Abstract
Reactive oxygen species, such as superoxide and hydrogen peroxide, are generated in all cells by mitochondrial and enzymatic sources. Left unchecked, these reactive species can cause oxidative damage to DNA, proteins, and membrane lipids. Glutathione peroxidase-1 (GPx-1) is an intracellular antioxidant enzyme that enzymatically reduces hydrogen peroxide to water to limit its harmful effects. Certain reactive oxygen species, such as hydrogen peroxide, are also essential for growth factor-mediated signal transduction, mitochondrial function, and maintenance of normal thiol redox-balance. Thus, by limiting hydrogen peroxide accumulation, GPx-1 also modulates these processes. This review explores the molecular mechanisms involved in regulating the expression and function of GPx-1, with an emphasis on the role of GPx-1 in modulating cellular oxidant stress and redox-mediated responses. As a selenocysteine-containing enzyme, GPx-1 expression is subject to unique forms of regulation involving the trace mineral selenium and selenocysteine incorporation during translation. In addition, GPx-1 has been implicated in the development and prevention of many common and complex diseases, including cancer and cardiovascular disease. This review discusses the role of GPx-1 in these diseases and speculates on potential future therapies to harness the beneficial effects of this ubiquitous antioxidant enzyme. Antioxid. Redox Signal. 15, 1957–1997.
-
III. Regulation of GPx‐1 Expression and Activity
VII. GPx‐1 and Future Directions for Therapeutic Applications
I. Introduction
Reactive oxygen species (ROS) are generated by all cells during normal oxidative respiration and, if left unchecked by antioxidant systems, can cause oxidative damage to DNA, proteins, and membrane lipids. Intracellularly, ROS are principally generated by mitochondrial respiration and redox enzymes, such as uncoupled nitric oxide synthase, cytochrome P-450 isoforms, and NADPH oxidase subtypes (NOXs), in the form of superoxide (Fig. 1) (222, 338). This short-lived ROS can combine with nitric oxide (NO · ) to form the highly reactive peroxynitrite (a reactive nitrogen species [RNS]) or can spontaneously or enzymatically be dismutated to form hydrogen peroxide and molecular oxygen (59, 232). Hydrogen peroxide can also be generated by the 2-electron reduction of oxygen by various oxidoreductases, including xanthine oxidase, which, recent findings suggest, predominately produces hydrogen peroxide (33, 195). Recent studies also suggest that NOX4 may preferentially produce hydrogen peroxide rather than superoxide anion, which is the major ROS produced by other NOX isoforms (97). Hydrogen peroxide has a longer half-life than superoxide, and unlike superoxide, hydrogen peroxide can transfer across lipid membranes by either diffusion or transport through channels, such as aquaporins (38).
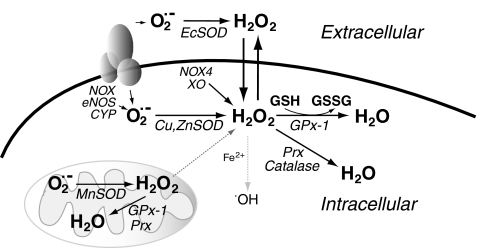
FIG. 1.
Modulation of cellular reactive oxygen species (ROS). Superoxide originates from normal mitochondrial respiration or from enzymatic sources, such as NADPH oxidases (NOX), uncoupled endothelial nitric oxide synthase (eNOS), or p-450 (CYP) isoforms. Superoxide is dismutated spontaneously or enzymatically to hydrogen peroxide. Extracellular superoxide dismutase (ECSOD), intracellular copper, zinc SOD (Cu, ZnSOD), or mitochondrially localized manganese SOD (MnSOD) are enzymatic sources of this conversion. Hydrogen peroxide can also be produced directly by xanthine oxidase and NADPH oxidase subtype 4. Under certain oxidative stress conditions such as ischemia-reperfusion, hydrogen peroxide can react with free iron to promote the formation of hydroxyl radical. Under normal cellular conditions the amount of free iron in the cell is low limiting the flux through this pathway (illustrated by light gray). Hydrogen peroxide is subsequently enzymatically reduced by glutathione peroxidases (GPxs), including GPx-1, as well as catalase and peroxiredoxins (Prxs). Catalase is primarily in the peroxisomes, whereas various Prxs localize to the mitochondria (e.g., Prx 3,5) or cytosol (such as Prx 1,2). Most Prxs utilize thioredoxin as a source of reducing equivalents, although Prx 6 appears to function as a reduced glutathione (GSH)-dependent peroxidase. Importantly, GPx-1 can be found in the cytosol, in mitochondria, and also in peroxisomes. GPx-1 utilizes GSH as a cofactor to reduce hydrogen peroxide, resulting in the formation of oxidized glutathione (GSSG). For simplicity, peroxisomes, thioredoxin, and mitochondrial GSH, are not represented in this figure.
Regulation and removal of hydrogen peroxide prevents the formation of the highly reactive and damaging hydroxyl radical, which can be formed by reaction of hydrogen peroxide with Fe2+ (Fenton reaction). Intracellularly, the Fenton reaction is limited, in part, by the lack of free transition metals in cells, but may play a role in oxidative damage after ischemia-reperfusion or under other oxidative stress conditions that involve accumulation of high levels of intracellular hydrogen peroxide and liberation of Fe2+ from intracellular storage sites (115, 288). Excess hydrogen peroxide can also lead to oxidation of susceptible cellular protein thiols to sulfenic (SOH) or sulfinic (SO2H) acid and irreversible oxidation to sulfonic (SO3H) acid (284). Low levels of hydrogen peroxide, however, maintain essential modifications of protein thiols including the formation of intra- and intermolecular disulfides (including mixed disulfides with low molecular weight thiols like reduced glutathione [GSH]) (133, 284, 390). Also, at low levels, hydrogen peroxide plays a role as a second messenger in signal transduction by modulating the oxidation state of redox-sensitive cysteines (Cys) to promote kinase function (133, 284, 390). Extracellular superoxide dismutase (SOD), cytosolic copper, zinc SOD, and mitochondrially located manganese SOD (MnSOD) play a major role in the formation of hydrogen peroxide, whereas glutathione peroxidases (GPxs), catalase, and peroxiredoxins all play a role in the enzymatic catabolism of this ROS. Catalase is principally limited to the peroxisomes, and peroxiredoxins are a family of enzymes with different subcellular distributions. GPxs are a family of enzymes homologous to the selenocysteine (Sec)-containing mammalian GPx-1 that uses GSH as an obligate cosubstrate in the reduction of hydrogen peroxide to water. Not all GPxs (defined by homology), however, use GSH, nor do they all contain Sec at the active site; rather, some of these enzymes are functionally identified as thioredoxin-dependent peroxidases containing a redox-active Cys in place of the Sec.
GPx-1 is one of the most abundant members of the GPx family of enzymes that include an epithelial-specific enzyme that is highly expressed in intestine (GPx-2); a secreted subtype (GPx-3); and GPx-4, which is widely expressed and differs in its substrate specificity compared to the other family members. Accordingly, GPx-1 is a crucial antioxidant enzyme involved in preventing the harmful accumulation of intracellular hydrogen peroxide. It is present in all cells; found in cytosolic, mitochondrial, and, in some cells, in peroxisomal compartments (113, 129, 225, 326, 331, 370); and has been found to be more effective than catalase at removing intracellular peroxides under many physiological conditions (11, 74). The relative effectiveness of peroxiredoxins versus GPxs in modulating intracellular hydrogen peroxide levels has been debated: peroxiredoxins are abundantly expressed and different peroxiredoxin isoforms are also found in the cytosol and mitochondria; however, some forms of peroxiredoxins are susceptible to oxidative inactivation at relatively low micromolar levels of hydrogen peroxide (133). GPx-1 can also reduce lipid hydroperoxides and other soluble hydroperoxides after their release from membrane lipids (248, 257), and may also reduce phospholipid-monoacylglycerol hydroperoxides, such as 1-linoleoyl lysophosphatidylcholine hydroperoxide (247), but not tri- or diacylglycerol hydroperoxides (247). These other membrane-associated phospholipids are, instead, reduced by GPx-4, which has a preferential association with membranes and appears to have a minimal effect on intracellular hydrogen peroxide tone (320, 359), although from an enzymological point of view, GPx-4 is no less efficient than GPx-1 in reducing hydrogen peroxide or fatty acid hydroperoxides. Recent findings suggest that peroxiredoxin 6 may also reduce phospholipid targets in cells (123). In addition, GPx-1 may also act as a peroxynitrite reductase (327), thereby, theoretically, modulating peroxynitrite-induced signaling pathways in vivo (325). To date, however, there has been no compelling evidence to indicate that GPx-1 modulates in vivo peroxynitrite flux; rather, there are studies to suggest that lack of GPx-1 enhances survival to peroxynitrite (136) by mechanisms that are not well understood.
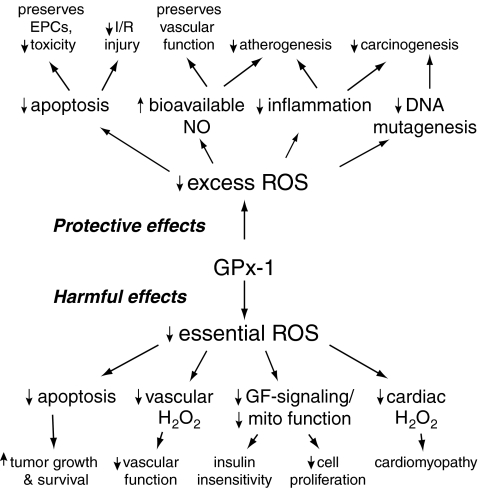
Disturbances of normal intracellular (and extracellular) redox balance contribute to susceptibility and/or pathology in many common and complex human diseases. Thus, the antioxidant GPx-1 has been studied for its effect in modulating processes in which oxidants play an essential role, including normal cellular growth and proliferative responses; adaptive pathological responses, such as apoptosis or inflammation; and disease/tissue injury processes, such as those involved in atherogenesis, drug toxicity, and ischemia-reperfusion injury. In addition, studies in human subjects implicate GPx-1 in some cancers and cardiovascular diseases. This review will summarize the current knowledge of the molecular determinants influencing the expression and function of GPx-1, with an emphasis on the role of GPx-1 in modulating cellular oxidant stress and redox-mediated signaling responses. Importantly, by regulating cellular hydroperoxides (and RNS), GPx-1 may protect against oxidative stress, but, in excess, GPx-1 may also have deleterious effects due to a lack of essential cellular oxidants (154, 251) that result in a reductive stress characterized by a lack of oxidants and/or excess reducing equivalents (297) (Fig. 2). Although reductive stress may appear to be a new concept, it has been known for some time that lack of cellular oxidants can diminish cell growth responses. Newer evidence points to additional cellular and physiological effects caused by lack of cellular oxidants and accumulation of excess reducing equivalents, including changes in protein disulfide bond formation, diminished mitochondrial function, and decreased cellular metabolism. Although, to date, a complete understanding of physiological conditions that may create reductive stress have not been elucidated, conditions, such as hypoxia, hyperglycemia, and other stresses that inhibit mitochondrial function, are known to cause excess accumulation of cellular reducing equivalents (199, 270, 358). Further, in some models of experimental cardiomyopathy, excess reducing equivalents and excess GPx-1 have been linked to the mechanism of cardiac dysfunction (297, 405). This review examines evidence for a role of GPx-1 in modulating cellular redox responses, summarizes the role of GPx-1 in human health and disease, and speculates on possible future therapeutic approaches in disease prevention and treatment.
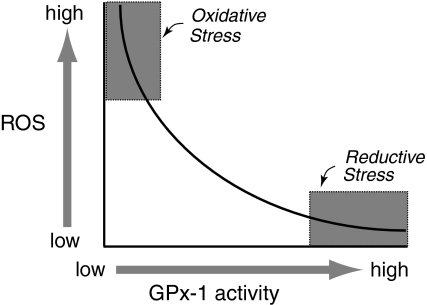
FIG. 2.
Modulation of oxidative and reductive stress by GPx-1. GPx-1 is one of several cellular enzymes that may modulate overall redox stress. Decreased GPx-1 activity can promote susceptibility to oxidative stress by allowing for the accumulation of harmful oxidants, whereas excess GPx-1 may promote reductive stress, characterized by a lack of essential ROS needed for cellular signaling processes. Excess oxidants or loss of essential ROS can each lead to diminished cell growth and promote apoptotic pathways.
II. GPx-1 Activity
A. Enzymatic mechanisms of GPx
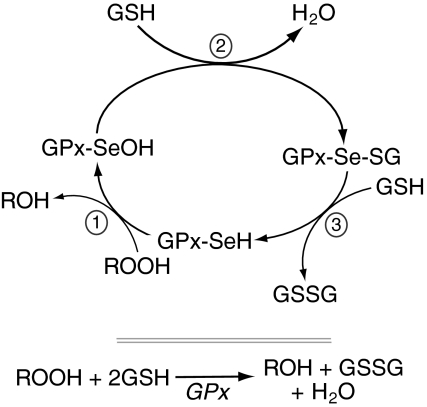
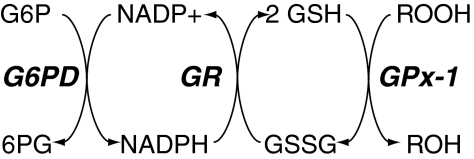
GPx-1 (glutathione:hydrogen-peroxide oxidoreductase; EC 1.11.1.9) was first characterized in 1957 as an erythrocytic enzyme that protects hemoglobin from oxidative damage (254). Subsequent analysis found that the trace mineral, selenium, in the form of the amino acid, Sec, is essential for the activity of GPx-1 (127, 203, 309, 312). Mechanistically, detoxification of peroxides by mammalian GPx-1 proceeds by way of a bi-substrate ping-pong-type enzymatic mechanism in which saturation kinetics are not observed. Enzymatic reduction of peroxides involves the formation of intermediate stable modifications to the Sec active site (127, 128, 204, 348, 369). After reacting with peroxide, a selenenic acid (Se-OH) forms at the selenol (Se-H) active site (Fig. 3). One molecule of GSH reduces the selenenic acid, leading to the formation of a glutathiolated selenol (Se-SG) intermediate (127, 204, 248). Evidence for the formation of the Se-SG intermediate was obtained by mass spectrometry analysis of GPx-4 reaction intermediates (250). A second GSH allows for reduction of the Se-SG bond, and results in the restoration of the active site with the formation of oxidized glutathione (GSSG). The subsequent resolution of GSSG involves the action of the NADPH-dependent glutathione reductase; recycling of NADP+/NADPH links the GSH pathway to glucose-6-phosphate dehydrogenase and the pentose-phosphate shunt (Fig. 4). Thus, by its enzymatic detoxification of nonradical hydroperoxides, GPx-1 regulates cellular oxidant status directly through elimination of hydroperoxides and via oxidation of GSH, the major low-molecular-weight thiol in cells. [Rotruck first established the selenium dependence of the GSH-peroxidase that relied on glucose-6-phosphate dehydrogenase-GSH-reductase–driven recycling of GSH to protect erythrocytes from oxidation (311, 312): in erythrocytes from selenium-deficient rats, glucose-mediated reduction of GSH had no protective effect against oxidation.] In fact, in studies of hydrogen peroxide metabolism in cultured cells from GPx-1-deficient and wild-type mice, exposure to hydrogen peroxide led to a transient increase in oxidized GSH in wild-type cells, whereas GSSG did not fluctuate in GPx-1-deficient cells (229). These data illustrate how the enzymatic action of GPx-1 links it to the intracellular GSH/GSSG redox couple transferring redox stress in the cell to the easily restored GSSG rather than reactive protein thiols (189, 196). Indirectly, GPx-1 is also linked to the NADP+/NADPH redox couple, which is involved in the restoration of normal GSH/GSSG ratios. GPx-1 oxidation of GSH may also influence the pentose-phosphate pathway, the activity of which is regulated by NADP+/NADPH ratios (116).
FIG. 3.
Reduction of hydrogen peroxide by GPx-1. The enzymatic inactivation of peroxides by GPx-1 involves the formation of several stable intermediary modifications to the active-site selenocysteine (Sec) (127, 204, 248). Thus, the selenol of GPx-SeH (with -SeH representing the Sec active site) forms a selenenic acid (Se-OH) after reacting with peroxides (no. 1 in the figure). One molecule of GSH reduces selenenic acid leading to the Se-SG intermediate (no. 2 in the figure), which is reduced by the second GSH, resulting in the formation of GSSG (no. 3 in the figure). The net reaction is shown in the lower part of the figure.
FIG. 4.
Redox pathways involved in maintaining the cofactors necessary for the activity of GPx-1. GPx-1 reductively inactivates hydrogen peroxide and lipid hydroperoxides at the expense of GSH, which is oxidized to form GSSG. The enzyme glutathione reductase (GR) recycles GSSG to GSH using NADPH as a source of reducing equivalents, and glucose-6-phosphate dehydrogenase (G6PD) maintains cellular stores of NADPH.
B. Structure and function: analysis of the active site
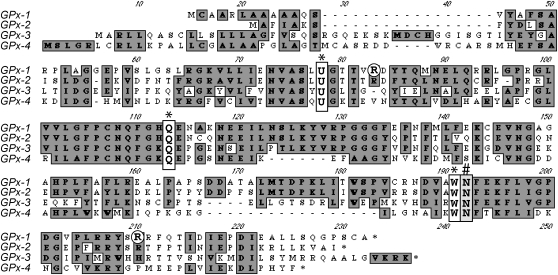
The molecular mass of the active purified mammalian GPx-1 has been estimated to be between 83 and 95 kDa, consisting of a tetramer of identical subunits of ∼22–23 kDa (17, 126, 257, 273). Amino acid sequence analysis of the bovine GPx-1 provided evidence for a 198 amino acid monomer with an approximate mass of 21,900 kDa (148). Sequence data from cDNA analysis indicate that GPx-1 monomers in humans are between 202 and 204 amino acids in length (depending on allelic variant), thus predicting a protein monomer of ∼21,800–21,950 kDa, in good agreement with the values obtained experimentally for purified human GPx-1 monomers. Protein sequencing and subsequent cDNA sequence analysis provided evidence for the presence of Sec in the GPx-1 protein and proof that it was encoded by a UGA (opal) stop codon in the gene transcript. Typically, in non-selenocysteine encoding transcripts, a UGA codon sequence terminates translation. Figure 5 shows a sequence comparison of human Sec-containing GPxs 1–4 based on cDNA sequence data (5, 112, 263, 340, 346).
FIG. 5.
Alignment of predicted human GPx proteins. Protein sequences for the Sec-containing GPxs-1–4 were aligned using the MacVector analysis program (version 8.1.2 from Accelyrs). Shown are single-letter amino acid codes for the precursor proteins derived from GenBank reference sequences NM_002083, NM_002084, and NM_002085 for GPx-2, 3, and 4, respectively, that were based on published cDNA sequences (5, 112, 346). The GPx-1 sequence was derived from a human cDNA (384) based on published GPx-1 sequences (263, 340). Conserved residues are boxed in gray, and conserved bases involved in the formation of the enzymatic active site are boxed in white and indicated with asterisks, including the Sec (represented with a U), Trp, and Gln residues (107). A conserved Asn residue that is part of the active site is marked with an “#” (359, 361). Arg residues involved in stabilizing the GSH and GPx-1 interactions are circled in white. Note that these Arg residues are conserved in the highly similar GPx-2 but not in GPx-3 and GPx-4.
One theory regarding the essential function of Sec at the active site of GPxs is that the presence of Sec instead of a Cys at the active site may enhance the rate of reaction with hydrogen peroxide because Sec is deprotonated at physiological pH. According to theoretical modeling studies, however, the pKa of Sec does not alone account for the catalytic difference between Sec and Cys-substituted enzymes; rather, the local tertiary structural environment and accessory amino acids are essential for the activity of the GPxs (54, 359). Strikingly, substitution of a Cys for Sec lowers the enzymatic activity of GPx-1 by orders of magnitude (309), leading to the suggestion that a different mechanism involving a second resolving Cys is essential for the enzymatic activity of normally occurring nonselenium (i.e., Cys-containing) forms of GPxs, such as the GPxs in yeast strains that lack a Sec incorporation mechanism (359). In some of these non-selenium-containing GPxs (which often function as thioredoxin-dependent peroxidases), the active-site Cys is also deprotonated, suggesting a redox-active site; however, there are also other amino acids such as a second resolving Cys that are necessary for their activity (348, 361). Regarding the catalytically advantageous nature of Sec, studies of Cys-substituted murine GPx-1 indicate that in the context of mammalian Sec-containing GPxs, the Sec active site may have the added advantage of being less sensitive to overoxidation and inactivation in the presence of peroxides compared to Cys (309). Although Sec in mammalian GPx-1 can be irreversibly inactivated by conversion to dehydroalanine (DHA) under excess oxidant exposure (69, 292), several studies suggest that abundant intracellular GSH normally protects GPx-1 from oxidative inactivation, illustrating the importance of the overall cellular redox state in maintaining GPx-1 function (280, 327, 373) (see also section III.C., “Post-translational regulation”).
The crystallographic structure of the bovine erythrocyte GPx-1 identified Gln and Trp amino acids that, with Sec, form a catalytic triad that is crucial for enzyme–substrate interactions (107). These residues are highly conserved in all mammalian Sec-containing GPxs (see Fig. 5 for a comparison of the primary sequence of human GPxs 1–4). Mutational analysis of these sites in GPx-4 provided proof that these residues are essential for catalytic function (242). Subsequent mutational analysis of a Drosophila GPx (DmGPx) also suggests a function for a conserved Asn residue that is necessary for the enzymatic function of the GPxs (361). Although this latter study using DmGPx was based on high-definition crystallographic data for GPx-4, molecular modeling suggests that the Asn may also be essential in GPx-1 (359) and that the functional catalytic site is actually a tetrad. However, DmGPx is a thioredoxin-dependent peroxidase that has a Cys in the active site rather than a Sec; therefore, there are other differences between this enzyme and its Sec-containing counterparts, including the involvement of a second Cys necessary to regenerate the active site. Analysis of the crystal structure also suggests that specific Arg residues in GPx-1 stabilize the interactions between GSH and GPx-1 (107). These residues are conserved between GPx-1 and the highly similar GPx-2, but are not found in GPx-3 or GPx-4. Other conserved Arg residues have also been suggested to be critically involved in enzymatic mechanisms (369).
C. Inhibitors of GPx
Owing to the structural similarities of the active site of GPx-1 and the other GPxs, there is no GPx-1-specific inhibitor. Mercaptosuccinate is one of the most effective and widely used of the related mercaptans that inhibit GPx-1 by competing with GSH for binding to the active-site Sec (62). Experimentally, mercaptosuccinate is not used as a GPx-4 inhibitor, but it will likely also inhibit GPx-2 and perhaps even GPx-3. Methylmercury (MeHg), another highly reactive compound that can promote neuronal death, has been shown to inactivate GPx-1 directly in purified enzyme preparations and in cerebellar granule cells grown in culture (119). Theoretically, inactivation of GPx-1 by MeHg involves a direct interaction of mercury with the selenol of GPx-1. Further, the ability of MeHg to inactivate GPx-1 at low nanomolar concentrations suggests that loss of GPx-1 activity may contribute to MeHg toxicity. Other agents, such as gold (19, 61), may similarly inhibit GPxs by reacting with the selenol. Similarly, lead may interact with and inactivate GPxs (3). Other nonspecific methods to inhibit GPxs include the use of L-buthionine sulfoximine to inhibit γ-glutamyl synthetase, an essential enzyme in GSH synthesis (298). This inhibition would affect total cellular GSH levels and the function of all GSH-dependent enzymes, including GPxs.
D. Comparison among mammalian GPxs 1–4
As discussed above, the active sites of the mammalian GPxs 1–4 are highly conserved. In addition, these GPxs have overlapping substrate specificities. Thus, activity assays may not alone be able to distinguish among activities for the various GPxs, especially GPx-1, −2, and −3 (330). For example, gastrointestinal samples will contain both the ubiquitous GPx-1 and the similar GPx-2, which has a more limited tissue distribution. As mentioned previously, GPx-3 is secreted and is the primary form measurable in plasma, but it is also expressed in lung tissue and kidney (the source of its plasma secretion).
GPx-4 is found in most cell types. Owing to its unique enzymatic properties, GPx-4 activity can be separately determined using a phospholipid hydroperoxide substrate in enzymatic assays (386). Possibly due to the ability of GPx-4 to reduce these membrane-associated hydroperoxides, it is the only subtype of mammalian GPxs for which the knockout is lethal (320), but the precise cause of the midgestational lethality caused by GPx-4 deficiency (401) is unknown, and may be unrelated to the reduction of phospholipid hydroperoxides by GPx-4. In addition, GPx-4 depletion in cell culture also promotes cell death by apoptotic mechanisms that could not be alleviated with water-soluble antioxidants, confirming that intracellularly GPx-4 mainly prevents lipid peroxidation, essentially antagonizing the actions of lipoxygenases and cycloogenase. Unlike the other GPxs, GPx-4 is a monomeric protein. In addition, GPx-4 has an essential role in spermatogenesis and sperm function (180).
GPx-1 knockout mice are viable, although they and GPx-1-deficient cells are more susceptible to oxidant-induced injury (65). GPx-2 knockout mice are also viable (111), as are the double knockout mice deficient in both GPx-1 and GPx-2 (111). There is an interesting synergy between GPx-2 and GPx-1 as they are very similar enzymatically, but they have different gene expression patterns, with the expression of GPx-2 in gastrointestinal epithelial cells thought to provide essential protection against gut pathogens and inflammation (72, 111, 114). GPx-2 may also be induced in other tissues as part of the stress response, such as in lung tissue in response to cigarette smoke and in breast cancer cells, and its gene expression is regulated directly by the antioxidant response element (ARE) (304, 330). Thus, GPx-2 serves a distinct function in antioxidant and cellular protection in some tissues that complements that of GPx-1. The roles of GPx-1 and GPx-2 in the antioxidant response and cancer are discussed further in sections III.A. and V.A., respectively. Experimental evidence suggests that the expression of GPx-2 and GPx-4 is less sensitive to variations in selenium levels compared to GPx-1 or GPx-3 (see section III).
III. Regulation of GPx-1 Expression and Activity
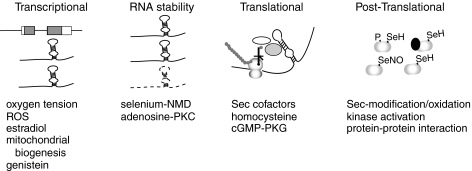
GPx-1 is subject to transcriptional, post-transcriptional, translational, and post-translational regulation (Fig. 6). These forms of regulation contribute to tissue and developmental patterns of expression, changes in activity in response to stress, and differences in expression between males and females (293, 294). A unique mechanism in the regulation of GPx-1, as a Sec-containing enzyme, is that of Sec incorporation (100, 334, 336). Essential for this mechanism is selenium, an essential nutrient that is normally acquired through the dietary consumption of plants and animals (387). In human populations, selenium deficiency, due to lack of selenium in the soil, has been found to cause Keshan disease (6, 138, 216, 305), a cardiomyopathy, and Kashin-Beck disease (287, 393), an osteoarthropathy, in part, due to reductions in GPx-1 expression. This section discusses the mechanisms of GPx-1 regulation by transcriptional and post-transcriptional means, including Sec incorporation and how GPx-1, in particular, is modulated by selenium restriction.
FIG. 6.
Regulation of GPx-1 expression and function. GPx-1 can be regulated by transcriptional, post-transcriptional, translational, or post-translational means. Shown is an overview of the factors that regulate the expression and activity of GPx-1. In addition to factors that regulate its transcription, GPx-1 can also be regulated post-transcriptionally by the presence or absence of selenium and cofactors involved in Sec biosynthesis and insertion. Represented in the figure is the stem loop structure or SECIS element formed in the 3′ untranslated region (UTR) of the GPx-1 transcript. Absence of selenium promotes RNA degradation due to nonsense-mediated decay (NMD) and/or the presence of SECIS binding factors that interfere with normal translation. Translation involves special SECIS binding factors, a Sec-specific tRNA (black cloverleaf structure), elongation factor (white oval), and SECIS binding protein 2 (SBP2) (gray oval). Post-translationally, Sec in GPx-1 (reduced form SeH in the figure) may be oxidatively inactivated by excess ROS or by excess NO (SeNO in the figure). In addition Hg and Pb may inactivate GPx-1 and specific protein–protein interactions (black oval represents regulatory protein that binds GPx-1) may also inhibit GPx-1 activity. Kinases, such as c-abl, may activate GPx-1 by phosphorylation (indicated by a P in the figure).
A. Transcriptional regulation
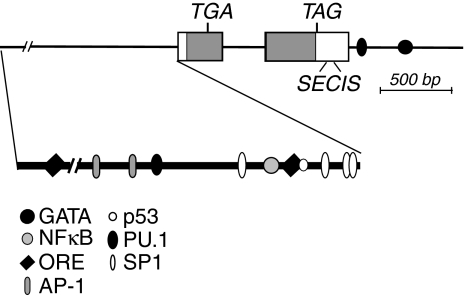
Comparison of mouse, rat, and human gene sequences indicates a conservation of the sequence and overall structure of the GPx-1 gene, with the protein coding region contained within two exons and the Sec codon in the first exon (Fig. 7) (58, 169, 259). The apparent promoter regions are conserved in these mammalian species with no apparent TATA or CCAAT box sequences, but several specificity protein 1 nuclear factor boxes. Additionally, the human GPx-1 gene is downstream of the 3′-end of the rhoH12 gene, which ends only ∼800 bp upstream of the transcriptional start site for GPx-1 and is flanked by Alu repeat sequences upstream and downstream (259). At least for the mouse GPx-1 gene, a 3′ enhancer region with GATA nuclear factor binding sites regulates transcription in erythroid cells, which have a higher rate of expression compared to tissues such as brain (271). The presence of sites with comparable effects has not been confirmed for the human GPx-1 gene. In addition, two PU.1 binding sites that are conserved in human and mouse GPx-1 genes have been proposed to play a role in the expression of GPx-1 in various myeloid and lymphoblastoid lineages, including neutrophils and macrophages (356). Although one of these sites is located in the 5′ promoter region and the other site in the 3′ flanking region, both sites are functional, as they have both been shown to bind the PU.1 nuclear factor in gel mobility shift assays, and both mediate transcription, as assessed by reporter gene assays.
FIG. 7.
Diagram of GPx-1 gene organization and nuclear factor binding sites. The GPx-1 gene consists of two exons (rectangular boxes). The 5′ and 3′ UTRs are shown as white boxes, whereas the protein coding region is shown as a gray box. The TGA Sec codon is in the first exon. Notably, the stop codon is a TAG nucleotide sequence (amber stop codon). The promoter region is expanded on the lower line to illustrate the position of nuclear factor binding sites thought to be important for GPx-1 transcription. The GATA and NFκB nuclear factor binding sites have primarily been studied in mice. All other sites are identified from studies with the human GPx-1 gene.
GPx-1 transcription is also regulated by oxygen tension. Specifically, the human GPx-1 gene has two oxygen response elements (OREs) that, under normoxic conditions, are important for transcription (78). The ORE sequences bind a nuclear complex that includes the nuclear factor, Ku (253). In cardiomyocytes grown in culture, chronic exposure to reduced oxygen tension decreased the p70 subunit association with the OREs, suggesting that this protein may modulate decreased GPx-1 expression in these cells during hypoxia. Further, it has been proposed that hypoxia-induced suppression of GPx-1 transcription may contribute to reperfusion injury after low oxygen tension in vivo (253); however, further analysis is necessary to confirm a role for the Ku antigen complex and ORE sequences in modulating hypoxic responses in vivo and in cells other than cardiomyocytes and to define the mechanism by which they respond to oxygen tension. Interestingly, hyperoxia enhances GPx-1 transcription in human umbilical-vein endothelial cells through a mechanism independent of the ORE (190), suggesting that GPx-1 transcription may be regulated in response to oxygen tension by more than one mechanism.
GPx-1 may also be transcriptionally upregulated as part of the cellular response to oxidative stress. Paraquat, a redox cycler that produces superoxide anion, has been reported to stimulate GPx-1 promoter activity (89), and p53 can upregulate GPx-1 transcription through its action at a classic p53-consensus binding site (177, 349). It is well known that p53 is a tumor suppressor: it modulates apoptosis, growth arrest, DNA repair, and cellular senescence in response to many types of cellular stress (178, 295). Recent data also suggest that hydroxyurea may upregulate GPx-1 expression in several cell lines and in circulating erythrocytes, in part, by a p53-dependent pathway, providing evidence that p53-mechanisms modulate GPx-1 expression in vivo (68).
Oxidative mechanisms may also regulate GPx-1 gene transcription via nuclear factor κB (NFκB) sites and activator protein 1 (AP-1) sites in the promoter. In fact, in skeletal muscle fibroblasts, oxidant-induced upregulation of GPx-1 expression was transcriptionally mediated by activation of NFκB (410). Interestingly, NFκB was also proposed to regulate the expression of GPx-1 in response to estradiol, as NFκB inhibitors attenuated estradiol-mediated upregulation of GPx-1 expression (42). Possibly, the ability of estradiol to upregulate GPx-1 transcription may contribute to the increased expression of GPx-1 in females compared to males (43, 294).
