Abstract
Spermiation is the process by which mature spermatids are released from Sertoli cells into the seminiferous tubule lumen prior to their passage to the epididymis. It takes place over several days at the apical edge of the seminiferous epithelium, and involves several discrete steps including remodelling of the spermatid head and cytoplasm, removal of specialized adhesion structures and the final disengagement of the spermatid from the Sertoli cell. Spermiation is accomplished by the co-ordinated interactions of various structures, cellular processes and adhesion complexes which make up the “spermiation machinery”. This review addresses the morphological, ultrastructural and functional aspects of mammalian spermiation. The molecular composition of the spermiation machinery, its dynamic changes and regulatory factors are examined. The causes of spermiation failure and their impact on sperm morphology and function are assessed in an effort to understand how this process may contribute to sperm count suppression during contraception and to phenotypes of male infertility.
Key words: spermiation, sperm, Sertoli cell, spermatid, spermiogenesis, adhesion, ectoplasmic specialization, tubulobulbar complex
Introduction
Spermiation is the process by which mature spermatids are released from the supporting somatic Sertoli cells into the lumen of the seminiferous tubule. It is a critical determinant of the number of sperm entering the epididymis, and thus the sperm content of the ejaculate. Spermiation is a protracted, complex process occurring over several days (∼82 hrs in the rat, see below), commencing at the beginning of stage VII in the rat and mouse1 with the alignment of elongated spermatids along the luminal edge of the seminiferous epithelium. Spermiation is completed towards the end of stage VIII, when spermatids are released into the tubule lumen, and the remainder of the spermatid cytoplasm, known as the residual body, is phagocytosed by the Sertoli cell. Although the primary goal of spermiation is to release the spermatid from the Sertoli cell, this process also leads to extensive restructuring and remodelling of the spermatid to produce a streamlined spermatozoan. Spermiation is a known target for hormone-based male contraceptives2 and, by virtue of its role in spermatid restructuring and release, is important for optimal fertility. The morphological and ultra-structural events associated with spermiation have been well described and appear conserved among rodents, monkeys and humans,1,3 however, the molecular control of spermiation is less understood.
The purpose of this review is to examine the morphological and molecular events involved in mammalian spermiation, together with their likely proteomic composition, in order to provide a better understanding of the spermiation machinery, i.e., the cellular processes and structures required for the successful completion of spermiation. Much of this information will necessarily come from studies in rodents, but will be applied to human spermiation where possible. The causes of spermiation failure and their impact on sperm morphology and function will also be examined in an effort to understand how this process may impact on male fertility.
Morphological, Ultrastructural and Functional Aspects of Spermiation
General concepts.
Much of what is known of the morphological and ultrastructural aspects of spermiation comes from the meticulous electron microscopic studies by the late Lonnie Russell, reviewed in reference 3–5. Spermiation is a multi-step process involving changes in both the spermatid and the Sertoli cell that ready the elongated spermatid, at the end of spermatogenesis, for its final release from the supporting Sertoli cell (Fig. 1). Key events are the remodeling of the spermatid nucleus and cytoplasm to produce the streamlined spermatozoan, removal of Sertoli cell “ectoplasmic specialization” (ES) junctions and retraction of Sertoli cell cytoplasm, and extension of the spermatid into the lumen. Spermiation ends with disengagement of the spermatid (now known as the spermatozoan4) into the lumen and the phagocytosis of the remainder of the residual body by the Sertoli cell (Fig. 1).
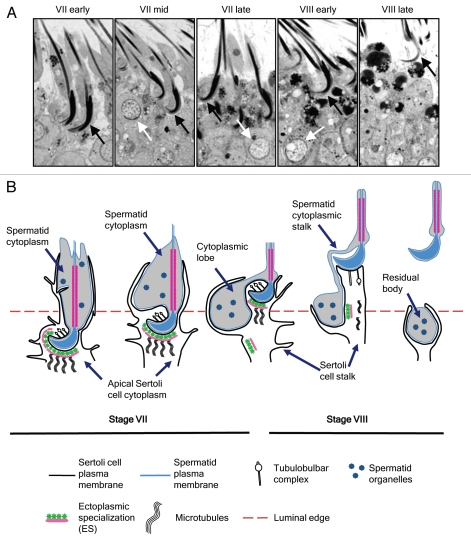
Figure 1.
The spermiation process. (A) Morphology of spermiation in the normal adult Sprague Dawley rat from early stage VII until late stage VIII immediately prior to disengagement. Elongated spermatids (black arrows) show progressive changes in nuclear and cytoplasmic morphology. Note the attainment of a hook-shaped nucleus (compare stage VII early to stage VII mid), and the reduction in spermatid cytoplasmic volume (compare stage VII early to stage VIII early). Tubules were staged based on round spermatid morphology according to published criteria.7 The beginning of stage VIII is characterized by the majority of round spermatid nuclei (white arrows) becoming oriented to the plasma membrane. (B) Diagram of the spermiation process, adapted from reference 1. At the initiation of spermiation in stage VII, the spermatid shows an extensive cytoplasm around the flagellum, and is largely enveloped by finger-like projections of the apical Sertoli cell cytoplasm (sometimes referred to as the apical process). Tubulobulbar complexes form in the ventral curvature of the spermatid head, in areas deficient in ES. As spermiation progresses, the Sertoli cell cytoplasm gradually recedes until it contacts only the dorsal surface in stage VIII. The spermatid head and flagellum is gradually extended further into the tubule lumen by the lengthening of the Sertoli cell stalk. As the spermatid head is extended, its cytoplasm condenses in volume and remains stationary within the epithelium; the net effect is the cytoplasm appears to flow downwards, until it is present below the level of the head. This is now referred to as the spermatid cytoplasmic lobe. Spermatid organelles become concentrated in the cytoplasmic lobe, which will ultimately form the residual body after disengagement. During the progression from stage VII to VIII, the ES structure disappears from the Sertoli cell plasma membrane opposite the spermatid head, and tracts of ES can be observed adjacent to the Sertoli cell plasma membrane within the Sertoli cell stalk. Tubulobulbar complexes also show changes in morphology as spermiation progresses; they are most numerous in the ventral curvature of the spermatid head in stage VII, but can be seen emanating from the dorsal curvature in stage VIII. Electron microscopic studies suggest these structures can lack bulbous components in stage VIII. Spermiation ends with the rapid disengagement of the spermatid head from the Sertoli cell, breakage of the thin cytoplasmic stalk between the perinuclear region of the spermatid and the residual body, and phagocytosis of the residual body by the Sertoli cell.
Ectoplasmic specialization.
Prior to spermiation, the elongated spermatid interacts with the Sertoli cell via an extensive structure known as the ectoplasmic specialization (ES). The ES is first seen in the Sertoli cell cytoplasm opposite round spermatids at the beginning of step 8,6 when the spermatid nucleus polarizes to one side of the cell.7 The ES remains associated with elongating spermatids until the beginning of spermiation.6 The ES associates with microtubules and motor proteins to translocate spermatids through the epithelium during spermiogenesis.8,9 During elongation, ES-mediated translocation facilitates downward movement of spermatids to their position in deep “crypts” within Sertoli cells.9 This maximises the surface area of spermatid-Sertoli cell contact and may facilitate communication between the two cells,4,9 perhaps via Sertoli cell “penetrating processes”, and may be a means by which the Sertoli cell can regulate the transcriptionally inactive spermatid.4 The degree to which spermatids are embedded in these crypts tends to be species-specific, with rat spermatids appearing to be embedded in deeper crypts compared to mice, suggesting that only a limited area of contact is needed between both cell types for such communication. Just prior to the beginning of spermiation, the elongated spermatids are rapidly translocated, in a microtubule-dependent mechanism8,10 from deep within the Sertoli cell crypts to the luminal edge.
Spermiation is initiated at the beginning of stage VII in the rat and mouse, which corresponds to stage II in the human, when the majority of late spermatids align along the luminal edge.1 At the beginning of spermiation (Fig. 1) the late elongated spermatid has a large cytoplasm that is enveloped by the Sertoli cell cytoplasm and the entire spermatid head associates with the extensive Sertoli cell ES. The term ectoplasmic specialization was devised to reflect the fact that it is “a surface modification of Sertoli cells that faces certain sites of cell-cell contact”.6 The composition of the ES has been extensively reviewed in reference 9, 11 and 12, it is comprised of hexagonally-packed actin filaments sandwiched between the Sertoli cell membrane and the underlying endoplasmic reticulum. ES forms at sites of intercellular adhesion between spermatids and Sertoli cells (apical ES) and also between Sertoli cells at the blood-testis-barrier (basal ES). As well as being involved in spermatid translocation, the ES stabilizes intercellular adhesion junctions9,13 and participates directly in intercellular adhesion. The latter was revealed by studies showing trypsin-sensitive adhesion elements present at sites of ES.14 For the purpose of this review, we use the term ES structure to describe the structural specialization of the Sertoli cell opposite the spermatid acrosome as visualized by electron microscopy6 and ES adhesion domain to collectively describe the intercellular adhesion elements likely associated with the ES structure.
A major goal of spermiation is to disassemble the apical ES in preparation for the eventual disengagement of spermatids into the lumen. The factors regulating apical ES disassembly have been the subject of intensive study, reviewed in references 12 and 15, and will not be considered further here. How the Sertoli cell disassembles the ES structure yet continues to “hold on” to the late spermatid is an important consideration. During spermiation, cohorts of spermatids are extended out into the tubule lumen while the Sertoli cell cytoplasm surrounding them gradually recedes (Fig. 1). It is likely that the spermatids are subjected to considerable shear forces from the seminiferous tubule fluid flow that carries the released spermatids towards the rete testis. This flow must be quite rapid since released spermatids rapidly disappear from the spermiation site. Thus the adhesion between spermatids and Sertoli cells during spermiation must be sufficiently strong to counter this force. It has long been noted that Sertoli cell nuclei become elongated and oriented perpendicular to the basement membrane during spermiation,16 and perhaps a reason for this is that the Sertoli cell may “stiffen” in response to shear stress placed upon it via its interaction with the spermatid, a phenomenon that has been observed in other cells subjected to fluid shear.17
Tubulobulbar complexes (TBCs).
Tubulobulbar complexes are fascinating structures that are most prominent between late spermatids and Sertoli cells during spermiation (Figs. 1 and 2),18,19 but also appear between Sertoli cells at the blood-testis barrier in a stage-specific manner.18 Between spermatids and Sertoli cells, TBCs first become evident at the beginning of spermiation and are observed until sperm are released.1,19,20 Up to 24 TBCs per spermatid can be observed in stage VII tubules early in spermiation.19 They first form preferentially at a site that is species-dependent; in rats and mice, which have falciform or sickle-shaped sperm heads, they form in the inner curvature of the spermatid head (Figs. 1 and 2). In species with spatulate sperm, such as human, TBCs largely form near the tip.21 In all cases, TBCs first form in areas devoid of the ES structure1,19–21 leading to the hypothesis that ES structure disassembly is linked to TBC formation.1
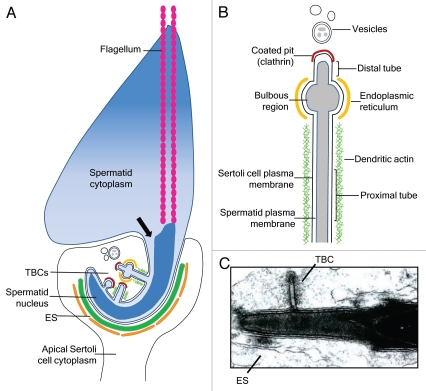
Figure 2.
Diagram of TBC formation and morphology during spermiation. (A) Diagram of a late spermatid during spermiation in stage VII (in the rat). The spermatid cytoplasm has started to condense; the arrow indicates the hypothesized route via which spermatid cytoplasmic contents may flow into the perinuclear region and, potentially, into TBCs.23 The spermatid head is encompassed by the apical Sertoli cell cytoplasm, and extensive ectoplasmic specialization (ES) structures are observed in the Sertoli cell cytoplasm closely opposed to the dorsal curvature of the spermatid head. Tubulobulbar complexes (TBCs) form in the ventral curvature of the spermatid head in areas deficient in ES. TBC formation begins with a small clathrin-coated pit which lengthens into a tubular structure, followed by formation of the dilated bulbous region. Vesicles are apparent near the ends of TBCs. (B) Diagram of the morphology of a mature TBC. The TBC contains both spermatid and Sertoli cell plasma membranes, which are closely opposed. The long proximal tube is surrounded by dendritic actin, however actin is not observed around the dilated bulbous region; this region is surrounded by endoplasmic reticulum. A short distal tube terminates in a coated pit containing clathrin. Near the TBCs are double-membraned vesicles which have formed from the “budding off” of the bulbous portion. (C) Electron micrograph of a TBC emanating from a spermatid head in late stage VII in the rat. A focal area of ES is also observed.
TBC formation is initiated when a small portion of spermatid cytoplasm begins to protrude into the Sertoli cell at the site of a bristle-coated pit on the Sertoli cell membrane, in an area deficient in, but often flanked by, Sertoli cell ES (Fig. 2).20 It is now understood that the bristle-coated pits contain clathrin,22 a protein involved in endocytosis. Clathrin may be involved in the initiation of TBC formation and recruitment of the actin cytoskeleton to the developing TBC.22 Narrow protrusions of spermatid cytoplasm then develop from the perinuclear region and invaginate the Sertoli cell cytoplasm forming a long, narrow, double-membraned tubular structure terminating in a bristle-coated pit20,23 and “capped” by clathrin.22 This tubule consists of Sertoli cell plasma membrane, cuffed by surrounding actin filaments,13 closely opposed to the spermatid plasma membrane (Fig. 2). The plasma membranes of the Sertoli cell and spermatid in this tubular structure are very closely opposed and electron microscopic observations suggested that they may differ from plasma membranes elsewhere, possibly lacking extracellular glycoproteins and membrane-associated particles.20,21 Recent work has demonstrated that the GTPase dynamin 3 is likely to play a major role in the formation of these tubular protrusions, since the transfection of dynamin 3 into MDCK cells stably expressing the Sertoli cell ES-associated adhesion protein nectin-2 promoted the formation of tubular structures labeled with nectin-2 near the cell periphery.24
As TBC development continues, a dilated region appears towards the end of the tubular structure (Fig. 2). When visualized by electron microscopy, this so-called “bulbar” portion contains finely granulated material that appears similar to the contents of the spermatid's perinuclear cytoplasm and is surrounded by Sertoli cell smooth endoplasmic reticulum (ER) but lacks an actin network (Fig. 2).20 A local loss of actin filaments at this site is hypothesized to be involved in formation of the bulbar portion,22 and proteins involved in actin polymerization, such as cortactin, are likely important for TBC dynamics.22,25 The Arp 2/3 complex is a seven-subunit protein that regulates the actin cytoskeleton by acting as a nucleation core for actin branching from mother filaments. Proteins associated with the Arp2/3 complex, as well as N-WASP which is involved in Arp2/3 activation, localize to TBCs and Arp2/3 complex-mediated dendritic actin assembly likely plays a major role in TBC elongation.22,24 Interestingly, intra-testicular injection of the N-WASP inhibitor, wiskostatin, caused late spermatids with TBCs to change their orientation at the luminal edge,26 pointing to a role for N-WASP activation of the Arp2/3 complex in actin dynamics during TBC formation.
Once the mature TBC has formed, the bulbar structure “buds off” and appears to fuse with lysosomes.20 The vesicularization of the bulbar structures may be mediated by dynamin 3, given the well known role of dynamins in “pinching off” vesicles from parent membranes.24 The vesicles produced also have a double-membrane and contain nectin-2 and -3,27 which are expressed by the Sertoli cell and the spermatid, respectively, at the ES adhesion domain.28 The vesicles also contain lysosomal markers27 and the early endosome antigen EEA-1.29 Thus, TBC structures appear to be involved in the internalization and degradation of spermatid-Sertoli cell plasma membranes during spermiation.27 Although TBCs are unique structures, they share structural and functional similarities with podosomes which form at sites of cell-substrate attachment in other tissues and are involved in extracellular matrix degradation.30
There have been various hypotheses put forward regarding the function of TBCs.1 During the period of TBC formation, there is a significant reduction in spermatid cytoplasm volume, around 50% in the rat, suggesting that these structures aid in the removal of spermatid cytoplasm.31 There appears to be a direct link between the spermatid's main cytoplasm near the flagellum and the perinuclear cytoplasm around the spermatid head. The perinuclear cytoplasm in turn connects directly with the tubular portion of TBCs forming in stage VII and is progressively reduced in size as stage VII continues.23 Russell proposed that the spermatid's cytoplasm flows through to the perinuclear region, via a narrow tube, and into TBCs, and that this tube may “filter out” the organelles which will remain in the cytoplasmic lobe and, eventually, in residual bodies (see Fig. 2).23 In support of this proposition, agents which are associated with disrupted TBC formation can cause swelling of the spermatid head and failure to eliminate cytoplasm (reviewed in ref. 1).
The most well characterized function of TBCs is their role in removing intercellular ES adhesion junctions. As TBCs first form in areas deficient in ES,27 Russell postulated that TBCs serve to remove junctional links between spermatid and Sertoli cell plasma membranes that were part of the adhesion domain of the ES.1 This proposition is supported by the demonstration that TBCs, and the endosomes associated with them, contain structural molecules associated with the ES (espin, myosin VIIa and Keap1) as well as components of the adhesion domain of ESs, such as nectin-2 and -3 and the nectin-actin linker protein afadin.27,29 Thus, TBCs participate in ES removal by internalizing intercellular adhesion junctions between late spermatids and Sertoli cells.27 Given that TBCs appear to share some molecular components of ES, it has been postulated that TBCs may initially develop from ES structures.27
TBCs may assist in shaping the spermatid head and acrosome during spermiation. In the rat, the late spermatid acrosome condenses during spermiation coincident with the formation of TBCs. Acrosomal material has been observed in the tubular structures of TBCs in the ventral curvature of the spermatid head,32 lending support to a role for TBCs in remodelling of the spermatid acrosome. TBCs have also been proposed to be involved in final head shaping, since they first form in the ventral aspect of the spermatid, coinciding with the formation of the “hooked” appearance of the head, reviewed in reference 33. The precise roles of TBCs, and whether they perform multiple functions, will be revealed by models targeting TBC formation in vivo.
