that observed by others when CD8+ T-cell clones specific for foreign antigens were treated with multimeric pMHC complexes (29, 39). CD28 costimulation helps to promote the survival of activated T cells (6), and this costimulatory signal is not provided by pMHC multimers. However, we observed cell death in only a fraction of the treated autoreactive 8.3 CTLs, and the remaining population appeared to exhibit hyporesponsiveness to antigen, rather than enhanced effector function. These findings are consistent with the reported induction of anergy in cognate self-reactive CD4+ T cells upon treatment with class II pMHC dimers (40–43), and suggest that scKd.IGRP tetramers use multiple mechanisms to interfere with CTL survival and function.
Investigation of the therapeutic potential of pMHC multimers in autoimmune disease has focused almost exclusively on the targeting of CD4+ T cells (41–44). A notable exception is the use of nanoparticles coated with class I pMHC complexes in NOD-based mouse models of type 1 diabetes (45). Nanoparticle treatment can delete cognate T cells and bring about the expansion of low-avidity memory T cells that have autoregulatory functions, resulting in achievement of both disease prevention and reversal (45). Class II pMHC dimers have also been shown to have a beneficial effect, at least in part, by the fostering of T-cell populations that have regulatory or immunosuppressive properties (41, 42, 44). As we continue in vivo studies of our single-chain pMHC tetramers, the activities of induction of apoptosis and hyporesponsiveness we observed in vitro will be evaluated, as will the impact on regulatory T-cell populations. Although 8.3 TCR-transgenic mice will be used for some of this future work, even standard NOD mice, in which disease is caused by a variety of antigen specificities (46), will be amenable to study, as they have a substantial population of CD8+ T cells reactive to the IGRP206–214 peptide (17, 19, 20). Regardless of the mechanisms at work, we have demonstrated that a single, readily produced reagent is capable of inducing apoptosis of naive peptide-specific CD8+ T cells and differentiated CTLs, while at the same time modulating CTL activity. These findings suggest the potential of such reagents for both early and late intervention in the course of autoimmune disease progression.
Materials and Methods
Mice.
Male 8.3 TCR-transgenic NOD mice (18) were obtained from The Jackson Laboratory and crossed with NOD mice bred in house to obtain female 8.3 TCR-transgenic NOD mice for our experiments. AI4 TCR-transgenic NOD mice (47) were bred in house. All animals were bred and maintained under specific pathogen-free conditions at the Albert Einstein College of Medicine in accordance with protocols approved by the institutional animal care and use committee.
Cloning, Expression, and Purification of Single-Chain pMHC Monomers.
The single-chain constructs of scKd.IGRP and scKd.TUM were expressed and purified as described in SI Materials and Methods.
Crystallization and Structure Determination.
The crystal structure of scKd.IGRP was solved and analyzed as described in SI Materials and Methods.
Tetramer Preparation.
A BirA biotinylation sequence (GLNDIFEAQKIEWHE) was added to the C terminus of the single-chain construct to generate streptavidin-mediated tetramers. The protein was expressed and purified as described earlier and biotinylated by using the BirA enzyme following the manufacturer’s protocol (Avidity ); free biotin was removed by size-exclusion chromatography. Tetramers were prepared by adding streptavidin or PE-labeled streptavidin (BD Biosciences) at a ratio of four molecules of biotinylated scKd.IGRP to one molecule of streptavidin. The formation of tetramers was analyzed by size-exclusion chromatography using a Superdex 200 HR 10/30 prepacked column (Amersham Biosciences). Tetramers of scKd.TUM were prepared and characterized in an identical fashion.
Flow Cytometry.
Flow cytometric studies were performed with a FACSCalibur or LSR II device (BD Biosciences) and analyzed by using FlowJo software (Treestar). Labeled monoclonal antibodies to murine CD8α (53-6.7) and CD62L (MEL-14) were purchased from BD Biosciences.
In Vitro 8.3 CTL Generation.