Regulation at AP-1 sites in the GPx-1 promoter may involve a number of nuclear factors. Some of these, such as c-jun and c-fos, are known to be redox sensitive and can be inactivated by oxidation at critical Cys residues necessary for their DNA-binding activity (1). In addition, AP-1 sites in the GPx-1 promoter region have been found to modulate transcriptional responses to phorbol esters in human umbilical-vein endothelial cells (190). Other evidence suggests that GPx-1 transcription may be regulated directly or indirectly by Nrf-nuclear factors downstream of the transcriptional coactivators peroxisome proliferator-activated receptor-γ coactivator-1α and −1β (PGC-1α and PGC-1β). PGC-1α or PGC-1β activate Nrf1 and Nrf2 nuclear factors to enhance the expression of nuclear genes involved in oxidative phosphorylation and mitochondrial biogenesis (396). In addition, Nrf2 is known to activate genes with AREs downstream of PGC-1-activation and after oxidant exposure (290, 291). AREs often overlap AP-1 sites and coordinately upregulate genes involved in cellular antioxidant-detoxifying responses; however, to date, the ability of Nrf1 or Nrf2 to regulate GPx-1 gene transcription has not been proven in reporter gene assays. Nonetheless, upregulation of GPx-1 in response to PGC-1 activation links GPx-1 expression to signals enhancing mitochondrial biogenesis (337). The concept that Nrf-nuclear factors may control GPx-1 expression (directly or indirectly) is supported by other studies that correlate Nrf1-responses with increased expression of GPx-1 (168). Further, in Nrf2 knockout mice, GPx-1 expression is downregulated in lung after exposure to cigarette smoke (330). Interestingly, Nrf2 clearly regulates the highly conserved GPx-2 at the level of transcription (23) and is responsible for its upregulation by cigarette smoke (330). Genistein, a soy isoflavone, is another effector that may control GPx-1 transcription by Nrf1-dependent mechanisms (168, 368). Genistein is considered protective against oxidants, in part, due to its effects on GPx-1 expression that ultimately prevent hydrogen peroxide-mediated cell death (168, 345). In addition, this isoflavone may augment glutathione synthesis by increasing the expression of γ-glutamylcysteine synthetase (264): the γ-glutamylcysteine synthetase gene is a well-studied target of Nrf-nuclear factors (both Nrf1 and Nrf2 have been implicated in its regulation) (353). Thus, these findings suggest that genistein (and possibly other Nrf-activating agents) coordinately upregulate both the cosubstrate (GSH) and enzyme (GPx-1 or GPx-2) necessary for hydrogen peroxide reduction, thereby contributing to the overall antioxidant capacity of the cell.
Taken together, transcriptional mechanisms may augment GPx-1 expression, in part, to provide increased resistance to oxidative stress from diverse sources, including mitochondrial biogenesis or direct oxidant exposure. To date, these responses appear to involve the ORE, AP-1, NFκB, and p53 sites. Other nuclear factor binding sites, such as the PU.1 and GATA sites, may modulate expression during cell differentiation. GPx-1 expression, however, is also highly regulated post-transcriptionally by translational mechanisms; thus, alterations in gene transcription may not always affect GPx-1 protein and enzyme activity levels to the extent suggested by the magnitude of transcriptional changes.
B. Post-transcriptional and translational regulation
1. Basic mechanisms of Sec incorporation
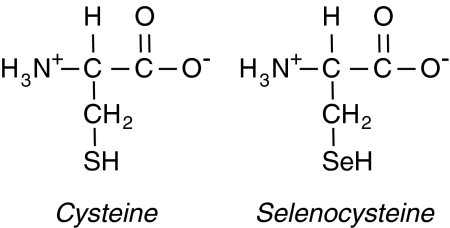
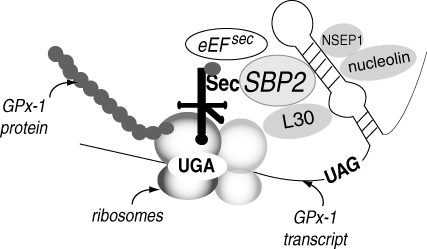
Many reviews have been written on the subject of Sec incorporation during translation of selenoproteins [see, e.g the reviews (100, 334, 336)]. Sec is the 21st amino acid (Fig. 8). Structurally, it is similar to Cys with Se substituting for S in the amino acid side chain. Functionally, like Cys, Sec is also redox active. Incorporation of Sec is limited to ∼25 proteins and their related isoforms in mammalian species (206) in a process that involves the recognition of a UGA (opal) stop codon as a site for Sec incorporation rather than termination of translation. Additional regions in the 3′ untranslated region (UTR) are essential for the incorporation process. These regions form stem-loop structures called Sec insertion sequences or SECIS elements (34). Although most UGA-containing transcripts do not facilitate Sec incorporation, transfer of a 3′ UTR with a SECIS element to heterologous gene transcripts with a UGA codon embedded in an open reading frame is sufficient to direct Sec incorporation (217, 219, 322). The role of the stem-loop structure in the SECIS element is to recruit specific RNA binding proteins that regulate Sec incorporation (63, 77, 323). One of the most important of these SECIS binding proteins is SECIS binding protein-2 (SBP2) (77). SBP2 binding to SECIS elements is thought to facilitate interactions with Sec elongation factor (eEFsec) (117) and specific Sec tRNA (tRNAsec) that are necessary for Sec incorporation (364, 403) (Fig. 9). SBP2 has been shown to contain a redox-active Cys, which regulates its subcellular localization and activity as oxidative conditions have been shown to sequester this protein in the nucleus and limit Sec incorporation into some selenoproteins (283). The importance of this mechanism in regulating GPx-1 expression under oxidative stress has not been established; nonetheless, SBP2 is an essential component in Sec incorporation. Other SECIS-binding proteins that may modulate Sec incorporation are ribosomal L30, nucleolin, NSEP1, and eIF4a3 (28, 49, 99). L30 and eIF4a3 may compete with SBP2, the latter in a manner that limits translation of GPx-1 under conditions of selenium restriction (49) (see also below). In addition to its recognition of the UGA codon (162, 210), the tRNAsec is unique in that it becomes aminoacylated with Ser, which is then enzymatically converted to Sec by Sec synthase using selenophosphate as a selenium donor. In mammalian cells, selenophosphate is specifically synthesized from selenide and ATP by selenophosphate synthetases (146, 237) in a process similar to that used by lower organisms (236). Thus, the mechanism of Sec incorporation is unique and may be modulated by the availability of Se, the oxidation state of SBP2 (76, 283), and the expression and function of factors involved in the biosynthesis and insertion of Sec discussed above. For example, novel SBP2 truncation mutations result in abnormal thyroid function, delayed bone development, congenital myopathy, and cognitive impairment in human subjects due to deficiencies in selenoproteins, including GPx-1 (121). Less severe defects in growth and thyroid function have been noted in other individuals carrying other functionally deficient mutations in SBP2 (104). These findings highlight the importance of the components of Sec incorporation and selenoproteins in normal health and development.
FIG. 8.
Comparison of cysteine (Cys) and Sec. Sec is the 21st amino acid. Its structure is similar to that of Cys, and, functionally, like Cys, Sec is also redox active.
FIG. 9.
Incorporation of Sec at UGA codons. The recognition of the UGA (opal) codon as a site for incorporation of Sec rather than a stop codon involves many specialized factors, such as SBP2, which binds to the stem-loop or Sec incorporation sequence (SECIS) element in the 3′ UTR of the GPx-1 transcript. SBP2 recruits the specific Sec tRNA and the Sec elongation factor (eEFsec) that are necessary for insertion of Sec at the ribosomes. Other factors, such as ribosomal L30, NSEP1, and nucleolin, may also bind to the SECIS element to modulate incorporation (28, 99). In the absence of selenium, eIF4a3 competes for SBP2 binding, essentially inhibiting GPx-1 transcription (not shown in figure). UAG (amber) stop codon specifies translational termination of GPx-1.
2. Selenium, nonsense-mediated decay of GPx-1 mRNA, and translational repression
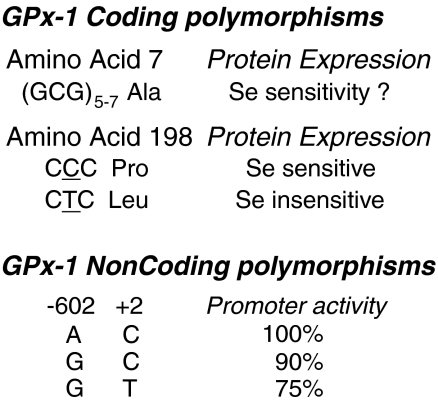
Dietary selenium restriction has an unequal effect on the expression of selenoproteins and their transcripts. One suggested theory by which to explain the unequal effect of selenium restriction is that this hierarchy of selenoprotein expression preserves the expression of some selenoproteins in cases of selenium depletion. GPx-1 is an example of a selenoprotein that falls low in the hierarchy, as its expression is diminished by selenium deficiency in cell culture systems as well as in in vivo studies (20, 31, 56, 342, 385). Importantly, these effects are not due to alterations in transcription, but have been thought to be due, in part, to nonsense-mediated mRNA decay and/or suppression of translation. Nonsense-mediated mRNA decay is a cotranslational mechanism that recognizes premature stop codons and targets such nonsense codon transcripts for degradation (328). One theory is that this process occurs specifically when a UGA is in the middle of an open reading frame and at least 50 nucleotides upstream from a splice junction. The TGA codon, encoding Sec, is found in the first exon of the human GPx-1 gene at amino acid 48 (of about 203 total), and it is located ∼105 nucleotides from the splice junction, suggesting that the GPx-1 gene transcript may be susceptible to nonsense-mediated decay. In some cells, however, selenium restriction reduces GPx-1 activity and protein to nearly undetectable levels with modest or no change in transcript levels (20, 56), suggesting that nonsense-mediated decay does not mediate suppression of GPx-1 protein expression under conditions of selenium restriction in all cells. Theoretically, GPx-4 would also meet the requirements for nonsense-mediated decay (NMD); yet, its transcript is considered to be relatively stable in the absence of selenium (32, 158, 385), although it has been suggested that GPx-4 transcripts may be susceptible to NMD in some cell culture systems (341). Similarly, GPx-2 transcripts are upregulated by selenium deficiency under conditions that diminish GPx-1 transcript levels (389). Taken together, these studies suggest that there may be cell-specific components that regulate the mRNA decay mechanisms compared to translational suppression of GPx-1; however, from these studies the requirements for NMD remain unresolved and NMD does not alone explain the in vivo hierarchy of selenoprotein expression.
Previous studies suggest that the hierarchy of selenoprotein expression is dependent on sequences in the 3′ UTR of Sec-gene transcripts, as an exchange of 3′ UTRs (including the SECIS elements) among various selenoprotein mRNAs altered their translational efficiency in selenium-depleted cells (30). Recent findings further clarify this mechanism by identifying a role for eukaryotic initiation factor 4a3 (eIF4a3) in the selective recognition of SECIS element subsequences that differ among various Sec transcripts (49). Essentially, two major forms of SECIS element exist, type 1 and type 2, which differ, in part, by the location of an AAR nucleotide motif in the stem-loop structure. Apparently, the type 1 SECIS found in GPx-1 interacts with eIF4a3, a factor that is induced in selenium deficiency. The interaction between eIF4a3 and the GPx-1-SECIS element limits GPx-1 expression by competing for the binding of SBP2, thereby selectively inhibiting GPx-1 translation under conditions of selenium restriction (49). Currently, it is unknown whether other conditions associated with alterations in GPx-1 translation and mRNA stability (see below) affect the expression or binding of eIF4a3 or other regulatory SECIS-binding factors.
3. Post-transcriptional upregulation of GPx-1
Factors other than selenium also modulate GPx-1 expression by post-transcriptional mechanisms. In human endothelial cells, adenosine causes an upregulation of GPx-1 expression and activity that depends on increased transcript stability rather than alterations in GPx-1 gene transcription (406). These findings may relate to protection during ischemic preconditioning when local concentrations of adenosine can increase up to 50-fold (262). Although the molecular determinants stabilizing GPx-1 transcripts have not yet been identified, protein kinase C (PKC)-mediated pathways are essential for adenosine-mediated GPx-1 upregulation, as bisindolylmaleimide-1, a nonspecific PKC-inhibitor, blocked upregulation. In addition, a binding site for nucleolin, a nucleic acid-binding protein that has been implicated in stabilizing transcripts (332), was identified in the 3′ UTR of the GPx-1 transcript. In support of a possible role for nucleolin in mediating GPx-1 transcript stability, nucleolin has been reported to bind to the 3′ UTR of the human GPx-1 transcript (394). Nucleolin is also activated by some forms of PKC (409), thereby suggesting a plausible link between PKC-pathways and mRNA stability. Further analysis, however, is necessary to determine whether nucleolin may specifically influence the stability or expression of GPx-1. More recent data suggest that nucleolin may preferentially regulate translation of other SECIS-containing transcripts and not GPx-1 (255). It is unclear whether these contradictory findings are a consequence of analyzing the rat GPx-1 SECIS in the latter study (255) rather than the human GPx-1 SECIS region that was analyzed in the earlier study (394).
Other post-translational mechanisms do not involve alterations in GPx-1 transcript stability. For example, cyclic guanosine monophosphate (cGMP) was recently shown to upregulate GPx-1 protein without affecting transcript levels (339). Interestingly, in this study, cGMP coordinately increased the expression of both GPx-1 and catalase in a manner dependent on protein kinase G-dependent mechanisms. Further, although antioxidant enzyme activity was not directly measured in this study, the cGMP-mediated upregulation of GPx-1 and catalase consequently enhanced protection against hydrogen peroxide-induced cell death (339). This cGMP-mediated increase in cellular antioxidant enzymes warrants further investigation to determine whether it plays a beneficial role in vivo, and whether it is relevant to the actions of receptor-mediated (particulate) guanylyl cyclase-activation pathways (e.g., through atrial natriuretic peptide) or soluble guanylyl cyclase (sGC) pathways (e.g., stimulated by NO· ) that activate protein kinase G.
4. Inhibition of GPx-1 translation
In general, Sec incorporation is an inefficient process that is affected by selenium concentration, the context of the UGA codon, the SECIS element structure and sequence, and the expression of cofactors involved in Sec-incorporation. Translation of GPx-1 may be even less robust than that of other Sec-transcripts (219, 385). Several studies have reported that a substitution of Cys for Sec in the context of a selenoprotein sequence increases translational efficiency concurrent with substantial decreases in enzyme-specific activity (36, 207, 214, 408). Thus, compared to other amino acid incorporation events, Sec incorporation may be less efficient, in part, due to limitations in the availability of cofactors involved in normal Sec-translation and the relative efficiency of normal translation termination events that occur at UGA stop codons. In support of this concept, cotransfection of cofactors, including SBP2 or tRNAsec, enhance the expression of various selenoproteins in different cell culture systems (35, 39).
Possibly owing to the inefficiencies of Sec incorporation, incorporation of this amino acid is also sensitive to amino acid substitution after exposure to aminoglycoside (AMG) antibiotics (153). AMGs are known to facilitate suppression of termination (at UGA or other stop codons) by altering proof-reading on ribosomes (246). In the context of GPx-1 expression, G418, an AMG, was found to augment GPx-1 protein expression while decreasing enzyme activity. Specifically, AMG treatment caused a misincorporation of Arg for Sec in GPx-1 as determined by mass spectrometry (153). These findings suggest that the AMG-mediated decrease in GPx-1-specific activity was caused by loss of a key amino acid at the enzymatic active site. Further, substitution of Arg for Sec may not be a random amino acid insertion, as two of the six Arg codons (CGA and AGA) have only a single base difference with the UGA codon. Interestingly, the effect of AMG on translation was enhanced during selenium restriction, suggesting that under conditions of adequate selenium, the presence of cofactors involved in Sec incorporation may protect against AMG-induced misincorporation. Subsequent studies support this hypothesis, as the presence of SBP2 in an in vitro translation system was found to decrease G418-mediated read-through of susceptible UGA-containing transcripts (151). These findings are especially of interest because AMG antibiotics are being considered as therapeutic agents to overcome nonsense mutations like those associated with some genetic variants causing cystic fibrosis, Duchenne's and Becker's muscular dystrophy, nephrogenic diabetes insipidus, and cardiac-specific arrhythmias (172, 193, 352, 388, 413). This class of drugs, however, is also associated with enhanced oxidative stress in susceptible cells. Specifically, they have been linked with oxidant-dependent ototoxicity and nephrotoxicity, and, in some studies, have been shown to decrease GPx-1 activity in vivo (108, 286, 321, 395). Taken together, these studies suggest the utility of monitoring Se and cofactor levels to minimize some of the deleterious consequences of AMG treatment.
Other studies have found that GPx-1 protein expression and activity are decreased by modest increases in homocysteine concentrations in vivo and in cell culture systems (155, 384). In vitro analysis suggests that this effect involves translational mechanisms that decrease the incorporation of Sec rather than alter transcript levels (155). These findings may, in part, explain the effects of modest (pathological) levels of homocysteine on the enhanced oxidative stress found in hyperhomocysteinemic mice (106). At high (millimolar) concentrations, homocysteine is known to cause endoplasmic reticulum stress, a process that alters the translational program of the cell and dramatically decreases GPx-1 transcript levels (279). It is possible that due to the inefficient nature of Sec incorporation, translation of GPx-1 is readily disrupted by homocysteine, before alterations in transcript stability or transcription. Alternatively, other effects of homocysteine on translation cannot be excluded.
C. Post-translational regulation
1. Sec oxidation
Several reports indicate that activity of GPx-1 may be modulated by post-translational modification. In vitro the Sec active site in GPx-1 can be oxidatively inactivated in the presence of millimolar concentrations of hydrogen peroxide (69, 292). GPx-1 can be irreversibly inactivated in vivo, as shown in recent studies of human isolated red blood cells (RBC). Interestingly, the degree of irreversible GPx-1 activation was increased in older circulating RBC and involved a loss of reactive selenol sites in GPx-1 rather than a decrease in protein levels. In fact, mass spectrometry (MS/MS) sequence analysis indicated that the underlying molecular cause of diminished GPx-1-specific activity was a conversion of Sec to dehydroalanine (DHA) by β-elimination (loss of H2SeO2) after excess hydrogen peroxide-induced oxidation of Se-OH to SeO2H (69). As described in this report, the irreversible conversion of Sec to DHA can be monitored using biotin-conjugated cysteamine to tag DHA residues. This method may prove useful for understanding the physiological and pathological conditions under which overoxidized, DHA-containing forms of GPx-1 may accumulate in cells other than RBC. Previous studies in keratinocytes indicated that adequate intracellular stores of GSH protect against peroxide-mediated loss of GPx-1 activity, as addition of GSH to cultures minimized enzymatic inactivation of GPx-1 by exposure to high concentrations of peroxides, whereas buthionine sulfoximine, an inhibitor of GSH biosynthesis, augmented sensitivity to peroxide-induced GPx-1 inactivation (373). Therefore, these findings suggest that peroxide-induced inactivation of GPx-1 is dependent on cellular redox state and availability of the GSH cosubstrate. Thus, RBC, which have a nominal ability for de novo protein synthesis, may accumulate oxidized proteins (69), including those necessary for maintaining NADPH stores (Fig. 4). Subsequently, over time or after chronic stress, RBC may exhaust their supply of reduced GSH, thereby promoting the susceptibility of GPx-1 to irreversible oxidation and loss of the Sec active site. Interestingly, in GPx-1-replete cells, compared to those with GPx-1 deficiency, the presence of GPx-1 can mediate a dramatic decrease in GSH and GSH/GSSG ratios in response to acute oxidative stress (136, 229). These findings suggest that accelerated loss of GSH can occur under oxidative stress, in part, due to GPx-1-mediated reduction of peroxides; however, this deficiency is eventually restored in cells that can recycle reduced GSH and synthesize new GSH to replenish GSH stores.
Other ROS and RNS can inactivate GPx-1. For example, GPx-1 can be inactivated by NO · generated by inducible NO · synthase (NOS) or by NO · donors, such as SNAP (S-nitroso-N-acetyl-D,L-penicillamine) (15, 16). Additionally, the reactive compounds, superoxide and peroxynitrite (ONOO−), can both inhibit GPx-1 (16, 41, 280). The molecular mechanisms involved in inactivation of GPx-1 by superoxide and peroxynitrite have not been completely elucidated, and it is not clear whether these reactive molecules inactivate GPx-1 in vivo. In in vitro studies, however, GPx-1 inactivation by peroxynitrite is prevented by GSH (280, 327), in the presence of which GPx-1 can act as a peroxynitrite reductase. Thus, similar to peroxide-mediated inactivation of GPx-1, the in vivo role of RNS-mediated pathways of GPx-1 inactivation may depend on the overall intracellular redox environment, including concentrations of GSH, the ratio of GSH/GSSG, and the amount of RNS produced.
2. Stimulation by signal transduction and/or protein–protein interactions
GPx-1 activity may also be enhanced by mechanisms such as phosphorylation or protein–protein interactions. For example, moderate concentrations of free fatty acids were found to augment GPx-1 activity in human ECV-304 and murine fibroblasts in a manner dependent on epidermal growth factor receptor (EGFR) activation (105). In these cells, increased GPx-1 activity was correlated with decreased ROS accumulation, illustrating a functional effect for increased GPx-1 activity. Although it is unclear how EGFR activation modulates GPx-1 activity, the increase in activity was rapid and peaked within 15 min, suggesting an event that required activation of existing GPx-1, possibly by phosphorylation or through alterations in protein–protein interactions. In proof of the concept that GPx-1 may be a target for kinase phosphorylation, GPx-1 was shown to be a substrate for the tyrosine kinases, c-Abl and Arg, which are important in the cellular response to oxidants (52). Specifically, recombinant tagged GPx-1 was found to coimmunoprecipitate with recombinant c-Abl or Arg; these kinases phosphorylated GPx-1 in cell culture and in in vitro assays with GST-fusion proteins. The specific Abl-family kinase inhibitor, ST15171, decreased cellular GPx-1 activity; and overexpression of recombinant c-Abl, but not a kinase- dead mutation, increased GPx-1-specific activity in cells (52). Taken together, these results suggest that GPx-1 activity may be modulated by the action of this class of nonreceptor tyrosine kinase enzymes. Functionally, the c-Abl kinases are known to mediate many cellular effects, including responses to oxidative stress, but, overall, they are believed to regulate pro-apoptotic pathways (227). Thus, the concerted upregulation of GPx-1 along with other pro-apoptotic pathways stimulated by c-Abl kinases may mitigate apoptosis under some circumstances. This last point is speculative; however, the finding that c-Abl modulates GPx-1 activity is notable in that it demonstrates regulation of GPx-1 activity by kinase-mediated phosphorylation.
Post-transcriptional mechanisms that regulate GPx-1 activity may prove to be crucial to maintaining cellular redox balance. In support of this view, a recent study suggests that protein–protein interactions between GPx-1 and selenium binding protein-1 may repress GPx-1 activity in some cancer cell lines (118). These findings highlight the need for additional studies to understand better the complex regulation of GPx-1 expression and function as alterations in GPx-1 activity may alter cellular redox, modulate tissue damage, and contribute to disease mechanisms.
IV. GPx-1 and Oxidant-Dependent Cellular Processes
Oxidative stress plays an essential role in modulating cell death in response to many stresses. In addition, oxidants play essential roles in cell signaling, growth, and proliferation. Thus, this section focuses on the importance of GPx-1 in modulating intracellular oxidant-driven pathways.
A. Oxidative damage and cell death, apoptosis, and injury
1. Role of oxidants in cell death and apoptosis
Overall, oxidative stress can contribute to cell death, and excess cellular oxidants play an important role in mediating the complex cascade of events leading to cell death via apoptotic pathways. Apoptosis is a process of programmed cell death that can contribute to pathological cell and tissue damage in cardiovascular and neurodegenerative diseases, and during the response to toxins (including chemotherapeutic agents), sepsis, or other environmental and physiological stimuli.
Briefly, there are two pathways of apoptotic cell death, the extrinsic and intrinsic pathways. The extrinsic pathway involves the activation of a protease cascade intitated by the activation of caspase 8 through the action of death receptors and their ligands, for example, FasL, and tumor necrosis factor-α (181). Once activated, caspase 8 cleaves downstream caspases 3 and 7, called effector caspases (48). These effectors mediate the pathways directly leading to cell death. In addition, caspase 8 can cleave Bid (Bcl-2 interacting domain), a pro-apoptotic factor that, once cleaved, activates the intrinsic pathway of apoptosis by interaction with pro-apoptotic factors Bak and Bax. Alternatively, the intrinsic pathway mediates the effects of ROS and many other stimuli, such as hypoxia, toxins, and ischemia-reperfusion injury, that may evoke an apoptotic response, in part, due to ROS generation. The intrinsic pathway involves mitochondrial release of pro-apoptotic factors (apoptogens), such as cytochrome c and apoptosis-inducing factor (AIF), from the intermembrane space and antiapoptotic Bcl-2 family members (Bcl-2, Bcl-XL) that prevent the apoptosis-associated release of apoptogens (277, 319). Released AIF translocates to the nucleus where it promotes chromatin condensation and DNA fragmentation. Other apoptogens, including cytochrome c, promote further caspase activation (48, 102, 223) to potentiate cell death.
ROS generated by apoptotic cytokines, such as tumor necrosis factor-α, simultaneously activate antiapoptotic pathways, such as Akt, mitogen-activated protein kinases (MAPK), and NFκB pathways, that promote cell survival (Fig. 10). Extracellular signal-related kinases 1 and 2, an MAPK, and NFκB specifically upregulate the expression of several IAP family members that attenuate apoptosis (137, 343). Further, NFκB modulates the pro-apoptotic actions of c-Jun-amino terminal (stress-activated) kinase, which is also activated by ROS, by inducing the expression of Gadd45β, an inhibitor of MAPK (282). Akt activation also attenuates apoptosis indirectly by antagonizing forkhead box subfamily O transcription factor 3a pathways to promote the expression of the caspase 8 inhibitor, FLICE-inhibitory protein (181, 333).
FIG. 10.
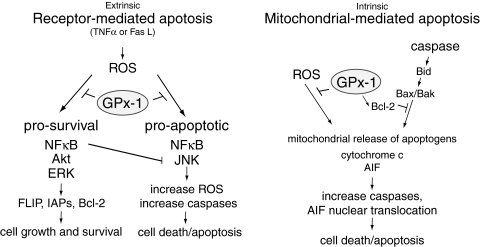
Role of GPx-1 in modulating apoptosis. The extrinsic pathway of apoptosis involves activation of caspase pathways that promote cell death. ROS activates both survival and apoptotic pathways. GPx-1 by modulating cellular hydrogen peroxide can inhibit both pro-survival and pro-apoptotic pathways. The end result (cell death or survival) may depend on the extent and levels of ROS generated. Interestingly, excess ROS as found in GPx-1 deficiency may alter nuclear factor κB (NFκB) signaling to promote pro-apoptotic responses. Normally, NFκB activation results in the upregulation of IAPs and other antiapoptotic genes. Similarly, extracellular signal-related kinase (ERK) and Akt activation can promote IAP or FLICE-inhibitory protein (FLIP) expression to inhibit caspase cascades. c-Jun-amino terminal (stress-activated) kinase (JNK) activation, which can be attenuated by NFκB, augments apoptotic pathways by further stimulating ROS production. GPx-1 overexpression has been shown to specifically suppress the activation of Akt and NFκB pathways. GPx-1 specifically blocks NFκB activation by preventing the degradation of the NFκB inhibitor inhibitor of κB. The intrinsic pathway of apoptosis involves the release of apoptogens like apoptosis-inducing factor (AIF) or cytochrome c from mitochondria. These pathways may be activated by ROS (including hydrogen and lipid hydroperoxides) and caspase cascades that promote Bid (Bcl-2 interacting domain) cleavage. GPx-1 has been shown to attenuate AIF release and enhance the expression of Bcl-2, an antiapoptotic factor.
2. Role of GPx-1 in cell death and apoptosis
In apoptotic cascades, there are several steps in which GPx-1 may influence phenotypic outcome, theoretically, by regulating oxidant accumulation. In cultured neurons isolated from GPx-1 knockout (GPx-1−/−) mice, enhanced susceptibility to hydrogen peroxide-induced apoptosis correlates with increased accumulation of intracellular ROS and decreased activation of the phosphatidylinositol 3-kinase (PI3K)/Akt survival pathway (89, 351). Similarly, aortic smooth muscle cells from GPx-1−/− mice exposed to low-density lipoprotein had significantly higher rates of apoptosis compared to similarly treated cells from wild-type mice (150). Other cells from GPx-1−/− mice, including astrocytes, fibroblasts, and endothelial progenitor cells (EPCs), also exhibit enhanced susceptibility to peroxide-mediated cell death and apoptosis (90, 139, 228, 371). These findings are consistent with those for other antioxidant enzymes: for example, lack of peroxiredoxin 3, a mitochondrially localized antioxidant enzyme, also augments apoptotic signaling in some cell types (60). Hydrogen peroxide-mediated toxicity is normally kept in check by several antioxidant enzymes that are essential in preserving cellular homeostasis. Absence of any of these enzymes may enhance susceptibility to cell death in response to excess oxidant accumulation. Interestingly, loss of GPx-4 promotes apoptotic signaling by allowing for the accumulation of oxidized membrane phospholipids, thereby stimulating AIF transmigration from mitochondria to the nucleus to initiate apoptotic signaling (320). In a separate analysis in neuronal cells, overexpression of GPx-1 and other antioxidant enzymes also blocks AIF-translocation from mitochondria to the nucleus in a model of ischemia-induced apoptosis (404), suggesting that intracellular oxidants can promote AIF transmigration. From these studies, it is unclear whether, ultimately, it is the accumulation of oxidized phospholipids that is essential for the nuclear transmigration of AIF or whether hydrogen peroxide stimulates apoptogen release by other mechanisms.
In general, increased expression of GPx-1 is protective against many apoptotic stimuli. This observation is consistent with the effects of antioxidants, such as N-acetyl-L-cysteine; MitoQ, a mitochondrially targeted antioxidant lipophilic triphenylphosphonium cation covalently bound to a ubiquinol antioxidant (2); and diphenylene iodonium, an inhibitor of NOX, in attenuating ROS-induced apoptosis. The protective effect of GPx-1 against oxidant-mediated stress was first shown in T47D breast cancer cells overexpressing recombinant GPx-1. These overexpressing cells had decreased oxidative modification of mitochondrial DNA and enhanced resistance to oxidant-induced cell death (215, 256). In subsequent studies, it was reported that GPx-1 could also protect against cell death in response to cytotoxic drugs, such as doxorubicin, and in response to direct ligand-mediated apoptosis, by ligand for CD95 receptor (CD95L) (142, 143). Mechanistically, GPx-1 was proposed to promote survival to CD95-induced apoptosis via reduction in cellular ROS. In support of this hypothesis, GPx-1 overexpression attenuated caspase 8 and caspase 3 activation and diminished release of cytochrome c into the cytosol, concomittant with decreased accumulation of ROS after CD95L activation. Further, consistent with the essential role of ROS in these pathways and GPx-1 as their modulator, inhibition of GSH synthesis blocked the protective action of GPx-1, whereas enhanced protection could be replicated by N-acetyl-L-cysteine treatment in nonoverexpressing cells. Similarly, in a model of neurotoxicity, excess GPx-1, as well as antioxidants, such as MitoQ, attenuated cellular ROS, thereby leading to a decrease in activation of the caspase cascade and a reduction in apoptotic cell death (191). Excess expression of other antioxidants, such as peroxidredoxin 3, can similarly attenuate apoptotic signaling (269).
GPx-1 overexpression, however, is protective against apoptosis only in circumstances where there is a disruption in normal redox balance favoring oxidation (i.e., under conditions of oxidative stress). Thus, in some tumor cells, doxorubicin-induced apoptosis apparently does not rely on oxidant generation, as excess GPx-1 overexpression fails to protect against apoptosis (379). Alternatively, the level of ROS-generated in these cells may not be overcome by only overexpressing a single antioxidant enzyme, or, perhaps, the basal level of hydrogen peroxide is low such that its removal may promote apoptosis. The latter option, that loss of oxidants can augment apoptosis, is supported by findings that correlate increased cellular hydrogen peroxide levels with resistance to apoptotic signaling in some cell systems (18, 86), as catalase overexpression in either cytoplasmic or mitochondrial compartments potentiated apoptosis, whereas inhibiting endogenous catalase promoted cell survival. Additional studies tied the catalase-induced decrease in hydrogen peroxide with diminished activation of nuclear factor κB (NFκB) survival pathways that are necessary to counteract apoptotic signaling (240). These findings with catalase suggest that excess GPx-1 may potentiate apoptosis as a consequence of the disruption of normal (adaptive) oxidant signaling (i.e., a reductive stress leading to lack of survival) and not by other properties of GPx-1. Among the molecular targets affected by increased expression of GPx-1, it has been shown that GPx-1 may modify the ratio of Bax:Bcl-2 to create a more antiapoptotic environment (120). Also, as discussed above and in the following sections, GPx-1 can regulate apoptogen-mediated signaling in apoptosis (404), alter the activation of NFκB (205, 224), and modulate Akt pathways (154, 351) to affect cellular proliferation and survival, suggesting that GPx-1 may have pleiotropic effects on apoptotic susceptibility. Given the role of hydrogen peroxide in promoting both protective and apoptotic pathways, GPx-1 modulation of intracellular hydrogen peroxide flux will ultimately regulate both apoptotic and survival pathways. The net result of manipulating GPx-1 expression on apoptosis will depend on levels of other intracellular antioxidant enzymes; regulation of oxidant producing enzymes, such as NOXs, and, possibly, the subcellular compartment in which ROS is produced.
3. GPx-1 and response to in vivo ROS
Consistent with increased apoptosis and cell death of isolated cells grown in culture, lack of GPx-1 in vivo sensitizes mice to death in response to the oxidant generators, diquat and paraquat (89, 135, 371). Thus, at doses that wild-type mice typically survive, there is a toxic, fatal response in GPx-1−/− mice within the first 24 h. Superficially, GPx-1-deficient mice appear normal; however, these mice are highly sensitive to oxidant generators. In addition, these mice are highly susceptible to injury after insults that augment in vivo oxidative stress. Thus, lack of GPx-1 enhances cell injury, apoptosis, and cell death in many in vivo models of disease and toxicity. For example, in cold-induced head trauma, brains from GPx-1−/− mice have more apoptotic cell loss than those from wild-type mice (125). Further, GPx-1−/− mice are more susceptible to injury in a cerebral ischemia-reperfusion model of stroke involving mid-cerebral artery (MCA) occlusion (83). In this model, cerebral injury correlated with an increase in oxidative stress markers and accelerated caspase 3 activation. Overall, GPx-1-deficient neurons are especially susceptible to ROS-mediated apoptosis after treatment in cell culture or in vivo after MCA-ischemia-reperfusion. In contrast, GPx-1-overexpressing mice are more protected against neuronal damage after MCA-ischemia-reperfusion than wild-type mice (383). GPx-1 was also found to be protective against traumatic brain injury. In this model, GPx-1 overexpression was found to improve subsequent spatial learning after brain injury in young mice, possibly due to the early reduction in oxidative injury in mice with excess GPx-1 compared to wild-type mice (363). Similarly, the use of ebselen, a GPx mimetic, attenuates cerebral ischemia-reperfusion injury in GPx-1−/−-deficient mice (392). Importantly, ebselen mimics the activities of all the selenium-dependent mammalian GPxs and has other effects on redox status (243, 249). Thus, its protective effects overlap those of GPx-1. Mechanistically, augmented ischemia-reperfusion injury in the absence of GPx-1 may be caused by increased NFκB activation, as pyrrolidine dithiocarbamate (PDTC), an NFκB inhibitor, partially protects against neurotoxicity in this model (82). This observation is interesting, as NFκB activation is considered antiapoptotic and pro-survival, in part, due to its role in augmenting the expression of IAPs, which attenuate caspase activation. PDTC, however, may improve neurotoxicity by other redox-active effects, such as its role as a metal chelator.
