Abstract
Megakaryocytes transfer a diverse and functional transcriptome to platelets during the final stages of thrombopoiesis. In platelets, these transcripts reflect the expression of their corresponding proteins and, in some cases, serve as a template for translation. It is not known, however, if megakaryocytes differentially sort mRNAs into platelets. Given their critical role in vascular remodeling and inflammation, we determined whether megakaryocytes selectively dispense transcripts for matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) into platelets. Next-generation sequencing (RNA-Seq) revealed that megakaryocytes express mRNA for 10 of the 24 human MMP family members. mRNA for all of these MMPs are present in platelets with the exception of MMP-2, 14, and 15. Megakaryocytes and platelets also express mRNA for TIMPs 1-3, but not TIMP-4. mRNA expression patterns predicted the presence and, in most cases, the abundance of each corresponding protein. Nonetheless, exceptions were observed: MMP-2 protein is present in platelets but not its transcript. In contrast, quiescent platelets express TIMP-2 mRNA but only traces of TIMP-2 protein. In response to activating signals, however, platelets synthesize significant amounts of TIMP-2 protein. These results demonstrate that megakaryocytes differentially express mRNAs for MMPs and TIMPs and selectively transfer a subset of these into platelets. Among the platelet messages, TIMP-2 serves as a template for signal-dependent translation.
Introduction
The biogenesis of platelets from megakaryocytes is a complex and intricate process that is incompletely understood. What is generally agreed on, however, is that platelets are assembled along intermediate pseudopodial-like extensions that derive from the cytoplasm of megakaryocytes.1 These extensions are referred to as proplatelets.1
Proplatelet formation has been modeled in vitro2 and more recently observed in vivo.3 Mechanisms that control proplatelet formation and platelet release are emerging, including the critical role of microtubules in driving the elongation of proplatelets.4 Microtubules are also thought to be involved in sorting mitochondria as well as α and dense granules into individual platelet buds.5,6 Granules readily track along microtubular-rich shafts of pro-platelets and distinct granule subpopulations are partitioned into platelets.6 This suggests that platelet biogenesis is under discrete control.
Protein synthesis also ramps up as proplatelets form. Adhesion molecules, secretome constituents, and other proteins are synthesized and packaged into discrete locations, including membranes and granules, so that platelets are loaded with requisite components before they are released into the bloodstream.7 In addition to proteins, megakaryocytes send thousands of mRNAs and miRNAs into platelets.8–10 The mRNAs are capped and polyadenylated on their 5′- and 3′-untranslated region (UTR), respectively, and code for protein when they are placed in in vitro translation systems.11,12 Intact platelets also translate mRNAs, both constitutively and in a signal-dependent fashion.13,14
Although not rigorously tested, it is generally assumed that mRNA expression patterns in megakaryocytes and platelets reflect one another. Here, we show by next-generation RNA sequencing that megakaryocytes differentially express and then sort specific matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinase (TIMP) transcripts into platelets. MMP and TIMP mRNA and protein expression often coincide with one another, but differences exist in platelets for MMP-2 and TIMP-2. Com-parative analyses also demonstrate that TIMP-2 is under translational control, providing the first piece of evidence that platelets accumulate this metalloproteinase inhibitor in response to activating signals.
Methods
Cell preparations
Research was approved by the University of Utah Institutional Review Board and by the Ethics Committee at the University of Perugia, and all human participants gave written informed consent to participate in the study in accordance with the Declaration of Helsinki. CD34+ cells were isolated from human umbilical cord blood and differentiated into megakaryocytes as previously described.15–18 At day 14 of differentiation, CD61-positive (CD61+) cells were separated by immunomagnetic beads using CD61 MicroBeads, Large Cell Columns, and a MiniMACS Separator (all by Miltenyi Biotec) according to the manufacturer's instructions. After purification, CD61+ cells were > 95% of the total as assessed by flow cytometry (data not shown).
Washed platelets were isolated from human blood as previously described.15,19 Five-day-old platelet concentrates were obtained from the Blood Transfusion Center of the Santa Maria della Misericordia Hospital (Perugia, Italy). CD45+ leukocytes were removed from every preparation,15,17 and the platelets were resuspended in M199 medium (4 × 108/mL). Unless otherwise indicated, platelets were treated with vehicle (M199) or thrombin (0.1 U/mL) for designated times. At select time intervals, the platelets were centrifuged (11 000g, 2 minutes) to collect cell-free supernatants that were immediately frozen (−80°C) for subsequent evaluation. The platelet pellets were lysed (40mM Tris-HCl, 0.3M NaCl, 1mM EDTA, 1mM Na3VO4, 1mM NaF, NaN3 0.05%, NP-40 1%, protease inhibitors 1 μL/mL, pH 7.4) and then centrifuged (20 000g, 4°C) for 20 minutes to remove cellular debris. The cleared lysates were stored (−80°C) for subsequent analyses.
The MEG-01 cell line20 (a kind gift of professor C. Van Geet and K. Freson, University of Leuven, Leuven, Belgium) was cultured in RPMI 1640 medium supplemented with 10% FBS, 2mM L-glutammine, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a humidified atmosphere of 5% CO2.
mRNA expression
Total RNA was isolated from differentiated CD61-positive megakaryocytes or leukocyte-depleted platelets as previously reported.15,17 The leukocyte-depleted platelet preparations were devoid of mRNA for CD45 (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). A total of 1-3 × 106 megakaryocytes or 1-3 × 109 platelets provided sufficient quantities of mRNA for next-generation RNA sequencing (RNA-seq) or PCR analyses (semi-quantitative or real-time).
For the deep sequencing studies, RNA was prepared according to protocols provided by Illumina (DNA vision). Briefly, total RNA was isolated from TRIzol as we have previously described.15,17 The RNA was treated with DNase for 25 minutes (37°C) according to the manufacturers protocol (Ambion), re-precipitated, and oligo dT–coated magnetic beads were used to isolate mRNA. The mRNA was fragmented and single-stranded cDNA was synthesized using Superscript II and random primers. Double-stranded cDNA was then synthesized using RNase H and DNA polymerase I. The cDNA ends were made blunt by repair with T4 and Klenow DNA polymerases and T4 polynucleotide kinase. Adenine 3′ tails were added with Klenow exo minus and adaptors added. After adaptor ligation, fragments of approximately 200 bp were size selected and collected by agarose gel resolution, excision, and spin column clean-up; isolated fragments were amplified by PCR and validated by an Agilent Bioanalyzer. Fragments were captured on an Illumina cell and paired-end sequencing (36 mer) was performed. Sequences were analyzed using USeq applications and visualized by Integrated Genome Browser (IGB).
Semi-quantitative and real-time PCR was performed according to our previously published protocols.15,17 Briefly, total RNA was isolated from megakaryocytes, MEG-01 cells or platelets, treated with DNase, cDNA was generated and then amplified by PCR. Primers that target sequences in exons that spanned one or more introns were designed to screen for pre-mRNA and spliced mRNA transcripts. In select studies, primers were designed within an exon. The primer sequences are listed in supplemental Table 1.