To generate 8.3 CTLs in vitro, splenocytes from 8.3 TCR-transgenic NOD mice were cultured in the presence of mitomycin C-treated NOD splenocytes and 10 nM NRP-A7 at a ratio of 1:4. After 6 d of culture, live 8.3 CTLs were purified with Ficoll and used for experiments.
Pancreatic Islet Isolation and Culture of Islet-Infiltrating CTLs.
Islets were isolated after perfusion of the pancreas with collagenase P and cultured for 6 d in RPMI medium (RPMI 1640 supplemented with 1 mM sodium pyruvate, 28 μM β-mercaptoethanol, and nonessential amino acids) supplemented with 10% FBS and 50 U/mL recombinant human IL-2 as described previously (48).
Cell Death Assay.
Splenocytes or CTLs (1 × 106 cells/mL) were resuspended in RPMI medium containing 10% FBS and incubated in 100-μL aliquots at 37 °C for 3 h with 25 nM tetramers of scKd.IGRP or scKd.TUM or left untreated. Cells were washed and stained with FITC-labeled Annexin V and 7-amino-actinomycin D (7-AAD) according to the manufacturer’s protocol (BD Biosciences) and analyzed by flow cytometry. Dead (i.e., 7-AAD–positive) cells were excluded from analysis.
IFN-γ ELISPOT.
Islet infiltrates from 8.3 TCR-transgenic NOD mice were collected after 6 d of culture, resuspended in RPMI medium containing 10% FBS, and incubated in 100-μL aliquots at 37 °C for 3 h with 25 nM tetramers of scKd.IGRP or scKd.TUM or left untreated. ELISPOT plates (MAHA S45 10; Millipore) were precoated with anti-mouse IFN-γ mAb R4-6A2 (BD Pharmingen) and blocked with 1% BSA (Fraction V; Sigma-Aldrich). RMA-S cells engineered to express the MHC class I molecule H-2Kd (RMA-S/Kd; originally obtained as a gift from M. Bevan, University of Washington, Seattle, WA) were plated at a density of 2 × 104 cells per well and pulsed with 1 μM NRP-V7 peptide for 1 h at 26 °C. Islet-infiltrating T cells were cocultured with the peptide-pulsed antigen-presenting cells at 103 cells per well for 40 h at 37 °C. IFN-γ secretion was detected with a second, biotinylated anti-mouse IFN-γ mAb XMG1.2 (BD Pharmingen) and spots were developed by using streptavidin–alkaline phosphatase (Zymed Laboratories) and 5-bromo-4-chloro-3-indolyl-phosphate/nitroblue tetrazolium substrate (Sigma-Aldrich). Spots were counted using an automated ELISPOT reader system (Autoimmun Diagnostika).
In Vivo Delivery of scKd.IGRP Tetramers.
Tetramers of scKd.IGRP or scKd.TUM were prepared with PE-labeled streptavidin for the purpose of visualization by flow cytometry after in vivo administration. The 8.3 TCR-transgenic NOD mice were injected i.v. with 200 μL of a 1-μM solution of PE-labeled tetramers of scKd.IGRP or scKd.TUM. After 4 h, single-cell suspensions of spleen and pancreas were prepared. To do this, spleens were gently ground between frosted glass slides. Pancreata were cut into small pieces in the presence of a protease inhibitor mixture (Sigma-Aldrich) and digested in RPMI medium containing 5% FBS, 1 mg/mL collagenase IV (Sigma-Aldrich), 2 U/mL DNase I (Roche), and 1.5 U/mL heparin (Sigma-Aldrich) for 10 min at 37°C. Digested pancreatic tissue was then passed through a 40-μm cell strainer. Single-cell suspensions of spleen and pancreas were stained with anti-CD8 and analyzed by flow cytometry for PE–tetramer binding.
