Abstract
Weight loss can have substantial health benefits for overweight or obese persons; however, the ratio of fat:lean tissue loss may be more important. We aimed to determine how daily exercise (resistance and/or aerobic) and a hypoenergetic diet varying in protein and calcium content from dairy foods would affect the composition of weight lost in otherwise healthy, premenopausal, overweight, and obese women. Ninety participants were randomized to 3 groups (n = 30/group): high protein, high dairy (HPHD), adequate protein, medium dairy (APMD), and adequate protein, low dairy (APLD) differing in the quantity of total dietary protein and dairy food-source protein consumed: 30 and 15%, 15 and 7.5%, or 15 and <2% of energy, respectively. Body composition was measured by DXA at 0, 8, and 16 wk and MRI (n = 39) to assess visceral adipose tissue (VAT) volume at 0 and 16 wk. All groups lost body weight (P < 0.05) and fat (P < 0.01); however, fat loss during wk 8–16 was greater in the HPHD group than in the APMD and APLD groups (P < 0.05). The HPHD group gained lean tissue with a greater increase during 8–16 wk than the APMD group, which maintained lean mass and the APLD group, which lost lean mass (P < 0.05). The HPHD group also lost more VAT as assessed by MRI (P < 0.05) and trunk fat as assessed by DXA (P < 0.005) than the APLD group. The reduction in VAT in all groups was correlated with intakes of calcium (r = 0.40; P < 0.05) and protein (r = 0.32; P < 0.05). Therefore, diet- and exercise-induced weight loss with higher protein and increased dairy product intakes promotes more favorable body composition changes in women characterized by greater total and visceral fat loss and lean mass gain.
Introduction
Weight loss in overweight and obese persons through diet and/or exercise can confer a host of metabolic benefits (1, 2). Although energy restriction alone often leads to weight loss, the tissue composition of the loss includes lean tissue (3–5), which could have deleterious metabolic consequences. The loss of lean tissue with weight loss may be responsible for the tendency toward a plateau in weight loss during, or weight regain following, a weight loss program (6, 7). Moreover, skeletal muscle plays a number of important roles in the regulation of glycemia (8) and lipidemia (9), suggesting that the loss of this tissue may have adverse effects on long-term metabolic health (10). We propose that weight loss strategies should be focused on the tissue composition of the weight lost and not based merely on scale weight; instead, weight loss interventions should be aimed toward loss of fat, especially visceral fat, and the preservation of muscle mass.
The optimal dietary macronutrient composition to achieve weight loss remains controversial. Several large clinical trials have shown that adherence to an energy-restricted diet, not the macronutrient composition per se, seems to be the primary factor driving weight loss (11–13). However, none of these studies addressed the tissue composition of the participants’ weight loss, especially lean mass loss, the sparing of which would actually reduce weight lost; thus, in these trials, the investigators potentially missed important effects of the different diets beyond weight change (11–13). For example, higher protein, lower carbohydrate, energy-restricted diets have been shown to help offset the lean mass loss observed with conventional (~55% of energy intake) carbohydrate diets (14–16), and pairing higher protein intakes with exercise, especially resistance exercise, worked synergistically to further maintain lean mass during weight loss (17–19). Insofar as weight maintenance is concerned, individuals who consumed higher protein (and reduced the glycemic index of their dietary carbohydrates) following 8 wk of weight loss were better able to maintain their initial loss for an additional 26 wk compared to those who consumed maintenance diets lower in protein (20).
Trials in humans have linked the consumption of dairy foods and dairy-derived calcium to greater weight loss and fat mass loss (21–25), a mechanism that is supported by in vitro experimental studies for the antiadipogenic effects of dairy foods (26–28). In young, recreationally active men and women, we observed an advantage of consuming low-fat milk with resistance exercise in promoting lean mass gains both acutely (29) and over time in conjunction with fat mass loss, despite participants being in positive energy balance (30, 31). Thus, as a strategy for weight loss and lean mass retention, dairy foods would appear advantageous.
In this study, we aimed to achieve weight loss with a high ratio of fat:lean mass loss. This pattern is important not only for short-term efficacy but also for long-term metabolic health and body weight maintenance (6, 7). Our approach was multifaceted and incorporated aspects shown in previous programs to help preserve or increase muscle mass and yet still promote fat mass loss (30–32). We combined energy restriction, higher dietary protein (lower carbohydrate), increased intakes of dairy foods, and exercise (aerobic and resistance) into one program. Our specific objectives were to test: the effect of consuming high-dairy protein and calcium compared to a typical dietary pattern, the effect of doubling the consumption of dietary (and dairy) protein as a percent of total energy intake, and the effect of protein source (dairy vs. nondairy) in diets that contain moderate amounts of protein. We hypothesized that the optimal condition for a high ratio of fat:lean mass loss would occur in the individuals who consumed higher protein and a greater amount of dairy foods.
Methods
Participants.
Our study, the Improving Diet, Exercise and Lifestyle (I.D.E.A.L.) for Women Study, was approved by the Research Ethics Board of the Hamilton Health Sciences and conformed to the most recent Canadian government tri-council funding policy statement on the use of human subjects in research (33). The study was conducted from June 2008 to May 2010. A total of 246 women were recruited from McMaster University and the surrounding Hamilton area through posters and local newspaper advertisements. Of those, 95 participants gave their written, informed consent to participate. Prior to beginning the trial, 5 women declined participation and 90 were randomized into 3 groups. Information regarding recruitment and participant flow is in Supplemental Figure 1.
Participants were all premenopausal and overweight or obese women (BMI between 27 and 40 kg/m2) between the ages of 19 and 45 y. Other general inclusion criteria were: low dairy product consumption, sedentary lifestyle, regular menstrual cycle, no vitamin or mineral supplementation, and otherwise healthy. Participants were deemed healthy and eligible to participate based on their responses to a short medical screening questionnaire that inquired about metabolic risk factors (cholesterol, insulin, and glucose concentrations and blood pressure), heart and other organ disease, orthopedic injury that would interfere with exercise, gastrointestinal disease, clinically diagnosed dairy protein allergy, clinically diagnosed lactose intolerance, and prescription medication use. Participants were excluded if they had any of the aforementioned conditions.
Prior to the study commencing, participants’ height and weight were measured (Healthweigh 140–10 Eye Level Digital Physician scale). They then completed a FFQ validated for calcium and dairy foods to verify their low consumption at baseline (as per the study inclusion criteria). Participants were also instructed on how to accurately complete a 7-d food record and were given their own set of measuring cups and spoons to use throughout the study.
Study protocol.