To test whether select mRNAs are stable in platelet precursors, differentiated megakaryocytes or MEG-01 cells were incubated (0-16 hours) with DMSO (vehicle) or 2 different transcriptional inhibitors that included actinomycin D (10 μg/mL) or α- amanitin (25mM). MMP-2 and MMP-14 mRNA was quantified by real-time PCR and normalized to β2-microglobulin (B2MG). To test transcript degradation in platelets, TIMP-1, TIMP-2, MMP-1, and MMP-9 mRNA was amplified by PCR from freshly isolated platelets or from 5-day-old platelet concentrates.
Presence of a mRNA-sorting machinery in megakaryocytes and platelets
Total RNA was isolated from white blood cells, megakaryocytes, MEG-01, and UT-7 cell lines, or platelets. cDNA was generated and then amplified by PCR. Primers were designed to screen for the exons common to all isoforms of STAU1, STAU2, CASC3, EIF4A3, and ZBP1 transcripts.
Protein expression
MMPs 1-3 and 9 and TIMPs 1-3 were measured in platelet lysates and in platelet supernatants by ELISA, according to the manufacturer's instructions (GE Healthcare Life Sciences and R&D Systems). There were no significant cross-reactivities of the metalloproteinase-specific ELISA kits with other unrelated MMPs; similarly, the TIMP-specific ELISAs did not cross-react with one another.
Immunocytochemistry for MMP-2 and MMP-9 was performed in megakaryocytes as previously described18,21,22; specimens were analyzed at 22°C, with an Olympus IX81 FV1000 inverted confocal microscope, using a PLAN APO 60×/1.45 NA oil objective. Abs directed against MMP-2 (ab52756) or MMP-9 (ab76003) were from Abcam. Rabbit IgG controls were run in parallel to adjust for nonspecific staining. Secondary Ab was a goat anti–rabbit Alexa Fluor 488 Dye (Molecular Probes; Invitrogen). Images were acquired using Fluoview ASW software.
Protein synthesis
Leukocyte-depleted platelets were suspended for 1 hour (37°C) in DMEM that lacked methionine and cysteine (MP Biomedical). [35S]methionine/cysteine (50 μCi) was subsequently added to the platelets and after a brief incubation period (15 minutes), the platelets were either processed immediately (0 hours) or activated with thrombin (0.1 U/mL) for designated time periods. At each sampling interval, platelets were centrifuged (20 000g, 5 minutes) and the cell pellets and supernatants were collected. Platelet pellets were lysed in RIPA buffer (1× PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, protease inhibitor cocktail) and soluble components of the lysates were separated from the cellular debris by centrifugation (20 800g, 4°C, 5 minutes). Unincorporated [35S]methionine/cysteine was removed from the supernatants by running the samples on 3-kDa spin columns. Rabiolabeled lysates and supernatants were placed in a TIMP-2–specific ELISA plate for 2 hours, which was previously blocked according to the manufacturer's instructions (GE Healthcare). At the end of this time period, the wells were washed 5 times with PBS to remove unbound material. Protein bound to TIMP-2 was subsequently removed from each well, placed in liquid scintillation fluid, and counted in a LS6500 liquid scintillation counter (Beckman Coulter).
Zymography
Zymography was performed in 10% polyacrylamide gels in the presence of 0.1% gelatin as previously described.20,23,24 Supernatants (20 μL) or intracellular lysates (20 μg) from proplatelet-producing megakaryocytes were loaded and run on gels without boiling. After electrophoresis, the gels were washed twice in 2.5% Triton X-100 for 30 minutes at room temperature to remove residual SDS. The gels were subsequently incubated at 37°C for 16 hours in development buffer (50mM Tris-HCl, 20mM NaCl, 5mM CaCl2, Brij-35 0.01%, pH 7.5), stained with 0.5% Coomassie Blue R250 in 40% methanol and 10% glacial acetic acid for 3 hours, and destained. Protein standards (pro– and active–MMP-2, kindly provided by Dr Rafael Fridman, Wayne State University, Detroit, MI, and pro–MMP-9, R&D Systems) were run in parallel. Light bands on the blue background were indicative of gelatinase activity.
Statistical analyses
Where appropriate, data are reported as the mean ± SEM. ANOVA was used for multiple group comparisons. If significant differences were identified, a Tukey multiple comparison post-hoc test was used to define the exact location of each difference. A P value of < .05 was considered statistically significant. Figures depicting immunocytochemistry, mRNA, or zymography experiments are representative of at least 3 indepen-dent studies.
Results
Megakaryocytes and platelets differentially express mRNAs for MMP and TIMP family members
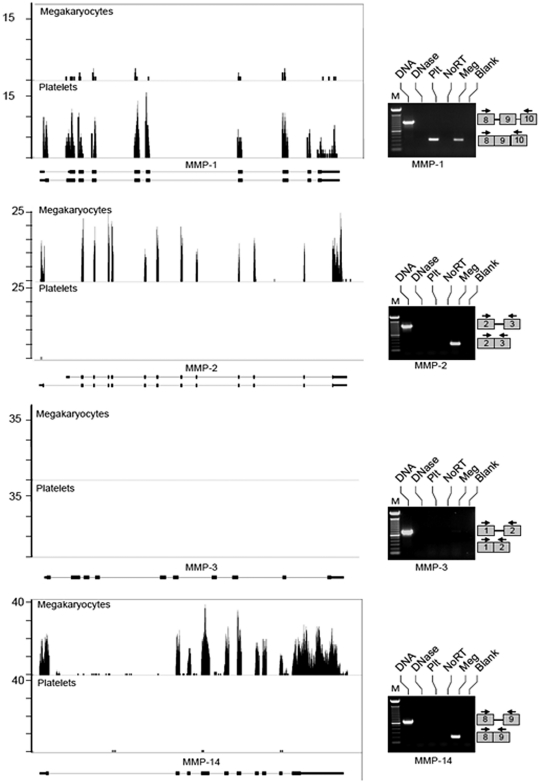
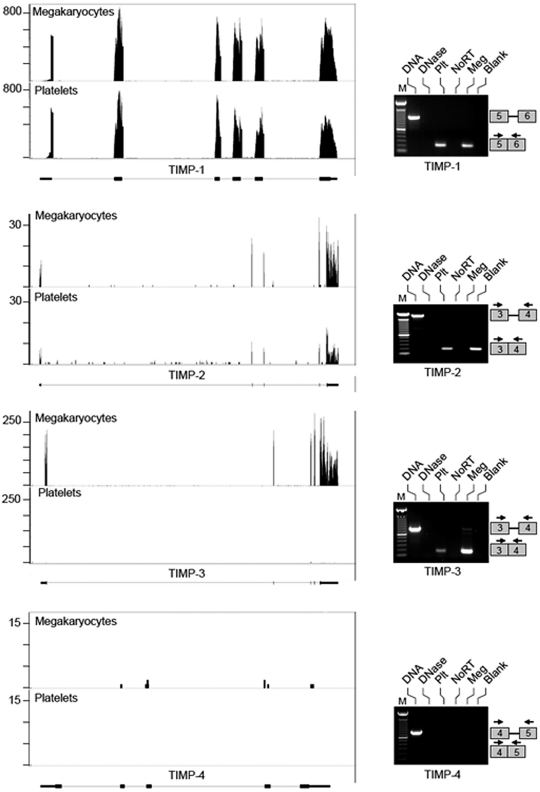
RNA-seq was used to comprehensively identify MMP and TIMP transcripts in human CD34+-derived megakaryocytes that were at the stage of proplatelet formation, which henceforth we refer to as megakaryocytes, and in freshly isolated platelets. We obtained 15-30 million 36-bp reads for each sample that mapped to unique sites in the human genome. We subsequently analyzed reads that mapped to MMP and TIMP genes and quantified transcript levels by using RPKM (reads per kilobase of exon model per million mapped reads). Tables 1 and 2 provide lists of MMP and TIMP transcripts present in megakaryocytes and platelets as well as their relative abundance. In regards to MMPs, we detected transcripts for 10 of 24 family members in megakaryocytes including MMP-1, 2, 9, 11, 14, 15, 17, 19, 24, and 25 (Table 1). Of these, MMP-17 yielded the highest RPKM value (21.62). Platelets expressed the majority of MMP transcripts that were detected in megakaryocytes with the exception of MMP-2, 14, and 15. RNA-seq also revealed that megakaryocytes expressed transcripts for TIMPs 1-3, but not TIMP-4 (Table 2). We did not detect mRNAs for MMP or TIMP family members that were exclusively in platelets (Tables 1–2). This indicates that our megakaryocyte model is a credible surrogate for examining events in proplatelet formation and thrombopoiesis.
