Abstract
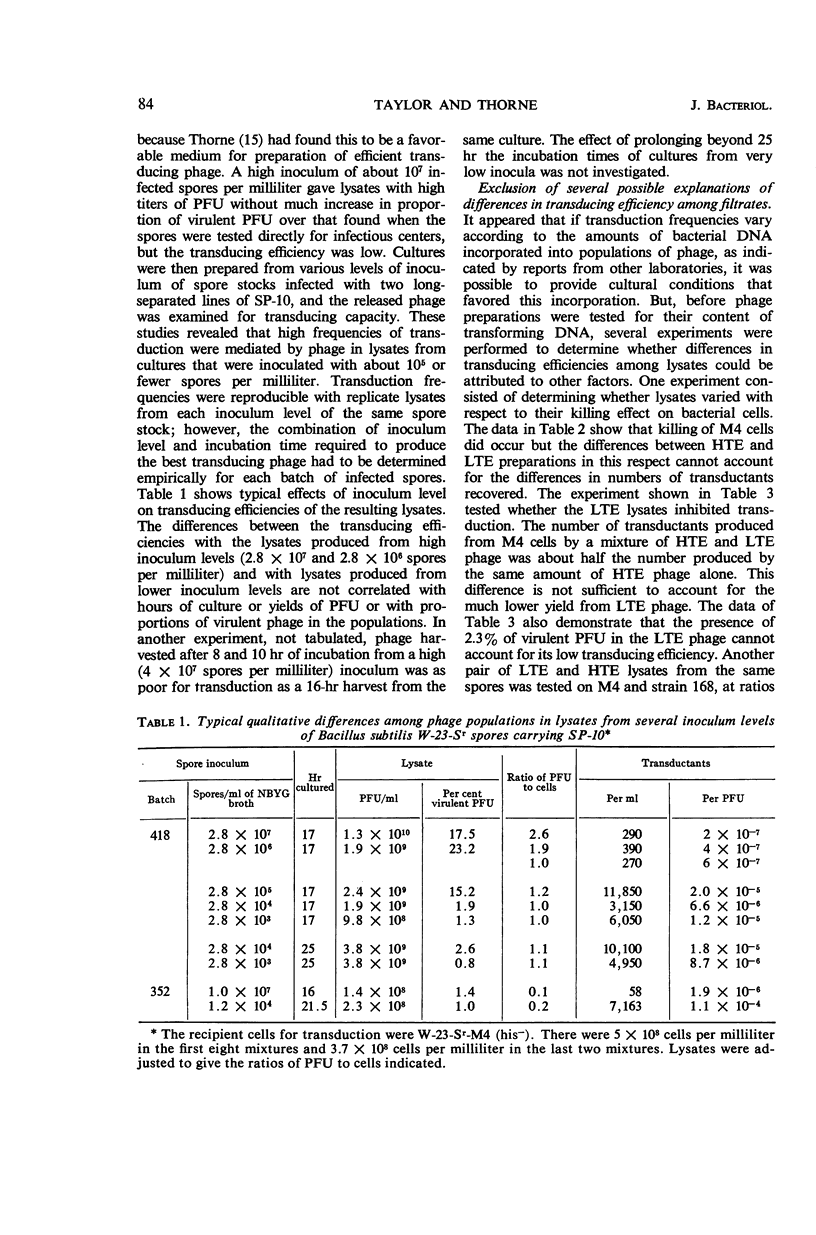
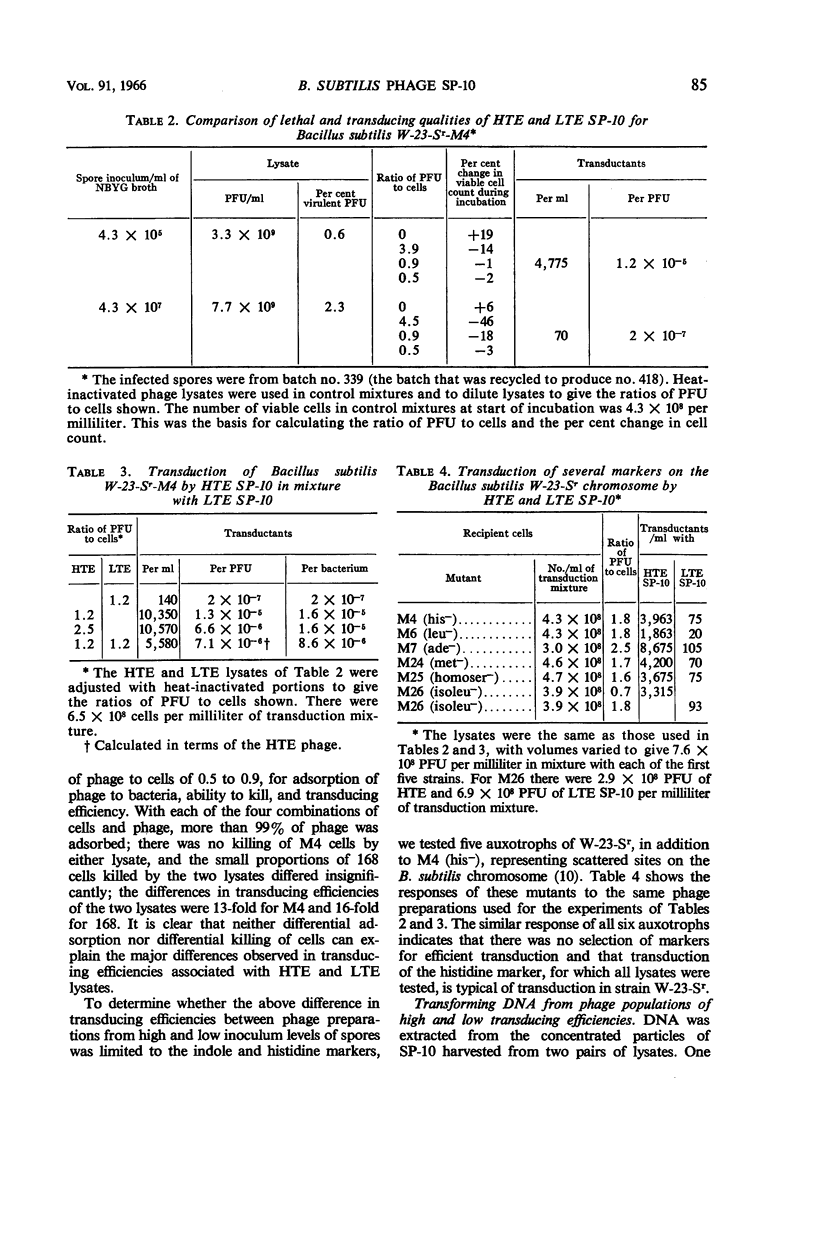
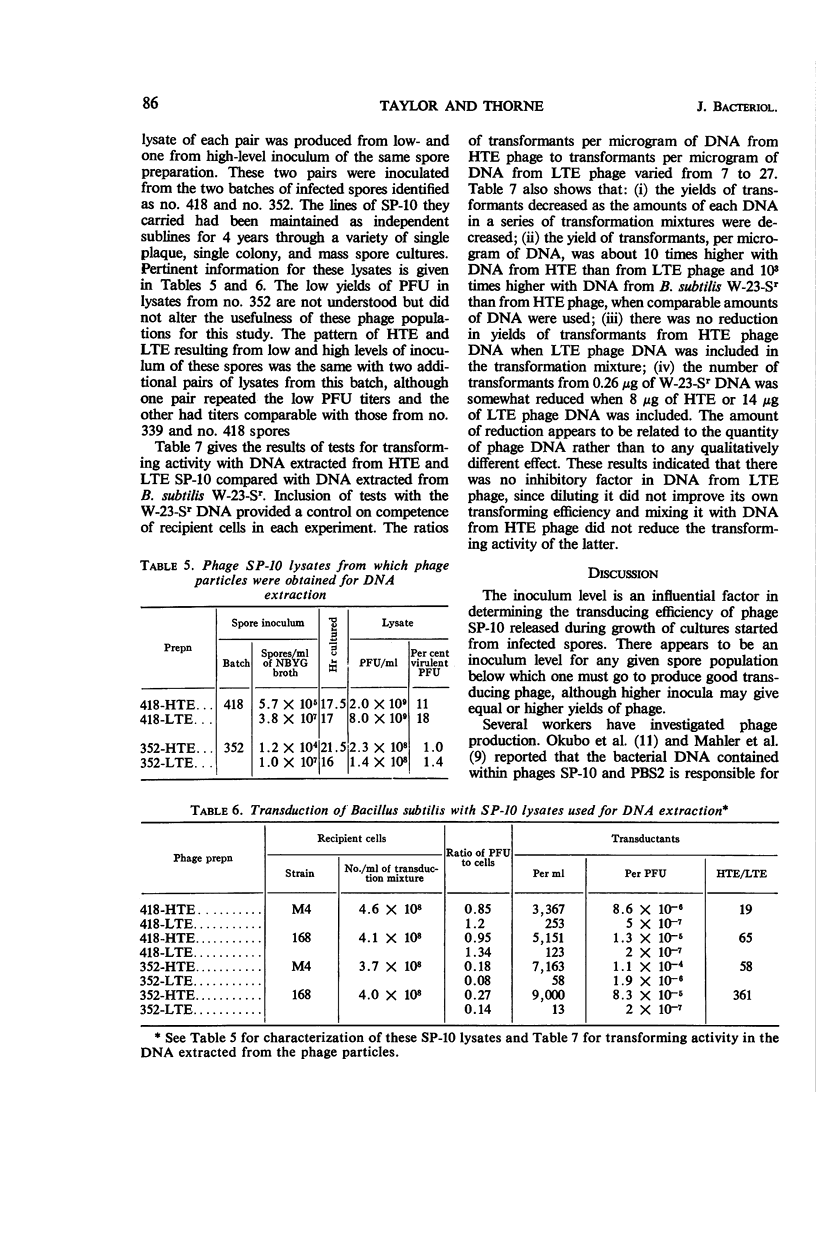
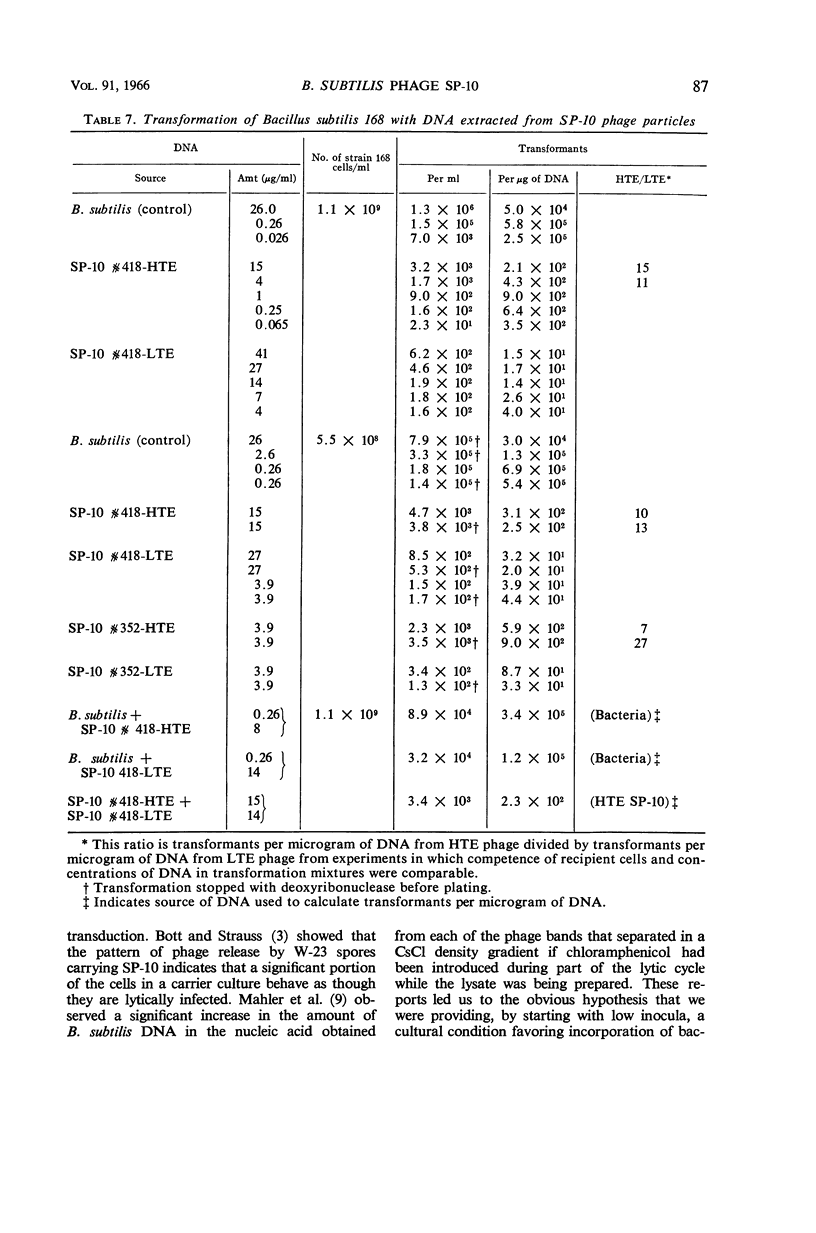
Taylor, Martha J. (Fort Detrick, Frederick, Md.), and Curtis B. Thorne. Concurrent changes in transducing efficiency and content of transforming deoxyribonucleic acid in Bacillus subtilis bacteriophage SP-10. J. Bacteriol. 91:81–88. 1966.—Spores of Bacillus subtilis W-23-Sr infected with transducing phage SP-10 served as convenient inocula for broth cultures from which transducing phage was harvested. Methods are described for producing highly infected spores. The inoculum level of infected spores in nutrient broth-yeast extract-glucose medium affected the transducing efficiency of SP-10 in lysates of these cultures. Phage in lysates of cultures inoculated with about 105 or fewer spores per milliliter transduced 20- to 350-fold more efficiently than did phage in lysates from cultures inoculated with 106 to 107 spores per milliliter. Transduction frequencies in the order of 10−5 per plaque-forming unit were obtained