Abstract
Background
No reviews have quantified the specific amounts of physical activity required for lower risks of coronary heart disease (CHD) when assessing the dose-response relation. Previous reviews have used, instead, qualitative estimates such as “low”, “moderate”, and “high” physical activity.
Methods and Results
We performed an aggregate data meta-analysis of epidemiologic studies investigating physical activity and primary prevention of CHD. We included prospective cohort studies published in English since 1995. After reviewing 3,194 abstracts, 33 studies were included. We used random-effects generalized least squares (GLST) spline models for trend estimation to derive pooled dose-response estimates. Among the 33 studies, 9 allowed quantitative estimates of leisure-time physical activity (LTPA). Individuals who engaged in the equivalent of 150 min/week of moderate-intensity LTPA (minimum amount, 2008 US federal guidelines) had a 14% lower CHD risk (relative risk (RR) = 0.86; 95% confidence interval (CI), 0.77–0.96), compared with those reporting no LTPA. Those engaging in the equivalent of 300 min/week of moderate-intensity LTPA (2008 US federal guidelines for additional benefits) had a 20% (RR = 0.80; 95% CI, 0.74–0.88) lower risk. At higher levels of physical activity, relative risks were modestly lower. Persons who were physically active at levels lower than the minimum amount recommended also had significantly lower risk of CHD. There was a significant interaction by gender (p=0.03); the association was stronger among women than men.
Conclusions
These findings provide quantitative data supporting US physical activity guidelines that stipulate “some physical activity is better than none” and “additional benefits occur with more physical activity”.
Keywords: Meta-analysis, physical activity, coronary heart disease, exercise, women
Introduction
Although prevalence and incidence rates of coronary heart disease (CHD) mortality have declined since the 1960s, it is estimated that ~17 million people in the United States are living with CHD in 2010.1 Coronary heart disease causes ~425,000 annual deaths in the US, making it the leading cause of mortality nation-wide.1 Identifying and characterizing modifiable risk factors for CHD remains important for public health and clinical medicine.
The independent role of physical activity in the primary prevention of CHD is well established, and has been assessed in numerous reviews or meta-analyses.2–8 Although all reviews agree that physical activity is associated with 20–30% lower risk of CHD7–8, no work to date has designated quantitative assessments of the amount of physical activity required for these lower risks, referring instead to qualitative levels of physical activity (e.g., “high” versus “low”).8 Public health guidelines on the amount of physical activity required for health benefits have relied on individual studies, rather than a systematic assessment of the overall evidence.9
Many early studies that assessed the relation between physical activity and CHD dichotomized participants according to their activity levels (e.g., active versus inactive); however, more recent studies have grouped participants into multiple, quantitatively designated categories of specific types of physical activity8 (e.g., quartiles of leisure-time physical activity [LTPA], in kilocalories/week [kcal/wk]), making it possible to assess and describe in detail the dose-response relation. The purpose of this meta-analysis is to pool results from prospective cohort studies to quantify the dose-response relationship between physical activity and risk of CHD, including both the amount of physical activity required as well as the magnitude of benefit to CHD risk.
Methods
We followed the Meta-Analysis of Observational Studies in Epidemiology (MOOSE)10 protocols throughout the design, implementation, analysis, and reporting for this study.
Literature Search Strategy
We searched for all prospective cohort studies that assessed potential associations among various types of physical activity and incidence of CHD in adults (age 18 or older at baseline). Searches were performed using electronic databases (MEDLINE and EMBASE) and were supplemented by manually searching through the reference lists of original publications as well as review articles. Key words included, among others: ‘physical activity’, ‘motor activity’, ‘energy expenditure’, ‘walking’, ‘exercise’, ‘coronary disease’, ‘heart disease’, ‘ischemic heart disease’, ‘myocardial infarction’, and ‘sudden death’ (full search terms available upon request). Searches were restricted to articles that focused on adults, published in English between January 1, 1995 and July 31, 2009. The 1995 cutoff was chosen to reflect likely changes in physical activity categorization for analyses by investigators following the 1995 US Centers for Disease Control and Prevention (CDC) / American College of Sports Medicine (ACSM) guideline,4 which allowed for moderate-intensity activities such as walking, in contrast to recommendations prior to 1995 which primarily recognized only vigorous-intensity activities as counting toward meeting guidelines.
Inclusion Criteria
The final collection of selected articles was chosen based on the following a priori inclusion criteria: the article, published in English between January 1, 1995 and July 31, 2009, reported a prospective cohort study among human adults that measured effect sizes (relative risks) of CHD (primary prevention) by level of physical activity (providing either confidence intervals [CIs] or standard errors [SEs]). All types of physical activity, including leisure-time physical activity (LTPA), time spent walking, walking pace, occupational physical activity, transport physical activity, ‘non-leisure’ physical activity, and ‘total’ physical activity were included. If multiple articles were published from the same cohort, we included the article with the most detailed report for each type of physical activity.
Selection of Articles
Initially, titles were reviewed to ascertain the potential fit to the inclusion criteria. If relevancy was doubted during the title review, a subsequent assessment was conducted. The list of potential articles was further shortened by reviewing abstracts and performing detailed evaluations of the methods and results of each remaining paper. Please refer to Supplemental Figure 1 for more detailed information regarding the progressive ‘flow’ of the study exclusion process. Decisions on inclusion were made and verified by two investigators [JS, JP], and disagreements were adjudicated by a third reader [IL].
Data Extraction
The following details were recorded for each study: author, year of publication, cohort/study name, geographic location of study (North America, Europe, other), and participants’ gender (male, female, combined), mean age at baseline, health at baseline (healthy, diabetic), and race. We also recorded CHD outcome (fatal, nonfatal, both), type of physical activity (e.g. leisure, walking, occupational, etc.), categorical physical activity level (e.g. 1,2,3), and where possible (and at each activity level) mean dose of physical activity (e.g. kilocalories per week [kcal/wk], MET-hours/week [MET-hr/wk], minutes per week [min/wk], kilometers per hour [km/h]), relative risk of CHD and confidence interval (or standard error), and number of cases and total subjects (or person time). We further noted variable assessment of confounding (crude, age-adjusted, multivariate, multivariate including plausible biologic intermediates; the multivariate model that included the most plausible confounders while excluding biologic intermediates was chosen for the primary analyses). Mean quantitative physical activity information was directly recorded, or inferred using the available cohort-specific or population norms (e.g. for height and weight). Data abstraction was conducted independently by two investigators [JS, JP], with disagreements adjudicated by a third reader [IL]. For articles where quantitative data regarding LTPA were unclear, we contacted authors for additional data. Please refer to Supplemental Table 1 for further detail.