Table 1.
Relative abundance of MMP messages
| Gene | Meg RPKM | Plt RPKM |
|---|---|---|
| MMP1 | + | ++ |
| MMP2 | ++ | − |
| MMP3 | − | − |
| MMP7 | +/− | − |
| MMP8 | − | − |
| MMP9 | ++ | +/− |
| MMP10 | − | − |
| MMP11 | + | +/− |
| MMP12 | − | − |
| MMP13 | − | − |
| MMP14 | +++ | − |
| MMP15 | + | − |
| MMP16 | − | − |
| MMP17 | +++ | +/− |
| MMP19 | + | +/− |
| MMP20 | − | − |
| MMP21 | − | − |
| MMP23A | − | − |
| MMP23B | − | − |
| MMP24 | ++ | ++ |
| MMP25 | ++ | + |
| MMP26 | − | − |
| MMP27 | − | − |
| MMP28 | − | +/− |
Scale RPKM range: ++++, ≥ 50; +++, ≥ 10 < 50; ++, ≥ 2 < 10; +, ≥ 0.5 < 2; +/−, ≥ 0.1 < 0.5; and −, < 0.1.
MMP indicates matrix metalloproteinase; Meg, megakaryocyte; Plt, platelet; and RPKM, reads per kilobase of exon model per million mapped reads.
Table 2.
Relative abundance of TIMP messages
| Gene | Meg RPKM | Plt RPKM |
|---|---|---|
| TIMP1 | ++++ | ++++ |
| TIMP2 | ++ | ++ |
| TIMP3 | ++++ | +/− |
| TIMP4 | +/− | − |
Scale RPKM range: ++++ indicates ≥ 50; +++, ≥ 10 < 50; ++, ≥ 2 < 10; +, ≥ 0.5 < 2; +/−, ≥ 0.1 < 0.5; and −, < 0.1.
MMP indicates matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase; Meg, megakaryocyte; Plt, platelet; and RPKM, reads per kilobase of exon model per million mapped reads.
Reads for each MMP and TIMP present in megakaryocytes and/or platelets demonstrate that the transcripts possess predicted 5′- and 3′-untranslated regions (Figures 1–2 and data not shown). Alternative splices were rare, but a variant for MMP-2 was prominent in megakaryocytes (Figure 1). We confirmed the differential expression of select MMP family members (ie, MMP-1, 2, 3, and 14) and all 4 TIMPs by semi-quantitative PCR. Similar to the RNA-seq data, we detected spliced MMP-1, 2, and 14 and TIMPs 1-3 in megakaryocytes (Figures 1–2). mRNA for MMP-3 and TIMP-4 was not observed in megakaryocytes or platelets under the conditions of our experiments (Figures 1–2). Unlike megakaryocytes, platelets did not express MMP-2 or MMP-14 transcripts. This suggested that MMP-2 and MMP-14 mRNA may be continually transcribed and degraded in megakaryocytes and, as a result, decline rapidly in platelets that lack transcriptional activity. However, MMP-2, MMP-14, TIMP-1, and TIMP-3 mRNAs remained stable in actinomycin D or α-amanitin–treated megakaryocytes (supplemental Figure 2). Similar results were obtained using the MEG-01 cell line (data not shown). Moreover TIMP-1, TIMP-2, MMP-1, and MMP-9 mRNA was detected both in freshly isolated and banked platelets (5-day old), showing that transcripts are stable in platelets stored for up to 5 days (supplemental Figure 3). Altogether, these data show that instability and/or degradation of mRNA in megakaryocytes or platelets was not responsible for differences in mRNA levels between megakaryocytes and platelets.
Figure 1.
Megakaryocytes and platelets differentially express MMP transcripts. mRNA was collected from megakaryocytes at the stage of proplatelet formation or freshly isolated, unactivated platelets and processed as described in “mRNA expression.” The left side of the figure shows RNA-seq analyses for MMP-1, MMP-2, MMP-3, and MMP-14 mRNA. Matched sequences are aligned to each target gene using the Integrated Genome Browser (IGB). Gene maps (bottom portion of each panel, oriented 5′-3′ direction) are represented by thick (exons) and thin (introns) lines. The bars above the gene (top portion of each panel) represent megakaryocyte or platelet transcripts that were fragmented, sequenced, and aligned to MMP-1, MMP-2, MMP-3, and MMP-14. If no bars are shown, then transcripts for these mRNAs were not detected under the conditions of these experiments. The right side of the figure shows semi-quantitative PCR analyses of mRNAs for MMPs 1-3 and MMP-14. From left to right, each lane represents: (1) amplification of DNA, which served as a positive control for primer efficiency; (2) DNA sample treated with DNase; (3) amplification of cDNA that was reverse transcribed from platelet (Plt) mRNA; (4) control reaction in platelet preparations that screened for residual genomic DNA contamination. In these studies, the reverse-transcription step was not performed (NoRT). (5) Amplification of cDNA that was reverse transcribed from megakaryocyte (Meg) mRNA; (6) negative control lane where the PCR was carried out without cDNA template (Blank). The PCR gels are representative of 5 or more independent experiments. The boxes on the right of each panel represent where the amplicons will migrate based on primer sequences specific for each exon.
Figure 2.
Megakaryocytes and platelets differentially express TIMP transcripts. mRNA was collected from megakaryocytes at the stage of proplatelet formation or freshly isolated, unactivated platelets and processed as described in “mRNA expression.” The left side of the figure shows RNA-seq analyses for TIMPs 1-4. Matched sequences are aligned to each target gene as described in Figure 1. The right side of the figures shows semi-quantitative PCR analyses of mRNAs for TIMPs 1-4. The lanes for these PCR gels are labeled as described in Figure 1. Each PCR gel is representative of 5 or more independent experiments. The boxes on the right of each panel represent where the amplicons will migrate based on primer sequences specific for each exon.