Participants were randomly assigned to 1 of 3 groups: adequate protein, low dairy (APLD)8, adequate protein, medium dairy (APMD); or high protein, high dairy (HPHD). The randomization was stratified by BMI (27–29, 30–34, and 35–40 kg/m2), the most commonly used criterion for classification of overweight/obesity, to ensure that an equal number of participants from each BMI category were in each treatment group. Our stratification scheme was adequate, because there were no between-group differences in any baseline variable. The 3 groups differed in the amount and type of protein consumed and their dietary macronutrient distribution. The APLD group maintained their stable baseline dairy food consumption at <2% energy/d from dairy protein corresponding to 0–1 serving/d [1 dairy serving represents 250 mL of milk, 50 g cheese, or 175 mL of yogurt according to Canada’s Food Guide (34), and 250 mL of milk or yogurt and 45 g of cheese according to the USDA Food Pyramid (35)]. The APMD group was instructed to consume 7.5% energy/d from dairy protein at 3–4 servings/d of dairy products and the HPHD group to consume 15% of energy/d from dairy protein at 6–7 servings/d of dairy products. All dairy products needed to control dairy and calcium intakes were provided to participants in the APMD and HPHD groups and were generously donated by Agropur Dairy Cooperative (Table 1). The APLD and APMD groups consumed a diet consisting of 55:30:15 (percent energy from carbohydrate, fat, and protein) and the HPHD group consumed a diet consisting of 40:30:30. Thus, the study was designed such that the HPHD group consumed twice the amount of protein (and lower carbohydrate) than the other 2 groups and twice the amount of dairy foods as the APMD group. The APMD group consumed the same amount of protein as the APLD group (15%), with one-half of that protein from dairy sources and the other one-half from high-quality nondairy sources such as lean red meat, eggs, fish, chicken, pork, legumes, soy, and wheat. The APLD group was counseled to consume all protein from the same nondairy sources mentioned above. All participants were prescribed the same exercise regimen and underwent individual biweekly dietary counseling during the 16 wk.
TABLE 1.
Nutritional information of the dairy foods provided to the participants in the adequate protein, medium dairy (APMD) and high protein, high dairy (HPHD) groups in the Improving Diet, Exercise and Lifestyle for Women Study, including the products used, the quantities of dairy foods consumed, and their nutritional contents
| Quantity | Energy1 | Calcium | Vitamin D | Protein | |
| kcal | mg | μg | g | ||
| APMD | |||||
| 1% chocolate milk (Sealtest)2 | 3 x 250 mL | 450 | 750 | 6.8 | 21 |
| Source yogurt (Yoplait) | 2 x 100 g | 70 | 200 | 1.5 | 8 |
| Total amount | 520 | 950 | 8.3 | 29 | |
| HPHD | |||||
| 1% Splenda-sweetened chocolate milk (Sealtest)2 | 3 x 250 mL | 300 | 750 | 6.8 | 21 |
| 1% white milk (Sealtest) | 1 x 250 mL | 100 | 300 | 2.3 | 9 |
| Source yogurt (Yoplait) | 4 x 100 g | 140 | 400 | 3.0 | 16 |
| Cheddar cheese (Bright Bites) | 2 x 21 g | 180 | 200 | 0.0 | 10 |
| Total amount | 720 | 1650 | 12.1 | 56 | |
1 kcal = 4.18 kJ.
Chocolate milk used as the study drinks for the APMD and HPHD groups.
Maintenance energy requirements were calculated per participant using the Mifflin St Jeor equation (36) with a sedentary activity factor. Once this energy level was determined, it was reduced by 500 kcal/d and used as the participant’s targeted total energy intake throughout the study; thus, all women consumed a hypoenergetic diet.
All groups consumed 2 study drinks/d; one immediately postexercise and another drink at least 5 h before or after exercise. Each drink was 375 mL and was prepared daily in opaque, reusable, plastic bottles. Bottles were prepared and labeled for each participant by study staff in our metabolic kitchen. All drinks provided similar energy and were chocolate flavored so that they looked, smelled, and tasted similar to keep the participants unaware of their contents. Known to study staff only, the drinks were either 1% chocolate milk (APMD); Splenda-sweetened 1% chocolate milk (HPHD) (Table 1); or a custom-blended, nondairy, vitamin D- and calcium-free, carbohydrate-based, chocolate-flavored beverage (APLD). Splenda-sweetened chocolate milk was provided to the HPHD group to keep their carbohydrate intake from study foods low. This enabled them to consume their restricted level of dietary carbohydrate (40%) from other food sources (e.g. fruits, vegetables, and whole grains).
All participants received individualized diet counseling by study dieticians and research nutritionists on a biweekly basis. The initial 7-d food record was analyzed using ESHA (Food Processor SQL, ESHA Research) (37) and it served as the starting point for which the diet counseling was based. Each participant was provided with an individualized plan outlining their required macronutrient intakes in grams corresponding to their new daily energy requirements and the respective macronutrient distribution of the group to which they were randomized. Every 2 wk thereafter, participants provided a 3-d food record to track compliance with the nutrition protocol. All 3-d food records were analyzed with ESHA and participants were provided with feedback in their next biweekly private counseling session. To ensure that the results were due as much as possible to the differing dietary components, participants were instructed not to take any vitamin or mineral supplements during the study.
Exercise training.
Participants exercised in our own exercise and testing center and at the main fitness center at McMaster University. They engaged in various modes of aerobic exercise every day, 5 d/wk with us and 2 d on their own on the weekend. At each workout participants were to expend 250 kcal. During the week (Monday–Friday), they reported to our study office daily and were often given a SenseWear Pro energy expenditure device (BodyMedia) to wear, programmed for them, so they could track their energy expenditure during their workout. Compliance with weekend workouts was assessed by having women take home and wear the SenseWear Pro device on random occasions. In addition to aerobic exercise, participants engaged in a progressive resistance training regimen 2 d/wk (upper body, lower body split) with trained study personnel (personal trainer or kinesiologist). Weight lifted by each participant was recorded every session and increased once they could complete 3 sets of 10 repetitions or more at any given weight. The majority (i.e. ~70%) of the aerobic exercise sessions were individually or small-group (i.e. 1 trainer to 2–3 participants) supervised and all of the resistance exercise sessions were individually supervised. Resistance exercise logs were completed by the trainer and aerobic exercise logs were completed by the participant and checked frequently by study personnel to ensure compliance.
Body composition.
Whole-body DXA scans (QDR-4500A; Hologic, software version 12.31) were carried out at wk 0, 8, and 16 to determine total body weight, fat mass, and (fat and bone free) lean mass. Women were scanned wearing light clothing at the same time of day and were instructed to follow the same prescan diet and exercise instructions before each scan. After study completion, pre- and poststudy DXA scans were further analyzed for the determination of trunk fat. The abdominal region of interest on all scans was isolated between the lumbar vertebrae L1–L4 by the same trained individual and fat mass was recorded. Investigators were unaware of the participants’ group assignment during analysis.
A subset of women (n = 39) underwent MR imaging (3T Signa Scanner, GE Healthcare) at wk 0 and 16 to directly ascertain the change in VAT volume during the intervention. Of these 39 women, 8/12 in the APLD group, 8/13 in the APMD group, and 9/14 in the HPHD group were Caucasian. MRI scans were performed on the participants’ torso region from the top of their liver to the iliac crest (determined by an axial scan), corresponding to 3 sectional scans of 10–12 slices each. Participants were instructed to hold their breath after exhalation for the duration of the sectional scan (~20 s each) to ensure that the movement of breathing did not adversely affect the quality of the image. The MR images were analyzed with Santesoft Medical Imaging software (Sante DICOM Editor) using a standardized tracing protocol. To reduce variability, all scans were analyzed by the same trained and blinded technician who, on random scans, showed an inter-scan variability of <5%.
Strength and aerobic fitness testing.
Strength was tested by determining each participant’s single lift voluntary maximal strength or 1 repetition maximum on the leg extension, hamstring curl, seated row, and chest press pre- and postintervention. All testing was done by trained study personnel using the same standardized protocol described elsewhere (31).
