ATP-sensitive K+ (KATP) channels are nucleotide-gated bioenergy sensors that enable high-fidelity feedback communication between cellular energy dynamics and membrane electrical activity.1-4 Particularly dense in cardiac sarcolemma where they are composed through heteromultimeric assembly of inwardly rectifying K+ channel pores, typically KCNJ11-encoded Kir6.2 proteins, and regulatory ATP-binding cassette proteins, namely ABCC9-encoded SUR2A subunits, KATP channels are increasingly recognized as integral to tissue energy conservation and optimization of energy use.5 Integrated with intracellular energy pathways, sarcolemmal KATP channels are established cardioprotectors implicated in the sustenance of wellness.6-8
Knockout of the Kir6.2 KATP channel pore induces inefficient cardiac energetics associated with altered metabolic fuel selection and remodeling of the myocardial proteome, highlighting a distinct role of the channel in heart energy homeostasis.9,10 KATP channel ablation alters the expression of one tenth of largely metabolism-related protein species, exposing within the KATP channel-deficient ventricle markers of cardiovascular disease susceptibility.9,11 Indeed, disruption of the Kir6.2 KATP channel compromises cardiac protection afforded by ischemic preconditioning, impairs myocardial tolerance to sympathetic surge, and aggravates the impact of endurance challenge or hemodynamic overload precipitating heart failure under stress.12 Conversely, overexpression of channel subunits generates a protective phenotype at cellular and organ levels.13,14
High-throughput molecular technologies applied to phenotypically characterized patient and population cohorts have contributed to the deciphering of human KATP channelopathies, disease entities caused by genetic disruption of ion channel function.15-19 In cardiovascular medicine, mutations in the regulatory SUR2A subunit have been linked to KATP channelopathy-associated electrical and cardiomyopathic disorders, namely the syndromes of adrenergic atrial fibrillation and dilated cardiomyopathy with tachycardia.19-21 Moreover, in clinical heart failure, a common polymorphism in the Kir6.2 KATP channel pore subunit has been identified as a robust biomarker for impaired performance in stress-test.22 Furthermore, the Kir6.2 K23 allele, present in over half the population, has been pinpointed as an independent risk factor for susceptibility to maladaptive cardiac remodeling in hypertension.23 Cardiovascular disorders associated with genetic variation in KATP channel genes also include myocardial infarction and ventricular fibrillation. Collectively, advances in molecular medicine have enabled a growing understanding of genetically-determined channel (mal)function, underscoring the broadening awareness of the impact of the KATP channel complex on individual and public cardiovascular health.
KATP channel complexes: Nucleotide-gated heteromultimers
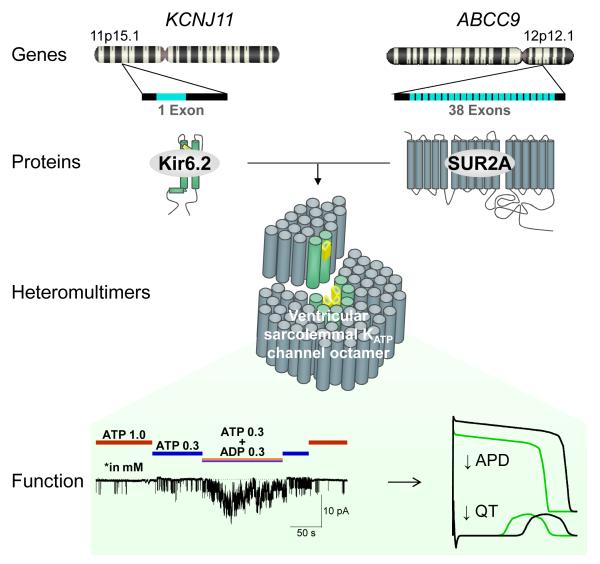
KATP channels were originally identified in the plasma membrane of cardiomyocytes,24 and shortly thereafter in other metabolically active tissues, such as pancreatic β-cells, as well as in subcellular compartments, including mitochondria.2,4,25,26 Plasmalemmal KATP channels, the focus of this overview, are defined as compulsory heteromultimers formed by octameric assembly of inward-rectifier K+ channels (Kir6.1/Kir6.2) with ATP-binding-cassette (ABC) proteins (SUR1/SUR2A/SUR2B).27-32 Human Kir6.1 and Kir6.2 genes – KCNJ8 and KCNJ11 – map to chromosome 12p11.23 and 11p15.1, and comprise two and one coding exons respectively. SUR genes, SUR1 (or ABCC8) at locus 11p15.1 and SUR2 (or ABBC9) at locus 12p12.1 comprise 39 and 38 exons respectively (Fig. 1). Alternative splicing gives rise to SUR2 protein variants, SUR2A and SUR2B. Tissue diversity, cellular distribution, and regulatory specificity are ensured by the assortment of subunit and isoform combinations. Biophysical properties are largely shared among plasmalemmal KATP channels, and include potassium selectivity and inward rectification imparted by the Kir protein, whereas responsiveness to cellular energetic signals is conferred by nucleotide interaction with both Kir and SUR channel subunits.33
Figure 1. Structure and function of ventricular sarcolemmal KATP channel complexes.
The pore-forming KCNJ11-encoded Kir6.2 subunit assembles with the regulatory ABCC9-encoded SUR2A protein to form heteromultimeric KATP channels abundantly expressed in the ventricular sarcolemma. The defining feature of KATP channel operation is adenine nucleotide-dependent gating, ensuring high-fidelity coupling between the cellular energetic state and membrane electrical activity. Intracellular ATP keeps KATP channels closed under normal conditions, while ADP promotes channel opening in response to metabolic challenge. KATP channel opening under stress translates into shortening of the cardiac action potential (↓APD) and accelerated repolarization (↓QT).