The progression of spermiation.
After the initiation of spermiation and TBC formation, there is a change in the relationship between the late spermatid and the Sertoli cell as spermiation progresses (Fig. 1). The spermatid head and flagellum are gradually further extended into the lumen of the seminiferous tubule, via the microtubule-mediated extension of the apical Sertoli cell cytoplasmic stalk.1 As the spermatid is gradually “pushed” into the lumen, the Sertoli cell cytoplasm surrounding it gradually recedes, so that by the end of stage VII, it contacts the spermatid head and is not present near the flagellum (Fig. 1). As spermiation progresses into stage VIII, the apical Sertoli cell cytoplasm retracts further to contact only a small portion of the spermatid head in most species. In rats and mice the Sertoli cell remains in contact with the dorsal surface only, reviewed in reference 1, see Figure 1.
During extension of the spermatid head and flagellum, the cytoplasm of the spermatid remains stationary within the epithelium. Thus instead of flowing towards the basement membrane, the cytoplasm remains “anchored” while the rest of the spermatid is pushed into the lumen (Fig. 1).1 By the end of stage VII, the spermatid cytoplasm is located below the level of the spermatid head (Fig. 1), and becomes more condensed, perhaps facilitated by TBCs.23,31 This relocated spermatid cytoplasm is now referred to as the cytoplasmic lobe (Fig. 1). The mechanism by which the cytoplasmic lobe remains “anchored” is unclear, but may depend on intercellular adhesion junctions and/or Sertoli cell penetrating processes, which are small extensions of Sertoli cell cytoplasm projecting into the spermatid cytoplasm.1 The orientation of the cytoplasmic lobe with respect to the spermatid is species specific: in the rat and mouse the cytoplasm shows a lobular shape (Fig. 1), whereas in monkeys it more closely resembles a hood over the spermatid head.1
A major change that takes place during spermiation is removal of the ES structure. This extensive structure has surrounded the entire spermatid nucleus during the elongation phase of spermiogenesis, and removal is essential for sperm release to occur.1 As discussed above, local dissolution of ES structures is linked to TBC formation.27 The precise triggers of this dissolution are unclear, however local activation of signaling molecules within the Sertoli cell may be involved, reviewed in reference 34 (see below). As spermiation progresses, large tracts of ES structures are observed in the apical Sertoli cell cytoplasm not associated with late spermatid nuclei;4–6,20 these are particularly evident in stage VII (our unpublished observations in the rat). This observation has led to the hypothesis that these actin-bundled structures may, to some extent, be recycled within the Sertoli cell, away from the late spermatid during spermiation to the newly elongating spermatid in step 8 below.1,4,6 This hypothesis is supported by localization of the ES marker espin, which bundles actin filaments into the characteristic hexagonally-packed arrangement in the ES35 (Fig. 3), and which marks the appearance of new ES structures opposite the acrosome of step 8 round spermatids that have just commenced the elongation phase.6,20 The extent of ES structure opposing step 8 round spermatid acrosome correlates with the degree of polarization of the round spermatid nucleus.36
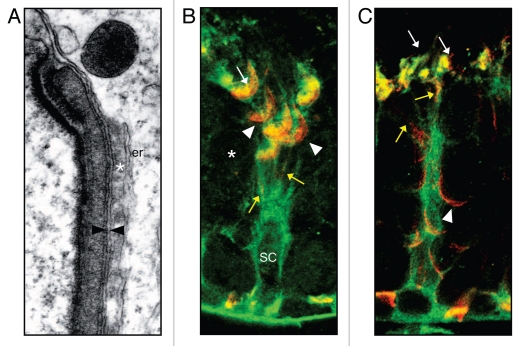
Figure 3.
Redistribution of the ES during spermiation. (A) Electron micrograph of the ES structure opposite a step 8 round spermatid. The spermatid and Sertoli cell plasma membranes are closely opposed (arrowheads). Actin bundles (asterix) are sandwiched between the Sertoli cell plasma membrane and an underlying layer of narrow endoplasmic reticulum (er). (B) Co-localization of vinculin (green) which labels the Sertoli cell cytoplasm and junctional structures and espin (red) (modified from ref. 36) which is present in the actin bundles of the ES structure.35 In stage VII, espin in the ES is concentrated around the late spermatid head (arrowheads) and is present in TBCs (arrows), presumably reflecting the role of these structures in ES dissolution. Step 7 round spermatids (asterix) below do not yet associate with an ES. SC = Sertoli cell nucleus. (C) Co-localization of vinculin (green) and espin (red) in stage VIII tubules. Espin becomes diffuse in the apical Sertoli cell cytoplasm (arrows) as it loses its association with late spermatids and becomes redistributed to step 8 round spermatids (arrowheads). In both stages VII and VIII, narrow lines of espin staining can be seen in the Sertoli cell cytoplasm (yellow arrows) perhaps reflecting recycling of ES structures.
The timing of ES removal varies between species; in the opossum the ES is lost very soon after the initiation of spermiation, whereas in the rat and mouse ES removal is more gradual.1 ES removal begins in the ventral curvature of the rat spermatid head, where TBCs form, during stage VII. As spermiation progresses into stage VIII, little ES remains in contact with the late spermatid.6,20 Many studies have shown the ES structures are removed some time prior to spermatid release, but the timing of this removal is unclear.1 We have used stereology37 to provide more precise information on the timing of spermiation in rats. Since the kinetics of the stages of spermatogenesis are known,7 determining the proportion of tubules with late spermatids lined along the luminal edge in the process of spermiation (i.e., in stages VII or VIII) provides a measure of the duration of spermiation. Using strict criteria for staging,7 we find that spermiation in the normal adult Sprague Dawley rat is 81.9 ± 1.9 hrs long (n = 5), suggesting that spermatids disengage from the Sertoli cell approximately 3.2 hrs before the end of stage VIII (Fig. 4). Using the immunohistochemical localization of espin, which correlates well with ES structure formation,35–37 we found that 72.6 ± 1.2% (n = 5 adult rats) of tubules in spermiation contain late spermatids associated with espin staining.37 Taken together, the findings suggest that late spermatids associate with ES structures for 59.4 hours (Fig. 4), approximately 3.4 hours into stage VIII. This further suggests that late spermatids remain attached to the Sertoli cell by an adhesion junction that does not involve the ES structure for approximately 22.5 hours prior to their disengagement from the Sertoli cell (see Fig. 4).
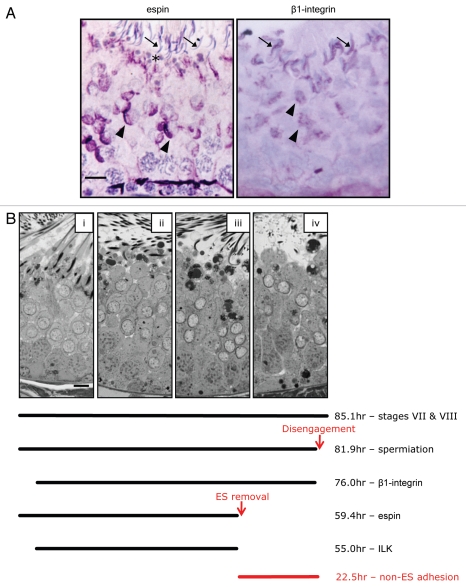
Figure 4.
Timing of events associated with spermiation. (A) Immunohistochemical localization of espin and β1-integrin in stage VIII tubules of the adult rat, as described previously in ref. 37. In stage VIII, espin (a marker of ES structures),35,36 is present in ES structures that have just formed opposite step 8 spermatids (arrowheads). It is not associated with elongated spermatids (arrows) prior to disengagement, but is present in the adluminal Sertoli cell cytoplasm near residual bodies (asterix). β1-integrin is also present in ES opposite step 8 round spermatids (arrowheads), and is concentrated around the dorsal curvature of the elongated spermatid prior to spermiation (arrows). (B) Micrographs of the seminiferous epithelium from the beginning of spermiation in stage VII (i) until after disengagement near the end of stage VIII (iv). The bar represents 10 µm. The total hours of stages VII and VIII combined is 85.1 hrs, based on published data.7 Stereological analysis of the proportion of stage VII and VIII tubules with elongated spermatids at the luminal edge out of the total number of tubules in stages VII and VIII, multiplied by 85.1 (described in Morphological, Ultrastructural and Functional Aspects of Spermiation: “The progression of spermiation”, also see refs. 37 and 55) indicates that the spermiation process is 81.9 hrs long, from its initiation in stage VII until disengagement (indicated by the red arrow) near the end of stage VIII. Using previously published information on the proportion of seminiferous tubules showing adluminal spermatids that were immuno-positive or immuno-negative for a particular protein,37,55 the lines indicate the time in hours that a particular protein is present during spermiation. Espin in ES structures is present from the beginning of spermiation, but is removed after 59.4 hrs37 (indicated by the red arrow). On the other hand, β1-integrin becomes visible very soon after the initiation of spermiation, and persists until disengagement.37 Integrin-linked kinase (ILK) also becomes visible soon after the initiation of spermiation, but is removed ∼55 hrs later, around the time of espin (and thus ES) removal.37 The removal of ES structures 59.4 hrs into the spermiation process suggests that a non-ES adhesion mechanism mediates spermatid-Sertoli cell adhesion for ∼22.5 hrs prior to disengagement (red text).
Endoplasmic reticulum (ER) is particularly enriched in the apical Sertoli cell process, and shows dynamic changes during spermiation.38,39 Narrow cisternae of ER, termed “flattened ER (fER)”, are markedly enriched in the apical process at the beginning of spermiation in rats.38 Some of this fER is associated with the ES structure, however a large proportion is not; instead it is observed in large, concentric layers in the apical Sertoli cell process enveloping the spermatid head. Surprisingly, some of this non-ES associated fER appears to be associated with bundles of actin and microtubules.38 The origin and function of this non-ES-associated fER is unclear however it shows a marked and progressive fenestration during stage VII and disappears from the apical process by the end of stage VII.38 Coincident with this disappearance is a marked increase in another type of ER referred to as tubular ER (tER).38,39 This tER (which is usually smooth, but shows occasional ribosomes),39 shows a striking pattern by specialized staining techniques, and is maximal in the apical process in late stage VII. It originates in the Sertoli cell stalk, and forms an extensive, continuous system in the apical process, surrounding both the dorsal and ventral aspects of the spermatid head, terminating at the ER associated with the bulbar portions of TBCs. Both fER and tER decrease as spermiation progresses into stage VIII, with only small areas of both present.38 Cyclic changes in ER during stages VII and VIII have been confirmed by other studies.39 The functional significance of these ER subtypes during spermiation remains to be established, however it is tempting to speculate that they have roles in TBC function, endocytosis and/or regulating adhesion junction dynamics. Of relevance to the latter proposition is emerging evidence of direct functional links between ER and adhesion (particularly focal adhesion) junctions in other systems (reviewed in ref. 40 and 41).
Electron microscopy studies suggest there are also changes in TBC morphology and location as spermiation progresses.20 As discussed above, TBCs first form and are most numerous, in the ventral curvature of the spermatid head during stage VII in the rat.19 However as the ES is removed and the apical Sertoli cell cytoplasm retracts away from the spermatid, TBCs start to form along the dorsal curvature of the spermatid head (Fig. 1), and are observed in this region until sperm are released.1,19 Indeed the fact that TBCs persist until the point of spermatid release led to the suggestion that they may have an anchoring function.1,19 Russell described changes in TBC morphology as spermiation progressed into stage VIII.20 In stage VIII there appeared to be fragmentation of the spermatid plasma membrane, but not the Sertoli cell membrane, within TBCs protruding from dorsal curvature.19,20 Also, the last remaining TBCs prior to disengagement often lack bulbous components1,19,20 (Fig. 1). These changes in TBC morphology are suggestive of changes in function as spermiation progresses.
Disengagement.
Spermiation ends when the spermatids disengage from the Sertoli cell and are released into the lumen of the seminiferous tubule, whereupon they are referred to as spermatozoa.4 Although the term spermiation is sometimes used to refer to this release event, it is more appropriate to use spermiation to describe the entire process, and the term disengagement to describe the moment when mature spermatids lose contact with the Sertoli cell.1 Disengagement occurs towards the end of stage VIII, approximately 81.9 hours after spermiation commenced (Fig. 4). Immediately prior to disengagement, only a small portion of Sertoli cell cytoplasm remains in contact with the outer dorsal surface of the spermatid head (Fig. 1). A small amount of spermatid cytoplasm remains attached to the midpiece of the spermatid; this cytoplasmic droplet contains a variety of proteins and is eventually lost during transit of the epididymis, reviewed in reference 42. The remainder of the spermatid's cytoplasmic lobe condenses to form the residual body that will be left behind after disengagement (Fig. 1). The spermatid is attached to the residual body via an elongated cytoplasmic stalk that becomes stretched just prior to release. It is not clear whether breakage of this stalk occurs prior to or during, disengagement.1,19
Disengagement is an extremely rapid event, and an entire population of sperm at any one site in a tubule are released simultaneously.1 Disengagement is rarely observed in testis sections,1 likely because it is an instantaneous event, and the disengaged sperm are rapidly swept away from the spermiation site in the seminiferous tubule fluid, to make their way to the rete testis and, subsequently, the epididymis. This transport may be facilitated by contractions of the peritubular myoid cells surrounding the tubules, since cytochalasin D, which promotes actin filament depolymerisation, caused changes in peritubular myoid cells and prevented released spermatozoa from traversing the duct system.10
In most mammals disengagement occurs once spermiation is complete, however in many non-mammals disengagement occurs in response to some stimulus, such as mating.1 In all cases, disengagement is likely to be mediated by the Sertoli cell, since the elongated spermatids have been transcriptionally inactive for some time and are thus likely to be passive in this process.1 Transplantation experiments have revealed that germ cells direct the duration of the spermatogenic stages43 and thus the timing of spermiation (e.g., the duration of stages VII and VIII) is likely controlled by germ cells, possibly by the entry of spermatogonia into meiosis and by the nuclear changes in steps 7 and 8 spermatids in these stages. Thus germ cells will contribute to the timing of the events associated with spermiation, however it seems likely that the final disengagement of the spermatid will largely be mediated by the Sertoli cell. The control of spermatid disengagement will be further discussed below.
After disengagement, the residual body is retained by the Sertoli cell and is transported to the base of the seminiferous epithelium, likely via microtubules.10,44 The residual body contains various organelles no longer needed by the spermatid, including the Golgi complex and ER.4 Linkages are seen between the Sertoli cell and residual bodies, suggesting residual bodies initially adhere to Sertoli cells via an adhesion junction.10,39 After disengagement, residual bodies undergo phagocytosis by Sertoli cells,4 and appear near the basement membrane in late VIII, early stage IX tubules. Sertoli cell lysosomes fuse with the residual bodies, transforming them into phagolysosomes;4 this integration of fluid-phase endocytosis and phagocytosis may be important for residual body elimination.39 The phagocytosis of residual bodies is associated with a peak in the number of lipid droplets at the base of the Sertoli cell, as observed in various species.39 Thus it is likely that residual body phagocytosis produces large quantities of lipids within the Sertoli cell. These are seemingly metabolized, but it is possible they could have other functions39 such as regulating blood-testis-barrier dynamics.45 It is established that residual bodies can influence Sertoli cell function46 and recent studies suggest the existence of a loop between endocytosis of proteins, such as laminin fragments, during spermiation and the control of remodelling of inter-Sertoli cell junctions at the blood-testis-barrier, reviewed in reference 47. Thus, endocytosis of components of the spermiation machinery and phagocytosis of residual bodies during spermiation may regulate other aspects of Sertoli cell function and hence, spermatogenesis, reviewed in reference 47.
Dynamic Changes in Adhesion Structures and Protein Localization during Spermiation
It is clear from the above description that spermiation is a multi-faceted process involving a variety of cellular structures and processes that we refer to as the spermiation machinery. It is now becoming clear that spermiation involves dynamic changes in the proteomic composition of the spermiation machinery as well as changes in adhesion junctions.
A growing number of studies reporting on the immunohistochemical localization of proteins in the testis have identified proteins at the site of spermiation. These include adhesion, signaling and structural proteins, as well as kinases, phosphatases and proteases (Table 1 and Sup. Table 1). When interpreting the likely functional role of a protein in the spermiation machinery, it is important to consider its precise localization in the context of the specific structures and the stage of spermatogenesis. Such information is often not reported. Localization of a protein to the inner (ventral or concave) curvature of the rat or mouse spermatid head in stage VII is likely to correlate to TBCs, endocytic vesicles or apical Sertoli cell cytoplasm which contains extensive ER. At this site, proteins may be present in a characteristic “spoke-like” pattern indicative of the tubular portion of TBCs,22,29 or may be present in focal “dots” indicating coated pits, bulbar regions and/or endocytic vesicles. Proteins may also be apparent in a diffuse staining pattern around the site of spermiation suggesting a presence in the apical Sertoli cell process. Proteins localized specifically to the outer dorsal curvature in stage VII are likely related to adhesion between the spermatid and the Sertoli cell, and may be associated with the apical ES structure which is still obvious at this time. Proteins that remain at this dorsal curvature into stage VIII, and are present immediately before disengagement, are likely to be associated with an adhesion junction between spermatids and Sertoli cells, however since the ES structure is not seen at this time (see above), these proteins are likely to be involved in non-ES-mediated adhesion. It has been our experience that the site of spermiation can be labeled non-specifically during immunohistochemical analysis, for unknown reasons, and thus it is extremely important to employ appropriate negative controls.
Table 1.
