Abstract
Objective
Epidural fibrosis and adhesion are the main reasons for post-laminectomy sustained pain and functional disability. In this study, the authors investigate the effect of irradiated freeze-dried human amniotic membrane on reducing epidural adhesion after laminectomy on a rat model.
Methods
A total of 20 rats were divided into two groups. The group A did not receive human amniotic membrane implantation after laminectomy and group B underwent human amniotic membrane implantation after laminectomy. Gross and microscopic findings were evaluated and compared at postoperative 1, 3 and 8 weeks.
Results
The amount of scar tissue and tenacity were reduced grossly in group of rats with human amniotic membrane implantation (group B). On a microscopic evaluation, there were less inflammatory cell infiltration and fibroblast proliferation in group B.
Conclusion
This experimental study shows that implantation of irradiated freeze-dried human amniotic membrane reduce epidural fibrosis and adhesion after spinal laminectomy in a rat model.
Keywords: Human amniotic membrane, Failed back surgery syndrome, Epidural adhesion, Laminectomy
INTRODUCTION
Failed back surgery syndrome (FBSS) is a condition characterized by persistent back pain with or without leg pain after lumbar spine surgery. FBSS occurs approximately about 5-40% in the literature7,14,28). Multiple factors may contribute to develop FBSS such as inadequate surgical decompression, spinal instability, recurrent disc herniation, and epidural nerve fibrosis15). However, many clinicians consider epidural fibrosis is one of major causes of persistent pain after lumbar spinal surgery8,20,27,28,35,40). The postoperative epidural fibrosis can cause extra-dural compression or dural tethering, which results persistent back and leg pain7,31,33). Therefore, various methods and researches were done to prevent or reduce the amount of scar formation. Implanting materials including free or pedicle fat graft, synthetic membrane, fibrin foam and Adcon-L have been tried13,17,18,21,24,29,32,40). However, results with these methods are variable and complications have been reported19,23,25,26,30).
Recently, many studies have been reported the effect of human amniotic membrane on reducing adhesion scar in various areas of surgical practices. Davis10) first reported the use of an amniotic membrane on skin transplantation on 1910. Since then it has been used to treat variable diseases such as skin ulcers4), vaginal atresia37), and plastic repair of conjunctiva defects12) and to reduce surgical adhesions in abdominal surgery and otolaryngology surgery38,43,44). Amniotic membrane may reduce postoperative adhesion by inhibit vascularization, reduce inflammation and suppress apoptosis in epithelial cells6,11,39).
The purpose of this experimental study was to assess the effect of implanting a human amniotic membrane around the nerve root to reduce epidural adhesion in a rat model.
MATERIALS AND METHODS
Animals
Six to eight weeks old Spraque-Dawley male rats (400-500 g) were acclimated to a housing facility (25±2℃ room temperature, 50% room humidity, 12 hours light-dark cycle) for 1 week under the permission of our animal experimental centre according to Korean National Veterinary Research and Quarantine Service Policy.
Amniotic membrane harvesting
Normal human amniotic membrane (AM) was obtained from one pregnant donor who agreed to informed consent of use of AM to animal experiment. The AM was obtained after cesarean section screened for HIV, hepatitis B and syphilis and washed with normal saline. The AM was separated from the placenta and attached to nitrocellulose paper and stored for one week under -80℃ in a mixed solution with Dulbecco modified eagle medium and glycerol (1 : 1 vol/vol). AM was melted on a room temperature before implantation. All AM were irradiated with 2.5 kGy radiation.
Grouping of rats
Total of 20 rats were used in this experiment. These rats were grouped into two groups. Groups A was the group of rats which AM was not implant after laminectomy (n=10) and implantation of freeze-dried AM was done after laminectomy in group B (n=10).
Surgical procedure and method
General anesthesia was induced with an intra-muscular injection of ketamine hydrochloride (0.1 g/kg). The experiment was done on thoraco-lumbar junction of rat, since thoraco-lumbar junction is largest spine in a rat model and has a least motion. A 3 cm skin incision was done on rat's thoraco-lumbar junction and the muscles dissected. Unilateral laminectomy was done to expose nerve root. Immediate muscle and skin closure were done using Dexon 3-0 and Nylon 3-0 after hemostasis around the exposed nerve root in group A. In group B, size of 0.4×0.8 cm freeze-dried AM was implanted before muscle and skin closure. All procedures were performed using loupe magnification of ×5.