Moreover, to exclude that the MMP-2 and MMP-14 mRNAs detection in megakaryocyte cultures was a false-positive signal generated by other cell types contaminating megakaryocyte cultures we assessed the expression of MMP-21, a transcript not expressed in megakaryocytes and platelets but present in white blood cells. Indeed, mRNA for MMP-21 was detected in white blood cells but not in purified megakaryocyte preparations. This result confirms the efficiency of our purification method (supplemental Figure 1B).
Megakaryocytes and platelets express mRNAs for mRNA-localization proteins
Given that our results suggest the existence of a sorting mechanism for megakaryocytes' mRNA to platelets, we assessed the expression of mRNAs for all the known mRNA-localization proteins described in mammalians: STAU1 and STAU2 (implicated in the transport of mRNA via the microtubule network in humans),25 CASC3 (a core component of the exon junction complex that interacts with STAU1),23 EIF4A3 (another exon junction complex component associated with neuronal mRNA localization),24 and ZBP1 (a zipcode-binding protein that localizes α-actin in human neurons).26 mRNAs for STAU-1, STAU-2, CASC-3, and EIF4A3 were expressed in all cells tested including megakaryocytes and platelets. In contrast, ZBP1 was expressed only in white blood cells (supplemental Figure 4).
Next, we used real-time PCR to quantify the relative abundance of transcripts that were present in platelets (eg, MMP-1, 9 and TIMPs 1-3). Real-time PCR revealed that TIMP-1, which had the highest RPKM value (167.47) in our RNA-seq analysis, was expressed at high levels in unactivated platelets (compare Table 2 with supplemental Figure 5). Similar quantitative correlations between RNA-seq and real-time PCR were observed for MMP-1, TIMP-2, and TIMP-3 transcripts (compare Tables 1 and 2 with supplemental Figure 5). Together, these results demonstrate that RNA-seq provides an unbiased approach for quantifying the abundance of MMP and TIMP transcripts in megakaryocytes and platelets.
Platelets differentially store and release MMP proteins on activation
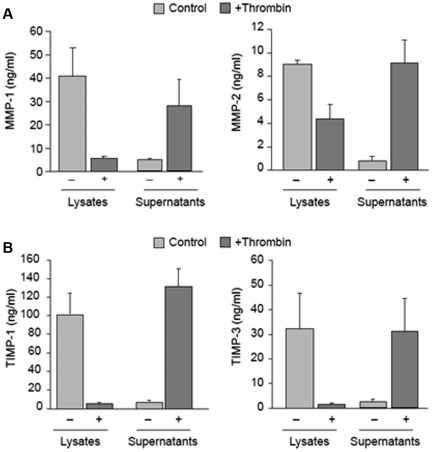
In-depth mRNA analyses indicated that transcripts for MMP and TIMP family members are differentially packaged into platelets. To determine whether the expression of mRNAs and their corresponding proteins parallel one another, protein products for each candidate were measured in platelets. As expected based on our previous studies,21,22,27,28 MMP-1 protein was readily detected in platelets as was MMP-2 (Figure 3A) and MMP-14 (data not shown). In contrast, we did not detect MMP-3 or pro-MMP-9 protein in unactivated platelet lysates (data not shown). In response to thrombin, platelets released MMP-1 and MMP-2 into the supernatant (Figure 3A). For the most part, the expression of MMP mRNAs and their corresponding proteins reflected one another (compare Figure 1 to Figure 3A). Nonetheless, this was not the case for MMP-2, which was readily detected in platelets in the absence of appreciable mRNA (compare Figure 1 to Figure 3A). We did find MMP-2 mRNA in megakaryocytes (Figure 1) and its protein localized to proplatelets (supplemental Figure 6). MMP-9 activity was observed in supernatants, but not lysates, of megakaryocytes (supplemental Figure 7).
Figure 3.
Platelets differentially express and release MMP and TIMP proteins. MMP and TIMP protein expression was analyzed as described in “Protein expression.” (A) Intracellular or released protein for MMPs 1 and 2 in unactivated or thrombin-activated (0.1 U/mL, 60 minutes) platelets. The bars represent the mean ± SEM of 5 or more independent experiments. (B) Intracellular or released TIMP-1 and TIMP-3 protein in unactivated or thrombin-activated (0.1 U/mL, 60 minutes) platelets. The bars represent the mean ± SEM of 5 or more independent experiments.
Platelets differentially express and synthesize TIMP proteins on activation
TIMP-1 protein was readily detected in unactivated platelets (Figure 3B), as was the transcript (Table 2). TIMP-3 protein was also found in platelets. In response to thrombin activation, platelets released TIMP-1 and TIMP-3 (Figure 3B). We did not detect TIMP-4 in platelets before or after activation (data not shown).
Unlike TIMP-1 and TIMP-3, unactivated platelets expressed mRNA for TIMP-2 but not its corresponding protein (compare Figure 2 with Figure 4A). Because platelets are capable of translating mRNA into protein, we assessed whether TIMP-2 is synthesized de novo by activated platelets. We found that TIMP-2 protein accumulated in platelet lysates and supernatants after 1 hour of activation (Figure 4A). Increases in TIMP-2 protein were observed after 30 minutes of activation and remained steady up to 18 hours (Figure 4B). To determine whether these increases were because of de novo synthesis, we incubated platelets with [35S]methionine and measured its incorporation into TIMP-2 protein. As shown in Figure 4C, radiolabeled methionine was readily incorporated into TIMP-2 protein in activated platelets, a response that increased over time.
Figure 4.