Supplementary Material
Acknowledgments
We thank the staff of the X29A beam line at the National Synchrotron Light Source; Hui Ziao and Wendy Zencheck for help with MS; and Jeffrey Babad, Eszter Lazar-Molnar, and Laura Santambrogio for their critical reading of the manuscript. This work was supported by National Institutes of Health (NIH) Grants AI007289 (to S.G.N. and S.C.A.), DK064315 (to T.P.D.), and DK020541 (to Albert Einstein College of Medicine’s Diabetes Research and Training Center). The flow cytometry facility at Albert Einstein College of Medicine is supported by NIH Cancer Center Grant CA013330.
Footnotes
The authors declare no conflict of interest.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 3NWM).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110971108/-/DCSupplemental.
References
- 1.Walter U, Santamaria P. CD8+ T cells in autoimmunity. Curr Opin Immunol. 2005;17:624–631. doi: 10.1016/j.coi.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Angelov GS, et al. Soluble MHC-peptide complexes containing long rigid linkers abolish CTL-mediated cytotoxicity. J Immunol. 2006;176:3356–3365. doi: 10.4049/jimmunol.176.6.3356. [DOI] [PubMed] [Google Scholar]
- 3.Cebecauer M, et al. Soluble MHC-peptide complexes induce rapid death of CD8+ CTL. J Immunol. 2005;174:6809–6819. doi: 10.4049/jimmunol.174.11.6809. [DOI] [PubMed] [Google Scholar]
- 4.Dal Porto J, et al. A soluble divalent class I major histocompatibility complex molecule inhibits alloreactive T cells at nanomolar concentrations. Proc Natl Acad Sci USA. 1993;90:6671–6675. doi: 10.1073/pnas.90.14.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Herrin SM, et al. Antigen-specific blockade of T cells in vivo using dimeric MHC peptide. J Immunol. 2001;167:2555–2560. doi: 10.4049/jimmunol.167.5.2555. [DOI] [PubMed] [Google Scholar]
- 6.Boise LH, et al. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. [PubMed] [Google Scholar]
- 7.Gimmi CD, Freeman GJ, Gribben JG, Gray G, Nadler LM. Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc Natl Acad Sci USA. 1993;90:6586–6590. doi: 10.1073/pnas.90.14.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 9.Van Parijs L, Ibraghimov A, Abbas AK. The roles of costimulation and Fas in T cell apoptosis and peripheral tolerance. Immunity. 1996;4:321–328. doi: 10.1016/s1074-7613(00)80440-9. [DOI] [PubMed] [Google Scholar]
- 10.Wang B, Maile R, Greenwood R, Collins EJ, Frelinger JA. Naive CD8+ T cells do not require costimulation for proliferation and differentiation into cytotoxic effector cells. J Immunol. 2000;164:1216–1222. doi: 10.4049/jimmunol.164.3.1216. [DOI] [PubMed] [Google Scholar]
- 11.Ge Q, et al. Soluble peptide-MHC monomers cause activation of CD8+ T cells through transfer of the peptide to T cell MHC molecules. Proc Natl Acad Sci USA. 2002;99:13729–13734. doi: 10.1073/pnas.212515299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schott E, Bertho N, Ge Q, Maurice MM, Ploegh HL. Class I negative CD8 T cells reveal the confounding role of peptide-transfer onto CD8 T cells stimulated with soluble H2-Kb molecules. Proc Natl Acad Sci USA. 2002;99:13735–13740. doi: 10.1073/pnas.212515399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitaksov V, et al. Structural engineering of pMHC reagents for T cell vaccines and diagnostics. Chem Biol. 2007;14:909–922. doi: 10.1016/j.chembiol.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu YY, Netuschil N, Lybarger L, Connolly JM, Hansen TH. Cutting edge: single-chain trimers of MHC class I molecules form stable structures that potently stimulate antigen-specific T cells and B cells. J Immunol. 2002;168:3145–3149. doi: 10.4049/jimmunol.168.7.3145. [DOI] [PubMed] [Google Scholar]