Aerobic fitness was measured using a modified Astrand submaximal fitness test protocol (38). Participants were asked to cycle on a stationary bicycle (Monark Ergomedic 828E, HealthCare International) at 2 consecutive workloads for 6 min each and HR was recorded every minute.
Anthropometry.
Waist and hip circumference was measured 3 times throughout the study: at wk 0, 8, and 16. We used the circumference of the waist at the participant’s umbilicus as the site of measure. Two separate research assistants took measurements independently with the same tape measure (Gulick II Tape Measure, FitnessMart) and the 2 measurements were averaged. The average difference between the 2 measures was always <1.5 cm.
Blood samples and laboratory analyses.
Blood samples were obtained on 2 occasions (pre- and postintervention) between 0630 and 1000 h after an overnight fast of 10–12 h in tubes containing either sodium heparin or no additives to obtain plasma and serum, respectively. Serum insulin (chemiluminescent microparticle immunoassay; Architect System, Abbott Laboratories) and lipids and plasma glucose and calcium (colorimetric assay; Roche COBAS MIRA Clinical Analyzer, Roche Diagnostics) were analyzed at the Core Laboratory at the McMaster University Medical Centre. The remaining samples were stored at −20°C for later analysis. At study completion, serum samples were batch-analyzed for IL-6 (high sensitivity ELISA; R&D Systems) and CRP (ELISA; ALPCO Immunoassays). All investigators and laboratory technicians were unaware of the participants’ group assignment during analysis.
Statistics.
Statistical analyses were performed using SPSS (version 18.0). Prior to hypothesis testing, primary endpoint data were examined for normality. Non-normally distributed variables were log-transformed before analysis. Analysis was conducted on all participants who completed the study as well as on those who did not complete (all of whom dropped out after 8 wk). In accordance with recently suggested practices for randomized weight loss trials (39), we conducted an intent-to-treat analysis where missing data values were imputed using model-based multiple imputation. Differences between groups in all baseline variables were compared by univariate ANOVA. Significance was set at P < 0.05. Data in the text are means ± SE.
Initial analysis was performed using a 2-way, repeated-measures ANCOVA with baseline bodyweight as the covariate for the primary outcome variables. Two-way, repeated-measures ANOVA was also performed on the change values (wk 0–8 and 8–16) for the primary outcome variables and waist circumference. Significant F ratios were further analyzed using planned contrast ANOVA (repeated measures or univariate) to determine differences both within and between groups, respectively. Significant differences were isolated with Tukey’s post hoc test with a Bonferroni correction for multiple comparisons. Pearson correlation coefficients were also calculated for several measures. The MRI portion of the study was carried out on a smaller cohort of women (n = 39; 12 in APLD, 13 in APMD, and 14 in HPHD); therefore, only these women were included in the MRI statistical analysis.
Results
Participants.
Ninety women participated in this study. Nine participants (6 in the APLD group and 3 in the HPHD group) dropped out after wk 8 (half-way) for reasons unrelated to the study (Supplemental Fig. 1). The participants represented a multiracial/multiethnic population with n = 15 (APLD), n = 13 (APMD), and n = 18 (HPHD) Caucasian women in each group. The other women were East Indian (n = 15), Asian (n = 9), African, African American, Native Canadian, Portuguese, European, or Hispanic and were equally distributed throughout the groups. None of the baseline values for any variables studied significantly differed between the groups (Table 2).
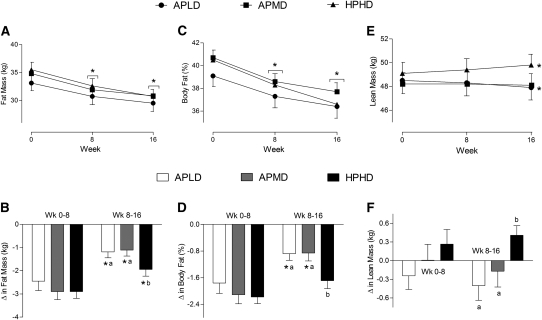
FIGURE 1.
Absolute (A,C,E) and relative-to-baseline (B,D,F) changes in body fat mass (A,B), percent body fat (C,D), and lean mass (E,F) in overweight and obese premenopausal women who underwent a 16-wk intervention of diet- and exercise-induced weight loss. Values are means ± SE, n = 90 (30/group). Means at a time without a common letter differ, P < 0.05. *Different from the preceding time point, P < 0.05.APLD, adequate protein, low dairy; APMD, adequate protein, medium dairy; HPHD, high protein, high dairy.
TABLE 2.
Baseline characteristics of participants in the Improving Diet, Exercise and Lifestyle for Women Study12
| Variable | APLD | APMD | HPHD |
| Body composition | |||
| BMI, kg/m2 | 31.5 ± 0.6 | 31.8 ± 0.6 | 31.4 ± 0.6 |
| Height, cm | 163 ± 1 | 164 ± 1 | 166 ± 1 |
| Age, y | 28 ± 1 | 26 ± 1 | 30 ± 1 |
| Body weight, kg | |||
| DXA | 84.0 ± 2.1 | 85.3 ± 2.1 | 87.1 ± 2.1 |
| Scale | 83.8 ± 2.1 | 85.2 ± 2.0 | 86.7 ± 2.2 |
| Fat mass, kg | 33.1 ± 1.4 | 34.8 ± 1.2 | 35.5 ± 1.3 |
| Body fat, % | 39.1 ± 0.9 | 40.6 ± 0.7 | 40.5 ± 0.6 |
| Lean mass, kg | 48.5 ± 1.1 | 48.2 ± 0.9 | 49.2 ± 0.9 |
| Waist circumference, cm | 99 ± 2.2 | 102 ± 1.80 | 102 ± 2.01 |
| Waist:hip ratio | 0.86 ± 0.01 | 0.88 ± 0.01 | 0.87 ± 0.01 |
| Trunk fat, DXA, kg | 3.3 ± 0.2 | 3.8 ± 0.2 | 4.0 ± 0.3 |
| Visceral fat,3MRI, cm3 | 655 ± 80.0 | 796 ± 77.2 | 666 ± 43.6 |
| HR in fitness test | |||
| 1st workload, HR, bpm | kp4 = 1.2; 138 ± 3 | kp = 1.2; 141 ± 3 | kp = 1.3; 139 ± 3 |
| 2nd workload, HR, bpm | kp = 1.9; 166 ± 3 | kp = 1.8; 163 ± 3 | kp = 2.1; 167 ± 3 |
| Daily dietary intakes5 | |||
| Carbohydrate | |||
| g | 234 ± 9 | 243 ± 8 | 250 ± 9 |
| % of total energy | 51 ± 1 | 53 ± 1 | 53 ± 1 |
| Protein | |||
| g | 69 ± 3 | 66 ± 3 | 69 ± 4 |
| g/kg body weight | 0.81 ± 0.05 | 0.78 ± 0.03 | 0.80 ± 0.05 |
| % of total energy | 16 ± 1 | 14 ± 1 | 15 ± 1 |
| Fat | |||
| g | 68 ± 4 | 65 ± 3 | 63 ± 4 |
| % total energy | 33 ± 1 | 31 ± 1 | 30 ± 1 |
| Energy | |||
| MJ (Mcal) | 7.66 ± 0.31 (1.83 ± 0.07) | 7.63 ± 0.22 (1.82 ± 0.05) | 7.69 ± 0.33 (1.84 ± 0.08) |
| kJ/kg body weight | 92 ± 4.1 | 90 ± 3.1 | 90 ± 4.4 |
| Calcium, mg | 555 ± 39 | 480 ± 26 | 520 ± 28 |
| Vitamin D, μg | 1.4 ± 0.2 | 1.7 ± 0.3 | 1.7 ± 0.3 |
| Blood analytes | |||
| Glucose, mmol/L | 4.9 ± 0.1 | 4.9 ± 0.1 | 4.9 ± 0.1 |
| Insulin, pmol/L | 57 ± 7 | 69 ± 8 | 51 ± 6 |
| Hemoglobin A1c, % | 5.6 ± 0.2 | 5.4 ± 0.1 | 5.4 ± 0.1 |
| Cholesterol, mmol/L | |||
| Total | 4.72 ± 0.15 | 4.48 ± 0.11 | 4.67 ± 0.15 |
| LDL | 2.75 ± 0.13 | 2.58 ± 0.09 | 2.77 ± 0.13 |
| HDL | 1.40 ± 0.05 | 1.39 ± 0.05 | 1.43 ± 0.05 |
| TG, mmol/L | 1.10 ± 0.07 | 1.11 ± 0.07 | 1.00 ± 0.07 |
Values are means ± SE, = 90 (30/group) unless otherwise noted.