Macroscopic and microscopic evaluations of epidural fibrosis were done on 1, 3, and 8 weeks post-operatively. Three rats were sacrificed at post-operative 1 and 3 week and 4 rats were sacrificed 8 weeks in both groups.
Both macroscopic and microscopic evaluation was done by one pathologist who was unaware of the operative details in a blinded manner to minimize the bias.
Macroscopic analysis
Macroscopic examination was done on a space between the dura mater and surrounding soft tissues. Quality of wound healing, possible adverse effects, and epidural adhesion were examined. Gross analysis of epidural adhesion was carried out by peel-off manually and the adhesion tenacity was scored using a visual 4-point qualitative scale proposed by Einhaus et al.13) (Table 1). The scale was consisted of four grades : 1) Grade 0 : no adhesion between dura, 2) Grade 1 : slight adhesions to dura and easily detached, 3) Grade 2 : moderate adhesions and detachable by moderate traction and 4) Grade 3 : tenacious adhesions and detachable on by sharp dissection.
Table 1.
Gross analysis scoring system
Histological analysis
The nerve and surround soft tissues were excised in 0.5 cm apart and fixed in 10% formalin for 48 hours and then decalcified for 1 hour. An axial slice of each nerve specimen with surrounding tissue was prepared on slides. For histological evaluation, the slides were stained with Hematoxylin-eosin (H&E). Masson-trichrome stain was done for evaluation of surrounding connective tissues.
For evaluation of differentiation of myofibroblast, an immunohistochemistry staining with alpha smooth muscle actin (α-SMA) (Dako, Denmark) antibody was carried out. For immunohistochemistry stain, avidin-biotin-peroxidase detection system was used with DAKO Envision Kit (Dako, Denmark). First, de-paraffin was done with 10% xylene and then dehydrated in 100%, 95%, 70% alcohol was done in sequence before rinsed on running water for 10 minutes. Peroxidase blocking was done using 0.3% hydrogen peroxide (H2O2) prior to primary antibody incubation to reduce nonspecific background staining. For blocking non-specific antigen, the specimens were incubated in 10 mmol/L citrate buffer (pH 6.0) and irradiated on microwave for 10 minutes. Primary antibody reaction was carried out with anti-mouse anti-serum α-SMA actin (Dako, Denmark) and secondary antibody reaction was done with avidin-biotin-peroxidase complex for one hour at room temperature. The slides were counter stained with Mayers hematoxylin and examined with microscope.
Grade of adhesions
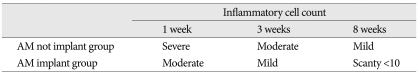
Histology grading method was used to standardize the quantity of adhesion between two groups. For slides that stained with H&E method, the number of inflammatory cells was counted per ×400 magnification field by a pathologist at post-operative 1, 3, and 8 weeks for both groups and classified into 4 groups : 1) scanty : less than 10 cells, 2) mild : 11-20 cells, 3) moderate : 21-30 cells, 4) severe : more than 31 cells.
The evaluation of the extend of myofibroblast differentiation was also performed under ×400 microscope magnification and scored into 4-points system : 1) point 1 : less than 10%, 2) point 2 : 11-50%, 3) point 3 : 51-75%, 4) point 4 : more than 76%.
Statistic analysis
Statistical difference of macroscopic and histological results between two groups was tested with Mann-Whitney test and statistically significant values were defined as p<0.05.
RESULTS
Macroscopic analysis
All specimens demonstrated epidural fibrosis and adhesion after re-exploration, especially on post-operative 3 and 8 weeks. Base on 4-point scale score system of amount and tenacity of epidural fibrosis and adhesion, the average score of AM not implant group (group A) was 1.8 and 1.5 at post-operative 1 week. However, the average score of AM implant group (group B) was 0 for both amount and tenacity of fibrosis and adhesion. The average score for group A was 2, 1.7 and 2, 2 at post-operative 3 and 8 weeks consequently for amount and tenacity of adhesion, whereas 0.5, 0.3 and 0.7, 1.0 at post-operative 3 and 8 weeks for group B. All results show statistically significant differences for scoring system of amount of fibrosis and tenacity of epidural adhesion at all post-operative week between two groups (p<0.05).