It is possible that in the context of GPx-1 deficiency, excess accumulation of cellular ROS alters cellular NFκB responses. In support of this concept, excess intracellular hydrogen peroxide has been shown to regulate differentially the expression of various NFκB component proteins. Alternatively, activation of NFκB in the presence of excess hydrogen peroxide has been shown to enhance the duration and intensity of the NFκB activation. These alterations in the composition or quantity of the NFκB dimer have been found to alter downstream target gene expression, contributing, in some cells, to increased expression of pro-inflammatory genes and a pro-apoptotic environment (170, 197, 275). In addition, intracellular hydrogen peroxide may differentially activate various components of the inhibitor of κB (IκB) kinase (IKK)-complex, as overexpression of GPx-1 in MCF-7 breast cancer cells inhibits ROS-mediated upregulation of IKKα and not IKKβ kinases (224). These findings are consistent with previous studies that implicated GPx-1 modulation of intracellular hydrogen peroxide with modulation of the phosphorylation of IκBα, the NFκB inhibitor, and one of the targets of IKK kinases (205). Phosphorylation of the NFκB inhibitor proteins, IκBα and IκBβ, by IKK kinases causes their subsequent proteasomal degradation and promotes the translocation of NFκB to the nucleus (275).
Other oxidant-dependent injury processes are enhanced in GPx-1-deficient mice. GPx-1−/− mice have enhanced susceptibility to neurotoxins, such as lead and methylmercury (MeHg), and to treatments that mimic damage found in neurodegenerative disease, such as Huntington and Parkinson diseases (37, 93, 119, 200). Thus, GPx-1-deficient mice have been found to be more sensitive to neuronal injury in response to a variety of neurotoxins, including the mitochondrial toxins; malonate and 3-nitropropionic acid, which inhibit succinate dehydrogenase; and methylphenyltetrahydropyridine, which inhibits complex I in the respiratory chain (200). In contrast, GPx-1-overexpressing mice are partially protected from dopaminergic damage in response to 6-hydroxydopamine-induced toxicity (29). Similarly, lentiviral overexpression of GPx-1 confers a beneficial effect to neuroblastoma cells in culture and to nigral dopaminergic neurons exposed to 6-hydroxydopamine in vivo (308). GPx-1 is also protective against amyloid beta peptide (Abeta-toxicity), which promotes intracellular ROS accumulation and has been proposed to have a central role in neurological dysfunction in Alzheimer disease pathology (24, 81). Thus, GPx-1-deficient neurons have enhanced cell death after exposure to Abeta, whereas ebselen or N-acetyl-L-cysteine treatment or overexpression of GPx-1 significantly attenuated Abeta-induced toxicity (200). In addition, GPx-1−/− mice are more susceptible to target tissue damage, such as liver parenchymal cell death, in acute models of inflammatory injury (185); hearing loss, in a noise-induced model (274); cataract formation (302); and enhanced cardiac injury in response to doxorubicin or ischemia-reperfusion (131, 231, 357, 402).
B. Redox-dependent cell signaling, growth, and survival
ROS may play an essential role in receptor activation and downstream signaling (64, 122, 141, 145). In fact, hydrogen peroxide can be generated directly by EGF binding to its cognate receptor (96), and overexpression of intracellular antioxidant enzymes, such as catalase, peroxiredoxins, or GPx-1, have been shown to interfere with cellular proliferation and growth factor-mediated responses by removing hydrogen peroxide essential for normal signaling responses (154, 269, 324). Relevant to understanding the role of GPx-1 in mediating cell signaling, modest (approximately twofold) overexpression of GPx-1 in permanently transfected cells was found to be sufficient to decrease accumulation of intracellular ROS and attenuate EGFR-mediated signal transduction in response to hydrogen peroxide or its cognate ligand, EGF. Further, diminished growth factor signaling under these conditions significantly attenuates cellular proliferation (154). Interestingly, loss of intracellular hydrogen peroxide or lipid hydroperoxides by overexpression of catalase or GPx-4, respectively, inhibits cell cycle progression from G0/G1- to S-phase (278, 377). In catalase-overexpressing cells, these antiproliferative responses were found to be the result of decreased activities in cyclin-dependent kinases (cdk) caused, in part, by the upregulation of Cdk inhibitors p21 and p27 (278). These findings with catalase suggest potential molecular targets that may be modulated by loss of intracellular hydrogen peroxide, although a role for GPx-1 in the regulation of cyclins and cell-cycle progression has not yet been shown.
Theoretically, hydrogen peroxide may modulate signal transduction through the (reversible) oxidation of proteins at redox-active Cyss, including free thiols in tyrosine kinase phosphatases (307). Reversible oxidation of phosphatases may be modulated by GPx-1, at least in some cells. Thus, in skeletal muscle of GPx-1−/− mice following a high-fat diet, insulin augmented phosphatase and tensin homolog deleted on chromosome 10 (PTEN) phosphatase oxidation (235). PTEN is a phosphatase that antagonizes PI3K-mediated signaling; thus, oxidative inactivation of PTEN would promote Akt activation, and, in the skeletal muscle cells, PTEN oxidation corresponded with enhanced Akt phosphorylation. Peroxiredoxin 1 has specifically been shown to protect PTEN from oxidative inactivation and may play an essential role in tumorigenesis by limiting the activation of growth and survival pathways (53). Other factors may, however, modulate ROS-induced Akt activation. Thus, in GPx-1-overexpressing cells grown in culture, excess GPx-1 failed to alter PTEN oxidation; rather, in these cells, attenuated growth factor-mediated Akt activation was attributable to alterations in mitochondrial ROS (154) (Fig. 11), consistent with a role for mitochondrial ROS in growth factor-mediated signaling (50). A role for GPx-1 in regulating mitochondrial oxidant output is also supported by previous studies that found liver mitochondria from GPx-1−/− mice generated more hydrogen peroxide than those from wild-type mice (110). GPx-1 is well suited to regulate oxidants generated from mitochondrial sources or other cellular sources as it localizes to the mitochondria, cytosol, and peroxisomes. GPx-1 may, therefore, diminish proliferative and survival signaling activated in response to cytokines, growth factors, or vasoactive substances that activate ROS-generating NOXs (9). Other antioxidants, such as catalase or peroxiredoxins, may similarly diminish the effects of ROS on proliferative and survival signaling (53, 154).
FIG. 11.
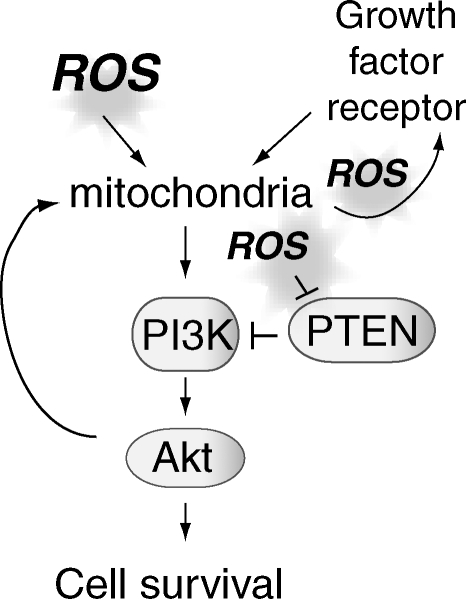
Role of ROS in cell signaling. Cellular ROS is necessary for the cellular responses to growth factor stimulation, the protective responses to excess ROS, and cell proliferation and growth. One of the ways ROS can mediate these pathways is by the oxidative inactivation of phosphatases, such as phosphatase and tensin homolog deleted on chromosome 10 (PTEN), which antagonizes the action of phosphatidylinositol-3-kinase (PI3K). Inactivation of PTEN promotes Akt signaling. In addition, ROS can modulate growth-factor-mediated trans-activation. ROS from mitochondria are essential in these signaling pathways.
Oxidants are also necessary for normal protein folding and disulfide bond formation that regulate the structure and function of many cellular proteins. Mitochondrial oxidants play an essential role in disulfide bond formation, as inhibitors of mitochondrial oxidant production dramatically decrease overall levels of cellular disulfide bonds in many cells, including Chang liver cells and bovine aortic endothelial cells, whereas other oxidant-generating cellular enzymes (such as NOXs) do not affect these pathways (400). Interestingly, GPx-1 overexpression also decreases this oxidant-dependent process in a manner dependent on its mitochondrial effects (154). This is consistent with the findings that overexpression of a mitochondrially targeted catalase decreases overall global disulfide bond formation (400), suggesting that these effects are due to the lack of mitochondrial oxidants and not other effects of GPx-1 overexpression (154). The significance of this reduction in global disulfide bond formations is not completely understood; however, alterations in protein oxidation state can alter the subcellular localization and function of proteins (45, 147, 266, 316). In the context of excess GPx-1, reduction of protein disulfides corresponded with diminished growth factor-mediated signaling and a reduction in mitochondrial function, characterized by a reduction in mitochondrial potential and a decrease in ATP generation (154). Taken together, these findings illustrate a role for GPx-1 in modulating mitochondrial generation of oxidants that regulate redox-dependent processes involved in growth factor signal transduction.
Interestingly, dysfunction caused by lack of GPx-1 also attenuates cell growth in some cell types. For example, fibroblasts isolated from GPx-1−/− mice had an overall slower rate of proliferation with reduced rates of DNA synthesis in response to growth factors and an increase in markers of senescence (90). In addition, isolated neurons from GPx-1−/− mice had significant reductions in the activation of the PI3K-Akt survival pathways after hydrogen peroxide or nerve growth factor stimulation (351). Similarly, the in vivo activation of these survival pathways was diminished after MCA-cerebral ischemia-reperfusion. Lack of Akt activation in these cells suggests that a lack of pro-survival pathways may contribute to the increased rates of cell death and apoptosis in GPx-1-deficient cells. Possibly, in the absence of GPx-1, accumulated oxidants may oxidatively damage proteins involved in signaling to suppress survival responses. Alternatively, some GPx-1-deficient cells may be overwhelmed by oxidants because they lack other partially compensatory antioxidant mechanisms, resulting in heightened sensitivity to ROS and a lack of adequate pro-survival signaling. Although further study is necessary to understand the reason for the lack of growth factor receptor responses in some but not all GPx-1-deficient cells, the end result of these attenuated pro-survival pathways would be decreased cell growth and augmented apoptosis. On the molecular level, Akt activation is part of the cell survival response. Strikingly, loss of Akt activation occurs in some GPx-1-deficient cells as well as in cells with diminished hydrogen peroxide accumulation caused by excess GPx-1. Taken together, these findings suggest a complex relationship among normal ROS flux, heightened ROS generation, and (patho)biological processes that can be regulated, in part, by GPx-1. Thus, excess GPx-1 can promote a reductive stress by limiting oxidant accumulation, whereas lack of GPx-1 can promote oxidative stress (Fig. 2). Consequently, either extreme may upset the crucial redox balance needed for normal functional phenotypes in cells: too much or too little ROS will interfere with cell growth and survival.
V. GPx-1 and Cancer
There are many studies linking GPx-1 to cancer development and risk. In epidemiological studies, low levels of dietary selenium correlate with increased risk of cancer (75, 184, 411). Owing to the selenoprotein hierarchy, GPx-1 is one of the selenoproteins most readily affected by selenium levels. Thus, several studies show decreased cancer risk after supplemental selenium intake (73); however, the beneficial effects of selenium supplementation have not been observed in all individuals, nor in all studies (233), and, in some studies, protection was only found among subjects who began in the bottom tertile of plasma selenium (75). In the latest prostate cancer prevention trial, selenium and vitamin E cancer prevention trial (SELECT), selenium supplementation had no effect on cancer risk (233). One possible explanation for these mixed effects of selenium supplementation is that different nutritional forms of selenium supplements were used in the recent negative study compared to previous studies with positive outcomes (163). Another possibility is that initial plasma selenium levels were higher in the SELECT population compared with previous studies. Relevant to a discussion of GPx-1 in cancer, evidence from experimental mouse models suggests that, at least for some cancers, selenium is protective, in part, through GPx-1, although expression of other selenoproteins will be modulated by a range of dietary Se concentrations (98, 182). Supplemental Se may also promote inhibition of cell growth and apoptosis by multiple mechanisms, including the enhanced accumulation of Se-metabolites that could be toxic to cells (75, 303). Antiproliferative effects of Se may not be limited to the accumulation of harmful Se-metabolites; as discussed above, excess antioxidant enzyme expression can similarly inhibit cell growth (154) and cause apoptosis under some circumstances, as well (18, 86). Another line of reasoning linking GPx-1 to cancer-causing mechanisms is its role in regulating cellular oxidants. Thus, GPx-1 may limit oxidant-induced cell mutagenesis and inflammatory responses that promote certain cancers. Loss of GPx-1 in early stages of carcinogenesis may contribute to cancer initiation, and, in later stages of cancer, GPx-1 deficiency may promote proliferative responses. In contrast, excess GPx-1 may prevent oxidative damage (such as DNA-oxidation) and inflammation, but may also block apoptotic cell death, leading to enhanced survival of transformed cells. Thus, GPx-1 has a complex effect on the development and progression of cancer due, in part, to its role modulating intracellular ROS (46). These findings are discussed further below, along with a summary of genetic studies that have analyzed the effects of GPx-1 gene polymorphisms on cancer risk in human subjects.
A. GPx-1 and the mechanisms of cancer susceptibility
GPx-1 has been implicated in both pro- and anticarcinogenic mechanisms in different experimental model systems. It has been suggested that decreased expression of GPx-1 may influence cancer susceptibility and development. Although its expression is not suppressed in all cancers, many cancers have reductions in GPx-1 expression (85, 140). Further, in cultured cells, manipulation of GPx-1 expression specifically alters the rate of UV-induced mutagenesis. Thus, selenium supplementation and GPx-1 overexpression were found to reduce DNA damage, as measured by micronuclei formation, and enhance cell survival in response to UV-irradiation (22), whereas knockdown of GPx-1 was found to increase UV-induced damage (21). These findings are consistent with in vivo studies using a transgenic mouse with a mutant tRNAsec to assess the role of selenoproteins in cancer development. In vivo, this mutant tRNAsec decreases the expression of selenoproteins with the greatest suppression found in the expression of GPx-1 (261). Concurrent with reductions in expression of GPx-1 (and other selenoproteins), these mice show enhanced susceptibility to precancerous changes in a model of prostate cancer and to preneoplastic alterations indicative of colon cancer susceptibility in another model system (98, 182).
Mice deficient solely in GPx-1 do not develop cancer; however, mice deficient in both GPx-1 and GPx-2 (double knockout mice) have increased bacterial-induced cancer of the colon and intestine that may be caused by increased inflammatory responses in these tissues (72). In fact, mice deficient in both of these GPxs (GPx-1−/−GPx-2−/−) spontaneously develop tumors. A small percentage of mice with only one copy of the GPx-1 allele and complete deficiency of GPx-2 develop mild ileocolitis (GPx-1+/−GPx-2−/−), whereas a single copy of the GPx-2 gene (GPx-1−/−GPx-2+/−) protects against inflammation, leading to the suggestion that GPx-2 has a greater protective effect in the intestine than GPx-1 (114), although an earlier study reported that one copy of either of these GPx genes was sufficient to prevent the development of intestinal cancer (72). Given the high degree of expression of GPx-2 in the colon and its overlapping activities with GPx-1, deletion of both GPxs is apparently necessary to develop robust inflammatory responses in the colon (111) that may promote neoplastic changes in this model (72). Subsequent studies have established that this overall deficiency in colonic GPx-1 and -2 results in higher DNA mutation rates in ileum and colonic epithelial cells; increased rates of epithelial cell proliferation, as measured by bromodeoxyuridine (BrdU) incorporation; and increased apoptosis in these tissues (212). Taken together, these findings support a role for GPx-1 (and GPx-2) in protecting against neoplastic transformation in intestinal cells.
As suggested by GPx-1's effect on cell growth and apoptosis reviewed in section IV, GPx-1 overexpression may have an antitumorogenic effect in some cancers theoretically by limiting oxidants (234), whereas it may allow for the survival of transformed cells in other cancers by limiting apoptotic mechanisms (238). Thus, GPx-1 overexpression was found to reduce the growth of some cancer cell lines in culture and decrease their tumorigencity after their injection into nude mice (234). In contrast, in a model of skin carcinogenesis, GPx-1-overexpressing mice have an accelerated pattern of lesion development, have more tumors per mouse, and have tumors with increased growth rates, as measured by BrdU labeling (238). Subsequent studies of this chemically induced skin cancer model implicate inflammation and apoptosis as crucial components modulating tumor development (198), and, in many studies, GPx-1 has been shown to attenuate both inflammation and apoptosis, suggesting that these effects may contribute to tumor development in some models. These and other data discussed above suggest that the overall redox state of the cell influences whether excess GPx-1 is pro- or antisurvival.
B. GPx-1 and genetic polymorphisms
The human GPx-1 gene localizes to human chromosome 3p21, a region that has been associated with loss of heterozygosity (LOH), a process that involves deletion of a chromosomal region from one of two autosomes leading to the detection of only one allelic variant in individuals who are (in all other tissues) heterozygous. Involvement of the GPx-1 gene locus in LOH events has been shown for cancers of the lung, breast, head and neck, and colon (159, 174–176, 260). Importantly, tumors with LOH have lower measurable GPx-1 enzyme activity and increased accumulation of 8-hydroxydeoxyguanosine, a pro-mutagenic DNA-oxidation product, suggesting that LOH results in oxidative stress in these tumors. The GPx-1 gene is not the only gene deleted in these LOH events, and a DNA repair gene, hOGG1, which may also repair oxidized DNA, is often deleted together with GPx-1. It is unclear whether loss of GPx-1 is causative or whether it occurs secondary to cellular transformation to promote subsequent tumor growth (presumably by enhanced hydrogen peroxide-mediated growth factor responses).
Overall, 38 single-nucleotide polymorphisms (SNPs) have been reported for the GPx-1 gene; most of these SNPs are in the 5′ and 3′ flanking region (134). Of the polymorphisms that alter the protein coding region, two major polymorphic sites of the GPx-1 gene are commonly studied due to their frequency and resulting alterations in the amino acid sequence of GPx-1 (Fig. 12). One of the polymorphisms involves a variable number of “GCG” tri-nucleotide repeats in the first exon of the human GPx-1 gene that results in five, six, or seven alanines near the N-terminus. The other common polymorphism involves a Leu substitution in place of the more common Pro at amino acid 198 due to a substitution of T (codon, CTC) for a C (CCC). Although one study reported an effect for the Ala repeats in modulating GPx-1 expression in response to selenium (412), most studies have analyzed the consequences of the Leu/Pro polymorphism on expression and activity. The Leu/Pro polymorphism (at SNP rs1050450, commonly referred to as Pro198Leu) shows an interesting gene–environment effect in that these allelic forms differ in their expression in response to selenium. Thus, in studies comparing the expression of recombinant forms of GPx-1 in breast cancer cells, the Leu allele was found to be less responsive to increased selenium levels than the Pro allele (175). This interaction between genotype and selenium was also verified in cardiomyocytes where selenium was found to have a diminished effect on stimulating the expression of the Leu variant (216). Further, in cardiomyocytes, the decreased GPx-1 activity of cells carrying the Leu variant was proposed to contribute to their increased apoptosis under serum restriction. These findings may be relevant to explaining the contribution of the Leu polymorphism to the development of a cardiomyopathy (Keshan disease) associated with regional selenium deficiency in China (see also below) (216). Similarly, reduced activity of the Leu allele compared to the Pro allele was reported in transfected bovine aortic endothelial cells, suggesting that differences between these polymorphic forms may affect GPx-1 activity in many different cell types (152).
FIG. 12.
GPx-1 gene polymorphisms. There are two common sets of polymorphisms in the GPx-1 protein coding region. One is at the N-terminal region and involves the presence of five, six, or seven in-frame GCG repeats that result in 5–7 Ala residues. The presence of seven Ala repeats may reduce the expression of the protein in response to selenium. Similarly, the single-nucleotide polymorphism at amino acid 198 involves a T for C change that results in a Leu-containing polypeptide (Leu encoded by CTC) with reduced expression in response to selenium compared to the Pro-containing form. The noncoding polymorphisms at −602 upstream of the start site and +2 from the start site in linkage disequilibrium: these pairs of linked changes apparently affect promoter activity in a reporter gene assay.
The effect of genotype on GPx-1 activity in vivo, however, may depend on many factors, including levels of selenium in the diet. Thus, although selenium can modulate GPx-1 expression in vivo, at a certain level of plasma selenium, the effects of this trace element on GPx-1 expression plateau (268). Nonetheless, the association of GPx-1 polymorphisms (specifically the Pro198Leu) with GPx-1 activity has been assessed in isolated blood cells from human subjects. In a study composed primarily of young, healthy, college-aged Caucasian and Asian/Pacific Islanders in the United States, an interaction between gender and genotype was found. Males with the TT genotype (homozygous for Leu variant) were reported to have the lowest GPx-1 activity (25), whereas genotype had no affect on GPx-1 activity level in females in this study. Although selenium was not measured in this study, adequate nutritional intake of selenium could potentially mask other differences between genotypes in this population. In a separate analysis of GPx-1 activity in red blood cells from young adult smokers and nonsmokers in Denmark, selenium levels were measured and found to be lower than that needed for optimal GPx-1 activity (244). In this population, GPx-1 activity was lower in males than in females, and smoking influenced GPx-1 activity. In contrast with the study from the United States, the effects of genotype on activity were significant in females rather than in males. Thus, in females from the Danish study, the highest levels of activity were associated with Pro homozygotes, and the lowest levels were found in Leu homozygotes. No interactions were found between GPx-1 genotypes and selenium levels in this population, although in males, the lowest quartile of serum selenium associated with the lowest level of GPx-1 activity by unadjusted linear regression analysis.
Other studies have reported correlations between genotype and activity (157, 301) or between selenium and GPx-1 activity (285, 355). Additionally, one study of over 400 human subjects found different regression profiles for selenium and GPx-1 activity for each genotype: the correlation of GPx-1 activity with plasma selenium concentrations was higher for the Pro/Pro genotype than the Leu/Leu genotype, providing evidence that GPx-1 genotypes can modulate the expression of GPx-1 in response to selenium in vivo (183).
In addition to the genetic polymorphisms that alter protein expression, other SNPs were found to influence GPx-1 promoter function (152). Thus, in a study of genetic variants in the GPx-1 locus in a Japanese population, a total of three major haplotypes were identified that involve two SNPs in transcriptional regulatory regions in combination with the Ala repeat and codon 198 polymorphism (152). Of the three haplotypes, the upstream genetic alterations that were in linkage disequilibrium with the Leu allele were also found to have the lowest level of activity in a transcription reporter gene assay, causing a 25% suppression in promoter activity (152). Taken together, these findings suggest a genetic basis for differences in GPx-1 gene expression, although many of these findings have not been replicated in other studies.
Taken together, these findings suggest that GPx-1 activity may be modulated in vivo by many interacting factors, including genotype, sex, smoking, and dietary selenium. The parameters affecting GPx-1 activity are even more complicated, as illustrated by findings from the SELGEN study. The purpose of SELGEN was to identify genetic polymorphisms in selenoprotein P (SelP) that may modulate in vivo selenium usage. Selenoprotein P is known to play a role in the delivery of selenium to cells throughout the body. Subsequently, in this analysis, an association was found between polymorphisms in SelP and the activity of Sec-containing proteins, including lymphocyte GPx-1 (252). Thus, these findings highlight the complex factors contributing to in vivo GPx-1 activity.
C. GPx-1: genetic polymorphisms and cancer risk
As discussed above, the association of GPx-1 gene polymorphisms with GPx-1 activity is not straightforward, as nutritional, environmental, and genetic factors can influence the expression and activity of this crucial antioxidant enzyme. Similarly, the biological effects of GPx-1 may depend on cell type and cellular redox-status and the regulation of other antioxidant enzymes and ROS-producing enzymes that are expressed (Fig. 1). As may be anticipated from these complex interactions, there is no consensus regarding an association of GPx-1 polymorphisms with cancer risk or predicted survival. Early studies pointed to a role for the Pro198Leu variant (i.e., Leu allele) in cancer risk; however, subsequent studies did not confirm this finding and other studies found that the Leu variant was protective rather than disease-associated (see Table 1 and discussion below for details). Some differences regarding which allele confers risk may be attributable to comparisons among studies of different cancers, and may reflect underlying differences in carcinogenic mechanisms and tissue-specific expression of other modifying factors, including other GPxs. Inconsistent results, however, were also found among studies of the same cancer. For example. in lung cancer, the presence of the Leu allele has been associated with increased risk (300) in one population and with protection in other populations (296, 310) (Table 1). Interestingly, within the same study on lung cancer risk, the effect of the Pro/Pro genotype in conferring risk or protection differed according to smoking status, but only in those diagnosed with cancer at an older age (399). These findings illustrate how nutritional and lifestyle differences may confound any genetic epidemiological analysis. In addition to these environmental factors, other population differences, such as linkage disequilibrium of the GPx-1 locus with other specific modifying genes, the influence of other gene variants that act to modulate GPx-1 activity [such as the SelP subtypes mentioned above (252)], and/or the presence of other gene variants that act in concert with GPx-1 to modify disease risk may also explain differences among studies performed in different populations. There is some evidence to support the latter concept that other gene variants can modulate the effects of GPx-1 polymorphisms, as two studies (one in breast cancer and one in bladder cancer) find significant risk associated with a combination of GPx-1 (Leu variant) and MnSOD (Ala variant) genotypes (80, 179). The MnSOD gene polymorphism (Val16Ala, also referred to as Val9Ala in some studies) results in an Ala variant at position 9 in the mature protein (position 16 in the precursor protein) that increases the efficiency at which MnSOD is transported into the mitochondria, theoretically promoting hydrogen peroxide generation in this organelle (381). Thus, a functional overexpression of MnSOD, caused by presence of the Ala variant, combined with a potential deficiency of GPx-1, caused by the Leu variant, could potentially create a redox imbalance leading to enhanced hydrogen peroxide flux. Further research is necessary to test whether a combination of these genetic polymorphisms alters intracellular ROS accumulation to explain the molecular basis for the gene–gene interactions. Overall, these findings suggest that additional analysis is necessary to understand the modifiers of GPx-1 expression and activity and the role of GPx-1 in modulating cellular growth and transformation. These also suggest the necessity for performing large studies, analyzing multiple genes in addition to GPx-1, and collecting detailed information on environmental and lifestyle factors to assess the true, likely complex role of GPx-1 in modifying cancer disease risk.
Table 1.
Glutathione Peroxidase-1 Polymorphisms and Cancer: Positive Association Studies with the Pro198Leu Varianta
| Cancer population subset | Cases | Controls | Genotypeb | ORc | 95% confidence interval | Comments | Reference |
|---|---|---|---|---|---|---|---|
| Breast | |||||||
| African American | 79 | 517 | Leu/Leu | 1.9 | 1.01–3.58 | Compared genotypes from cancer tissue (cases) to population-based lymphocytes (controls) | 175 |
| US | 1038 | 1088 | nsd | Long Island Breast Cancer Study Project, population case–control, overall ns. | 4 | ||
| Parity | Pro/Leu | 1.48 | 0.99–2.23 | Subgroup comparison null parous to parous significant with variant gene copy number effect | |||
| Leu/Leu | 2.12 | 1.01–4.48 | |||||
| Danes | 377 | 377 | Pro/Leu + Leu/Leu | 1.43 | 1.07–1.92 | Diet, cancer, health prospective case–control combination one or two variant genes | 301 |
| US Combined with MnSOD genotype | 1262 | 1533 | ns. | 1.09–3.19 | Nurses Heatlth Study, nested case–control | 79, 80 | |
| Leu/Leu + MnSOD Ala | 1.87 | Follow up gene X gene interaction study | |||||
| Leu/Leu combined with variant MnSODAla allele confers risk | |||||||
| Lung | |||||||
| Finlanders | 315 | 315 | Pro/Leu | 1.8 | 1.2–2.8 | α-Tocopherol, β-Carotene Cancer Prevention Study, nested case–control | 300 |
| Leu/Leu | 2.3 | 1.3–3.8 | |||||
| US elderly | 67 | 69 | ns | Age diagnosis >80 years, not significant overall. Also not significant in younger patients. | 399 | ||
| Smokers | Pro/Pro | 3.3 | 1.3–8.4 | Subgroup analysis of smokers vs. nonsmokers same genotype had opposite effects on risk | |||
| Nonsmokers | Pro/Pro | 0.12 | 0.02–0.7 | ||||
| Korean | 200 | 200 | Pro/Leu + Leu/Leu | 2.29 | 1.44–3.62 | 211 | |
| Danes | 432 | 798 | Pro/Leu | 0.75e | 0.54–1.04 | Diet, Cancer, Health Study nested case–control | 296 |
| Leu/Leu | 0.6e | 0.35–1.05 | |||||
| German | 246 | 223 | Pro/Leu + Leu/Leu | 0.6 | 0.4–0.8 | Case–control, Age of diagnosis < 51; subgroup heavy smokers Leu variant more protection (OR = 0.3, 0.1–0.8) | 310 |
| Prostate | |||||||
| Macedonians | 82 | 123 | Leu/Pro | 0.38 | 0.2–0.75 | 14 | |
| Bladder | |||||||
| Japanese Combined with MnSOD | 213 | 209 | Pro/Leu | 2.63 | 1.07–6.18 | No Leu/Leu genotypes were found in this population | 179 |
| Pro/Leu +MnSOD Ala | 6.3 | 1.28–31.24 | Possible gene-gene interaction with MnSOD Ala enhances risk of in individuals carrying a variant Leu198 | ||||
| US | 224 | Analyzed recurrence in individuals with bladder cancer, overall ns. |
407 | ||||
| Caucasian | Pro/Leu +Leu/Leu | 0.63f | 0.42–0.96 | Subgroup analysis of Caucasians showed variant allele was protective | |||
| Colorectal | |||||||
| Danes | 375 | 779 | ns | Overall no significant association was found. Evidence for lifestyle modifiers: | 157 | ||
| Drinkers | Leu/Leu | 1.45 | 1.17–1.81 | Subgroup with 10 g alcohol per day | |||
| Smokers | Leu/Leu | 2.56 | 0.99–6.61 | Subgroup smokers | |||
These studies and negative studies are discussed further in the text.
Glutathione peroxidase-1 Pro198Leu genotype associated with risk or protection is given, along with MnSOD genotype in applicable gene–gene interaction studies.
OR are provided as given in the reference, unless otherwise noted by dns, indicating not significant overall; erelative risk or fhazard ratio. “ns” studies are included because of positive subgroup analysis, which are also listed.
MnSOD, manganese superoxide dismutase; OR, odds ratio.
1. Breast cancer
Initial studies of breast cancer risk associated with GPx-1 Pro198Leu polymorphisms reported a higher frequency of the Leu/Leu genotype in breast cancer tissues from African American women than the Pro/Pro genotype compared to the distribution of these genotypes in normal cancer free individuals (175). This study, however, compared genotypes taken from cancerous tissue to those found in lymphocytes from cancer-free individuals; thus, other factors, such as LOH, may contribute to the increased frequency of Leu/Leu in tumors compared to normal cells. Overall, there are inconsistent results regarding the association between the Leu allele and breast cancer risk. In support of a role for the GPx-1 polymorphisms in breast cancer risk, a nested case–control study from Denmark found that Leu carriers (i.e heterozygous, Leu/Pro, plus homozygous, Leu/Leu) had significantly increased risk of breast cancer compared to Pro/Pro homozygotes (301). Other studies, however, failed to find an association between the GPx-1 Pro198Leu polymorphisms and risk of breast cancer, including one study in a Canadian population (201), one from the United Kingdom (SEARCH) (55) and two studies from the United States (4, 79), including a study from the large prospective Nurses Health Study and a large case–control study, the Long Island Breast Cancer Study (79). Interestingly, although the Long Island Breast Cancer Study yielded an overall negative result, in a subgroup analysis from this study, nulliparous women homozygous for the Leu allele had significant increased risk compared to homozygous Pro parous women. Not surprisingly, a meta-analysis combining the outcomes of these major studies failed to find an overall significant association between the Leu allele and breast cancer risk (173). A subgroup analysis from the meta-analysis, however, found that the Leu allele was significantly associated with risk in African Americans, using either an additive (odds ratio = 1.91, 95% confidence interval [CI]: 1.02–3.58) or recessive (odds ratio = 2.09, 95% CI: 1.16–3.76) model, suggesting the need for additional studies, as the numbers of cases in this subgroup were small (173). Similar to the lack of overall breast cancer risk with GPx-1 genotype, no significant association was found between GPx-1 genetic variations, including the Pro198Leu, and prognosis in individuals with breast cancer in a large case–control study involving SEARCH participants (366). In contrast, as mentioned above in a follow-up nested case–control study from the Nurses Health Study, it was reported that a combination of a polymorphism in the MnSOD gene together with the Leu/Leu genotype in the GPx-1 gene, confers a significant increased risk of breast cancer (80).