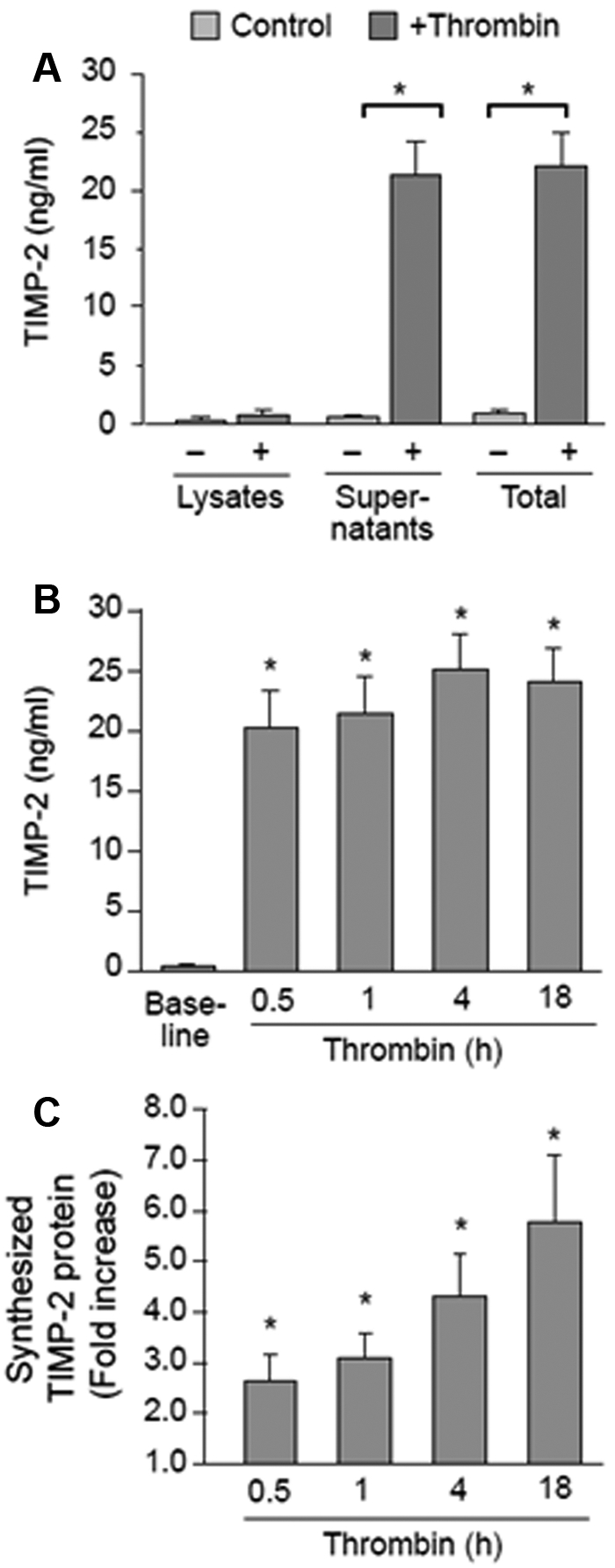
Activated platelets synthesize TIMP-2 protein. (A) Intracellular or released TIMP-2 protein in unactivated or thrombin-activated (0.1 U/mL, 60 minutes) platelets. The bars represent the mean ± SEM of 8 independent experiments. (B) TIMP-2 protein that is secreted into the supernatants of thrombin-activated platelets over 16 hours. The bars represent the mean ± SEM of 8 independent experiments. (C) Incorporation of [35S]methionine/cysteine into TIMP-2 protein (intracellular and secreted) in thrombin-activated platelets. The bars, which display the mean ± SEM of 5 independent experiments, represent the fold increase over baseline of [35S]methionine/cysteine that incorporates into TIMP-2 protein.
Discussion
Two major conclusions are drawn from our studies. First, megakaryocytes differentially express mRNAs and proteins for MMP and TIMP family members and deliver a subset of these mRNAs into platelets. Most MMP and TIMP mRNAs are represented by their corresponding proteins in platelets, but mismatches for MMP-2 and TIMP-2 exist. This indicates that megakaryocytes selectively transfer, rather than randomly dispense, mRNAs and proteins into platelets. Second, platelets translate TIMP-2 mRNA into protein in a signal-dependent fashion. This is the first demonstration that activated platelets synthesize TIMP-2, which has a critical role in MMP-2 activation.29,30 In addition, translational control of TIMP-2 expression has not previously been reported.
The “life cycle” of mRNAs in megakaryocytes presumably mirrors other eukaryotic cells: mRNAs are transcribed and processed in the nucleus, transported to the cytoplasm, and then translated by ribosomes. These events are increased during the final stages of proplatelet biogenesis and the newly synthesized proteins are transferred to platelets.7 Although the fate of mRNAs has received less attention, it is now appreciated that platelets receive thousands of messages from megakaryocytes.8,10,31 The MMP and TIMP dataset presented here demonstrates that megakaryocytes transfer some, but not all, of their mRNAs to platelets. Megakaryocytes expressed 10 of 24 MMPs and 3 of 4 TIMPs; some of these have been reported at the mRNA and/or protein level in platelets (eg, MMP 1, 2, 9, and 14 and TIMPs 1-3) while others have not (eg, MMPs 11, 15, 17, 19, 24, and 25). Several of the mRNAs that were substantially expressed by megakaryocytes (ie, RPKM > 2) were missing or barely detectable in platelets, including MMPs 2, 9, 14, and 17 and TIMP-3. Moreover, the abundance of a transcript in megakaryocytes did not uniformly predict its expression level in platelets. For example, TIMP-1 mRNA is expressed at high levels in both megakaryocytes and platelets while TIMP-3 mRNA is abundantly expressed in megakaryocytes only.
Discordant expression of mRNAs between megakaryocytes and platelets may be because of increased degradation of specific transcripts during proplatelet formation or release. Inhibition of transcription in megakaryocyte-producing proplatelets, however, did not destabilize MMP-2 or MMP-14 mRNA. These data show that MMP-2 and 14 messages are not actively degraded in megakaryocytes as they produce proplatelets. Whether localized mRNA destabilization, a mechanism used by yeast and Drosophila to protect mRNAs in one area and degrade them elsewhere,32 occurs in circulating platelets is not known. However, mRNAs expressed in platelets (such as TIMP-1, TIMP-2, MMP-1, and MMP-9) were detected both in freshly prepared and in 5-day-old platelets making it unlikely that a significant degradation process for these mRNAs takes place in circulating platelets.
Another possibility is that megakaryocytes selectively transfer mRNAs to platelets. Dedicated mRNA transport pathways exist in asymmetric cells where the cytoplasm far exceeds the diameter of the nucleus. For example, mRNAs travel great lengths in neurons as they sort themselves into axons and dendrites.33 In neurons, mRNAs have sequences (ie, zip codes) that facilitate their movement as they track along microtubules.32,34 It is not known whether similar mRNA trafficking systems are active in proplatelets. However, microtubules control the formation of proplatelets, which have average lengths of 250-500 μm, and direct the trafficking of α-granule subtypes to platelet buds.6,35 The actin-rich cytoskeleton also regulates mRNA transport32 and previous studies from our group demonstrate that platelet transcripts distribute to cytoskeletal scaffolds.36 Moreover, in the present study, we showed that megakaryocytes and platelets possess mRNA sorting machinery that is present in other mammalian cells, such as STAU1, STAU2, CASC3, and EIF4A3.23–26
More and more, investigators are profiling the platelet transcriptome to identify and characterize novel proteins or screen for differential expression of mRNAs in thrombotic and inflammatory diseases.37–39 The basis behind these strategies is that the platelet transcriptome and proteome mirror one another. Indeed, McRedmond and colleagues40 demonstrated that approximately 70% of proteins in human platelets are detected at the mRNA level. Results from the current study generally agree with this premise, but divergent expression of mRNAs and their corresponding proteins were observed. Although platelets lacked mRNA for MMP-2, they constitutively expressed MMP-2 protein and released it on activation, consistent with published work.27,28,41 Results from our studies indicate that platelets are invested with MMP-2 protein by megakaryocytes during proplatelet formation rather than endocytosing it from the circulation. The physiologic activator of MMP-2, MMP-14, has a similar expression pattern: platelets express protein, but not mRNA, for MMP-14. The expression of MMP-14 has been studied in a variety of cell types and is well correlated with activation of pro-MMP-230,42. The role of MMP-14 in regulating the activation of pro-MMP-2 in concert with TIMP-2 is also recognized.30,43 TIMP-2 interacts with the catalytic site of MMP-14 and the C-terminal domain of MMP-2, forming a trimolecular complex that controls cleavage of pro-MMP-2. Low concentrations of TIMP-2 facilitate MMP-2 activation whereas high concentrations inactivate MMP-2. The concentration-dependent activities of TIMP-2 may explain why this protein, in contrast to others in the metalloproteinase family that are constitutively expressed by platelets, is synthesized in a signal-dependent fashion. Initially, low levels of TIMP-2 present in quiescent platelets may facilitate activation of MMP-2, which primes platelet aggregation and degrades type IV collagenases27,28,41,44,45. Accumulation and secretion of increased amounts of synthesized TIMP-2 protein by stimulated platelets would eventually terminate MMP-2 activity, providing a regulated check-and-balance mechanism.