Kir6.2 is integral in the make-up of myocellular KATP channels, and targeted disruption of KCNJ11 generates a Kir6.2-deficient state characterized by lack of functional KATP channels in ventricular myocytes.34 Sarcolemmal KATP channels in the ventricle are composed primarily through co-assembly of Kir6.2 and SUR2A subunits (Fig. 1). Indeed, recombinant Kir6.2/SUR2A channels match features of native ventricular myocyte KATP channels.35 The molecular channel architecture may however exhibit plasticity, in particular in response to metabolic challenge. Kir6.1 mRNA expression, for example, is significantly elevated following ischemia or hypoxia, raising the possibility that Kir6.1 may be upregulated in myocytes in response to stress.36 Moreover, a disparate structure of atrial compared to ventricular KATP channels has been proposed, implicating SUR1-based channels in atrial specific functions, such as coupling atrial stretch with secretion of the atrial natriuretic peptide.37,38
Crosstalk of cardiac KATP channel proteins with myocellular energetics is facilitated by privileged associations of channel subunits with phosphotransfer and glycolytic enzymes.39 Metabolism-related proteins with established links to the cardiac KATP channel include creatine kinase, adenylate kinase, glyceraldehyde-3-phosphate dehydrogenase, lactate dehydrogenase, long chain acyl-CoA dehydrogenase, pyruvate kinase, and triosephosphate isomerase.40-45 More broadly, over 100 KATP channel-dependent proteins have been identified through deconvolution of the KATP channel knockout heart proteome.8-10 Categorization of proteomic changes in the KATP channel-deficient myocardium have demonstrated a highly represented metabolic theme that includes members of the tricarboxylic acid cycle, fatty acid β-oxidation, glycolysis, as well as amino acid and nucleic acid metabolism.8-10
ATP/ADP modulation of channel function is a defining property of KATP channels as metabolic sensors (Fig. 1).5,8,33,46 The interface between Kir6.2 subunits is critical for ATP-mediated pore inhibition.47 Nucleotide binding domains (NBD1 and NBD2) of SUR2A harbor intrinsic ATPase activity, endowing this regulatory KATP channel subunit with the ability to modulate ATP-induced Kir6.2 pore inhibition and thereby K+ efflux to reduce myocyte excitability.48-52 It is thought, that by relying on the intactness of cooperative NBD1/2 interaction, a stabilized ADP-bound post-hydrolytic conformation at NBD2 of SUR in the presence of Mg2+, promotes KATP channel opening.1,53-55 Furthermore, the nucleotide-bound conformations of SUR mediate the regulation of KATP channel activity by pharmacological agents, such as sulfonylurea inhibitors and potassium channel openers (KC0).33,55
KATP channels: Stress without distress
Vital in the adaptive response to metabolic stress, cardioprotective KATP channels are a recognized energy-sparing system that limits muscle energy expenses during the propagation of action potentials.7,8,33,56 Defined as the most densely expressed K+ channels in the myocardium, KATP channels are critical endogenous elements for cardiac energy homeostasis and electrical stability across a spectrum of stress conditions, including acute ischemia, the “fight-or-flight” response, chronic exertion, and heart failure.12,19,57
Ischemic stress
In ischemia, KATP channel opening shortens the duration of cardiac action potential (Fig. 1) and controls Ca2+ influx.58,59 Sarcolemmal KATP channel activation is apparently responsible for the electrical current that underlies ST-segment elevation of transmural ischemic injury, and has been implicated in protection afforded by ischemic preconditioning.12 Ablation of KATP channels disrupts the homeostatic mechanism integral to energetic myocardial stability under ischemic stress. Specifically, in the Kir6.2-knockout mouse following transmural anterior myocardial infarction, absence of significant and sustained ST-segment change has been documented and contrasts the wild-type counterpart that demonstrates prompt and pronounced ST-segment elevation following ischemic injury.60 KATP channel activity in ischemia appears to have a diagnostic implication of clinical significance. In particular, patients with diabetes mellitus presenting with acute myocardial infarction demonstrate attenuated ST-segment elevation when taking sulfonylureas, inhibitors of KATP channel activity, resulting in a failure to meet criteria for emergent revascularization therapy and, as a consequence, inappropriate withholding of proven beneficial therapy.12 Moreover, KATP channels have been implicated in the ischemic preconditioning mechanism by which exposure to brief ischemia preceding a sustained ischemic insult reduces subsequent infarct size.61 Analogous to ischemic preconditioning, pharmacologic activation of the channel by KATP channel openers has also protective benefit.62,63 Both ischemic and pharmacologic preconditioning are abolished in the absence of Kir6.2-containing KATP channels.64,65 Absence of sarcolemmal KATP channel activity has negative effects on cardiac relaxation and contractility under acute ischemic stress. In parallel, knockout of Kir6.2 negates protection afforded by ischemic preconditioning on myocardial energy generation, transfer and utilization.66 Total ATP turnover, a global parameter of energy demand, fails to increase in the ischemic-preconditioned KATP channel knockout as opposed to the wild-type, correlating with failure of preconditioned hearts lacking KATP channels to functionally recover.66 The KATP channel-dependent cardioprotective potential is underscored by improved myocardial function during post-ischemic reperfusion following introduction of the SUR2A transgene into the SUR2 null cardiomyocyte.67
Fight-or-flight response
Beyond protection against the insult of ischemia, KATP channels serve as guarantors of metabolic and ionic homeostasis, appearing central to cardiac participation in the general adaptation syndrome.57 In the “fight-or-flight” response, metabolic adaptations in bodily functions achieve a superior level of performance necessary to cope with the demands of imposed stress. The sustenance of augmented performance requires energy-controlling mechanisms, such as the KATP channel, ensuring that the reaction to stress itself does not become harmful, i.e., “stress without distress”.57 A common trigger of the general adaptation syndrome is systemic sympathetic stimulation that augments cardiac contractility and heart rate, and thereby provides the necessary higher cardiac output to meet demand. Enhanced cardiac output imposes a significant metabolic load on the heart. A compensatory increase in outward K+ current activates when the sustained augmentation of heart muscle performance competes with the ability of cellular energetics to maintain contractile and electrical stability. The KATP channel-mediated action potential shortening limits actomyosin ATP consumption by reducing Ca2+ influx, thereby restraining energy utilization to ensure functional and structural cellular integrity.68 Under sympathetic distress, hearts without KATP channels lack stress-induced cardiac action potential shortening predisposing to cytosolic Ca2+ overload associated with development of contractile dysfunction, and possibly death.68 On autopsy, contraction bands, pathognomonic of cytosolic Ca2+ loading, are visible throughout the myocardium of the Kir6.2-knockout but not wildtype.68 Measurement of oxygen consumption has revealed that increased workloads produce only moderate elevation of energy expenditure, in line with KATP channel-dependent shortening of action potential duration.56 Conversely, absence of KATP channel-driven action potential shortening in KATP channel-deficient hearts precipitates significant elevation in oxygen consumption.56
Under catecholamine challenge, action potential prolongation remains uncompensated in the absence of KATP channel function predisposing the myocardium to early afterdepolarizations.69 This deficit in repolarization reserve translates into a high risk for induction of triggered activity and ventricular dysrhythmia.69 Intact KATP channel function appears thus mandatory for adequate repolarization under sympathetic stress providing electrical tolerance against triggered arrhythmia. Proarrhythmogenic features and lack of adaptation to stress in transgenic mice with cardiac myocyte-specific ablation of KATP channels verifies that these features are intrinsic to the myocardium, and that KATP channel function has an essential role in protecting the heart from lethal arrhythmias ensuring stress adaptation.70 Moreover KATP channels may provide a vital feedback element for cardiovascular tolerance in sepsis, where the systemic inflammatory response to infection imposes a high demand for bodily adaptation.71 In a model of acute septic shock, induced by endotoxin lipopolysaccharide challenge, knockout of the KCNJ8 gene encoding the typically vascular Kir6.1 KATP channel pore predisposes to an early and profound survival disadvantage. The exaggerated susceptibility provoked by disruption of Kir6.1-containing channels was linked to progressive deterioration in cardiac activity, ischemic myocardial damage, and contractile dysfunction.71 KATP channels, harnessing the ability to recognize alterations in the cellular energy state and to translate this information into changes in membrane excitability, provide therefore a link necessary for maintaining myocardial well being in the face of stress-induced energy demanding augmentation in performance.