2. Lung cancer
Tobacco smoke is a known risk factor for lung cancer development, and antioxidant enzymes, such as GPx-1, may play essential roles in eliminating pro-carcinogenic oxidants caused by smoke inhalation. Similar to breast cancer, lung cancer is also known to have LOH and reduced expression of GPx-1, suggesting that decreased intracellular GPx-1 activity may contribute to malignant transformation. In a Finish study of male smokers, there was a significant association of the Leu allele with increased risk of lung cancer (300). Consistent with a role of the Leu allele in lung cancer risk, a case–control study of lung cancer from Korea found that individuals with at least one Leu allele had increased levels of urinary 8-hydroxydeoxyguanosine, the mutagenized DNA oxidation product compared to Pro/Pro (211), as well as a greater risk for lung cancer. However, not all studies report increased risk with the Leu allele. In fact, in a study from Denmark, there was a trend for a protective effect for the variant (Leu) allele in lung cancer cases compared to controls (296), and, similarly, other studies have found a protective effect for the Leu allele (144, 399). In a study of genetic polymorphisms in lung cancer in young (average age diagnosis 42.9 ± 5.5) versus old (average age diagnosis 83.2 ± 2.1) subjects from the Mayo Clinic in the United States, there was a distinct difference in the risk allele in these age groups (399). Thus, in a comparison of 165 cases and 170 controls for early onset cancer, there was no significant effect of the Pro/Pro genotype on lung cancer risk in smokers or those who never smoked. In contrast, in the older age group, comparing 69 cases to 67 controls, the Pro/Pro genotype conferred significant risk for disease in smokers but had a protective effect in those who never smoked. The different mechanisms in lung cancer development in older versus younger individuals may explain some of these findings; however, in Germany, a risk reduction was found in carriers of the Leu-allele in young lung cancer patients (age of onset <50 years) (144). This latter study did not examine risk in the elderly; however, these findings are not entirely consistent with the U.S. study that found no association of the Pro198Leu polymorphism with lung cancer risk in the young. There is no single explanation for these inconsistencies in the at-risk allele from these various studies. Instead, it has been suggested that modifiers of GPx-1 activity and expression, such as nutritional effects (dietary selenium), other gene variants (regulating GSH levels, for example, or those in linkage disequilibrium with the 198 polymorphism in different populations), or other lifestyle differences (fitness, alcohol consumption), may modulate the effects of GPx-1 polymorphism in disease risk (144). One possibility not analyzed in any of these studies is the significance of GPx-3 or GPx-2 polymorphisms. Both GPx-3 and GPx-2 are also expressed in lung, and, at least in mouse models, GPx-2 was found to be upregulated in response to cigarette smoke. Thus, these other GPxs may also be crucial in lung protection.
3. Prostate cancer
Epidemiological studies suggest an inverse correlation between selenium and prostate cancer risk; however, as mentioned above, the most recent prevention trial (SELECT) has not found the same protective effects as earlier studies. Importantly, earlier studies found the greatest beneficial effect of selenium supplementation in individuals with the lowest plasma selenium levels (103). Regarding any protective effect of selenium, there is not always a correlation between GPx-1 activity and plasma selenium concentrations. Thus, in a study in normal, healthy individuals with adequate selenium levels (347) [that is, all individuals had plasma selenium concentrations above the threshold values that modulate in vivo circulating GPx activity (268)], plasma selenium concentrations were found to correlate with prostate selenium levels, but neither of these selenium measurements correlated with prostate GPx-1 activity. Further analysis of these parameters in individuals with low selenium levels, in combination with genotype analysis, may provide a better understanding of the prostate-specific modulation of GPx-1 activity.
Similar to other cancer association studies, there have been inconsistent results regarding the association of GPx-1 polymorphisms with prostate cancer risk. One published report on GPx-1 and risk of prostate cancer found a protective effect for the Leu/Pro genotype compared to Pro/Pro genotype in subjects from Macedonia. In this study, overall erythrocyte GPx-1 activity was also significantly lower in subjects with prostate cancer compared with controls, although there was no effect of the GPx-1 polymorphism on GPx-1 activity (14). One study that analyzed prostate cancer risk and the GCG copy number (Ala) polymorphism in a group of young-onset prostate cancer patients (202) also found no significant association between Ala copy number and prostate cancer risk. Similarly, in a separate analysis of the Pro198Leu polymorphisms and prostate cancer risk in heavy smokers, no associated risk was found for any GPx-1 genotype (70). Failure to find consistent association with GPx-1 in prostate cancer may be attributable to linkage disequilibrium with other disease SNPs, differences among populations, including environmental factors, and other factors that regulate prostate GPx-1 levels.
4. Bladder cancer
In a small case–control study from Poland comparing 33 bladder cancer cases to 47 controls, no associations were found with GPx-1 genotype; however, in this study, transcript levels of several selenoprotein genes, including GPx-1, were found to be downregulated in the leukocytes of these cancer patients compared to those from controls (306). The significance of this finding is not clear, but could relate to alterations in leukocyte populations and cancer-related immune responses that may warrant further analysis in patients with bladder and other cancers. Another larger study from Japan, comparing 213 cases with 209 controls, found that the presence of the Leu allele was associated with an increased risk of bladder cancer (179). Interestingly, the Pro/Leu genotype was also significantly associated with more advanced stages of disease, and, further, the risk was greater in the presence of a MnSOD Ala9 variant (179). A previous study of recurrence of bladder cancer from the United States followed recurrence rates over 36 months in 224 bladder cancer patients after tumor resection and treatment for superficial bladder cancer (407). This study found an opposite effect in the at-risk allele, in that individuals with the Pro/Pro genotype had reduced overall cancer-free survival compared with those carrying the Leu allele. These opposite findings as to the at-risk allele in disease risk and recurrence may either relate to different mechanisms of cancer initiation versus recurrence, or could be the result of other genetic and lifestyle differences in the groups assessed.
5. Other cancers
Several studies find no association of GPx-1 polymorphisms with risk for other cancers such as basal-cell carcinoma (374) or colorectal cancer, as reported from the Prostate, Lung, Colorectal and Ovarian Trial in the United States in which a nested analysis of over 700 cases and controls was performed (289). These negative findings are consistent with those from Norwegian and Danish populations (156, 157). In contrast, interactions of GPx-1 genotypes with lifestyle factors may combine to create increased risks for some cancers. For example, an interesting genotype–environment effect was reported in the nested case–control study from Denmark in that alcohol consumption and smoking modulated colorectal cancer risk in Leu/Leu homozygous individuals (157). In this study, risk was found to be higher in Leu/Leu homozygotes according to their alcohol consumption or among homozygous Leu/Leu smokers. This study also found a significant effect of gender, diet, smoking, and alcohol on GPx-1 activity by univariate analysis (157), consistent with a role of these factors in modulating GPx-1. Other studies similarly report that the GPx-1 genotype may interact with environmental factors to modify the effects of lead exposure on adult brain tumors, such as glioblastoma and meningioma (37). Another study reported that antioxidant gene profile may predict carcinoma development secondary to cirrhosis (344): in patients with alcohol-induced cirrhosis, individuals with at least one Ala variant for MnSOD and at least one Leu variant for GPx-1 had a hazard ratio of 2.0 (95% CI: 1.311–3.052).
VI. GPx-1, Diabetes, and Cardiovascular Disease
Oxidative, and, in some cases reductive, stress have a role in the pathogenesis of various forms of cardiovascular disease. This section will review experimental clinical and genetic studies analyzing the contribution of GPx-1 to diabetes, angiogenesis, endothelial dysfunction, atherogenesis, and cardiac dysfunction (a discussion of GPx-1 in stroke and neurotoxicity may be found in section IV.A.3., above.)
A. GPx-1 and the mechanisms of susceptibility to diabetes and cardiovascular disease
1. Diabetes mellitus
One of the unexpected consequences of GPx-1 overexpression in mice is their development of insulin resistance, hyperinsulinemia, and obesity, which attenuates normal insulin-mediated Akt signaling (251). Loss of cellular ROS most likely causes this phenotype, as excess GPx-1 and lack of cellular ROS similarly attenuates growth-factor-mediated responses in cell culture models (154). In contrast, knockdown of GPx-1 in cells grown in culture augments receptor-mediated activation, theoretically by increasing oxidant-mediated responses (154). In mice, GPx-1 deficiency protects against high-fat-induced insulin resistance, with enhanced insulin-mediated ROS production, increased insulin-mediated Akt signaling, and preserved glucose uptake in muscle (235). These findings suggest that under some circumstances, decreased expression of GPx-1 and enhanced production of ROS (and hydrogen peroxide in particular) may be beneficial (235).
The concept that excess GPx-1 may lead to development of a insulin resistance phenotype, whereas GPx-1 deficiency may prevent this phenotype, is surprising, as oxidative mechanisms are thought to play a crucial role in the development of insulin resistance, a common feature in type 2 diabetes and the metabolic syndrome. One common mechanism that may connect insulin resistance in GPx-1-overexpressing mouse and oxidative stress-mediated mechanisms of insulin resistance is loss of normal mitochondrial oxidative phosphorylation and decreased production of ATP. In the case of GPx-1 overexpression, this phenotype results, in part, from decreased cellular ROS that suppresses mitochondrial function and blunts growth-factor-mediated signaling (154). Although there are some studies to the contrary, decreased mitochondrial function and/or content are associated with insulin resistance in animal models as well as in human subjects (44, 171, 194, 365).
In many cases, insulin resistance also correlates with increases in mitochondrial ROS generation, possibly due to excess glucose or fatty acid substrates. Thus, in high-fat-induced insulin resistance, excess ROS emanating from mitochondria contribute to insulin insensitivity in skeletal muscle, as overexpression of a mitochondrially targeted catalase in skeletal muscle or acute treatments with antioxidants improve insulin-mediated glucose uptake (7). In contrast, GPx-1-deficient mice on a high-fat diet are spared from insulin resistance, and antioxidants make them less sensitive to insulin (235). Perhaps the protective nature of GPx-1 deficiency in the context of the high-fat diet has to do with overall energy expenditure and resistance to obesity in these mice, which is caused, in part, by their increased sensitivity to growth factor-mediated signaling (235), which may subsequently alter the efficiency of mitochondrial substrate oxidation. Although the findings from the GPx-1-deficient and GPx-1-overexpressing mice provide complementary data regarding insulin sensitivity and insensitivity, these findings are counterintuitive with the concept that oxidative stress plays a causative role in insulin resistance. Possibly, the deleterious or protective effects of excess oxidants on metabolic regulation may depend on multiple factors, including their local concentrations, time course and sites of production, and duration of their accumulation.
Interestingly, targeted overexpression of GPx-1 solely in pancreatic β-cells is protective against diabetes in some animal models (160). Specifically, overexpression of GPx-1 in pancreatic β-cells (under the regulation of the insulin promoter) protects these cells from streptozotocin-induced diabetes by preserving islets and reducing hyperglycemia. Streptozotocin normally induces diabetes by selectively destroying β-cells through a process dependent, in part, on oxidative stress in the β-cells, which lack high levels of endogenous antioxidant enzymes. Further, in the context of db/db mice, targeted overexpression of GPx-1 reduces pancreatic β-cell loss and attenuates hyperglycemia (160). Similarly, previous studies suggested a protective role for excess antioxidant enzymes in limiting ROS damage to β-cells in mouse islet grafts (265). It should be mentioned that GPx-1 transgenic overexpressing mice, which express GPx-1 under its native promoter, also have increased β-cell mass. In this context, their enhanced glucose-stimulated insulin secretion has been suggested to contribute to chronic hyperinsulinemia (382). Of course, in this global overexpression model, the lack of receptor-mediated responses in skeletal muscles and liver also contributes to the overall phenotype in these mice (i.e., hyperinsulinemia, hyperglycemia, and obesity). Nonetheless, these findings suggest the utility of further studies to understand the role of excess GPx-1 in modulating β-cell homeostasis. Thus, although it seems that GPx-1 overexpression is protective in β-cells, which are highly susceptible to oxidant-mediated cell death, in other cells, such as skeletal muscle and liver, excess GPx-1 can be detrimental by suppressing essential ROS necessary for signal transduction, contributing to an insulin-resistant phenotype.
2. Cardiac dysfunction and toxicity
Similar to the findings with insulin resistance, evidence exists for a negative effect of reductive stress in cardiac tissue, characterized by excess GSH, NAD(P)H, reduced ROS-accumulation, and upregulation of antioxidant enzymes. Specifically, reductive stress was found to contribute to cardiomyopathy in response to an accumulation of a mutant form of the αβ-crystallin protein in transgenic αβ-crystallin mutant mice. Subsequent upregulation of heat shock protein (Hsp)25 in these mutant mice contributed to the reductive phenotype, which was characterized by increased levels of cardiac NADPH and GSH, and excess expression of glucose-6-phosphate dehydrogenase (G6PD) and the antioxidant enzymes catalase and GPx-1 (297). Although ROS were not measured in these mice, excess G6PD has previously been shown to decrease effectively intracellular ROS accumulation (218). Crosses between these αβ-crystallin mutant mice and G6PD-deficient mice reduced NADPH production and rescued the cardiomyopathy, confirming the idea that the disease pathology was caused by reductive stress. In a subsequent study, cardiac overexpression of a different Hsp, Hsp27, similarly caused reductive stress with elevated GSH, increased GSH/GSSG ratio and increased cardiac GPx-1 protein and activity that contributed to cardiomyopathy (405). Importantly, in this latter study it was shown that these redox alterations correlated with diminished cardiac ROS accumulation. Accordingly, treatment with mercaptosuccinate, an inhibitor of GPx-1, improved cardiac function and decreased heart weight in the transgenic Hsp27 mice. Although the molecular mechanisms by which reductive stress leads to cardiomyopathy are not yet understood, these studies confirm that shifting the normal cellular redox balance in either direction can be detrimental.
A link between selenium deficiency and heart disease has been recognized for some time, as selenium deficiency is associated with Keshan disease, an endemic cardiomyopathy that is found in regions of China with low soil selenium and is characterized by mitochondrial insufficiency and reductions in GPx-1 activity (66, 138). Notably, selenium supplementation has substantially lessened the incidence of disease in these regions; however, there may also be an infectious component to the disease, possibly due to Coxsackie virus infection. Studies in mouse models confirm that selenium deficiency enhances the sensitivity to developing myocarditis when infected with virulent strains of Coxsackie. There may also be a role for other selenoproteins that are suppressed during selenium restriction in the pathogenesis of myocarditis and cardiomyopathy. Other studies, however, suggest that GPx-1-deficient mice developed myocarditis in response to avirulent strains of Coxsackie virus that were found to mutate readily in the GPx-1-deficient hosts, similar to the findings in selenium deficiency (26, 220). Taken together, these studies suggest that GPx-1 may protect against virally induced cardiac inflammation, which may be a possible contributor to cardiomyopathy in Keshan disease.
Similarly, GPx-1 is protective against agents that mediate cardiotoxicity by augmenting mitochondrial generation of oxidants. Thus, doxorubicin, an anthracycline antibiotic and antitumor drug, has cardiotoxic side effects that are believed to involve superoxide generation (47) by mechanisms associated with disruption of mitochondrial respiration. In cells grown in culture, excess GPx-1 specifically decreased doxorubicin-induced NFκB-activation and apoptosis (380). Similarly, in mouse models, cardiac-specific GPx-1 overexpression protects against doxorubicin-induced cardiac dysfunction, specifically attenuating mitochondrial dysfunction and impairing contractile function (397). In contrast, lack of GPx-1, in GPx-1−/− mice, potentiates doxorubicin-induced cardiac injury leading to increased impairment of contractility and diastolic function, deficiencies in coronary blood flow, and suppressed heart rate. These functional cardiac changes correspond with enhanced apoptosis in this model and suggest a crucial role of GPx-1 in modulating cardiomyocyte oxidative toxicity.
3. Ischemia/reperfusion injury, angiogenesis, and EPC function
Other studies suggest that GPx-1 preserves cardiac function after ischemia/reperfusion injury. In support of this idea, older mice with heterozygous gene knockout of GPx-1 (GPx-1+/−) were reported to have structural abnormalities of the myocardial vasculature, including changes consistent with augmented fibrosis and intimal thickening. These structural changes may contribute to the diastolic dysfunction that develops after myocardial ischemia-reperfusion injury (131). Subsequent analysis suggests that hearts from male but not female GPx-1-deficient mice are susceptible to decreased myocardial recovery after ischemia-reperfusion, due, in part, to substantial differences in intracellular thiols, levels of reduced ascorbate, and nitrate/nitrite concentrations in GPx-1−/− females after ischemia-reperfusion injury. These findings suggest that females, but not males, compensate for the loss of GPx-1 by upregulation of other antioxidant systems. Previous studies have reported sex-specific differences in hydrogen peroxide, GSH, and GPx-1 levels, with females having the more protective phenotype (lower peroxide, higher GSH, and increased GPx-1) (43, 186). These findings may partially explain the lower rates of ischemic heart disease in premenopausal women.
Mechanistically, reduced recovery in GPx-1−/− mice after cardiac ischemia-reperfusion may be a result of increased mitochondrial injury caused by excess oxidants. Thus, in a study that only examined male mice in a model of hypoxia reperfusion injury, GPx-1−/− mice had augmented mitochondrial oxidant production, increased mitochondrial DNA damage, and decreased expression of mitochondrial proteins, leading to a reduction in NADH and ATP (357). Similar to previous studies of liver mitochondria (110), baseline production of hydrogen peroxide was also augmented in mitochondria from GPx-1-deficient hearts. These data suggest a protective role for GPx-1 in modulating mitochondrially generated oxidants at baseline and during hypoxia and reoxygenation. In support of these findings, GPx-1 deficiency was found to increase hypoxia-induced ROS accumulation in mouse pulmonary artery smooth muscle cells, whereas excess GPx-1 or catalase decreased ROS accumulation during hypoxia (378).
GPx-1 deficiency appears to attenuate neovascularization in a model of hindlimb ischemia by mechanisms that suggest enhanced sensitivity of EPCs to oxidant-mediated cell death. The defect in EPC function is characterized by a lack of in vivo proliferative response to ischemic injury or to direct vascular endothelial growth factor injections in GPx-1−/− mice (139). Thus, in response to hindlimb ischemia, GPx-1−/− mice have significantly decreased recovery of hindlimb blood flow over 28 days with apparent reductions in CD31-positive cells in the ischemic tissue, indicative of reduced capillary density. EPC from GPx-1−/− mice also show decreased ability to migrate or form angiogenic networks compared to EPCs from wild-type mice. In addition, GPx-1-deficient cells are more sensitive to ROS, as they accumulate more intracellular oxidants and have enhanced apoptosis in response to hydrogen peroxide compared to wild-type EPCs. It has previously been proposed that EPCs are subject to high levels of oxidative stress accounting for the relatively high levels of antioxidant enzymes in these cells compared to endothelial cells (95, 165). Thus, lack of GPx-1 in EPCs may leave them susceptible to oxidative stress-induced apoptosis. Similarly, muscle progenitor cells from GPx-1−/− mice also have decreased survival responses with deficiencies in proliferation, impairments in differentiation, and augmented rates of apoptosis (213). Interestingly, a recent study found differences in the apoptotic rates of human EPCs isolated from older subjects (average age 72 years) compared to younger subjects (average age 24 years) after their exposure to hydrogen peroxide (164). Overall, EPCs from older subjects had reduced survival and increased apoptosis to hydrogen peroxide stress, possibly due to the substantial decrease of GPx-1 activity and protein in these EPCs compared with EPCs from younger subjects. Thus, these findings suggest a role of enhanced oxidative stress in EPCs that correlates with the age-related decrease in GPx-1 expression. These findings are of interest because loss of EPC function may promote vascular dysfunction and diseases of aging, such as atherosclerosis (167, 313).
4. Endothelial dysfunction and vascular tone
Endothelial cells play a crucial role in regulating vascular homeostasis, in part, through their production of NO· (281). Under normal physiological conditions, NO· is formed in these cells after activation of the endothelial NOS (eNOS) by mechanical stress or agonists such as bradykinin and acetylcholine (124). Nitric oxide mediates many of the actions of the endothelium, including stimulation of sGC, which, in the vascular smooth muscle cells, promotes relaxation (94). Nitric oxide also attenuates platelet activation and smooth muscle cell growth, and inhibits cytokine-mediated adhesion molecule expression. Thus, normal production of NO· is antithrombotic and antiatherogenic (372). Oxidative stress can neutralize these protective actions by promoting endothelial dysfunction.
Endothelial dysfunction implies a loss of normal endothelial function characterized by a decrease in bioavailable NO· and a loss in normal endothelium-dependent vasorelaxation responses (161). Mechanistically, NO· may become inactivated in response to ROS by the diffusion-limited reaction of NO· with superoxide to form peroxynitrite, a highly reactive RNS that further enhances oxidant production; modifies DNA, proteins, and lipids; and has other cytotoxic effects. ROS, including hydrogen peroxide, can also contribute to endothelial dysfunction by a number of mechanisms that may ultimately influence bioavailable NO·. Thus, excess hydrogen peroxide can augment superoxide production by stimulating NOX activation (226). Additionally, ROS can inactivate enzymes such as dihydrofolate reductase that are necessary to generate eNOS cofactors, leading to eNOS uncoupling (and superoxide generation) (57). Alternatively, hydrogen peroxide may cause an increase in the intracellular labile iron pool, which promotes a loss of bioavailable NO · through transition metal-dependent redox inactivation (354). Importantly, endothelial dysfunction is considered a marker for increased cardiovascular risk (51, 84).
Owing to the antioxidant actions of GPx-1 in removing intracellular hydrogen peroxide, GPx-1 plays an essential role in preserving endothelial function and NO · bioavailability. In mice, GPx-1 deficiency (as is found in homozygous GPx-1−/− or heterozygous GPx-1+/− knockout mice) alters vascular responses to agents that normally elicit endothelium-dependent vasorelaxation (131, 132). Thus, unlike the vasodilatory response of normal mesenteric vessels, vessels from GPx-1-deficient mice contract in response to bradykinin or acetylcholine. Responses to sodium nitroprusside, an NO· generator, are, however, preserved in GPx-1-deficient mice. These findings suggest that vascular smooth muscle cells are capable of generating cGMP in response to NO· by activation of sGC, but that endothelium-dependent responses are deficient, due to loss of endothelial generated NO· needed to activate sGC. In support of this concept, isolated aortic rings from deficient mice have a significant reduction in acetylcholine-induced cGMP generation (132) and other studies have reported similar deficiencies in relaxation for carotid arteries from GPx-1−/− mice (71). Further, in studies of spontaneous hypertensive rats compared to normotensive control rats, endothelial dysfunction was similarly associated with decreased expression of GPx-1 and other antioxidant enzymes. Importantly, in this model, there was no detectable change in the expression or activation of eNOS (367), suggesting a crucial role for antioxidant enzymes, such as GPx-1, in modulating NO · bioavailability. In the context of increased ROS and preserved eNOS expression, as in the GPx-1-deficient mice, correction of the redox imbalance is sufficient to restore normal agonist-induced production of cGMP and in vivo vasoactive responses in GPx-1-deficient mice (131, 132). In contrast, when eNOS expression is directly downregulated, overexpression of GPx-1 is not sufficient to correct endothelial dysfunction (187).
GPx-1 may also play a role in modulating the effects of hyperhomocysteinemia on endothelial function and cardiovascular risk. Modest increases in homocysteine can reduce GPx-1 expression in cell culture as well as in in vivo models (384) due to decreased translation of GPx-1 transcripts (155). Thus, in heterozygous cystathionine-beta-synthase knockout mice, hyperhomocysteinemia was found to lead to endothelial dysfunction (106), which could be corrected by overexpression of GPx-1 to increase bioavailable NO and restore normal endothelial function (384). Similarly, in other studies, endothelial function in aortae from GPx-1−/− mice was diminished by hyperhomocysteinemia (88). Although there is controversy regarding the importance of modest elevations in homocysteine to cardiovascular risk (10), in clinical studies, homocysteine and GPx-1 activity measurements have been found to be predictors of cardiovascular risk in coronary artery disease patients (314). Further, in this population, subjects with the lowest GPx-1 activity and highest homocysteine had a nearly threefold increased risk for cardiovascular events, whereas homocysteine had no influence on risk on those with the highest levels of GPx-1 activity (314).
Additional studies suggest that GPx-1 protects against vascular dysfunction in response to ROS-inducing agents, such as angiotensin II (AII) (71). AII is a bioactive product of the renin-angiotensin pathway that plays a major role in promoting vascular remodeling, inflammation, and tissue organ damage found in hypertension, atherosclerosis, and diabetes (166). In vascular cells, AII can stimulate ROS through the activation of NOX, leading to a reduction in bioavailable NO and subsequent endothelial dysfunction in mouse models and in human subjects (8, 209, 245, 376). Thus, consistent with a beneficial role of GPx-1 in preserving endothelial function, GPx-1 deficiency was found to augment endothelial dysfunction in response to AII in carotid arteries of heterozygous GPx-1+/− mice, whereas catalase treatment or GPx-1 overexpression decreased the harmful effects of AII on endothelium-dependent relaxation (71). These findings suggest that modest alterations in GPx-1 expression can significantly alter endothelial function by modulating the accumulation of intracellular oxidants in response to AII. This observation may be significant for human subjects, as well, as GPx-1 activity was inversely correlated with endothelium-dependent vasodilation in a recent study measuring acetylcholine-induced vasodilation in human hypertensive subjects (92).
By affecting blood pressure, vascular remodeling, and left ventricular hypertrophy, AII promotes cardiac dysfunction. GPx-1 appears to be protective against these deleterious effects of this vasoactive peptide. Thus, by echocardiography, hearts from GPx-1−/− and wild-type mice are not substantially different at baseline; however, after 7 days of systemic AII infusion, GPx-1-deficient hearts have increased left ventricular hypertrophy and dysfunction (13). Interestingly, there were no differences in vascular parameters after short-term AII administration: AII exposure raised blood pressure in GPx-1−/− mice to a similar extent as in the wild-type mice, and there were no apparent differences in vascular remodeling between these treated groups.
Overall, GPx-1 deficiency contributes to endothelial dysfunction, whereas GPx-1 overexpression is protective, presumably by modulating hydrogen peroxide in endothelial cells to preserve NO. Paradoxically, arachidonic acid mediates vascular tone by producing hydrogen peroxide in some vascular beds (101, 276). In support of a role for hydrogen peroxide in mediating arachidonic acid-induced vasodilation, exogenous catalase blocked ∼50% of maximal dilatory response to arachidonic acid in the basilar cerebral arterioles in an in vivo model of vasoactivity (258). In addition, similar attenuated responses to arachidonic acid-induced vasorelaxation were found in cerebral vessels from GPx-1-overexpressing mice compared to control mice. Further, excess GPx-1 attenuated direct hydrogen peroxide-mediated vasodilation (using 10 micromolar hydrogen peroxide) in isolated vessels analyzed in vitro. Taken together, these data are another example of reductive stress characterized by loss of oxidants that are essential for a biological response. In support of the significance of these pathways, hydrogen peroxide has been shown to cause relaxation of preconstricted vessels from a variety of vascular beds by endothelium-dependent and endothelium-independent mechanisms (12). Overall, the effect of hydrogen peroxide on the vasculature may depend on a myriad of factors, such as the source of hydrogen peroxide, the levels of hydrogen peroxide, the status of K+ channels, eNOS-activation state, and the specific vascular bed.
5. Inflammation and atherogenesis
Owing to the importance of oxidative stress to inflammatory pathways (317), several studies have analyzed atherogenic susceptibility in GPx-1-deficient mice. Modest atherogenic lesions can be induced in the aortic sinus of C57Bl/6 mice fed a high-fat diet over 20 weeks. Surprisingly, GPx-1−/− mice on a C57Bl/6 background showed decreased development of lesions compared with wild-type C57Bl/6 mice fed this atherogenic diet (91). This unexpected effect may be due to protective lipid profiles in the GPx-1−/− mice compared to the wild-type mice. Thus, at the end of the 20 weeks of high-fat diet, GPx-1−/− mice had decreased triacylglycerol and higher high-density lipoprotein than the wild-type control mice. Further, GPx-1−/− mice fed a high-fat diet had a compensatory increase in the expression of glutaredoxin-2 that was not found in control mice after dietary treatment. Glutaredoxin-2 may play a significant role in preserving thiol function by modulating reversible protein thiol-glutathionylation in the mitochondria and nucleus (27, 188), and its actions may serve to limit oxidative damage and decrease apoptosis (230). In contrast with this study, deficiency of GPx-1 has been shown to augment atherosclerosis in susceptible mice with combined apolipoprotein E (ApoE)/GPx-1 deficiency (ApoE−/−/GPx-1−/−) in two independent studies, one that used a Western diet and one that induced diabetes with streptozotocin to promote atherogenic lesion development (221, 360). Unlike the lesions in C57Bl/6 mice fed a high-fat diet, in the context of ApoE deficiency, robust atherosclerotic lesions develop throughout the aorta over time on a normal chow diet; notably, a Western diet or diabetes accelerates lesion development. Thus, in the context of ApoE deficiency, GPx-1 deficiency caused a significant increase in atherosclerotic lesion area and augmented markers associated with oxidative stress (221, 360). In addition, macrophages from ApoE−/−/GPx-1−/− mice had increased proliferative responses compared with those from ApoE−/− mice and, in the context of streptozotocin-induced diabetes, ApoE−/−/GPx-1−/− mice had increased expression of pro-inflammatory and pro-fibrotic markers compared with diabetic ApoE−/− mice. Taken together, these findings suggest that GPx-1 deficiency promotes inflammatory responses that augment atherogenesis in susceptible models. In support of a role for GPx-1 in protecting against atherogenesis, subsequent studies showed that ebselen treatment substantially reduced total aortic lesions in diabetic ApoE−/− mice (67). Interestingly, although aortic lesion area was reduced with this treatment, lesion development within the aortic sinus region was not improved by ebselen. Oxidative stress markers were decreased in both the aorta and sinus region by ebselen treatment, as measured by decreased expression of NOX subunits and decreased nitrotyrosine staining. In contrast, pro-atherogenic markers, such as the receptor for advanced glycation end-products, were reduced in the aorta but not in the aortic sinus. These findings are consistent with those using the antioxidant probucol as an antiatherogenic treatment: probucol also had a regional effect on attenuating lesion formation, which was attributed, in part, to regional hemodynamic factors (391). It is important to note that ebselen also mimics the activity of other GPxs, including the phospholipid GPx-4 (243), and that overexpression of GPx-4 can also slow the progression of atherogenesis in ApoE−/− mice possibly due to its effects in reducing the accumulation of oxidized phospholipids (149). Nonetheless, the effects of ebselen are consistent with the enhancement of atherosclerosis promoted by GPx-1 deficiency in ApoE−/− mice.
Other studies also support a beneficial role of GPx-1 in protecting against pro-atherogenic alterations in the vasculature. One recent study suggested that upregulation of GPx-1 expression in response to biomechanical forces in the vessel wall attenuates pro-atherogenic gene expression in human and mouse endothelial cells. Thus, knockdown of GPx-1 expression in these cells resulted in an augmented expression of the vascular cell adhesion molecule-1 and monocyte chemoattractant protein-1 after cyclic stretch (375). These findings suggest a crucial role for GPx-1 in modulating pro-inflammatory responses. Similarly, in human endothelial cells, GPx-1 was found to be protective against endotoxin-induced expression of inflammatory mediators, such as vascular cell adhesion molecule-1 and intercellular adhesion molecule-1, in part, by mechanisms that regulated intracellular ROS and the expression of CD14 (239). These findings also suggest a role for GPx-1 in modulating innate immune responses by modulating endothelial levels of CD14, an essential factor for Toll-like receptor 4 activation by endotoxin.
GPx-1 activity may similarly modulate atherogenic susceptibility in human subjects. In patients with suspected coronary artery disease, erythrocyte GPx-1 activity was found to correlate inversely with the extent of atherosclerosis and, in this population, cardiovascular event rates were inversely associated with GPx-1 activity with a hazard ratio of 2.3 (95% CI: 1.4–4.0) for the lowest compared with the highest tertile of GPx-1 (109). Further, risk is greatest in individuals with the lowest GPx-1 activity and greatest extent of atherosclerosis (109). These findings suggest that GPx-1 can modify vascular risk in the context of atherogenesis.
B. Epidemiologic and genetic studies of GPx-1 and cardiovascular disease
Several studies implicate GPx-1 as a factor contributing to cardiovascular disease risk in human subjects. In the AtheroGene prospective study of 636 patients with coronary heart disease, GPx-1 activity was one of the strongest univariate predictors of future cardiovascular events, and the risk of future cardiovascular events was inversely related to erythrocyte GPx-1 activity levels (40). In particular, GPx-1 was found to be protective, with individuals in the top quartile of GPx-1 activity almost threefold less likely to have an event than those with the lowest levels of GPx-1. In a follow-up study, individuals with high GPx-1 activity were found to be more protected from the deleterious effects of elevated homocysteine (314). Further, it was found that those with the lowest levels of GPx-1 and the most extensive atherosclerosis were at the greatest risk for cardiovascular events (109). In support of the importance of GPx-1 (and other antioxidant enzymes) in preventing cardiovascular disease, a meta-analysis that examined 42 case–control and three prospective studies found that increased cellular GPx, SOD, and catalase activity levels are all protective against coronary heart disease. (Although this study used the generic term “GPx,” many of these studies assayed GPx activity from erythrocytes, platelets, or lymphocytes, cell sources that would mostly express GPx-1 and not the related GPx-2 nor the extracellular GPx-3. Thus, in these lysates, GPx-1 activity would be the predominant determinant of assayed activity, although some assays may also detect GPx-4 activity as well.) Overall, modest increases in “GPx” activity correlated with a pooled odds ratio of 0.51 (95% CI: 0.35–0.75) (130).
Other studies in human subjects examined whether there is a genetic component modulating the effects of GPx-1 on cardiovascular disease risk. In the case of Keshan disease, a cardiomyopathy primarily due to the dietary deficiency of selenium, presence of the GPx-1 Leu variant (Pro198Leu) was found to associate with selenium deficiency and impaired GPx-1 activity (216). As discussed above, theoretically, the Leu variant may result in decreased GPx-1 activity, especially under inadequate selenium conditions. Not all studies, however, found this association between genotype and activity in samples from human subjects, and the significance of these polymorphic forms to in vivo effects is not totally understood. Nonetheless, unlike the studies of genetic risk and cancer, many of the studies of cardiovascular risk and GPx-1 genotype report the Leu allele to be associated with increased disease risk (Table 2).
Table 2.