Meta-Analysis Statistical Techniques
Relative risks (RRs) of CHD were reported for each category of physical activity. For studies that allowed quantitative estimates of physical activity, the mean (7 studies) or median (2 studies) of each category was used to define the median physical activity level for that category. For studies with an open-ended highest physical activity category, we assumed that the difference from the lowest range of this category to its median was equivalent to the difference between the lowest range of the closest adjacent category and its median.11 We excluded from the main analysis studies wherein all participants were diabetic at baseline, selecting rather from those wherein adults were at usual risk of CHD. We did not assess or adjust for quality score, as there has not been uniform agreement that correction for study quality impacts results. Additionally, we believe that the detailed level of data required to be included in the present analysis was such that study quality would be generally high and uniform among the studies included.
In an initial ‘qualitative’ analysis intended for comparison with results of previous reviews, we assessed the random effects summary relative risk of CHD by comparing the highest to lowest categories of physical activity across studies, for each type of activity reported. Random effects models allowed for heterogeneity between studies. Study-specific plots (trend lines) were then constructed to graphically depict the dose-response relation among levels of physical activity (assessed categorically, normalized to five levels of physical activity) and relative risk of CHD.
We used generalized least squares (GLST) regression models12 to assess the pooled dose-response relation between physical activity and risk of CHD across prospective cohort studies that had heterogeneous categorizations of physical activity. This modeling technique allows for the estimation of a weighted average of the log RRs across all studies, with the weight depending in part on the inverse of the variance of the log of the relative risk (i.e., larger studies carry more weight). Random effects methods were used in order to take into account heterogeneity among study results.
All ‘quantitative’ studies (those that allowed quantitative estimates of physical activity levels) were eligible for inclusion in GLST analyses. However, we were only able to apply GLST methods to assess LTPA, as there were too few (≤2) studies that allowed quantitative estimates of other physical activity types, or too much heterogeneity among studies (such as for walking time, where confidence intervals were too wide for reasonable estimates). We used spline models to conduct GLST analyses for LTPA, which allowed the relation between physical activity and CHD to vary across the range of physical activity dose in kcal/wk, but assumed linear relations between designated doses.
Guidelines from the 2008 US Physical Activity Guidelines were used to assign the first two doses of physical activity at which to assess relative risk.9 These guidelines recommend 150 minutes of moderate intensity (3–<6 METs) physical activity per week as a minimum amount for health enhancement (referred to hereafter as “basic”), and 300 minutes per week for additional health benefits (”advanced”). Alternatively, guidelines recommend equivalent expenditure from vigorous intensity (≥6 METs) physical activity (75 and 150 minutes per week, respectively); or any combination of moderate and vigorous intensity activity that results in energy expenditure equivalent to either regimen. The cutoffs associated with the basic and advanced guidelines, converted into approximate units of kcal/wk, were 550 and 1100 kcal/wk, respectively, for both genders combined; 600 and 1200 kcal/wk for men; and 500 and 1000 kcal/wk for women (based on population norms for weight). These intervals were used as a guide to extend analyses to higher levels of LTPA in order to fit the available data; higher doses were assigned to balance model parsimony and goodness of fit.
In a sensitivity analysis, we examined lower doses of physical activity (e.g., 275 kcal/wk for both genders combined), to test the statement in the 2008 US guidelines, which, in addition to recommended levels of physical activity, also state that “All adults should avoid inactivity. Some physical activity is better than none, and adults who participate in any amount of activity gain some health benefits.”9
We also assessed pre-specified potential interaction by geographic region (North America, Europe, Middle East); adjustment for confounding (multivariate, multivariate inclusive of intermediates); and CHD outcome (fatal, non-fatal, combined) using GLST spline models, evaluating p-values for interaction terms with indicator variables. We were unable to assess potential interaction by age (<65/≥65 at baseline) or race (white, black, other), as there was insufficient variation among included studies. Because assessment of interaction with spline models had less power (due to multiple degrees of freedom), as a secondary analysis, we assessed potential interaction using quadratic models. When appropriate, we performed GLST analyses restricted to strata of potential effect modifiers. Potential publication bias was assessed using Begg’s Test and a funnel plot.13
All analysis were performed using STATA 10.0 (College Station, TX), with two-tailed alpha set at p<0.05 for statistical significance.
Results
Search Results
The initial search produced 1545 articles using PubMed and 1649 articles using EMBASE; 87 and 129 studies were selected for further evaluation from PubMed and EMBASE, respectively. Based on information from abstracts, 68 studies warranted further assessment. Inclusion or exclusion was determined following a detailed evaluation of the study design, population, physical activity assessment, and CHD assessment. An additional 7 studies were identified by hand-searching through references of recent reviews.7, 8 Finally, 33 prospective cohort studies were selected for analysis14–46 (see Supplemental Figure 1 for selection ‘flow’, and Supplemental Tables 1 & 2 for characteristics of all studies selected for analysis) from which 30 assessments of LTPA were analyzed, 10 of which provided quantitative estimates of LTPA categories.
Binary Analysis
To relate our findings to past reviews, we first compared the highest to the lowest (or referent) categories of physical activity, for each type of physical activity, using random effects pooled relative risks (Table 1). The majority of physical activity types were associated with significantly lower risks of CHD, which varied between 6% and 51%. The summary risk among all studies that assessed LTPA indicated a 26% risk reduction (RR = 0.74; 95% CI, 0.69–0.78).
Table 1.