Chronic exertion
Exercise training elicits an array of metabolic responses that underlie fitness. Mice lacking KATP channels when challenged with a regimented training protocol failed to manifest improved exercise capacity.72 Repetitive exercise-stress unmasks a survival disadvantage in the Kir6.2-knockout associated with cardiac damage, implicating KATP channel activity in achieving physiologic benefits of exercise training without accumulating deficits.72 Even modest stress following a repetitive physical exertion program provokes significant mortality in the Kir6.2-knockout with death occurring during or suddenly in the immediate post-exercise period,72 perhaps representing manifestation of stress-induced dysrhythmia, to which Kir6.2-knockout are predisposed.69,70 Exercise intolerance has also been documented in the setting of ablation of the regulatory SUR2 subunit,73 underscoring the role of KATP channels in ensuring optimal performance. Beyond physical exercise, in distinct hyperadrenergic states exemplified by cocaine abuse, KATP channel deletion amplifies poor cardiovascular outcome while promotion of channel activity by potassium channel opening drugs improves survival.74,75
Experimental heart failure
Intact KATP channels prevent the transition from a state of disease risk to that of overt organ failure. In experimental hypertension, induced by volume overload, knockout of Kir6.2 KATP channel predisposes to heart failure and death.76 Defective decoding of hypertension-induced metabolic distress signals in the KATP channel knockout sets in motion pathological Ca2+ overload and aggravates cardiac remodeling through a calcium/calcineurin-dependent cyclosporine-sensitive pathway, implicating intact channel activity as a required safety element preventing hypertension-induced heart failure.76 Similarly, in experimental models of pressure overload, such as that imposed by transverse aortic constriction, compromised KATP channel function renders the heart vulnerable to poor outcome.77 The constricted KATP channel knockout displays fulminant biventricular congestive heart failure, characterized by exercise intolerance, cardiac contractile dysfunction, hepatopulmonary congestion and death. Surviving KATP channel knockouts develop sequelae, including exaggerated fibrotic myocardial hypertrophy associated with nuclear up-regulation of calcium-dependent pro-remodeling MEF2 and NF-AT pathways, precipitating chamber dilatation.77 Moreover, it has been documented that disease-induced KATP channel metabolic dysregulation, even in the absence of channel gene defect, is a contributor to the pathobiology of heart failure, illustrating a mechanism for acquired channelopathy.78 Thus, operational KATP channels appear mandatory in securing cardiac adaptation and protecting against heart failure.12
Human cardiac KATP channelopathies
Genetically-determined KATP channel malfunction has been originally linked to insulin secretory disorders, namely congenital hyperinsulinism and neonatal diabetes.4,17-19,79,80 Beyond isolated failure of pancreatic β-cells, KATP channel mutations are also pathogenic in the DEND syndrome, characterized by varying degrees of delayed speech/motor development, epilepsy, neonatal diabetes, muscle hypotonia, and balance issues.17-19 An even broader role in disease pathogenesis has been realized with the discovery of KATP channel malfunction in human skeletal myopathies.81,82 In cardiovascular medicine, KATP channelopathies have been associated with atrial fibrillation and dilated cardiomyopathy with tachycardia, as well as phenotypic modifiers of preclinical and overt heart disease (Fig. 2).19
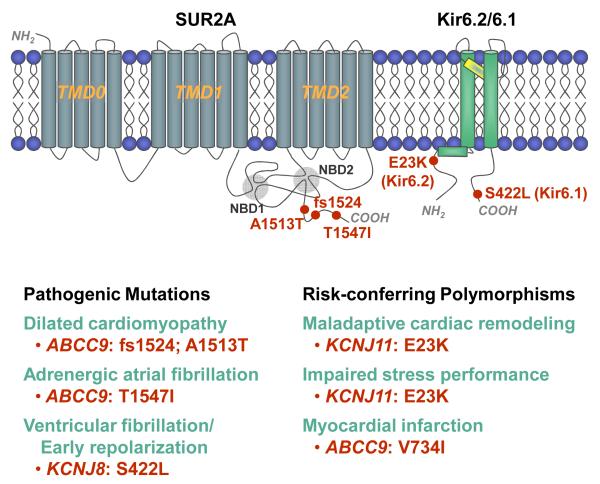
Figure 2. Pathogenic mutations and risk-conferring polymorphisms in KATP channel genes associated with human cardiac disorders.
Topology of Kir6.2/Kir6.1 and SUR2A (with nucleotide binding domain NBD1/NBD2 and transmembrane domains TMD0-TMD1-TMD2) subunits, with mapped locations of variant sites underlying KATP channelopathies.
Adrenergic atrial fibrillation
Atrial fibrillation is increasingly recognized as having genetic underpinnings.83,84 A case in point are the early onset cases in a subset of patients attributable to monogenic defects. The paradigm of a heritable basis for atrial fibrillation is exemplified by reports of familial disease attributed to gain-of-function or loss-of-function mutations in ion channel genes predicted to accelerate or slow repolarization. In these cases, channel malfunction creates an arrhythmogenic substrate of re-entry or triggered activity caused by reduced electrical refractoriness or after-depolarization, respectively. Initially, channelopathy-based atrial fibrillation predicted shortening of the action potential duration and proarrhythmogenic reduction in refractory period as mechanisms of arrhythmia.85,86 An alternative mechanism for atrial fibrillation, namely increased propensity for prolongation of action potential duration and triggered activity in the human atrium, was identified for a loss-of-function mutation in KCNA5, encoding the voltage-dependent Kv1.5 channel.87 A possibly equivalent mechanism has been reported in the case of a KATP channel mutation conferring risk for adrenergic atrial fibrillation originating from the vein of Marshall.20 The mutation was identified in a middle-aged patient who, in the absence of identifiable risk factors, presented with long-standing atrial fibrillation precipitated by activity and refractory to medical therapy. In this patient with early-onset atrial fibrillation and an overtly normal heart, adrenergic stress as a possible trigger was investigated using a candidate gene approach and invasive electrophysiologic testing under sympathomimetic challenge.20 The focal source of rapidly firing electrical activity was mapped to the vein of Marshall, a remnant of the left superior vena cava rich in sympathetic fibers and a recognized source for adrenergic atrial fibrillation. Although this potentially arrhythmogenic veno-atrial interface is present in the population at large, it does not trigger arrhythmia in the majority of individuals despite comparable environmental stress exposure. It was postulated that the patient was vulnerable to adrenergic atrial fibrillation due to an inherent defect in electrical stability.20
Molecular genetic investigation demonstrated a missense mutation in ABCC9, encoding the regulatory subunit of cardiac KATP channels (Fig. 2).20 Identified in exon 38, specific for the cardiac splice variant of SUR2A, this heterozygous c.4640C>T transition caused substitution of the threonine residue at amino acid position 1547 with isoleucine (T1547I). Protein alignments revealed that the missense substitution altered the amino acid sequence of the evolutionarily conserved carboxy-terminal tail. Homology modeling mapped the defect adjacent to the signature Walker motifs of the nucleotide binding domain, required for coordination of adenine nucleotides in the nucleotide binding pocket. Removal of the polar threonine (T1547) and replacement with the larger aliphatic and highly hydrophobic isoleucine, as would occur in this patient, predicted compromised nucleotide-dependent KATP channel gating.20
Patch-clamp recording demonstrated that the T1547I substitution compromised adenine nucleotide-dependent induction of KATP channel current.20 Mutant T1547I SUR2A, co-expressed with the KCNJ11-encoded Kir6.2 pore, generated an aberrant channel that retained ATP-induced inhibition of potassium current, but demonstrated a blunted response to ADP. A deficit in nucleotide gating, resulting from the T1547I mutation, would compromise the homeostatic role of the KATP channel required for proper readout of cellular distress and maintenance of electrical stability.
The pathogenic link between channel malfunction and adrenergic atrial fibrillation was verified, at the whole organism level, in a murine knockout model deprived of operational KATP channels. Compared with the normal atrium, resistant to arrhythmia under adrenergic provocation, vulnerability to atrial fibrillation was recapitulated in the setting of a KATP channel deficit.20 Thus a lack of intact KATP channels, either due to a naturally occurring mutation affecting channel regulation or a targeted disruption of the channel complex, is a substrate for atrial electrical instability under stress, and a molecular risk factor for adrenergic atrial fibrillation.
Once the vein of Marshall had been isolated by radiofrequency ablation, atrial fibrillation could no longer be provoked by programmed electrical stimulation and burst pacing with or without isoproterenol infusion.20 This case demonstrates that vulnerability to arrhythmia can be caused by an inability of mutant KATP channels to safeguard against adrenergic stress-induced ectopy. The apparently curative outcome was achieved by disrupting the gene-environment substrate for arrhythmia conferred by the underlying KATP channelopathy.19
Risk factor for electrical instability
While the case underscores heritable channel dysfunction in lone atrial fibrillation, KATP channel deficit could play a broader role in the pathogenesis of electrical instability. Gene expression and electrophysiological studies in patients with atrial fibrillation demonstrate altered atrial ion channel mRNA transcription and post-translational activity, including downregulation of the KATP channel pore and associated current.88,89 Moreover, structural heart disease and/or atrial dilation may compromise metabolic and mechanosensitive gating of KATP channels,90-92 precipitating a suboptimal repolarization reserve and providing a substrate for the more common acquired form of atrial fibrillation.