Association of Glutathione Peroxidase-1 Polymorphisms with Cardiovascular Diseasea
| Disease population subgroup | Cases | Controls | Genotype | OR | 95% confidence interval | Comments | Reference |
|---|---|---|---|---|---|---|---|
| Coronary artery disease | |||||||
| Chinese | 256 | 265 | Pro/Leu + Leu/Leu | 2.02 | 1.27–3.22 | Overall | 350 |
| < 64 Years | Pro/Leu + Leu/Leu | 2.41 | 1.16–4.98 | Stratified by age | |||
| Males | Pro/Leu + Leu/Leu | 1.86 | 1.09–3.18 | Within males | |||
| Nonsmokers | Pro/Leu + Leu/Leu | 2.40 | 1.15–5.01 | Nonsmokers | |||
| Coronary artery calcification | |||||||
| Japanese type 2 diabetics | 11 | 80 | Pro/Leu | 3.61 | 0.97–13.42 | Case–control, assessment of calcification score in 91 subjects with type 2 diabetes | 267 |
| Coronary artery restenosis | |||||||
| Japanese coronary stent patients | 107 | 354 | Pro/Leu + Leu/Leu | 1.9 | 1.0–3.4 | Consecutive bare-metal stent implants; quantitative coronary angiogram at 6 months of follow-up | 272 |
| Metabolic syndrome | |||||||
| Japanese | 312 | 1871 | nsb | Cross-sectional population study, overall ns. | 208 | ||
| Males | Pro/Leu + Leu/Leu | 2.02 | 1.3–3.15 | Subgroup analysis of males only, risk associated with variant allele. | |||
| Aortic aneurysm | |||||||
| Japanese hypertensives | 88 | 1243 | Pro/Leu + Leu/Leu | 0.34 | 0.12–0.78 | Protective effect of variant allele. | 192 |
Shown are studies with an association between glutathione peroxidase-1 genotype and cardiovascular disease risk.
ns, not significant in overall group. NS studies are included because of positive subgroup analysis, which are also listed.
Owing to the importance of oxidative stress in the development and pathobiology of type 2 diabetes mellitus and the metabolic syndrome, many studies have performed association analyses for polymorphisms in antioxidant genes and the associated cardiovascular consequences of these common disorders. In one of these studies in Japan, the GPx-1 Leu variant was found to associate with macrovascular disease, including increased intima-media thickness of the carotid arteries, coronary heart disease, and peripheral vascular disease in a small cohort of type 2 diabetics in Japan (152). Similarly, in another small study of 91 type 2 diabetic subjects in Japan, coronary artery calcification showed a trend of association with the Pro/Leu genotype (267). In a larger, cross-sectional analysis of 1128 males and 1105 females in a Japanese population, the Leu variant was associated with the metabolic syndrome in men but not in women (208). Similarly, in other cardiovascular studies, the Leu variant was significantly associated with an increased risk for restenosis after stenting in a Japapanese study (272) and increased risk of coronary artery disease in a case–control study from China (350). The molecular basis for the Leu variants' effect on disease risk are unclear, but may relate to differences in GPx-1 expression levels, which were not tested in these populations, or other functional differences in the regulation or function of the Leu variant not yet recognized.
In contrast to the above studies associating cardiovascular risk with the Leu variant of GPx-1, in a study of thoracic aortic aneurysm (TAA) in 1351 hypertensive patients, the Leu variant was associated with reduced risk of TAA (192). Similarly, in a study analyzing the genetic components of longevity, the Leu allele conferred protection in the oldest old (over 92 years of age) (335). Additionally, in these old subjects, the Leu allele was highly synergistic with the MnSOD Val6Ala variant that has theoretically more activity in the mitochondria. Thus, in this aged population, the combination of GPx-1 Leu and MnSOD Ala significantly reduced mortality (HR = 0.76, 95% CI: 0.647–0.894) (335). Although there may be other functional differences between the GPx-1 Pro and Leu alleles that are as of yet unknown, it is possible that excess oxidants may be beneficial under some circumstances.
VII. GPx-1 and Future Directions for Therapeutic Applications
Although overall GPx-1 can be considered protective against oxidative stress, it is important to consider that changes in redox balance in either direction, oxidative or reductive, may also influence the protective or harmful roles of GPx-1. Under some circumstances, enhanced expression of GPx-1 may promote reductive stress by removing essential oxidants resulting in detrimental physiological effects, such as diminished growth factor-mediated signaling (which may contribute to insulin resistance), decreased cellular proliferation, and, in some cases, enhanced apoptosis. In addition, as discussed in the previous section, excess GPx-1 may contribute to some forms of cardiomyopathies (Fig. 13). Other than these cardiomyopathies that have been caused by genetic manipulation of mice, physiological or pathological conditions that contribute to upregulation of GPx-1 to the extent that it causes reductive stress are unclear and most likely will depend on the overall redox state. In lower eurkaryotic organisms, reductive stress has been suggested to reduce lifespan and alter protein folding (299, 329, 362), as antioxidant treatments decreased lifespan in nematodes (318). Thus, the consequences of excess GPx-1 should be considered in any therapeutic scheme. Nonetheless, therapeutically, the most likely successful strategies would be those that target GPx-1 overexpression to modulate excess oxidative stress associated with disease pathobiology. Selenium supplementation is clearly of consideration in this regard. In fact, as has been shown in human populations with lower concentrations of plasma selenium (i.e., below the threshold for saturation of GPx-1 activity), supplemental selenium can augment GPx-1 expression (and possibly that of other selenoproteins). This was recently shown for coronary artery disease patients, in that 12 weeks of supplemental sodium selenite increased GPx-1 activity in a dose-dependent manner (315). The protective nature of this supplementation, however, was unclear from this short-term treatment, as there were no apparent improvements in either flow-mediated endothelial function or other biomarkers of oxidative stress after this short-term treatment. One consideration regarding supplementation is whether the form of selenium administered may affect efficacy (163). Thus, SELECT, a trial that found no protective anticancer effect of selenium supplementation, used selenium in the form of purified L-selenomethionine, whereas previous studies that suggested protective effects of selenium in reducing risk of prostate cancer used selenite or selenium-enriched baker's yeast as sources of supplemental selenium (73, 163, 233). Of course, selenium treatment may also upregulate other selenoproteins and may have other nonselenoprotein effects. GPx-1, however, is one of the selenoproteins most likely to be affected by selenium supplementation or restriction; therefore, this antioxidant enzyme may play an essential role in any physiological effect of selenium supplementation. In Keshan disease, for example, addition of selenium in table salt essentially eliminates the incidence of this cardiomyopathy and increases GPx-1 activity (66).
FIG. 13.
Protective and harmful effects of GPx. This diagram illustrates some of the protective effects (top half) and harmful actions (bottom half) of GPx-1 that are mediated by decreasing cellular ROS. Thus, elimination of excess ROS can protect against apoptotic cell loss or injury during ischemia-reperfusion (including coronary and neuronal); protect against cell toxicity to drugs (preserving neurons, cardiomyocytes, and other cells); and protect cells and susceptible cells against high ROS, including endothelial progenitor cells (EPCs), and β-cells in pancreatic islets. In addition, removal of excess ROS can preserve bioavailable NO, preserving vascular function, which also decreases thrombosis and is antiatherogenic. GPx-1 has also been shown to protect against inflammatory stimuli that may promote a proatherogenic state and foster carcinogenesis. In addition, GPx-1 prevents DNA mutagenesis, which also decreases carcinogenic potential. Harmful effects of GPx-1. Note that many of the harmful effects of GPx-1 occur under conditions of excess GPx-1, which may be induced by physiological conditions such as mechanical stress in the vasculature (375) or under pathological conditions such as in certain cardiomyopathies (297, 405). Overall, by reducing excess oxidants, GPx-1 may allow for tumor survival and growth. Further, removal of essential oxidants can create a reductive stress characterized by loss of normal physiological responses, including reduced vascular responses to hydrogen peroxide and arachidonic acid; decreased mitochondrial function and growth factor (GF) signaling that can cause insulin insensitivity and decreased cell proliferation; and development of cardiomyopathies by mechanisms not completely understood but presumed to involve a reduction in essential levels of hydrogen peroxide.
Similarly, antioxidant thiols such as N-acetyl-L-cysteine have been shown to be beneficial against inflammation, apoptosis, and ischemia-reperfusion injury (N-acetyl-L-cysteine is commonly given as a therapy for liver toxicity caused by acetaminophen overdose and its corrective effects are partially due to a regeneration of liver GSH stores). Thus, along with its properties as an antioxidant, N-acetyl-L-cysteine will also increase GSH, possibly preserving or enhancing GPx-1 function.
A more targeted way to eliminate hydrogen peroxide is through the use of small molecule GPx-1 mimics, such as the selenium-containing ebselen. Mechanistically, ebselen is thought to detoxify hydrogen peroxide, lipid, and phospholipid hydroperoxides, the latter substrates for GPx-4, by utilizing small molecular thiols like GSH as a cofactor (243). Ebselen may also mediate its beneficial effects, in part, by reducing peroxynitrite (249, 325). In addition, it may bind directly to protein thiols, possibly affecting their function (87). Although there is an active field of research into developing more potent GPx-1 mimics, ebselen has been successfully used to reduce damaging oxidants in several models involving oxidative injury. Ebselen has been shown to prevent noise-induced hearing loss (241), lessen atherosclerosis (67), and reduce neurotoxicity in a variety of experimental animal models (81, 392) in which GPx-1 deficiency was previously shown to have the opposite effect. Clinically, ebselen has also been shown to improve neurological outcomes after ischemic stroke in human subjects (398); however, to date, the utility of this compound to treat human disease is not clear. Further, ebselen mimics the activities of all the selenium-dependent mammalian GPxs and has other effects on redox status. Thus, its protective effects overlap those of GPx-1. Nonetheless, similar therapies to augment GPx-like activity could potentially improve outcomes in a variety of disease pathologies that involve oxidative stress. In addition, notwithstanding current issues with vector-based therapies, gene-targeting therapies to overexpress GPx-1 may similarly provide future mechanisms by which to reduce oxidative stress and lessen tissue damage in certain pathological disease states. However, additional analysis is needed to develop further and test the in vivo utility of any GPx-specific treatments.
Much has been learned about GPx-1 redox biology since its discovery as a crucial antioxidant enzyme that inactivates peroxides. Since then, GPx-1 has been studied for its role in cancer susceptibility and prevention, and as a protective agent in neurological and cardiovascular diseases. Importantly, GPx-1 has many cellular functions: to protect cells from oxidative damage; to regulate metabolism and mitochondrial function; and to control cellular processes, such as apoptosis, growth, and signaling by modulating intracellular levels of hydrogen peroxide and the overall intracellular redox balance. In fact, the complex effects of GPx-1 in biological systems appear to stem from the delicate balance between intracellular oxidants and antioxidants and the deleterious effects of shifting the balance too far in either direction, which can result in an oxidative or reductive stress. The findings to date suggest the usefulness of further studies into the mechanisms regulating the expression and function of this crucial antioxidant enzyme, as well as the need for future studies to understand better the mechanisms by which GPx-1 contributes to health and disease.
Abbreviations Used
- Abeta
amyloid beta peptide
- AIF
apoptosis inducing factor
- AII
angiotensin II
- AMG
aminoglycoside
- AP-1
activator protein 1
- ApoE
apolipoprotein E
- ARE
antioxidant response element
- Bid
Bcl-2 interacting domain
- CD95L
ligand for CD95 receptor
- cGMP
cyclic guanosine monophosphate
- Cu, ZnSOD
copper, zinc superoxide dismutase
- Cys
cysteine
- DHA
dehydroalanine
- ECSOD
extracellular superoxide dismutase
- eEFsec
selenocysteine elongation factor
- EGFR
epidermal growth factor receptor
- eNOS
endothelial nitric oxide synthase
- EPC
endothelial progenitor cell
- ERK1/2
extracellular signal-related kinases 1 and 2
- FLIP
FLICE-inhibitory protein
- GPx
glutathione peroxidase
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- Hsp
heat shock protein
- IκB
inhibitor of κB
- IκK
IκB kinase
- JNK
Jun-amino terminal (stress-activated) kinase
- LOH
loss of heterozygosity
- MAPK
mitogen-activated protein kinase
- MCA
mid-cerebral artery
- MnSOD
manganese superoxide dismutase
- NFκB
nuclear factor κB
- NMD
nonsense-mediated decay
- NO ·
nitric oxide
- NOX
NADPH oxidase
- ORE
oxygen response element
- PGC
peroxisome proliferators-activated receptor-γ coactivator
- PI3K
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SBP2
SECIS binding protein-2
- Sec
selenocysteine
- SELECT
selenium and vitamin E cancer prevention trial
- sGC
soluble guanylate cyclase
- SNP
single-nucleotide polymorphism
- SOD
superoxide dismutase
- tRNAsec
specific Sec tRNA
- UAG
nucleotide sequence of amber stop codon in RNA, TAG in DNA
- UGA
nucleotide sequence of opal stop codon in non selenocysteine encoding RNA, Sec codon in selenocysteine transcripts, TGA in DNA
- UTR
untranslated region
Acknowledgments
The authors wish to thank Stephanie Tribuna for her expert editorial assistance. This work was supported by Deutsche Forschungsgemeinschaft grant LU 1452/1-1, and LU 1452/2-1 (E.L.). This work was also supported by NIH grants HL61795, HL081587, and HL089734 (J.L. and D.E.H.).
References
- 1.Abate C. Patel L. Rauscher FJ., 3rd Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990;249:1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- 2.Adlam VJ. Harrison JC. Porteous CM. James AM. Smith RA. Murphy MP. Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 3.Ahamed M. Siddiqui MK. Environmental lead toxicity and nutritional factors. Clin Nutr. 2007;26:400–408. doi: 10.1016/j.clnu.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Ahn J. Gammon MD. Santella RM. Gaudet MM. Britton JA. Teitelbaum SL. Terry MB. Neugut AI. Ambrosone CB. No association between glutathione peroxidase Pro198Leu polymorphism and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:2459–2461. doi: 10.1158/1055-9965.EPI-05-0459. [DOI] [PubMed] [Google Scholar]
- 5.Akasaka M. Mizoguchi J. Takahashi K. A human cDNA sequence of a novel glutathione peroxidase-related protein. Nucleic Acids Res. 1990;18:4619. doi: 10.1093/nar/18.15.4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alfthan G. Xu GL. Tan WH. Aro A. Wu J. Yang YX. Liang WS. Xue WL. Kong LH. Selenium supplementation of children in a selenium-deficient area in China: blood selenium levels and glutathione peroxidase activities. Biol Trace Elem Res. 2000;73:113–125. doi: 10.1385/BTER:73:2:113. [DOI] [PubMed] [Google Scholar]
- 7.Anderson EJ. Lustig ME. Boyle KE. Woodlief TL. Kane DA. Lin CT. Price JW., 3rd Kang L. Rabinovitch PS. Szeto HH. Houmard JA. Cortright RN. Wasserman DH. Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119:573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson TJ. Elstein E. Haber H. Charbonneau F. Comparative study of ACE-inhibition, angiotensin II antagonism, and calcium channel blockade on flow-mediated vasodilation in patients with coronary disease (BANFF study) J Am Coll Cardiol. 2000;35:60–66. doi: 10.1016/s0735-1097(99)00537-9. [DOI] [PubMed] [Google Scholar]
- 9.Anilkumar N. Weber R. Zhang M. Brewer A. Shah AM. Nox4 and nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arterioscler Thromb Vasc Biol. 2008;28:1347–1354. doi: 10.1161/ATVBAHA.108.164277. [DOI] [PubMed] [Google Scholar]
- 10.Antoniades C. Antonopoulos AS. Tousoulis D. Marinou K. Stefanadis C. Homocysteine and coronary atherosclerosis: from folate fortification to the recent clinical trials. Eur Heart J. 2009;30:6–15. doi: 10.1093/eurheartj/ehn515. [DOI] [PubMed] [Google Scholar]
- 11.Antunes F. Han D. Cadenas E. Relative contributions of heart mitochondria glutathione peroxidase and catalase to H(2)O(2) detoxification in in vivo conditions. Free Radic Biol Med. 2002;33:1260–1267. doi: 10.1016/s0891-5849(02)01016-x. [DOI] [PubMed] [Google Scholar]
- 12.Ardanaz N. Pagano PJ. Hydrogen peroxide as a paracrine vascular mediator: regulation and signaling leading to dysfunction. Exp Biol Med (Maywood) 2006;231:237–251. doi: 10.1177/153537020623100302. [DOI] [PubMed] [Google Scholar]
- 13.Ardanaz N. Yang XP. Cifuentes ME. Haurani MJ. Jackson KW. Liao TD. Carretero OA. Pagano PJ. Lack of glutathione peroxidase 1 accelerates cardiac-specific hypertrophy and dysfunction in angiotensin II hypertension. Hypertension. 2010;55:116–123. doi: 10.1161/HYPERTENSIONAHA.109.135715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arsova-Sarafinovska Z. Matevska N. Eken A. Petrovski D. Banev S. Dzikova S. Georgiev V. Sikole A. Erdem O. Sayal A. Aydin A. Dimovski AJ. Glutathione peroxidase 1 (GPX1) genetic polymorphism, erythrocyte GPX activity, and prostate cancer risk. Int Urol Nephrol. 2009;41:63–70. doi: 10.1007/s11255-008-9407-y. [DOI] [PubMed] [Google Scholar]
- 15.Asahi M. Fujii J. Suzuki K. Seo HG. Kuzuya T. Hori M. Tada M. Fujii S. Taniguchi N. Inactivation of glutathione peroxidase by nitric oxide. Implication for cytotoxicity. J Biol Chem. 1995;270:21035–21039. doi: 10.1074/jbc.270.36.21035. [DOI] [PubMed] [Google Scholar]
- 16.Asahi M. Fujii J. Takao T. Kuzuya T. Hori M. Shimonishi Y. Taniguchi N. The oxidation of selenocysteine is involved in the inactivation of glutathione peroxidase by nitric oxide donor. J Biol Chem. 1997;272:19152–19157. doi: 10.1074/jbc.272.31.19152. [DOI] [PubMed] [Google Scholar]
- 17.Awasthi YC. Beutler E. Srivastava SK. Purification and properties of human erythrocyte glutathione peroxidase. J Biol Chem. 1975;250:5144–5149. [PubMed] [Google Scholar]
- 18.Bai J. Cederbaum AI. Overexpression of catalase in the mitochondrial or cytosolic compartment increases sensitivity of HepG2 cells to tumor necrosis factor-alpha-induced apoptosis. J Biol Chem. 2000;275:19241–19249. doi: 10.1074/jbc.M000438200. [DOI] [PubMed] [Google Scholar]
- 19.Baker MA. Tappel AL. Effects of ligands on gold inhibition of selenium glutathione peroxidase. Biochem Pharmacol. 1986;35:2417–2422. doi: 10.1016/0006-2952(86)90470-3. [DOI] [PubMed] [Google Scholar]
- 20.Baker RD. Baker SS. LaRosa K. Whitney C. Newburger PE. Selenium regulation of glutathione peroxidase in human hepatoma cell line Hep3B. Arch Biochem Biophys. 1993;304:53–57. doi: 10.1006/abbi.1993.1320. [DOI] [PubMed] [Google Scholar]
- 21.Baliga MS. Diwadkar-Navsariwala V. Koh T. Fayad R. Fantuzzi G. Diamond AM. Selenoprotein deficiency enhances radiation-induced micronuclei formation. Mol Nutr Food Res. 2008;52:1300–1304. doi: 10.1002/mnfr.200800020. [DOI] [PubMed] [Google Scholar]
- 22.Baliga MS. Wang H. Zhuo P. Schwartz JL. Diamond AM. Selenium and GPx-1 overexpression protect mammalian cells against UV-induced DNA damage. Biol Trace Elem Res. 2007;115:227–242. doi: 10.1007/BF02685998. [DOI] [PubMed] [Google Scholar]
- 23.Banning A. Deubel S. Kluth D. Zhou Z. Brigelius-Flohe R. The GI-GPx gene is a target for Nrf2. Mol Cell Biol. 2005;25:4914–4923. doi: 10.1128/MCB.25.12.4914-4923.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barkats M. Millecamps S. Abrioux P. Geoffroy MC. Mallet J. Overexpression of glutathione peroxidase increases the resistance of neuronal cells to Abeta-mediated neurotoxicity. J Neurochem. 2000;75:1438–1446. doi: 10.1046/j.1471-4159.2000.0751438.x. [DOI] [PubMed] [Google Scholar]
- 25.Bastaki M. Huen K. Manzanillo P. Chande N. Chen C. Balmes JR. Tager IB. Holland N. Genotype-activity relationship for Mn-superoxide dismutase, glutathione peroxidase 1 and catalase in humans. Pharmacogenet Genomics. 2006;16:279–286. doi: 10.1097/01.fpc.0000199498.08725.9c. [DOI] [PubMed] [Google Scholar]
- 26.Beck MA. Esworthy RS. Ho YS. Chu FF. Glutathione peroxidase protects mice from viral-induced myocarditis. FASEB J. 1998;12:1143–1149. doi: 10.1096/fasebj.12.12.1143. [DOI] [PubMed] [Google Scholar]
- 27.Beer SM. Taylor ER. Brown SE. Dahm CC. Costa NJ. Runswick MJ. Murphy MP. Glutaredoxin 2 catalyzes the reversible oxidation and glutathionylation of mitochondrial membrane thiol proteins: implications for mitochondrial redox regulation and antioxidant defense. J Biol Chem. 2004;279:47939–47951. doi: 10.1074/jbc.M408011200. [DOI] [PubMed] [Google Scholar]
- 28.Bellinger FP. Raman AV. Reeves MA. Berry MJ. Regulation and function of selenoproteins in human disease. Biochem J. 2009;422:11–22. doi: 10.1042/BJ20090219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bensadoun JC. Mirochnitchenko O. Inouye M. Aebischer P. Zurn AD. Attenuation of 6-OHDA-induced neurotoxicity in glutathione peroxidase transgenic mice. Eur J Neurosci. 1998;10:3231–3236. doi: 10.1046/j.1460-9568.1998.00345.x. [DOI] [PubMed] [Google Scholar]
- 30.Bermano G. Arthur JR. Hesketh JE. Role of the 3′ untranslated region in the regulation of cytosolic glutathione peroxidase and phospholipid-hydroperoxide glutathione peroxidase gene expression by selenium supply. Biochem J. 1996;320(Pt 3):891–895. doi: 10.1042/bj3200891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bermano G. Arthur JR. Hesketh JE. Selective control of cytosolic glutathione peroxidase and phospholipid hydroperoxide glutathione peroxidase mRNA stability by selenium supply. FEBS Lett. 1996;387:157–160. doi: 10.1016/0014-5793(96)00493-0. [DOI] [PubMed] [Google Scholar]
- 32.Bermano G. Nicol F. Dyer JA. Sunde RA. Beckett GJ. Arthur JR. Hesketh JE. Tissue-specific regulation of selenoenzyme gene expression during selenium deficiency in rats. Biochem J. 1995;311(Pt 2):425–430. doi: 10.1042/bj3110425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berry CE. Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol. 2004;555:589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berry MJ. Banu L. Chen YY. Mandel SJ. Kieffer JD. Harney JW. Larsen PR. Recognition of UGA as a selenocysteine codon in type I deiodinase requires sequences in the 3′ untranslated region. Nature. 1991;353:273–276. doi: 10.1038/353273a0. [DOI] [PubMed] [Google Scholar]
- 35.Berry MJ. Harney JW. Ohama T. Hatfield DL. Selenocysteine insertion or termination: factors affecting UGA codon fate and complementary anticodon:codon mutations. Nucleic Acids Res. 1994;22:3753–3759. doi: 10.1093/nar/22.18.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berry MJ. Maia AL. Kieffer JD. Harney JW. Larsen PR. Substitution of cysteine for selenocysteine in type I iodothyronine deiodinase reduces the catalytic efficiency of the protein but enhances its translation. Endocrinology. 1992;131:1848–1852. doi: 10.1210/endo.131.4.1396330. [DOI] [PubMed] [Google Scholar]
- 37.Bhatti P. Stewart PA. Hutchinson A. Rothman N. Linet MS. Inskip PD. Rajaraman P. Lead exposure, polymorphisms in genes related to oxidative stress, and risk of adult brain tumors. Cancer Epidemiol Biomarkers Prev. 2009;18:1841–1848. doi: 10.1158/1055-9965.EPI-09-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bienert GP. Moller AL. Kristiansen KA. Schulz A. Moller IM. Schjoerring JK. Jahn TP. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem. 2007;282:1183–1192. doi: 10.1074/jbc.M603761200. [DOI] [PubMed] [Google Scholar]
- 39.Bierl C. Voetsch B. Jin RC. Handy DE. Loscalzo J. Determinants of human plasma glutathione peroxidase (GPx-3) expression. J Biol Chem. 2004;279:26839–26845. doi: 10.1074/jbc.M401907200. [DOI] [PubMed] [Google Scholar]
- 40.Blankenberg S. Rupprecht HJ. Bickel C. Torzewski M. Hafner G. Tiret L. Smieja M. Cambien F. Meyer J. Lackner KJ. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med. 2003;349:1605–1613. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- 41.Blum J. Fridovich I. Inactivation of glutathione peroxidase by superoxide radical. Arch Biochem Biophys. 1985;240:500–508. doi: 10.1016/0003-9861(85)90056-6. [DOI] [PubMed] [Google Scholar]
- 42.Borras C. Gambini J. Gomez-Cabrera MC. Sastre J. Pallardo FV. Mann GE. Vina J. 17beta-oestradiol up-regulates longevity-related, antioxidant enzyme expression via the ERK1 and ERK2[MAPK]/NFkappaB cascade. Aging Cell. 2005;4:113–118. doi: 10.1111/j.1474-9726.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 43.Borras C. Sastre J. Garcia-Sala D. Lloret A. Pallardo FV. Vina J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med. 2003;34:546–552. doi: 10.1016/s0891-5849(02)01356-4. [DOI] [PubMed] [Google Scholar]
- 44.Boudina S. Sena S. Theobald H. Sheng X. Wright JJ. Hu XX. Aziz S. Johnson JI. Bugger H. Zaha VG. Abel ED. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–2466. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 45.Brennan JP. Bardswell SC. Burgoyne JR. Fuller W. Schroder E. Wait R. Begum S. Kentish JC. Eaton P. Oxidant-induced activation of type I protein kinase A is mediated by RI subunit interprotein disulfide bond formation. J Biol Chem. 2006;281:21827–21836. doi: 10.1074/jbc.M603952200. [DOI] [PubMed] [Google Scholar]
- 46.Brigelius-Flohe R. Kipp A. Glutathione peroxidases in different stages of carcinogenesis. Biochim Biophys Acta. 2009;1790:1555–1568. doi: 10.1016/j.bbagen.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 47.Bristow MR. Thompson PD. Martin RP. Mason JW. Billingham ME. Harrison DC. Early anthracycline cardiotoxicity. Am J Med. 1978;65:823–832. doi: 10.1016/0002-9343(78)90802-1. [DOI] [PubMed] [Google Scholar]
- 48.Budihardjo I. Oliver H. Lutter M. Luo X. Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269–290. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 49.Budiman ME. Bubenik JL. Miniard AC. Middleton LM. Gerber CA. Cash A. Driscoll DM. Eukaryotic initiation factor 4a3 is a selenium-regulated RNA-binding protein that selectively inhibits selenocysteine incorporation. Mol Cell. 2009;35:479–489. doi: 10.1016/j.molcel.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc Res. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 51.Cai H. Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 52.Cao C. Leng Y. Li C. Kufe D. Functional interaction between the c-Abl and Arg protein-tyrosine kinases in the oxidative stress response. J Biol Chem. 2003;278:12961–12967. doi: 10.1074/jbc.M300058200. [DOI] [PubMed] [Google Scholar]
- 53.Cao J. Schulte J. Knight A. Leslie NR. Zagozdzon A. Bronson R. Manevich Y. Beeson C. Neumann CA. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 2009;28:1505–1517. doi: 10.1038/emboj.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cardey B. Enescu M. Selenocysteine versus cysteine reactivity: a theoretical study of their oxidation by hydrogen peroxide. J Phys Chem A. 2007;111:673–678. doi: 10.1021/jp0658445. [DOI] [PubMed] [Google Scholar]
- 55.Cebrian A. Pharoah PD. Ahmed S. Smith PL. Luccarini C. Luben R. Redman K. Munday H. Easton DF. Dunning AM. Ponder BA. Tagging single-nucleotide polymorphisms in antioxidant defense enzymes and susceptibility to breast cancer. Cancer Res. 2006;66:1225–1233. doi: 10.1158/0008-5472.CAN-05-1857. [DOI] [PubMed] [Google Scholar]
- 56.Chada S. Whitney C. Newburger PE. Post-transcriptional regulation of glutathione peroxidase gene expression by selenium in the HL-60 human myeloid cell line. Blood. 1989;74:2535–2541. [PubMed] [Google Scholar]
- 57.Chalupsky K. Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2005;102:9056–9061. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chambers I. Frampton J. Goldfarb P. Affara N. McBain W. Harrison PR. The structure of the mouse glutathione peroxidase gene: the selenocysteine in the active site is encoded by the “termination” codon, TGA. EMBO J. 1986;5:1221–1227. doi: 10.1002/j.1460-2075.1986.tb04350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chance B. Sies H. Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 60.Chang TS. Cho CS. Park S. Yu S. Kang SW. Rhee SG. Peroxiredoxin III, a mitochondrion-specific peroxidase, regulates apoptotic signaling by mitochondria. J Biol Chem. 2004;279:41975–41984. doi: 10.1074/jbc.M407707200. [DOI] [PubMed] [Google Scholar]
- 61.Chaudiere J. Tappel AL. Interaction of gold(I) with the active site of selenium-glutathione peroxidase. J Inorg Biochem. 1984;20:313–325. doi: 10.1016/0162-0134(84)85030-8. [DOI] [PubMed] [Google Scholar]
- 62.Chaudiere J. Wilhelmsen EC. Tappel AL. Mechanism of selenium-glutathione peroxidase and its inhibition by mercaptocarboxylic acids and other mercaptans. J Biol Chem. 1984;259:1043–1050. [PubMed] [Google Scholar]
- 63.Chavatte L. Brown BA. Driscoll DM. Ribosomal protein L30 is a component of the UGA-selenocysteine recoding machinery in eukaryotes. Nat Struct Mol Biol. 2005;12:408–416. doi: 10.1038/nsmb922. [DOI] [PubMed] [Google Scholar]
- 64.Chen K. Keaney J. Reactive oxygen species-mediated signal transduction in the endothelium. Endothelium. 2004;11:109–121. doi: 10.1080/10623320490482655. [DOI] [PubMed] [Google Scholar]
- 65.Cheng WH. Ho YS. Valentine BA. Ross DA. Combs GF., Jr. Lei XG. Cellular glutathione peroxidase is the mediator of body selenium to protect against paraquat lethality in transgenic mice. J Nutr. 1998;128:1070–1076. doi: 10.1093/jn/128.7.1070. [DOI] [PubMed] [Google Scholar]
- 66.Cheng YY. Qian PC. The effect of selenium-fortified table salt in the prevention of Keshan disease on a population of 1.05 million. Biomed Environ Sci. 1990;3:422–428. [PubMed] [Google Scholar]
- 67.Chew P. Yuen DY. Koh P. Stefanovic N. Febbraio MA. Kola I. Cooper ME. de Haan JB. Site-specific antiatherogenic effect of the antioxidant ebselen in the diabetic apolipoprotein E-deficient mouse. Arterioscler Thromb Vasc Biol. 2009;29:823–830. doi: 10.1161/ATVBAHA.109.186619. [DOI] [PubMed] [Google Scholar]
