Abstract
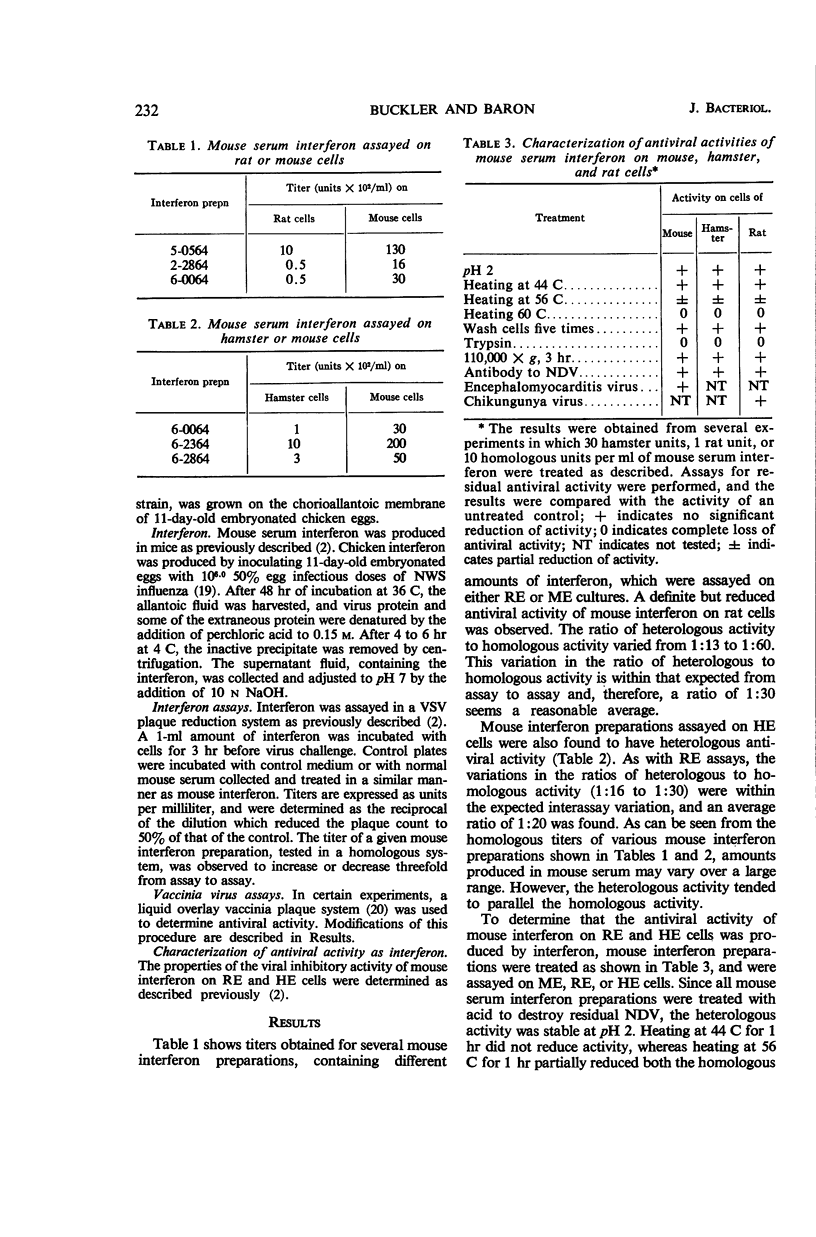
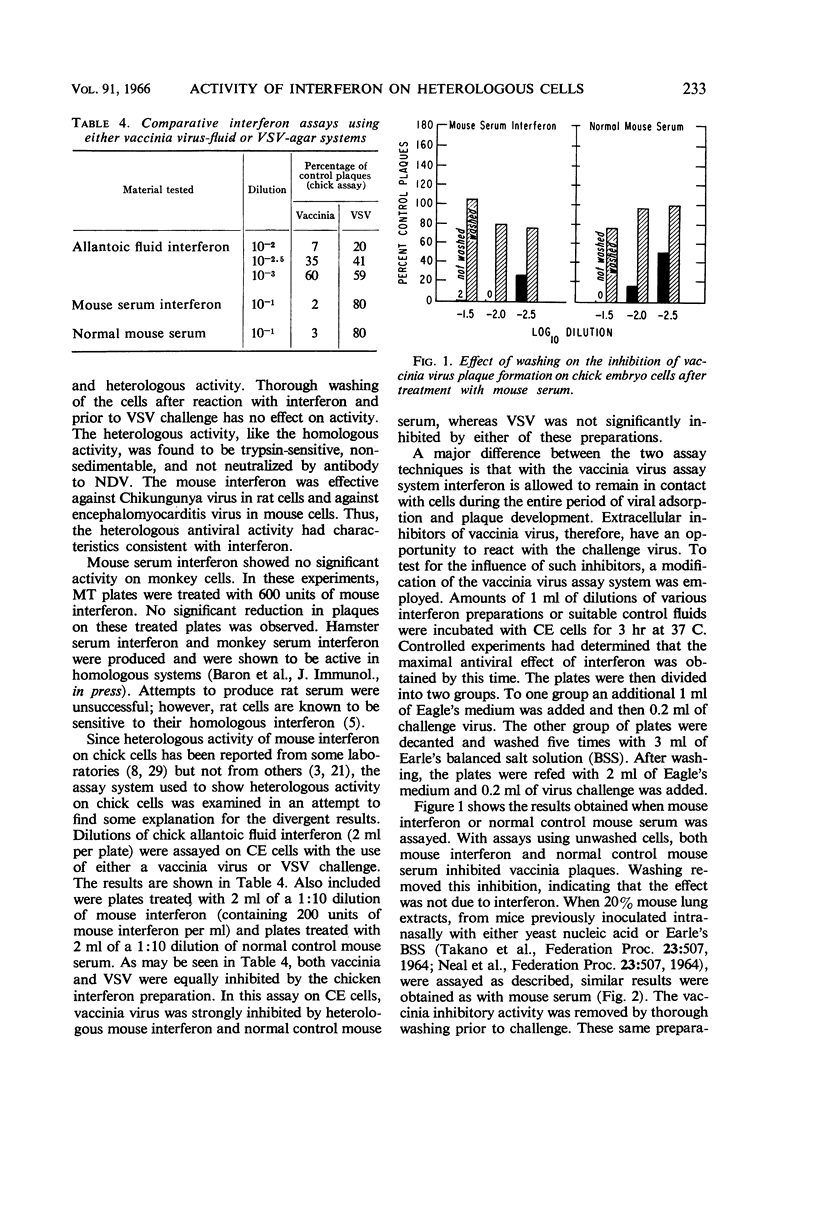
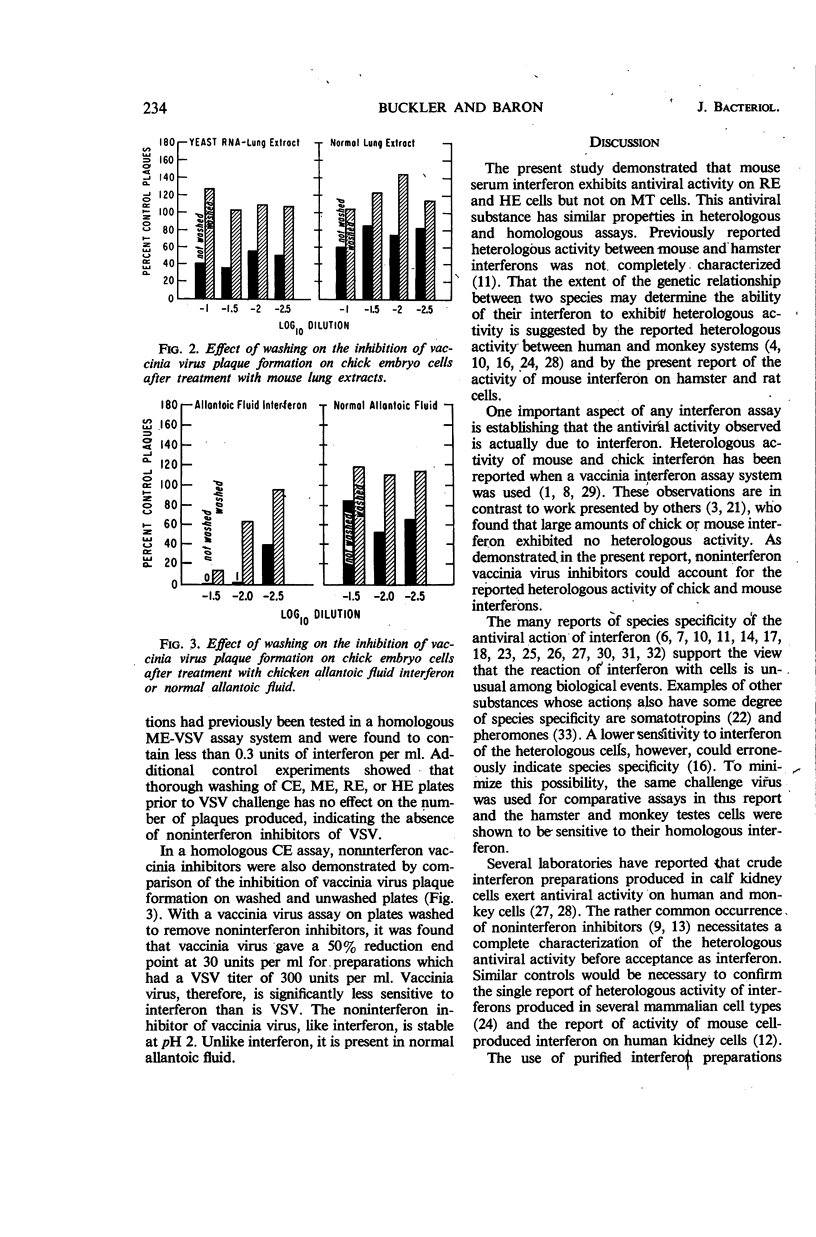
Buckler, Charles E. (National Institute of Allergy and Infectious Diseases, Bethesda, Md.), and Samuel Baron. Antiviral action of mouse interferon in heterologous cells. J. Bacteriol. 91:231–235. 1966.—The antiviral action of mouse interferon in cell cultures of mouse, hamster, rat, chicken, and monkey origin was investigated. Using a vesicular stomatitis virus (VSV) plaque reduction test, we found that mouse serum interferon, assayed on closely related rat or hamster cells, exerted 5% of its homologous antiviral activity. This activity was characterized as interferon by its temperature of inactivation, trypsin sensitivity, nonsedimentability, stability at pH 2, lack of inactivation by antibody to virus, and inability to be washed off cells. In the more distantly related chicken and monkey cells, mouse interferon had less than 0.1% of its homologous activity. Conflicting reports of heterologous activity of chicken and mouse interferon preparations may result in part from the observed action of noninterferon inhibitors of vaccinia virus. These inhibitors, like interferon, are stable at pH 2. They are present in mouse serum, mouse lung extracts, and allantoic fluid, and they prevent the development of vaccinia plaques when allowed to remain in contact with cells during virus growth. Unlike interferon the inhibitors are removed by adequate washing of cells prior to virus challenge, and they are not active in the VSV assay system. These findings reemphasize the need for thorough characterization of interferon preparations.