Pooled Relative Risks of CHD Comparing ‘Highest’ to ‘Lowest’ Physical Activity Categories
| Type of Activity | Gender | Studies | Relative Risk (95% CI) | I squared (%) | # Studies* |
|---|---|---|---|---|---|
| LTPA | Combined | All Studies | 0.74 (0.69, 0.78) | 28.3 | 26 |
| Quant | 0.71 (0.63, 0.80) | 39.8 | 9 | ||
| Men | All Studies | 0.78 (0.73, 0.82) | 0 | 15 | |
| Quant | 0.79 (0.72, 0.86) | 0 | 5 | ||
| Women | All Studies | 0.67 (0.61, 0.74) | 12.5 | 11 | |
| Quant | 0.64 (0.52, 0.79) | 40.6 | 5 | ||
| Walking Time | Combined | All Studies | 0.71 (0.59, 0.84) | 44.7 | 7 |
| Quant | 0.67 (0.57, 0.79) | 37.2 | 5 | ||
| Men | All Studies | 0.63 (0.34, 1.17) | 76.9 | 2 | |
| Quant | 0.63 (0.34, 1.17) | 76.9 | 2 | ||
| Women | All Studies | 0.65 (0.55, 0.76) | 0 | 4 | |
| Quant | 0.64 (0.54, 0.76) | 0 | 3 | ||
| Walking Pace | Combined | All Studies | 0.53 (0.43, 0.66) | 0 | 3 |
| Quant | 0.51 (0.35, 0.74) | 0 | 2 | ||
| Men | All Studies | 0.53 (0.42, 0.67) | 0 | 2 | |
| Women | All Studies | 1 | |||
| Occupational PA | Combined | All Studies | 0.84 (0.79, 0.90) | 0 | 4 |
| Men | All Studies | 0.87 (0.81, 0.99) | 0 | 3 | |
| Women | All Studies | 1 | |||
| Transport PA | Combined | All Studies | 0.87 (0.74, 1.02) | 81.0 | 4 |
| Men | All Studies | 0.93 (0.85, 1.02) | 25.9 | 3 | |
| Women | All Studies | 0.74 (0.57, 0.97) | 73.2 | 2 | |
| ‘Total’ PA | Combined | All Studies | 0.74 (0.62, 0.90) | 0 | 3 |
| Men | All Studies | 0.79 (0.59, 1.07) | 18.9 | 2 | |
| Women | All Studies | 0.66 (0.44, 0.99) | 0 | 2 | |
| Non-Specific PA | Combined | All Studies | 1 | ||
| All Studies | Combined | All Studies | 0.75 (0.71, 0.79) | 47.6 | 33** |
| LTPA | Leisure-time physical activity |
| All Studies | Includes studies that characterized physical activity qualitatively or quantitatively |
| Quant | Includes only those studies that categorized physical activity quantitatively |
| I squared | Percentage of variation across studies that is due to heterogeneity rather than chance |
Actual number of comparisons included was greater than the number of studies for several types of physical activity, as some studies provided comparisons for both genders. In particular, the 26 studies of LTPA provided 30 comparisons, and the 9 quantitative studies of LTPA provided 10 comparisons.
These 33 studies included 56 physical activity-type-specific assessments; many studies included comparisons from multiple physical activity types (e.g., LTPA, walking time, and walking pace assessed in the same study), and/or comparisons from both genders.
Within each type of physical activity, pooled relative risks were also provided for each gender (where there were ≥2 studies for each gender). For the majority of physical activity types, the relative risk among the most active women was lower than the corresponding value among men by approximately 0.10. Among all studies that assessed LTPA, those conducted in men showed a 22% lower risk (RR = 0.78; 95% CI, 0.73–0.82) comparing most with least active; in women, a 33% lower risk (RR = 0.67; 95% CI, 0.61–0.74).
Within each type of physical activity relative risks were also provided for the subset of studies that included quantitative assessments of physical activity (where there were ≥2 studies). These quantitative studies tended to demonstrate relative risks of magnitudes similar to those observed when all studies were included (Table 1).
Dose-Response Analysis
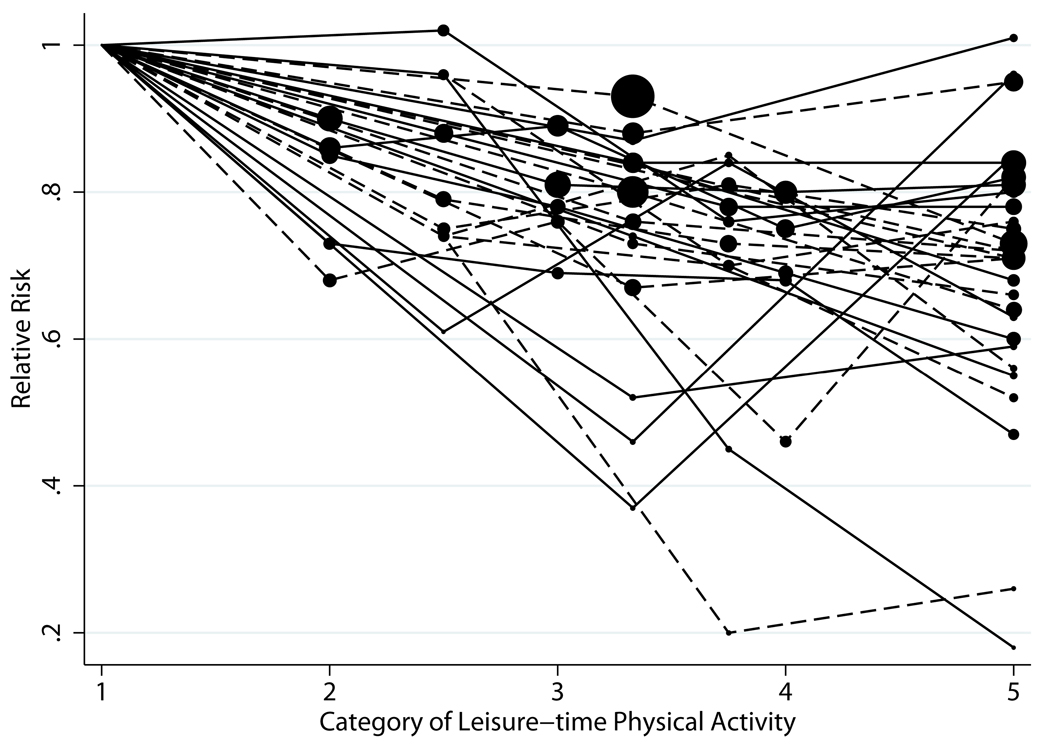
Plots of the dose-response relation between LTPA, assessed categorically, and CHD risk (30 comparisons [26 studies], out of 56 comparisons [33 studies], included data on LTPA) are shown in Figure 1. Studies that allowed quantitative estimates of LTPA demonstrated similar trends as studies that only assessed LTPA qualitatively.
Figure 1.