- 68.Cho CS. Kato GJ. Yang SH. Bae SW. Lee JS. Gladwin MT. Rhee SG. Hydroxyurea-induced expression of glutathione peroxidase 1 in red blood cells of individuals with sickle cell anemia. Antioxid Redox Signal. 2010;13:1–11. doi: 10.1089/ars.2009.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cho CS. Lee S. Lee GT. Woo HA. Choi EJ. Rhee SG. Irreversible inactivation of glutathione peroxidase 1 and reversible inactivation of peroxiredoxin II by H2O2 in red blood cells. Antioxid Redox Signal. 2010;12:1235–1246. doi: 10.1089/ars.2009.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choi JY. Neuhouser ML. Barnett M. Hudson M. Kristal AR. Thornquist M. King IB. Goodman GE. Ambrosone CB. Polymorphisms in oxidative stress-related genes are not associated with prostate cancer risk in heavy smokers. Cancer Epidemiol Biomarkers Prev. 2007;16:1115–1120. doi: 10.1158/1055-9965.EPI-07-0040. [DOI] [PubMed] [Google Scholar]
- 71.Chrissobolis S. Didion SP. Kinzenbaw DA. Schrader LI. Dayal S. Lentz SR. Faraci FM. Glutathione peroxidase-1 plays a major role in protecting against angiotensin II-induced vascular dysfunction. Hypertension. 2008;51:872–877. doi: 10.1161/HYPERTENSIONAHA.107.103572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chu FF. Esworthy RS. Chu PG. Longmate JA. Huycke MM. Wilczynski S. Doroshow JH. Bacteria-induced intestinal cancer in mice with disrupted Gpx1 and Gpx2 genes. Cancer Res. 2004;64:962–968. doi: 10.1158/0008-5472.can-03-2272. [DOI] [PubMed] [Google Scholar]
- 73.Clark LC. Combs GF., Jr. Turnbull BW. Slate EH. Chalker DK. Chow J. Davis LS. Glover RA. Graham GF. Gross EG. Krongrad A. Lesher JL., Jr. Park HK. Sanders BB., Jr. Smith CL. Taylor JR. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. Jama. 1996;276:1957–1963. [PubMed] [Google Scholar]
- 74.Cohen G. Hochstein P. Glutathione peroxidase: the primary agent for the elimination of hydrogen peroxide in erythrocytes. Biochemistry. 1963;2:1420–1428. doi: 10.1021/bi00906a038. [DOI] [PubMed] [Google Scholar]
- 75.Combs GF., Jr Status of selenium in prostate cancer prevention. Br J Cancer. 2004;91:195–199. doi: 10.1038/sj.bjc.6601974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Copeland PR. Driscoll DM. Purification, redox sensitivity, and RNA binding properties of SECIS-binding protein 2, a protein involved in selenoprotein biosynthesis. J Biol Chem. 1999;274:25447–25454. doi: 10.1074/jbc.274.36.25447. [DOI] [PubMed] [Google Scholar]
- 77.Copeland PR. Fletcher JE. Carlson BA. Hatfield DL. Driscoll DM. A novel RNA binding protein, SBP2, is required for the translation of mammalian selenoprotein mRNAs. EMBO J. 2000;19:306–314. doi: 10.1093/emboj/19.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cowan DB. Weisel RD. Williams WG. Mickle DA. Identification of oxygen responsive elements in the 5′-flanking region of the human glutathione peroxidase gene. J Biol Chem. 1993;268:26904–26910. [PubMed] [Google Scholar]
- 79.Cox DG. Hankinson SE. Kraft P. Hunter DJ. No association between GPX1 Pro198Leu and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2004;13:1821–1822. [PubMed] [Google Scholar]
- 80.Cox DG. Tamimi RM. Hunter DJ. Gene x Gene interaction between MnSOD and GPX-1 and breast cancer risk: a nested case-control study. BMC Cancer. 2006;6:217. doi: 10.1186/1471-2407-6-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Crack PJ. Cimdins K. Ali U. Hertzog PJ. Iannello RC. Lack of glutathione peroxidase-1 exacerbates Abeta-mediated neurotoxicity in cortical neurons. J Neural Transm. 2006;113:645–657. doi: 10.1007/s00702-005-0352-y. [DOI] [PubMed] [Google Scholar]
- 82.Crack PJ. Taylor JM. Ali U. Mansell A. Hertzog PJ. Potential contribution of NF-kappaB in neuronal cell death in the glutathione peroxidase-1 knockout mouse in response to ischemia-reperfusion injury. Stroke. 2006;37:1533–1538. doi: 10.1161/01.STR.0000221708.17159.64. [DOI] [PubMed] [Google Scholar]
- 83.Crack PJ. Taylor JM. Flentjar NJ. de Haan J. Hertzog P. Iannello RC. Kola I. Increased infarct size and exacerbated apoptosis in the glutathione peroxidase-1 (Gpx-1) knockout mouse brain in response to ischemia/reperfusion injury. J Neurochem. 2001;78:1389–1399. doi: 10.1046/j.1471-4159.2001.00535.x. [DOI] [PubMed] [Google Scholar]
- 84.Creager MA. Cooke JP. Mendelsohn ME. Gallagher SJ. Coleman SM. Loscalzo J. Dzau VJ. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest. 1990;86:228–234. doi: 10.1172/JCI114688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cullen JJ. Mitros FA. Oberley LW. Expression of antioxidant enzymes in diseases of the human pancreas: another link between chronic pancreatitis and pancreatic cancer. Pancreas. 2003;26:23–27. doi: 10.1097/00006676-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 86.Dasgupta J. Subbaram S. Connor KM. Rodriguez AM. Tirosh O. Beckman JS. Jourd'Heuil D. Melendez JA. Manganese superoxide dismutase protects from TNF-alpha-induced apoptosis by increasing the steady-state production of H2O2. Antioxid Redox Signal. 2006;8:1295–1305. doi: 10.1089/ars.2006.8.1295. [DOI] [PubMed] [Google Scholar]
- 87.Day BJ. Catalase and glutathione peroxidase mimics. Biochem Pharmacol. 2009;77:285–296. doi: 10.1016/j.bcp.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dayal S. Brown KL. Weydert CJ. Oberley LW. Arning E. Bottiglieri T. Faraci FM. Lentz SR. Deficiency of glutathione peroxidase-1 sensitizes hyperhomocysteinemic mice to endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2002;22:1996–2002. doi: 10.1161/01.atv.0000041629.92741.dc. [DOI] [PubMed] [Google Scholar]
- 89.de Haan JB. Bladier C. Griffiths P. Kelner M. O'Shea RD. Cheung NS. Bronson RT. Silvestro MJ. Wild S. Zheng SS. Beart PM. Hertzog PJ. Kola I. Mice with a homozygous null mutation for the most abundant glutathione peroxidase, Gpx1, show increased susceptibility to the oxidative stress-inducing agents paraquat and hydrogen peroxide. J Biol Chem. 1998;273:22528–22536. doi: 10.1074/jbc.273.35.22528. [DOI] [PubMed] [Google Scholar]
- 90.de Haan JB. Bladier C. Lotfi-Miri M. Taylor J. Hutchinson P. Crack PJ. Hertzog P. Kola I. Fibroblasts derived from Gpx1 knockout mice display senescent-like features and are susceptible to H2O2-mediated cell death. Free Radic Biol Med. 2004;36:53–64. doi: 10.1016/j.freeradbiomed.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 91.de Haan JB. Witting PK. Stefanovic N. Pete J. Daskalakis M. Kola I. Stocker R. Smolich JJ. Lack of the antioxidant glutathione peroxidase-1 does not increase atherosclerosis in C57BL/J6 mice fed a high-fat diet. J Lipid Res. 2006;47:1157–1167. doi: 10.1194/jlr.M500377-JLR200. [DOI] [PubMed] [Google Scholar]
- 92.de la Sierra A. Larrousse M. Endothelial dysfunction is associated with increased levels of biomarkers in essential hypertension. J Hum Hypertens. 2010;24:373–379. doi: 10.1038/jhh.2009.91. [DOI] [PubMed] [Google Scholar]
- 93.de Moura MB. dos Santos LS. Van Houten B. Mitochondrial dysfunction in neurodegenerative diseases and cancer. Environ Mol Mutagen. 2010;51:391–405. doi: 10.1002/em.20575. [DOI] [PubMed] [Google Scholar]
- 94.Denninger JW. Marletta MA. Guanylate cyclase and the NO/cGMP signaling pathway. Biochim Biophys Acta. 1999;1411:334–350. doi: 10.1016/s0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 95.Dernbach E. Urbich C. Brandes RP. Hofmann WK. Zeiher AM. Dimmeler S. Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood. 2004;104:3591–3597. doi: 10.1182/blood-2003-12-4103. [DOI] [PubMed] [Google Scholar]
- 96.DeYulia GJ., Jr. Carcamo JM. Borquez-Ojeda O. Shelton CC. Golde DW. Hydrogen peroxide generated extracellularly by receptor-ligand interaction facilitates cell signaling. Proc Natl Acad Sci U S A. 2005;102:5044–5049. doi: 10.1073/pnas.0501154102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dikalov SI. Dikalova AE. Bikineyeva AT. Schmidt HH. Harrison DG. Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Diwadkar-Navsariwala V. Prins GS. Swanson SM. Birch LA. Ray VH. Hedayat S. Lantvit DL. Diamond AM. Selenoprotein deficiency accelerates prostate carcinogenesis in a transgenic model. Proc Natl Acad Sci U S A. 2006;103:8179–8184. doi: 10.1073/pnas.0508218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Donovan J. Copeland PR. Threading the needle: getting selenocysteine into proteins. Antioxid Redox Signal. 2010;12:881–892. doi: 10.1089/ars.2009.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Driscoll DM. Copeland PR. Mechanism and regulation of selenoprotein synthesis. Annu Rev Nutr. 2003;23:17–40. doi: 10.1146/annurev.nutr.23.011702.073318. [DOI] [PubMed] [Google Scholar]
- 101.Drouin A. Thorin-Trescases N. Hamel E. Falck JR. Thorin E. Endothelial nitric oxide synthase activation leads to dilatory H2O2 production in mouse cerebral arteries. Cardiovasc Res. 2007;73:73–81. doi: 10.1016/j.cardiores.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 102.Du C. Fang M. Li Y. Li L. Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 103.Duffield-Lillico AJ. Dalkin BL. Reid ME. Turnbull BW. Slate EH. Jacobs ET. Marshall JR. Clark LC. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the nutritional prevention of cancer trial. BJU Int. 2003;91:608–612. doi: 10.1046/j.1464-410x.2003.04167.x. [DOI] [PubMed] [Google Scholar]
- 104.Dumitrescu AM. Di Cosmo C. Liao XH. Weiss RE. Refetoff S. The syndrome of inherited partial SBP2 deficiency in humans. Antioxid Redox Signal. 2010;12:905–920. doi: 10.1089/ars.2009.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Duval C. Auge N. Frisach MF. Casteilla L. Salvayre R. Negre-Salvayre A. Mitochondrial oxidative stress is modulated by oleic acid via an epidermal growth factor receptor-dependent activation of glutathione peroxidase. Biochem J. 2002;367:889–894. doi: 10.1042/BJ20020625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eberhardt RT. Forgione MA. Cap A. Leopold JA. Rudd MA. Trolliet M. Heydrick S. Stark R. Klings ES. Moldovan NI. Yaghoubi M. Goldschmidt-Clermont PJ. Farber HW. Cohen R. Loscalzo J. Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia. J Clin Invest. 2000;106:483–491. doi: 10.1172/JCI8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Epp O. Ladenstein R. Wendel A. The refined structure of the selenoenzyme glutathione peroxidase at 0.2-nm resolution. Eur J Biochem. 1983;133:51–69. doi: 10.1111/j.1432-1033.1983.tb07429.x. [DOI] [PubMed] [Google Scholar]
- 108.Erdem A. Gundogan NU. Usubutun A. Kilinc K. Erdem SR. Kara A. Bozkurt A. The protective effect of taurine against gentamicin-induced acute tubular necrosis in rats. Nephrol Dial Transplant. 2000;15:1175–1182. doi: 10.1093/ndt/15.8.1175. [DOI] [PubMed] [Google Scholar]
- 109.Espinola-Klein C. Rupprecht HJ. Bickel C. Schnabel R. Genth-Zotz S. Torzewski M. Lackner K. Munzel T. Blankenberg S. Glutathione peroxidase-1 activity, atherosclerotic burden, and cardiovascular prognosis. Am J Cardiol. 2007;99:808–812. doi: 10.1016/j.amjcard.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 110.Esposito LA. Kokoszka JE. Waymire KG. Cottrell B. MacGregor GR. Wallace DC. Mitochondrial oxidative stress in mice lacking the glutathione peroxidase-1 gene. Free Radic Biol Med. 2000;28:754–766. doi: 10.1016/s0891-5849(00)00161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Esworthy RS. Aranda R. Martin MG. Doroshow JH. Binder SW. Chu FF. Mice with combined disruption of Gpx1 and Gpx2 genes have colitis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G848–G855. doi: 10.1152/ajpgi.2001.281.3.G848. [DOI] [PubMed] [Google Scholar]
- 112.Esworthy RS. Doan K. Doroshow JH. Chu FF. Cloning and sequencing of the cDNA encoding a human testis phospholipid hydroperoxide glutathione peroxidase. Gene. 1994;144:317–318. doi: 10.1016/0378-1119(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 113.Esworthy RS. Ho YS. Chu FF. The Gpx1 gene encodes mitochondrial glutathione peroxidase in the mouse liver. Arch Biochem Biophys. 1997;340:59–63. doi: 10.1006/abbi.1997.9901. [DOI] [PubMed] [Google Scholar]
- 114.Esworthy RS. Yang L. Frankel PH. Chu FF. Epithelium-specific glutathione peroxidase, Gpx2, is involved in the prevention of intestinal inflammation in selenium-deficient mice. J Nutr. 2005;135:740–745. doi: 10.1093/jn/135.4.740. [DOI] [PubMed] [Google Scholar]
- 115.Eto M. Kajihara N. Morita S. Tominaga R. A novel electron paramagnetic resonance spin-probe technique demonstrates the relation between the production of hydroxyl radicals and ischemia-reperfusion injury. Eur J Cardiothorac Surg. 2010 doi: 10.1016/j.ejcts.2010.08.011. [Epub ahead of print]; [DOI] [PubMed] [Google Scholar]
- 116.Fabregat I. Vitorica J. Satrustegui J. Machado A. The pentose phosphate cycle is regulated by NADPH/NADP ratio in rat liver. Arch Biochem Biophys. 1985;236:110–118. doi: 10.1016/0003-9861(85)90610-1. [DOI] [PubMed] [Google Scholar]
- 117.Fagegaltier D. Hubert N. Yamada K. Mizutani T. Carbon P. Krol A. Characterization of mSelB, a novel mammalian elongation factor for selenoprotein translation. EMBO J. 2000;19:4796–4805. doi: 10.1093/emboj/19.17.4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fang W. Goldberg ML. Pohl NM. Bi X. Tong C. Xiong B. Koh TJ. Diamond AM. Yang W. Functional and physical interaction between the selenium-binding protein 1 (SBP1) and the glutathione peroxidase 1 selenoprotein. Carcinogenesis. 2010;31:1360–1366. doi: 10.1093/carcin/bgq114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Farina M. Campos F. Vendrell I. Berenguer J. Barzi M. Pons S. Sunol C. Probucol increases glutathione peroxidase-1 activity and displays long-lasting protection against methylmercury toxicity in cerebellar granule cells. Toxicol Sci. 2009;112:416–426. doi: 10.1093/toxsci/kfp219. [DOI] [PubMed] [Google Scholar]
- 120.Faucher K. Rabinovitch-Chable H. Cook-Moreau J. Barriere G. Sturtz F. Rigaud M. Overexpression of human GPX1 modifies Bax to Bcl-2 apoptotic ratio in human endothelial cells. Mol Cell Biochem. 2005;277:81–87. doi: 10.1007/s11010-005-5075-8. [DOI] [PubMed] [Google Scholar]
- 121.Ferreira Azevedo M. Barra GB. Naves LA. Ribeiro Velasco LF. Godoy Garcia Castro P. de Castro LC. Amato AA. Miniard A. Driscoll D. Schomburg L. de Assis Rocha Neves F. Selenoprotein-Related Disease in a Young Girl Caused by Nonsense Mutations in the SBP2 Gene. J Clin Endocrinol Metab. 2010;95:4066–4071. doi: 10.1210/jc.2009-2611. [DOI] [PubMed] [Google Scholar]
- 122.Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- 123.Fisher AB. Peroxiredoxin 6: A bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. Antioxid Redox Signal. 2011;15:831–844. doi: 10.1089/ars.2010.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fleming I. Molecular mechanisms underlying the activation of eNOS. Pflugers Arch. 2010;459:793–806. doi: 10.1007/s00424-009-0767-7. [DOI] [PubMed] [Google Scholar]
- 125.Flentjar NJ. Crack PJ. Boyd R. Malin M. de Haan JB. Hertzog P. Kola I. Iannello R. Mice lacking glutathione peroxidase-1 activity show increased TUNEL staining and an accelerated inflammatory response in brain following a cold-induced injury. Exp Neurol. 2002;177:9–20. doi: 10.1006/exnr.2002.7927. [DOI] [PubMed] [Google Scholar]
- 126.Flohe L. Eisele B. Wendel A. [Glutathion peroxidase. I. Isolation and determinations of molecular weight] Hoppe Seylers Z Physiol Chem. 1971;352:151–158. [PubMed] [Google Scholar]
- 127.Flohe L. Gunzler WA. Schock HH. Glutathione peroxidase: a selenoenzyme. FEBS Lett. 1973;32:132–134. doi: 10.1016/0014-5793(73)80755-0. [DOI] [PubMed] [Google Scholar]
- 128.Flohe L. Loschen G. Gunzler WA. Eichele E. Glutathione peroxidase, V. The kinetic mechanism. Hoppe Seylers Z Physiol Chem. 1972;353:987–999. doi: 10.1515/bchm2.1972.353.1.987. [DOI] [PubMed] [Google Scholar]
- 129.Flohe L. Schlegel W. [Glutathione peroxidase. IV. Intracellular distribution of the glutathione peroxidase system in the rat liver] Hoppe Seylers Z Physiol Chem. 1971;352:1401–1410. [PubMed] [Google Scholar]
- 130.Flores-Mateo G. Carrillo-Santisteve P. Elosua R. Guallar E. Marrugat J. Bleys J. Covas MI. Antioxidant enzyme activity and coronary heart disease: meta-analyses of observational studies. Am J Epidemiol. 2009;170:135–147. doi: 10.1093/aje/kwp112. [DOI] [PubMed] [Google Scholar]
- 131.Forgione MA. Cap A. Liao R. Moldovan NI. Eberhardt RT. Lim CC. Jones J. Goldschmidt-Clermont PJ. Loscalzo J. Heterozygous cellular glutathione peroxidase deficiency in the mouse: abnormalities in vascular and cardiac function and structure. Circulation. 2002;106:1154–1158. doi: 10.1161/01.cir.0000026820.87824.6a. [DOI] [PubMed] [Google Scholar]
- 132.Forgione MA. Weiss N. Heydrick S. Cap A. Klings ES. Bierl C. Eberhardt RT. Farber HW. Loscalzo J. Cellular glutathione peroxidase deficiency and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2002;282:H1255–H1261. doi: 10.1152/ajpheart.00598.2001. [DOI] [PubMed] [Google Scholar]
- 133.Forman HJ. Maiorino M. Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49:835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Foster CB. Aswath K. Chanock SJ. McKay HF. Peters U. Polymorphism analysis of six selenoprotein genes: support for a selective sweep at the glutathione peroxidase 1 locus (3p21) in Asian populations. BMC Genet. 2006;7:56. doi: 10.1186/1471-2156-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fu Y. Cheng WH. Ross DA. Lei X. Cellular glutathione peroxidase protects mice against lethal oxidative stress induced by various doses of diquat. Proc Soc Exp Biol Med. 1999;222:164–169. doi: 10.1046/j.1525-1373.1999.d01-127.x. [DOI] [PubMed] [Google Scholar]
- 136.Fu Y. Sies H. Lei XG. Opposite roles of selenium-dependent glutathione peroxidase-1 in superoxide generator diquat- and peroxynitrite-induced apoptosis and signaling. J Biol Chem. 2001;276:43004–43009. doi: 10.1074/jbc.M106946200. [DOI] [PubMed] [Google Scholar]
- 137.Furusu A. Nakayama K. Xu Q. Konta T. Kitamura M. MAP kinase-dependent, NF-kappaB-independent regulation of inhibitor of apoptosis protein genes by TNF-alpha. J Cell Physiol. 2007;210:703–710. doi: 10.1002/jcp.20881. [DOI] [PubMed] [Google Scholar]
- 138.Fuyu Y. Keshan disease and mitochondrial cardiomyopathy. Sci China C Life Sci. 2006;49:513–518. doi: 10.1007/s11427-006-2041-y. [DOI] [PubMed] [Google Scholar]
- 139.Galasso G. Schiekofer S. Sato K. Shibata R. Handy DE. Ouchi N. Leopold JA. Loscalzo J. Walsh K. Impaired angiogenesis in glutathione peroxidase-1-deficient mice is associated with endothelial progenitor cell dysfunction. Circ Res. 2006;98:254–261. doi: 10.1161/01.RES.0000200740.57764.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gladyshev VN. Factor VM. Housseau F. Hatfield DL. Contrasting patterns of regulation of the antioxidant selenoproteins, thioredoxin reductase, and glutathione peroxidase, in cancer cells. Biochem Biophys Res Commun. 1998;251:488–493. doi: 10.1006/bbrc.1998.9495. [DOI] [PubMed] [Google Scholar]
- 141.Goldstein BJ. Mahadev K. Wu X. Zhu L. Motoshima H. Role of insulin-induced reactive oxygen species in the insulin signaling pathway. Antioxid Redox Signal. 2005;7:1021–1031. doi: 10.1089/ars.2005.7.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gouaze V. Andrieu-Abadie N. Cuvillier O. Malagarie-Cazenave S. Frisach MF. Mirault ME. Levade T. Glutathione peroxidase-1 protects from CD95-induced apoptosis. J Biol Chem. 2002;277:42867–42874. doi: 10.1074/jbc.M203067200. [DOI] [PubMed] [Google Scholar]
- 143.Gouaze V. Mirault ME. Carpentier S. Salvayre R. Levade T. Andrieu-Abadie N. Glutathione peroxidase-1 overexpression prevents ceramide production and partially inhibits apoptosis in doxorubicin-treated human breast carcinoma cells. Mol Pharmacol. 2001;60:488–496. [PubMed] [Google Scholar]
- 144.Gresner P. Gromadzinska J. Wasowicz W. Polymorphism of selected enzymes involved in detoxification and biotransformation in relation to lung cancer. Lung Cancer. 2007;57:1–25. doi: 10.1016/j.lungcan.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 145.Griendling KK. Sorescu D. Lassegue B. Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2000;20:2175–2183. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 146.Guimaraes MJ. Peterson D. Vicari A. Cocks BG. Copeland NG. Gilbert DJ. Jenkins NA. Ferrick DA. Kastelein RA. Bazan JF. Zlotnik A. Identification of a novel selD homolog from eukaryotes, bacteria, and archaea: is there an autoregulatory mechanism in selenocysteine metabolism? Proc Natl Acad Sci U S A. 1996;93:15086–15091. doi: 10.1073/pnas.93.26.15086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gulshan K. Rovinsky SA. Coleman ST. Moye-Rowley WS. Oxidant-specific folding of Yap1p regulates both transcriptional activation and nuclear localization. J Biol Chem. 2005;280:40524–40533. doi: 10.1074/jbc.M504716200. [DOI] [PubMed] [Google Scholar]
- 148.Gunzler WA. Steffens GJ. Grossmann A. Kim SM. Otting F. Wendel A. Flohe L. The amino-acid sequence of bovine glutathione peroxidase. Hoppe Seylers Z Physiol Chem. 1984;365:195–212. doi: 10.1515/bchm2.1984.365.1.195. [DOI] [PubMed] [Google Scholar]
- 149.Guo Z. Ran Q. Roberts LJ., 2nd Zhou L. Richardson A. Sharan C. Wu D. Yang H. Suppression of atherogenesis by overexpression of glutathione peroxidase-4 in apolipoprotein E-deficient mice. Free Radic Biol Med. 2008;44:343–352. doi: 10.1016/j.freeradbiomed.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Guo Z. Van Remmen H. Yang H. Chen X. Mele J. Vijg J. Epstein CJ. Ho YS. Richardson A. Changes in expression of antioxidant enzymes affect cell-mediated LDL oxidation and oxidized LDL-induced apoptosis in mouse aortic cells. Arterioscler Thromb Vasc Biol. 2001;21:1131–1138. doi: 10.1161/hq0701.092092. [DOI] [PubMed] [Google Scholar]
- 151.Gupta M. Copeland PR. Functional analysis of the interplay between translation termination, selenocysteine codon context, and selenocysteine insertion sequence-binding protein 2. J Biol Chem. 2007;282:36797–36807. doi: 10.1074/jbc.M707061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hamanishi T. Furuta H. Kato H. Doi A. Tamai M. Shimomura H. Sakagashira S. Nishi M. Sasaki H. Sanke T. Nanjo K. Functional variants in the glutathione peroxidase-1 (GPx-1) gene are associated with increased intima-media thickness of carotid arteries and risk of macrovascular diseases in japanese type 2 diabetic patients. Diabetes. 2004;53:2455–2460. doi: 10.2337/diabetes.53.9.2455. [DOI] [PubMed] [Google Scholar]
- 153.Handy DE. Hang G. Scolaro J. Metes N. Razaq N. Yang Y. Loscalzo J. Aminoglycosides decrease glutathione peroxidase-1 activity by interfering with selenocysteine incorporation. J Biol Chem. 2006;281:3382–3388. doi: 10.1074/jbc.M511295200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Handy DE. Lubos E. Yang Y. Galbraith JD. Kelly N. Zhang YY. Leopold JA. Loscalzo J. Glutathione peroxidase-1 regulates mitochondrial function to modulate redox-dependent cellular responses. J Biol Chem. 2009;284:11913–11921. doi: 10.1074/jbc.M900392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Handy DE. Zhang Y. Loscalzo J. Homocysteine down-regulates cellular glutathione peroxidase (GPx1) by decreasing translation. J Biol Chem. 2005;280:15518–15525. doi: 10.1074/jbc.M501452200. [DOI] [PubMed] [Google Scholar]
- 156.Hansen R. Saebo M. Skjelbred CF. Nexo BA. Hagen PC. Bock G. Bowitz Lothe IM. Johnson E. Aase S. Hansteen IL. Vogel U. Kure EH. GPX Pro198Leu and OGG1 Ser326Cys polymorphisms and risk of development of colorectal adenomas and colorectal cancer. Cancer Lett. 2005;229:85–91. doi: 10.1016/j.canlet.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 157.Hansen RD. Krath BN. Frederiksen K. Tjonneland A. Overvad K. Roswall N. Loft S. Dragsted LO. Vogel U. Raaschou-Nielsen O. GPX1 Pro(198)Leu polymorphism, erythrocyte GPX activity, interaction with alcohol consumption and smoking, and risk of colorectal cancer. Mutat Res. 2009;664:13–19. doi: 10.1016/j.mrfmmm.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 158.Hara S. Shoji Y. Sakurai A. Yuasa K. Himeno S. Imura N. Effects of selenium deficiency on expression of selenoproteins in bovine arterial endothelial cells. Biol Pharm Bull. 2001;24:754–759. doi: 10.1248/bpb.24.754. [DOI] [PubMed] [Google Scholar]
- 159.Hardie LJ. Briggs JA. Davidson LA. Allan JM. King RF. Williams GI. Wild CP. The effect of hOGG1 and glutathione peroxidase I genotypes and 3p chromosomal loss on 8-hydroxydeoxyguanosine levels in lung cancer. Carcinogenesis. 2000;21:167–172. doi: 10.1093/carcin/21.2.167. [DOI] [PubMed] [Google Scholar]
- 160.Harmon JS. Bogdani M. Parazzoli SD. Mak SS. Oseid EA. Berghmans M. Leboeuf RC. Robertson RP. beta-Cell-specific overexpression of glutathione peroxidase preserves intranuclear MafA and reverses diabetes in db/db mice. Endocrinology. 2009;150:4855–4862. doi: 10.1210/en.2009-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Harrison DG. Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest. 1997;100:2153–2157. doi: 10.1172/JCI119751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hatfield D. Portugal FH. Seryl-tRNA in mammalian tissues: chromatographic differences in brain and liver and a specific response to the codon, UGA. Proc Natl Acad Sci U S A. 1970;67:1200–1206. doi: 10.1073/pnas.67.3.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hatfield DL. Gladyshev VN. The outcome of selenium and vitamin E cancer prevention trial (SELECT) reveals the need for better understanding of selenium biology. Mol Interv. 2009;9:18–21. doi: 10.1124/mi.9.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.He T. Joyner MJ. Katusic ZS. Aging decreases expression and activity of glutathione peroxidase-1 in human endothelial progenitor cells. Microvasc Res. 2009;78:447–452. doi: 10.1016/j.mvr.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.He T. Peterson TE. Holmuhamedov EL. Terzic A. Caplice NM. Oberley LW. Katusic ZS. Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arterioscler Thromb Vasc Biol. 2004;24:2021–2027. doi: 10.1161/01.ATV.0000142810.27849.8f. [DOI] [PubMed] [Google Scholar]
- 166.Heeneman S. Sluimer JC. Daemen MJ. Angiotensin-converting enzyme and vascular remodeling. Circ Res. 2007;101:441–454. doi: 10.1161/CIRCRESAHA.107.148338. [DOI] [PubMed] [Google Scholar]
- 167.Heiss C. Keymel S. Niesler U. Ziemann J. Kelm M. Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45:1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 168.Hernandez-Montes E. Pollard SE. Vauzour D. Jofre-Montseny L. Rota C. Rimbach G. Weinberg PD. Spencer JP. Activation of glutathione peroxidase via Nrf1 mediates genistein's protection against oxidative endothelial cell injury. Biochem Biophys Res Commun. 2006;346:851–859. doi: 10.1016/j.bbrc.2006.05.197. [DOI] [PubMed] [Google Scholar]
- 169.Ho YS. Howard AJ. Cloning and characterization of the rat glutathione peroxidase gene. FEBS Lett. 1992;301:5–9. doi: 10.1016/0014-5793(92)80198-p. [DOI] [PubMed] [Google Scholar]
- 170.Hoffmann A. Leung TH. Baltimore D. Genetic analysis of NF-kappaB/Rel transcription factors defines functional specificities. EMBO J. 2003;22:5530–5539. doi: 10.1093/emboj/cdg534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hojlund K. Wrzesinski K. Larsen PM. Fey SJ. Roepstorff P. Handberg A. Dela F. Vinten J. McCormack JG. Reynet C. Beck-Nielsen H. Proteome analysis reveals phosphorylation of ATP synthase beta -subunit in human skeletal muscle and proteins with potential roles in type 2 diabetes. J Biol Chem. 2003;278:10436–10442. doi: 10.1074/jbc.M212881200. [DOI] [PubMed] [Google Scholar]
- 172.Howard MT. Anderson CB. Fass U. Khatri S. Gesteland RF. Atkins JF. Flanigan KM. Readthrough of dystrophin stop codon mutations induced by aminoglycosides. Ann Neurol. 2004;55:422–426. doi: 10.1002/ana.20052. [DOI] [PubMed] [Google Scholar]
- 173.Hu J. Zhou GW. Wang N. Wang YJ. GPX1 Pro198Leu polymorphism and breast cancer risk: a meta-analysis. Breast Cancer Res Treat. 2010;124:425–431. doi: 10.1007/s10549-010-0841-z. [DOI] [PubMed] [Google Scholar]
- 174.Hu Y. Benya RV. Carroll RE. Diamond AM. Allelic loss of the gene for the GPX1 selenium-containing protein is a common event in cancer. J Nutr. 2005;135:3021S–3024S. doi: 10.1093/jn/135.12.3021S. [DOI] [PubMed] [Google Scholar]
- 175.Hu YJ. Diamond AM. Role of glutathione peroxidase 1 in breast cancer: loss of heterozygosity and allelic differences in the response to selenium. Cancer Res. 2003;63:3347–3351. [PubMed] [Google Scholar]
- 176.Hu YJ. Dolan ME. Bae R. Yee H. Roy M. Glickman R. Kiremidjian-Schumacher L. Diamond AM. Allelic loss at the GPx-1 locus in cancer of the head and neck. Biol Trace Elem Res. 2004;101:97–106. doi: 10.1385/BTER:101:2:097. [DOI] [PubMed] [Google Scholar]
- 177.Hussain SP. Amstad P. He P. Robles A. Lupold S. Kaneko I. Ichimiya M. Sengupta S. Mechanic L. Okamura S. Hofseth LJ. Moake M. Nagashima M. Forrester KS. Harris CC. p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res. 2004;64:2350–2356. doi: 10.1158/0008-5472.can-2287-2. [DOI] [PubMed] [Google Scholar]
- 178.Hussain SP. Harris CC. p53 biological network: at the crossroads of the cellular-stress response pathway and molecular carcinogenesis. J Nippon Med Sch. 2006;73:54–64. doi: 10.1272/jnms.73.54. [DOI] [PubMed] [Google Scholar]
- 179.Ichimura Y. Habuchi T. Tsuchiya N. Wang L. Oyama C. Sato K. Nishiyama H. Ogawa O. Kato T. Increased risk of bladder cancer associated with a glutathione peroxidase 1 codon 198 variant. J Urol. 2004;172:728–732. doi: 10.1097/01.ju.0000130942.40597.9d. [DOI] [PubMed] [Google Scholar]
- 180.Imai H. Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med. 2003;34:145–169. doi: 10.1016/s0891-5849(02)01197-8. [DOI] [PubMed] [Google Scholar]
- 181.Imanishi T. Murry CE. Reinecke H. Hano T. Nishio I. Liles WC. Hofsta L. Kim K. O'Brien KD. Schwartz SM. Han DK. Cellular FLIP is expressed in cardiomyocytes and down-regulated in TUNEL-positive grafted cardiac tissues. Cardiovasc Res. 2000;48:101–110. doi: 10.1016/s0008-6363(00)00154-1. [DOI] [PubMed] [Google Scholar]
- 182.Irons R. Carlson BA. Hatfield DL. Davis CD. Both selenoproteins and low molecular weight selenocompounds reduce colon cancer risk in mice with genetically impaired selenoprotein expression. J Nutr. 2006;136:1311–1317. doi: 10.1093/jn/136.5.1311. [DOI] [PubMed] [Google Scholar]
- 183.Jablonska E. Gromadzinska J. Reszka E. Wasowicz W. Sobala W. Szeszenia-Dabrowska N. Boffetta P. Association between GPx1 Pro198Leu polymorphism, GPx1 activity and plasma selenium concentration in humans. Eur J Nutr. 2009;48:383–386. doi: 10.1007/s00394-009-0023-0. [DOI] [PubMed] [Google Scholar]
- 184.Jacobs ET. Jiang R. Alberts DS. Greenberg ER. Gunter EW. Karagas MR. Lanza E. Ratnasinghe L. Reid ME. Schatzkin A. Smith-Warner SA. Wallace K. Martinez ME. Selenium and colorectal adenoma: results of a pooled analysis. J Natl Cancer Inst. 2004;96:1669–1675. doi: 10.1093/jnci/djh310. [DOI] [PubMed] [Google Scholar]