KATP channel alteration may also impact predisposition towards ventricular vulnerability to arrhythmia. In this regard, a case of ventricular fibrillation with prominent early repolarization was recently reported in a young patient who was resuscitated following an episode of sudden death. Subsequent, unrelenting ventricular fibrillation was unresponsive to several classes of antiarrhythmics prior to rhythm restoration with quinidine.93 Myopathic and coronary heart disease were excluded, and a KATP channel subunit amino acid substitution, namely the S422L variant of the KCNJ8 gene encoding Kir6.1, identified (Fig. 2).93 The gain-of-function KATP channel variant was further linked to the pathogenic substrate of pleiotropic J-wave abnormalities, in single Brugada syndrome and early repolarization syndrome cases.94
Dilated cardiomyopathy with tachycardia
Beyond isolated arrhythmias, KATP channelopathy has been implicated in a syndrome of cardiomyopathy with ventricular arrhythmia. The ontological spectrum of cardiomyopathy-associated mutant gene products has encompassed the fundamental components of excitation-contraction coupling such as contractile, cytoskeletal, and myocellular ion regulatory proteins.95 Human molecular genetic studies have also linked KATP channel defects and aberrant homeostatic stress response in the pathogenesis of dilated cardiomyopathy.19 These defects, identified in the regulatory KATP channel subunit, impair channel-dependent decoding of cellular metabolic state, establishing a previously unrecognized mechanism in human heart failure.18
The cardiomyopathic-arrhythmia syndrome characterized by the triad of dilated cardiomyopathy, ventricular arrhythmia, and ABCC9 KATP channel mutations has been designated CMD1O (OMIM #608569; Fig. 2),21 and salient phenotypic traits were reproduced by KATP channel knockout under imposed stress.77 Clinically, this entity was reported in middle-aged patients with marked left ventricular enlargement, severe systolic dysfunction, and ventricular tachycardia. In these patients, heterozygous mutations were identified in exon 38 of ABCC9, which encodes the C-terminal domain of the SUR2A channel subunit, specific to the cardiac splice variant. DNA sequencing of a mutated allele identified a 3-bp deletion and 4-bp insertion mutation (c.4570-4572delTTAinsAAAT), causing a frameshift at L1524 and introducing four anomalous terminal residues followed by a premature stop codon (fs1524).21 Another mutated allele harbored a missense mutation (c.4537G>A) causing the amino acid substitution A1513T. The identified frameshift and missense mutations occurred in evolutionarily conserved domains of SUR2A, and neither mutation was present in unrelated control individuals.21
The identified missense and frameshift mutations were mapped to domains bordering the catalytic ATPase pocket within SUR2A. Structural molecular dynamics simulation showed that residues A1513 and L1524 flank the C-terminal β-strand in close proximity to the signature Walker A motif, required for coordination of nucleotides in the catalytic pocket of ATP-binding cassette proteins.21 Replacement of A1513 with a sterically larger and more hydrophilic threonine residue or truncation of the C terminus caused by the fs1524 mutation would disrupt folding of the C-terminal β-strand and, thus, the tertiary organization of the adjacent second nucleotide binding domain (NBD2) pocket in SUR2A (Fig. 2). Indeed, ATP-induced KATP channel gating was aberrant in channel mutants, suggesting that structural alterations induced by the mutations A1513T and fs1524 of SUR2A distorted ATP-dependent pore regulation.21 Thus, the mutations A1513T and fs1524 compromise ATP hydrolysis at SUR2A NBD2, generating distinct reaction kinetic defects. Aberrant catalytic properties in the A1513T and fs1524 mutants translated into abnormal interconversion of discrete conformations in the NBD2 ATPase cycle. Alterations in hydrolysis-driven SUR2A conformational probability induced by A1513T and fs1524 perturbed intrinsic catalytic properties of the SUR2A ATPase, compromising proper translation of cellular energetic signals into KATP channel-mediated membrane electrical events. Traditionally linked to defects in ligand interaction, subunit trafficking or pore conductance, human cardiac KATP channel dysfunction provoked by alterations in the catalytic module of the channel complex establishes a new mechanism for channelopathy.
Risk factor for cardiac remodeling
Susceptibility or resistance to heart failure, despite apparently similar risk load, is attributable to individual variation in homeostatic reserve. Following identification of mutations within a KATP channel gene in patients with dilated cardiomyopathy,21 the relationship between the common Kir6.2 E23K polymorphism (rs5219) and subclinical heart disease was investigated (Fig. 2).23 A community-based cross-sectional cohort of 2,031 predominantly Caucasian adults was utilized, for which detailed clinical and prospective echocardiographic data were available. Genotype frequencies were in Hardy–Weinberg equilibrium (EE = 44%; EK = 47%; KK = 9%) and similar to previously reported control populations. In the group at large, there was no significant association between genotypes and measures of cardiac structure/function (left ventricular dimensions, mass, and ejection fraction), electrical instability (atrial and ventricular arrhythmias), or metabolism (fasting glucose, diabetes, and body mass index) at enrollment. However, among individuals with documented hypertension at the time of echocardiography (n = 1,187), the KK genotype was significantly associated with greater left ventricular dimension and volume in both diastole and systole.23 A synergistic effect on left ventricular size of KK genotype and left ventricular mass, a marker of chronic cardiac stress load, further validated the impact of Kir6.2 E23K on cardiac structure in hypertension. From a public health perspective, hypertension is the most common risk factor for congestive heart failure, and left ventricular enlargement is an established precursor of symptomatic ventricular dysfunction. The Kir6.2 K23 allele, present in over half the population, is thus implicated as a risk factor for transition from hypertensive stress load to subclinical maladaptive cardiac remodeling.23 These findings, consistent with previous human and animal studies,19,76 uncover an interactive KATP channel gene-environment substrate that confers cardiac disease risk. Determining the overall impact of Kir6.2 E23K across ethnic groups and on long-term clinical outcome, i.e., progression to left ventricular enlargement and clinical heart failure, will require further study.
Biomarker for impaired stress performance
The translational significance of the Kir6.2 E23K polymorphism in human cardiac physiology was more recently explored in a cohort of patients with heart failure who underwent comprehensive exercise stress testing.22 The frequency of the minor K23 allele was found over-represented in the 115 subjects with congestive heart failure compared to the 2,031 community-based controls described above (69 vs. 56%, P < 0.001). Moreover, the KK genotype, present in 18% of heart failure patients, was associated with abnormal cardiopulmonary exercise stress test results.22 In spite of similar baseline heart rates at rest among genotypic subgroups, subjects with the KK genotype had a significantly reduced heart rate increase at matched workloads. Molecular modeling of the tetrameric Kir6.2 pore structure revealed the E23 residue within the functionally relevant intracellular slide helix region.22 Substitution of the wild-type E residue with an oppositely charged, bulkier K residue would potentially result in a significant structural rearrangement and disrupted interactions with neighboring Kir6.2 subunits, providing a basis for altered high-fidelity KATP channel gating, particularly in the homozygous state. Blunted heart rate response during exercise is a risk factor for mortality in patients with heart failure, establishing the clinical relevance of Kir6.2 E23K as a biomarker for impaired performance under exercise stress underscoring the essential role of KATP channels in human cardiac physiology.22
Association with myocardial infarction
Experimental evidence has also suggested that KATP channels could be involved in the pathogenesis of coronary vasomotor dysfunction and ischemic heart disease.96,97 The potential clinical significance of such a premise was documented in a cohort of patients with myocardial infarction at an early age, whereby a rare missense variant V734I in the ABCC9 SUR2A KATP channel gene was found overepresented (Fig. 2).98 Statistical significance was demonstrated after controlling for multiple established risk factors for coronary artery disease.98
Summary
Deficient cellular energetics set by aberrant KATP channel function is increasingly implicated in a spectrum of conditions underlying metabolic imbalance and electrical instability.5 Indeed, cardiac KATP channelopathies are emerging as a recognized disease entity underlying heart failure and arrhythmia.19 Understanding the molecular structure and function of KATP channel subunits,8 and their relationship to cellular metabolic signaling,99 has been instrumental in interpreting the pathophysiology of channel malfunction associated with heart disease predisposition (Fig. 3).12 From individual patients to populations, variants in KATP channel genes have now been documented in human dilated cardiomyopathy21, atrial fibrillation,20 and as risk factors for electrical instability,93,94 adverse cardiac remodeling,23 impaired performance under stress22 or myocardial infarction.98 Beyond the initial deciphering of genotype-phenotype relationships, development and application of high-throughput platforms to screen for disrupted coding and/or regulatory sequences in cardioprotective KATP channel genes, as well as diagnose corrupted interactions within the cellular milieu, would advance current knowledge regarding this homeostatic channel complex and its implications in cardiovascular medicine. In particular, deconvolution of altered metabolic pathways and signaling cascades associated with pathogenic KATP channel mutation may offer unique opportunities to pinpoint lesions that stratify the consequences of genetic variation on disease traits.18 In this regard, it can be anticipated that systems biology and network medicine strategies will be increasingly deployed to resolve the KATP channel interactome.11 Mapping of the systems integration of molecules and their respective biological networks in health versus disease will, in turn, guide the judicious development of prognostic discriminators of disease variability and selection of treatment response predictors.100-102 Advances in the molecular medicine of KATP channelopathies are thus poised to offer new perspectives in the diagnosis and therapy of individuals and populations.103-107
Figure 3. Advances in KATP channelopathies.