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews R. D. Specificity of Interferon. Br Med J. 1961 Jun 17;1(5241):1728–1730. doi: 10.1136/bmj.1.5241.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARON S., BARBAN S., BUCKLER C. E. HOST CELL SPECIES SPECIFICITY OF MOUSE AND CHICKEN INTERFERONS. Science. 1964 Aug 21;145(3634):814–814. doi: 10.1126/science.145.3634.814. [DOI] [PubMed] [Google Scholar]
- BARON S., BUCKLER C. E. CIRCULATING INTERFERON IN MICE AFTER INTRAVENOUS INJECTION OF VIRUS. Science. 1963 Sep 13;141(3585):1061–1063. doi: 10.1126/science.141.3585.1061. [DOI] [PubMed] [Google Scholar]
- CHANY C. An interferon-like inhibitor of viral multiplication from malignant cells (the viral autoinhibition phenomenon). Virology. 1961 Apr;13:485–492. doi: 10.1016/0042-6822(61)90279-3. [DOI] [PubMed] [Google Scholar]
- FALCOFF E., FAUCONNIER B. IN VITRO PRODUCTION OF AN INTERFERON-LIKE INHIBITOR OF VIRAL MULTIPLICATION BY A POIKILOTHERMIC ANIMAL CELL, THE TORTOISE (TESTUDO GRECA). Proc Soc Exp Biol Med. 1965 Mar;118:609–612. doi: 10.3181/00379727-118-29918. [DOI] [PubMed] [Google Scholar]
- FORCE E. E., STEWART R. C., HAFF R. F. DEVELOPMENT OF INTERFERON IN RABBIT DERMIS AFTER INFECTION WITH HERPES SIMPLEX VIRUS. Virology. 1965 Feb;25:322–325. doi: 10.1016/0042-6822(65)90210-2. [DOI] [PubMed] [Google Scholar]