routinely, and some infected-spore preparations yielded phage that gave frequencies as high as 10−4. The combination of inoculum level and incubation time required to produce the best transducing phage had to be determined empirically for each batch of infected spores. Several possible explanations for the difference between lysates having high (HTE) and those having low (LTE) transducing efficiency were ruled out by special experiments. The hypothesis is presented that some cultural condition resulting from a relatively low inoculum of phage-infected spores favors the incorporation by phage particles of bacterial deoxyribonucleic acid (DNA) in the manner required for the production of transducing phage. Support for this hypothesis is a demonstration, through transformation experiments with DNA extracted from HTE and LTE phage particles, that populations of HTE phage particles yielded significantly more (7 to 27 times) transforming activity per microgram of DNA than did populations of LTE phage.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOTT K., STRAUSS B. THE CARRIER STATE OF BACILLUS SUBTILIS INFECTED WITH THE TRANSDUCING BACTERIOPHAGE SP10. Virology. 1965 Feb;25:212–225. doi: 10.1016/0042-6822(65)90200-x. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GWINN D. D., THORNE C. B. TRANSFORMATION OF BACILLUS LICHENIFORMIS. J Bacteriol. 1964 Mar;87:519–526. doi: 10.1128/jb.87.3.519-526.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVANOVICS G., CSISZAR K. Isolation and some characteristics of subtilis pages with transdducing activity. Acta Microbiol Acad Sci Hung. 1962;9:209–218. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LWOFF A. Lysogeny. Bacteriol Rev. 1953 Dec;17(4):269–337. doi: 10.1128/br.17.4.269-337.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAHLER I., CAHOON M., MARMUR J. BACILLUS SUBTILIS DEOXYRIBONUCLEIC ACID TRANSFER IN PBS2 TRANSDUCTION. J Bacteriol. 1964 Jun;87:1423–1428. doi: 10.1128/jb.87.6.1423-1428.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OISHI M., YOSHIKAWA H., SUEOKA N. SYNCHRONOUS AND DICHOTOMOUS REPLICATIONS OF THE BACILLUS SUBTILIS CHROMOSOME DURING SPORE GERMINATION. Nature. 1964 Dec 12;204:1069–1073. doi: 10.1038/2041069a0. [DOI] [PubMed] [Google Scholar]
- OKUBO S., STODOLSKY M., BOTT K., STRAUSS B. SEPARATION OF THE TRANSFORMING AND VIRAL DEOXYRIBONUCLEIC ACIDS OF A TRANSDUCING BACTERIOPHAGE OF BACILLUS SUBTILIS. Proc Natl Acad Sci U S A. 1963 Oct;50:679–686. doi: 10.1073/pnas.50.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMIG W. R. Infection of Bacillus subtilis with phenol-extracted bacteriophages. Virology. 1962 Apr;16:452–459. doi: 10.1016/0042-6822(62)90226-x. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI I. Transducing phages for Bacillus subtilis. J Gen Microbiol. 1963 May;31:211–217. doi: 10.1099/00221287-31-2-211. [DOI] [PubMed] [Google Scholar]
- TAYLOR M. J., THORNE C. B. TRANSDUCTION OF BACILLUS LICHENIFORMIS AND BACILLUS SUBTILIS BY EACH OF TWO PHAGES. J Bacteriol. 1963 Sep;86:452–461. doi: 10.1128/jb.86.3.452-461.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNE C. B. Transduction in Bacillus subtilis. J Bacteriol. 1962 Jan;83:106–111. doi: 10.1128/jb.83.1.106-111.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]