Plot of Relative Risks of CHD by Category* of LTPA
*All study categories were standardized to five categories for ease of comparison. The size of the data point corresponds to the study size; the larger the dot, the larger the sample size.
| Dashed lines | Studies with physical activity categorized quantitatively |
| Solid lines | Studies with physical activity categorized categorically |
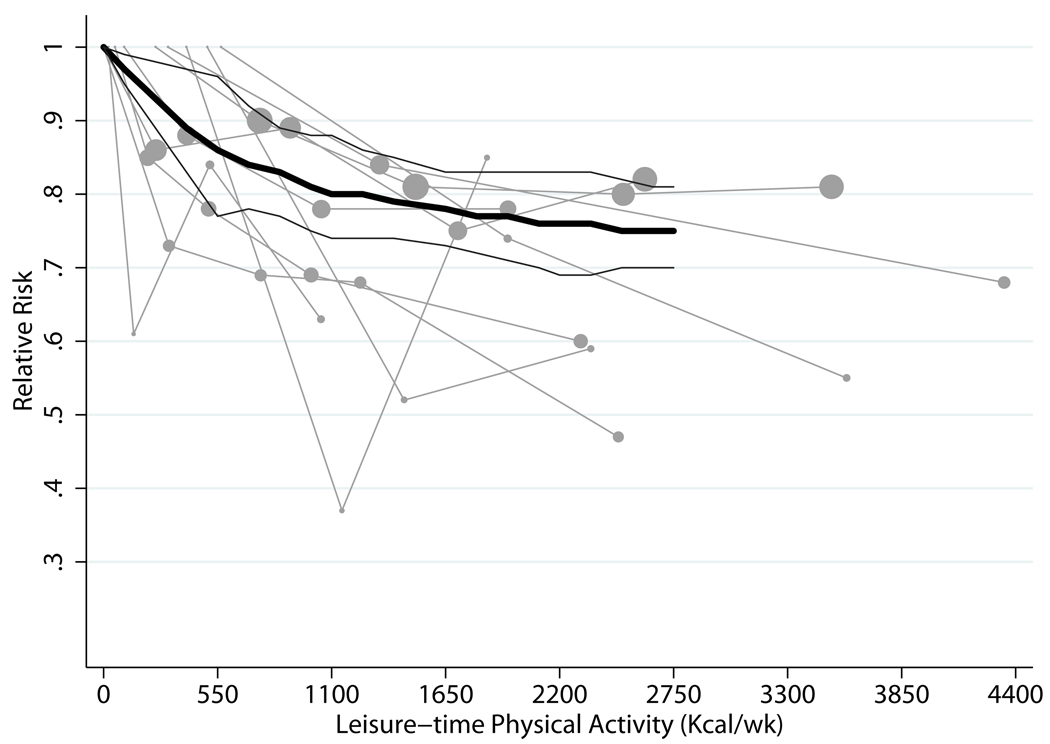
Plots of the dose-response relation between quantitative estimates of LTPA, in kcal/wk, and CHD risk14–22 (10 comparisons; 9 studies), including a trend line derived from random effects, one stage GLST spline analysis for both genders combined, is shown in Figure 2. Pooled results indicated the expected inverse relation between LTPA and CHD risk. Individuals who met the basic guideline had a 14% lower risk of CHD than those who engaged in no LTPA (RR = 0.86; 95% CI, 0.77–0.96), whereas those who met the advanced guideline had a 20% lower risk (RR = 0.80; 95% CI, 0.74–0.88). Additionally lower risks, of moderate magnitude, were observed among those with higher physical activity levels; e.g., there was a 25% lower risk for those active at five times the basic guideline. Among persons who were physically active at half the basic guideline level (275 kcal/wk), we found a 14% lower risk of CHD (RR = 0.86; 95% CI, 0.76–0.97).
Figure 2.
| Kcal/wk* | Relative Risk (95% CI) |
|---|---|
| 550 vs. 0 | 0.86 (0.77, 0.96) |
| 1100 vs. 0 | 0.80 (0.74, 0.88) |
| 2200 vs. 0 | 0.76 (0.69, 0.83) |
| 2750 vs. 0 | 0.75 (0.70, 0.81) |
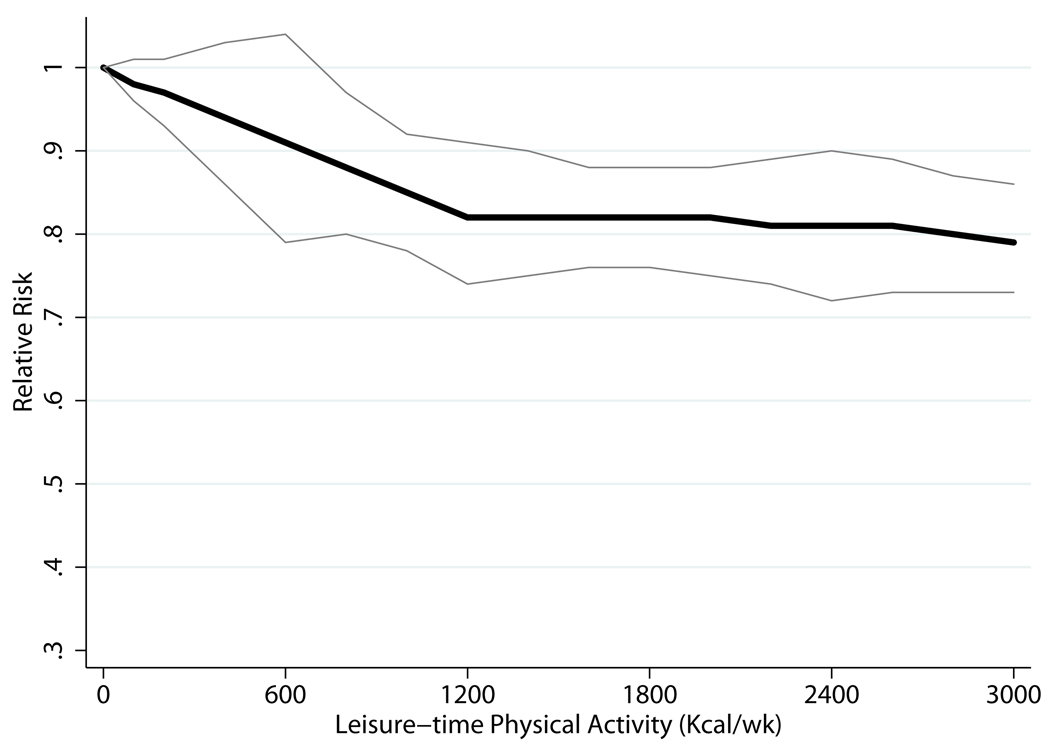
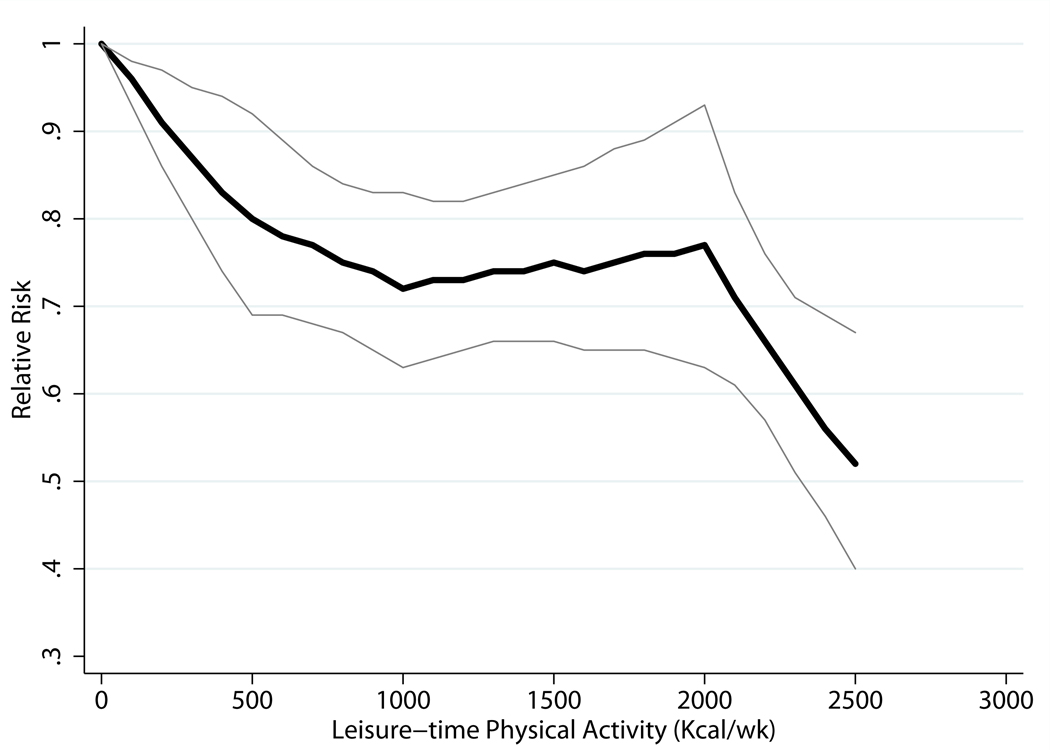
Using GLST spline models, we observed significant interaction by gender (P = 0.03). Figure 3 shows trend lines from gender-specific GLST spline analysis. Men who met the basic and advanced guidelines were at 9% (RR = 0.91; 95% CI, 0.79–1.04) and 18% (RR = 0.82; 95% CI, 0.74–0.91) lower risk of CHD, respectively, than men with no LTPA. Minimally lower risk was observed among men who participated in higher levels of LTPA; e.g., there was a 21% lower risk among men who were physically active at five times the basic guideline. Women who met the basic guideline were at 20% lower risk (RR = 0.80; 95% CI, 0.69–0.92) of CHD than women who engaged in no LTPA; women who met the advanced guideline, 28% lower risk (RR = 0.72; 95% CI, 0.63–0.83). Among women, no added lower risks were observed at higher levels of LTPA, until five times the basic guideline, which was associated with a 48% lower risk (RR = 0.52; 95% CI, 0.40–0.67).
Figure 3.
| Men | Women | ||
|---|---|---|---|
| Kcal/wk* | Relative Risk (95% CI) | Kcal/wk | Relative Risk (95% CI) |
| 600 vs.0 | 0.91 (0.79, 1.04) | 5001 vs.0 | 0.80 (0.69, 0.92) |
| 1200 vs.0 | 0.82 (0.74, 0.91) | 10002 vs.0 | 0.72 (0.63, 0.83) |
| 2400 vs.0 | 0.81 (0.73, 0.90) | 2000 vs.0 | 0.77 (0.63, 0.93) |
| 3000 vs.0 | 0.79 (0.73, 0.86) | 2500 vs.0 | 0.52 (0.40, 0.67) |
We observed no interaction by geographic region, adjustment strategy for confounding variables, or CHD outcome (data not shown). Because the interaction assessment using spline models had low power, as a secondary analysis, we assessed potential interaction using quadratic models. We found significant interaction by gender, adjustment for confounding, and CHD outcome (all P < 0.05). Despite the low power, we found that among studies that controlled for plausible biologic intermediates (e.g. BMI, hypertension, and diabetes), CHD relative risks were higher by approximately 0.1 than those from studies that did not, indicating that additional adjustment for plausible intermediates attenuated the observed associations. We observed no effect modification by geography. We found no evidence for publication bias using Begg’s test (with funnel plot) (P=0.21)