- 185.Jaeschke H. Ho YS. Fisher MA. Lawson JA. Farhood A. Glutathione peroxidase-deficient mice are more susceptible to neutrophil-mediated hepatic parenchymal cell injury during endotoxemia: importance of an intracellular oxidant stress. Hepatology. 1999;29:443–450. doi: 10.1002/hep.510290222. [DOI] [PubMed] [Google Scholar]
- 186.Jang YM. Kendaiah S. Drew B. Phillips T. Selman C. Julian D. Leeuwenburgh C. Doxorubicin treatment in vivo activates caspase-12 mediated cardiac apoptosis in both male and female rats. FEBS Lett. 2004;577:483–490. doi: 10.1016/j.febslet.2004.10.053. [DOI] [PubMed] [Google Scholar]
- 187.Jiang X. Yang F. Tan H. Liao D. Bryan RM., Jr. Randhawa JK. Rumbaut RE. Durante W. Schafer AI. Yang X. Wang H. Hyperhomocystinemia impairs endothelial function and eNOS activity via PKC activation. Arterioscler Thromb Vasc Biol. 2005;25:2515–2521. doi: 10.1161/01.ATV.0000189559.87328.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Johansson C. Lillig CH. Holmgren A. Human mitochondrial glutaredoxin reduces S-glutathionylated proteins with high affinity accepting electrons from either glutathione or thioredoxin reductase. J Biol Chem. 2004;279:7537–7543. doi: 10.1074/jbc.M312719200. [DOI] [PubMed] [Google Scholar]
- 189.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jornot L. Junod AF. Hyperoxia, unlike phorbol ester, induces glutathione peroxidase through a protein kinase C-independent mechanism. Biochem J. 1997;326(Pt 1):117–123. doi: 10.1042/bj3260117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kalivendi SV. Konorev EA. Cunningham S. Vanamala SK. Kaji EH. Joseph J. Kalyanaraman B. Doxorubicin activates nuclear factor of activated T-lymphocytes and Fas ligand transcription: role of mitochondrial reactive oxygen species and calcium. Biochem J. 2005;389:527–539. doi: 10.1042/BJ20050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kato K. Oguri M. Kato N. Hibino T. Yajima K. Yoshida T. Metoki N. Yoshida H. Satoh K. Watanabe S. Yokoi K. Murohara T. Yamada Y. Assessment of genetic risk factors for thoracic aortic aneurysm in hypertensive patients. Am J Hypertens. 2008;21:1023–1027. doi: 10.1038/ajh.2008.229. [DOI] [PubMed] [Google Scholar]
- 193.Keeling KM. Brooks DA. Hopwood JJ. Li P. Thompson JN. Bedwell DM. Gentamicin-mediated suppression of Hurler syndrome stop mutations restores a low level of alpha-L-iduronidase activity and reduces lysosomal glycosaminoglycan accumulation. Hum Mol Genet. 2001;10:291–299. doi: 10.1093/hmg/10.3.291. [DOI] [PubMed] [Google Scholar]
- 194.Kelley DE. He J. Menshikova EV. Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 195.Kelley EE. Khoo NK. Hundley NJ. Malik UZ. Freeman BA. Tarpey MM. Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic Biol Med. 2010;48:493–498. doi: 10.1016/j.freeradbiomed.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kemp M. Go YM. Jones DP. Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology. Free Radic Biol Med. 2008;44:921–937. doi: 10.1016/j.freeradbiomed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Khan N. Rahim SS. Boddupalli CS. Ghousunnissa S. Padma S. Pathak N. Thiagarajan D. Hasnain SE. Mukhopadhyay S. Hydrogen peroxide inhibits IL-12 p40 induction in macrophages by inhibiting c-rel translocation to the nucleus through activation of calmodulin protein. Blood. 2006;107:1513–1520. doi: 10.1182/blood-2005-04-1707. [DOI] [PubMed] [Google Scholar]
- 198.Kim DJ. Kataoka K. Sano S. Connolly K. Kiguchi K. DiGiovanni J. Targeted disruption of Bcl-xL in mouse keratinocytes inhibits both UVB- and chemically induced skin carcinogenesis. Mol Carcinog. 2009;48:873–885. doi: 10.1002/mc.20527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kim JW. Gao P. Dang CV. Effects of hypoxia on tumor metabolism. Cancer Metastasis Rev. 2007;26:291–298. doi: 10.1007/s10555-007-9060-4. [DOI] [PubMed] [Google Scholar]
- 200.Klivenyi P. Andreassen OA. Ferrante RJ. Dedeoglu A. Mueller G. Lancelot E. Bogdanov M. Andersen JK. Jiang D. Beal MF. Mice deficient in cellular glutathione peroxidase show increased vulnerability to malonate, 3-nitropropionic acid, and 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. J Neurosci. 2000;20:1–7. doi: 10.1523/JNEUROSCI.20-01-00001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Knight JA. Onay UV. Wells S. Li H. Shi EJ. Andrulis IL. Ozcelik H. Genetic variants of GPX1 and SOD2 and breast cancer risk at the Ontario site of the Breast Cancer Family Registry. Cancer Epidemiol Biomarkers Prev. 2004;13:146–149. doi: 10.1158/1055-9965.epi-03-0164. [DOI] [PubMed] [Google Scholar]
- 202.Kote-Jarai Z. Durocher F. Edwards SM. Hamoudi R. Jackson RA. Ardern-Jones A. Murkin A. Dearnaley DP. Kirby R. Houlston R. Easton DF. Eeles R. Association between the GCG polymorphism of the selenium dependent GPX1 gene and the risk of young onset prostate cancer. Prostate Cancer Prostatic Dis. 2002;5:189–192. doi: 10.1038/sj.pcan.4500569. [DOI] [PubMed] [Google Scholar]
- 203.Kraus RJ. Foster SJ. Ganther HE. Identification of selenocysteine in glutathione peroxidase by mass spectroscopy. Biochemistry. 1983;22:5853–5858. doi: 10.1021/bi00294a026. [DOI] [PubMed] [Google Scholar]
- 204.Kraus RJ. Prohaska JR. Ganther HE. Oxidized forms of ovine erythrocyte glutathione peroxidase. Cyanide inhibition of a 4-glutathione:4-selenoenzyme. Biochim Biophys Acta. 1980;615:19–26. doi: 10.1016/0005-2744(80)90004-2. [DOI] [PubMed] [Google Scholar]
- 205.Kretz-Remy C. Mehlen P. Mirault ME. Arrigo AP. Inhibition of I kappa B-alpha phosphorylation and degradation and subsequent NF-kappa B activation by glutathione peroxidase overexpression. J Cell Biol. 1996;133:1083–1093. doi: 10.1083/jcb.133.5.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kryukov GV. Castellano S. Novoselov SV. Lobanov AV. Zehtab O. Guigo R. Gladyshev VN. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 207.Kuiper GG. Klootwijk W. Visser TJ. Substitution of cysteine for selenocysteine in the catalytic center of type III iodothyronine deiodinase reduces catalytic efficiency and alters substrate preference. Endocrinology. 2003;144:2505–2513. doi: 10.1210/en.2003-0084. [DOI] [PubMed] [Google Scholar]
- 208.Kuzuya M. Ando F. Iguchi A. Shimokata H. Glutathione peroxidase 1 Pro198Leu variant contributes to the metabolic syndrome in men in a large Japanese cohort. Am J Clin Nutr. 2008;87:1939–1944. doi: 10.1093/ajcn/87.6.1939. [DOI] [PubMed] [Google Scholar]
- 209.Lassegue B. Sorescu D. Szocs K. Yin Q. Akers M. Zhang Y. Grant SL. Lambeth JD. Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2001;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 210.Lee BJ. Worland PJ. Davis JN. Stadtman TC. Hatfield DL. Identification of a selenocysteyl-tRNA(Ser) in mammalian cells that recognizes the nonsense codon, UGA. J Biol Chem. 1989;264:9724–9727. [PubMed] [Google Scholar]
- 211.Lee CH. Lee KY. Choe KH. Hong YC. Noh SI. Eom SY. Ko YJ. Zhang YW. Yim DH. Kang JW. Kim H. Kim YD. [Effects of oxidative DNA damage and genetic polymorphism of the glutathione peroxidase 1 (GPX1) and 8-oxoguanine glycosylase 1 (hOGG1) on lung cancer] J Prev Med Public Health. 2006;39:130–134. [PubMed] [Google Scholar]
- 212.Lee DH. Esworthy RS. Chu C. Pfeifer GP. Chu FF. Mutation accumulation in the intestine and colon of mice deficient in two intracellular glutathione peroxidases. Cancer Res. 2006;66:9845–9851. doi: 10.1158/0008-5472.CAN-06-0732. [DOI] [PubMed] [Google Scholar]
- 213.Lee S. Shin HS. Shireman PK. Vasilaki A. Van Remmen H. Csete ME. Glutathione-peroxidase-1 null muscle progenitor cells are globally defective. Free Radic Biol Med. 2006;41:1174–1184. doi: 10.1016/j.freeradbiomed.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 214.Lee SR. Bar-Noy S. Kwon J. Levine RL. Stadtman TC. Rhee SG. Mammalian thioredoxin reductase: oxidation of the C-terminal cysteine/selenocysteine active site forms a thioselenide, and replacement of selenium with sulfur markedly reduces catalytic activity. Proc Natl Acad Sci U S A. 2000;97:2521–2526. doi: 10.1073/pnas.050579797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Legault J. Carrier C. Petrov P. Renard P. Remacle J. Mirault ME. Mitochondrial GPx1 decreases induced but not basal oxidative damage to mtDNA in T47D cells. Biochem Biophys Res Commun. 2000;272:416–422. doi: 10.1006/bbrc.2000.2800. [DOI] [PubMed] [Google Scholar]
- 216.Lei C. Niu X. Wei J. Zhu J. Zhu Y. Interaction of glutathione peroxidase-1 and selenium in endemic dilated cardiomyopathy. Clin Chim Acta. 2009;399:102–108. doi: 10.1016/j.cca.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 217.Leonard JL. Leonard DM. Shen Q. Farwell AP. Newburger PE. Selenium-regulated translation control of heterologous gene expression: normal function of selenocysteine-substituted gene products. J Cell Biochem. 1996;61:410–419. doi: 10.1002/(sici)1097-4644(19960601)61:3<410::aid-jcb8>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 218.Leopold JA. Zhang YY. Scribner AW. Stanton RC. Loscalzo J. Glucose-6-phosphate dehydrogenase overexpression decreases endothelial cell oxidant stress and increases bioavailable nitric oxide. Arterioscler Thromb Vasc Biol. 2003;23:411–417. doi: 10.1161/01.ATV.0000056744.26901.BA. [DOI] [PubMed] [Google Scholar]
- 219.Lesoon A. Mehta A. Singh R. Chisolm GM. Driscoll DM. An RNA-binding protein recognizes a mammalian selenocysteine insertion sequence element required for cotranslational incorporation of selenocysteine. Mol Cell Biol. 1997;17:1977–1985. doi: 10.1128/mcb.17.4.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Levander OA. Beck MA. Interacting nutritional and infectious etiologies of Keshan disease. Insights from coxsackie virus B-induced myocarditis in mice deficient in selenium or vitamin E. Biol Trace Elem Res. 1997;56:5–21. doi: 10.1007/BF02778980. [DOI] [PubMed] [Google Scholar]
- 221.Lewis P. Stefanovic N. Pete J. Calkin AC. Giunti S. Thallas-Bonke V. Jandeleit-Dahm KA. Allen TJ. Kola I. Cooper ME. de Haan JB. Lack of the antioxidant enzyme glutathione peroxidase-1 accelerates atherosclerosis in diabetic apolipoprotein E-deficient mice. Circulation. 2007;115:2178–2187. doi: 10.1161/CIRCULATIONAHA.106.664250. [DOI] [PubMed] [Google Scholar]
- 222.Li JM. Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- 223.Li P. Nijhawan D. Budihardjo I. Srinivasula SM. Ahmad M. Alnemri ES. Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 224.Li Q. Sanlioglu S. Li S. Ritchie T. Oberley L. Engelhardt JF. GPx-1 gene delivery modulates NFkappaB activation following diverse environmental injuries through a specific subunit of the IKK complex. Antioxid Redox Signal. 2001;3:415–432. doi: 10.1089/15230860152409068. [DOI] [PubMed] [Google Scholar]
- 225.Li S. Yan T. Yang JQ. Oberley TD. Oberley LW. The role of cellular glutathione peroxidase redox regulation in the suppression of tumor cell growth by manganese superoxide dismutase. Cancer Res. 2000;60:3927–3939. [PubMed] [Google Scholar]
- 226.Li WG. Miller FJ., Jr. Zhang HJ. Spitz DR. Oberley LW. Weintraub NL. H(2)O(2)-induced O(2) production by a non-phagocytic NAD(P)H oxidase causes oxidant injury. J Biol Chem. 2001;276:29251–29256. doi: 10.1074/jbc.M102124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Liberatore RA. Goff SP. Nunes I. NF-kappaB activity is constitutively elevated in c-Abl null fibroblasts. Proc Natl Acad Sci U S A. 2009;106:17823–17828. doi: 10.1073/pnas.0905935106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Liddell JR. Dringen R. Crack PJ. Robinson SR. Glutathione peroxidase 1 and a high cellular glutathione concentration are essential for effective organic hydroperoxide detoxification in astrocytes. Glia. 2006;54:873–879. doi: 10.1002/glia.20433. [DOI] [PubMed] [Google Scholar]
- 229.Liddell JR. Hoepken HH. Crack PJ. Robinson SR. Dringen R. Glutathione peroxidase 1 and glutathione are required to protect mouse astrocytes from iron-mediated hydrogen peroxide toxicity. J Neurosci Res. 2006;84:578–586. doi: 10.1002/jnr.20957. [DOI] [PubMed] [Google Scholar]
- 230.Lillig CH. Lonn ME. Enoksson M. Fernandes AP. Holmgren A. Short interfering RNA-mediated silencing of glutaredoxin 2 increases the sensitivity of HeLa cells toward doxorubicin and phenylarsine oxide. Proc Natl Acad Sci U S A. 2004;101:13227–13232. doi: 10.1073/pnas.0401896101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lim CC. Bryan NS. Jain M. Garcia-Saura MF. Fernandez BO. Sawyer DB. Handy DE. Loscalzo J. Feelisch M. Liao R. Glutathione peroxidase deficiency exacerbates ischemia-reperfusion injury in male but not female myocardium: insights into antioxidant compensatory mechanisms. Am J Physiol Heart Circ Physiol. 2009;297:H2144–H2153. doi: 10.1152/ajpheart.00673.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Liochev SI. Fridovich I. The effects of superoxide dismutase on H2O2 formation. Free Radic Biol Med. 2007;42:1465–1469. doi: 10.1016/j.freeradbiomed.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 233.Lippman SM. Klein EA. Goodman PJ. Lucia MS. Thompson IM. Ford LG. Parnes HL. Minasian LM. Gaziano JM. Hartline JA. Parsons JK. Bearden JD., 3rd Crawford ED. Goodman GE. Claudio J. Winquist E. Cook ED. Karp DD. Walther P. Lieber MM. Kristal AR. Darke AK. Arnold KB. Ganz PA. Santella RM. Albanes D. Taylor PR. Probstfield JL. Jagpal TJ. Crowley JJ. Meyskens FL., Jr. Baker LH. Coltman CA., Jr Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the selenium and vitamin E cancer prevention trial (SELECT) Jama. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Liu J. Hinkhouse MM. Sun W. Weydert CJ. Ritchie JM. Oberley LW. Cullen JJ. Redox regulation of pancreatic cancer cell growth: role of glutathione peroxidase in the suppression of the malignant phenotype. Hum Gene Ther. 2004;15:239–250. doi: 10.1089/104303404322886093. [DOI] [PubMed] [Google Scholar]
- 235.Loh K. Deng H. Fukushima A. Cai X. Boivin B. Galic S. Bruce C. Shields BJ. Skiba B. Ooms LM. Stepto N. Wu B. Mitchell CA. Tonks NK. Watt MJ. Febbraio MA. Crack PJ. Andrikopoulos S. Tiganis T. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009;10:260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Low SC. Berry MJ. Knowing when not to stop: selenocysteine incorporation in eukaryotes. Trends Biochem Sci. 1996;21:203–208. [PubMed] [Google Scholar]
- 237.Low SC. Harney JW. Berry MJ. Cloning and functional characterization of human selenophosphate synthetase, an essential component of selenoprotein synthesis. J Biol Chem. 1995;270:21659–21664. doi: 10.1074/jbc.270.37.21659. [DOI] [PubMed] [Google Scholar]
- 238.Lu YP. Lou YR. Yen P. Newmark HL. Mirochnitchenko OI. Inouye M. Huang MT. Enhanced skin carcinogenesis in transgenic mice with high expression of glutathione peroxidase or both glutathione peroxidase and superoxide dismutase. Cancer Res. 1997;57:1468–1474. [PubMed] [Google Scholar]
- 239.Lubos E. Mahoney CE. Leopold JA. Zhang YY. Loscalzo J. Handy DE. Glutathione peroxidase-1 modulates lipopolysaccharide-induced adhesion molecule expression in endothelial cells by altering CD14 expression. FASEB J. 2010;24:2525–2532. doi: 10.1096/fj.09-147421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Lupertz R. Chovolou Y. Kampkotter A. Watjen W. Kahl R. Catalase overexpression impairs TNF-alpha induced NF-kappaB activation and sensitizes MCF-7 cells against TNF-alpha. J Cell Biochem. 2008;103:1497–1511. doi: 10.1002/jcb.21538. [DOI] [PubMed] [Google Scholar]
- 241.Lynch ED. Kil J. Compounds for the prevention and treatment of noise-induced hearing loss. Drug Discov Today. 2005;10:1291–1298. doi: 10.1016/S1359-6446(05)03561-0. [DOI] [PubMed] [Google Scholar]
- 242.Maiorino M. Aumann KD. Brigelius-Flohe R. Doria D. van den Heuvel J. McCarthy J. Roveri A. Ursini F. Flohe L. Probing the presumed catalytic triad of a selenium-containing peroxidase by mutational analysis. Z Ernahrungswiss. 1998;37(Suppl 1):118–121. [PubMed] [Google Scholar]
- 243.Maiorino M. Roveri A. Coassin M. Ursini F. Kinetic mechanism and substrate specificity of glutathione peroxidase activity of ebselen (PZ51) Biochem Pharmacol. 1988;37:2267–2271. doi: 10.1016/0006-2952(88)90591-6. [DOI] [PubMed] [Google Scholar]
- 244.Malling TH. Sigsgaard T. Andersen HR. Frischknecht L. Deguchi Y. Skadhauge L. Sherson D. Thomsen G. Baelum J. Pedersen JK. Omland O. Sex determines the influence of smoking and gene polymorphism on glutathione peroxidase activity in erythrocytes. Scand J Clin Lab Invest. 2009;69:295–302. doi: 10.1080/00365510802632155. [DOI] [PubMed] [Google Scholar]
- 245.Mancini GB. Henry GC. Macaya C. O'Neill BJ. Pucillo AL. Carere RG. Wargovich TJ. Mudra H. Luscher TF. Klibaner MI. Haber HE. Uprichard AC. Pepine CJ. Pitt B. Angiotensin-converting enzyme inhibition with quinapril improves endothelial vasomotor dysfunction in patients with coronary artery disease. The TREND (trial on reversing endothelial dysfunction) study. Circulation. 1996;94:258–265. doi: 10.1161/01.cir.94.3.258. [DOI] [PubMed] [Google Scholar]
- 246.Manuvakhova M. Keeling K. Bedwell DM. Aminoglycoside antibiotics mediate context-dependent suppression of termination codons in a mammalian translation system. RNA. 2000;6:1044–1055. doi: 10.1017/s1355838200000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Marinho HS. Antunes F. Pinto RE. Role of glutathione peroxidase and phospholipid hydroperoxide glutathione peroxidase in the reduction of lysophospholipid hydroperoxides. Free Radic Biol Med. 1997;22:871–883. doi: 10.1016/s0891-5849(96)00468-6. [DOI] [PubMed] [Google Scholar]
- 248.Martinez JI. Garcia RD. Galarza AM. The kinetic mechanism of glutathione peroxidase from human platelets. Thromb Res. 1982;27:197–203. doi: 10.1016/0049-3848(82)90199-2. [DOI] [PubMed] [Google Scholar]
- 249.Masumoto H. Sies H. The reaction of ebselen with peroxynitrite. Chem Res Toxicol. 1996;9:262–267. doi: 10.1021/tx950115u. [DOI] [PubMed] [Google Scholar]
- 250.Mauri P. Benazzi L. Flohe L. Maiorino M. Pietta PG. Pilawa S. Roveri A. Ursini F. Versatility of selenium catalysis in PHGPx unraveled by LC/ESI-MS/MS. Biol Chem. 2003;384:575–588. doi: 10.1515/BC.2003.065. [DOI] [PubMed] [Google Scholar]
- 251.McClung JP. Roneker CA. Mu W. Lisk DJ. Langlais P. Liu F. Lei XG. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci U S A. 2004;101:8852–8857. doi: 10.1073/pnas.0308096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Meplan C. Crosley LK. Nicol F. Beckett GJ. Howie AF. Hill KE. Horgan G. Mathers JC. Arthur JR. Hesketh JE. Genetic polymorphisms in the human selenoprotein P gene determine the response of selenoprotein markers to selenium supplementation in a gender-specific manner (the SELGEN study) FASEB J. 2007;21:3063–3074. doi: 10.1096/fj.07-8166com. [DOI] [PubMed] [Google Scholar]
- 253.Merante F. Altamentova SM. Mickle DA. Weisel RD. Thatcher BJ. Martin BM. Marshall JG. Tumiati LC. Cowan DB. Li RK. The characterization and purification of a human transcription factor modulating the glutathione peroxidase gene in response to oxygen tension. Mol Cell Biochem. 2002;229:73–83. doi: 10.1023/a:1017921110363. [DOI] [PubMed] [Google Scholar]
- 254.Mills GC. Hemoglobin catabolism. I. Glutathione peroxidase, an erythrocyte enzyme which protects hemoglobin from oxidative breakdown. J Biol Chem. 1957;229:189–197. [PubMed] [Google Scholar]
- 255.Miniard AC. Middleton LM. Budiman ME. Gerber CA. Driscoll DM. Nucleolin binds to a subset of selenoprotein mRNAs and regulates their expression. Nucleic Acids Res. 2010;38:4807–4820. doi: 10.1093/nar/gkq247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Mirault ME. Tremblay A. Beaudoin N. Tremblay M. Overexpression of seleno-glutathione peroxidase by gene transfer enhances the resistance of T47D human breast cells to clastogenic oxidants. J Biol Chem. 1991;266:20752–20760. [PubMed] [Google Scholar]
- 257.Miwa T. Adachi T. Ito Y. Hirano K. Sugiura M. Purification and properties of glutathione peroxidase from human liver. Chem Pharm Bull (Tokyo) 1983;31:179–185. doi: 10.1248/cpb.31.179. [DOI] [PubMed] [Google Scholar]
- 258.Modrick ML. Didion SP. Lynch CM. Dayal S. Lentz SR. Faraci FM. Role of hydrogen peroxide and the impact of glutathione peroxidase-1 in regulation of cerebral vascular tone. J Cereb Blood Flow Metab. 2009;29:1130–1137. doi: 10.1038/jcbfm.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Moscow JA. Morrow CS. He R. Mullenbach GT. Cowan KH. Structure and function of the 5′-flanking sequence of the human cytosolic selenium-dependent glutathione peroxidase gene (hgpx1) J Biol Chem. 1992;267:5949–5958. [PubMed] [Google Scholar]
- 260.Moscow JA. Schmidt L. Ingram DT. Gnarra J. Johnson B. Cowan KH. Loss of heterozygosity of the human cytosolic glutathione peroxidase I gene in lung cancer. Carcinogenesis. 1994;15:2769–2773. doi: 10.1093/carcin/15.12.2769. [DOI] [PubMed] [Google Scholar]
- 261.Moustafa ME. Carlson BA. El-Saadani MA. Kryukov GV. Sun QA. Harney JW. Hill KE. Combs GF. Feigenbaum L. Mansur DB. Burk RF. Berry MJ. Diamond AM. Lee BJ. Gladyshev VN. Hatfield DL. Selective inhibition of selenocysteine tRNA maturation and selenoprotein synthesis in transgenic mice expressing isopentenyladenosine-deficient selenocysteine tRNA. Mol Cell Biol. 2001;21:3840–3852. doi: 10.1128/MCB.21.11.3840-3852.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mullane K. Bullough D. Harnessing an endogenous cardioprotective mechanism: cellular sources and sites of action of adenosine. J Mol Cell Cardiol. 1995;27:1041–1054. doi: 10.1016/0022-2828(95)90073-x. [DOI] [PubMed] [Google Scholar]
- 263.Mullenbach GT. Tabrizi A. Irvine BD. Bell GI. Hallewell RA. Sequence of a cDNA coding for human glutathione peroxidase confirms TGA encodes active site selenocysteine. Nucleic Acids Res. 1987;15:5484. doi: 10.1093/nar/15.13.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Myhrstad MC. Carlsen H. Nordstrom O. Blomhoff R. Moskaug JO. Flavonoids increase the intracellular glutathione level by transactivation of the gamma-glutamylcysteine synthetase catalytical subunit promoter. Free Radic Biol Med. 2002;32:386–393. doi: 10.1016/s0891-5849(01)00812-7. [DOI] [PubMed] [Google Scholar]
- 265.Mysore TB. Shinkel TA. Collins J. Salvaris EJ. Fisicaro N. Murray-Segal LJ. Johnson LE. Lepore DA. Walters SN. Stokes R. Chandra AP. O'Connell PJ. d'Apice AJ. Cowan PJ. Overexpression of glutathione peroxidase with two isoforms of superoxide dismutase protects mouse islets from oxidative injury and improves islet graft function. Diabetes. 2005;54:2109–2116. doi: 10.2337/diabetes.54.7.2109. [DOI] [PubMed] [Google Scholar]
- 266.Nakajima H. Amano W. Fujita A. Fukuhara A. Azuma YT. Hata F. Inui T. Takeuchi T. The active site cysteine of the proapoptotic protein glyceraldehyde-3-phosphate dehydrogenase is essential in oxidative stress-induced aggregation and cell death. J Biol Chem. 2007;282:26562–26574. doi: 10.1074/jbc.M704199200. [DOI] [PubMed] [Google Scholar]
- 267.Nemoto M. Nishimura R. Sasaki T. Hiki Y. Miyashita Y. Nishioka M. Fujimoto K. Sakuma T. Ohashi T. Fukuda K. Eto Y. Tajima N. Genetic association of glutathione peroxidase-1 with coronary artery calcification in type 2 diabetes: a case control study with multi-slice computed tomography. Cardiovasc Diabetol. 2007;6:23. doi: 10.1186/1475-2840-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Neve J. Human selenium supplementation as assessed by changes in blood selenium concentration and glutathione peroxidase activity. J Trace Elem Med Biol. 1995;9:65–73. doi: 10.1016/S0946-672X(11)80013-1. [DOI] [PubMed] [Google Scholar]
- 269.Nonn L. Berggren M. Powis G. Increased expression of mitochondrial peroxiredoxin-3 (thioredoxin peroxidase-2) protects cancer cells against hypoxia and drug-induced hydrogen peroxide-dependent apoptosis. Mol Cancer Res. 2003;1:682–689. [PubMed] [Google Scholar]
- 270.Nyengaard JR. Ido Y. Kilo C. Williamson JR. Interactions between hyperglycemia and hypoxia: implications for diabetic retinopathy. Diabetes. 2004;53:2931–2938. doi: 10.2337/diabetes.53.11.2931. [DOI] [PubMed] [Google Scholar]
- 271.O'Prey J. Ramsay S. Chambers I. Harrison PR. Transcriptional up-regulation of the mouse cytosolic glutathione peroxidase gene in erythroid cells is due to a tissue-specific 3′ enhancer containing functionally important CACC/GT motifs and binding sites for GATA and Ets transcription factors. Mol Cell Biol. 1993;13:6290–6303. doi: 10.1128/mcb.13.10.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Oguri M. Kato K. Hibino T. Yokoi K. Segawa T. Matsuo H. Watanabe S. Nozawa Y. Murohara T. Yamada Y. Genetic risk for restenosis after coronary stenting. Atherosclerosis. 2007;194:e172–e178. doi: 10.1016/j.atherosclerosis.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 273.Oh SH. Ganther HE. Hoekstra WG. Selenium as a component of glutathione periodase isolated from ovine erythrocytes. Biochemistry. 1974;13:1825–1829. doi: 10.1021/bi00706a008. [DOI] [PubMed] [Google Scholar]
- 274.Ohlemiller KK. McFadden SL. Ding DL. Lear PM. Ho YS. Targeted mutation of the gene for cellular glutathione peroxidase (Gpx1) increases noise-induced hearing loss in mice. J Assoc Res Otolaryngol. 2000;1:243–254. doi: 10.1007/s101620010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Oliveira-Marques V. Marinho HS. Cyrne L. Antunes F. Role of hydrogen peroxide in NF-kappaB activation: from inducer to modulator. Antioxid Redox Signal. 2009;11:2223–2243. doi: 10.1089/ars.2009.2601. [DOI] [PubMed] [Google Scholar]
- 276.Oltman CL. Kane NL. Miller FJ., Jr. Spector AA. Weintraub NL. Dellsperger KC. Reactive oxygen species mediate arachidonic acid-induced dilation in porcine coronary microvessels. Am J Physiol Heart Circ Physiol. 2003;285:H2309–H2315. doi: 10.1152/ajpheart.00456.2003. [DOI] [PubMed] [Google Scholar]
- 277.Oltvai ZN. Milliman CL. Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 278.Onumah OE. Jules GE. Zhao Y. Zhou L. Yang H. Guo Z. Overexpression of catalase delays G0/G1- to S-phase transition during cell cycle progression in mouse aortic endothelial cells. Free Radic Biol Med. 2009;46:1658–1667. doi: 10.1016/j.freeradbiomed.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Outinen PA. Sood SK. Pfeifer SI. Pamidi S. Podor TJ. Li J. Weitz JI. Austin RC. Homocysteine-induced endoplasmic reticulum stress and growth arrest leads to specific changes in gene expression in human vascular endothelial cells. Blood. 1999;94:959–967. [PubMed] [Google Scholar]
- 280.Padmaja S. Squadrito GL. Pryor WA. Inactivation of glutathione peroxidase by peroxynitrite. Arch Biochem Biophys. 1998;349:1–6. doi: 10.1006/abbi.1997.0407. [DOI] [PubMed] [Google Scholar]
- 281.Palmer RM. Ferrige AG. Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 282.Papa S. Zazzeroni F. Bubici C. Jayawardena S. Alvarez K. Matsuda S. Nguyen DU. Pham CG. Nelsbach AH. Melis T. De Smaele E. Tang WJ. D'Adamio L. Franzoso G. Gadd45 beta mediates the NF-kappa B suppression of JNK signalling by targeting MKK7/JNKK2. Nat Cell Biol. 2004;6:146–153. doi: 10.1038/ncb1093. [DOI] [PubMed] [Google Scholar]
- 283.Papp LV. Lu J. Striebel F. Kennedy D. Holmgren A. Khanna KK. The redox state of SECIS binding protein 2 controls its localization and selenocysteine incorporation function. Mol Cell Biol. 2006;26:4895–4910. doi: 10.1128/MCB.02284-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Paulsen CE. Carroll KS. Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem Biol. 2010;5:47–62. doi: 10.1021/cb900258z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Pearson DJ. Suarez-Mendez VJ. Day JP. Miller PF. Selenium status in relation to reduced glutathione peroxidase activity in aspirin-sensitive asthma. Clin Exp Allergy. 1991;21:203–208. doi: 10.1111/j.1365-2222.1991.tb00831.x. [DOI] [PubMed] [Google Scholar]
- 286.Pedraza-Chaverri J. Maldonado PD. Medina-Campos ON. Olivares-Corichi IM. Granados-Silvestre MA. Hernandez-Pando R. Ibarra-Rubio ME. Garlic ameliorates gentamicin nephrotoxicity: relation to antioxidant enzymes. Free Radic Biol Med. 2000;29:602–611. doi: 10.1016/s0891-5849(00)00354-3. [DOI] [PubMed] [Google Scholar]
- 287.Peng A. Yang C. Rui H. Li H. Study on the pathogenic factors of Kashin-Beck disease. J Toxicol Environ Health. 1992;35:79–90. doi: 10.1080/15287399209531597. [DOI] [PubMed] [Google Scholar]
- 288.Perron NR. Brumaghim JL. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys. 2009;53:75–100. doi: 10.1007/s12013-009-9043-x. [DOI] [PubMed] [Google Scholar]
- 289.Peters U. Chatterjee N. Hayes RB. Schoen RE. Wang Y. Chanock SJ. Foster CB. Variation in the selenoenzyme genes and risk of advanced distal colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2008;17:1144–1154. doi: 10.1158/1055-9965.EPI-07-2947. [DOI] [PubMed] [Google Scholar]
- 290.Piantadosi CA. Suliman HB. Mitochondrial transcription factor A induction by redox activation of nuclear respiratory factor 1. J Biol Chem. 2006;281:324–333. doi: 10.1074/jbc.M508805200. [DOI] [PubMed] [Google Scholar]
- 291.Pickett CBPD. Nguyen T. Nioi P. The Nrf2-ARE signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Pigeolet E. Corbisier P. Houbion A. Lambert D. Michiels C. Raes M. Zachary MD. Remacle J. Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech Ageing Dev. 1990;51:283–297. doi: 10.1016/0047-6374(90)90078-t. [DOI] [PubMed] [Google Scholar]
- 293.Pinto RE. Bartley W. The effect of age and sex on glutathione reductase and glutathione peroxidase activities and on aerobic glutathione oxidation in rat liver homogenates. Biochem J. 1969;112:109–115. doi: 10.1042/bj1120109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Pinto RE. Bartley W. The nature of the sex-linked differences in glutathione peroxidase activity and aerobic oxidation of glutathione in male and female rat liver. Biochem J. 1969;115:449–456. doi: 10.1042/bj1150449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Polyak K. Xia Y. Zweier JL. Kinzler KW. Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 296.Raaschou-Nielsen O. Sorensen M. Hansen RD. Frederiksen K. Tjonneland A. Overvad K. Vogel U. GPX1 Pro198Leu polymorphism, interactions with smoking and alcohol consumption, and risk for lung cancer. Cancer Lett. 2007;247:293–300. doi: 10.1016/j.canlet.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 297.Rajasekaran NS. Connell P. Christians ES. Yan LJ. Taylor RP. Orosz A. Zhang XQ. Stevenson TJ. Peshock RM. Leopold JA. Barry WH. Loscalzo J. Odelberg SJ. Benjamin IJ. Human alphaB-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice. Cell. 2007;130:427–439. doi: 10.1016/j.cell.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Rajasekaran NS. Devaraj NS. Devaraj H. Modulation of rat erythrocyte antioxidant defense system by buthionine sulfoximine and its reversal by glutathione monoester therapy. Biochim Biophys Acta. 2004;1688:121–129. doi: 10.1016/j.bbadis.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 299.Ralser M. Benjamin IJ. Reductive stress on life span extension in C. elegans. BMC Res Notes. 2008;1:19. doi: 10.1186/1756-0500-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Ratnasinghe D. Tangrea JA. Andersen MR. Barrett MJ. Virtamo J. Taylor PR. Albanes D. Glutathione peroxidase codon 198 polymorphism variant increases lung cancer risk. Cancer Res. 2000;60:6381–6383. [PubMed] [Google Scholar]