Histological analysis
H&E stain
At postoperative 1 week, predominant lymphocyte infiltration was shown in group A and predominant neutrophil infiltration was shown in group B (Fig. 1). Mixed lymphoplasma cells were shown at post-operative 3 weeks and small amount of lymphocytes were observed at post-operative 8 week in both groups.
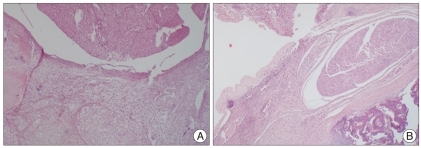
Fig. 1.
In the human amniotic membrane in group without implant shows severe amount of acute inflammatory cell (A) and moderate inflammatory cell infiltration are shown in the human amniotic membrane implant group (B) at post-operative 1 week (H&E stain; ×400).
At post-operative 1 week, moderate number of inflammatory cells was shown in group B and severe number of inflammatory cells in group A. Mild and scanty number of inflammatory cells was shown at 3 and 8 weeks consequently in group B. However, moderate number of inflammatory cells was observed at post-operative 3 weeks and mild for 3 specimens and scanty for one specimen at post-operative 8 weeks in group A (Table 2).
Table 2.
Cell component and inflammatory cell count on H&E stain
AM : amniotic membrane
Masson-trichrome stain
Muscle fibers and fibroblast stained in red, and collagen tissues or fibrosis were stained in blue in Masson-trichrome stain. Group B shows only scanty amount of collagen tissues, in contrast of group A which showed profuse amount of collagen tissues at post-operative 1, 3 and 8 weeks (Fig. 2).
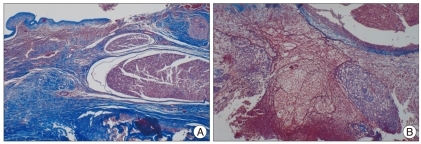
Fig. 2.
Dense and thick fibrotic band (stained in blue) around dura are show in group without in group with human amniotic membrane (AM) implantation (A). In contrast, thin and loose fibrotic adhesions around the dura are shown in human AM implant group at post-operative 8 weeks (Masson-trichrome stain, ×400) (B).
Immunohistochemistry staining with α-SMA
The score for evaluation of extend of myofibroblast differentiation was two at post-operative one week and one at post-operative 3 and 8 weeks in each specimens of group B. However, each specimens score was 4 at post-operative one week and 4, 3, 3 point for each specimens at post-operative 3 weeks and 1, 2, 2, 1 point for each specimens at post-operative 8 weeks (Table 3) (Fig. 3). These results also show statistically significant differences (p<0.05).
Table 3.
Smooth Muscle Actin Score
AM : amniotic membrane
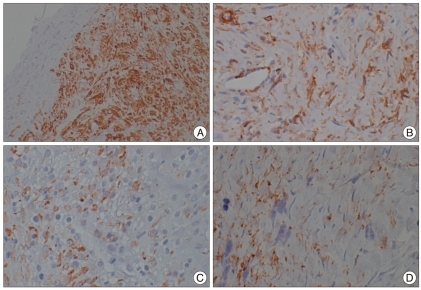
Fig. 3.
At an alpha smooth muscle actin immunohistochemical stain, section from human amniotic membrane in group without implant shows strong positive reaction around the nerve root at post-operative 1 week (A) and reduced immnunohistochemical reaction at post-operative 3 weeks (B). In human amniotic membrane implant group shows weak positive reaction under amniotic membrane at post-operative 1 week (C) and also reduced immnunohistochemical reaction at post-operative 3 weeks (D).