Discussion
This meta-analysis is the first to quantify the dose-response relation between physical activity and CHD risk with regard to both physical activity amount and magnitude of lower CHD risk. We found that individuals who met the basic US physical activity guideline for health9 had a 14% lower risk of CHD, compared to those with no leisure-time physical activity. Those meeting the advanced guideline had a 20% lower risk of CHD. At higher levels of physical activity, modest increments of risk reduction were observed. We also noted lower relative risks among persons who were physically active below the basic guideline, supporting the guideline’s assertion that some physical activity is better than none.
Interestingly, we observed a significant interaction by gender, such that the association of physical activity and CHD risk was stronger in women than in men. We were unable to assess whether the association differed by race or age, because of insufficient variation among studies. Geographic region of origin did not influence the association.
It is unclear why we observed a significant interaction by gender. Possible explanations include biologic differences, methodologic considerations, or some combination of both. Previous evidence does not support more favorable effects of habitual physical activity on CHD risk factors (including blood pressure, lipid levels, vascular indicators, cardio-respiratory fitness, and metabolic syndrome) among women compared to men.8 The type or intensity of physical activity contributing to total LTPA energy expenditure may differ between men and women (e.g., men favor vigorous activities while women are more likely to engage in moderate activities).17, 43 However, this does not explain the stronger effects in women, as there are limited data suggesting that vigorous-intensity physical activity may be associated with additional cardiovascular benefits, beyond its contribution to energy expenditure.47
Methodological issues may explain a portion of the difference. For instance, women have lower CHD rates1; thus, the presence of imprecisely measured or unmeasured plausible confounders (such as smoking habit and diet) may have a smaller effect in women than men.
There may be gender differences in the reporting of physical activities. However, it is unlikely that such misclassification would be greater among men than women since vigorous-intensity activities (in which men are more likely to engage) tend to be better reported than activities of lesser intensity.48 Of the studies included, longer duration of follow-up was more likely in studies of men, leading to greater potential for misclassification of energy expenditure. However, analyzing a subset of studies with comparable follow-up in men and women did not change our main results.
The primary strength of this study was the quantification of physical activity amount in analyses, enabling assessments of the risk associated with specific quantitative levels of LTPA. We chose to quantify physical activity in units of kilocalories per week (and accounting for the different average weights of men and women) as they were more frequently reported in studies, and are a more easily understood unit. We also assessed potential effect modification by numerous variables, and reported gender specific results.