APLD, adequate protein, low dairy; APMD, adequate protein, medium dairy; HPHD, high protein, high dairy; HR, heart rate.
= 39: 12 in APLD, 13 in APMD, 14 in HPHD.
kp = Kilopond; 1 kp at 50 rpm = 50 watts; 1.5 kp = 75 watts; 2 kp = 100 watts.
From baseline 7-d food records.
Body composition (DXA).
Total body mass decreased by similar amounts across all groups over time with no differences between groups (pooled mean change over time: −4.3 ± 0.7 kg; P < 0.05). The loss of body fat in all groups was significant when expressed in kilograms and percent of initial weight (P < 0.01), but the fat mass and percent fat reductions in the latter half of the study (wk 8–16) in the HPHD group were significantly greater than in the other 2 groups (Fig. 1). The loss of percent fat expressed as the change from baseline in the HPHD group was similar in the first and second halves of the study. Lean mass increased over the 16 wk in the HPHD group (0.7 ± 0.3 kg; P < 0.05), remained unchanged in the APMD group (−0.2 ± 0.2 kg), and decreased in the APLD group (−0.7 ± 0.3 kg; P < 0.05). During the latter half of the study, the accretion of lean mass in the HPHD group was greater than in the APLD (P < 0.01) and APMD groups (P < 0.05). DXA-determined trunk fat mass [regional analysis of L1–L4; a surrogate measure of VAT (40)] decreased in all groups (P < 0.05), with the HPHD group losing more trunk fat than the APLD group (P < 0.005; Fig. 2A).
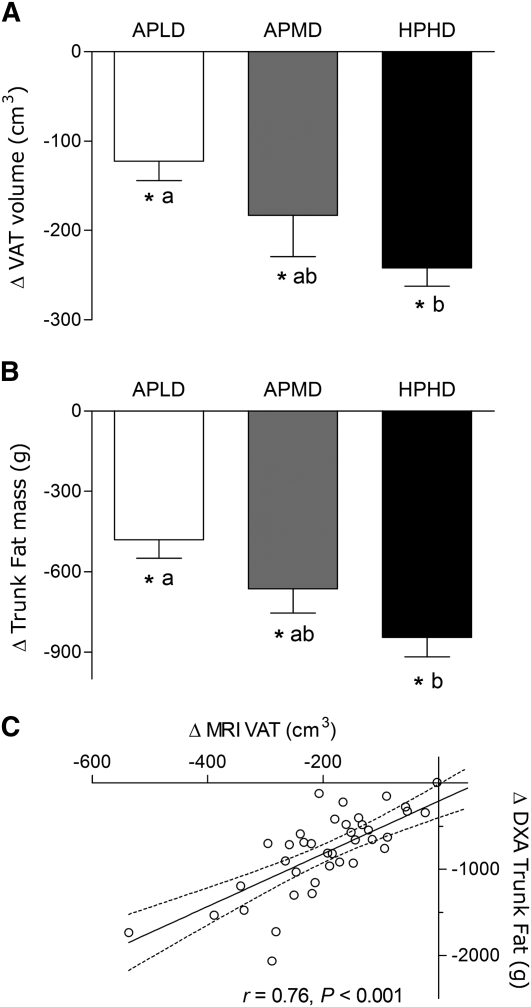
FIGURE 2.
Relative to baseline changes in trunk fat mass measured by DXA (A) and visceral fat volume measured by MRI (B) in overweight and obese premenopausal women who underwent a 16-wk intervention of diet- and exercise-induced weight loss, and the correlation between the 2 measures (C). Values are means ± SE, n = 39. Means without a common letter differ, P < 0.05. *Different from wk 0, P < 0.05. APLD, adequate protein, low dairy; APMD, adequate protein, medium dairy; HPHD, high protein, high dairy; VAT, visceral adipose tissue.
VAT volume (MRI).
All groups had a reduction in VAT volume over time (P < 0.05); however, the HPHD group lost more VAT than the APLD group (P < 0.05; Fig. 2B). The change in trunk fat mass, obtained by DXA, was correlated with the change in MRI VAT measured in the same women (Fig. 2C). This indicates that determination of changes in trunk fat mass by DXA in this population provides a reasonable proxy measure for changes in VAT.
Strength and fitness.
All groups improved their strength for all exercises over time (Table 3). The HPHD group had greater strength at wk 16 than the APLD group in the seated row and the hamstring curl (P < 0.05) and a tendency for greater strength in the chest press (P = 0.059). Cardiovascular fitness improved similarly in all groups with a mean HR reduction pre- to postintervention of ~15 bpm (P < 0.05).
TABLE 3.
Single repetition voluntary strength test results from overweight and obese premenopausal women before and after a 16-wk intervention of diet- and exercise-induced weight loss12
| APLD |
APMD |
HPHD |
||||
| Variable | wk 0 | wk 16 | wk 0 | wk 16 | wk 0 | wk 16 |
| kg | ||||||
| Seated row | 34 ± 1 | 36 ± 1a* | 35 ± 1 | 40 ± 1b* | 35 ± 1 | 41 ± 1b* |
| Chest press | 28 ± 2 | 30 ± 1* | 28 ± 2 | 33 ± 1* | 28 ± 1 | 34 ± 1* |
| Leg extension | 77 ± 3 | 89 ± 3* | 79 ± 3 | 89 ± 3* | 80 ± 3 | 95 ± 3* |
| Hamstring curl | 46 ± 2 | 52 ± 2a* | 49 ± 2 | 55 ± 2a,b* | 49 ± 2 | 58 ± 2b* |
Values are means ± SE, = 90 (30/group). Means at a time with superscripts without a common letter differ, P < 0.05. *Different from wk 0, P < 0.01.
APLD, adequate protein, low dairy; APMD, adequate protein, medium dairy; HPHD, high protein, high dairy.