- GIFFORD G. E. STUDIES ON THE SPECIFICITY OF INTERFERON. J Gen Microbiol. 1963 Dec;33:437–443. doi: 10.1099/00221287-33-3-437. [DOI] [PubMed] [Google Scholar]
- GINSBERG H. S. Serum and tissue inhibitors of virus. Bacteriol Rev. 1960 Mar;24(1):141–150. doi: 10.1128/br.24.1.141-150.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRESSER I. Induction by Sendal virus of non-transmissible cytopathic changes associated with rapid and marked production of interferon. Proc Soc Exp Biol Med. 1961 Nov;108:303–307. doi: 10.3181/00379727-108-26921. [DOI] [PubMed] [Google Scholar]
- HENLE G., HENLE W. DIFFERENCES IN RESPONSE OF HAMSTER TUMOR CELLS INDUCED BY POLYOMA VIRUS TO INTERFERING VIRUS AND INTERFERON. J Natl Cancer Inst. 1963 Jul;31:143–153. [PubMed] [Google Scholar]
- HENLE W., HENLE G., DEINHARDT F., BERGS V. V. Studies on persistent infections of tissue cultures. IV. Evidence for the production of an interferon in MCN cells by myxoviruses. J Exp Med. 1959 Oct 1;110:525–541. doi: 10.1084/jem.110.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HO M., ENDERS J. F. Further studies on an inhibitor of viral activity appearing in infected cell cultures and its role in chronic viral infections. Virology. 1959 Nov;9:446–477. doi: 10.1016/0042-6822(59)90135-7. [DOI] [PubMed] [Google Scholar]
- HO M. IDENTIFICATION AND "INDUCTION" OF INTERFERON. Bacteriol Rev. 1964 Dec;28:367–381. doi: 10.1128/br.28.4.367-381.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISAACS A. INTERFERON. Adv Virus Res. 1963;10:1–38. [PubMed] [Google Scholar]