DISCUSSION
The formation of epidural fibrosis and adhesion is an inevitable sequel of spinal laminectomy. Persistent pain, peresthesia and neurological deficit such as motor weakness can occur after lumbar laminectomy due to epidural fibrosis and adhesion27,31,35). Although there are multiple factors that may cause FBSS, epidural fibrosis plays a major role in as many as 24% of cases33). Persistent or recurrent symptom and sign by epidural adhesion are due to direct mechanical tethering of nerve root or the dura, a powerful mediator of inflammation, phospholipase A2, prostaglandin E1 and E2, leukotriene B, and impaired axoplasmic transport and nutritional or conduction disturbance as well as restricted arterial supply and venous return1,7,31,34). Smith et al.36) reported that that the nerve roots of this region moved 0.5 to 5 mm distally and laterally during a straight leg raising test, depending on vertebral level on human cadavers study. Thus, a postoperative anterolateral epidural and periradicular scar situated in critical proximity to a lumbar nerve root might induce dynamic neural tension in a patient's everyday activities. Additionally, chronic compression of nerve root by epidural fibrosis may lead to markedly increase mechanical sensitivity1,24).
The main reasons for epidural fibrosis formation are epidural fat destruction, hematoma, and paraspinal muscle fiber invasion22,29). The extent of fibrosis depends primarily on the extent of the surgical procedure, especially on the degree of hemostasis3). Preventing the migration of the fibroblasts into the exposed dura in the early healing phase may be the most important step to reduce epidural fibrosis formation. Thus, the interposition of a physical barrier to limit cell migration is considered an effective strategy to reduce scar formation.
Various biological and synthetic materials used to prevent scar formation have been evaluated, such as hemostatic sponges, free fat grafts, silastic, hyaluronic acid, polyactic acid, carboxymethylcellulose gels, a mixture of dextran sulfate and gelatin (Adcon-L, Adba), 5-fluorouracil, cyclosporine, and radiation therapy5,13,18,21,40-42). But, the results show only limited success. Some complications from use of Adcon-L have been reported such as cerebrospinal fluid leakage and infection19,23). Also, seroma formation, dimpling of the scar, and cauda equina syndrome were reported as complications from free fat graft25,30).
In this study, we assess the ability of human amniotic membrane to reduce postlaminectomy epidural adhesion in a rat model. Amniotic membrane has been used clinically in prevention of ocular disease12), vaginal reconstruction surgery37), pelvic and abdominal adhesions38), repairing omphaloceles16), and skin lesions including burn, ulceration and trauma2,4,9). AM is a thin tissue sized about 0.2-0.5 mm that creates the inner layer of the amniotic sac. AM consists of a single layer of ectodermally derived columnar cells firmly fixed to an underlying layer of mesenchyme which contains large amounts of collagen. AM protects fetus from maternal infection and immune-reaction. AM modulates levels of cytokine and growth factor levels, and have also been shown to have other key properties such as pain reduction, antifibroblastic activity, and anti-bacterial effect9). The membrane also contains a host of growth factors (epidermal growth factor, hepatocyte growth factor, nerve growth factor), anti-inflammatory cytokines (interleukin-6) and antivasculogenic factors (thrombospondin and tissue inhibitors of metalloproteases) which promote wound healing and suppress inflammation and neovascularization11,12). Moreover, AM does not express HLA-A, B, C, or DR antigen or beta-2 microglobulin which immunological reaction after implantation does not occur11,12).
In this study, we used irradiated freeze-dried AM to reduce immune reaction and infection. Our experiment shows that implanting of a human AM after laminectomy significantly reduces the epidural fibrosis on macroscopic evaluation. On histological evaluation, less amount of inflammatory cell infiltration was shown in group of human AM implantation on H&E stain. There were definite differences between two groups in which group A showed profound amount of collagen tissue staining compare to group B on Masson-trichrome staining. This implies that the group without AM implantation formed more fibrosis between dura and surrounding tissues. Also, less extent of myofibroblast differentiation was observed in group B compared to group A in immunohistochemistry staining with α-SMA.
These results are due to anti-inflammatory reaction by down-regulation of the cytokine such as interleukin-1, 8 and polymorphonuclear neutrophil elastase, suppression of epithelial apoptosis and antifibrotic effect by inducing a down-regulation of transforming growth factor-β which prevent differentiation of fibroblast into myofibroblast11,12). In summary, our experiment suggest that AM can be used to reduce post-laminectomy epidural adhesion by inhibiting inflammation, antifibrotic effect and mechanical barrier between nerve root and surrounding structures.