Over the last three decades, science has increasingly defined KATP channel structure and function at molecular, cellular, organ and organism levels. Today, new knowledge in KATP channelopathies informs the practice of cardiovascular medicine expanding the understanding of cardioprotection in health and disease.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health (HL064822, HL071225, HL083439), and Marriott Heart Disease Research Program.
Footnotes
DISCLOSURES None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alekseev AE, Hodgson DM, Karger AB, Park S, Zingman LV, Terzic A. ATP-sensitive K+ channel channel/enzyme multimer: Metabolic gating in the heart. J Mol Cell Cardiol. 2005;38:895–905. doi: 10.1016/j.yjmcc.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miki T, Seino S. Roles of KATP channels as metabolic sensors in acute metabolic changes. J Mol Cell Cardiol. 2005;38:917–925. doi: 10.1016/j.yjmcc.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- 4.Ashcroft FM. ATP-sensitive K+ channels and disease: from molecule to malady. Am J Physiol Endocrinol Metab. 2007;293:E880–E889. doi: 10.1152/ajpendo.00348.2007. [DOI] [PubMed] [Google Scholar]
- 5.Reyes S, Park S, Terzic A, Alekseev AE. KATP channels process nucleotide signals in muscle thermogenic response. Crit Rev Biochem Mol Biol. 2010;45:506–519. doi: 10.3109/10409238.2010.513374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorenz E, Terzic A. Physical association between recombinant cardiac ATP-sensitive K+ channel subunits Kir6.2 and SUR2A. J Mol Cell Cardiol. 1999;31:425–434. doi: 10.1006/jmcc.1998.0876. [DOI] [PubMed] [Google Scholar]
- 7.Zingman LV, Alekseev AE, Hodgson-Zingman DM, Terzic A. ATP-sensitive potassium channels: metabolic sensing and cardioprotection. J Appl Physiol. 2007;103:1888–1893. doi: 10.1152/japplphysiol.00747.2007. [DOI] [PubMed] [Google Scholar]
- 8.Flagg TP, Enkvetchakul D, Koster JC, Nichols CG. Muscle KATP channels: recent insights to energy sensing and myoprotection. Physiol Rev. 2010;90:799–829. doi: 10.1152/physrev.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arrell DA, Zlatkovic J, Kane GC, Yamada S, Terzic A. ATP-sensitive K+ channel knockout induces cardiac proteome remodeling predictive of heart disease susceptibility. J Proteome Res. 2009;8:4823–4834. doi: 10.1021/pr900561g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zlatkovic J, Arrell DK, Kane GC, Miki T, Seino S, Terzic A. Proteomic profiling of KATP channel-deficient hypertensive hearts maps risk for maladaptive cardiomyopathic outcome. Proteomics. 2009;9:1314–1325. doi: 10.1002/pmic.200800718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arrell DA, Lindor J Zlatkovic, Yamada S, Terzic A. KATP channel-dependent metaboproteome decoded. Cardiovasc Res. 2011 doi: 10.1093/cvr/cvr046. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kane GC, Liu XK, Yamada S, Olson TM, Terzic A. Cardiac KATP channels in health and disease. J Mol Cell Cardiol. 2005;38:937–943. doi: 10.1016/j.yjmcc.2005.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jovanovic N, Jovanovic S, Jovanovic A, Terzic A. Gene delivery of Kir6.2/SUR2A in conjunction with pinacidil handles intracellular Ca2+ homeostasis under metabolic stress. FASEB J. 1999;13:923–929. doi: 10.1096/fasebj.13.8.923. [DOI] [PubMed] [Google Scholar]
- 14.Du Q, Jovanovic S, Clelland A, Sukhodub A, Budas G, Phelan K, Murray-Tait V, Malone L, Jovanovic A. Overexpression of SUR2A generates a cardiac phenotype resistant to ischemia. FASEB J. 2006;20:1131–1141. doi: 10.1096/fj.05-5483com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ashcroft FM. ATP-sensitive potassium channelopathies: focus on insulin secretion. J Clin Invest. 2005;115:2047–2058. doi: 10.1172/JCI25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ashcroft FM. From molecule to malady. Nature. 2006;440:440–447. doi: 10.1038/nature04707. [DOI] [PubMed] [Google Scholar]
- 17.Remedi MS, Koster JC. KATP channelopathies in the pancreas. Pflugers Arch. 2010;460:307–320. doi: 10.1007/s00424-009-0756-x. [DOI] [PubMed] [Google Scholar]
- 18.Terzic A, Perez-Terzic C. Channelopathies: decoding disease pathogenesis. Sci Transl Med. 2010;2:42ps37. doi: 10.1126/scitranslmed.3001433. [DOI] [PubMed] [Google Scholar]
- 19.Olson TM, Terzic A. Human KATP channelopathies: diseases of metabolic homeostasis. Pflugers Arch. 2010;460:295–306. doi: 10.1007/s00424-009-0771-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olson TM, Alekseev AE, Moreau C, Liu XK, Zingman LV, Miki T, Seino S, Asirvatham SJ, Jahangir A, Terzic A. KATP channel mutation confers risk for vein of Marshall adrenergic atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2007;4:110–116. doi: 10.1038/ncpcardio0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bienengraeber M, Olson TM, Selivanov VA, Kathmann EC, O’Cochlain F, Gao F, Karger AB, Ballew JD, Hodgson DM, Zingman LV, Pang YP, Alekseev AE, Terzic A. ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nat Genet. 2004;36:382–387. doi: 10.1038/ng1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reyes S, Park S, Johnson BD, Terzic A, Olson TM. KATP channel Kir6.2 E23K variant overrepresented in human heart failure is associated with impaired exercise stress response. Hum Genet. 2009;126:779–189. doi: 10.1007/s00439-009-0731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reyes S, Terzic A, Mahoney DW, Redfield MM, Rodeheffer RJ, Olson TM. KATP channel polymorphism is associated with left ventricular size in hypertensive individuals: a large-scale community-based study. Hum Genet. 2008;123:665–667. doi: 10.1007/s00439-008-0519-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 25.Ardehali H, O’Rourke B. Mitochondrial KATP channels in cell survival and death. J Mol Cell Cardiol. 2005;39:7–16. doi: 10.1016/j.yjmcc.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi NQ, Ye B, Makielski JC. Function and distribution of the SUR isoforms and splice variants. J Mol Cell Cardiol. 2005;39:51–60. doi: 10.1016/j.yjmcc.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 27.Inagaki N, Gonoi T, Clement JP, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of I-KATP: An inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 28.Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- 29.Chutkow WA, Simon MC, LeBeau MM, Burant CF. Cloning, tissue expression, and chromosomal localization of SUR2, the putative drug-binding subunit of cardiac, skeletal muscle, and vascular KATP channels. Diabetes. 1996;45:1439–1445. doi: 10.2337/diab.45.10.1439. [DOI] [PubMed] [Google Scholar]