Proteins localized to the site of spermiationa
| ES throughout spermiogenesis and early spermiationb | 14-3-3, actin, afadin, Cdc42, Csk, Cathepsin L, desmoglein, espin, FAKTyr397, fimbrin, fyn, galectin 1, ILK, α6β1 integrin, Keap1, laminin 3, MT-MMP1, Nectin 2&3, Par6, PI3 kinase, PKB, PTEN, Rab 4a, 12 & 13, Src, testin, vinculin, zyxin |
| Entire spermiation processc | actin, β-catenin, Cdc42, cortactin, Csk, ERK (phospho and total), FAKTyr397, fyn, galectin 1, laminin 333, α6β1 integrin, MEK, MT-MMP1, MMP-2, pan-cad, PI3 kinase, PTEN, Rab4a, Src, Timp2 |
| Tubulobulbar complex | actin, amphiphysin 1, Arp2/3, clathrin, cofilin, cortactin, dynamin 2&3, Eps8, Eps15, N-WASP, paxillin |
Proteins were included where there was appropriate information on their localization in stage VII and VIII tubules. See Sup. Table 1 for further information and references.
Protein localized at or near the site of ES between elongating spermatids and Sertoli cells during spermiogenesis (step 8 and beyond) and present during early spermiation.
Protein localized to spermiation in stage VII and VIII tubules, until spermatid disengagement.
As discussed, removal of the ES structure necessarily occurs when the spermatid is present in a somewhat precarious position at the luminal edge and thus strong adhesion must be maintained during, and after, ES removal. There is evidence to suggest that adhesion during TBC formation, ES structure removal and spermatid remodelling is achieved by an integrin-based junction with similarities to a Focal Adhesion (FA). Focal adhesions are large, macromolecular complexes which mediate adhesion between cells and extracellular matrix (ECM). They are capable of rapid adhesion formation and disassembly (such as observed in migrating cells), and also of tight, stable adhesion. They first form as small focal adhesion complexes but, upon various triggers such as tensile force, mature into a stable FA with considerable adhesive strength, and are highly dynamic in terms of their structure and protein composition.48–50 The well-known ability of FAs to act as sensors to the extracellular environment is conferred by integrins, which are key transmembrane adhesion receptors in FAs.51 The cytoplasmic tails of integrin receptors in FAs interact via linker proteins with actin filaments within the cell, and bind to ECM components on their extracellular surface.49 FAs show an extraordinary degree of molecular complexity.48,49
The ES adhesion domain that is present between Sertoli cells and elongating spermatids throughout spermiogenesis appears to comprise a number of different adhesion junction components, including adherens junction proteins52 and FA components,9,11,53,54 (see Sup. Table 1). As the ES structure forms opposite step 8 round spermatids, α6β1 integrins,53 integrin-linked kinase (ILK),53 and phosphorylated FAK55,56 can be seen in the vicinity of the developing ES, and α6β1 integrins are present opposite elongating spermatids throughout spermiogenesis.57,58 Sertoli cells express α6β1 integrin, and the likely ligand on elongating and elongated spermatids is laminin 333.56,59 Thus the ES adhesion domain throughout spermiogenesis includes junctions with properties of FAs.
This FA-type junction is also likely to be an integral component of the spermiation machinery whilst the ES structure is removed. When spermatids are translocated to the luminal edge at the beginning of spermiation, α6β1 integrin becomes concentrated on the outer dorsal curvature of the spermatid head, along with phosphorylated FAK and laminin subunits37,55,56,59 as well as other FA-related proteins (Sup. Table 1). Integrins can be visualised in TBCs, indicating that they are internalized during ES removal.29 However, α6β1 integrin immunostaining also persists after ES removal, and remains at the dorsal surface of the spermatid head until disengagement (Fig. 4).37,55 Integrin-linked Kinase (ILK) appears to be removed along with the ES and is not present immediately prior to disengagement (see Fig. 4).37 Taken together, these observations suggest that a subset of integrins may be removed along with the ES structure, yet adhesion between the Sertoli cell and the spermatid is mediated by an integrin-containing junction prior to disengagement. At least 8 different integrin subunits are expressed in the seminiferous epithelium during spermiation (our unpublished data) leading to the possibility that there are dynamic changes in integrin receptor subtypes as spermiation progresses. Consistent with the concept that a FA type junction is a major component of the spermiation machinery is the fact that many FA proteins localize to this site, such as paxillin, Src and FAK (see Table 1 and Sup. Table 1). In addition, the majority of the components of the integrin “adhesome”49 are expressed in the seminiferous epithelium during spermiation, as assessed by microarray analysis (our unpublished data and reviewed in ref. 60).
Integrin clustering is well known to be triggered by an extracellular mechanical force51 thus, when spermatids are first translocated to the luminal edge at the beginning of spermiaton, shear forces on the spermatid from seminiferous tubule fluid flow could initiate clustering of integrins (either further clustering of α6β1 integrin and/or clustering of other integrins). Precise nanoclustering of integrins in FAs regulates FA functions and integrin-mediated signaling.51 Our working hypothesis is that the initiation of spermiation causes a further strengthening, and likely a change in proteomic composition, of the FA-type junction present in the ES adhesion domain. As ES structures are disassembled, so are some of the components of the ES adhesion domain, including nectin-mediated adhesion,27 as well as some FA components.29,37 However, tight adhesion is maintained by an integrin-FAK mediated complex, likely binding to laminin 333 on the spermatid,59 until the point of disengagement.37,55 Given that FAs are in a constant state of flux as proteins enter and exit,48,49 it is reasonable to assume that a FA in the spermiation machinery undergoes proteomic and structural changes as spermiation progresses. Interestingly, the geometry of the adhesive field in a FA controls integrin distribution and specific gene expression programs,51 leading to the possibility that changes in spermatid head shape during spermiation may influence FA structure/function in the Sertoli cell. The composition of this FA may also be modulated by endocytosis, perhaps via TBCs29 or fluid-phase endocytosis,4 and may be part of a regulatory feedback loop between spermiation and Sertoli cell function.47
Comparative studies on spermiation in non-mammalian vertebrates and invertebrates have been reviewed in reference 1, and will not be considered in detail here. However it is pertinent to note that a recent study investigated the dynamics of spermiation in Drosophila as compared to mammals.61 The observations suggest a dynamic F-actin based adhesion junction is present in somatic cyst cells and adheres to spermatids prior to their release in this species. This junction, known as an “actin cap” is not morphologically discernible at the electron microscopic level, but includes filopodia-type components and adhesion-associated proteins including-catenin, DE-cadherin, myosin subtypes and WASP as well as clathrin-associated vesicles.61 Interestingly, dynamin was shown to be required for normal spermiation in Drosophila.61 The spermiation-associated junction in Drosophila was shown to be highly dynamic and complex, suggesting that dynamic changes in the spermiation machinery may be a conserved feature of spermiation.
The complexity of the spermiation process in terms of cellular structures and processes is reflected in dynamic changes in adhesion proteins and proteomic composition as spermiation progresses. Information presented in Table 1 and Sup. Table 1 reveals some of the components of individual processes, such as TBCs and the FA present during spermiation. Many proteins that associate with the apical ES structure and adhesion domain during spermiogenesis are localized to the spermatid-Sertoli cell interaction during stage VII (Table 1 and Sup. Table 1). Some of these proteins are removed along with the ES (e.g., espin, nectins and afadin), whereas others persist until the point of disengagement (e.g., α6β1 integrin, FAK, Src and galectin 1). Identifying the components of each structure within the spermiation machinery, and understanding their changes in localization and function as spermiation progresses, will allow a better understanding of how the various aspects of spermiation are regulated.
Defects in the Spermiation Process
Consistent with the multi-faceted nature of the spermiation process, various defects to spermiation can be observed, the most common of which is a failure of spermatids to be released from Sertoli cells (Fig. 5).3 Spermatids that fail to be released or disengaged, are phagocytosed by the Sertoli cell,3,62 and spermatid heads can be observed at the base of the seminiferous tubule in stages VIII through to about XIII in the rat (Fig. 5A).63 This results in the appearance of seminiferous tubules with elongated spermatids where none would normally be seen, leading some investigators to erroneously use the phrase “delayed sperm release”, however it has been argued that a true delay of spermiation is unlikely.3 Some spermatids fail to be released even in normal animals,3,62,63 however a marked increase in spermatid retention occurs in response to various insults, reviewed in reference 3 and below.
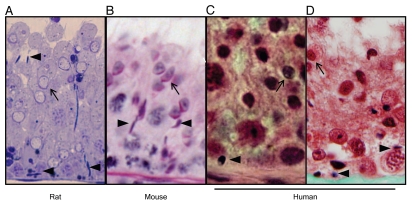
Figure 5.
Histology of spermiation failure in rats, mice and humans. (A) Spermiation failure in adult rat during acute androgen and FSH suppression, as previously described in reference 37 and 63. (B) Spermiation failure in a genetically modified mouse, as previously described in reference 65. (C) Spermiation failure in adult human undergoing androgen and progestin-based contraceptive for 6 weeks, as previously described in reference 68. (D) Spermiation failure in infertile adult human. In rats and mice, spermatid disengagement occurs towards the end of stage VIII, whereas in humans disengagement occurs in stage II. The appearance of mature spermatids in the epithelium in stage IX onwards in rats and mice, and in stages III–IV in the human is indicative of spermiation failure. In all micrographs, retained mature spermatid heads (arrowheads) are visible within the seminiferous epithelium at a stage of spermatogenesis where no mature spermatids should be seen, as indicated by the presence of early elongating spermatids (arrows).
Careful examination of the retained spermatids can yield information on the specific site at which spermiation is disrupted. If elongated spermatids are apparent at the base of the epithelium in stage VII and early VIII, this could be caused by a failure of spermatids to initiate spermiation (i.e., to be translocated to the luminal edge) rather than a failure to disengage. Unsuccessful spermiation could also be caused by a failure early in the process (such as in stage VII), followed by spermatid phagocytosis.64 In contrast, spermatids that are retained from late stage VIII onwards are likely to be a result of the failure of spermatid disengagement at the end of spermiation.37 The appearance of the retained spermatid at the electron microscopic level and immunohistochemical analyses can be used to pinpoint the site at which spermiation is disrupted (reviewed in ref. 20, 37, 64–66). If retained spermatids lack ES structures and significant cytoplasm, then it is likely that the spermatids have completed the earlier phases of spermiation (including TBC formation and cytoplasm remodelling) but disengagement has failed (reviewed in ref. 37). Conversely, the presence of ES structure and/or excess cytoplasm on retained spermatids suggests failure of TBC formation, ES disassembly and/or spermatid remodelling. Further assessment of spermiation using appropriate immunohistochemical markers (see Table 1 and Sup. Table 1) can then be carried out to more precisely examine the mechanism of spermiation failure.37,64,67
The quantitative impact of spermiation failure on spermatid output can be difficult to establish. Figure 5A shows a micrograph of a rat stage IX tubule with spermiation failure induced by acute androgen and FSH suppression.63 These animals fail to release more than half of their spermatids as assessed by stereological analysis, meaning that sperm counts would be effectively halved within a week. However, only modest changes in seminiferous epithelial architecture are apparent. Spermiation failure can be difficult to detect in human biopsies as well, given the helical arrangement of spermatogenesis which requires accurate staging of spermatids in relation to the Sertoli cell. Figure 5C shows a testis biopsy from a man given a testosterone and progestin-based contraceptive for 6 weeks. This regime resulted in azoospermia (zero sperm in the ejaculate) yet normal numbers of elongated spermatid populations were present in the testis as determined by stereological analysis,68 suggesting a near complete failure of spermiation. Careful examination of the biopsy revealed retained spermatids in the stage immediately after spermiation, as characterized by the morphology of other cells in that portion of the epithelium.
The appearance of late spermatids undergoing phagocytosis by Sertoli cells is often an acute response of the testis to a variety of insults, including hormone manipulation and reproductive toxicant administration (reviewed in ref. 3 and see below). Many of these treatments will cause other defects in spermatogenesis, such as spermatocyte and spermatid loss/apoptosis. Thus, spermiation failure may be observed in combination with other spermatogenic defects. Indeed, chronic treatments can disrupt spermatogenesis to the extent that spermiation failure will not be observed, as elongated spermatids fail to be produced. For example, testicular testosterone suppression in the adult rat causes spermiation failure within 1 week, however elongating spermatid production gradually ceases due to a defect in mid-spermiogenesis, until spermatids are no longer available for spermiation and retained spermatids are not observed.63 This example highlights the importance of assessing both acute (within a week) and chronic time points when considering the impact of an agent on spermatogenesis.
Genetically-modified mouse models often show multiple defects to spermatogenesis in conjunction with spermiation failure (reviewed in ref. 65) and thus careful histological examination of the testis is required to determine whether spermiation failure is occurring. To this end, it is imperative to have good testicular histology (quality sections, preferably resin or plastic), appropriate staining methods such as the periodic acid-Schiffs (PAS),69 technique to facilitate accurate staging and high resolution micrographs. The presence of mature spermatids in the testis, but an absence in the epididymis usually indicates spermiation failure, and should prompt closer examination of the seminiferous epithelium. A simple comparison between the number of detergent-resistant spermatids in the testis vs. epididymis70 of wildtype and mutant tissues should also provide useful information. A discrepancy (expressed as % of wildtype) would point to a defect in spermiation. Spermiation failure will be evident as retained step 16 spermatids within the basal portion of the epithelium in stages VII–XII (see Fig. 5B). An examination of the stage at which spermatids are retained (see above), the morphology of the retained spermatids by electron microscopy, immunohistochemistry of appropriate markers of the spermiation machinery (Table 1) and markers of spermatid cytoplasm such as aquaporin 11,71 will provide insight into the mechanism of spermiation failure.
It is anticipated that some defects in spermiation may not impair spermatid disengagement, but would result in the release of sperm with abnormal morphology, reviewed in reference 3. In particular, spermatids in which head shape is relatively normal (indicating normal spermiogenesis and manchette formation) yet there is residual cytoplasm around the flagella, would be suggestive of defects to the spermiation process (see the Regulation of Spermiation section under “Spermatid cytoplasm elimination”). A careful assessment of when the sperm abnormality arises in the testis, e.g., early in spermiogenesis or at the end of maturation, will provide important insights.
Abnormal spermiation may also have an adverse impact on earlier germ cell development. Compromised spermiation could cause abnormal signaling within the Sertoli cell (e.g., adhesion-related signaling pathways), the failure of residual body phagocytosis which would impact on Sertoli cell function, and importantly, the added requirement for the Sertoli cell to phagocytose large numbers of spermatogenic cells. All of these events would promote changes in Sertoli cell function which could in turn impact on their ability to support germ cell development in general. This proposition is supported by transgenic mouse models demonstrating spermiation failure in younger animals and a progressive decline in other aspects of spermatogenesis (reviewed in ref. 72).
Regulation of Spermiation
Spermiation failure can be a sign that “all is not well” in the testis. Lonnie Russell reported that failure of sperm release was observed in ∼50% of all the abnormal treatments/conditions he examined in common laboratory species, and that it was usually an early feature of many forms of testicular damage.3 It is highly sensitive to certain disruptions, particularly reproductive toxicants and is often the first indication of spermatogenic damage, although subsequent defects to spermatogenesis will follow (reviewed in ref. 3, 63 and 73). We have observed spermiation failure in combination with other spermatogenic defects in a number of genetically-modified mouse models (O'Donnell L and O'Bryan MK, unpublished observations). This suggests that spermiation is regulated at many levels and by a variety of signal transduction pathways.
Endocrine and paracrine regulation.
It is well known that spermiation is responsive to changes in testicular hormones. FSH and androgen, acting on their receptors in Sertoli cells, are major endocrine regulators of spermiation.2 Ablation of both hormones by hypophysectomy causes retention of step 19 spermatids in adult rats.62 The suppression of either FSH (by immunoneutralization) or androgen (by LH suppression resulting from low dose exogenous androgen and estradiol administration) alone causes spermiation failure, however suppression of both has a synergistic effect.63 Using this model of FSH and androgen suppression, approximately 50% of spermatids in the testis failed to be released after 1 week.63 The addition of the androgen receptor antagonist flutamide to this model, to block residual androgen action, caused more than 90% of spermatids to fail to be released after 4 days.60 Thus acute androgen and FSH blockade in the testis causes rapid and near-complete spermiation failure before other major defects in spermatogenesis become evident.60
Analysis of the spermiation failure phenotype during androgen and FSH suppression demonstrates that the earlier phases of spermiation, such as ES and spermatid cytoplasm removal, occur normally, but there is a failure of spermatids to disengage from Sertoli cells at the end of spermiation.37 Spermatids retained in late VIII/early stage IX tubules remained associated with α6β1 integrin and phosphorylated FAK (FAKTyr397),37,55 suggesting that failure to disengage may be due to failure of an integrin-based FA to “let go” of the spermatid at the end of the spermiation process. These results suggest that FSH and androgens act on signaling pathways within Sertoli cells to modulate the function of the FA at the spermiation machinery.
FSH and androgen act on distinct signaling pathways within Sertoli cells74 and have independent effects on spermatogenesis. Yet they also act co-operatively to control germ cell survival and spermiation2 and it has long been speculated they may regulate common signaling pathways in Sertoli cells.75 Transcriptional changes occurring in stages VII and VIII in the androgen and FSH suppression rat model involved genes associated with lysosome function and lipid metabolism in Sertoli cells, likely reflecting the requirement of Sertoli cells to phagocytose spermatids that fail to be released. Changes in various cell adhesion molecules were also seen, such as a marked downregulation of Lgals1 which encodes the cell adhesion-associated protein galectin 1. Galectin 1 is present in Sertoli cells at the site of spermiation76 and is involved in β1 integrin activation,77 and thus is a potential mediator of the androgen and FSH-mediated failure of spermatid disengagement.