In the previous clinical use of human AM in skin ulcers and ocular diseases, low incidences of clinical complications have been reported. Even though there is no clinical report on use of human AM on spine operation, it may be a potentially attractive material to prevent epidural adhesion after spine operation. Since mass production of human AM in tissue bank is possible, the price is affordable compared to alternative anti-adhesion products. It also may be used in patients with very thin body or undergoing long level spinal decompression instead of using fat graft. However, this experimental study has limitation in that a small number of animals have been used. In macroscopic evaluation, using visual 4-point scale system is subjective rather than objective and can lead to bias. Before clinical use of AM, more number with longer period animal experimental study and larger animal model study should be done.
CONCLUSION
This experimental study demonstrates that human amniotic membrane is an effective material to reduce epidural fibrosis and adhesion after spinal laminectomy in a rat model and also suggests the potential use of human amniotic membrane as an anti-adhesion technique in clinical practice.
Acknowledgements
This paper was supported by the Dong-A University Fund.
References
- 1.Abitbol JJ, Lincoln TL, Lind BI, Amiel D, Akeson WH, Garfin SR. Preventing postlaminectomy adhesion. A new experimental model. Spine (Phila Pa 1976) 1994;19:1809–1814. doi: 10.1097/00007632-199408150-00004. [DOI] [PubMed] [Google Scholar]
- 2.Bennett JP, Matthews R, Faulk WP. Treatment of chronic ulceration of the legs with human amnion. Lancet. 1980;1:1153–1156. doi: 10.1016/s0140-6736(80)91616-5. [DOI] [PubMed] [Google Scholar]
- 3.Benoist M, Ficat C, Baraf P, Cauchoix J. Postoperative lumbar epiduro-arachnoiditis. Diagnostic and therapeutic aspects. Spine (Phila Pa 1976) 1980;5:432–436. doi: 10.1097/00007632-198009000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Boc SF, Chairman EL, Freed EL. Implications for the use of amnion and chorion in podiatric medicine and surgery. J Foot Surg. 1985;24:236–242. [PubMed] [Google Scholar]
- 5.Bora H, Aykol SV, Akyurek N, Akmansu M, Ataoglu O. Inhibition of epidural scar tissue formation after spinal surgery : external irradiation vs. spinal membrane application. Int J Radiat Oncol Biol Phys. 2001;51:507–513. doi: 10.1016/s0360-3016(01)01647-9. [DOI] [PubMed] [Google Scholar]
- 6.Boudreau N, Werb Z, Bissell MJ. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc Natl Acad Sci U S A. 1996;93:3509–3513. doi: 10.1073/pnas.93.8.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton CV, Kirkaldy-Willis WH, Yong-Hing K, Heithoff KB. Causes of failure of surgery on the lumbar spine. Clin Orthop Relat Res. 1981:191–199. [PubMed] [Google Scholar]
- 8.Cauchoix J, Ficat C, Girard B. Repeat surgery after disc excision. Spine (Phila Pa 1976) 1978;3:256–259. doi: 10.1097/00007632-197809000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Colocho G, Graham WP, 3rd, Greene AE, Matheson DW, Lynch D. Human amniotic membrane as a physiologic wound dressing. Arch Surg. 1974;109:370–373. doi: 10.1001/archsurg.1974.01360030022006. [DOI] [PubMed] [Google Scholar]
- 10.Davis JW. Skin transplantation with a review of 550 cases at the Johns Hopkins hospital. Johns Hopkins Med J. 1910;15:307. [Google Scholar]
- 11.Dua HS, Azuara-Blanco A. Amniotic membrane transplantation. Br J Ophthalmol. 1999;83:748–752. doi: 10.1136/bjo.83.6.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dua HS, Gomes JA, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49:51–77. doi: 10.1016/j.survophthal.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Einhaus SL, Robertson JT, Dohan FC, Jr, Wujek JR, Ahmad S. Reduction of peridural fibrosis after lumbar laminotomy and discectomy in dogs by a resorbable gel (ADCON-L) Spine (Phila Pa 1976) 1997;22:1440–1446. doi: 10.1097/00007632-199707010-00003. discussion 1446-1447. [DOI] [PubMed] [Google Scholar]