Anthropometry.
BMI decreased across all groups over time with no differences between groups (pooled mean change over time: −1.8 ± 0.3 kg/m2; P < 0.05). Waist circumference also decreased in all groups throughout the study (pooled mean change over time: −4.9 ± 0.9 cm; P < 0.05). When expressed as change from baseline, that in the APLD group during the latter half of the study (−0.6 ± 0.8 cm; wk 8–16) was less than that during the first half (−4.2 ± 1.0 cm; wk 0–8; P < 0.05). On the other hand, both the APMD (−3.0 ± 0.7 and −1.8 ± 0.7 cm) and HPHD (−3.2 ± 0.8 and −1.9 ± 0.7 cm) groups showed similar significant reductions in waist circumference in the earlier and later halves of the study.
Blood results.
IL-6 decreased over time in the APMD group (−0.39 ± 0.27 ng/L; P < 0.05) and tended to decline in the HPHD group (−0.37 ± 0.12 ng/L; P = 0.071). There was no change in the APLD group (−0.10 ± 0.14 ng/L). CRP decreased in all groups over time with no differences between groups (pooled mean change over time: −2.32 ± 0.66 mg/L; P < 0.05). Baseline levels in all groups for glucose, insulin, cholesterol (total, LDL, and HDL), and TG were within normal ranges (41, 42) and did not significantly differ from one another (Table 2). During the study, total cholesterol, LDL cholesterol, and TG decreased in the HPHD and APLD groups (P < 0.05). Insulin levels decreased in the APMD group (P < 0.05). Plasma calcium remained unchanged during the study in all groups (Supplemental Table 1).
Diet.
Baseline diets were analyzed from the prestudy 7-d food records. Calcium intake at the start of the study was approximately one-half of the recommended intake (43) (Table 2) and did not differ between groups. At study entrance, participants were consuming close to the RDA for protein (44) (Table 2), which corresponded to a mean intake of 15% of daily energy from protein across all groups. Carbohydrate consumption in all groups was ~52% and fat was ~31% (Table 2).
Calcium intake at wk 16 (which reflected dietary intakes throughout the study; Table 4) correlated with changes in percent body fat (r = 0.21; P < 0.05), trunk fat mass (r = 0.30; P < 0.01), and VAT volume (r = 0.40; P < 0.01). Protein consumption at wk 16 (expressed as a percentage of daily energy intake; Table 4) was also correlated with changes in body fat mass (r = 0.22; P < 0.05), percent body fat (r = 0.25; P < 0.05), trunk fat mass (r = 0.33; P < 0.005), and VAT volume (r = 0.32; P < 0.05).
TABLE 4.
Dietary intakes from overweight and obese premenopausal women consuming hypoenergetic diets during a 16-wk intervention of diet- and exercise-induced weight loss12
| Dietary variable | APLD | APMD | HPHD |
| Energy intake,3kcal/d | 1320 ± 40a | 1430 ± 42a,b | 1500 ± 36b |
| Energy restriction achieved,3kcal /d | −498 ± 41 | −477 ± 42 | −435 ± 37 |
| Protein | |||
| %/d | 16 ± 1a | 18 ± 1b | 28 ± 1c |
| g/d | 55 ± 1a | 66 ± 2b | 108 ± 3c |
| g/(kg⋅d) | 0.72 ± 0.02a | 0.84 ± 0.02b | 1.33 ± 0.04c |
| Fat, %/d | 28 ± 1a | 24 ± 1b | 31 ± 1c |
| Carbohydrate, %/d | 56 ± 1a | 58 ± 1a | 41 ± 1b |
| Dietary fiber, g/d | 21 ± 1 | 18 ± 1 | 16 ± 1 |
| Calcium, mg/d | 299 ± 22a | 1200 ± 19b | 1840 ± 13c |
| Vitamin D, μg/d | 0.7 ± 0.1a | 9.8 ± 0.3b | 13.2 ± 0.2c |
| Vitamin C, mg/d | 119 ± 19a | 79 ± 6a,b | 76 ± 6b |
| Vitamin A, mg of RAE/d | 1.71 ± 0.22a | 1.85 ± 0.16a | 2.55 ± 0.18b |
| Iron, mg/d | 10 ± 0.6a | 15 ± 0.5b | 11 ± 0.6a |
Values are means ± SE, = 90 (30/group). Data were taken from post study 7-d food records. Means in a row with superscripts without a common letter differ, P < 0.05.
APLD, adequate protein, low dairy; APMD, adequate protein, medium dairy; HPHD, high protein, high dairy; RAE, retinol activity equivalent.
1 kcal = 4.18 kJ.
Discussion
In overweight and obese premenopausal women, significantly greater fat loss accompanied by a gain in lean (muscle) mass was achieved during a 16-wk hypoenergetic period, mediated by diet (−500 kcal/d) and exercise (−250 kcal/d), that provided higher dietary protein (30% of total energy intake) within the AMDR (44), with one-half of the total dietary protein intake from dairy foods. Although all treatment groups lost a significant amount of trunk fat, higher dairy food and dietary protein consumption were associated with significantly greater reductions in trunk fat mass and VAT volume than those consuming no dairy foods and lower protein despite undergoing the identical exercise regimen. Reductions in trunk fat mass were reflected in reduced waist circumferences, which were also greater in the APMD and HPHD groups compared to the APLD group. The observed greater fat loss and the increase in lean mass in the HPHD group, in the face of similar weight loss across all groups, highlights the beneficial body composition changes in the HPHD diet intervention in support of our original hypothesis. Because all groups performed resistance exercise (2 d of 7 d/wk), which is a potent stimulus for skeletal muscle anabolism (32, 45, 46), our results likely represent a loss of skeletal muscle (lean) tissue that was minimized in the APLD and APMD groups (3, 32) but that translated into a gain, with a measurably greater strength gain, in the HPHD group.
Favorable associations between dairy food consumption and adiposity, weight loss, body compositional change, prevention of weight gain, and a decreased incidence of insulin resistance and other metabolic syndrome risk factors have been reported in observational studies and population surveys (47–49). Data from a recent meta-analysis also emphasizes the benefit of calcium on body weight in premenopausal women (25), and clinical trials highlight a possible threshold effect for calcium intake such that those with habitual calcium intake levels > ~600–700 mg/d do not seem to derive the same body composition effect from calcium consumption as those with lower baseline intakes (50, 51). Thus, as an entrance criterion, using a FFQ and baseline food records, we measured and verified that our participants were consuming <555 mg/d calcium at study entry. As such, our data can still be generalized to North American women, of whom a large percentage, according to the NHANES (52, 53) and the Canadian Community Health Survey (54), do not consume adequate calcium and are overweight or obese (55).
The decision to use dairy foods in this trial was premised on evidence demonstrating that consumption of calcium from dairy foods as opposed to elemental calcium, with energy restriction, has a greater effect on weight loss and body composition in humans (56, 57). The greater fat loss observed with dairy foods compared to calcium supplementation may relate to the fact that dairy foods contain other bioactive components acting independently or synergistically with calcium and vitamin D to enhance fat loss. In premenopausal obese women consuming low-fat milk (providing 1200 mg/d calcium) on an 8-wk energy-restricted diet, greater reductions were observed in weight, BMI, and waist:hip ratio than in groups who consumed calcium carbonate or a soy beverage with equal amounts of calcium (56). The authors concluded that the additional effect observed with milk consumption likely related to other bioactive components such as the protein fraction rich in branched-chain amino acids (leucine, isoleucine, and valine) (58). Other effects of calcium and dairy foods that could potentially be contributing to a greater fat loss include an increased fecal fat excretion (59), a decrease in fat absorption (60), increase in fat oxidation (61), and an increased thermic effect of food (62).