Recent studies show that estrogen can also regulate spermiation in the rat.64,78 Exogenous estradiol administration (100 µg/kg/day for 10 days) suppressed FSH and intratesticular testosterone levels (to a lesser extent than the model of FSH and androgen suppression described above) and caused a 5 fold elevation of testicular estradiol.78 This regime increased germ cell apoptosis in stages VII and VIII and caused a marked induction of spermiation failure.78 Characterization of the mechanism of spermiation failure revealed an interesting series of defects; some spermatids failed to be released at the end of spermiation and were immunopositive for α6β1 integrin,64 as observed during androgen and FSH suppression.60 Failure to initiate spermiation was also evident, with some spermatids failing to translocate to the luminal edge at the beginning of stage VII.64 Strikingly, TBCs, as assessed by electron microscopy and localization of markers such as ARP3 and actin, failed to form.64 Microarray analyses revealed a reduction in the expression of genes associated with the Arp 2/3 complex (Arpc1b and Arpc5l), which is present in TBCs along with its activator N-WASP22,24 as well as genes associated with endocytic function in Sertoli cells (such as Stx8, Ralbp1, Trappc1, Lamp2).79 Collectively, this suggests that intra-testicular estradiol targets the expression of genes important for TBC formation and function. Interestingly, in our androgen and FSH suppression model we observed no change or a slight increase, in the expression of these genes and of other Arp2/3 subunits (reviewed in ref. 60 and our unpublished data), suggesting this effect on TBCs is mediated by estrogen rather than androgen. Estradiol also caused stage-specific changes in Sertoli cell microtubules, which may underlie the failure to initiate spermiation.64 The results of these studies may explain why failed TBC formation, together with defective spermatid cytoplasm removal, was observed in hypophysectomized rats given large doses of exogenous testosterone;66 the 100 cm testosterone-filled silastic implants given in this study would have likely produced high intratesticular estradiol levels.
Retinoic acid (RA), metabolized from retinol (Vitamin A), exerts its effects by binding to the nuclear retinoid receptors (RARα, β and γ) and retinoid X receptors (RXRα, β and γ). These receptors heterodimerize to control the expression of RA responsive genes, reviewed in reference 80. An elegant series of studies on a number of transgenic mouse models have revealed that RA acting on a RARα/RXRβ heterodimer expressed in Sertoli cells is essential for spermiation (reviewed in ref. 73, 81–85 and Table 2). RARα is activated by RA but ligand-dependent activation of RXRβ is not required for spermiation.82,84 Spermiation failure is an acute feature of and is highly sensitive to, a lack of RA signaling and is manifested by retained spermatids in stages IX–X and a reduced epididymal sperm count. Some spermatids fail to initiate spermiation in stage VII83,84 whereas others may fail to disengage. RARα/RXRβ in Sertoli cells may co-operate to some degree with AR signaling and/or regulate the expression of adhesion junction components (reviewed in ref. 84 and references therein).
Table 2.
Examples of genetically-modified rodent models with a potential phenotype of spermiation failure
| Gene | Function | Cella | Model | Spermiation phenotypeb |
| Sox8 | Transcription factor | SC | Null | Retained spermatids, failure of ES removal (EM and immunohistochemistry). Sub-fertility.65 |
| AR | Androgen receptor | SC | Hypomorph (partial androgen insensitivity) | Reduction in epididymal sperm counts, retained spermatids.216 |
| Clusterin | Glycoprotein with various functions | SC | Null | Retained spermatids in stage IX onwards. Fertile.217 |
| RARα Retinoic acid receptor α | Receptor for retinoic acid | SC | Null, and SC-specific ablation | Failure of spermatids to “line up” at luminal edge, retained spermatids in stages VIII and IX. Suggests at least some sperm fail to initiate spermiation. Reduced sperm content in epididymis. Other defects in spermiogenesis. Infertility.83,218 |
| RXRβ Retinoid X receptor β | Cellular response to retinoic acid, heterodimerizes with other nuclear receptors | SC | Null (whole body, and SC-specific ablation) | Retained spermatids in stages VII–IX, some likely due to failure to initiate spermiation. Few eST in epididymis. Other defects in sperm morphology. Infertility.81,84 |
| Rbp4; Retinol binding protein | Bioavailability of retinol to tissues | NA | Retinol deficiency when fed vitamin A deficient diet | Retained mature spermatids in stages IX–X, described as partial spermiation failure. Reduced sperm content in epididymis. Other spermiogenic defects.73 |
| γ-tubulin | Microtubule nucleation (this model disrupts microtubules) | All | Adenoviral-vector mediated over expression in SC | Retained spermatids observed in stages VII–VIII indicative of failure to initiate spermiation. Increased numbers of retained spermatids and residual bodies in stages IX–XIV indicates defective disengagement and residual body processing.92 |
| Amphiphysin I | Roles in clathrin-mediated endocytosis, actin dynamics & membrane curvature sensing | SC | Null | Reported reduction in TBC formation. Retained spermatids in stage VIII.99 |
| Ehd1 | Endocytic recycling of receptors | SC and GC | Null | Presence of elongated spermatids but no sperm in epididymis. Retained spermatids in stages VIII and IX. Evidence of failure to remove ES and spermatid cytoplasm. Other defects including spermiogenic defects. Infertility.72 |
| Spem1 | Unknown | elST | Null | Infertility due to defective removal of spermatid cytoplasm in late spermatids. Retained spermatids not described.107 |
| Capza3 | Regulates actin dynamics | elST | Missense mutation (Repro23) | Delayed spermiation; sperm apparently released in stage X, retained spermatids not described, abnormal removal of cytoplasm during spermiation. Other defects in sperm morphology. Infertility.106 |
| Slx/Slx1 | Unknown, gene expressed on X chromosome | rST | Transgenic delivery of shRNA | Retained spermatids in stages IX–XI, reduced epididymal sperm count. Abnormalities in spermatid morphology from step 9 onwards, abnormal sperm in epididymis. Infertility.219 |
| Tarbp2 | Encodes Prbp protein; in spermatids this controls translational activation of protamines | rST | Null | Retained spermatids in stages VIII–XI, few mature sperm in epididymis. Other abnormalities in spermiogenesis including abnormal head shaping and loss of immature spermatids. Infertility.108 |
Seminiferous epithelial cell type in which the protein is expressed: SC, Sertoli cell; rST, round spermatid; elST, elongating spermatid; eST, elongated spermatid.
Descriptions of fertility status are included where information was provided.
Oxytocin is first produced by Leydig cells at a time that coincides with the initiation of spermiation in the pubertal rat86 and stimulates seminiferous tubule contractility in a stage-specific manner, with spermiation-stage tubules showing the greatest response.87 Transgenic mice overexpressing oxytocin showed earlier spermiation, as evidenced by the appearance of residual bodies and of sperm in the epididymis, compared to wildtype mice during the first wave of spermatogenesis.88 Conversely, pubertal mice deficient in oxytocin showed a delay in spermiation and the appearance of sperm in the epididymis.88 These observations confirm previous studies suggesting a role for oxytocin in spermiation and sperm transport.86 Oxytocin may regulate spermiation by specific intracellular signaling pathways leading to release, and/or by influencing seminiferous tubule contractility or fluid production which in turn modulates spermatid release and their subsequent transport to the epididymis.
Interestingly, spermiation failure was observed in rats treated with drugs that interfere with potassium channels.89 These drugs also reduced seminiferous tubule fluid secretion,89 suggesting that disturbances in tubule fluid flow and/or potassium channels in Sertoli cells may influence spermatid release. Disturbances in androgens and estrogens can also influence fluid dynamics within the testis (reviewed in ref. 90 and 91). The concept that fluid flow may influence aspects of spermiation is important, as fluid forces on the FA between the spermatid and the Sertoli cell may influence adhesion, as described in the “Dynamic Changes in Adhesion Structures and Protein Localization during Spermiation” section and other systems.51
From the above discussion it is clear that different aspects of spermiation are targeted by multiple signaling pathways under endocrine and paracrine control, and disturbances in these pathways cause spermiation failure. Observations from geneticallymodified mouse models (Table 2) and studies on individual processes are starting to provide new information regarding the regulation of specific aspects of the spermiation machinery.
Initiation of spermiation.
The failure to initiate spermiation at the beginning of stage VII is commonly observed (see “Endocrine and paracrine regulation” under Regulation of Spermiation section and Table 2). Translocation of spermatids to the luminal edge, and hence the initiation of spermiation, depends on microtubule dynamics in Sertoli cells. Agents that impair microtubule dynamics, such as colchicine and taxol, prevent the translocation of spermatids to the luminal edge.10,13,44 Spermatid translocation is facilitated by microtubules and associated motor proteins, which interact with the cytoplasmic face of the ES structure within the Sertoli cell.8 Adenoviral-mediated overexpression of γ-tubulin (which nucleates microtubules) in Sertoli cells caused an increase in tubulin protein around the heads of step 19 spermatids in rats, and was associated with a failure to initiate spermiation in stage VII (see Table 2).92 Furthermore, exogenous estradiol administration (see above) caused a failure to initiate spermiation, and coincided with disturbances in microtubule localization in stage VII.64 Hence, pathways within Sertoli cells that regulate microtubule dynamics, polarity and association with proteins and molecular motors, are critical for the successful initiation of spermiation.
ES disassembly and TBC formation.
As discussed in the Morphological, Ultrastructural and Functional Aspects of Spermiation section [“Tubulobulbar complexes (TBCs)”], dissolution of ES structures and TBC formation are linked. It seems likely that independent mechanisms will modulate each process, which occur simultaneously at the dorsal and ventral surfaces of the spermatid head. Such mechanisms include local regulation of actin dynamics, signal transduction pathways and endocytic pathways involved in trafficking proteins to either recycling or degradative pathways.12,47,93
An emerging picture of the composition of TBCs, and the endocytic vesicles that arise from these structures (reviewed in ref. 22, 24, 27 and 29 and see the “Tubulobulbar complexes (TBCs)” subsection and Table 1), provides clues as to the potential regulators of their formation and function. TBC formation is preceded by the appearance of clathrin-coated pits,22 which facilitate the sorting and packaging of post-translationally-tagged cargo proteins in plasma membranes into early endosomes. Elucidation of the clathrin-mediated endocytosis interactome highlights the complexity of this process and identifies key “hubs”, such as the adaptor protein complex AP2 and accessory proteins that regulate endocytotic pathways, for future research attention.94 The C-terminal Eps15 homology domain-containing (EHD) protein Ehd1 participates in clathrin-mediated endocytosis by regulating the rate of endocytic recycling to the cell surface,72 but can also participate in the Arf6-mediated pathway for bulk recycling of plasma membrane proteins through non-clathrin-associated intermediates.95 Cell lines lacking Ehd1 show defective endocytic recycling of β1 integrin, resulting in slower FA disassembly and the formation of larger, more prominent FAs.96 Ehd1-deficient mice are infertile, and exhibit spermatid retention and defective removal of ES and spermatid cytoplasm (Table 2).72 TBC structures were not directly assessed in this model, however it is possible that Ehd1 is an early component of TBCs and that this will be a useful model for investigating regulation of TBC formation and adhesion junction dynamics during spermiation.
Amphiphysin I and N-WASP are likely to be key regulators of TBC formation and ES disassembly. N-WASP, found in TBCs22 and the Sertoli cell cytoplasm during spermiation,26 is a member of the WASP family of proteins which activate the Arp2/3 complex to promote actin nucleation. The ability of N-WASP to activate the Arp2/3 complex is conferred by WASP Interacting Proteins (WIPs) and by binding to phosphatidylinositol 4,5-bisphosphate.97 CR16 (WIPF3) is one such N-WASP-interacting protein that localizes to the site of spermiation.98 Mice lacking this protein are sterile due to abnormal sperm morphology arising during the final stages of maturation (thus potentially during spermiation, although this was not directly assessed) and have reduced levels of N-WASP in the testis.98 Interestingly, inhibition of N-WASP activity in the testis was associated with the loss of proper orientation of late spermatids during spermiation.25 Amphiphysin I, present in TBCs,99 has binding sites for clathrin and dynamin and was recently shown to be an N-WASP-interacting protein in Sertoli cells.97 This interaction promoted actin polymerization in vitro,97 revealing a mechanism whereby amphiphysin I can modulate actin dynamics.97 Amphiphysin I null mice are reported to have reduced numbers of TBCs and an increase in retained spermatids in stage VIII (Table 2).99 The authors speculated that amphiphysin I may participate in TBC formation rather than endocytosis, since it did not modulate endocytic transferrin uptake by Sertoli cells in vitro,99 supporting the concept that it may regulate actin dynamics via N-WASP within TBCs. Collectively, these studies demonstrate that the regulation of actin dynamics by the Arp2/3 complex, N-WASP and interacting proteins are likely to be critical for TBC formation and ES disassembly. Furthermore, actin polymerization is required for successful completion of spermiation, in support of early studies demonstrating TBC formation was prevented by cytochalasin D.10
Mechanisms of apical ES disassembly in the rat have been extensively studied using the pharmacological agent, adjudin, which affects adhesion junctions in the seminiferous epithelium, reviewed in reference 12 and 47. Adjudin treatment does not cause spermatid retention but instead causes a rapid (within 4 hr) loss of elongated spermatids from the epithelium, followed by a loss of round spermatids within 24 hr.100 These germ cells are found in the tubule lumen, suggesting that this model may initially be one of premature dissolution of apical ES junctions. Notably, elongating and elongated spermatids are depleted from the epithelium in all spermatogenic stages100 indicating that this treatment acutely interferes with the ES during spermiogenesis, but a specific impairment of spermiation is not seen. Longer term treatment (>2 weeks) causes a loss of pachytene spermatocytes100 and degenerating germ cells,101 resulting in the presence of only spermatogonia and Sertoli cells.100 Electron microscopy reveals vacuolization of Sertoli cells and widening of the intercellular space between germ cells and Sertoli cells.101 While this model is not specific for spermiation, it has provided interesting insights into the events that may be involved in ES dissolution during spermiation. This information has been recently reviewed in reference 12, 34 and 47, so will not be considered in detail here, however it is worth noting that these studies point to the roles of numerous signal transduction pathways, including the phosphatidylinositol-3-102 and ERK MAP-103 kinase pathways, the activation of proteases such as matrix metalloproteases104 and factors regulating cell polarity and actin polymerization26,105 in apical ES disassembly.
Taken together, the above studies and the information discussed in the Morphological, Ultrastructural and Functional Aspects of Spermiation section suggest that dissolution of the ES and TBC formation in the initial phases of spermiation involve a complex series of events including endocytic pathways, regulation of actin dynamics, signal transduction events and regulation of adhesion structures. A better understanding of each of these processes, and their inter-dependence, will be gained from the generation of models that specifically inhibit each process during spermiation.
Spermatid cytoplasm elimination.
TBCs are thought to participate in spermatid cytoplasm removal, however this has not been conclusively proven and the factors regulating remodelling of the spermatid during spermiation remain elusive. While it seems likely that excess cytoplasm is removed principally by the Sertoli cell during spermiation (see Morphological, Ultrastructural and Functional Aspects of Spermiation), emerging evidence from transgenic mouse models (Table 2) suggests that spermatids may influence cytoplasm removal by producing proteins essential for dynamic changes in their cytoskeleton. For example, a missense mutation in the Capza3 gene in mice results in infertility due to abnormal sperm morphology and a failure to shed excess cytoplasm during spermiation.106 Capza3 is localized in elongated spermatid cytoplasm and is an actin-capping protein involved in the regulation of F-actin dynamics. Spermatids within the Capza3 mutant epididymis have a “bag” of excess cytoplasm around their heads, together with other flagellar and structural abnormalities that likely arose earlier in the spermiogenic process. The retention and phagocytosis of spermatids within Sertoli cells has not been described in these mice, however, there is evidence to suggest that spermatids with abnormal cytoplasm persist longer in the epithelium, into stage IX, before eventually being released.106 This fascinating phenotype points to two novel concepts: (1) that a delay of disengagement may be possible, as the Sertoli cell tries unsuccessfully to remodel the abnormal spermatid (contradicting earlier reviews stating that such a delay is unlikely1) and (2) that the spermatid may influence its own ability to undergo successful spermiation.
This latter concept is supported by observations in mice deficient in the Spermatid Maturation 1 (Spem1) gene (see Table 2).107 SPEM1 is localized in the cytoplasm of late (step 14–16) spermatids in mice, however its function is unknown.107 Ablation of this gene results in infertility due to abnormal sperm morphology arising during the final steps of maturation, likely during spermiation. Sperm in the epididymis exhibit gross cytoplasmic abnormalities, with the cytoplasm remaining attached to and connecting the head and middle piece of the tail, so that sperm heads are bent back onto the flagella.107 This phenotype of sperm abnormality is also observed in a subset of mice deficient in a variety of genes important in late spermiogenesis (including Tarbp2,108 see Table 2), raising the intriguing possibility that various defects during spermiogenesis may contribute to the failure of cytoplasmic removal during spermiation (reviewed in ref. 107). It is thus possible that abnormalities in the spermatid physically restrict the movement of the cytoplasm and/or hinder the Sertoli cell's ability to “strip off ” the cytoplasmic lobe.
Loss of water from the spermatid cytoplasm likely contributes to the loss of cytoplasmic “bulk” during spermiation.31 Aquaporins are water-selective channels that allow transport of water across plasma membranes. Aquaporin 11 is found in mature elongated spermatid cytoplasm and has been proposed to play a role in concentration of the spermatid cytoplasmic lobe.106 However, knockout mice die before a reproductive phenotype can be revealed, thus the role of aquaporins in cytoplasmic lobe condensation remains unclear.109
In summary, it now seems likely that the spermatid can modulate its own ability to be remodelled by the Sertoli cell, and thus to undergo successful spermiation, by the co-ordinated expression of genes required for normal sperm morphology and cytoplasm remodelling.
Adhesion during spermiation and the final disengagement.
Control of adhesion dynamics during spermiation allows the Sertoli cell to adhere to the spermatid during its final remodelling, and for the ultimate disengagement of the mature spermatid. There is little understanding of how adhesion is maintained while the ES is removed, although the participation of a FA-type junction seems likely (see Dynamic Changes in Adhesion Structures and Protein Localization during Spermiation).