- 14.Fager CA, Freidberg SR. Analysis of failures and poor results of lumbar spine surgery. Spine (Phila Pa 1976) 1980;5:87–94. doi: 10.1097/00007632-198001000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Fritsch EW, Heisel J, Rupp S. The failed back surgery syndrome : reasons, intraoperative findings, and long-term results : a report of 182 operative treatments. Spine (Phila Pa 1976) 1996;21:626–633. doi: 10.1097/00007632-199603010-00017. [DOI] [PubMed] [Google Scholar]
- 16.Gharib M. [Repair of prenatally ruptured omphalocele and the paraumbilical abdominal wall defect with the infant's own fetal membranes (author's transl).] MMW Munch Med Wochenschr. 1975;117:1555–1558. [PubMed] [Google Scholar]
- 17.Gill GG, Sakovich L, Thompson E. Pedicle fat grafts for the prevention of scar formation after laminectomy. An experimental study in dogs. Spine (Phila Pa 1976) 1979;4:176–186. doi: 10.1097/00007632-197903000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Gill GG, Scheck M, Kelley ET, Rodrigo JJ. Pedicle fat grafts for the prevention of scar in low-back surgery. A preliminary report on the first 92 cases. Spine (Phila Pa 1976) 1985;10:662–667. doi: 10.1097/00007632-198509000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Hieb LD, Stevens DL. Spontaneous postoperative cerebrospinal fluid leaks following application of anti-adhesion barrier gel : case report and review of the literature. Spine (Phila Pa 1976) 2001;26:748–751. doi: 10.1097/00007632-200104010-00009. [DOI] [PubMed] [Google Scholar]
- 20.Hurme M, Katevuo K, Nykvist F, Aalto T, Alaranta H, Einola S. CT five years after myelographic diagnosis of lumbar disk herniation. Acta Radiol. 1991;32:286–289. [PubMed] [Google Scholar]
- 21.Jacobs RR, McClain O, Neff J. Control of postlaminectomy scar formation : an experimental and clinical study. Spine (Phila Pa 1976) 1980;5:223–229. doi: 10.1097/00007632-198005000-00004. [DOI] [PubMed] [Google Scholar]
- 22.LaRocca H, Macnab I. The laminectomy membrane. Studies in its evolution, characteristics, effects and prophylaxis in dogs. J Bone Joint Surg Br. 1974;56B:545–550. [PubMed] [Google Scholar]
- 23.Le AX, Rogers DE, Dawson EG, Kropf MA, De Grange DA, Delamarter RB. Unrecognized durotomy after lumbar discectomy : a report of four cases associated with the use of ADCON-L. Spine (Phila Pa 1976) 2001;26:115–117. doi: 10.1097/00007632-200101010-00020. discussion 118. [DOI] [PubMed] [Google Scholar]
- 24.Lee CK, Alexander H. Prevention of postlaminectomy scar formation. Spine (Phila Pa 1976) 1984;9:305–312. doi: 10.1097/00007632-198404000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Martin-Ferrer S. Failure of autologous fat grafts to prevent postoperative epidural fibrosis in surgery of the lumbar spine. Neurosurgery. 1989;24:718–721. doi: 10.1227/00006123-198905000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Mayer PJ, Jacobsen FS. Cauda equina syndrome after surgical treatment of lumbar spinal stenosis with application of free autogenous fat graft. A report of two cases. J Bone Joint Surg Am. 1989;71:1090–1093. [PubMed] [Google Scholar]
- 27.Merrild U, Søgaard I. Sciatica caused by perifibrosis of the sciatic nerve. J Bone Joint Surg Br. 1986;68:706. doi: 10.1302/0301-620X.68B5.3782227. [DOI] [PubMed] [Google Scholar]