In vitro mechanisms have been shown to exist in adipocytes that support an antiadipogenic and prolipolytic effect of dairy components, including leucine and calcium (26, 63, 64). Similar effects have been demonstrated in rodents (65, 66). These mechanisms may help to explain the greater total and visceral fat mass loss experienced by those women consuming the most dairy foods and dairy-derived calcium (the HPHD group). Interestingly, it may not require an energy deficit to see such effects. We have reported similar effects under anabolic conditions (i.e. energy surplus with intense resistance training) in young women consuming an additional 1 L/d of low-fat milk (31). Our trial design featured changes in consumption of whole foods with varying contents of macronutrients to affect fat loss as opposed to isolated individual nutrients that comprise dairy; thus, the significance of calcium per se as a causative factor in fat loss is hard to determine. However, we also observed that dietary calcium intakes during the study were significantly correlated with changes in percent body fat and visceral fat assessed by both DXA and MRI, which is similar to the relationships between calcium intake and fat loss during energy restriction reported by others (26, 63, 64). Reductions in inflammatory biomarkers (CRP and IL-6), which also may relate to visceral fat loss, were seen in all groups, with the HPHD and APMD groups having greater reductions in IL-6 than the APLD group.
The consumption of higher protein (lower carbohydrate) compared to conventional protein (i.e. 15% of energy) with energy restriction has yielded mixed results with respect to weight loss and several studies have shown that the macronutrient composition of a weight loss diet does not seem to matter as much as compliance to the regimen itself (11–13). However, when assessing the effect of weight loss diets with different macronutrient ratios on hypoenergetic-induced changes in body composition, higher protein diets appear to be advantageous. In a meta-analysis (14), energy restriction (without exercise) with higher protein intakes [125% of the protein RDA (44)] were associated with a greater lean mass retention than studies where protein consumption was at the RDA (44). In our study, the HPHD group consumed ~1.3 g/(kg⋅d) protein for 16 wk (Table 4), which could have contributed to the lean mass gain in this group. In addition, the protein and carbohydrate intakes of the HPHD group (~30 and 40% daily energy intake, respectively) were not as excessive as those employed in more extreme protocols (11, 12) and they were close to intakes recommended in the AMDR (44). Of note (and not by design), during the study the APLD group was consuming protein at a level slightly below the RDA (44) (Table 4) and an additional 0.08 g/(kg⋅d) corresponding to ~8 g/d of protein would have rectified this. However, it has been suggested that even protein intakes around the RDA may be insufficient during weight loss to spare muscle mass (16). Nevertheless, protein intake in the APLD group did correspond to 16% of their daily energy intake, which is within the AMDR (10–35%) (44) and at the appropriate a priori level per the study protocol. Other studies have assessed the combined effect of high protein and physical activity during a period of hypoenergetic weight loss (17, 19) and reported that exercise does not actually affect total weight loss; however, exercise increases fat mass loss and also promotes lean mass retention greater than that seen with the intake of higher protein alone (17), which parallels our observations (17, 19).
With 16 wk of training composed of both aerobic and resistance exercise, strength and fitness increased substantially in all groups. HR reductions at the same absolute workload averaged 15 bpm, which is indicative of a clinically meaningful increase in aerobic fitness. Improvements in cardiovascular fitness show a strong negative association with all-cause mortality in women (67); therefore, the importance of the increase in fitness shown here should not be overlooked. Insofar as muscular strength is concerned, although gains were evident in all groups for all exercises performed, the HPHD group had greater increases in strength in various exercises (seated row, hamstring curl) compared to the APLD group (Table 3). It is entirely possible that these strength gains relate to the body composition changes in that the group that lost muscle mass (the APLD group, albeit minimal) had the most modest improvements in strength, whereas those that lost no muscle (the APMD group) or even gained muscle (the HPHD group) showed greater improvements. The improvement or even preservation of muscle function with weight loss could have important implications in populations such as the elderly, in whom muscle mass preservation is critical. Similar results for strength gains were demonstrated in a previous study performed in our laboratory in women consuming milk compared to an isoenergetic carbohydrate-containing drink post resistance exercise (31).
In summary, higher intakes of dietary protein and dairy foods, during dietary energy restriction combined with an exercise intervention, improved the composition of weight lost compared to those who consumed diets lower in protein and lower or devoid of dairy foods. We observed what we view as a highly beneficial profile of weight loss in the HPHD group: greater total fat and visceral fat losses, greater lean mass gains, and increases in strength despite identical body weight loss. Correlations between self-reported dietary calcium and protein intakes with total fat, and DXA- and MRI-derived trunk/visceral fat losses indicate potential mechanisms of action for these nutrients in fat loss. Our data suggest that higher protein intakes with an emphasis on increased intakes from dairy foods, modest energy restriction, and combined aerobic and resistance training result in favorable body composition, strength, and fitness changes that are not captured by simple measures of body weight or BMI. These data provide evidence for the promotion of low-fat dairy food consumption not only to ensure adequate calcium intakes in populations previously deficient (52–54), but to promote the provision of high-quality protein that can aid in fat loss and lean mass retention during energy restriction.
Supplementary Material
Acknowledgments
We thank our laboratory manager T. Prior and the research assistants, dietitians/nutritionists and thesis students who helped run the study: P. Kocsis, J. Wood, L. Wright, G. Zubic, S. French, K. Theeuwen, J. Clark, A. Paashuis, R. Robinson, K. Booker, J. Jackson, H. Zoschke, L. Bellamy, S. Losier, M. Labouesse, K. Howarth, D. Gallo, H. Robertshaw, and M. Marcinow. A.R.J., S.A.A., M.A.T., and S.M.P. designed the research project; A.R.J. and S.M.P. conducted the research; A.R.J. and S.M.P. conducted the statistical analysis; and A.R.J., S.A.A., M.A.T., and S.M.P. helped write the final manuscript. All authors read and approved the final manuscript.
Footnotes
Supported by the Dairy Farmers of Canada, The Dairy Research Institute, and the Canadian Institutes of Health Research.
This trial was registered at clinicaltrials.gov as NCT00710398.
Supplemental Figure 1 and Supplemental Table 1 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at jn.nutrition.org.
Abbreviations used: AMDR, acceptable macronutrient distribution range; APLD, adequate protein, low dairy; APMD, adequate protein, medium dairy; CRP, C-reactive protein; HPHD, high protein, high dairy; HR, heart rate; I.D.E.A.L., Improving Diet, Exercise, and Lifestyle for Women Study; VAT, visceral adipose tissue.