Although the selection of studies that included quantitative estimates of physical activity allowed for this more quantitative approach, it also limited the number of studies that could be included. In a secondary analysis we included several additional studies for which we were able to crudely estimate quantitative levels of LTPA; findings were similar to the main analyses. We also examined the potential influence of single studies, and found that no one study changed results.
This study was limited by inclusion of only English language studies, possibly resulting in bias since statistically significant results may be more likely to be published in English. However, it is unclear whether inclusion of only English language papers does cause bias.49,50 By designating meta-analytic methods a priori, we aimed to minimize any potential investigator bias due to preconceptions. However, it is possible that the a priori designations, as well as subsequent interpretations, were subject to personal biases. Because this is a meta-analysis of observational studies, the potential for residual confounding and bias cannot be addressed through pooling. A primary source of potential residual confounding is likely to stem from confounding variables which were either unmeasured or insufficiently measured in the individual studies themselves. For instance, dietary intake was rarely assessed in the studies reviewed. In all studies included, physical activity was assessed by self-report; some misclassification of activity levels is probable and quantitative characterizations should therefore be considered approximate in nature.
We were only able to conduct our primary analysis on LTPA on 9 of 26 of potential studies. As result, there were insufficient data to assess potential interaction by several important factors (e.g., baseline age and race). Among women alone, it appeared that there was a marked and sudden decline in risk at five times the minimally recommended level of physical activity (Figure 3). However, this data point was based on only 2 studies.
We contacted the authors of the remaining 17 studies to request unpublished quantitative physical activity data; however, little additional usable information was obtained, as many of these studies used qualitative categories to assess physical activity. The inclusion of only the 9 studies for quantitative analyses was unlikely to have biased results, since these 9 studies appeared representative of the broader group of 26 eligible studies. In initial analyses comparing “high” versus “low” physical activity, which included all 26 studies, findings were similar to those including only the 9 studies. Further, in comparing our findings with previous reviews, which quantified only the magnitude of lower relative risks but not the amount of physical activity required, the results are comparable. Our comparison of “high” versus “low” physical activity yielded a relative risk of 0.75 for CHD, similar in magnitude to several past reviews.2, 3, 7, 8
In conclusion, the present study provides quantitative data supporting the 2008 Physical Activity Guidelines for Americans which recommends the equivalent of 150 min/week of moderate-intensity physical activity for health, and 300 min/week for additional health benefits, as well as encouraging any amount of activity for those unable to meet the minimum. Future studies that quantitatively assess the dose-response relation between LTPA, as well as other types and features of physical activity, and CHD risk will help clarify the upper end of the dose-response curve and enable additional quantitative evaluations in future reviews, such as exploring potential differences by age and race. Additionally, individual participant meta-analyses conducted via collaboration among research groups, though resource intensive, can make use of existing studies to further clarify dose-response relationships.51
Clinical Summary.
Physical activity clearly has been shown to decrease the risk of developing coronary heart disease (CHD). However, the dose-response relation (How much activity is needed? What level of risk reduction is associated with specified levels of activity? Does the risk continue to decrease at higher levels of activity?) is less clear. This is the first meta-analysis of epidemiologic studies to quantify the dose-response relation, examining both the specific amounts of physical activity and associated risk reductions for CHD (previous meta-analyses have quantified only risk reductions, but not the specific doses of activity required). We found that individuals who engaged in the equivalent of 150 min/week of moderate-intensity leisure-time physical activity (corresponding to the minimum amount recommended by the 2008 US federal guidelines) had a 14% lower CHD risk, compared with those reporting no LTPA. Those engaging in the equivalent of 300 min/week of moderate-intensity leisure-time activity had a 20% lower risk. At higher levels of physical activity, relative risks were modestly lower; for example, at five times the minimum recommended, there was a 25% lower risk. Persons who were physically active at levels lower than the minimum amount recommended also had a significantly lower risk of CHD. These findings provide quantitative data that support the 2008 US physical activity guidelines. They indicate that the “biggest bang for the buck” for CHD risk reduction occurs at the lower end of the activity spectrum: very modest, achievable levels of physical activity.
Supplementary Material
Acknowledgements
We would also like to acknowledge Mieke Van Hemelrijck and Renata Micha for their contributions to this work, as well as the authors who we contacted to request additional information.
Funding Sources
This research was supported by grants NIH T32 (Brigham and Women’s Hospital) and the Donald and Sue Pritzker Scholarship Fund. Funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Author JS had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures:
Jacob Sattelmair is an employee at Dossia.
Jeremy Pertman has no potential conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript.
Eric Ding is supported by a fellowship from the American Diabetes Association.
Bill Kohl has no potential conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript.
Bill Haskell has no potential conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript.
I-Min Lee has received investigator-initiated research funding from the National Institutes of Health; she serves as a consultant to Virgin HealthMiles and sits on their Scientific Advisory Board.
Contributor Information
Jacob Sattelmair, Department of Epidemiology, Harvard School of Public Health, sattelmair@post.harvard.edu.
Jeremy Pertman, Department of Epidemiology, Harvard School of Public Health, jpertman@post.harvard.edu.
Eric L. Ding, Channing Laboratory, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School; Department of Nutrition, Harvard School of Public Health, eding@post.harvard.edu.
Harold W. Kohl, III, Division of Epidemiology, Genetics and Environmental Health Sciences, University of Texas Health Science Center – Houston; Department of Kinesiology and Health Education, University of Texas at Austin, harold.w.kohl@uth.tmc.edu.
William Haskell, Stanford Center for Research in Disease Prevention, Stanford Medical School, whaskell@stanford.edu.