- 301.Ravn-Haren G. Olsen A. Tjonneland A. Dragsted LO. Nexo BA. Wallin H. Overvad K. Raaschou-Nielsen O. Vogel U. Associations between GPX1 Pro198Leu polymorphism, erythrocyte GPX activity, alcohol consumption and breast cancer risk in a prospective cohort study. Carcinogenesis. 2006;27:820–825. doi: 10.1093/carcin/bgi267. [DOI] [PubMed] [Google Scholar]
- 302.Reddy VN. Giblin FJ. Lin LR. Dang L. Unakar NJ. Musch DC. Boyle DL. Takemoto LJ. Ho YS. Knoernschild T. Juenemann A. Lutjen-Drecoll E. Glutathione peroxidase-1 deficiency leads to increased nuclear light scattering, membrane damage, and cataract formation in gene-knockout mice. Invest Ophthalmol Vis Sci. 2001;42:3247–3255. [PubMed] [Google Scholar]
- 303.Redman C. Scott JA. Baines AT. Basye JL. Clark LC. Calley C. Roe D. Payne CM. Nelson MA. Inhibitory effect of selenomethionine on the growth of three selected human tumor cell lines. Cancer Lett. 1998;125:103–110. doi: 10.1016/s0304-3835(97)00497-7. [DOI] [PubMed] [Google Scholar]
- 304.Reeves MA. Hoffmann PR. The human selenoproteome: recent insights into functions and regulation. Cell Mol Life Sci. 2009;66:2457–2478. doi: 10.1007/s00018-009-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Reeves WC. Marcuard SP. Willis SE. Movahed A. Reversible cardiomyopathy due to selenium deficiency. JPEN J Parenter Enteral Nutr. 1989;13:663–665. doi: 10.1177/0148607189013006663. [DOI] [PubMed] [Google Scholar]
- 306.Reszka E. Gromadzinska J. Jablonska E. Wasowicz W. Jablonowski Z. Sosnowski M. Level of selenoprotein transcripts in peripheral leukocytes of patients with bladder cancer and healthy individuals. Clin Chem Lab Med. 2009;47:1125–1132. doi: 10.1515/CCLM.2009.261. [DOI] [PubMed] [Google Scholar]
- 307.Rhee SG. Chae HZ. Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med. 2005;38:1543–1552. doi: 10.1016/j.freeradbiomed.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 308.Ridet JL. Bensadoun JC. Deglon N. Aebischer P. Zurn AD. Lentivirus-mediated expression of glutathione peroxidase: neuroprotection in murine models of Parkinson's disease. Neurobiol Dis. 2006;21:29–34. doi: 10.1016/j.nbd.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 309.Rocher C. Lalanne JL. Chaudiere J. Purification and properties of a recombinant sulfur analog of murine selenium-glutathione peroxidase. Eur J Biochem. 1992;205:955–960. doi: 10.1111/j.1432-1033.1992.tb16862.x. [DOI] [PubMed] [Google Scholar]
- 310.Rosenberger A. Illig T. Korb K. Klopp N. Zietemann V. Wolke G. Meese E. Sybrecht G. Kronenberg F. Cebulla M. Degen M. Drings P. Groschel A. Konietzko N. Kreymborg KG. Haussinger K. Hoffken G. Jilge B. Ko YD. Morr H. Schmidt C. Schmidt EW. Tauscher D. Bickeboller H. Wichmann HE. Do genetic factors protect for early onset lung cancer? A case control study before the age of 50 years. BMC Cancer. 2008;8:60. doi: 10.1186/1471-2407-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Rotruck JT. Pope AL. Ganther HE. Hoekstra WG. Prevention of oxidative damage to rat erythrocytes by dietary selenium. J Nutr. 1972;102:689–696. doi: 10.1093/jn/102.5.689. [DOI] [PubMed] [Google Scholar]
- 312.Rotruck JT. Pope AL. Ganther HE. Swanson AB. Hafeman DG. Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 313.Scheubel RJ. Zorn H. Silber RE. Kuss O. Morawietz H. Holtz J. Simm A. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2003;42:2073–2080. doi: 10.1016/j.jacc.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 314.Schnabel R. Lackner KJ. Rupprecht HJ. Espinola-Klein C. Torzewski M. Lubos E. Bickel C. Cambien F. Tiret L. Munzel T. Blankenberg S. Glutathione peroxidase-1 and homocysteine for cardiovascular risk prediction: results from the AtheroGene study. J Am Coll Cardiol. 2005;45:1631–1637. doi: 10.1016/j.jacc.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 315.Schnabel R. Lubos E. Messow CM. Sinning CR. Zeller T. Wild PS. Peetz D. Handy DE. Munzel T. Loscalzo J. Lackner KJ. Blankenberg S. Selenium supplementation improves antioxidant capacity in vitro and in vivo in patients with coronary artery disease The selenium therapy in coronary artery disease patients (SETCAP) study. Am Heart J. 2008;156(1201):e1201–e1211. doi: 10.1016/j.ahj.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Schroder E. Brennan JP. Eaton P. Cardiac peroxiredoxins undergo complex modifications during cardiac oxidant stress. Am J Physiol Heart Circ Physiol. 2008;295:H425–H433. doi: 10.1152/ajpheart.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Schroder K. Zhou R. Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010;327:296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- 318.Schulz TJ. Zarse K. Voigt A. Urban N. Birringer M. Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 319.Sedlak TW. Oltvai ZN. Yang E. Wang K. Boise LH. Thompson CB. Korsmeyer SJ. Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc Natl Acad Sci U S A. 1995;92:7834–7838. doi: 10.1073/pnas.92.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Seiler A. Schneider M. Forster H. Roth S. Wirth EK. Culmsee C. Plesnila N. Kremmer E. Radmark O. Wurst W. Bornkamm GW. Schweizer U. Conrad M. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008;8:237–248. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 321.Servais H. Ortiz A. Devuyst O. Denamur S. Tulkens PM. Mingeot-Leclercq MP. Renal cell apoptosis induced by nephrotoxic drugs: cellular and molecular mechanisms and potential approaches to modulation. Apoptosis. 2008;13:11–32. doi: 10.1007/s10495-007-0151-z. [DOI] [PubMed] [Google Scholar]
- 322.Shen Q. Chu FF. Newburger PE. Sequences in the 3′-untranslated region of the human cellular glutathione peroxidase gene are necessary and sufficient for selenocysteine incorporation at the UGA codon. J Biol Chem. 1993;268:11463–11469. [PubMed] [Google Scholar]
- 323.Shen Q. Fan L. Newburger PE. Nuclease sensitive element binding protein 1 associates with the selenocysteine insertion sequence and functions in mammalian selenoprotein translation. J Cell Physiol. 2006;207:775–783. doi: 10.1002/jcp.20619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Shi M. Yang H. Motley ED. Guo Z. Overexpression of Cu/Zn-superoxide dismutase and/or catalase in mice inhibits aorta smooth muscle cell proliferation. Am J Hypertens. 2004;17:450–456. doi: 10.1016/j.amjhyper.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 325.Sies H. Arteel GE. Interaction of peroxynitrite with selenoproteins and glutathione peroxidase mimics. Free Radic Biol Med. 2000;28:1451–1455. doi: 10.1016/s0891-5849(00)00253-7. [DOI] [PubMed] [Google Scholar]
- 326.Sies H. Moss KM. A role of mitochondrial glutathione peroxidase in modulating mitochondrial oxidations in liver. Eur J Biochem. 1978;84:377–383. doi: 10.1111/j.1432-1033.1978.tb12178.x. [DOI] [PubMed] [Google Scholar]
- 327.Sies H. Sharov VS. Klotz LO. Briviba K. Glutathione peroxidase protects against peroxynitrite-mediated oxidations. A new function for selenoproteins as peroxynitrite reductase. J Biol Chem. 1997;272:27812–27817. doi: 10.1074/jbc.272.44.27812. [DOI] [PubMed] [Google Scholar]
- 328.Silva AL. Romao L. The mammalian nonsense-mediated mRNA decay pathway: to decay or not to decay! Which players make the decision? FEBS Lett. 2009;583:499–505. doi: 10.1016/j.febslet.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 329.Simons JF. Ferro-Novick S. Rose MD. Helenius A. BiP/Kar2p serves as a molecular chaperone during carboxypeptidase Y folding in yeast. J Cell Biol. 1995;130:41–49. doi: 10.1083/jcb.130.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Singh A. Rangasamy T. Thimmulappa RK. Lee H. Osburn WO. Brigelius-Flohe R. Kensler TW. Yamamoto M. Biswal S. Glutathione peroxidase 2, the major cigarette smoke-inducible isoform of GPX in lungs, is regulated by Nrf2. Am J Respir Cell Mol Biol. 2006;35:639–650. doi: 10.1165/rcmb.2005-0325OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Singh AK. Dhaunsi GS. Gupta MP. Orak JK. Asayama K. Singh I. Demonstration of glutathione peroxidase in rat liver peroxisomes and its intraorganellar distribution. Arch Biochem Biophys. 1994;315:331–338. doi: 10.1006/abbi.1994.1508. [DOI] [PubMed] [Google Scholar]
- 332.Singh K. Laughlin J. Kosinski PA. Covey LR. Nucleolin is a second component of the CD154 mRNA stability complex that regulates mRNA turnover in activated T cells. J Immunol. 2004;173:976–985. doi: 10.4049/jimmunol.173.2.976. [DOI] [PubMed] [Google Scholar]
- 333.Skurk C. Maatz H. Kim HS. Yang J. Abid MR. Aird WC. Walsh K. The Akt-regulated forkhead transcription factor FOXO3a controls endothelial cell viability through modulation of the caspase-8 inhibitor FLIP. J Biol Chem. 2004;279:1513–1525. doi: 10.1074/jbc.M304736200. [DOI] [PubMed] [Google Scholar]
- 334.Small-Howard AL. Berry MJ. Unique features of selenocysteine incorporation function within the context of general eukaryotic translational processes. Biochem Soc Trans. 2005;33:1493–1497. doi: 10.1042/BST0331493. [DOI] [PubMed] [Google Scholar]
- 335.Soerensen M. Christensen K. Stevnsner T. Christiansen L. The Mn-superoxide dismutase single nucleotide polymorphism rs4880 and the glutathione peroxidase 1 single nucleotide polymorphism rs1050450 are associated with aging and longevity in the oldest old. Mech Ageing Dev. 2009;130:308–314. doi: 10.1016/j.mad.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Squires JE. Berry MJ. Eukaryotic selenoprotein synthesis: mechanistic insight incorporating new factors and new functions for old factors. IUBMB Life. 2008;60:232–235. doi: 10.1002/iub.38. [DOI] [PubMed] [Google Scholar]
- 337.St-Pierre J. Drori S. Uldry M. Silvaggi JM. Rhee J. Jager S. Handschin C. Zheng K. Lin J. Yang W. Simon DK. Bachoo R. Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 338.Staniek K. Nohl H. H(2)O(2) detection from intact mitochondria as a measure for one-electron reduction of dioxygen requires a non-invasive assay system. Biochim Biophys Acta. 1999;1413:70–80. doi: 10.1016/s0005-2728(99)00083-3. [DOI] [PubMed] [Google Scholar]
- 339.Stephens RS. Rentsendorj O. Servinsky LE. Moldobaeva A. Damico R. Pearse DB. cGMP increases antioxidant function and attenuates oxidant cell death in mouse lung microvascular endothelial cells by a protein kinase G-dependent mechanism. Am J Physiol Lung Cell Mol Physiol. 2010;299:L323–L333. doi: 10.1152/ajplung.00442.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Sukenaga Y. Ishida K. Takeda T. Takagi K. cDNA sequence coding for human glutathione peroxidase. Nucleic Acids Res. 1987;15:7178. doi: 10.1093/nar/15.17.7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Sun X. Li X. Moriarty PM. Henics T. LaDuca JP. Maquat LE. Nonsense-mediated decay of mRNA for the selenoprotein phospholipid hydroperoxide glutathione peroxidase is detectable in cultured cells but masked or inhibited in rat tissues. Mol Biol Cell. 2001;12:1009–1017. doi: 10.1091/mbc.12.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Sunde RA. Raines AM. Barnes KM. Evenson JK. Selenium status highly regulates selenoprotein mRNA levels for only a subset of the selenoproteins in the selenoproteome. Biosci Rep. 2009;29:329–338. doi: 10.1042/BSR20080146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Sung B. Pandey MK. Aggarwal BB. Fisetin, an inhibitor of cyclin-dependent kinase 6, down-regulates nuclear factor-kappaB-regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of TAK-1 and receptor-interacting protein-regulated IkappaBalpha kinase activation. Mol Pharmacol. 2007;71:1703–1714. doi: 10.1124/mol.107.034512. [DOI] [PubMed] [Google Scholar]
- 344.Sutton A. Nahon P. Pessayre D. Rufat P. Poire A. Ziol M. Vidaud D. Barget N. Ganne-Carrie N. Charnaux N. Trinchet JC. Gattegno L. Beaugrand M. Genetic polymorphisms in antioxidant enzymes modulate hepatic iron accumulation and hepatocellular carcinoma development in patients with alcohol-induced cirrhosis. Cancer Res. 2006;66:2844–2852. doi: 10.1158/0008-5472.CAN-05-2566. [DOI] [PubMed] [Google Scholar]
- 345.Suzuki K. Koike H. Matsui H. Ono Y. Hasumi M. Nakazato H. Okugi H. Sekine Y. Oki K. Ito K. Yamamoto T. Fukabori Y. Kurokawa K. Yamanaka H. Genistein, a soy isoflavone, induces glutathione peroxidase in the human prostate cancer cell lines LNCaP and PC-3. Int J Cancer. 2002;99:846–852. doi: 10.1002/ijc.10428. [DOI] [PubMed] [Google Scholar]
- 346.Takahashi K. Akasaka M. Yamamoto Y. Kobayashi C. Mizoguchi J. Koyama J. Primary structure of human plasma glutathione peroxidase deduced from cDNA sequences. J Biochem. 1990;108:145–148. doi: 10.1093/oxfordjournals.jbchem.a123172. [DOI] [PubMed] [Google Scholar]
- 347.Takata Y. Morris JS. King IB. Kristal AR. Lin DW. Peters U. Correlation between selenium concentrations and glutathione peroxidase activity in serum and human prostate tissue. Prostate. 2009;69:1635–1642. doi: 10.1002/pros.21012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Takebe G. Yarimizu J. Saito Y. Hayashi T. Nakamura H. Yodoi J. Nagasawa S. Takahashi K. A comparative study on the hydroperoxide and thiol specificity of the glutathione peroxidase family and selenoprotein P. J Biol Chem. 2002;277:41254–41258. doi: 10.1074/jbc.M202773200. [DOI] [PubMed] [Google Scholar]
- 349.Tan M. Li S. Swaroop M. Guan K. Oberley LW. Sun Y. Transcriptional activation of the human glutathione peroxidase promoter by p53. J Biol Chem. 1999;274:12061–12066. doi: 10.1074/jbc.274.17.12061. [DOI] [PubMed] [Google Scholar]
- 350.Tang NP. Wang LS. Yang L. Gu HJ. Sun QM. Cong RH. Zhou B. Zhu HJ. Wang B. Genetic variant in glutathione peroxidase 1 gene is associated with an increased risk of coronary artery disease in a Chinese population. Clin Chim Acta. 2008;395:89–93. doi: 10.1016/j.cca.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 351.Taylor JM. Ali U. Iannello RC. Hertzog P. Crack PJ. Diminished Akt phosphorylation in neurons lacking glutathione peroxidase-1 (Gpx1) leads to increased susceptibility to oxidative stress-induced cell death. J Neurochem. 2005;92:283–293. doi: 10.1111/j.1471-4159.2004.02863.x. [DOI] [PubMed] [Google Scholar]
- 352.Teng S. Gao L. Paajanen V. Pu J. Fan Z. Readthrough of nonsense mutation W822X in the SCN5A gene can effectively restore expression of cardiac Na+ channels. Cardiovasc Res. 2009;83:473–480. doi: 10.1093/cvr/cvp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Thimmulappa RK. Mai KH. Srisuma S. Kensler TW. Yamamoto M. Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 354.Thomas SR. Schulz E. Keaney JF., Jr Hydrogen peroxide restrains endothelium-derived nitric oxide bioactivity—role for iron-dependent oxidative stress. Free Radic Biol Med. 2006;41:681–688. doi: 10.1016/j.freeradbiomed.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 355.Thomson CD. Rea HM. Doesburg VM. Robinson MF. Selenium concentrations and glutathione peroxidase activities in whole blood of New Zealand residents. Br J Nutr. 1977;37:457–460. doi: 10.1079/bjn19770049. [DOI] [PubMed] [Google Scholar]
- 356.Throm SL. Klemsz MJ. PU.1 regulates glutathione peroxidase expression in neutrophils. J Leukoc Biol. 2003;74:111–117. doi: 10.1189/jlb.0203061. [DOI] [PubMed] [Google Scholar]
- 357.Thu VT. Kim HK. Ha SH. Yoo JY. Park WS. Kim N. Oh GT. Han J. Glutathione peroxidase 1 protects mitochondria against hypoxia/reoxygenation damage in mouse hearts. Pflugers Arch. 2010;460:55–68. doi: 10.1007/s00424-010-0811-7. [DOI] [PubMed] [Google Scholar]
- 358.Tilton RG. Diabetic vascular dysfunction: links to glucose-induced reductive stress and VEGF. Microsc Res Tech. 2002;57:390–407. doi: 10.1002/jemt.10092. [DOI] [PubMed] [Google Scholar]
- 359.Toppo S. Flohe L. Ursini F. Vanin S. Maiorino M. Catalytic mechanisms and specificities of glutathione peroxidases: variations of a basic scheme. Biochim Biophys Acta. 2009;1790:1486–1500. doi: 10.1016/j.bbagen.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 360.Torzewski M. Ochsenhirt V. Kleschyov AL. Oelze M. Daiber A. Li H. Rossmann H. Tsimikas S. Reifenberg K. Cheng F. Lehr HA. Blankenberg S. Forstermann U. Munzel T. Lackner KJ. Deficiency of glutathione peroxidase-1 accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:850–857. doi: 10.1161/01.ATV.0000258809.47285.07. [DOI] [PubMed] [Google Scholar]
- 361.Tosatto SC. Bosello V. Fogolari F. Mauri P. Roveri A. Toppo S. Flohe L. Ursini F. Maiorino M. The catalytic site of glutathione peroxidases. Antioxid Redox Signal. 2008;10:1515–1526. doi: 10.1089/ars.2008.2055. [DOI] [PubMed] [Google Scholar]
- 362.Trotter EW. Grant CM. Thioredoxins are required for protection against a reductive stress in the yeast Saccharomyces cerevisiae. Mol Microbiol. 2002;46:869–878. doi: 10.1046/j.1365-2958.2002.03216.x. [DOI] [PubMed] [Google Scholar]
- 363.Tsuru-Aoyagi K. Potts MB. Trivedi A. Pfankuch T. Raber J. Wendland M. Claus CP. Koh SE. Ferriero D. Noble-Haeusslein LJ. Glutathione peroxidase activity modulates recovery in the injured immature brain. Ann Neurol. 2009;65:540–549. doi: 10.1002/ana.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Tujebajeva RM. Copeland PR. Xu XM. Carlson BA. Harney JW. Driscoll DM. Hatfield DL. Berry MJ. Decoding apparatus for eukaryotic selenocysteine insertion. EMBO Rep. 2000;1:158–163. doi: 10.1093/embo-reports/kvd033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Turner N. Heilbronn LK. Is mitochondrial dysfunction a cause of insulin resistance? Trends Endocrinol Metab. 2008;19:324–330. doi: 10.1016/j.tem.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 366.Udler M. Maia AT. Cebrian A. Brown C. Greenberg D. Shah M. Caldas C. Dunning A. Easton D. Ponder B. Pharoah P. Common germline genetic variation in antioxidant defense genes and survival after diagnosis of breast cancer. J Clin Oncol. 2007;25:3015–3023. doi: 10.1200/JCO.2006.10.0099. [DOI] [PubMed] [Google Scholar]
- 367.Ulker S. McMaster D. McKeown PP. Bayraktutan U. Impaired activities of antioxidant enzymes elicit endothelial dysfunction in spontaneous hypertensive rats despite enhanced vascular nitric oxide generation. Cardiovasc Res. 2003;59:488–500. doi: 10.1016/s0008-6363(03)00424-3. [DOI] [PubMed] [Google Scholar]
- 368.Ullmann K. Wiencierz AM. Muller C. Thierbach R. Steege A. Toyokuni S. Steinberg P. A high-throughput reporter gene assay to prove the ability of natural compounds to modulate glutathione peroxidase, superoxide dismutase and catalase gene promoters in V79 cells. Free Radic Res. 2008;42:746–753. doi: 10.1080/10715760802337273. [DOI] [PubMed] [Google Scholar]
- 369.Ursini F. Maiorino M. Brigelius-Flohe R. Aumann KD. Roveri A. Schomburg D. Flohe L. Diversity of glutathione peroxidases. Methods Enzymol. 1995;252:38–53. doi: 10.1016/0076-6879(95)52007-4. [DOI] [PubMed] [Google Scholar]
- 370.Utsunomiya H. Komatsu N. Yoshimura S. Tsutsumi Y. Watanabe K. Exact ultrastructural localization of glutathione peroxidase in normal rat hepatocytes: advantages of microwave fixation. J Histochem Cytochem. 1991;39:1167–1174. doi: 10.1177/39.9.1918936. [DOI] [PubMed] [Google Scholar]
- 371.Van Remmen H. Qi W. Sabia M. Freeman G. Estlack L. Yang H. Mao Guo Z. Huang TT. Strong R. Lee S. Epstein CJ. Richardson A. Multiple deficiencies in antioxidant enzymes in mice result in a compound increase in sensitivity to oxidative stress. Free Radic Biol Med. 2004;36:1625–1634. doi: 10.1016/j.freeradbiomed.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 372.Vanhoutte PM. Endothelial dysfunction: the first step toward coronary arteriosclerosis. Circ J. 2009;73:595–601. doi: 10.1253/circj.cj-08-1169. [DOI] [PubMed] [Google Scholar]
- 373.Vessey DA. Lee KH. Inactivation of enzymes of the glutathione antioxidant system by treatment of cultured human keratinocytes with peroxides. J Invest Dermatol. 1993;100:829–833. doi: 10.1111/1523-1747.ep12476735. [DOI] [PubMed] [Google Scholar]
- 374.Vogel U. Olsen A. Wallin H. Overvad K. Tjonneland A. Nexo BA. No association between GPX Pro198Leu and risk of basal cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2004;13:1412–1413. [PubMed] [Google Scholar]
- 375.Wagner AH. Kautz O. Fricke K. Zerr-Fouineau M. Demicheva E. Guldenzoph B. Bermejo JL. Korff T. Hecker M. Upregulation of glutathione peroxidase offsets stretch-induced proatherogenic gene expression in human endothelial cells. Arterioscler Thromb Vasc Biol. 2009;29:1894–1901. doi: 10.1161/ATVBAHA.109.194738. [DOI] [PubMed] [Google Scholar]
- 376.Wang HD. Xu S. Johns DG. Du Y. Quinn MT. Cayatte AJ. Cohen RA. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ Res. 2001;88:947–953. doi: 10.1161/hh0901.089987. [DOI] [PubMed] [Google Scholar]
- 377.Wang HP. Schafer FQ. Goswami PC. Oberley LW. Buettner GR. Phospholipid hydroperoxide glutathione peroxidase induces a delay in G1 of the cell cycle. Free Radic Res. 2003;37:621–630. doi: 10.1080/1071576031000088283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Wang QS. Zheng YM. Dong L. Ho YS. Guo Z. Wang YX. Role of mitochondrial reactive oxygen species in hypoxia-dependent increase in intracellular calcium in pulmonary artery myocytes. Free Radic Biol Med. 2007;42:642–653. doi: 10.1016/j.freeradbiomed.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Wang S. Konorev EA. Kotamraju S. Joseph J. Kalivendi S. Kalyanaraman B. Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms. intermediacy of H(2)O(2)- and p53-dependent pathways. J Biol Chem. 2004;279:25535–25543. doi: 10.1074/jbc.M400944200. [DOI] [PubMed] [Google Scholar]
- 380.Wang S. Kotamraju S. Konorev E. Kalivendi S. Joseph J. Kalyanaraman B. Activation of nuclear factor-kappaB during doxorubicin-induced apoptosis in endothelial cells and myocytes is pro-apoptotic: the role of hydrogen peroxide. Biochem J. 2002;367:729–740. doi: 10.1042/BJ20020752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Wang S. Wang F. Shi X. Dai J. Peng Y. Guo X. Wang X. Shen H. Hu Z. Association between manganese superoxide dismutase (MnSOD) Val-9Ala polymorphism and cancer risk—a meta-analysis. Eur J Cancer. 2009;45:2874–2881. doi: 10.1016/j.ejca.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 382.Wang XD. Vatamaniuk MZ. Wang SK. Roneker CA. Simmons RA. Lei XG. Molecular mechanisms for hyperinsulinaemia induced by overproduction of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia. 2008;51:1515–1524. doi: 10.1007/s00125-008-1055-3. [DOI] [PubMed] [Google Scholar]
- 383.Weisbrot-Lefkowitz M. Reuhl K. Perry B. Chan PH. Inouye M. Mirochnitchenko O. Overexpression of human glutathione peroxidase protects transgenic mice against focal cerebral ischemia/reperfusion damage. Brain Res Mol Brain Res. 1998;53:333–338. doi: 10.1016/s0169-328x(97)00313-6. [DOI] [PubMed] [Google Scholar]
- 384.Weiss N. Zhang YY. Heydrick S. Bierl C. Loscalzo J. Overexpression of cellular glutathione peroxidase rescues homocyst(e)ine-induced endothelial dysfunction. Proc Natl Acad Sci U S A. 2001;98:12503–12508. doi: 10.1073/pnas.231428998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Weiss Sachdev S. Sunde RA. Selenium regulation of transcript abundance and translational efficiency of glutathione peroxidase-1 and −4 in rat liver. Biochem J. 2001;357:851–858. doi: 10.1042/0264-6021:3570851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Weitzel F. Ursini F. Wendel A. Phospholipid hydroperoxide glutathione peroxidase in various mouse organs during selenium deficiency and repletion. Biochim Biophys Acta. 1990;1036:88–94. doi: 10.1016/0304-4165(90)90018-r. [DOI] [PubMed] [Google Scholar]
- 387.Whanger PD. Selenocompounds in plants and animals and their biological significance. J Am Coll Nutr. 2002;21:223–232. doi: 10.1080/07315724.2002.10719214. [DOI] [PubMed] [Google Scholar]
- 388.Wilschanski M. Yahav Y. Yaacov Y. Blau H. Bentur L. Rivlin J. Aviram M. Bdolah-Abram T. Bebok Z. Shushi L. Kerem B. Kerem E. Gentamicin-induced correction of CFTR function in patients with cystic fibrosis and CFTR stop mutations. N Engl J Med. 2003;349:1433–1441. doi: 10.1056/NEJMoa022170. [DOI] [PubMed] [Google Scholar]
- 389.Wingler K. Bocher M. Flohe L. Kollmus H. Brigelius-Flohe R. mRNA stability and selenocysteine insertion sequence efficiency rank gastrointestinal glutathione peroxidase high in the hierarchy of selenoproteins. Eur J Biochem. 1999;259:149–157. doi: 10.1046/j.1432-1327.1999.00012.x. [DOI] [PubMed] [Google Scholar]
- 390.Winterbourn CC. Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 391.Witting PK. Pettersson K. Letters J. Stocker R. Site-specific antiatherogenic effect of probucol in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:E26–E33. doi: 10.1161/01.atv.20.8.e26. [DOI] [PubMed] [Google Scholar]
- 392.Wong CH. Bozinovski S. Hertzog PJ. Hickey MJ. Crack PJ. Absence of glutathione peroxidase-1 exacerbates cerebral ischemia-reperfusion injury by reducing post-ischemic microvascular perfusion. J Neurochem. 2008;107:241–252. doi: 10.1111/j.1471-4159.2008.05605.x. [DOI] [PubMed] [Google Scholar]
- 393.Wu J. Xu GL. Plasma selenium content, platelet glutathione peroxidase and superoxide dismutase activity of residents in Kashin-Beck disease affected area in China. J Trace Elem Electrolytes Health Dis. 1987;1:39–43. [PubMed] [Google Scholar]
- 394.Wu R. Shen Q. Newburger PE. Recognition and binding of the human selenocysteine insertion sequence by nucleolin. J Cell Biochem. 2000;77:507–516. doi: 10.1002/(sici)1097-4644(20000601)77:3<507::aid-jcb15>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 395.Wu WJ. Sha SH. Schacht J. Recent advances in understanding aminoglycoside ototoxicity and its prevention. Audiol Neurootol. 2002;7:171–174. doi: 10.1159/000058305. [DOI] [PubMed] [Google Scholar]
- 396.Wu Z. Puigserver P. Andersson U. Zhang C. Adelmant G. Mootha V. Troy A. Cinti S. Lowell B. Scarpulla RC. Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 397.Xiong Y. Liu X. Lee CP. Chua BH. Ho YS. Attenuation of doxorubicin-induced contractile and mitochondrial dysfunction in mouse heart by cellular glutathione peroxidase. Free Radic Biol Med. 2006;41:46–55. doi: 10.1016/j.freeradbiomed.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 398.Yamaguchi T. Sano K. Takakura K. Saito I. Shinohara Y. Asano T. Yasuhara H. Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Ebselen Study Group. Stroke. 1998;29:12–17. doi: 10.1161/01.str.29.1.12. [DOI] [PubMed] [Google Scholar]
- 399.Yang P. Bamlet WR. Ebbert JO. Taylor WR. de Andrade M. Glutathione pathway genes and lung cancer risk in young and old populations. Carcinogenesis. 2004;25:1935–1944. doi: 10.1093/carcin/bgh203. [DOI] [PubMed] [Google Scholar]
- 400.Yang Y. Song Y. Loscalzo J. Regulation of the protein disulfide proteome by mitochondria in mammalian cells. Proc Natl Acad Sci U S A. 2007;104:10813–10817. doi: 10.1073/pnas.0702027104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Yant LJ. Ran Q. Rao L. Van Remmen H. Shibatani T. Belter JG. Motta L. Richardson A. Prolla TA. The selenoprotein GPX4 is essential for mouse development and protects from radiation and oxidative damage insults. Free Radic Biol Med. 2003;34:496–502. doi: 10.1016/s0891-5849(02)01360-6. [DOI] [PubMed] [Google Scholar]
- 402.Yoshida T. Maulik N. Engelman RM. Ho YS. Magnenat JL. Rousou JA. Flack JE., 3rd Deaton D. Das DK. Glutathione peroxidase knockout mice are susceptible to myocardial ischemia reperfusion injury. Circulation. 1997;96:II-216–II-220. [PubMed] [Google Scholar]
- 403.Zavacki AM. Mansell JB. Chung M. Klimovitsky B. Harney JW. Berry MJ. Coupled tRNA(Sec)-dependent assembly of the selenocysteine decoding apparatus. Mol Cell. 2003;11:773–781. doi: 10.1016/s1097-2765(03)00064-9. [DOI] [PubMed] [Google Scholar]
- 404.Zemlyak I. Brooke SM. Singh MH. Sapolsky RM. Effects of overexpression of antioxidants on the release of cytochrome c and apoptosis-inducing factor in the model of ischemia. Neurosci Lett. 2009;453:182–185. doi: 10.1016/j.neulet.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 405.Zhang X. Min X. Li C. Benjamin IJ. Qian B. Zhang X. Ding Z. Gao X. Yao Y. Ma Y. Cheng Y. Liu L. Involvement of reductive stress in the cardiomyopathy in transgenic mice with cardiac-specific overexpression of heat shock protein 27. Hypertension. 2010;55:1412–1417. doi: 10.1161/HYPERTENSIONAHA.109.147066. [DOI] [PubMed] [Google Scholar]
- 406.Zhang Y. Handy DE. Loscalzo J. Adenosine-dependent induction of glutathione peroxidase 1 in human primary endothelial cells and protection against oxidative stress. Circ Res. 2005;96:831–837. doi: 10.1161/01.RES.0000164401.21929.CF. [DOI] [PubMed] [Google Scholar]
- 407.Zhao H. Liang D. Grossman HB. Wu X. Glutathione peroxidase 1 gene polymorphism and risk of recurrence in patients with superficial bladder cancer. Urology. 2005;66:769–774. doi: 10.1016/j.urology.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 408.Zhong L. Holmgren A. Essential role of selenium in the catalytic activities of mammalian thioredoxin reductase revealed by characterization of recombinant enzymes with selenocysteine mutations. J Biol Chem. 2000;275:18121–18128. doi: 10.1074/jbc.M000690200. [DOI] [PubMed] [Google Scholar]
- 409.Zhou G. Seibenhener ML. Wooten MW. Nucleolin is a protein kinase C-zeta substrate. Connection between cell surface signaling and nucleus in PC12 cells. J Biol Chem. 1997;272:31130–31137. doi: 10.1074/jbc.272.49.31130. [DOI] [PubMed] [Google Scholar]
- 410.Zhou LZ. Johnson AP. Rando TA. NF kappa B and AP-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Free Radic Biol Med. 2001;31:1405–1416. doi: 10.1016/s0891-5849(01)00719-5. [DOI] [PubMed] [Google Scholar]
- 411.Zhuo H. Smith AH. Steinmaus C. Selenium and lung cancer: a quantitative analysis of heterogeneity in the current epidemiological literature. Cancer Epidemiol Biomarkers Prev. 2004;13:771–778. [PubMed] [Google Scholar]
- 412.Zhuo P. Goldberg M. Herman L. Lee BS. Wang H. Brown RL. Foster CB. Peters U. Diamond AM. Molecular consequences of genetic variations in the glutathione peroxidase 1 selenoenzyme. Cancer Res. 2009;69:8183–8190. doi: 10.1158/0008-5472.CAN-09-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Zsembery A. Jessner W. Sitter G. Spirli C. Strazzabosco M. Graf J. Correction of CFTR malfunction and stimulation of Ca-activated Cl channels restore HCO3- secretion in cystic fibrosis bile ductular cells. Hepatology. 2002;35:95–104. doi: 10.1053/jhep.2002.30423. [DOI] [PubMed] [Google Scholar]