TIMP-2 adds to the growing list of proteins that are synthesized by platelets.46 Although platelets constitutively synthesize some proteins, TIMP-2 falls into the category of mRNAs whose translation is controlled by signal-dependent events.14 Several mechanisms control signal-dependent translation in platelets, including pre-mRNA splicing15,17 and activation of the mammalian target of rapamycin (mTOR).12,18 Whether either of these mechanisms controls translation of TIMP-2 mRNA in platelets is not known. However, only trace amounts of TIMP-2 pre-mRNA were detected reducing the likelihood that platelets use pre-mRNA splicing pathways to regulate TIMP-2 synthesis. mTOR may be involved because the 5′-UTR of TIMP-2 has significant secondary structure (eg, −150 kcal/mol), a predictive feature of transcripts that rely on mTOR for translation.18 The role of mTOR in regulating TIMP-2 is currently being investigated by our group, as are other translational control pathways. In this regard, the 3′-UTR of TIMP-2 has a K-box motif that is complimentary to the 5′-end of several miRNAs. This type of “box:seed” region controls the expression of Notch target genes, which regulate gene expression in megakaryocytes.47,48 The 3′-UTR of TIMP-2 also has a consensus 15-LOX-DICE site, which is involved in translational silencing.49 A role for K-box or 15-LOX DICE motifs in regulating the translation of TIMP-2 mRNA, in platelets and other cell types, has not previously been considered.
For the most part, this comprehensive analysis of MMP and TIMP expression agrees with previous studies and extends our understanding of metalloproteinase biology in megakaryocytes and platelets.21,22,27,28,30,41,45,50 However, some differences are worth noting. We did not detect MMP-9 protein in unstimulated or stimulated platelets (data not shown), a result that coincides with previous work from our group22 but is incongruent with others.51,52 Unlike its corresponding protein, MMP-9 mRNA was present in platelets, albeit at expression levels far below those observed in megakaryocytes. We also detected MMP-9 activity in megakaryocyte releasates, but not lysates. This result suggests that protein for MMP-9 may be secreted during proplatelet formation in lieu of being retained in mature platelets. Similar to MMP-9, we did not find MMP-3 or TIMP-4 in platelets while others have.50,53 The precise reasons for these variances are not clear, but it is possible that these proteins escaped the detection limit of our assays. Another explanation is that residual leukocytes, which express MMPs and TIMPs, were present in other studies while they were more efficiently cleared from our platelet preparations (see “Cell preparations”).
A strength of the current approach is that it provides quantitative information regarding MMP and TIMP transcript expression in platelets. We can infer that MMP-1 is more abundant (ie, ∼ 10- to 20-fold) than MMP-2 and exceeds MMP-3 and 9, if they are indeed present in platelets. The physiologic significance of this finding is unknown, but new roles for MMP-1 in activating PAR-1 have recently emerged.54 It is also obvious that TIMP-1 transcript and protein are the most abundant of the metalloproteinase/TIMP gene products in human platelets. Why platelets receive and/or retain so much TIMP-1 is not clear. Because of its critical roles in preventing apoptosis in endothelium, it is possible that platelets continually release TIMP-1 to maintain vascular integrity.55 If this is the case, high levels of TIMP-1 mRNA may be required so that platelets can replenish their protein pool via constitutive translation. Quantitative assessment of TIMP-2 also provides new insight into the functions of TIMP-2 in regulating platelet responses, inhibiting metalloproteinases, and maintaining tissue homeostasis.
In summary, characterization of MMP and TIMP expression in this study indicates that regulated mechanisms determine the mRNAs and proteins that megakaryocytes deliver to platelets. A key question is whether this transfer process is altered in disease situations and, as a result, yields platelets with distinct phenotypes. Indeed, the platelet transcriptomes of patients with cardiovascular disease, lupus, or sickle cell anemia differ from healthy subjects.37–39 Together, these studies suggest that megakaryocytes transcribe a different set of messages that they subsequently furnish to platelets under pathologic conditions. Dysregulated transfer of MMPs and TIMPs to platelets may also occur. If so, resultant alterations in the metalloproteinase proteome of platelets in these syndromes may contribute to plaque instability and favor thrombus formation.45,56
Supplementary Material
Acknowledgments
The authors are grateful for the editorial assistance of Dr Sara Orsini and Diana Lim for formatting the figures and tables.
This study was supported, in part, by grants from Fondazione Cassa di Risparmio di Perugia (protocol no. 2010.020.161 and no. 2009.010.0478; P.G.), and also by grants from the National Institutes of Health (HL66227; A.S.W.).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: L.C. performed the majority of experiments and drafted the manuscript; N.D.T. performed mRNA expression-based and protein synthetic experiments; N.M. performed immunocytochemistry experiments; L.B. performed rtPCR experiments, and participated in the design of PCR primers and in the analysis of data; A.S.W. codirected all aspects of the study and prepared the manuscript; and P.G. codirected all aspects of the study and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Paolo Gresele, MD, PhD, Division of Internal and Cardiovascular Medicine, Department of Internal Medicine, University of Perugia, Via E dal Pozzo, 06126 Perugia, Italy; e-mail: grespa@unipg.it.