I-Min Lee, Division of Preventive Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School; Department of Epidemiology, Harvard School of Public Health, ilee@rics.bwh.harvard.edu.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart Disease and Stroke Statistics--2010 Update: A Report From the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Berlin JA, Colditz GA. A meta-analysis of physical activity in the prevention of coronary heart disease. Am J Epidemiol. 1990;132:612–628. doi: 10.1093/oxfordjournals.aje.a115704. [DOI] [PubMed] [Google Scholar]
- 3.Eaton CB. Relation of physical activity and cardiovascular fitness to coronary heart disease, Part I: A meta-analysis of the independent relation of physical activity and coronary heart disease. J Am Board Fam Pract. 1992;5:31–42. [PubMed] [Google Scholar]
- 4.Pate RP, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, Buchner D, Ettinger W, Heath GW, King AC, Kriska A, Leon AS, Marcus BH, Morris J, Paffenbarger RS, Jr, Patrick K, Pollock ML, Rippe JM, Sallis J, Wilmore JH. A recommendation from the Centers for Disease control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 5.Williams PT. Physical fitness and activity as separate heart disease risk factors: a meta-analysis. Med Sci Sports Exerc. 2001;33:754–761. doi: 10.1097/00005768-200105000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oguma Y, Shinoda-Tagawa T. Physical activity decreases cardiovascular disease risk in women: Review and meta-analysis. American Journal of Preventive Medicine. 2004;26:407–418. doi: 10.1016/j.amepre.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Sofi F, Capalbo A, Cesari F, Abbate R, Gensini GF. Physical activity during leisure time and primary prevention of coronary heart disease: an updated meta-analysis of cohort studies. Eur J Cardiovasc Prev Rehabil. 2008;15:247–257. doi: 10.1097/HJR.0b013e3282f232ac. [DOI] [PubMed] [Google Scholar]
- 8.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: 2008. [Google Scholar]
- 9.US Department of Health and Human Services. Physical Activity Guidelines for Americans. Office of Disease Prevention and Health Promotion; 2008. Oct, (Publication No. U0036). [Google Scholar]
- 10.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis Of Observational Studies in Epidemiology: A Proposal for Reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 11.Micha R, Wallace SK, Mozaffarian D. Red and Processed Meat Consumption and Risk of Incident Coronary Heart Disease, Stroke, and Diabetes Mellitus: A Systematic Review and Meta-Analysis. Circulation. 2010;121:2271–2283. doi: 10.1161/CIRCULATIONAHA.109.924977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenland S, Longnecker MP. Methods for Trend Estimation from Summarized Dose-Response Data, with Applications to Meta-Analysis. Am. J. Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 13.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 14.Chiuve SE, McCullough ML, Sacks FM, Rimm EB. Healthy lifestyle factors in the primary prevention of coronary heart disease among men: Benefits among users and nonusers of lipid-lowering and antihypertensive medications. Circulation. 2006;114:160–167. doi: 10.1161/CIRCULATIONAHA.106.621417. [DOI] [PubMed] [Google Scholar]
- 15.Haapanen-Niemi N, Miilunpalo S, Pasanen M, Vuori I, Oja P, Malmberg J. Body mass index, physical inactivity and low level of physical fitness as determinants of all-cause and cardiovascular disease mortality--16 y follow-up of middle-aged and elderly men and women. Int J Obes Relat Metab Disord. 2000;24:1465–1474. doi: 10.1038/sj.ijo.0801426. [DOI] [PubMed] [Google Scholar]
- 16.Manson JE, Greenland P, LaCroix AZ, Stefanick ML, Mouton CP, Oberman A, Perri MG, Sheps DS, Pettinger MB, Siscovick DS. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716–725. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 17.Manson JE, Hu FB, Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, Speizer FE, Hennekens CH. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med. 1999;341:650–658. doi: 10.1056/NEJM199908263410904. [DOI] [PubMed] [Google Scholar]
- 18.Kaprio J, Kujala UM, Koskenvuo M, Sarna S. Physical activity and other risk factors in male twin-pairs discordant for coronary heart disease. Atherosclerosis. 2000;150:193–200. doi: 10.1016/s0021-9150(99)00368-8. [DOI] [PubMed] [Google Scholar]
- 19.Sesso HD, Paffenbarger RS, Jr, Lee IM. Physical activity and coronary heart disease in men: The Harvard Alumni Health Study. Circulation. 2000;102:975–980. doi: 10.1161/01.cir.102.9.975. [DOI] [PubMed] [Google Scholar]
- 20.Weinstein AR, Sesso HD, Lee IM, Rexrode KM, Cook NR, Manson JE, Buring JE, Gaziano JM. The joint effects of physical activity and body mass index on coronary heart disease risk in women. Archives of Internal Medicine. 2008;168:884–890. doi: 10.1001/archinte.168.8.884. [DOI] [PubMed] [Google Scholar]
- 21.Yu S, Yarnell JW, Sweetnam PM, Murray L. What level of physical activity protects against premature cardiovascular death? The Caerphilly study. Heart. 2003;89:502–506. doi: 10.1136/heart.89.5.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weller I, Corey P. The Impact of Excluding Non-Leisure Energy Expenditure on the Relation between Physical Activity and Mortality in Women. Epidemiology. 1998;9:632–635. [PubMed] [Google Scholar]
- 23.Akesson A, Weismayer C, Newby PK, Wolk A. Combined effect of low-risk dietary and lifestyle behaviors in primary prevention of myocardial infarction in women. Arch Intern Med. 2007;167:2122–2127. doi: 10.1001/archinte.167.19.2122. [DOI] [PubMed] [Google Scholar]
- 24.Batty GD, Shipley MJ, Marmot M, Smith GD. Physical activity and cause-specific mortality in men: further evidence from the Whitehall study. Eur J Epidemiol. 2001;17:863–869. doi: 10.1023/a:1015609909969. [DOI] [PubMed] [Google Scholar]
- 25.Batty GD, Shipley MJ, Marmot M, Smith GD. Physical activity and cause-specific mortality in men with Type 2 diabetes/impaired glucose tolerance: evidence from the Whitehall study. Diabet Med. 2002;19:580–588. doi: 10.1046/j.1464-5491.2002.00748.x. [DOI] [PubMed] [Google Scholar]
- 26.Bijnen FC, Caspersen CJ, Feskens EJ, Saris WH, Mosterd WL, Kromhout D. Physical activity and 10-year mortality from cardiovascular diseases and all causes: The Zutphen Elderly Study. Arch Intern Med. 1998;158:1499–1505. doi: 10.1001/archinte.158.14.1499. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Millar WJ. Health effects of physical activity. Health Rep. 1999;11:21–30. [PubMed] [Google Scholar]
