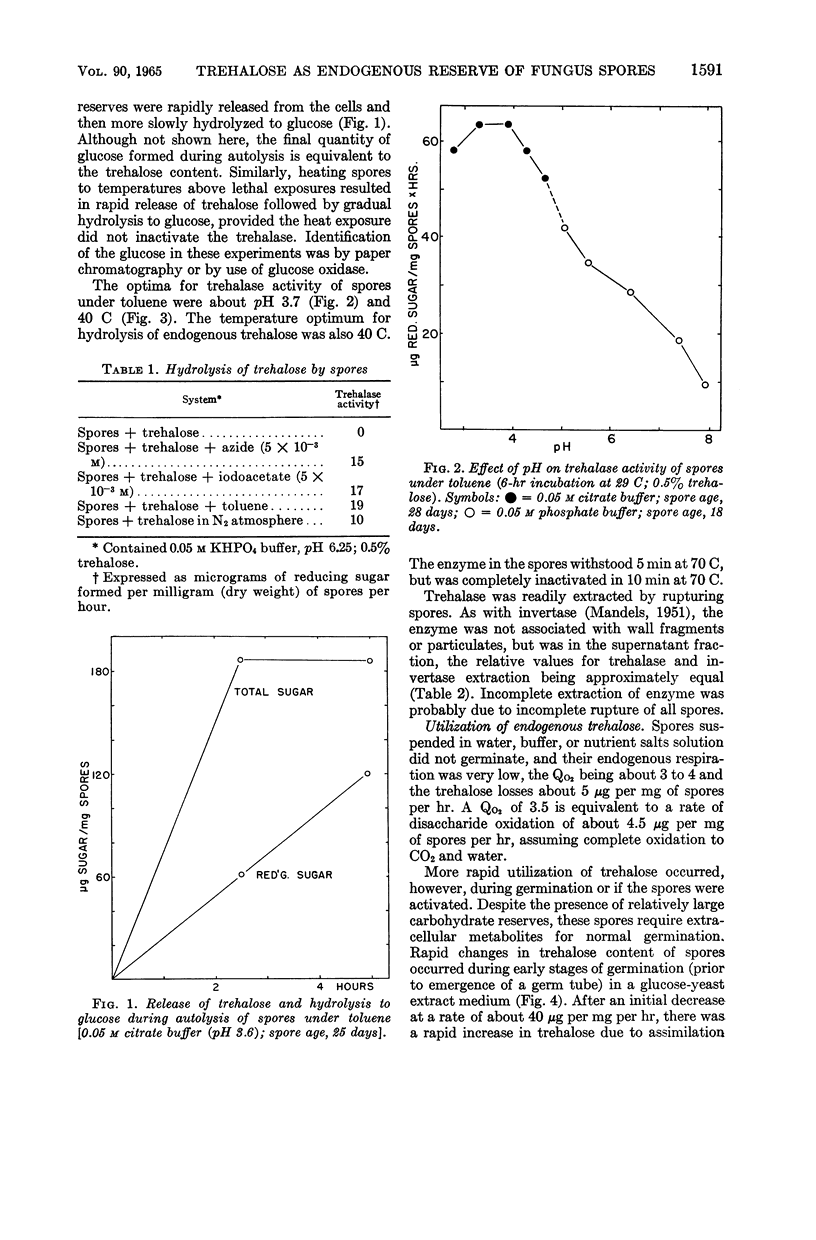
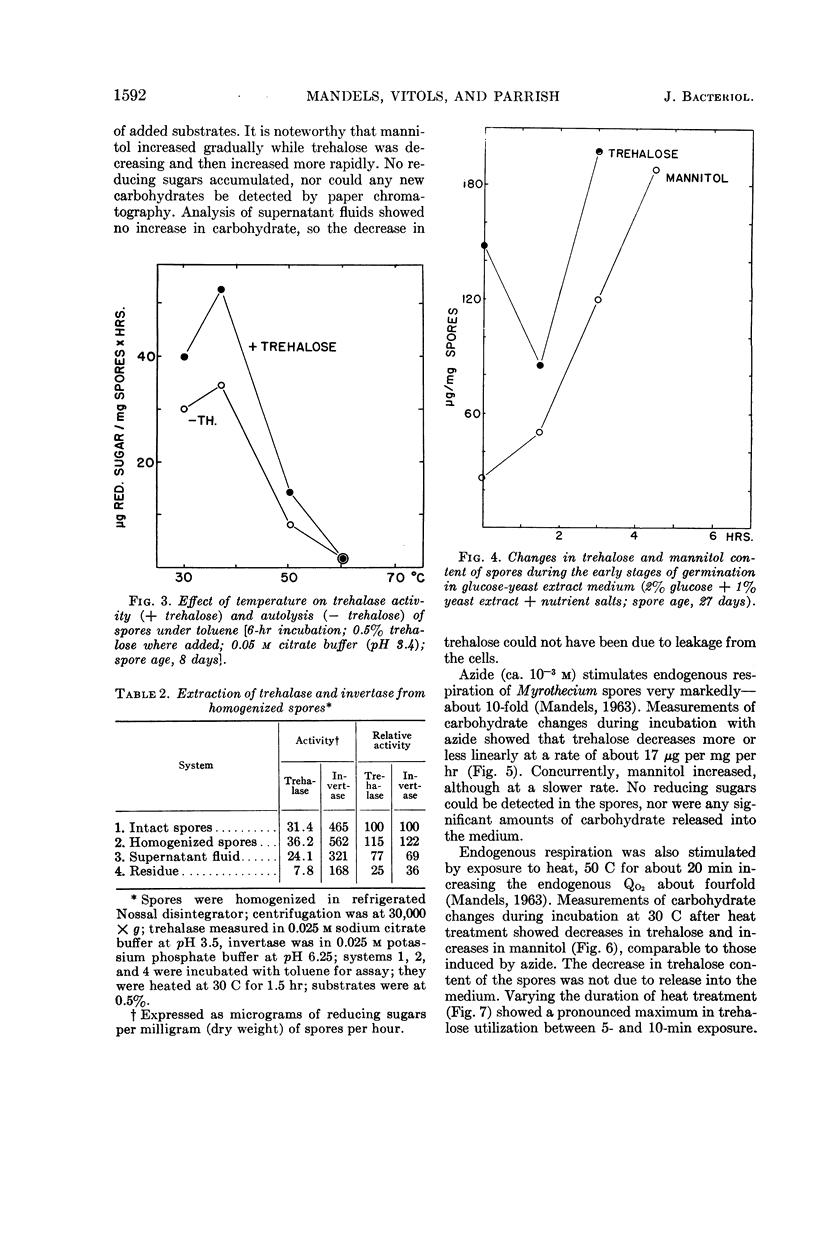
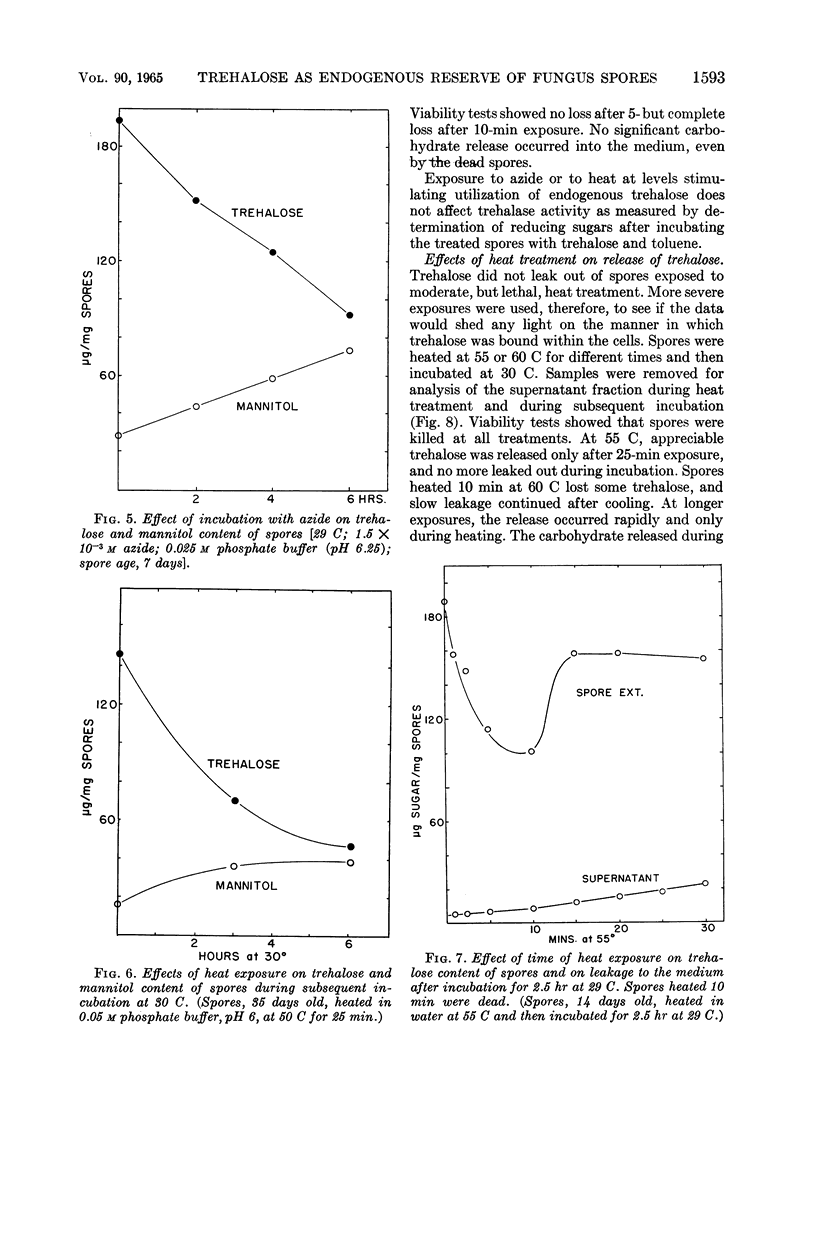
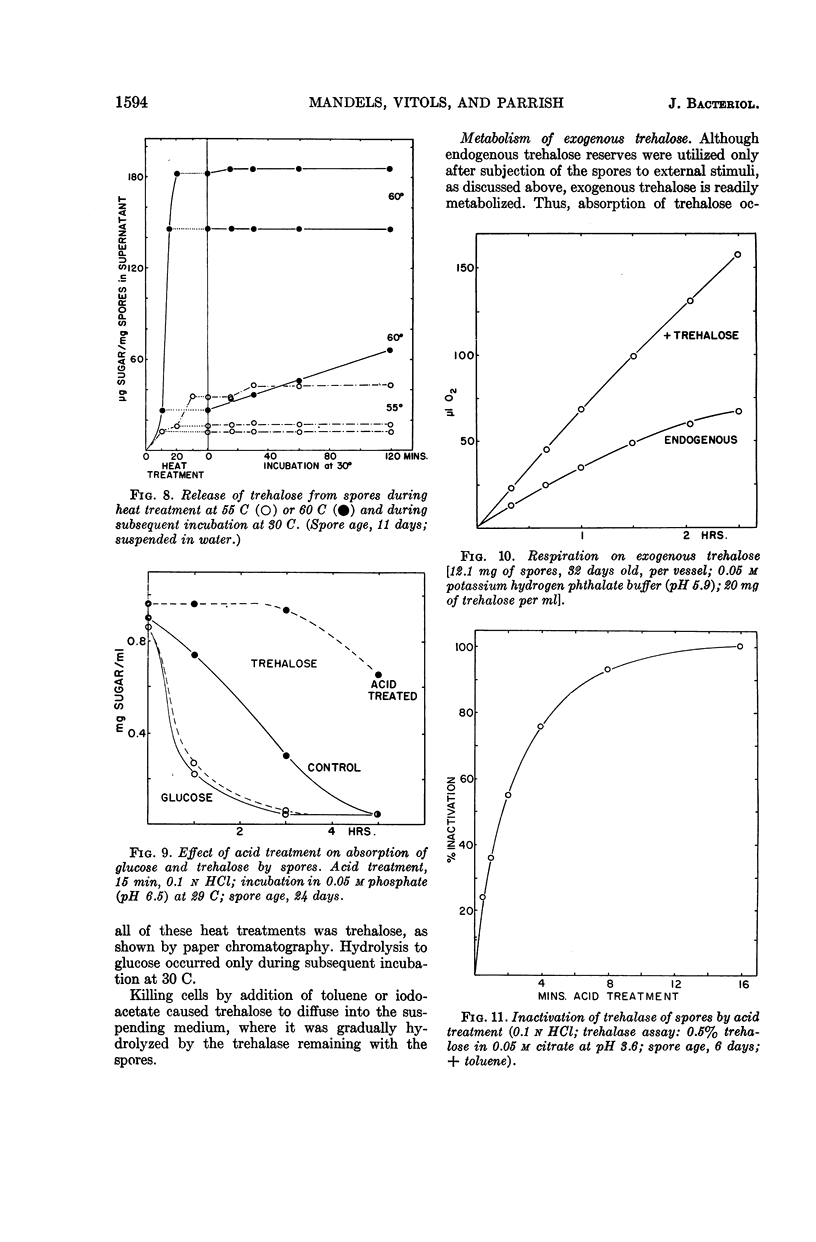
Abstract
Mandels, G. R. (U.S. Army Natick Laboratories, Natick, Mass.), Rasma Vitols, and Frederick W. Parrish. Trehalose as an endogenous reserve in spores of the fungus Myrothecium verrucaria. J. Bacteriol. 90:1589–1598. 1965.—Gross analysis of Myrothecium verrucaria spores showed approximately 3% fat, 33% carbohydrate, and 9.5% nitrogen. The water-soluble carbohydrates were trehalose, glucose, mannitol, and an unidentified phosphorylated compound. Water-soluble amino acids include leucine or norleucine (or both), valine, γ-amino-n-butyric acid, β-amino-n-butyric acid, ergothionine, glutamic acid, glutamine, glycine, aspartic acid, asparagine, cystine, and cystathionine. Ergosterol was also present. αα-Trehalose is the major reserve (20% of the dry weight), although approximately 30% of it appeared to be at the spore surface and was released by nonlethal