References
- 1.Italiano JE, Jr, Shivdasani RA. Megakaryocytes and beyond: the birth of platelets. J Thromb Haemost. 2003;1(6):1174–1182. doi: 10.1046/j.1538-7836.2003.00290.x. [DOI] [PubMed] [Google Scholar]
- 2.Italiano JE, Jr, Lecine P, Shivdasani RA, Hartwig JH. Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocytes. J Cell Biol. 1999;147(6):1299–1312. doi: 10.1083/jcb.147.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Junt T, Schulze H, Chen Z, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317(5845):1767–1770. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 4.Italiano JE, Jr, Patel-Hett S, Hartwig JH. Mechanics of proplatelet elaboration. J Thromb Haemost. 2007;5(suppl 1):18–23. doi: 10.1111/j.1538-7836.2007.02487.x. [DOI] [PubMed] [Google Scholar]
- 5.van Nispen tot Pannerden H, de Haas F, Geerts W, Posthuma G, van Dijk S, Heijnen HF. The platelet interior revisited: electron tomography reveals tubular alpha-granule subtypes. Blood. 2010;116(7):1147–1156. doi: 10.1182/blood-2010-02-268680. [DOI] [PubMed] [Google Scholar]
- 6.Italiano JE, Jr, Battinelli EM. Selective sorting of alpha-granule proteins. J Thromb Haemost. 2009;7(suppl 1):173–176. doi: 10.1111/j.1538-7836.2009.03387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Italiano JE., Jr . The structure and production of blood platelets. In: Gresele P, Fuster V, Lòpez JA, Page CP, Vermylen J, editors. Platelets in Hematological and Cardiovascular Disorders. Cambridge, United Kingdom: Cambridge University Press; 2008. pp. 1–20. [Google Scholar]
- 8.Gnatenko DV, Dunn JJ, McCorkle SR, Weissmann D, Perrotta PL, Bahou WF. Transcript profiling of human platelets using microarray and serial analysis of gene expression. Blood. 2003;101(6):2285–2293. doi: 10.1182/blood-2002-09-2797. [DOI] [PubMed] [Google Scholar]
- 9.Landry P, Plante I, Ouellet DL, Perron MP, Rousseau G, Provost P. Existence of a microRNA pathway in anucleate platelets. Nat Struct Mol Biol. 2009;16(9):961–966. doi: 10.1038/nsmb.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dittrich M, Birschmann I, Pfrang J, et al. Analysis of SAGE data in human platelets: features of the transcriptome in an anucleate cell. Thromb Haemost. 2006;95(4):643–651. [PubMed] [Google Scholar]
- 11.Weyrich AS, Lindemann S, Tolley ND, et al. Change in protein phenotype without a nucleus: translational control in platelets. Semin Thromb Hemost. 2004;30(4):493–500. doi: 10.1055/s-2004-833484. [DOI] [PubMed] [Google Scholar]
- 12.Weyrich AS, Dixon DA, Pabla R, et al. Signal-dependent translation of a regulatory protein, Bcl-3, in activated human platelets. Proc Natl Acad Sci U S A. 1998;95(10):5556–5561. doi: 10.1073/pnas.95.10.5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thon JN, Devine DV. Translation of glycoprotein IIIa in stored blood platelets. Transfusion. 2007;47(12):2260–2270. doi: 10.1111/j.1537-2995.2007.01455.x. [DOI] [PubMed] [Google Scholar]
- 14.Zimmerman GA, Weyrich AS. Signal-dependent protein synthesis by activated platelets: new pathways to altered phenotype and function. Arterioscler Thromb Vasc Biol. 2008;28(3):s17–s24. doi: 10.1161/ATVBAHA.107.160218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denis MM, Tolley ND, Bunting M, et al. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122(3):379–391. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foulks JM, Marathe GK, Michetti N, et al. PAF-acetylhydrolase expressed during megakaryocyte differentiation inactivates PAF-like lipids. Blood. 2009;113(26):6699–6706. doi: 10.1182/blood-2008-11-186312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwertz H, Tolley ND, Foulks JM, et al. Signal-dependent splicing of tissue factor pre-mRNA modulates the thrombogenecity of human platelets. J Exp Med. 2006;203(11):2433–2440. doi: 10.1084/jem.20061302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weyrich AS, Denis MM, Schwertz H, et al. mTOR-dependent synthesis of Bcl-3 controls the retraction of fibrin clots by activated human platelets. Blood. 2007;109(5):1975–1983. doi: 10.1182/blood-2006-08-042192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weyrich AS, Elstad MR, McEver RP, et al. Activated platelets signal chemokine synthesis by human monocytes. J Clin Invest. 1996;97(6):1525–1534. doi: 10.1172/JCI118575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogura M, Morishima Y, Ohno R, et al. Establishment of a novel human megakaryoblastic leukemia cell line, MEG-01, with positive Philadelphia chromosome. Blood. 1985;66(6):1384–1392. [PubMed] [Google Scholar]
- 21.Galt SW, Lindemann S, Allen L, et al. Outside-in signals delivered by matrix metalloproteinase-1 regulate platelet function. Circ Res. 2002;90(10):1093–1099. doi: 10.1161/01.res.0000019241.12929.eb. [DOI] [PubMed] [Google Scholar]
- 22.Galt SW, Lindemann S, Medd D, et al. Differential regulation of matrix metalloproteinase-9 by monocytes adherent to collagen and platelets. Circ Res. 2001;89(6):509–516. doi: 10.1161/hh1801.096339. [DOI] [PubMed] [Google Scholar]
- 23.Macchi P, Kroening S, Palacios IM, et al. Barentsz, a new component of the Staufen-containing ribonucleoprotein particles in mammalian cells, interacts with Staufen in an RNA-dependent manner. J Neurosci. 2003;23(13):5778–5788. doi: 10.1523/JNEUROSCI.23-13-05778.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giorgi C, Yeo GW, Stone ME, et al. The EJC factor eIF4AIII modulates synaptic strength and neuronal protein expression. Cell. 2007;130(1):179–191. doi: 10.1016/j.cell.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 25.Villacé P, Marión RM, Ortín J. The composition of Staufen-containing RNA granules from human cells indicates their role in the regulated transport and translation of messenger RNAs. Nucleic Acids Res. 2004;32(8):2411–2420. doi: 10.1093/nar/gkh552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huttelmaier S, Zenklusen D, Lederer M, et al. Spatial regulation of b-actin translation by Src-dependent phosphorylation of ZBP1. Nature. 2005;438(7067):512–515. doi: 10.1038/nature04115. [DOI] [PubMed] [Google Scholar]
- 27.Falcinelli E, Guglielmini G, Torti M, Gresele P. Intraplatelet signaling mechanisms of the priming effect of matrix metalloproteinase-2 on platelet aggregation. J Thromb Haemost. 2005;3(11):2526–2535. doi: 10.1111/j.1538-7836.2005.01614.x. [DOI] [PubMed] [Google Scholar]
- 28.Falcinelli E, Giannini S, Boschetti E, Gresele P. Platelets release active matrix metalloproteinase-2 in vivo in humans at a site of vascular injury: lack of inhibition by aspirin. Br J Haematol. 2007;138(2):221–230. doi: 10.1111/j.1365-2141.2007.06632.x. [DOI] [PubMed] [Google Scholar]
- 29.Shofuda K, Moriyama K, Nishihashi A, et al. Role of tissue inhibitor of metalloproteinases-2 (TIMP-2) in regulation of pro-gelatinase A activation catalyzed by membrane-type matrix metalloproteinase-1 (MT1-MMP) in human cancer cells. J Biochem. 1998;124(2):462–470. doi: 10.1093/oxfordjournals.jbchem.a022136. [DOI] [PubMed] [Google Scholar]