- 30.Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Horio Y, Matsuzawa Y, Kurachi Y. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J Biol Chem. 1996;271:24321–24324. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- 31.Aguilar-Bryan L, Clement JP, Gonzalez G, Kunjilwar K, Babenko A, Bryan J. Toward understanding the assembly and structure of KATP channels. Physiol Rev. 1998;78:227–245. doi: 10.1152/physrev.1998.78.1.227. [DOI] [PubMed] [Google Scholar]
- 32.Bryan J, Muñoz A, Zhang X, Düfer M, Drews G, Krippeit-Drews P, Aguilar-Bryan L. ABCC8 and ABCC9: ABC transporters that regulate K+ channels. Pflugers Arch. 2007;453:703–718. doi: 10.1007/s00424-006-0116-z. [DOI] [PubMed] [Google Scholar]
- 33.Vivaudou M, Moreau CJ, Terzic A. Structure and function of ATP-sensitive K+ channels. In: Kew J, Davies C, editors. Ion channels: From structure to function. Oxford University Press; Oxford, United Kingdom: 2009. pp. 454–473. [Google Scholar]
- 34.Suzuki M, Li RA, Miki T, Uemura H, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Ogura T, Seino S, Marbán E, Nakaya H. Functional roles of cardiac and vascular ATP-sensitive potassium channels clarified by Kir6.2-knockout mice. Circ Res. 2001;88:570–577. doi: 10.1161/01.res.88.6.570. [DOI] [PubMed] [Google Scholar]
- 35.Babenko AP, Gonzalez G, Aguilar-Bryan L, Bryan J. Reconstituted human cardiac KATP channels: Functional identity with the native channels from the sarcolemma of human ventricular cells. Circ Res. 1998;83:1132–1143. doi: 10.1161/01.res.83.11.1132. [DOI] [PubMed] [Google Scholar]
- 36.Melamed-Frank M, Terzic A, Carrasco AJ, Nevo E, Avivi A, Levy AP. Reciprocal regulation of expression of pore-forming KATP channel genes by hypoxia. Mol Cell Biochem. 2001;225:145–150. doi: 10.1023/a:1012286624993. [DOI] [PubMed] [Google Scholar]
- 37.Flagg TP, Kurata HT, Masia R, Caputa G, Magnuson MA, Lefer DJ, Coetzee WA, Nichols CG. Differential structure of atrial and ventricular KATP channels. Circ Res. 2008;103:1458–1465. doi: 10.1161/CIRCRESAHA.108.178186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen HH, Oh KY, Terzic A, Burnett JC., Jr. The modulating actions of sulfonylurea on atrial natriuretic peptide release in experimental acute heart failure. Eur J Heart Fail. 2000;2:33–40. doi: 10.1016/s1388-9842(99)00074-4. [DOI] [PubMed] [Google Scholar]
- 39.Dzeja PP, Terzic A. Phosphotransfer reactions in the regulation of ATP-sensitive K+ channels. FASEB J. 1998;12:523–529. doi: 10.1096/fasebj.12.7.523. [DOI] [PubMed] [Google Scholar]
- 40.Abraham MR, Selivanov VA, Hodgson DM, Pucar D, Zingman LV, Wieringa B, Dzeja PP, Alekseev AE, Terzic A. Coupling of cell energetics with membrane metabolic sensing. Integrative signaling through creatine kinase phosphotransfer disrupted by M-CK gene knock-out. J Biol Chem. 2002;277:24427–24434. doi: 10.1074/jbc.M201777200. [DOI] [PubMed] [Google Scholar]
- 41.Crawford RM, Ranki HJ, Botting CH, Budas GR, Jovanovic A. Creatine kinase is physically associated with the cardiac ATP-sensitive K+ channel in vivo. FASEB J. 2002;16:102–104. doi: 10.1096/fj.01-0466fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carrasco AJ, Dzeja PP, Alekseev AE, Pucar D, Zingman LV, Abraham MR, Hodgson D, Bienengraeber M, Puceat M, Janssen E, Wieringa B, Terzic A. Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc Natl Acad Sci USA. 2001;98:7623–7628. doi: 10.1073/pnas.121038198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jovanovic S, Du Q, Crawford RM, Budas GR, Stagljar I, Jovanovic A. Glyceraldehyde 3-phosphate dehydrogenase serves as an accessory protein of the cardiac sarcolemmal KATP channel. EMBO Rep. 2005;6:848–852. doi: 10.1038/sj.embor.7400489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dhar-Chowdhury P, Harrell MD, Han SY, Jankowska D, Parachuru L, Morrissey A, Srivastava S, Liu W, Malester B, Yoshida H, Coetzee WA. The glycolytic enzymes, glyceraldehyde-3-phosphate dehydrogenase, triose-phosphate isomerase, and pyruvate kinase are components of the KATP channel macromolecular complex and regulate its function. J Biol Chem. 2005;280:38464–38470. doi: 10.1074/jbc.M508744200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu GX, Hanley PJ, Ray J, Daut J. Long-chain acyl-coenzyme A esters and fatty acids directly link metabolism to KATP channels in the heart. Circ Res. 2001;88:918–924. doi: 10.1161/hh0901.089881. [DOI] [PubMed] [Google Scholar]
- 46.Terzic A, Jahangir A, Kurachi Y. Cardiac ATP-sensitive K+ channels: Regulation by intracellular nucleotides and K+ channel-opening drugs. Am J Physiol. 1995;269:C525–C545. doi: 10.1152/ajpcell.1995.269.3.C525. [DOI] [PubMed] [Google Scholar]
- 47.Antcliff JF, Haider S, Proks P, Sansom MS, Ashcroft FM. Functional analysis of a structural model of the ATP-binding site of the KATP channel Kir6.2 subunit. EMBO J. 2005;24:229–239. doi: 10.1038/sj.emboj.7600487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bienengraeber M, Alekseev AE, Abraham MR, Carrasco AJ, Moreau C, Vivaudou M, Dzeja PP, Terzic A. ATPase activity of the sulfonylurea receptor: A catalytic function for the KATP channel complex. FASEB J. 2000;14:1943–1952. doi: 10.1096/fj.00-0027com. [DOI] [PubMed] [Google Scholar]
- 49.Zingman LV, Hodgson DM, Bienengraeber M, Karger AB, Kathmann EC, Alekseev AE, Terzic A. Tandem function of nucleotide binding domains confers competence to sulfonylurea receptor in gating ATP-sensitive K+ channels. J Biol Chem. 2002;277:14206–14210. doi: 10.1074/jbc.M109452200. [DOI] [PubMed] [Google Scholar]
- 50.Park S, Lim BB, Perez-Terzic C, Mer G, Terzic A. Interaction of asymmetric ABCC9-encoded nucleotide binding domains determines KATP channel SUR2A catalytic activity. J Proteome Res. 2008;7:1721–1728. doi: 10.1021/pr7007847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dabrowski M, Tarasov A, Ashcroft FM. Mapping the architecture of the ATP-binding site of the KATP channel subunit Kir6.2. J Physiol. 2004;557:347–354. doi: 10.1113/jphysiol.2003.059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dupuis JP, Revilloud J, Moreau CJ, Vivaudou M. Three C-terminal residues from SUR contribute to the functional coupling between the KATP channel subunits SUR2A and Kir6.2. J Physiol. 2008;586:3075–3085. doi: 10.1113/jphysiol.2008.152744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zingman LV, Alekseev AE, Bienengraeber M, Hodgson D, Karger AB, Dzeja PP, Terzic A. Signaling in channel/enzyme multimers: ATPase transitions in SUR module gate ATP-sensitive K+ conductance. Neuron. 2001;31:233–245. doi: 10.1016/s0896-6273(01)00356-7. [DOI] [PubMed] [Google Scholar]
