Abstract
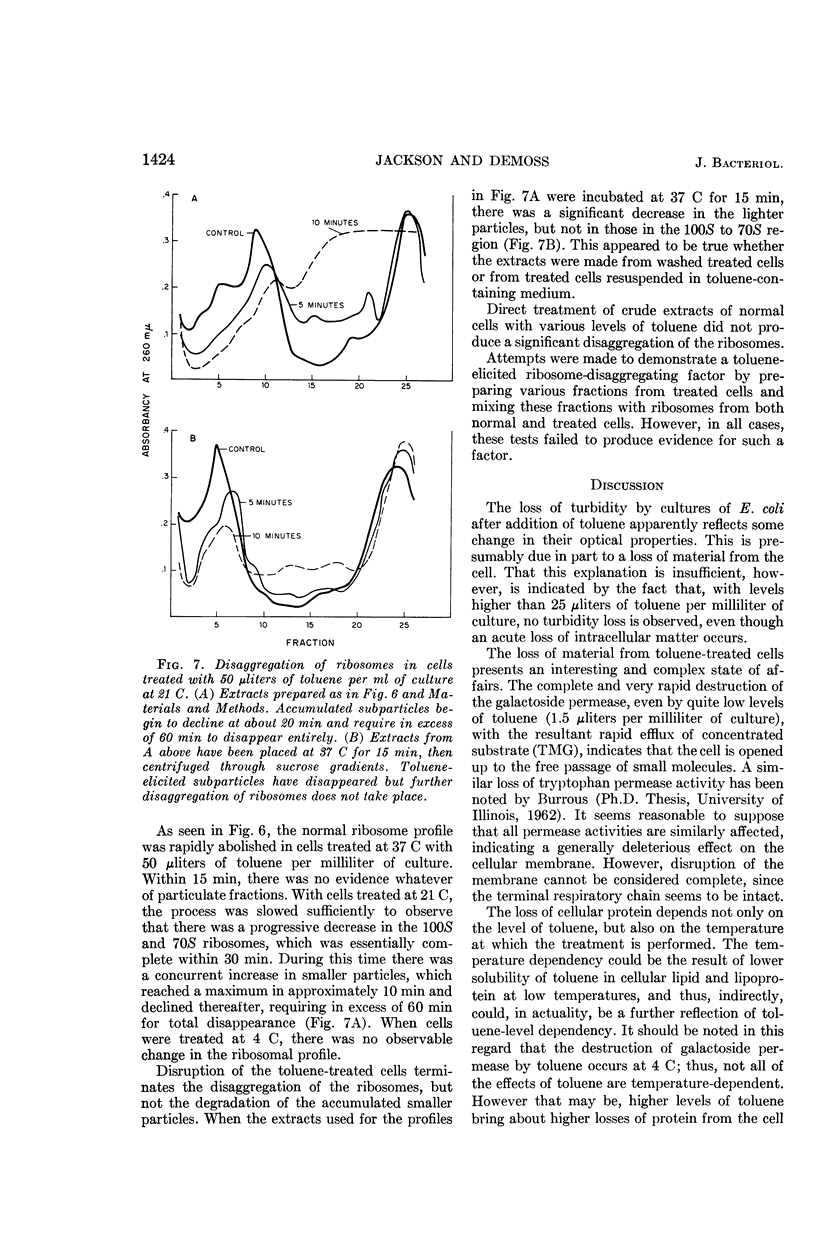
Jackson, Robert W. (University of California, San Diego, La Jolla), and J. A. DeMoss. Effects of toluene on Escherichia coli. J. Bacteriol. 90:1420–1425. 1965.—When toluene is added at appropriate levels to exponentially growing cultures of Escherichia coli, a time-dependent loss of turbidity is observed which is concurrent with a loss of material to the medium and with unmasking of β-galactosidase. In addition, the galactoside permease system is totally destroyed. Electron micrographs confirm the indications that the cells are not being lysed by toluene, although the cytoplasm collapses to the interior of the cell. Included in the material lost from the cell after toluene treatment is 85% of the total ribonucleic acid (RNA), the principal source of which appears to be the ribosomes. The loss of RNA is temperature-dependent. Protein is also lost to the medium as a function of both temperature and available toluene. Up to 25% of the total protein is found in the medium, the precise amount depending on the level of toluene employed. Zone centrifugation studies of extracts from treated cells indicate that toluene elicits a rapid disaggregation of ribosomes that is terminated, at any stage, by disruption of the cells. The disaggregation is temperature-dependent and does not occur at 4 C. It appears to be distinct from the actual degradation of ribosomal RNA and is accompanied by an accumulation of small particles during the initial phases of treatment at 21 C. Toluene added to crude extracts of normal E. coli cells is unable to cause detectable ribosome destruction.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DOBROGOSZ W. J., DEMOSS R. D. INDUCTION AND REPRESSION OF L-ARABINOSE ISOMERASE IN PEDIOCOCCUS PENTOSACEUS. J Bacteriol. 1963 Jun;85:1350–1355. doi: 10.1128/jb.85.6.1350-1355.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERZENBERG L. A. Studies on the induction of beta-galactosidase in a cryptic strain of Escherichia coli. Biochim Biophys Acta. 1959 Feb;31(2):525–538. doi: 10.1016/0006-3002(59)90029-0. [DOI] [PubMed] [Google Scholar]
- LEDERBERG J. The beta-d-galactosidase of Escherichia coli, strain K-12. J Bacteriol. 1950 Oct;60(4):381–392. doi: 10.1128/jb.60.4.381-392.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINTHAL C., SIGNER E. R., FETHEROLF K. Reactivation and hybridization of reduced alkaline phosphatase. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1230–1237. doi: 10.1073/pnas.48.7.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sypherd P. S., Strauss N. CHLORAMPHENICOL-PROMOTED REPRESSION OF beta-GALACTOSIDASE SYNTHESIS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1963 Mar;49(3):400–407. doi: 10.1073/pnas.49.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]