treatment with 0.1 n HCl. Treatment with toluene or exposure to heat sufficient to kill the spores (20 min at 60 C) caused rapid liberation of all of the trehalose. Although spores could utilize exogenous trehalose with no appreciable lag, some stimulus, such as exposure to heat (10 min at 55 C), incubation with azide, or germination on exogenous substrates, was necessary to effect utilization of trehalose reserves. Spores have trehalase, but it is apparently at the spore surface, since it is inactivated by acid treatment which does not kill the spores. The metabolic pathway for utilization of trehalose is not known, but presumably it is not mediated by trehalase. The involvement of mannitol is indicated, since it tends to increase as trehalose decreases, although the changes are not quantitatively equivalent.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BALLIO A., DIVITTORIO V., RUSSI S. THE ISOLATION OF TREHALOSE AND POLYOLS FROM THE CONIDIA OF PENICILLIUM CHRYSOGENUM THOM. Arch Biochem Biophys. 1964 Aug;107:177–183. doi: 10.1016/0003-9861(64)90319-4. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BIRCH G. G. TREHALOSES. Adv Carbohydr Chem. 1963;18:201–225. doi: 10.1016/s0096-5332(08)60243-x. [DOI] [PubMed] [Google Scholar]
- HILL E. P., SUSSMAN A. S. DEVELOPMENT OF TREHALASE AND INVERTASE ACTIVITY IN NEUROSPORA. J Bacteriol. 1964 Dec;88:1556–1566. doi: 10.1128/jb.88.6.1556-1566.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa B. T., Sussman A. S. Endogenous Substrates of Dormant, Activated and Germinating Ascospores of Neurospora Tetrasperma. Plant Physiol. 1959 Jul;34(4):466–472. doi: 10.1104/pp.34.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELS G. R. Localization of carbohydrases at the surface of fungus spores by acid treatment. Exp Cell Res. 1953 Sep;5(1):48–55. doi: 10.1016/0014-4827(53)90093-7. [DOI] [PubMed] [Google Scholar]
- MANDELS G. R. Properties and surface location of a sulfhydryl oxidizing enzyme in fungus spores. J Bacteriol. 1956 Aug;72(2):230–234. doi: 10.1128/jb.72.2.230-234.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAMURA S., WAKEYAMA T. Distribution of trypsin inhibitors in the sera of various animals. Nature. 1961 Dec 16;192:1077–1077. doi: 10.1038/1921077a0. [DOI] [PubMed] [Google Scholar]
- REISENER H. J., GOLDSCHMID H. R., LEDINGHAM G. A., PERLIN A. S. Formation of trehalose and polyols by wheat stem rust (Puccinia graminis tritici) uredospores. Can J Biochem Physiol. 1962 Sep;40:1248–1251. [PubMed] [Google Scholar]
- Rimington C. Seromucoid and the bound carbohydrate of the serum proteins. Biochem J. 1940 Jun;34(6):931–940. doi: 10.1042/bj0340931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman A. S., Lingappa B. T. Role of Trehalose in Ascospores of Neurospora Tetrasperma. Science. 1959 Nov 13;130(3385):1343–1343. doi: 10.1126/science.130.3385.1343. [DOI] [PubMed] [Google Scholar]
- ULTMANN J. E., FEIGELSON P. The interaction between xanthine oxidase and its antibody. Ann N Y Acad Sci. 1963 May 8;103:724–734. doi: 10.1111/j.1749-6632.1963.tb53729.x. [DOI] [PubMed] [Google Scholar]