- 54.Park S, Terzic A. Quaternary structure of KATP channel SUR2A nucleotide binding domains resolved by synchrotron radiation X-ray scattering. J Struct Biol. 2010;169:243–251. doi: 10.1016/j.jsb.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karger AB, Park S, Reyes S, Bienengraeber M, Dyer RB, Terzic A, Alekseev AE. Role for SUR2A ED domain in allosteric coupling within the KATP channel complex. J Gen Physiol. 2008;131:185–196. doi: 10.1085/jgp.200709852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alekseev AE, Reyes S, Yamada S, Hodgson-Zingman DM, Sattiraju S, Zhu Z, Sierra A, Gerbin M, Coetzee WA, Goldhamer DJ, Terzic A, Zingman LV. Sarcolemmal ATP-sensitive K+ channels control energy expenditure determining body weight. Cell Metabolism. 2010;11:58–69. doi: 10.1016/j.cmet.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zingman LV, Hodgson DM, Alekseev AE, Terzic A. Stress without distress: homeostatic role for KATP channels. Mol Psychiatry. 2003;8:253–254. doi: 10.1038/sj.mp.4001323. [DOI] [PubMed] [Google Scholar]
- 58.Nichols CG, Lederer WJ. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol. 1991;261:H1675–H1686. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- 59.Gumina RJ, O’Cochlain DF, Kurtz CE, Bast P, Pucar D, Mishra P, Miki T, Seino S, Macura S, Terzic A. KATP channel knockout worsens myocardial calcium stress load in vivo and impairs recovery in stunned heart. Am J Physiol. 2007;292:H1706–H1713. doi: 10.1152/ajpheart.01305.2006. [DOI] [PubMed] [Google Scholar]
- 60.Li RA, Leppo M, Miki T, Seino S, Marban E. Molecular basis of electrocardiographic ST-segment elevation. Circ Res. 2000;87:837–839. doi: 10.1161/01.res.87.10.837. [DOI] [PubMed] [Google Scholar]
- 61.Gross GJ. ATP-sensitive potassium channels and myocardial preconditioning. Basic Res Cardiol. 1995;90:85–88. doi: 10.1007/BF00789438. [DOI] [PubMed] [Google Scholar]
- 62.Grover GJ, Garlid KD. ATP-sensitive potassium channels: a review of their cardioprotective pharmacology. J Mol Cell Cardiol. 2000;32:677–695. doi: 10.1006/jmcc.2000.1111. [DOI] [PubMed] [Google Scholar]
- 63.Jahangir A, Terzic A. KATP channel therapeutics at the bedside. J Mol Cell Cardiol. 2005;39:99–112. doi: 10.1016/j.yjmcc.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, Marbán E, Nakaya H. Role of sarcolemmal KATP channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109:509–516. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suzuki M, Saito T, Sato T, Tamagawa M, Miki T, Seino S, Nakaya H. Cardioprotective effect of diazoxide is mediated by activation of sarcolemmal but not mitochondrial ATP-sensitive potassium channels in mice. Circulation. 2003;107:682–685. doi: 10.1161/01.cir.0000055187.67365.81. [DOI] [PubMed] [Google Scholar]
- 66.Gumina RJ, Pucar D, Bast P, Hodgson DM, Kurtz CE, Dzeja PP, Miki T, Seino S, Terzic A. Knockout of Kir6.2 negates ischemic preconditioning-induced protection of myocardial energetics. Am J Physiol. 2003;284:H2106–H2113. doi: 10.1152/ajpheart.00057.2003. [DOI] [PubMed] [Google Scholar]
- 67.Stoller DA, Fahrenbach JP, Chalupsky K, Tan BH, Aggarwal N, Metcalfe J, Hadhazy M, Shi NQ, Makielski JC, McNally EM. Cardiomyocyte sulfonylurea receptor 2-KATP channel mediates cardioprotection and ST segment elevation. Am J Physiol Heart Circ Physiol. 2010;299:H1100–H1108. doi: 10.1152/ajpheart.00084.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, Pucar D, Bienengraeber M, Dzeja PP, Miki T, Seino S, Alekseev AE, Terzic A. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci USA. 2002;99:13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu XK, Yamada S, Kane GC, Alekseev AE, Hodgson DM, O’Cochlain F, Jahangir A, Miki T, Seino S, Terzic A. Genetic disruption of Kir6.2, the pore-forming subunit of ATP-sensitive K+ channel, predisposes to catecholamine-induced ventricular dysrhythmia. Diabetes. 2004;53:S165–S168. doi: 10.2337/diabetes.53.suppl_3.s165. [DOI] [PubMed] [Google Scholar]
- 70.Tong X, Porter LM, Liu G, Dhar-Chowdhury P, Srivastava S, Pountney DJ, Yoshida H, Artman M, Fishman GI, Yu C, Iyer R, Morley GE, Gutstein DE, Coetzee WA. Consequences of cardiac myocyte-specific ablation of KATP channels in transgenic mice expressing dominant negative Kir6 subunits. Am J Physiol. 2006;291:H543–H551. doi: 10.1152/ajpheart.00051.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kane GC, Lam CF, O’Cochlain F, Hodgson DM, Reyes S, Liu XK, Miki T, Seino S, Katusic ZS, Terzic A. Gene knockout of the KCNJ8-encoded Kir6.1 KATP channel imparts fatal susceptibility to endotoxemia. FASEB J. 2006;20:2271–2280. doi: 10.1096/fj.06-6349com. [DOI] [PubMed] [Google Scholar]
- 72.Kane GC, Behfar A, Yamada S, Perez-Terzic C, O’Cochlain F, Reyes S, Dzeja PP, Miki T, Seino S, Terzic A. ATP-sensitive K+ channel knockout compromises the metabolic benefit of exercise training, resulting in cardiac deficits. Diabetes. 2004;53:S169–S175. doi: 10.2337/diabetes.53.suppl_3.s169. [DOI] [PubMed] [Google Scholar]
- 73.Stoller D, Pytel P, Katz S, Earley JU, Collins K, Metcalfe J, Lang RM, McNally EM. Impaired exercise tolerance and skeletal muscle myopathy in sulfonylurea receptor-2 mutant mice. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1144–R1153. doi: 10.1152/ajpregu.00081.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reyes S, Kane GC, Zingman LV, Yamada S, Terzic A. Targeted disruption of KATP channels aggravates cardiac toxicity in cocaine abuse. Clin Transl Sci. 2009;2:361–365. doi: 10.1111/j.1752-8062.2009.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reyes S, Kane GC, Miki T, Seino S, Terzic A. KATP channels confer survival advantage in cocaine overdose. Mol Psychiatry. 2007;12:1060–1061. doi: 10.1038/sj.mp.4002083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kane GC, Behfar A, Dyer RB, O’Cochlain DF, Liu XK, Hodgson DM, Reyes S, Miki T, Seino S, Terzic A. KCNJ11 gene knockout of the Kir6.2 KATP channel causes maladaptive remodeling and heart failure in hypertension. Hum Mol Genet. 2006;15:2285–2297. doi: 10.1093/hmg/ddl154. [DOI] [PubMed] [Google Scholar]
- 77.Yamada S, Kane GC, Behfar A, Liu XK, Dyer RB, Faustino RS, Miki T, Seino S, Terzic A. Protection conferred by myocardial ATP-sensitive K+ channels in pressure overload-induced congestive heart failure revealed in KCNJ11 Kir6.2-null mutant. J Physiol. 2006;577:1053–1065. doi: 10.1113/jphysiol.2006.119511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hodgson DM, Zingman LV, Kane GC, Perez-Terzic C, Bienengraeber M, Ozcan C, Gumina RJ, Pucar D, O’Coclain F, Mann DL, Alekseev AE, Terzic A. Cellular remodeling in heart failure disrupts KATP channel-dependent stress tolerance. EMBO J. 2003;22:1732–1742. doi: 10.1093/emboj/cdg192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abraham MR, Jahangir A, Alekseev AE, Terzic A. Channelopathies of inwardly rectifying potassium channels. FASEB J. 1999;13:1901–1910. doi: 10.1096/fasebj.13.14.1901. [DOI] [PubMed] [Google Scholar]
- 80.Dunne MJ, Cosgrove KE, Shepherd RM, Aynsley-Green A, Lindley KJ. Hyperinsulinism in infancy: From basic science to clinical disease. Physiol Rev. 2004;84:239–275. doi: 10.1152/physrev.00022.2003. [DOI] [PubMed] [Google Scholar]