- ISAACS A., PORTERFIELD J. S., BARON S. The influence of oxygenation on virus growth. II. Effect on the antiviral action of interferon. Virology. 1961 Aug;14:450–455. doi: 10.1016/0042-6822(61)90337-3. [DOI] [PubMed] [Google Scholar]
- ISAACS A., WESTWOOD M. A. Inhibition by interferon of the growth of vaccinia virus in the rabbit skin. Lancet. 1959 Sep 12;2(7098):324–325. doi: 10.1016/s0140-6736(59)91361-3. [DOI] [PubMed] [Google Scholar]
- KONO Y., HO M. THE ROLE OF THE RETICULOENDOTHELIAL SYSTEM IN INTERFERON FORMATION IN THE RABBIT. Virology. 1965 Jan;25:163–166. doi: 10.1016/0042-6822(65)90268-0. [DOI] [PubMed] [Google Scholar]
- LAMPSON G. P., TYTELL A. A., NEMES M. M., HILLEMAN M. R. CHARACTERIZATION OF CHICK EMBRYO INTERFERON INDUCED BY A DNA VIRUS. Proc Soc Exp Biol Med. 1965 Feb;118:441–448. doi: 10.3181/00379727-118-29870. [DOI] [PubMed] [Google Scholar]
- LINDENMANN J., GIFFORD G. E. Studies on vaccinia virus plaque formation and its inhibition by interferon. III. A simplified plaque inhibition assay of interferon. Virology. 1963 Mar;19:302–309. doi: 10.1016/0042-6822(63)90068-0. [DOI] [PubMed] [Google Scholar]
- MERIGAN T. C. PURIFIED INTERFERONS: PHYSICAL PROPERTIES AND SPECIES SPECIFICITY. Science. 1964 Aug 21;145(3634):811–813. doi: 10.1126/science.145.3634.811-a. [DOI] [PubMed] [Google Scholar]
- POLLIKOFF R., DONIKIAN M. A., PADRON A., LIU O. C. Tissue specificity of interferon prepared in various tissue cultures. Proc Soc Exp Biol Med. 1962 Jun;110:232–234. doi: 10.3181/00379727-110-27476. [DOI] [PubMed] [Google Scholar]