- 28.North RB, Campbell JN, James CS, Conover-Walker MK, Wang H, Piantadosi S, et al. Failed back surgery syndrome : 5-year follow-up in 102 patients undergoing repeated operation. Neurosurgery. 1991;28:685–690. discussion 690-691. [PubMed] [Google Scholar]
- 29.Petrie JL, Ross JS. Use of ADCON-L to inhibit postoperative peridural fibrosis and related symptoms following lumbar disc surgery : a preliminary report. Eur Spine J. 1996;5(Suppl 1):S10–S17. doi: 10.1007/BF00298567. [DOI] [PubMed] [Google Scholar]
- 30.Prusick VR, Lint DS, Bruder WJ. Cauda equina syndrome as a complication of free epidural fat-grafting. A report of two cases and a review of the literature. J Bone Joint Surg Am. 1988;70:1256–1258. [PubMed] [Google Scholar]
- 31.Robertson JT. Role of peridural fibrosis in the failed back : a review. Eur Spine J. 1996;5(Suppl 1):S2–S6. doi: 10.1007/BF00298565. [DOI] [PubMed] [Google Scholar]
- 32.Robertson JT, Meric AL, Dohan FC, Jr, Schweitzer JB, Wujek JR, Ahmad S. The reduction of postlaminectomy peridural fibrosis in rabbits by a carbohydrate polymer. J Neurosurg. 1993;79:89–95. doi: 10.3171/jns.1993.79.1.0089. [DOI] [PubMed] [Google Scholar]
- 33.Ross JS, Robertson JT, Frederickson RC, Petrie JL, Obuchowski N, Modic MT, et al. ADCON-L European Study Group. Association between peridural scar and recurrent radicular pain after lumbar discectomy : magnetic resonance evaluation. Neurosurgery. 1996;38:855–861. discussion 861-863. [PubMed] [Google Scholar]
- 34.Saal JS. The role of inflammation in lumbar pain. Spine (Phila Pa 1976) 1995;20:1821–1827. doi: 10.1097/00007632-199508150-00013. [DOI] [PubMed] [Google Scholar]
- 35.Siqueira EB, Kranzler LI, Dharkar DD. Fibrosis of the dura mater. A cause of "failed back" syndrome. Surg Neurol. 1983;19:168–170. doi: 10.1016/0090-3019(83)90418-4. [DOI] [PubMed] [Google Scholar]
- 36.Smith SA, Massie JB, Chesnut R, Garfin SR. Straight leg raising. Anatomical effects on the spinal nerve root without and with fusion. Spine. 1993;18:992–999. [PubMed] [Google Scholar]
- 37.Tancer ML, Katz M, Veridiano NP. Vaginal epithelialization with human amnion. Obstet Gynecol. 1979;54:345–349. [PubMed] [Google Scholar]
- 38.Trelford-Sauder M, Dawe EJ, Trelford JD. Use of allograft amniotic membrane for control of intra-abdominal adhesions. J Med. 1978;9:273–284. [PubMed] [Google Scholar]
- 39.von Versen-Höynck F, Syring C, Bachmann S, Möller DE. The influence of different preservation and sterilisation steps on the histological properties of amnion allografts--light and scanning electron microscopic studies. Cell Tissue Bank. 2004;5:45–56. doi: 10.1023/b:catb.0000022276.47180.96. [DOI] [PubMed] [Google Scholar]
- 40.Wujek JR, Ahmad S, Harel A, Maier KH, Roufa D, Silver J. A carbohydrate polymer that effectively prevents epidural fibrosis at laminectomy sites in the rat. Exp Neurol. 1991;114:237–245. doi: 10.1016/0014-4886(91)90040-j. [DOI] [PubMed] [Google Scholar]
- 41.Yildiz KH, Gezen F, Is M, Cukur S, Dosoglu M. Mitomycin C, 5-fluorouracil, and cyclosporin A prevent epidural fibrosis in an experimental laminectomy model. Eur Spine J. 2007;16:1525–1530. doi: 10.1007/s00586-007-0344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoon DH, Kim KN, Shin DA, Lee JE, Lee JG, Kim DH. Preventive effect of anti-adhesion barrier gel for peridural fibrosis in rat laminectomy model. J Korean Neurosurg Soc. 2003;34:456–460. [Google Scholar]
- 43.Young RL, Cota J, Zund G, Mason BA, Wheeler JM. The use of an amniotic membrane graft to prevent postoperative adhesions. Fertil Steril. 1991;55:624–628. doi: 10.1016/s0015-0282(16)54197-1. [DOI] [PubMed] [Google Scholar]
- 44.Zohar Y, Talmi YP, Finkelstein Y, Shvili Y, Sadov R, Laurian N. Use of human amniotic membrane in otolaryngologic practice. Laryngoscope. 1987;97:978–980. [PubMed] [Google Scholar]