- 30.Kazes I, Elalamy I, Sraer JD, Hatmi M, Nguyen G. Platelet release of trimolecular complex components MT1-MMP/TIMP2/MMP2: involvement in MMP2 activation and platelet aggregation. Blood. 2000;96(9):3064–3069. [PubMed] [Google Scholar]
- 31.Macaulay IC, Carr P, Gusnanto A, Ouwehand WH, Fitzgerald D, Watkins NA. Platelet genomics and proteomics in human health and disease. J Clin Invest. 2005;115(12):3370–3377. doi: 10.1172/JCI26885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin KC, Ephrussi A. mRNA localization: gene expression in the spatial dimension. Cell. 2009;136(4):719–730. doi: 10.1016/j.cell.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu Z, McLaren RS, Winters CA, Ralston E. Ribosome association contributes to restricting mRNAs to the cell body of hippocampal neurons. Mol Cell Neurosci. 1998;12(6):363–375. doi: 10.1006/mcne.1998.0723. [DOI] [PubMed] [Google Scholar]
- 34.Fusco D, Accornero N, Lavoie B, et al. Single mRNA molecules demonstrate probabilistic movement in living mammalian cells. Curr Biol. 2003;13(2):161–167. doi: 10.1016/s0960-9822(02)01436-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel SR, Hartwig JH, Italiano JE., Jr The biogenesis of platelets from megakaryocyte proplatelets. J Clin Invest. 2005;115(12):3348–3354. doi: 10.1172/JCI26891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindemann S, Tolley ND, Eyre JR, Kraiss LW, Mahoney TM, Weyrich AS. Integrins regulate the intracellular distribution of eukaryotic initiation factor 4E in platelets. A checkpoint for translational control. J Biol Chem. 2001;276(36):33947–33951. doi: 10.1074/jbc.M104281200. [DOI] [PubMed] [Google Scholar]
- 37.Raghavachari N, Xu X, Harris A, et al. Amplified expression profiling of platelet transcriptome reveals changes in arginine metabolic pathways in patients with sickle cell disease. Circulation. 2007;115(12):1551–1562. doi: 10.1161/CIRCULATIONAHA.106.658641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lood C, Amisten S, Gullstrand B, et al. Platelet transcriptional profile and protein expression in patients with systemic lupus erythematosus: up-regulation of the type I interferon system is strongly associated with vascular disease. Blood. 2010;116(1):1951–1957. doi: 10.1182/blood-2010-03-274605. [DOI] [PubMed] [Google Scholar]
- 39.Healy AM, Pickard MD, Pradhan AD, et al. Platelet expression profiling and clinical validation of myeloid-related protein-14 as a novel determinant of cardiovascular events. Circulation. 2006;113(19):2278–2284. doi: 10.1161/CIRCULATIONAHA.105.607333. [DOI] [PubMed] [Google Scholar]
- 40.McRedmond JP, Park SD, Reilly DF, et al. Integration of proteomics and genomics in platelets: a profile of platelet proteins and platelet-specific genes. Mol Cell Proteomics. 2004;3(2):133–144. doi: 10.1074/mcp.M300063-MCP200. [DOI] [PubMed] [Google Scholar]
- 41.Sawicki G, Salas E, Murat J, Miszta-Lane H, Radomski MW. Release of gelatinase A during platelet activation mediates aggregation. Nature. 1997;386(6625):616–619. doi: 10.1038/386616a0. [DOI] [PubMed] [Google Scholar]
- 42.Tokuraku M, Sato H, Murakami S, Okada Y, Watanabe Y, Seiki M. Activation of the precursor of gelatinase A/72 kDa type IV collagenase/MMP-2 in lung carcinomas correlates with the expression of membrane-type matrix metalloproteinase (MT-MMP) and with lymph node metastasis. Int J Cancer. 1995;64(5):355–359. doi: 10.1002/ijc.2910640513. [DOI] [PubMed] [Google Scholar]
- 43.Seiki M. Membrane-type matrix metalloproteinases. APMIS. 1999;107(1):137–143. doi: 10.1111/j.1699-0463.1999.tb01536.x. [DOI] [PubMed] [Google Scholar]
- 44.Gresele P, Falcinelli E, Momi S. Potentiation and priming of platelet activation: a potential target for antiplatelet therapy. Trends Pharmacol Sci. 2008;29(7):352–360. doi: 10.1016/j.tips.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Momi S, Falcinelli E, Giannini S, et al. Loss of matrix metalloproteinase 2 in platelets reduces arterial thrombosis in vivo. J Exp Med. 2009;206(11):2365–2379. doi: 10.1084/jem.20090687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schubert P, Devine DV. De novo protein synthesis in mature platelets: a consideration for transfusion medicine. Vox Sang. 2010;99(2):112–122. doi: 10.1111/j.1423-0410.2010.01333.x. [DOI] [PubMed] [Google Scholar]
- 47.Lai EC, Tam B, Rubin GM. Pervasive regulation of Drosophila Notch target genes by GY-box-, Brd-box-, and K-box-class microRNAs. Genes Dev. 2005;19(9):1067–1080. doi: 10.1101/gad.1291905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mercher T, Cornejo MG, Sears C, et al. Notch signaling specifies megakaryocyte development from hematopoietic stem cells. Cell Stem Cell. 2008;3(3):314–326. doi: 10.1016/j.stem.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reimann I, Huth A, Thiele H, Thiele BJ. Suppression of 15-lipoxygenase synthesis by hnRNP E1 is dependent on repetitive nature of LOX mRNA 3′-UTR control element DICE. J Mol Biol. 2002;315(5):965–974. doi: 10.1006/jmbi.2001.5315. [DOI] [PubMed] [Google Scholar]
- 50.Villeneuve J, Block A, Le Bousse-Kerdiles MC, et al. Tissue inhibitors of matrix metalloproteinases in platelets and megakaryocytes: a novel organization for these secreted proteins. Exp Hematol. 2009;37(7):849–856. doi: 10.1016/j.exphem.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 51.Fernandez-Patron C, Martinez-Cuesta MA, Salas E, et al. Differential regulation of platelet aggregation by matrix metalloproteinases-9 and -2. Thromb Haemost. 1999;82(6):1730–1735. [PubMed] [Google Scholar]
- 52.Sheu JR, Fong TH, Liu CM, et al. Expression of matrix metalloproteinase-9 in human platelets: regulation of platelet activation in in vitro and in vivo studies. Br J Pharmacol. 2004;143(1):193–201. doi: 10.1038/sj.bjp.0705917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Radomski A, Jurasz P, Sanders EJ, et al. Identification, regulation and role of tissue inhibitor of metalloproteinases-4 (TIMP-4) in human platelets. Br J Pharmacol. 2002;137(8):1330–1338. doi: 10.1038/sj.bjp.0704936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trivedi V, Boire A, Tchernychev B, et al. Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell. 2009;137(2):332–343. doi: 10.1016/j.cell.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boulday G, Fitau J, Coupel S, Soulillou JP, Charreau B. Exogenous tissue inhibitor of metalloproteinase-1 promotes endothelial cell survival through activation of the phosphatidylinositol 3-kinase/Akt pathway. Ann N Y Acad Sci. 2004;1030:28–36. doi: 10.1196/annals.1329.004. [DOI] [PubMed] [Google Scholar]
- 56.Gresele P, Falcinelli E, Loffredo F, et al. Platelets release matrix metalloproteinase-2 in the coronary circulation of patients with acute coronary syndromes: possible role in sustained platelet activation. Eur Heart J. 2011;32(3):316–325. doi: 10.1093/eurheartj/ehq390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.