The contribution of microtubules and actin filaments to the process of disengagement can be inferred from models employing the administration of pharmacological agents. Spermatid disengagement is not prevented by agents, such as taxol, that interfere with microtubule dynamics, suggesting that microtubules are not involved in this process.10,44 These observations are consistent with the finding that microtubules show no particular association with the spermatid head just prior to disengagement.55 Although FAs are actin-dependent structures48 the finding that the addition of an inhibitor of actin polymerization, cytochalasin D, does not impair disengagement suggests that actin polymerization is not directly involved in disengagement.10
Given that the junction likely to control disengagement is an integrin-based FA-type junction (see Dynamic Changes in Adhesion Structures and Protein Localization during Spermiation) and that disengagement is likely an instantaneous “loss of adhesion” event (see Morphological, Ultrastructural and Functional Aspects of Spermiation), future research could be directed towards elucidating the molecular mechanisms by which the Sertoli cell FA loses its adhesive properties. Perhaps clues can be gained from studies investigating FAs at the trailing edge of migrating cells, reviewed in reference 110, where there is a rapid loss of adhesion and FA disassembly. Key triggers of FA disassembly in migrating cells include FAK-Src signaling, local regulation of ERK,111 calpain-mediated proteolysis of FA components112 and the reversal of certain processes involved in FA maturation.110 Proteins that remain between the spermatid and the Sertoli cell until the point of disengagement (Table 1 and Sup. Table 1) are all potential candidates for regulating sperm release. These include adhesion related proteins such as α6β1 integrin and galectin 1, signaling proteins (including phosphorylated FAK, Src, Cdc42, Csk, PTEN, PI3kinase and ERK) and proteases, such as MT-MMP1 and MMP2.
Most of our understanding of the regulation of spermatid disengagement comes from in vitro studies investigating the release of spermatids from cultured stage VIII tubules.113 Sperm released from these tubules have normal morphology and lack excess cytoplasm (our unpublished observations) suggesting that they are released at the end of spermiation at or near the time of disengagement by normal means. These studies demonstrated that protein phosphorylation, particularly serine-threonine phosphorylation, is important for disengagement, as various pharmacological modulators of kinase or phosphatase activity were able to stimulate or inhibit sperm release.113 Bacitracin, at a dose that can inhibit β1 integrin-mediated adhesion, stimulated sperm release, consistent with the concept that disengagement is integrin-mediated.113 Immunoprecipitation studies on protein interactions before and after spermatid disengagement suggest that ERK phosphorylation and 14-3-3 signaling may play important roles in spermatid release.113
Clinical Relevance of Spermiation: Roles in Contraception and Infertility
Current strategies for the development of an effective and reversible male contraceptive focus on the administration of hormones that feedback on the pituitary to inhibit LH and FSH release and thereby suppress intratesticular androgen production, reviewed in reference 114. FSH and testicular testosterone suppression in non-human primates and men causes an acute induction of spermiation failure which underlies the rapid suppression of sperm counts, followed by an impairment of spermatogenesis at the level of spermatogonial development.2,68,115–117 Heterogeneity of suppression of germ cell populations between men are observed, with some individuals maintaining significant levels of elongated spermatid production in the testis after 2–3 months of contraceptive administration.68,116,118,119 In some subjects, elongated spermatid production is evident yet they are azoospermic, while others maintain higher levels of sperm output, suggesting that the induction of spermiation failure can influence the attainment of azoospermia.118 This proposition is supported by studies in primates, which showed an inverse correlation between the number of retained spermatids in the testis and sperm output during androgen-based contraceptive administration.115
These studies demonstrate that spermiation is a target for hormone-based contraceptives in men, and that the induction of spermiation failure is, at least in part, important for achieving rapid and adequate sperm count suppression. Contraceptive agents that target spermiation would be particularly beneficial as an adjunct to other contraceptives targeting earlier phases of germ cell development, as they would hasten sperm count suppression and likely provide extra contraceptive cover during the longer term. Given that spermiation likely co-operates with other aspects of germ cell development (see the “Disengagement” subsection under “Morphological, Ultrastructural and Functional Aspects of Spermiation,” and reviewed in ref. 47), agents that specifically impair spermiation may eventually result in other defects in spermatogenesis that would improve contraceptive efficacy in the longer term. While hormone-based contraceptives show considerable promise, there continue to be issues related to the safety of long term hormone administration, and to the fact that some men consistently fail to achieve adequate sperm count suppression. Thus it is generally agreed that testis-specific, non-hormonal methods should also be explored in the hope they can show efficacy, safety and acceptability. The identification of novel agents that specifically disrupt spermiation would be of considerable clinical benefit in a contraceptive setting.
To our knowledge, defects in spermiation in isolation have not been found to cause human male infertility. This is likely due to inherent difficulties in assessing a causal relationship between human sperm phenotypes and spermiation, including a paucity of testicular biopsy material, and the fact that current guidelines for semen analysis do not provide a means to determine the quality of sperm as produced within the testis.120 Nevertheless, several features of spermiation suggest defects in this process are likely to contribute to infertility or sub-fertility in humans. Given the inherent vulnerability of spermiation to disruption in many species by environmental toxicants and endocrine disruptors (see above and reviewed in ref. 3), a variety of environmental exposures in humans could adversely affect spermiation and sperm output, whether it be a transient or long term effect. Disruption to spermiation, whether from exogenous or genetic causes, could impact on sperm number in the ejaculate and could thus underlie azoospermia, oligospermia or variability in sperm counts in a clinical setting.
The contribution of defects in the spermiation process to abnormal sperm forms in the ejaculate is potentially clinically significant. Spermiation is essential for the final remodelling of the spermatid cytoplasm and abnormalities arising during spermiogenesis or spermiation have been shown to result in ineffective removal of excess cytoplasm in mice. DNA damage in sperm is associated with a number of poor reproductive outcomes, including infertility and increased rates of miscarriage, and is likely caused by increased generation of Reactive Oxygen Species (ROS) predominantly by the sperm mitochondria, but potentially other sites as well, within the epididymis.121 Abnormal sperm with excess residual cytoplasm may be an important source of ROS, which in turn may adversely impact sperm DNA integrity.121 Further basic and clinical research is required to establish links between defects in the spermiation process and the production of abnormal sperm with poor reproductive outcomes.
Unresolved Questions
The information presented in this review demonstrates the advances made in our understanding of the ultrastructural, morphological and functional aspects of spermiation. Recent developments in the field have given us a preliminary understanding of the composition of the spermiation machinery and the molecular mechanisms regulating their formation and function. But many unresolved questions remain. Such questions include: (1) what signals from Sertoli cells trigger key events during spermiation; TBC formation, ES dissolution and disengagement? (2) What is the relationship between TBC formation and ES dissolution; does local ES dissolution trigger TBC formation or vice versa? (3) Do TBCs have functions other than internalization of intercellular adhesion junctions? (4) What controls the removal of spermatid cytoplasm, and how do the spermatid and the Sertoli cell co-operate to control these processes? (5) What adhesion and associated structural molecules mediate the disengagement process? (6) How do Sertoli cells co-ordinate signals to regulate the composition and function of the adhesion junction as it remodels during spermiation? (7) What is the proteomic composition of the adhesion junction present during spermiation and how does it change as spermiation progresses? (8) Are components of the ES recycled to newly formed ESs opposite round spermatids? (9) How much autonomy does the spermatid have in spermiation? (10) Do defects in spermiation contribute to poor reproductive outcomes in men? (11) Which steps in spermiation are most amenable to inhibition as a means of contraception?
A final point to consider is whether spermiation can act as a “checkpoint” for spermatogenesis, and can act as a sensor for adverse conditions. This idea has merit from the Sertoli cell's perspective, as it can obviously retain and phagocytose spermatids the moment it “feels unwell”.1,3 Thus Sertoli cells may induce spermatid retention as a means of preventing fertility when the environmental conditions are unfavourable. However spermiation is unlikely to be a true checkpoint for sperm quality. Some evidence from transgenic models suggests that Sertoli cells have the capacity to retain spermatids that are abnormal, yet it seems unlikely that the Sertoli cell can detect a large range of abnormalities within spermatids and prevent their release. Perhaps Sertoli cells have the ability to detect certain structural defects, which may explain why some retained spermatids are observed in normal adult animals (reviewed in ref. 20 and our unpublished observations).
Conclusions
Spermiation is a fascinating process that is well documented at the ultrastructural level, with various structures and adhesion complexes working together to make up the spermiation machinery that facilitates the efficient release of normal sperm. Many proteins have been localized to this site, and we are beginning to unravel the molecular composition of aspects of the spermiation machinery. Research over the past decade has provided important insights into the composition of TBCs and associated endocytic vesicles, and their roles in adhesion junction and ES structure removal, however whether they play other roles in spermiation is unclear. Spermiation requires the efficient dissolution of the ES structure that has facilitated spermatid translocation throughout spermiogenesis and new insights into the mechanisms of ES disassembly are emerging.
An integrin-based adhesion junction maintains tight adhesion to the spermatid during ES dissolution. This allows the Sertoli cell to grip the spermatid head and extend it into the seminiferous tubule lumen as the spermatid's cytoplasm is removed. The composition of this adhesion junction changes as spermiation progresses. Some adhesion-related proteins only become apparent at the initiation of spermiation, while others have remained at the spermatid-Sertoli cell interface during the elongation phase of spermiogenesis. As the ES structure is removed, so are a subset of adhesion-associated proteins. Other proteins persist until disengagement, and are likely to mediate the instantaneous release of spermatids into the lumen. Thus the adhesion junction present within the spermiation machinery is constantly undergoing transformation as the various aspects of spermiation are completed and the Sertoli cell prepares the spermatid for release.
Since spermiation is the final maturation step of spermatogenesis and influences final head shaping and cytoplasm removal, this process contributes to sperm morphology and thus understanding it has important implications for optimizing male fertility. As a known target for contraception and perhaps infertility, agents designed to impair or promote spermiation would be of therapeutic benefit in male fertility regulation.
Acknowledgements
The authors wish to thank Ms. Georgia Balourdos for excellent technical assistance, Dr. Amanda Beardsley for providing images, and Professor Wayne Vogl for helpful comments and review of the manuscript. The authors were supported by a research grant from the NHMRC of Australia (Program Grant #494802) and NHMRC Research Fellowships (#545805 Moira K. O'Bryan and #441103 Robert I. McLachlan), and by the Victorian Government's Operational Infrastructure Support Program. PHI data audit #10–34.
Supplementary Material
References
- 1.Russell L. Role in spermiation. In: Russell LD, Griswold MD, editors. The Sertoli cell. Clearwater FL: Cache River Press; 1993. pp. 269–302. [Google Scholar]
- 2.O'Donnell L, Meachem SJ, Stanton PG, McLachlan RI. Endocrine regulation of spermatogenesis. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. San Diego, CA: Elsevier; 2006. pp. 1017–1069. [Google Scholar]
- 3.Russell LD. The perils of sperm release: Let my children go. Int J Androl. 1991;14:307–311. doi: 10.1111/j.1365-2605.1991.tb01097.x. [DOI] [PubMed] [Google Scholar]
- 4.de Kretser DM, Kerr JB. The cytology of the testis. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press Ltd.; 1988. pp. 837–932. [Google Scholar]
- 5.Ross MH. The Sertoli cell junctional specialization during spermiogenesis and at spermiation. Anat Rec. 1976;186:79–104. [Google Scholar]
- 6.Russell L. Observations on rat Sertoli ectoplasmic (‘junctional’) specializations in their association with germ cells of the rat testis. Tissue Cell. 1977;9:475–498. doi: 10.1016/0040-8166(77)90007-6. [DOI] [PubMed] [Google Scholar]
- 7.Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED. Histological and histopathological evaluation of the testis. Clearwater, Florida: Cache River Press; 1990. [Google Scholar]
- 8.Guttman J, Kimel G, Vogl AW. Dyein and plus-end microtubule-dependent motors are associaed with specialized Sertoli cell junction plaques (ectoplasmic specializations) J Cell Sci. 2000;113:2167–2176. doi: 10.1242/jcs.113.12.2167. [DOI] [PubMed] [Google Scholar]
- 9.Vogl AW, Pfeiffer DC, Mulholland D, Kimel G, Guttman J. Unique and multifunctional adhesion junctions in the testis: ectoplasmic specializations. Arch Histol Cytol. 2000;63:1–15. doi: 10.1679/aohc.63.1. [DOI] [PubMed] [Google Scholar]
- 10.Russell LD, Saxena NK, Turner TT. Cytoskeletal involvement in spermiation and sperm transport. Tissue Cell. 1989;21:361–379. doi: 10.1016/0040-8166(89)90051-7. [DOI] [PubMed] [Google Scholar]
- 11.Toyama Y, Maekawa M, Yuasa S. Ectoplasmic specializations in the Sertoli cell: new vistas based on genetic defects and testicular toxicology. Anat Sci Int. 2003;78:1–16. doi: 10.1046/j.0022-7722.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- 12.Wong EW, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochim Biophys Acta. 2008;1778:692–708. doi: 10.1016/j.bbamem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogl AW. Distribution and function of organized concentrations of actin filaments in mammalian spermatogenic cells and Sertoli cells. Int Rev Cytol. 1989;119:1–56. doi: 10.1016/s0074-7696(08)60648-8. [DOI] [PubMed] [Google Scholar]
- 14.Romrell LJ, Ross MH. Characterization of Sertoli cell-germ cell junctional specializations in dissociated testicular cells. Anat Rec. 1979;193:23–41. doi: 10.1002/ar.1091930103. [DOI] [PubMed] [Google Scholar]
- 15.Yan HH, Mruk DD, Cheng CY. Junction restructuring and spermatogenesis: The biology, regulation and implication in male contraceptive development. Curr Top Dev Biol. 2008;80:57–92. doi: 10.1016/S0070-2153(07)80002-0. [DOI] [PubMed] [Google Scholar]
- 16.Leblond CP, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann NY Acad Sci. 1952:548–573. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee JS, Panorchan P, Hale CM, Khatau SB, Kole TP, Tseng Y, Wirtz D. Ballistic intracellular nanorheology reveals ROCK-hard cytoplasmic stiffening response to fluid flow. J Cell Sci. 2006;119:1760–1768. doi: 10.1242/jcs.02899. [DOI] [PubMed] [Google Scholar]