Literature Cited
- 1.Oreopoulos A, Padwal R, McAlister FA, Ezekowitz J, Sharma AM, Kalantar-Zadeh K, Fonarow GC, Norris CM. Association between obesity and health-related quality of life in patients with coronary artery disease. Int J Obes (Lond). 2010;34:1434–41 [DOI] [PubMed] [Google Scholar]
- 2.Sharma AM, Padwal R. Obesity is a sign over-eating is a symptom: an aetiological framework for the assessment and management of obesity. Obes Rev. 2010;11:362–70 [DOI] [PubMed] [Google Scholar]
- 3.Campbell WW, Haub MD, Wolfe RR, Ferrando AA, Sullivan DH, Apolzan JW, Iglay HB. Resistance training preserves fat-free mass without impacting changes in protein metabolism after weight loss in older women. Obesity (Silver Spring). 2009;17:1332–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunter GR, Byrne NM, Sirikul B, Fernandez JR, Zuckerman PA, Darnell BE, Gower BA. Resistance training conserves fat-free mass and resting energy expenditure following weight loss. Obesity (Silver Spring). 2008;16:1045–51 [DOI] [PubMed] [Google Scholar]
- 5.Weinheimer EM, Sands LP, Campbell WW. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: implications for sarcopenic obesity. Nutr Rev. 2010;68:375–88 [DOI] [PubMed] [Google Scholar]
- 6.Hunter GR, Brock DW, Byrne NM, Chandler-Laney PC, Del Corral P, Gower BA. Exercise training prevents regain of visceral fat for 1 year following weight loss. Obesity (Silver Spring). 2010;18:690–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strychar I, Lavoie ME, Messier L, Karelis AD, Doucet E, Prud'homme D, Fontaine J, Rabasa-Lhoret R. Anthropometric, metabolic, psychosocial, and dietary characteristics of overweight/obese postmenopausal women with a history of weight cycling: a MONET (Montreal Ottawa New Emerging Team) study. J Am Diet Assoc. 2009;109:718–24 [DOI] [PubMed] [Google Scholar]
- 8.Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23:201–29 [DOI] [PubMed] [Google Scholar]
- 10.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475–82 [DOI] [PubMed] [Google Scholar]
- 11.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA. 2005;293:43–53 [DOI] [PubMed] [Google Scholar]
- 12.Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, Kraemer HC, King AC. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA. 2007;297:969–77 [DOI] [PubMed] [Google Scholar]
- 13.Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo N, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krieger JW, Sitren HS, Daniels MJ, Langkamp-Henken B. Effects of variation in protein and carbohydrate intake on body mass and composition during energy restriction: a meta-regression 1. Am J Clin Nutr. 2006;83:260–74 [DOI] [PubMed] [Google Scholar]
- 15.Abete I, Astrup A, Martinez JA, Thorsdottir I, Zulet MA. Obesity and the metabolic syndrome: role of different dietary macronutrient distribution patterns and specific nutritional components on weight loss and maintenance. Nutr Rev. 2010;68:214–31 [DOI] [PubMed] [Google Scholar]
- 16.Layman DK. Protein quantity and quality at levels above the RDA improves adult weight loss. J Am Coll Nutr. 2004;23:S631–6 [DOI] [PubMed] [Google Scholar]
- 17.Layman DK, Evans E, Baum JI, Seyler J, Erickson DJ, Boileau RA. Dietary protein and exercise have additive effects on body composition during weight loss in adult women. J Nutr. 2005;135:1903–10 [DOI] [PubMed] [Google Scholar]
- 18.Mettler S, Mitchell N, Tipton KD. Increased protein intake reduces lean body mass loss during weight loss in athletes. Med Sci Sports Exerc. 2010;42:326–37 [DOI] [PubMed] [Google Scholar]
- 19.Meckling KA, Sherfey R. A randomized trial of a hypocaloric high-protein diet, with and without exercise, on weight loss, fitness, and markers of the metabolic syndrome in overweight and obese women. Appl Physiol Nutr Metab. 2007;32:743–52 [DOI] [PubMed] [Google Scholar]
- 20.Larsen TM, Dalskov SM, van Baak M, Jebb SA, Papadaki A, Pfeiffer AF, Martinez JA, Handjieva-Darlenska T, Kunesova M, Pihlsgard M, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363:2102–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Major GC, Chaput JP, Ledoux M, St-Pierre S, Anderson GH, Zemel MB, Tremblay A. Recent developments in calcium-related obesity research. Obes Rev. 2008;9:428–45 [DOI] [PubMed] [Google Scholar]
- 22.Zemel MB, Miller SL. Dietary calcium and dairy modulation of adiposity and obesity risk. Nutr Rev. 2004;62:125–31 [DOI] [PubMed] [Google Scholar]
- 23.Zemel MB, Richards J, Mathis S, Milstead A, Gebhardt L, Silva E. Dairy augmentation of total and central fat loss in obese subjects. Int J Obes (Lond). 2005;29:391–7 [DOI] [PubMed] [Google Scholar]
- 24.Shahar DR, Schwarzfuchs D, Fraser D, Vardi H, Thiery J, Fiedler GM, Bluher M, Stumvoll M, Stampfer MJ, Shai I. Dairy calcium intake, serum vitamin D, and successful weight loss. Am J Clin Nutr. 2010;92:1017–22 [DOI] [PubMed] [Google Scholar]
- 25.Onakpoya IJ, Perry R, Zhang J, Ernst E. Efficacy of calcium supplementation for management of overweight and obesity: systematic review of randomized clinical trials. Nutr Rev. 2011;69:335–43 [DOI] [PubMed] [Google Scholar]
- 26.Parikh SJ, Yanovski JA. Calcium intake and adiposity. Am J Clin Nutr. 2003;77:281–7 [DOI] [PubMed] [Google Scholar]
- 27.Zemel MB. The role of dairy foods in weight management. J Am Coll Nutr. 2005;24:S537–46 [DOI] [PubMed] [Google Scholar]
- 28.Zemel MB. Mechanisms of dairy modulation of adiposity. J Nutr. 2003;133:S252–6 [DOI] [PubMed] [Google Scholar]
- 29.Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr. 2007;85:1031–40 [DOI] [PubMed] [Google Scholar]
- 30.Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, Phillips SM. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr. 2007;86:373–81 [DOI] [PubMed] [Google Scholar]
- 31.Josse AR, Tang JE, Tarnopolsky MA, Phillips SM. Body composition and strength changes in women with milk and resistance exercise. Med Sci Sports Exerc. 2010;42:1122–30 [DOI] [PubMed] [Google Scholar]
- 32.Garthe I, Raastad T, Refsnes PE, Koivisto A, Sundgot-Borgen J. Effect of two different weight-loss rates on body composition and strength and power-related performance in elite athletes. Int J Sport Nutr Exerc Metab. 2011;21:97–104 [DOI] [PubMed] [Google Scholar]
- 33.Government of Canada Tri-council policy statement: ethical conduct for research involving humans [cited January, 15, 2011]. Available from: http://www.pre.ethics.gc.ca/eng/index/
- 34.Health Canada Eating Well with Canada's Food Guide [cited June 1, 2011]. Available from: http://wwwhc-scgcca/fn-an/food-guide-aliment/index-engphp
- 35.USDA Food Guide Pyramid [cited June 1, 2011]. Available from: http://wwwlifecliniccom/focus/nutrition/food-pyramidasp#serving