- 15.Nagata M, Santamaria P, Kawamura T, Utsugi T, Yoon JW. Evidence for the role of CD8+ cytotoxic T cells in the destruction of pancreatic β-cells in nonobese diabetic mice. J Immunol. 1994;152:2042–2050. [PubMed] [Google Scholar]
- 16.DiLorenzo TP, Serreze DV. The good turned ugly: immunopathogenic basis for diabetogenic CD8+ T cells in NOD mice. Immunol Rev. 2005;204:250–263. doi: 10.1111/j.0105-2896.2005.00244.x. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman SM, et al. Identification of the β cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc Natl Acad Sci USA. 2003;100:8384–8388. doi: 10.1073/pnas.0932778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verdaguer J, et al. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J Exp Med. 1997;186:1663–1676. doi: 10.1084/jem.186.10.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lieberman SM, et al. Individual nonobese diabetic mice exhibit unique patterns of CD8+ T cell reactivity to three islet antigens, including the newly identified widely expressed dystrophia myotonica kinase. J Immunol. 2004;173:6727–6734. doi: 10.4049/jimmunol.173.11.6727. [DOI] [PubMed] [Google Scholar]
- 20.Trudeau JD, et al. Prediction of spontaneous autoimmune diabetes in NOD mice by quantification of autoreactive T cells in peripheral blood. J Clin Invest. 2003;111:217–223. doi: 10.1172/JCI16409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarchum I, Nichol L, Trucco M, Santamaria P, DiLorenzo TP. Identification of novel IGRP epitopes targeted in type 1 diabetes patients. Clin Immunol. 2008;127:359–365. doi: 10.1016/j.clim.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mallone R, et al. CD8+ T-cell responses identify β-cell autoimmunity in human type 1 diabetes. Diabetes. 2007;56:613–621. doi: 10.2337/db06-1419. [DOI] [PubMed] [Google Scholar]
- 23.Standifer NE, et al. Identification of Novel HLA-A*0201-restricted epitopes in recent-onset type 1 diabetic subjects and antibody-positive relatives. Diabetes. 2006;55:3061–3067. doi: 10.2337/db06-0066. [DOI] [PubMed] [Google Scholar]
- 24.Wallny HJ, et al. Identification and quantification of a naturally presented peptide as recognized by cytotoxic T lymphocytes specific for an immunogenic tumor variant. Int Immunol. 1992;4:1085–1090. doi: 10.1093/intimm/4.10.1085. [DOI] [PubMed] [Google Scholar]
- 25.Mitaksov V, Fremont DH. Structural definition of the H-2Kd peptide-binding motif. J Biol Chem. 2006;281:10618–10625. doi: 10.1074/jbc.M510511200. [DOI] [PubMed] [Google Scholar]
- 26.Wilson IA, Fremont DH. Structural analysis of MHC class I molecules with bound peptide antigens. Semin Immunol. 1993;5:75–80. doi: 10.1006/smim.1993.1011. [DOI] [PubMed] [Google Scholar]
- 27.Anderson B, Park BJ, Verdaguer J, Amrani A, Santamaria P. Prevalent CD8+ T cell response against one peptide/MHC complex in autoimmune diabetes. Proc Natl Acad Sci USA. 1999;96:9311–9316. doi: 10.1073/pnas.96.16.9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amrani A, et al. Expansion of the antigenic repertoire of a single T cell receptor upon T cell activation. J Immunol. 2001;167:655–666. doi: 10.4049/jimmunol.167.2.655. [DOI] [PubMed] [Google Scholar]
- 29.Guillaume P, et al. Soluble major histocompatibility complex-peptide octamers with impaired CD8 binding selectively induce Fas-dependent apoptosis. J Biol Chem. 2003;278:4500–4509. doi: 10.1074/jbc.M208863200. [DOI] [PubMed] [Google Scholar]
- 30.Krishnamurthy B, et al. Autoimmunity to both proinsulin and IGRP is required for diabetes in nonobese diabetic 8.3 TCR transgenic mice. J Immunol. 2008;180:4458–4464. doi: 10.4049/jimmunol.180.7.4458. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, et al. In situ β cell death promotes priming of diabetogenic CD8 T lymphocytes. J Immunol. 2002;168:1466–1472. doi: 10.4049/jimmunol.168.3.1466. [DOI] [PubMed] [Google Scholar]