- 18.Russell LD. Observations on the inter-relationships of Sertoli cells at the level of the blood-testis barrier: evidence for formation and resorption of Sertoli-Sertoli tubulobulbar complexes during the spermatogenic cycle of the rat. Am J Anat. 1979;155:259–279. doi: 10.1002/aja.1001550208. [DOI] [PubMed] [Google Scholar]
- 19.Russell L, Clermont Y. Anchoring device between Sertoli cells and late spermatids in rat seminiferous tubules. Anat Rec. 1976;185:259–278. doi: 10.1002/ar.1091850302. [DOI] [PubMed] [Google Scholar]
- 20.Russell LD. Further observations on tubulobulbar complexes formed by late spermatids and Sertoli cells in the rat testis. Anat Rec. 1979;194:213–232. doi: 10.1002/ar.1091940204. [DOI] [PubMed] [Google Scholar]
- 21.Russell LD, Malone JP. A study of Sertoli-spermatid tubulobulbar complexes in selected mammals. Tissue Cell. 1980;12:263–285. doi: 10.1016/0040-8166(80)90005-1. [DOI] [PubMed] [Google Scholar]
- 22.Young JS, Guttman JA, Vaid KS, Shahinian H, Vogl AW. Cortactin (CTTN), N-WASP (WASL) and clathrin (CLTC) are present at podosome-like tubulobulbar complexes in the rat testis. Biol Reprod. 2009;80:153–161. doi: 10.1095/biolreprod.108.070615. [DOI] [PubMed] [Google Scholar]
- 23.Russell LD. Spermatid-Sertoli tubulobulbar complexes as devices for elimination of cytoplasm from the head region late spermatids of the rat. Anat Rec. 1979;194:233–246. doi: 10.1002/ar.1091940205. [DOI] [PubMed] [Google Scholar]
- 24.Vaid KS, Guttman JA, Babyak N, Deng W, McNiven MA, Mochizuki N, et al. The role of dynamin 3 in the testis. J Cell Physiol. 2007;210:644–654. doi: 10.1002/jcp.20855. [DOI] [PubMed] [Google Scholar]
- 25.Guttman JA, Obinata T, Shima J, Griswold M, Vogl AW. Non-muscle cofilin is a component of tubulobulbar complexes in the testis. Biol Reprod. 2004;70:805–812. doi: 10.1095/biolreprod.103.022723. [DOI] [PubMed] [Google Scholar]
- 26.Lie PP, Chan AY, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA. 2010;107:11411–11416. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guttman JA, Takai Y, Vogl AW. Evidence that tubulobulbar complexes in the seminiferous epithelium are involved with internalization of adhesion junctions. Biol Reprod. 2004;71:548–559. doi: 10.1095/biolreprod.104.028803. [DOI] [PubMed] [Google Scholar]
- 28.Ozaki-Kuroda K, Nakanishi H, Ohta H, Tanaka H, Kurihara H, Mueller S, et al. Nectin couples cell-cell adhesion and the actin scaffold at heterotypic testicular junctions. Curr Biol. 2002;12:1145–1150. doi: 10.1016/s0960-9822(02)00922-3. [DOI] [PubMed] [Google Scholar]
- 29.Young JS, Guttman JA, Vaid KS, Vogl AW. Tubulobulbar complexes are intercellular podosome-like structures that internalize intact intercellular junctions during epithelial remodeling events in the rat testis. Biol Reprod. 2009;80:162–174. doi: 10.1095/biolreprod.108.070623. [DOI] [PubMed] [Google Scholar]
- 30.Block MR, Badowski C, Millon-Fremillon A, Bouvard D, Bouin AP, Faurobert E, et al. Podosome-type adhesions and focal adhesions, so alike yet so different. Eur J Cell Biol. 2008;87:491–506. doi: 10.1016/j.ejcb.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 31.Sprando RL, Russell LD. Comparative study of cytoplasmic elimination in spermatids of selected mammalian species. Am J Anat. 1987;178:72–80. doi: 10.1002/aja.1001780109. [DOI] [PubMed] [Google Scholar]
- 32.Tanii I, Yoshinaga K, Toshimori K. Morphogenesis of the acrosome during the final steps of rat spermiogenesis with special reference to tubulobulbar complexes. Anat Rec. 1999;256:195–201. doi: 10.1002/(SICI)1097-0185(19991001)256:2<195::AID-AR10>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 33.Kierszenbaum AL, Tres LL. The acrosome-acroplaxome-manchette complex and the shaping of the spermatid head. Arch Histol Cytol. 2004;67:271–284. doi: 10.1679/aohc.67.271. [DOI] [PubMed] [Google Scholar]
- 34.Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- 35.Bartles JR, Wierda A, Zheng L. Identification and characterization of espin, an actin-binding protein localized to the F-actin-rich junctional plaques of Sertoli cell ectoplasmic specializations. J Cell Sci. 1996;109:1229–1239. doi: 10.1242/jcs.109.6.1229. [DOI] [PubMed] [Google Scholar]
- 36.O'Donnell L, Stanton PG, Bartles JR, Robertson DM. Sertoli cell ectoplasmic specializations in the seminiferous epithelium of the testosterone-suppressed adult rat. Biol Reprod. 2000;63:99–108. doi: 10.1095/biolreprod63.1.99. [DOI] [PubMed] [Google Scholar]
- 37.Beardsley A, O'Donnell L. Characterization of normal spermiation and spermiation failure induced by hormone suppression in adult rats. Biol Reprod. 2003;68:1299–1307. doi: 10.1095/biolreprod.102.009811. [DOI] [PubMed] [Google Scholar]
- 38.Clermont Y, McCoshen J, Hermo L. Evolution of the endoplasmic reticulum in the Sertoli cell cytoplasm encapsulating the heads of late spermatids in the rat. Anat Rec. 1980;196:83–99. doi: 10.1002/ar.1091960109. [DOI] [PubMed] [Google Scholar]
- 39.Morales C, Clermont Y. Structural changes of the Sertoli cell during the cycle of the seminiferous epithelium. In: Russell LD, Griswold MD, editors. The Sertoli Cell. Clearwater FL: Cache River Press; 1993. pp. 305–329. [Google Scholar]
- 40.Villagomez M, Szabo E, Podcheko A, Feng T, Papp S, Opas M. Calreticulin and focal-contact-dependent adhesion. Biochem Cell Biol. 2009;87:545–556. doi: 10.1139/o09-016. [DOI] [PubMed] [Google Scholar]
- 41.Wang Q, Rajshankar D, Branch DR, Siminovitch KA, Herrera Abreu MT, Downey GP, et al. Protein-tyrosine phosphatase-alpha and Src functionally link focal adhesions to the endoplasmic reticulum to mediate interleukin-1-induced Ca2+ signaling. J Biol Chem. 2009;284:20763–20772. doi: 10.1074/jbc.M808828200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history and genes/proteins expressed by testicular germ cells. Part 3: developmental changes in spermatid flagellum and cytoplasmic droplet and interaction of sperm with the zona pellucida and egg plasma membrane. Microsc Res Tech. 2010;73:320–363. doi: 10.1002/jemt.20784. [DOI] [PubMed] [Google Scholar]
- 43.Franca LR, Ogawa T, Avarbock MR, Brinster RL, Russell LD. Germ cell genotype controls cell cycle during spermatogenesis in the rat. Biol Reprod. 1998;59:1371–1377. doi: 10.1095/biolreprod59.6.1371. [DOI] [PubMed] [Google Scholar]
- 44.Vogl AW, Linck RW, Dym M. Colchicine-induced changes in the cytoskeleton of the golden-mantled ground squirrel (Spermophilus lateralis) Sertoli cells. Am J Anat. 1983;168:99–108. doi: 10.1002/aja.1001680110. [DOI] [PubMed] [Google Scholar]
- 45.Komljenovic D, Sandhoff R, Teigler A, Heid H, Just WW, Gorgas K. Disruption of blood-testis barrier dynamics in ether-lipid-deficient mice. Cell Tissue Res. 2009;337:281–299. doi: 10.1007/s00441-009-0809-7. [DOI] [PubMed] [Google Scholar]
- 46.Jegou B. Spermatids are regulators of Sertoli cell function. Ann NY Acad Sci. 1991;637:340–353. doi: 10.1111/j.1749-6632.1991.tb27321.x. [DOI] [PubMed] [Google Scholar]
- 47.Cheng CY, Mruk DD. An intracellular trafficking pathway in the seminiferous epithelium regulating spermatogenesis: a biochemical and molecular perspective. Crit Rev Biochem Mol Biol. 2009;44:245–263. doi: 10.1080/10409230903061207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lo SH. Focal adhesions: what's new inside. Dev Biol. 2006;294:280–291. doi: 10.1016/j.ydbio.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 49.Zaidel-Bar R, Itzkovitz S, Ma'ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaidel-Bar R, Cohen M, Addadi L, Geiger B. Hierarchical assembly of cell-matrix adhesion complexes. Biochem Soc Trans. 2004;32:416–420. doi: 10.1042/BST0320416. [DOI] [PubMed] [Google Scholar]
- 51.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 52.Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- 53.Mulholland DJ, Dedhar S, Vogl AW. Rat seminiferous epithelium contains a unique junction (Ectoplasmic specialization) with signaling properties both of cell/cell and cell/matrix junctions. Biol Reprod. 2001;64:396–407. doi: 10.1095/biolreprod64.1.396. [DOI] [PubMed] [Google Scholar]
- 54.Kopera IA, Bilinska B, Cheng CY, Mruk DD. Sertoli-germ cell junctions in the testis: a review of recent data. Philos Trans R Soc Lond B Biol Sci. 2010;365:1593–1605. doi: 10.1098/rstb.2009.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beardsley A, Robertson DM, O'Donnell L. A complex containing alpha6beta1-integrin and phosphorylated focal adhesion kinase between Sertoli cells and elongated spermatids during spermatid release from the seminiferous epithelium. J Endocrinol. 2006;190:759–770. doi: 10.1677/joe.1.06867. [DOI] [PubMed] [Google Scholar]
- 56.Siu MK, Mruk DD, Lee WM, Cheng CY. Adhering junction dynamics in the testis are regulated by an interplay of beta1-integrin and focal adhesion complex-associated proteins. Endocrinology. 2003;144:2141–2163. doi: 10.1210/en.2002-221035. [DOI] [PubMed] [Google Scholar]
- 57.Palombi F, Salanova M, Tarone G, Farini D, Stefanini M. Distribution of beta1 integrin subunit in rat seminiferous epithelium. Biol Reprod. 1992;47:1173–1182. doi: 10.1095/biolreprod47.6.1173. [DOI] [PubMed] [Google Scholar]
- 58.Salanova M, Stefanini M, De Curtis I, Palombi F. Integrin receptor alpha6beta1 is localized at specific sites of cell-to-cell contact in rat seminiferous epithelium. Biol Reprod. 1995;52:79–87. doi: 10.1095/biolreprod52.1.79. [DOI] [PubMed] [Google Scholar]
- 59.Yan HH, Cheng CY. Laminin alpha 3 forms a complex with beta3 and gamma3 chains that serves as the ligand for alpha6beta1-integrin at the apical ectoplasmic specialization in adult rat testes. J Biol Chem. 2006;281:17286–17303. doi: 10.1074/jbc.M513218200. [DOI] [PubMed] [Google Scholar]
- 60.O'Donnell L, Pratis K, Wagenfeld A, Gottwald U, Muller J, Leder G, et al. Transcriptional profiling of the hormone-responsive stages of spermatogenesis reveals cell-, stage- and hormone-specific events. Endocrinology. 2009;150:5074–5084. doi: 10.1210/en.2009-0755. [DOI] [PubMed] [Google Scholar]
- 61.Desai BS, Shirolikar S, Ray K. F-actin-based extensions of the head cyst cell adhere to the maturing spermatids to maintain them in a tight bundle and prevent their premature release in Drosophila testis. BMC Biol. 2009;7:19. doi: 10.1186/1741-7007-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Russell LD, Clermont Y. Degeneration of germ cells in normal, hypophysectomized and hormone treated hypophysectomized rats. Anat Rec. 1977;187:347–366. doi: 10.1002/ar.1091870307. [DOI] [PubMed] [Google Scholar]
- 63.Saito K, O'Donnell L, McLachlan RI, Robertson DM. Spermiation failure is a major contributor to early spermatogenic suppression caused by hormone withdrawal in adult rats. Endocrinology. 2000;141:2779–2785. doi: 10.1210/endo.141.8.7628. [DOI] [PubMed] [Google Scholar]
- 64.D'Souza R, Pathak S, Upadhyay R, Gaonkar R, D'Souza S, Sonawane S, et al. Disruption of tubulobulbar complex by high intratesticular estrogens leading to failed spermiation. Endocrinology. 2009;150:1861–1869. doi: 10.1210/en.2008-1232. [DOI] [PubMed] [Google Scholar]
- 65.O'Bryan MK, Takada S, Kennedy CL, Scott G, Harada S, Ray MK, et al. Sox8 is a critical regulator of adult Sertoli cell function and male fertility. Dev Biol. 2008;316:359–370. doi: 10.1016/j.ydbio.2008.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Russell LD. Deformities in the head region of late spermatids of hypophysectomized-hormone-treated rats. Anat Rec. 1980;197:21–31. doi: 10.1002/ar.1091970103. [DOI] [PubMed] [Google Scholar]
- 67.Wine RN, Chapin RE. Adhesion and signaling proteins spatiotemporally associated with spermiation in the rat. J Androl. 1999;20:198–213. [PubMed] [Google Scholar]
- 68.McLachlan RI, O'Donnell L, Stanton PG, Balourdos G, Frydenberg M, de Kretser DM, et al. Effects of testosterone plus medroxyprogesterone acetate on semen quality, reproductive hormones and germ cell populations in normal young men. J Clin Endocrinol Metab. 2002;87:546–556. doi: 10.1210/jcem.87.2.8231. [DOI] [PubMed] [Google Scholar]
- 69.Leblond CP, Clermont Y. Spermiogenesis of rat, mouse, hamster and guinea pig as revealed by the periodic acid-fuchsin sulfurous acid technique. Am J Anat. 1952;90:167–215. doi: 10.1002/aja.1000900202. [DOI] [PubMed] [Google Scholar]
- 70.O'Donnell L, McLachlan RI, Wreford NG, de Kretser DM, Robertson DM. Testosterone withdrawal promotes stage-specific detachment of round spermatids from the rat seminiferous epithelium. Biol Reprod. 1996;55:895–901. doi: 10.1095/biolreprod55.4.895. [DOI] [PubMed] [Google Scholar]
- 71.Yeung CH. Aquaporins in spermatozoa and testicular germ cells: identification and potential role. Asian J Androl. 12:490–499. doi: 10.1038/aja.2010.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rainey MA, George M, Ying G, Akakura R, Burgess DJ, Siefker E, et al. The endocytic recycling regulator EHD1 is essential for spermatogenesis and male fertility in mice. BMC Dev Biol. 2010;10:37. doi: 10.1186/1471-213X-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ghyselinck NB, Vernet N, Dennefeld C, Giese N, Nau H, Chambon P, et al. Retinoids and spermatogenesis: lessons from mutant mice lacking the plasma retinol binding protein. Dev Dyn. 2006;235:1608–1622. doi: 10.1002/dvdy.20795. [DOI] [PubMed] [Google Scholar]
- 74.Holdcraft RW, Braun RE. Hormonal regulation of spermatogenesis. Int J Androl. 2004;27:335–342. doi: 10.1111/j.1365-2605.2004.00502.x. [DOI] [PubMed] [Google Scholar]
- 75.Russell LD, Corbin TJ, Borg KE, De Franca LR, Grasso P, Bartke A. Recombinant human follicle-stimulating hormone is capable of exerting a biological effect in the adult hypophysectomized rat by reducing the numbers of degenerating germ cells. Endocrinology. 1993;133:2062–2070. doi: 10.1210/endo.133.5.8404654. [DOI] [PubMed] [Google Scholar]
- 76.Dettin L, Rubinstein N, Aoki A, Rabinovich GA, Maldonado CA. Regulated expression and ultrastructural localization of galectin-1, a proapoptotic beta-galactoside-binding lectin, during spermatogenesis in rat testis. Biol Reprod. 2003;68:51–59. doi: 10.1095/biolreprod.102.006361. [DOI] [PubMed] [Google Scholar]
- 77.Elola MT, Chiesa ME, Alberti AF, Mordoh J, Fink NE. Galectin-1 receptors in different cell types. J Biomed Sci. 2005;12:13–29. doi: 10.1007/s11373-004-8169-5. [DOI] [PubMed] [Google Scholar]
- 78.D'Souza R, Gill-Sharma MK, Pathak S, Kedia N, Kumar R, Balasinor N. Effect of high intratesticular estrogen on the seminiferous epithelium in adult male rats. Mol Cell Endocrinol. 2005;241:41–48. doi: 10.1016/j.mce.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 79.Balasinor NH, D'Souza R, Nanaware P, Idicula-Thomas S, Kedia-Mokashi N, He Z, et al. Effect of high intratesticular estrogen on global gene expression and testicular cell number in rats. Reprod Biol Endocrinol. 2010;8:72. doi: 10.1186/1477-7827-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chambon P. The nuclear receptor superfamily: a personal retrospect on the first two decades. Mol Endocrinol. 2005;19:1418–1428. doi: 10.1210/me.2005-0125. [DOI] [PubMed] [Google Scholar]
- 81.Kastner P, Mark M, Leid M, Gansmuller A, Chin W, Grondona JM, et al. Abnormal spermatogenesis in RXR beta mutant mice. Genes Dev. 1996;10:80–92. doi: 10.1101/gad.10.1.80. [DOI] [PubMed] [Google Scholar]
- 82.Mascrez B, Ghyselinck NB, Watanabe M, Annicotte JS, Chambon P, Auwerx J, et al. Ligand-dependent contribution of RXRbeta to cholesterol homeostasis in Sertoli cells. EMBO Rep. 2004;5:285–290. doi: 10.1038/sj.embor.7400094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vernet N, Dennefeld C, Guillou F, Chambon P, Ghyselinck NB, Mark M. Prepubertal testis development relies on retinoic acid but not rexinoid receptors in Sertoli cells. EMBO J. 2006;25:5816–5825. doi: 10.1038/sj.emboj.7601447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vernet N, Dennefeld C, Klopfenstein M, Ruiz A, Bok D, Ghyselinck NB, et al. Retinoid X receptor beta (RXRB) expression in Sertoli cells controls cholesterol homeostasis and spermiation. Reproduction. 2008;136:619–626. doi: 10.1530/REP-08-0235. [DOI] [PubMed] [Google Scholar]
- 85.Vernet N, Dennefeld C, Rochette-Egly C, Oulad-Abdelghani M, Chambon P, Ghyselinck NB, et al. Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology. 2006;147:96–110. doi: 10.1210/en.2005-0953. [DOI] [PubMed] [Google Scholar]
- 86.Frayne J, Townsend D, Nicholson HD. Effects of oxytocin on sperm transport in the pubertal rat. J Reprod Fertil. 1996;107:299–306. doi: 10.1530/jrf.0.1070299. [DOI] [PubMed] [Google Scholar]
- 87.Harris GC, Nicholson HD. Stage-related differences in rat seminiferous tubule contractility in vitro and their response to oxytocin. J Endocrinol. 1998;157:251–257. doi: 10.1677/joe.0.1570251. [DOI] [PubMed] [Google Scholar]