- ROTEM Z., BERWALD Y., SACHS L. INHIBITION OF INTERFERON PRODUCTION IN HAMSTER CELLS TRANSFORMED IN VITRO WITH CARCINOGENIC HYDROCARBONS. Virology. 1964 Nov;24:483–486. doi: 10.1016/0042-6822(64)90189-8. [DOI] [PubMed] [Google Scholar]
- SELLERS R. F., FITZPATRICK M. An assay of interferon produced in rhesus monkey and calf kidney tissue cultures using bovine enterovirus M6 as challenge. Br J Exp Pathol. 1962 Dec;43:674–683. [PMC free article] [PubMed] [Google Scholar]
- TAKANO K., JENSEN K. E., WARREN J. PASSIVE INTERFERON PROTECTION IN MOUSE INFLUENZA. Proc Soc Exp Biol Med. 1963 Nov;114:472–475. doi: 10.3181/00379727-114-28706. [DOI] [PubMed] [Google Scholar]
- TYRRELL D. A. Interferon produced by cultures of calf kidney cells. Nature. 1959 Aug 8;184(Suppl 7):452–453. doi: 10.1038/184452a0. [DOI] [PubMed] [Google Scholar]
- WAGNER R. R. Biological studies of interferon. I. Suppression of cellular infection with eastern equine encephalomyelitis virus. Virology. 1961 Mar;13:323–337. doi: 10.1016/0042-6822(61)90152-0. [DOI] [PubMed] [Google Scholar]
- Wagner R. R. VIRAL INTERFERENCE. SOME CONSIDERATIONS OF BASIC MECHANISMS AND THEIR POTENTIAL RELATIONSHIP TO HOST RESISTANCE. Bacteriol Rev. 1960 Mar;24(1):151–166. doi: 10.1128/br.24.1.151-166.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson E. O. Chemical Communication in the Social Insects. Science. 1965 Sep 3;149(3688):1064–1071. doi: 10.1126/science.149.3688.1064. [DOI] [PubMed] [Google Scholar]