- 36.Frankenfield D, Roth-Yousey L, Compher C. Comparison of predictive equations for resting metabolic rate in healthy nonobese and obese adults: a systematic review. J Am Diet Assoc. 2005;105:775–89 [DOI] [PubMed] [Google Scholar]
- 37.Bazzano LA, He J, Ogden LG, Loria CM, Vupputuri S, Myers L, Whelton PK. Agreement on nutrient intake between the databases of the First National Health and Nutrition Examination Survey and the ESHA Food Processor. Am J Epidemiol. 2002;156:78–85 [DOI] [PubMed] [Google Scholar]
- 38.Astrand PO, Ryhming I. A nomogram for calculation of aerobic capacity (physical fitness) from pulse rate during sub-maximal work. J Appl Physiol. 1954;7:218–21 [DOI] [PubMed] [Google Scholar]
- 39.Elobeid MA, Padilla MA, McVie T, Thomas O, Brock DW, Musser B, Lu K, Coffey CS, Desmond RA, St-Onge MP, et al. Missing data in randomized clinical trials for weight loss: scope of the problem, state of the field, and performance of statistical methods. PLoS ONE. 2009;4:e6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glickman SG, Marn CS, Supiano MA, Dengel DR. Validity and reliability of dual-energy X-ray absorptiometry for the assessment of abdominal adiposity. J Appl Physiol. 2004;97:509–14 [DOI] [PubMed] [Google Scholar]
- 41.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421 [PubMed] [Google Scholar]
- 42.American Diabetes Association Executive summary: standards of medical care in diabetes–2011. Diabetes Care. 2011;34 Suppl 1:S4–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dietary Reference Intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: Institute of Medicine. National Academies Press; 1997 [PubMed] [Google Scholar]
- 44.Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: Institute of Medicine. National Academies Press; 2005 [DOI] [PubMed] [Google Scholar]
- 45.Burd NA, Tang JE, Moore DR, Phillips SM. Exercise training and protein metabolism: influences of contraction, protein intake, and sex-based differences. J Appl Physiol. 2009;106:1692–701 [DOI] [PubMed] [Google Scholar]
- 46.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol. 1997;273:E99–107 [DOI] [PubMed] [Google Scholar]
- 47.Caan B, Neuhouser M, Aragaki A, Lewis CB, Jackson R, LeBoff MS, Margolis KL, Powell L, Uwaifo G, Whitlock E, et al. Calcium plus vitamin D supplementation and the risk of postmenopausal weight gain. Arch Intern Med. 2007;167:893–902 [DOI] [PubMed] [Google Scholar]
- 48.Liu S, Song Y, Ford ES, Manson JE, Buring JE, Ridker PM. Dietary calcium, vitamin D, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care. 2005;28:2926–32 [DOI] [PubMed] [Google Scholar]
- 49.McCarron DA, Morris CD, Henry HJ, Stanton JL. Blood pressure and nutrient intake in the United States. Science. 1984;224:1392–8 [DOI] [PubMed] [Google Scholar]
- 50.Wagner G, Kindrick S, Hertzler S, DiSilvestro RA. Effects of various forms of calcium on body weight and bone turnover markers in women participating in a weight loss program. J Am Coll Nutr. 2007;26:456–61 [DOI] [PubMed] [Google Scholar]
- 51.Major GC, Alarie FP, Dore J, Tremblay A. Calcium plus vitamin D supplementation and fat mass loss in female very low-calcium consumers: potential link with a calcium-specific appetite control. Br J Nutr. 2009;101:659–63 [DOI] [PubMed] [Google Scholar]
- 52.Ervin RB, Wang CY, Wright JD, Kennedy-Stephenson J. Dietary intake of selected minerals for the United States population: 1999–2000. NHANES Database. Advance data from vital and health statistics; no 341. Hyattsville (MD): National Center for Health Statistics; 2004 [Google Scholar]
- 53.Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Annu Rev Nutr. 2004;24:401–31 [DOI] [PubMed] [Google Scholar]
- 54.Nutrient intakes from food. Canadian Community Health Survey, Cycle 2.2 (2004, published 2007). Health Canada [cited: January 15, 2011]. Available from: http://www.hc-sc.gc.ca/fn-an/surveill/nutrition/commun/index-eng.php
- 55.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41 [DOI] [PubMed] [Google Scholar]
- 56.Faghih S, Abadi AR, Hedayati M, Kimiagar SM. Comparison of the effects of cows’ milk, fortified soy milk, and calcium supplement on weight and fat loss in premenopausal overweight and obese women. Nutr Metab Cardiovasc Dis. 2010;21:499–503 [DOI] [PubMed] [Google Scholar]
- 57.Zemel MB, Thompson W, Milstead A, Morris K, Campbell P. Calcium and dairy acceleration of weight and fat loss during energy restriction in obese adults. Obes Res. 2004;12:582–90 [DOI] [PubMed] [Google Scholar]
- 58.Layman DK, Walker DA. Potential importance of leucine in treatment of obesity and the metabolic syndrome. J Nutr. 2006;136:S319–23 [DOI] [PubMed] [Google Scholar]
- 59.Jacobsen R, Lorenzen JK, Toubro S, Krog-Mikkelsen I, Astrup A. Effect of short-term high dietary calcium intake on 24-h energy expenditure, fat oxidation, and fecal fat excretion. Int J Obes (Lond). 2005;29:292–301 [DOI] [PubMed] [Google Scholar]
- 60.Lorenzen JK, Nielsen S, Holst JJ, Tetens I, Rehfeld JF, Astrup A. Effect of dairy calcium or supplementary calcium intake on postprandial fat metabolism, appetite, and subsequent energy intake. Am J Clin Nutr. 2007;85:678–87 [DOI] [PubMed] [Google Scholar]
- 61.Melanson EL, Donahoo WT, Dong F, Ida T, Zemel MB. Effect of low- and high-calcium dairy-based diets on macronutrient oxidation in humans. Obes Res. 2005;13:2102–12 [DOI] [PubMed] [Google Scholar]
- 62.Shi H, Dirienzo D, Zemel MB. Effects of dietary calcium on adipocyte lipid metabolism and body weight regulation in energy-restricted aP2-agouti transgenic mice. FASEB J. 2001;15:291–3 [DOI] [PubMed] [Google Scholar]
- 63.Sun X, Zemel MB. Leucine and calcium regulate fat metabolism and energy partitioning in murine adipocytes and muscle cells. Lipids. 2007;42:297–305 [DOI] [PubMed] [Google Scholar]
- 64.Zemel MB, Shi H, Greer B, Dirienzo D, Zemel PC. Regulation of adiposity by dietary calcium. FASEB J. 2000;14:1132–8 [PubMed] [Google Scholar]
- 65.Sun X, Zemel MB. Calcium and dairy products inhibit weight and fat regain during ad libitum consumption following energy restriction in Ap2-agouti transgenic mice. J Nutr. 2004;134:3054–60 [DOI] [PubMed] [Google Scholar]
- 66.Marsset-Baglieri A, Fromentin G, Tome D, Bensaid A, Makkarios L, Even PC. Increasing the protein content in a carbohydrate-free diet enhances fat loss during 35% but not 75% energy restriction in rats. J Nutr. 2004;134:2646–52 [DOI] [PubMed] [Google Scholar]
- 67.Farrell SW, Fitzgerald SJ, McAuley PA, Barlow CE. Cardiorespiratory fitness, adiposity, and all-cause mortality in women. Med Sci Sports Exerc. 2010;42:2006–12 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.