- 88.Assinder SJ, Rezvani A, Nicholson HD. Oxytocin promotes spermiation and sperm transfer in the mouse. Int J Androl. 2002;25:19–27. doi: 10.1046/j.1365-2605.2002.0318a.x. [DOI] [PubMed] [Google Scholar]
- 89.Setchell BP, Ploen L, Ritzen EM. Reduction in fluid secretion by rat testis by drugs that block potassium channels. J Reprod Fertil. 1998;112:87–94. doi: 10.1530/jrf.0.1120087. [DOI] [PubMed] [Google Scholar]
- 90.Hess RA, Bunick D, Lee KH, Bahr J, Taylor JA, Korach KS, et al. A role for oestrogens in the male reproductive system. Nature. 1997;390:509–512. doi: 10.1038/37352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sharpe RM, Kerr JB, McKinnell C, Millar M. Temporal relationship between androgen-dependent changes in the volume of seminiferous tubule fluid, lumen size and seminiferous tubule protein secretion in rats. J Reprod Fertil. 1994;101:193–198. doi: 10.1530/jrf.0.1010193. [DOI] [PubMed] [Google Scholar]
- 92.Fleming SL, Shank PR, Boekelheide K. gamma-Tubulin overexpression in Sertoli cells in vivo. II: Retention of spermatids, residual bodies and germ cell apoptosis. Biol Reprod. 2003;69:322–330. doi: 10.1095/biolreprod.102.011817. [DOI] [PubMed] [Google Scholar]
- 93.Cheng CY, Wong EW, Yan HH, Mruk DD. Regulation of spermatogenesis in the microenvironment of the seminiferous epithelium: New insights and advances. Mol Cell Endocrinol. 2010;315:49–56. doi: 10.1016/j.mce.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schmid EM, McMahon HT. Integrating molecular and network biology to decode endocytosis. Nature. 2007;448:883–888. doi: 10.1038/nature06031. [DOI] [PubMed] [Google Scholar]
- 95.Caplan S, Naslavsky N, Hartnell LM, Lodge R, Polishchuk RS, Donaldson JG, et al. A tubular EHD1-containing compartment involved in the recycling of major histocompatibility complex class I molecules to the plasma membrane. EMBO J. 2002;21:2557–2567. doi: 10.1093/emboj/21.11.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jovic M, Naslavsky N, Rapaport D, Horowitz M, Caplan S. EHD1 regulates beta1 integrin endosomal transport: effects on focal adhesions, cell spreading and migration. J Cell Sci. 2007;120:802–814. doi: 10.1242/jcs.03383. [DOI] [PubMed] [Google Scholar]
- 97.Yamada H, Padilla-Parra S, Park SJ, Itoh T, Chaineau M, Monaldi I, et al. Dynamic interaction of amphiphysin with N-WASP regulates actin assembly. J Biol Chem. 2009;284:34244–34256. doi: 10.1074/jbc.M109.064204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Suetsugu S, Banzai Y, Kato M, Fukami K, Kataoka Y, Takai Y, et al. Male-specific sterility caused by the loss of CR16. Genes Cells. 2007;12:721–733. doi: 10.1111/j.1365-2443.2007.01088.x. [DOI] [PubMed] [Google Scholar]
- 99.Kusumi N, Watanabe M, Yamada H, Li SA, Kashiwakura Y, Matsukawa T, et al. Implication of amphiphysin1 and dynamin2 in tubulobulbar complex formation and spermatid release. Cell Struct Funct. 2007;32:101–113. doi: 10.1247/csf.07024. [DOI] [PubMed] [Google Scholar]
- 100.Chen YM, Lee NP, Mruk DD, Lee WM, Cheng CY. Fer kinase/FerT and adherens junction dynamics in the testis: an in vitro and in vivo study. Biol Reprod. 2003;69:656–672. doi: 10.1095/biolreprod.103.016881. [DOI] [PubMed] [Google Scholar]
- 101.Lee NP, Mruk DD, Conway AM, Cheng CY. Zyxin, axin and Wiskott-Aldrich syndrome protein are adaptors that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J Androl. 2004;25:200–215. doi: 10.1002/j.1939-4640.2004.tb02780.x. [DOI] [PubMed] [Google Scholar]
- 102.Siu MK, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280:25029–25047. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- 103.Xia W, Cheng CY. TGFbeta3 regulates anchoring junction dynamics in the seminiferous epithelium of the rat testis via the Ras/ERK signaling pathway: An in vivo study. Dev Biol. 2005;280:321–343. doi: 10.1016/j.ydbio.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 104.Siu MK, Cheng CY. Interactions of proteases, protease inhibitors and the beta1 integrin/laminin gamma3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol Reprod. 2004;70:945–964. doi: 10.1095/biolreprod.103.023606. [DOI] [PubMed] [Google Scholar]
- 105.Wong EW, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:9657–9662. doi: 10.1073/pnas.0801527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Geyer CB, Inselman AL, Sunman JA, Bornstein S, Handel MA, Eddy EM. A missense mutation in the Capza3 gene and disruption of F-actin organization in spermatids of repro32 infertile male mice. Dev Biol. 2009;330:142–152. doi: 10.1016/j.ydbio.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zheng H, Stratton CJ, Morozumi K, Jin J, Yanagimachi R, Yan W. Lack of Spem1 causes aberrant cytoplasm removal, sperm deformation and male infertility. Proc Natl Acad Sci USA. 2007;104:6852–6857. doi: 10.1073/pnas.0701669104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhong J, Peters AH, Lee K, Braun RE. A double-stranded RNA binding protein required for activation of repressed messages in mammalian germ cells. Nat Genet. 1999;22:171–174. doi: 10.1038/9684. [DOI] [PubMed] [Google Scholar]
- 109.Yeung CH, Cooper TG. Aquaporin AQP11 in the testis: molecular identity and association with the processing of residual cytoplasm of elongated spermatids. Reproduction. 2010;139:209–216. doi: 10.1530/REP-09-0298. [DOI] [PubMed] [Google Scholar]
- 110.Broussard JA, Webb DJ, Kaverina I. Asymmetric focal adhesion disassembly in motile cells. Curr Opin Cell Biol. 2008;20:85–90. doi: 10.1016/j.ceb.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 111.Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, et al. FAK-Src signaling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. doi: 10.1038/ncb1094. [DOI] [PubMed] [Google Scholar]
- 112.Franco SJ, Rodgers MA, Perrin BJ, Han J, Bennin DA, Critchley DR, et al. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat Cell Biol. 2004;6:977–983. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- 113.Chapin RE, Wine RN, Harris MW, Borchers CH, Haseman JK. Structure and control of a cell-cell adhesion complex associated with spermiation in rat seminiferous epithelium. J Androl. 2001;22:1030–1052. doi: 10.1002/j.1939-4640.2001.tb03444.x. [DOI] [PubMed] [Google Scholar]
- 114.Wang C, Swerdloff RS. Hormonal approaches to male contraception. Curr Opin Urol. 2010;20:520–524. doi: 10.1097/MOU.0b013e32833f1b4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.O'Donnell L, Narula A, Balourdos G, Gu YQ, Wreford NG, Robertson DM, et al. Impairment of spermatogonial development and spermiation after testosterone-induced gonadotropin suppression in adult monkeys (Macaca fascicularis) J Clin Endocrinol Metab. 2001;86:1814–1822. doi: 10.1210/jcem.86.4.7400. [DOI] [PubMed] [Google Scholar]
- 116.Matthiesson KL, McLachlan RI, O'Donnell L, Frydenberg M, Robertson DM, Stanton PG, et al. The relative roles of follicle-stimulating hormone and luteinizing hormone in maintaining spermatogonial maturation and spermiation in normal men. J Clin Endocrinol Metab. 2006;91:3962–3969. doi: 10.1210/jc.2006-1145. [DOI] [PubMed] [Google Scholar]
- 117.Walton MJ, Bayne RA, Wallace I, Baird DT, Anderson RA. Direct effect of progestogen on gene expression in the testis during gonadotropin withdrawal and early suppression of spermatogenesis. J Clin Endocrinol Metab. 2006;91:2526–2533. doi: 10.1210/jc.2006-0222. [DOI] [PubMed] [Google Scholar]
- 118.Matthiesson KL, Stanton PG, O'Donnell L, Meachem SJ, Amory JK, Berger R, et al. Effects of testosterone and levonorgestrel combined with a 5alpha-reductase inhibitor or gonadotropin-releasing hormone antagonist on spermatogenesis and intratesticular steroid levels in normal men. J Clin Endocrinol Metab. 2005;90:5647–5655. doi: 10.1210/jc.2005-0639. [DOI] [PubMed] [Google Scholar]
- 119.Zhengwei Y, Wreford NG, Royce P, de Kretser DM, McLachlan RI. Stereological evaluation of human spermatogenesis after suppression by testosterone treatment: heterogeneous pattern of spermatogenic impairment. J Clin Endocrinol Metab. 1998;83:1284–1291. doi: 10.1210/jcem.83.4.4724. [DOI] [PubMed] [Google Scholar]
- 120.Amann RP. Tests to measure the quality of spermatozoa at spermiation. Asian J Androl. 2010;12:71–78. doi: 10.1038/aja.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Aitken RJ, De Iuliis GN. On the possible origins of DNA damage in human spermatozoa. Mol Hum Reprod. 2010;16:3–13. doi: 10.1093/molehr/gap059. [DOI] [PubMed] [Google Scholar]
- 122.Toshima JY, Toshima J, Watanabe T, Mizuno K. Binding of 14-3-3beta regulates the kinase activity and subcellular localization of testicular protein kinase 1. J Biol Chem. 2001;276:43471–43481. doi: 10.1074/jbc.M104620200. [DOI] [PubMed] [Google Scholar]
- 123.Chaudhary J, Skinner MK. Characterization of a novel transcript of 14-3-3theta in Sertoli cells. J Androl. 2000;21:730–738. [PubMed] [Google Scholar]
- 124.Wong EW, Sun S, Li MW, Lee WM, Cheng CY. 14-3-3 Protein regulates cell adhesion in the seminiferous epithelium of rat testes. Endocrinology. 2009;150:4713–4723. doi: 10.1210/en.2009-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sun S, Wong EW, Li MW, Lee WM, Cheng CY. 14-3-3 and its binding partners are regulators of protein-protein interactions during spermatogenesis. J Endocrinol. 2009;202:327–336. doi: 10.1677/JOE-09-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chapin RE, Wine RN, Harris MW, Borchers CH, Haseman JK. Structure and control of a cell-cell adhesion complex associated with spermiation in rat seminiferous epithelium. J Androl. 2001;22:1030–1052. doi: 10.1002/j.1939-4640.2001.tb03444.x. [DOI] [PubMed] [Google Scholar]
- 127.Russell LD, Goh JC, Rashed RM, Vogl AW. The consequences of actin disruption at Sertoli ectoplasmic specialization sites facing spermatids after in vivo exposure of rat testis to cytochalasin D. Biol Reprod. 1988;39:105–118. doi: 10.1095/biolreprod39.1.105. [DOI] [PubMed] [Google Scholar]
- 128.Masri BA, Russell LD, Vogl AW. Distribution of actin in spermatids and adjacent Sertoli cell regions of the rat. Anat Rec. 1987;218:20–26. doi: 10.1002/ar.1092180105. [DOI] [PubMed] [Google Scholar]
- 129.Nakanishi A, Abe T, Watanabe M, Takei K, Yamada H. Dynamin 2 cooperates with amphiphysin 1 in phagocytosis in sertoli cells. Acta Med Okayama. 2008;62:385–391. doi: 10.18926/AMO/30954. [DOI] [PubMed] [Google Scholar]
- 130.Takei K, Slepnev VI, Haucke V, De Camilli P. Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat Cell Biol. 1999;1:33–39. doi: 10.1038/9004. [DOI] [PubMed] [Google Scholar]
- 131.Murakami N, Xie W, Lu RC, Chen-Hwang MC, Wieraszko A, Hwang YW. Phosphorylation of amphiphysin I by minibrain kinase/dual-specificity tyrosine phosphorylation-regulated kinase, a kinase implicated in Down syndrome. J Biol Chem. 2006;281:23712–23724. doi: 10.1074/jbc.M513497200. [DOI] [PubMed] [Google Scholar]
- 132.Goley ED, Welch MD. The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol Cell Biol. 2006;7:713–726. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- 133.D'Souza R, Pathak S, Upadhyay R, Gaonkar R, D'Souza S, Sonawane S, et al. Disruption of Tubulobulbar Complex by High Intratesticular Estrogens Leading to Failed Spermiation. Endocrinology. 2008;150:1861–1869. doi: 10.1210/en.2008-1232. [DOI] [PubMed] [Google Scholar]
- 134.Anahara R, Toyama Y, Maekawa M, Yoshida M, Kai M, Ishino F, et al. Anti-estrogen ICI 182.780 and anti-androgen flutamide induce tyrosine phosphorylation of cortactin in the ectoplasmic specialization between the Sertoli cell and spermatids in the mouse testis. Biochem Biophys Res Commun. 2006;346:276–280. doi: 10.1016/j.bbrc.2006.05.125. [DOI] [PubMed] [Google Scholar]
- 135.Anahara R, Yoshida M, Toyama Y, Maekawa M, Kai M, Ishino F, et al. Estrogen agonists, 17beta-estradiol, bisphenol A and diethylstilbestrol, decrease cortactin expression in the mouse testis. Arch Histol Cytol. 2006;69:101–107. doi: 10.1679/aohc.69.101. [DOI] [PubMed] [Google Scholar]
- 136.Su L, Mruk DD, Lee WM, Cheng CY. Differential effects of testosterone and TGFbeta3 on endocytic vesicle-mediated protein trafficking events at the blood-testis barrier. Exp Cell Res. 2010;316:2945–2960. doi: 10.1016/j.yexcr.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Goossens S, van Roy F. Cadherin-mediated cell-cell adhesion in the testis. Front Biosci. 2005;10:398–419. doi: 10.2741/1537. [DOI] [PubMed] [Google Scholar]
- 138.Zabludoff SD, Karzai AW, Wright WW. Germ cell-Sertoli cell interactions: the effect of testicular maturation on the synthesis of cyclic protein-2 by rat Sertoli cells. Biol Reprod. 1990;43:25–33. doi: 10.1095/biolreprod43.1.25. [DOI] [PubMed] [Google Scholar]
- 139.Wright WW. Germ cell-Sertoli cell interactions: analysis of the biosynthesis and secretion of cyclic protein-2. Dev Biol. 1988;130:45–56. doi: 10.1016/0012-1606(88)90412-5. [DOI] [PubMed] [Google Scholar]
- 140.Penttila TL, Hakovirta H, Mali P, Wright WW, Parvinen M. Follicle-stimulating hormone regulates the expression of cyclic protein-2/cathepsin L messenger ribonucleic acid in rat Sertoli cells in a stage-specific manner. Mol Cell Endocrinol. 1995;113:175–181. doi: 10.1016/0303-7207(95)03629-l. [DOI] [PubMed] [Google Scholar]
- 141.Wright WW, Smith L, Kerr C, Charron M. Mice that express enzymatically inactive cathepsin L exhibit abnormal spermatogenesis. Biol Reprod. 2003;68:680–687. doi: 10.1095/biolreprod.102.006726. [DOI] [PubMed] [Google Scholar]
- 142.Igdoura SA, Morales CR, Hermo L. Differential expression of cathepsins B and D in testis and epididymis of adult rats. J Histochem Cytochem. 1995;43:545–557. doi: 10.1177/43.5.7730593. [DOI] [PubMed] [Google Scholar]
- 143.Anway MD, Wright WW, Zirkin BR, Korah N, Mort JS, Hermo L. Expression and localization of cathepsin k in adult rat sertoli cells. Biol Reprod. 2004;70:562–569. doi: 10.1095/biolreprod.103.018291. [DOI] [PubMed] [Google Scholar]
- 144.Burdan F, Szumilo J, Dudka J, Dabrowski A, Maciejewski R, Wojtowicz Z. The activity and immunoexpression of cathepsin D in rat male reproductive organs. Folia Morphol (Warsz) 2006;65:111–115. [PubMed] [Google Scholar]
- 145.Charron M, Chern JY, Wright WW. The cathepsin L first intron stimulates gene expression in rat sertoli cells. Biol Reprod. 2007;76:813–824. doi: 10.1095/biolreprod.106.057851. [DOI] [PubMed] [Google Scholar]
- 146.Gye MC, Kim ST. Expression of cathepsin L in human testis under diverse infertility conditions. Arch Androl. 2004;50:187–191. doi: 10.1080/01485010490425223. [DOI] [PubMed] [Google Scholar]
- 147.Wong EW, Mruk DD, Lee WM, Cheng CY. Regulation of blood-testis barrier dynamics by TGFbeta3 is a Cdc42-dependent protein trafficking event. Proc Natl Acad Sci USA. 2010;107:11399–11404. doi: 10.1073/pnas.1001077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Miki H, Sasaki T, Takai Y, Takenawa T. Induction of filopodium formation by a WASP-related actin-depolymerizing protein N-WASP. Nature. 1998;391:93–96. doi: 10.1038/34208. [DOI] [PubMed] [Google Scholar]
- 149.Xia W, Wong EW, Mruk DD, Cheng CY. TGFbeta3 and TNFalpha perturb blood-testis barrier (BTB) dynamics by accelerating the clathrin-mediated endocytosis of integral membrane proteins: a new concept of BTB regulation during spermatogenesis. Dev Biol. 2009;327:48–461. doi: 10.1016/j.ydbio.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, et al. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 151.Toshima J, Toshima JY, Amano T, Yang N, Narumiya S, Mizuno K. Cofilin phosphorylation by protein kinase testicular protein kinase 1 and its role in integrin-mediated actin reorganization and focal adhesion formation. Mol Biol Cell. 2001;12:1131–1145. doi: 10.1091/mbc.12.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Toshima J, Toshima JY, Takeuchi K, Mori R, Mizuno K. Cofilin phosphorylation and actin reorganization activities of testicular protein kinase 2 and its predominant expression in testicular Sertoli cells. J Biol Chem. 2001;276:31449–31458. doi: 10.1074/jbc.M102988200. [DOI] [PubMed] [Google Scholar]
- 153.Lui WY, Lee WM, Cheng CY. Sertoli-germ cell adherens junction dynamics in the testis are regulated by RhoB GTPase via the ROCK/LIMK signaling pathway. Biol Reprod. 2003;68:2189–2206. doi: 10.1095/biolreprod.102.011379. [DOI] [PubMed] [Google Scholar]
- 154.Brehm R, Zeiler M, Ruttinger C, Herde K, Kibschull M, Winterhager E, et al. A sertoli cell-specific knockout of connexin43 prevents initiation of spermatogenesis. Am J Pathol. 2007;171:19–31. doi: 10.2353/ajpath.2007.061171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Carette D, Weider K, Gilleron J, Giese S, Dompierre J, Bergmann M, et al. Major involvement of connexin 43 in seminiferous epithelial junction dynamics and male fertility. Dev Biol. 2010;346:54–67. doi: 10.1016/j.ydbio.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 156.Li MW, Mruk DD, Lee WM, Cheng CY. Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proc Natl Acad Sci USA. 2009;106:10213–10218. doi: 10.1073/pnas.0901700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee NP, Cheng CY. Protein kinases and adherens junction dynamics in the seminiferous epithelium of the rat testis. J Cell Physiol. 2005;202:344–360. doi: 10.1002/jcp.20119. [DOI] [PubMed] [Google Scholar]