- 81.Tricarico D, Servidei S, Tonali P, Jurkat-Rott K, Camerino DC. Impairment of skeletal muscle adenosine triphosphate-sensitive K+ channels in patients with hypokalemic periodic paralysis. J Clin Invest. 1999;103:675–682. doi: 10.1172/JCI4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jovanovic S, Du Q, Mukhopadhyay S, Swingler R, Buckley R, McEachen J, Jovanovic A. A patient suffering from hypokalemic periodic paralysis is deficient in skeletal muscle ATP-sensitive K+ channels. Clin Transl Sci. 2008;1:71–74. doi: 10.1111/j.1752-8062.2008.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Darbar D, Herron KJ, Ballew JD, Jahangir A, Gersh BJ, Shen WK, Hammill SC, Packer DL, Olson TM. Familial atrial fibrillation is a genetically heterogeneous disorder. J Am Coll Cardiol. 2003;41:2185–2192. doi: 10.1016/s0735-1097(03)00465-0. [DOI] [PubMed] [Google Scholar]
- 84.Ellinor PT, Yoerger DM, Ruskin JN, MacRae CA. Familial aggregation in lone atrial fibrillation. Hum Genet. 2005;118:179–184. doi: 10.1007/s00439-005-0034-8. [DOI] [PubMed] [Google Scholar]
- 85.Fatkin D, Otway R, Vandenberg JI. Genes and atrial fibrillation. A new look at an old problem. Circulation. 2007;116:782–792. doi: 10.1161/CIRCULATIONAHA.106.688889. [DOI] [PubMed] [Google Scholar]
- 86.Lubitz SA, Yi B, Ellinor P. Genetics of atrial fibrillation. Cardiol Clin. 2009;27:25–33. doi: 10.1016/j.ccl.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Olson TM, Alekseev AE, Liu XK, Park S, Zingman LV, Bienengraeber M, Sattiraju S, Ballew JD, Jahangir A, Terzic A. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum Mol Genet. 2006;15:2185–2191. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- 88.Balana B, Dobrev D, Wettwer E, Christ T, Knaut M, Ravens U. Decreased ATP-sensitive K+ current density during chronic human atrial fibrillation. J Mol Cell Cardiol. 2003;35:1399–1405. doi: 10.1016/s0022-2828(03)00246-3. [DOI] [PubMed] [Google Scholar]
- 89.Brundel BJ, Van Gelder IC, Henning RH, Tuinenburg AE, Wietses M, Grandjean JG, Wilde AA, Van Gilst WH, Crijns HJ. Alterations in potassium channel gene expression in atria of patients with persistent and paroxysmal atrial fibrillation: differential regulation of protein and mRNA levels for K+ channels. J Am Coll Cardiol. 2001;37:926–932. doi: 10.1016/s0735-1097(00)01195-5. [DOI] [PubMed] [Google Scholar]
- 90.Van Wagoner DR. Mechanosensitive gating of atrial ATP-sensitive potassium channels. Circ Res. 1993;72:973–983. doi: 10.1161/01.res.72.5.973. [DOI] [PubMed] [Google Scholar]
- 91.Brady PA, Alekseev AE, Aleksandrova LA, Gomez LA, Terzic A. A disrupter of actin microfilaments impairs sulfonylurea-inhibitory gating of cardiac KATP channels. Am J Physiol. 1996;271:H2710–H2716. doi: 10.1152/ajpheart.1996.271.6.H2710. [DOI] [PubMed] [Google Scholar]
- 92.Ravens U. Mechano-electric feedback and arrhythmias. Prog Biophys Mol Biol. 2003;82:255–266. doi: 10.1016/s0079-6107(03)00026-9. [DOI] [PubMed] [Google Scholar]
- 93.Haïssaguerre M, Chatel S, Sacher F, Weerasooriya R, Probst V, Loussouarn G, Horlitz M, Liersch R, Schulze-Bahr E, Wilde A, Kääb S, Koster J, Rudy Y, Le Marec H, Schott JJ. Ventricular fibrillation with prominent early repolarization associated with a rare variant of KCNJ8/KATP channel. J Cardiovasc Electrophysiol. 2009;20:93–98. doi: 10.1111/j.1540-8167.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 94.Medeiros-Domingo A, Tan BH, Crotti L, Tester DJ, Eckhardt L, Cuoretti A, Kroboth SL, Song C, Zhou Q, Kopp D, Schwartz PJ, Makielski JC, Ackerman MJ. Gain-of-function mutation S422L in the KCNJ8-encoded cardiac KATP channel Kir6.1 as a pathogenic substrate for J-wave syndromes. Heart Rhythm. 2010;7:1466–1471. doi: 10.1016/j.hrthm.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ahmad F, Seidman JG, Seidman CE. The genetic basis for cardiac remodeling. Annu Rev Genomics Hum Genet. 2005;6:185–216. doi: 10.1146/annurev.genom.6.080604.162132. [DOI] [PubMed] [Google Scholar]
- 96.Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, Koseki H, Iwanaga T, Nakaya H, Seino S. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6. 1. Nat Med. 2002;8:466–472. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- 97.Chutkow WA, Pu J, Wheeler MT, Wada T, Makielski JC, Burant CF, McNally EM. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 KATP channels. J Clin Invest. 2002;110:203–208. doi: 10.1172/JCI15672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Minoretti P, Falcone C, Aldeghi A, Olivieri V, Mori F, Emanuele E, Calcagnino M, Geroldi D. A novel Val734Ile variant in the ABCC9 gene associated with myocardial infarction. Clin Chim Acta. 2006;370:124–128. doi: 10.1016/j.cca.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 99.Selivanov VA, Alekseev AE, Hodgson DM, Dzeja PP, Terzic A. Nucleotide-gated KATP channels integrated with creatine and adenylate kinases: Amplification, tuning and sensing of energetic signals in the compartmentalized cellular environment. Mol Cell Biochem. 2004;256:243–256. doi: 10.1023/b:mcbi.0000009872.35940.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Waldman SA, Terzic A. Molecular therapeutics from knowledge to delivery. Clin Pharmacol Ther. 2010;87:619–623. doi: 10.1038/clpt.2010.61. [DOI] [PubMed] [Google Scholar]
- 101.Arrell DK, Terzic A. Network systems biology for drug discovery. Clin Pharmacol Ther. 2010;88:120–125. doi: 10.1038/clpt.2010.91. [DOI] [PubMed] [Google Scholar]
- 102.Terzic A, Waldman SA. Translational medicine: path to personalized and public health. Biomark Med. 2010;4:787–790. doi: 10.2217/bmm.10.101. [DOI] [PubMed] [Google Scholar]
- 103.Sattiraju S, Reyes S, Kane GC, Terzic A. KATP channel pharmacogenomics: from bench to bedside. Clin Pharmacol Ther. 2008;83:354–357. doi: 10.1038/sj.clpt.6100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nelson TJ, Martinez-Fernandez A, Terzic A. KCNJ11 knockout morula re-engineered by stem cell diploid aggregation. Philos Trans R Soc Lond B Biol Sci. 2009;364:269–276. doi: 10.1098/rstb.2008.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yamada S, Nelson TJ, Crespo-Diaz RJ, Perez-Terzic C, Liu XK, Miki T, Seino S, Behfar A, Terzic A. Embryonic stem cell therapy of heart failure in genetic cardiomyopathy. Stem Cells. 2008;26:2644–2653. doi: 10.1634/stemcells.2008-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Waldman SA, Terzic A. Clinical and translational science: From bench-bedside to global village. Clin Translal Sci. 2010;3:254–257. doi: 10.1111/j.1752-8062.2010.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Waldman SA, Terzic A. Clinical translational science 2020: disruptive innovation redefines the discovery-application enterprise. Clin Transl Sci. 2011;4:69–71. doi: 10.1111/j.1752-8062.2011.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]