- 28.Eaton CB, Medalie JH, Flocke SA, Zyzanski SJ, Yaari S, Goldbourt U. Self-reported physical activity predicts long-term coronary heart disease and all-cause mortalities. Twenty-one-year follow-up of the Israeli Ischemic Heart Disease Study. Arch Fam Med. 1995;4:323–329. doi: 10.1001/archfami.4.4.323. [DOI] [PubMed] [Google Scholar]
- 29.Folsom AR, Arnett DK, Hutchinson RG, Liao F, Clegg LX, Cooper LS. Physical activity and incidence of coronary heart disease in middle-aged women and men. Med Sci Sports Exerc. 1997;29:901–909. doi: 10.1097/00005768-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Hakim AA, Curb JD, Petrovitch H, Rodriguez BL, Yano K, Ross GW, White LR, Abbott RD. Effects of walking on coronary heart disease in elderly men: the Honolulu Heart Program. Circulation. 1999;100:9–13. doi: 10.1161/01.cir.100.1.9. [DOI] [PubMed] [Google Scholar]
- 31.Hillsdon M, Thorogood M, Murphy M, Jones L. Can a simple measure of vigorous physical activity predict future mortality? Results from the OXCHECK study. Public Health Nutrition. 2004;7:557–562. doi: 10.1079/PHN2003548. [DOI] [PubMed] [Google Scholar]
- 32.Hu G, Jousilahti P, Borodulin K, Barengo NC, Lakka TA, Nissinen A, Tuomilehto J. Occupational, commuting and leisure-time physical activity in relation to coronary heart disease among middle-aged Finnish men and women. Atherosclerosis. 2007;194:490–497. doi: 10.1016/j.atherosclerosis.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 33.Inoue M, Iso H, Yamamoto S, Kurahashi N, Iwasaki M, Sasazuki S, Tsugane S. Daily total physical activity level and premature death in men and women: results from a large-scale population-based cohort study in Japan (JPHC study) Ann Epidemiol. 2008;18:522–530. doi: 10.1016/j.annepidem.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Knoops KT, de Groot LC, Kromhout D, Perrin AE, Moreiras-Varela O, Menotti A, van Staveren WA. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. Jama. 2004;292:1433–1439. doi: 10.1001/jama.292.12.1433. [DOI] [PubMed] [Google Scholar]
- 35.Lee IM, Rexrode KM, Cook NR, Manson JE, Buring JE. Physical activity and coronary heart disease in women: is "no pain, no gain" passe? Jama. 2001;285:1447–1454. doi: 10.1001/jama.285.11.1447. [DOI] [PubMed] [Google Scholar]
- 36.Leon AS, Myers MJ, Connett J. Leisure time physical activity and the 16-year risks of mortality from coronary heart disease and all-causes in the Multiple Risk Factor Intervention Trial (MRFIT) Int J Sports Med. 1997;18:S208–S215. doi: 10.1055/s-2007-972717. [DOI] [PubMed] [Google Scholar]
- 37.Meisinger C, Lowel H, Heier M, Kandler U, Doring A. Association of sports activities in leisure time and incident myocardial infarction in middle-aged men and women from the general population: The MONICA/KORA Augsburg cohort study. European Journal of Cardiovascular Prevention and Rehabilitation. 2007;14:788–792. doi: 10.1097/HJR.0b013e32828641be. [DOI] [PubMed] [Google Scholar]
- 38.Pedersen JO, Heitmann BL, Schnohr P, Gronbaek M. The combined influence of leisure-time physical activity and weekly alcohol intake on fatal ischaemic heart disease and all-cause mortality. Eur Heart J. 2008;29:204–212. doi: 10.1093/eurheartj/ehm574. [DOI] [PubMed] [Google Scholar]
- 39.Qvist Jx, Johansson SE, Johansson LM. Multivariate analyses of mortality from coronary heart disease due to biological and behavioural factors. Scand J Soc Med. 1996;24:67–76. doi: 10.1177/140349489602400111. [DOI] [PubMed] [Google Scholar]
- 40.Rosengren A, Wilhelmsen L. Physical activity protects against coronary death and deaths from all causes in middle-aged men. Evidence from a 20-year follow-up of the primary prevention study in Goteborg. Ann Epidemiol. 1997;7:69–75. doi: 10.1016/s1047-2797(96)00106-8. [DOI] [PubMed] [Google Scholar]
- 41.Smith TC, Wingard DL, Smith B, Kritz-Silverstein D, Barrett-Connor E. Walking decreased risk of cardiovascular disease mortality in older adults with diabetes. J Clin Epidemiol. 2007;60:309–317. doi: 10.1016/j.jclinepi.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sundquist K, Qvist J, Johansson SE, Sundquist J. The long-term effect of physical activity on incidence of coronary heart disease: a 12-year follow-up study. Prev Med. 2005;41:219–225. doi: 10.1016/j.ypmed.2004.09.043. [DOI] [PubMed] [Google Scholar]
- 43.Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. Jama. 2002;288:1994–2000. doi: 10.1001/jama.288.16.1994. [DOI] [PubMed] [Google Scholar]
- 44.Virkkunen H, Harma M, Kauppinen T, Tenkanen L. Shift work, occupational noise and physical workload with ensuing development of blood pressure and their joint effect on the risk of coronary heart disease. Scand J Work Environ Health. 2007;33:425–434. doi: 10.5271/sjweh.1170. [DOI] [PubMed] [Google Scholar]
- 45.Wagner A, Simon C, Evans A, Ferrieres J, Montaye M, Ducimetiere P, Arveiler D. Physical activity and coronary event incidence in Northern Ireland and France: the Prospective Epidemiological Study of Myocardial Infarction (PRIME) Circulation. 2002;105:2247–2252. doi: 10.1161/01.cir.0000016345.58696.4f. [DOI] [PubMed] [Google Scholar]
- 46.Wannamethee SG, Shaper AG, Alberti KG. Physical activity, metabolic factors, and the incidence of coronary heart disease and type 2 diabetes. Arch Intern Med. 2000;160:2108–2116. doi: 10.1001/archinte.160.14.2108. [DOI] [PubMed] [Google Scholar]
- 47.Swain DP, Franklin BA. Comparison of Cardioprotective Benefits of Vigorous Versus Moderate Intensity Aerobic Exercise. The American Journal of Cardiology. 2006;97:141–147. doi: 10.1016/j.amjcard.2005.07.130. [DOI] [PubMed] [Google Scholar]
- 48.Lee I, Blair SN, Manson J, Paffenbarger RSJ. Epidemiologic Methods in Physical Activity Studies. New York, NY: Oxford University Press; 2009. [Google Scholar]
- 49.Egger E, Zellwegerzahner T, Schneider M, Junker C, Lengeler C, Antes G. Language bias in randomised controlled trials published in English and German. Lancet. 1997;350:326–329. doi: 10.1016/S0140-6736(97)02419-7. [DOI] [PubMed] [Google Scholar]
- 50.Juni P, Holenstein F, Sterne J, Bartlett C, Egger M. Direction and impact of language bias in meta- analyses of controlled trials: empirical study. Int J Epidemiol. 2002;31:115–123. doi: 10.1093/ije/31.1.115. [DOI] [PubMed] [Google Scholar]
- 51.Riley RD, Lambert PC, Abo-Zaid G. Meta-Analysis of individual participant data: rationale, conduct and reporting. British Medical Journal. 2010;340:c221. doi: 10.1136/bmj.c221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.