- 158.Gye MC, Choi JK, Ahn HS, Kim YS. Expression of p50 C-terminal Src kinase (Csk) in mouse testis. Arch Androl. 2004;50:287–293. doi: 10.1080/01485010490448714. [DOI] [PubMed] [Google Scholar]
- 159.Lie PP, Cheng CY, Mruk DD. Crosstalk between desmoglein-2/desmocollin-2/Src kinase and coxsackie and adenovirus receptor/ZO-1 protein complexes, regulates blood-testis barrier dynamics. Int J Biochem Cell Biol. 42:975–986. doi: 10.1016/j.biocel.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Otsuka A, Abe T, Watanabe M, Yagisawa H, Takei K, Yamada H. Dynamin 2 is required for actin assembly in phagocytosis in Sertoli cells. Biochem Biophys Res Commun. 2009;378:478–482. doi: 10.1016/j.bbrc.2008.11.066. [DOI] [PubMed] [Google Scholar]
- 161.Disanza A, Mantoani S, Hertzog M, Gerboth S, Frittoli E, Steffen A, et al. Regulation of cell shape by Cdc42 is mediated by the synergic actin-bundling activity of the Eps8-IRSp53 complex. Nat Cell Biol. 2006;8:1337–1347. doi: 10.1038/ncb1502. [DOI] [PubMed] [Google Scholar]
- 162.Scita G, Nordstrom J, Carbone R, Tenca P, Giardina G, Gutkind S, et al. EPS8 and E3B1 transduce signals from Ras to Rac. Nature. 1999;401:290–293. doi: 10.1038/45822. [DOI] [PubMed] [Google Scholar]
- 163.Lie PP, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23:2555–2567. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wunsch A, Strothmann K, Simoni M, Gromoll J, Nieschlag E, Luetjens CM. Epidermal growth factor receptor pathway substrate 8 (Eps8) expression in maturing testis. Asian J Androl. 2004;6:195–203. [PubMed] [Google Scholar]
- 165.Wong CH, Cheng CY. Mitogen-activated protein kinases, adherens junction dynamics and spermatogenesis: a review of recent data. Dev Biol. 2005;286:1–15. doi: 10.1016/j.ydbio.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 166.Chen B, Li A, Wang D, Wang M, Zheng L, Bartles JR. Espin contains an additional actin-binding site in its N terminus and is a major actin-bundling protein of the Sertoli cell-spermatid ectoplasmic specialization junctional plaque. Mol Biol Cell. 1999;10:4327–4339. doi: 10.1091/mbc.10.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zheng L, Sekerkova G, Vranich K, Tilney LG, Mugnaini E, Bartles JR. The deaf jerker mouse has a mutation in the gene encoding the espin actin-bundling proteins of hair cell stereocilia and lacks espins. Cell. 2000;102:377–385. doi: 10.1016/s0092-8674(00)00042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sluka P, O'Donnell L, Bartles JR, Stanton PG. FSH regulates the formation of adherens junctions and ectoplasmic specialisations between rat Sertoli cells in vitro and in vivo. J Endocrinol. 2006;189:381–395. doi: 10.1677/joe.1.06634. [DOI] [PubMed] [Google Scholar]
- 169.Grove BD, Vogl AW. Sertoli cell ectoplasmic specializations: a type of actin-associated adhesion junction? J Cell Sci. 1989;93:309–323. doi: 10.1242/jcs.93.2.309. [DOI] [PubMed] [Google Scholar]
- 170.Maekawa M, Toyama Y, Yasuda M, Yagi T, Yuasa S. Fyn tyrosine kinase in Sertoli cells is involved in mouse spermatogenesis. Biol Reprod. 2002;66:211–221. doi: 10.1095/biolreprod66.1.211. [DOI] [PubMed] [Google Scholar]
- 171.Deschildre C, Ji JW, Chater S, Dacheux F, Selva J, Albert M, et al. Expression of galectin-3 and its regulation in the testes. Int J Androl. 2007;30:28–40. doi: 10.1111/j.1365-2605.2006.00707.x. [DOI] [PubMed] [Google Scholar]
- 172.Timmons PM, Rigby PW, Poirier F. The murine seminiferous epithelial cycle is pre-figured in the Sertoli cells of the embryonic testis. Development. 2002;129:635–647. doi: 10.1242/dev.129.3.635. [DOI] [PubMed] [Google Scholar]
- 173.Velichkova M, Guttman J, Warren C, Eng L, Kline K, Vogl AW, et al. A human homologue of Drosophila kelch associates with myosin-VIIa in specialized adhesion junctions. Cell Motil Cytoskeleton. 2002;51:147–164. doi: 10.1002/cm.10025. [DOI] [PubMed] [Google Scholar]
- 174.Salanova M, Ricci G, Boitani C, Stefanini M, De Grossi S, Palombi F. Junctional contacts between Sertoli cells in normal and aspermatogenic rat seminiferous epithelium contain alpha6beta1 integrins, and their formation is controlled by follicle-stimulating hormone. Biol Reprod. 1998;58:371–378. doi: 10.1095/biolreprod58.2.371. [DOI] [PubMed] [Google Scholar]
- 175.Gliki G, Ebnet K, Aurrand-Lions M, Imhof BA, Adams RH. Spermatid differentiation requires the assembly of a cell polarity complex downstream of junctional adhesion molecule-C. Nature. 2004;431:320–324. doi: 10.1038/nature02877. [DOI] [PubMed] [Google Scholar]
- 176.Mirza M, Hreinsson J, Strand ML, Hovatta O, Soder O, Philipson L, et al. Coxsackievirus and adenovirus receptor (CAR) is expressed in male germ cells and forms a complex with the differentiation factor JAM-C in mouse testis. Exp Cell Res. 2006;312:817–830. doi: 10.1016/j.yexcr.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 177.Longin J, Guillaumot P, Chauvin MA, Morera AM, Le Magueresse-Battistoni B. MT1-MMP in rat testicular development and the control of Sertoli cell proMMP-2 activation. J Cell Sci. 2001;114:2125–2134. doi: 10.1242/jcs.114.11.2125. [DOI] [PubMed] [Google Scholar]
- 178.Dalmolin RJ, Zanotto-Filho A, De Oliveira RB, Duarte RF, Pasquali MA, Moreira JC. Retinol and retinoic acid increase MMP-2 activity by different pathways in cultured Sertoli cells. Free Radic Res. 2007;41:1338–1347. doi: 10.1080/10715760701717427. [DOI] [PubMed] [Google Scholar]
- 179.Robinson LL, Sznajder NA, Riley SC, Anderson RA. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in human fetal testis and ovary. Mol Hum Reprod. 2001;7:641–648. doi: 10.1093/molehr/7.7.641. [DOI] [PubMed] [Google Scholar]
- 180.Lin YF, Yeh TS, Chen SF, Tsai YH, Chou CM, Yang YY, et al. Nonmuscle myosin IIA (myosin heavy polypeptide 9): a novel class of signal transducer mediating the activation of Galpha h/phospholipase C-delta1 pathway. Endocrinology. 2010;151:876–885. doi: 10.1210/en.2009-0722. [DOI] [PubMed] [Google Scholar]
- 181.Hasson T, Walsh J, Cable J, Mooseker MS, Brown SD, Steel KP. Effects of shaker-1 mutations on myosin-VIIa protein and mRNA expression. Cell Motil Cytoskeleton. 1997;37:127–138. doi: 10.1002/(SICI)1097-0169(1997)37:2<127::AID-CM5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 182.Bouchard MJ, Dong Y, McDermott B, Jr, Lam DH, Brown KR, Shelanski M, et al. Defects in nuclear and cytoskeletal morphology and mitochondrial localization in spermatozoa of mice lacking nectin-2, a component of cell-cell adherens junctions. Mol Cell Biol. 2000;20:2865–2873. doi: 10.1128/mcb.20.8.2865-2873.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Inagaki M, Irie K, Ishizaki H, Tanaka-Okamoto M, Miyoshi J, Takai Y. Role of cell adhesion molecule nectin-3 in spermatid development. Genes Cells. 2006;11:1125–1132. doi: 10.1111/j.1365-2443.2006.01006.x. [DOI] [PubMed] [Google Scholar]
- 184.Toyama Y, Suzuki-Toyota F, Maekawa M, Ito C, Toshimori K. Disruption of ectoplasmic specializations between Sertoli cells and maturing spermatids by anti-nectin-2 and anti-nectin-3 antibodies. Asian J Androl. 2008;10:577–584. doi: 10.1111/j.1745-7262.2008.00357.x. [DOI] [PubMed] [Google Scholar]
- 185.Ciraolo E, Morello F, Hobbs RM, Wolf F, Marone R, Iezzi M, et al. Essential role of the p110beta subunit of phosphoinositide-3-OH kinase in male fertility. Mol Biol Cell. 2010;21:704–711. doi: 10.1091/mbc.E09-08-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Guttman JA, Janmey P, Vogl AW. Gelsolin: Evidence for a role in turnover of junction-related actin filaments in Sertoli cells. J Cell Sci. 2002;115:499–505. doi: 10.1242/jcs.115.3.499. [DOI] [PubMed] [Google Scholar]
- 187.Riera MF, Galardo MN, Pellizzari EH, Meroni SB, Cigorraga SB. Participation of phosphatidyl inositol-3-kinase/protein kinase B and ERK1/2 pathways in interleukin-1beta stimulation of lactate production in Sertoli cells. Reproduction. 2007;133:763–773. doi: 10.1530/rep.1.01091. [DOI] [PubMed] [Google Scholar]
- 188.Meroni SB, Riera MF, Pellizzari EH, Cigorraga SB. Regulation of rat Sertoli cell function by FSH: possible role of phosphatidylinositol-3-kinase/protein kinase B pathway. J Endocrinol. 2002;174:195–204. doi: 10.1677/joe.0.1740195. [DOI] [PubMed] [Google Scholar]
- 189.McDonald CA, Millena AC, Reddy S, Finlay S, Vizcarra J, Khan SA, et al. Follicle-stimulating hormone-induced aromatase in immature rat Sertoli cells requires an active phosphatidylinositol-3-kinase pathway and is inhibited via the mitogen-activated protein kinase signaling pathway. Mol Endocrinol. 2006;20:608–618. doi: 10.1210/me.2005-0245. [DOI] [PubMed] [Google Scholar]
- 190.Khan SA, Ndjountche L, Pratchard L, Spicer LJ, Davis JS. Follicle-stimulating hormone amplifies insulin-like growth factor I-mediated activation of AKT/protein kinase B signaling in immature rat Sertoli cells. Endocrinology. 2002;143:2259–2267. doi: 10.1210/endo.143.6.8838. [DOI] [PubMed] [Google Scholar]
- 191.Meroni SB, Riera MF, Pellizzari EH, Galardo MN, Cigorraga SB. FSH activates phosphatidylinositol-3-kinase/protein kinase B signaling pathway in 20 day-old Sertoli cells independently of IGF-I. J Endocrinol. 2004;180:257–265. doi: 10.1677/joe.0.1800257. [DOI] [PubMed] [Google Scholar]
- 192.Hixon ML, Boekelheide K. Expression and localization of total Akt1 and phosphorylated Akt1 in the rat seminiferous epithelium. J Androl. 2003;24:891–898. doi: 10.1002/j.1939-4640.2003.tb03141.x. [DOI] [PubMed] [Google Scholar]
- 193.Moon C, Kim JS, Jang H, Lee HJ, Kim SH, Kang SS, et al. Activation of Akt/protein kinase B and extracellular signal-regulated kinase in rats with acute experimental testicular torsion. J Vet Med Sci. 2008;70:337–341. doi: 10.1292/jvms.70.337. [DOI] [PubMed] [Google Scholar]
- 194.Zhang XS, Zhang ZH, Jin X, Wei P, Hu XQ, Chen M, et al. Dedifferentiation of adult monkey Sertoli cells through activation of extracellularly regulated kinase 1/2 induced by heat treatment. Endocrinology. 2006;147:1237–1245. doi: 10.1210/en.2005-0981. [DOI] [PubMed] [Google Scholar]
- 195.Ballester M, Molist J, Lopez-Bejar M, Sanchez A, Santalo J, Folch JM, et al. Disruption of the mouse phospholipase C-beta1 gene in a beta-lactoglobulin transgenic line affects viability, growth and fertility in mice. Gene. 2004;341:279–289. doi: 10.1016/j.gene.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 196.Dupont J, Musnier A, Decourtye J, Boulo T, Lecureuil C, Guillou H, et al. FSH-stimulated PTEN activity accounts for the lack of FSH mitogenic effect in prepubertal rat Sertoli cells. Mol Cell Endocrinol. 2010;315:271–276. doi: 10.1016/j.mce.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 197.Di Vizio D, Cito L, Boccia A, Chieffi P, Insabato L, Pettinato G, et al. Loss of the tumor suppressor gene PTEN marks the transition from intratubular germ cell neoplasias (ITGCN) to invasive germ cell tumors. Oncogene. 2005;24:1882–1894. doi: 10.1038/sj.onc.1208368. [DOI] [PubMed] [Google Scholar]
- 198.Roland JT, Kenworthy AK, Peranen J, Caplan S, Goldenring JR. Myosin Vb interacts with Rab8a on a tubular network containing EHD1 and EHD3. Mol Biol Cell. 2007;18:2828–2837. doi: 10.1091/mbc.E07-02-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mruk DD, Lau AS, Sarkar O, Xia W. Rab4A GTPase catenin interactions are involved in cell junction dynamics in the testis. J Androl. 2007;28:742–754. doi: 10.2164/jandrol.106.002204. [DOI] [PubMed] [Google Scholar]
- 200.Lau AS, Mruk DD. Rab8B GTPase and junction dynamics in the testis. Endocrinology. 2003;144:1549–1563. doi: 10.1210/en.2002-220893. [DOI] [PubMed] [Google Scholar]
- 201.Mruk DD, Lau AS. RAB13 participates in ectoplasmic specialization dynamics in the rat testis. Biol Reprod. 2009;80:590–601. doi: 10.1095/biolreprod.108.071647. [DOI] [PubMed] [Google Scholar]
- 202.Segretain D, Gilleron J, Carette D, Denizot JP, Pointis G. Differential time course of FSH/FSH receptor complex endocytosis within sertoli and germ cells during rat testis development. Dev Dyn. 2010;239:1113–1123. doi: 10.1002/dvdy.22261. [DOI] [PubMed] [Google Scholar]
- 203.Iida H, Noda M, Kaneko T, Doiguchi M, Mori T. Identification of rab12 as a vesicle-associated small GTPase highly expressed in Sertoli cells of rat testis. Mol Reprod Dev. 2005;71:178–185. doi: 10.1002/mrd.20294. [DOI] [PubMed] [Google Scholar]
- 204.Cheng J, Watkins SC, Walker WH. Testosterone activates mitogen-activated protein kinase via Src kinase and the epidermal growth factor receptor in sertoli cells. Endocrinology. 2007;148:2066–2074. doi: 10.1210/en.2006-1465. [DOI] [PubMed] [Google Scholar]
- 205.Wang W, Wine RN, Chapin RE. Rat testicular Src: Normal distribution and involvement in ethylene glycol monomethyl ether-induced apoptosis. Toxicol Appl Pharmacol. 2000;163:125–134. doi: 10.1006/taap.1999.8870. [DOI] [PubMed] [Google Scholar]
- 206.Gye MC, Choi JK, Ahn HS, Kim YS. Postnatal changes in the expression of p60c-Src in mouse testes. Dev Growth Differ. 2005;47:233–242. doi: 10.1111/j.1440-169X.2005.00799.x. [DOI] [PubMed] [Google Scholar]
- 207.Garvalov BK, Higgins TE, Sutherland JD, Zettl M, Scaplehorn N, Kocher T, et al. The conformational state of Tes regulates its zyxin-dependent recruitment to focal adhesions. J Cell Biol. 2003;161:33–39. doi: 10.1083/jcb.200211015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Griffith E, Coutts AS, Black DM. RNAi knockdown of the focal adhesion protein TES reveals its role in actin stress fibre organisation. Cell Motil Cytoskeleton. 2005;60:140–152. doi: 10.1002/cm.20052. [DOI] [PubMed] [Google Scholar]
- 209.Grima J, Wong CC, Zhu LJ, Zong SD, Cheng CY. Testin secreted by Sertoli cells is associated with the cell surface, and its expression correlates with the disruption of Sertoli-germ cell junctions but not the inter-Sertoli tight junction. J Biol Chem. 1998;273:21040–21053. doi: 10.1074/jbc.273.33.21040. [DOI] [PubMed] [Google Scholar]
- 210.Drusco A, Zanesi N, Roldo C, Trapasso F, Farber JL, Fong LY, et al. Knockout mice reveal a tumor suppressor function for Testin. Proc Natl Acad Sci USA. 2005;102:10947–10951. doi: 10.1073/pnas.0504934102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zong SD, Bardin CW, Phillips D, Cheng CY. Testins are localized to the junctional complexes of rat Sertoli and epididymal cells. Biol Reprod. 1992;47:568–572. doi: 10.1095/biolreprod47.4.568. [DOI] [PubMed] [Google Scholar]
- 212.Zong SD, Zhu LJ, Grima J, Aravindan GR, Bardin CW, Cheng CY. Cyclic and postnatal developmental changes of testin in the rat seminiferous epithelium—an immunohistochemical study. Biol Reprod. 1994;51:843–851. doi: 10.1095/biolreprod51.5.843. [DOI] [PubMed] [Google Scholar]
- 213.Pfeiffer DC, Vogl AW. Evidence that vinculin is co-distributed with actin bundles in ectoplasmic (“junctional”) specializations of mammalian Sertoli cells. Anat Rec. 1991;231:89–100. doi: 10.1002/ar.1092310110. [DOI] [PubMed] [Google Scholar]
- 214.Wu X, Suetsugu S, Cooper LA, Takenawa T, Guan JL. Focal adhesion kinase regulation of N-WASP subcellular localization and function. J Biol Chem. 2004;279:9565–9576. doi: 10.1074/jbc.M310739200. [DOI] [PubMed] [Google Scholar]
- 215.Tomasevic N, Jia Z, Russell A, Fujii T, Hartman JJ, Clancy S, et al. Differential regulation of WASP and N-WASP by Cdc42, Rac1, Nck and PI(4,5)P2. Biochemistry. 2007;46:3494–3502. doi: 10.1021/bi062152y. [DOI] [PubMed] [Google Scholar]
- 216.Holdcraft RW, Braun RE. Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development. 2004;131:459–467. doi: 10.1242/dev.00957. [DOI] [PubMed] [Google Scholar]
- 217.Bailey RW, Aronow B, Harmony JA, Griswold MD. Heat shock-initiated apoptosis is accelerated and removal of damaged cells is delayed in the testis of clusterin/ApoJ knock-out mice. Biol Reprod. 2002;66:1042–1053. doi: 10.1095/biolreprod66.4.1042. [DOI] [PubMed] [Google Scholar]
- 218.Chung SS, Wang X, Wolgemuth DJ. Male sterility in mice lacking retinoic acid receptor alpha involves specific abnormalities in spermiogenesis. Differentiation. 2005;73:188–198. doi: 10.1111/j.1432-0436.2005.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Cocquet J, Ellis PJ, Yamauchi Y, Riel JM, Karacs TP, Rattigan A, et al. Deficiency in the multicopy Sycp3 like X-linked genes Slx and Slxl1 causes major defects in spermatid differentiation. Mol Biol Cell. 2010;21:3497–3505. doi: 10.1091/mbc.E10-07-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.