- 32.Podojil JR, Miller SD. Molecular mechanisms of T-cell receptor and costimulatory molecule ligation/blockade in autoimmune disease therapy. Immunol Rev. 2009;229:337–355. doi: 10.1111/j.1600-065X.2009.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schiff M. Abatacept treatment for rheumatoid arthritis. Rheumatology (Oxford) 2011;50:437–449. doi: 10.1093/rheumatology/keq287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen TH, Connolly JM, Gould KG, Fremont DH. Basic and translational applications of engineered MHC class I proteins. Trends Immunol. 2010;31:363–369. doi: 10.1016/j.it.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishioka GY, et al. Failure to demonstrate long-lived MHC saturation both in vitro and in vivo. Implications for therapeutic potential of MHC-blocking peptides. J Immunol. 1994;152:4310–4319. [PubMed] [Google Scholar]
- 36.Liu E, et al. Anti-peptide autoantibodies and fatal anaphylaxis in NOD mice in response to insulin self-peptides B:9-23 and B:13-23. J Clin Invest. 2002;110:1021–1027. doi: 10.1172/JCI15488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fife BT, et al. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203:2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niens M, et al. Prevention of “humanized” diabetogenic CD8 T-cell responses in HLA-transgenic NOD mice by a multipeptide coupled-cell approach. Diabetes. 2011;60:1229–1236. doi: 10.2337/db10-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu XN, et al. A novel approach to antigen-specific deletion of CTL with minimal cellular activation using α3 domain mutants of MHC class I/peptide complex. Immunity. 2001;14:591–602. doi: 10.1016/s1074-7613(01)00133-9. [DOI] [PubMed] [Google Scholar]
- 40.Appel H, Seth NP, Gauthier L, Wucherpfennig KW. Anergy induction by dimeric TCR ligands. J Immunol. 2001;166:5279–5285. doi: 10.4049/jimmunol.166.8.5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casares S, et al. Down-regulation of diabetogenic CD4+ T cells by a soluble dimeric peptide-MHC class II chimera. Nat Immunol. 2002;3:383–391. doi: 10.1038/ni770. [DOI] [PubMed] [Google Scholar]
- 42.Masteller EL, et al. Peptide-MHC class II dimers as therapeutics to modulate antigen-specific T cell responses in autoimmune diabetes. J Immunol. 2003;171:5587–5595. doi: 10.4049/jimmunol.171.10.5587. [DOI] [PubMed] [Google Scholar]
- 43.Zuo L, et al. A single-chain class II MHC-IgG3 fusion protein inhibits autoimmune arthritis by induction of antigen-specific hyporesponsiveness. J Immunol. 2002;168:2554–2559. doi: 10.4049/jimmunol.168.5.2554. [DOI] [PubMed] [Google Scholar]
- 44.Li L, Yi Z, Wang B, Tisch R. Suppression of ongoing T cell-mediated autoimmunity by peptide-MHC class II dimer vaccination. J Immunol. 2009;183:4809–4816. doi: 10.4049/jimmunol.0901616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsai S, et al. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity. 2010;32:568–580. doi: 10.1016/j.immuni.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 46.Chaparro RJ, DiLorenzo TP. An update on the use of NOD mice to study autoimmune (Type 1) diabetes. Expert Rev Clin Immunol. 2010;6:939–955. doi: 10.1586/eci.10.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graser RT, et al. Identification of a CD8 T cell that can independently mediate autoimmune diabetes development in the complete absence of CD4 T cell helper functions. J Immunol. 2000;164:3913–3918. doi: 10.4049/jimmunol.164.7.3913. [DOI] [PubMed] [Google Scholar]
- 48.Jarchum I, Takaki T, DiLorenzo TP. Efficient culture of CD8+ T cells from the islets of NOD mice and their use for the study of autoreactive specificities. J Immunol Methods. 2008;339:66–73. doi: 10.1016/j.jim.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.