Abstract
Neural tube defects (NTDs), a common birth defect in humans, result from the failure of the embryonic neural tube (NT) to close properly. NT closure is a complex, poorly understood morphogenetic process influenced by genes and environment. The most effective environmental influence in decreasing the risk for NTDs is folic acid (FA) fortification and supplementation, and these findings led to the recommendation of periconceptual FA intake and mandatory fortification of the US grain supply in 1998. To explore the relationship between genetics and responsiveness to FA supplementation, we used five mouse NTDs models—Zic2, Shroom3, Frem2, Grhl2 (Grainyhead-like 2) and L3P (Line3P)—and a long-term generational FA supplementation scheme. Contrary to expectations, we find that three genetic mutants respond adversely to FA supplementation with increased incidence of NTDs in homozygous mutants, occurrence of NTDs in heterozygous embryos and embryonic lethality prior to NT closure. Because of these unexpected responses, we examined NTD risk after short-term FA supplementation. Our results indicate that, for the same genetic allele, NTD risk can depend on the length of FA exposure. Our data indicate that, depending on the gene mutation, FA supplementation may adversely influence embryonic development and NT closure.
INTRODUCTION
The embryonic neural tube (NT) forms the brain and spinal cord. Defects in NT closure cause an opening in the central nervous system and can result in anencephaly and spina bifida [NT defects (NTDs) in the cranial or caudal regions, respectively]. Infants born with NTDs have increased risk of mortality within the first year of life, and survivors face life-long morbidities including neurologic, cognitive, urologic and gastrointestinal complications. In humans, most genetic causes of NTDs remain unknown. Environmental conditions experienced in utero can also influence NTD risk (1,2). Periconceptual folic acid (FA) fortification and supplementation is well documented to reduce NTD incidence in humans, ranging from 30–40% reduction in the general population to 70% for women given high levels of FA following a previous NTD pregnancy (3,4). These data prompted the mandatory fortification of grains in the USA to ensure that women of childbearing age would consume a higher amount of FA to prevent NTDs (3,5).
In mice, over 240 genes are known to be important in NT closure, and mouse models of NTDs are beginning to provide insight into the genetic causes and developmental origins of NTDs in humans (6). Moreover, mouse NTD models have the potential to aid in understanding the genetics underlying FA responsiveness or non-responsiveness in NTD prevention, as well as to identify alternative approaches to prevent FA-resistant NTDs (7). However, this potential has been minimally exploited as only 14 mouse NTD models have been tested for FA responsiveness. Here, we substantially increase this data set by testing FA responsiveness in five unique mouse lines generated through a N-ethyl-N-nitrosourea (ENU) mutagenesis screen for NTDs (8). Of the previously tested models, FA supplementation reduced the incidence of NTDs (six cases), was not beneficial (seven cases) or exacerbated NTDs (one case) (7,9). Previous mouse studies have used short-term diet regimes (7), which are less representative of the current long-term and generational trend in FA exposure occurring in the US population. Therefore, we used long-term and multi-generational exposure to dietary FA (Fig. 1) to more closely approximate the trend in FA exposure occurring in the US population as periconceptual FA supplementation has been recommended since the early 1990s and mandatory FA fortification began in 1998.
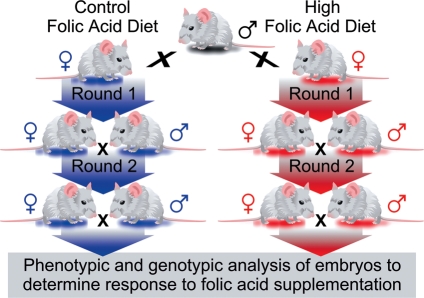
Figure 1.
Long-term folic acid (FA) experimental design. The lines were bred and maintained on two diets that only differ in the concentration of FA (2 p.p.m. for the control FA diet or 10 p.p.m. for the high FA diet). Following two generations on the control or high FA diet, heterozygous males and females from the same diet were timed-mated and embryos recovered and scored for phenotype and genotype.
Here, we find, contrary to expectations, that three mouse lines respond detrimentally to long-term dietary FA. Comparison between two diets that differ only in FA concentration shows that increased dietary FA can result in an increased frequency of NTDs in homozygous mutant embryos; NTDs in heterozygous embryos on high FA diet, whereas on control FA diet NTDs were not observed; and embryonic lethality prior to the time of NT closure. To determine whether the response depended on the length of exposure to FA diet, we used a short-term FA diet similar to previous mouse studies. We find that, depending on the genetic allele, the response can differ between short- and long-term diet. Overall, our results suggest that the response to FA fortification and supplementation may be more complex than previously suggested and that the genetics of an individual may determine whether FA provides a beneficial outcome.
RESULTS
We tested FA responsiveness in mice with mutations in five different genes—Zic2, Frem2, Shroom3, Grhl2 (Grainyhead-like 2) and L3P (Line3P)—that have not been previously examined for FA responsiveness. Splotch (Sp2H), a mouse line that carries a mutation in the Pax3 gene was used as a positive control as it shows decreased NTD risk on short-term FA exposure (10). Table 1 summarizes the gene functions, which vary broadly in their control of NT development. These genes have not been implicated in FA uptake, metabolism or utilization. The ENU lines, which were generated in the Niswander laboratory, include a new allele of Zic2 (an A-to-T change in Exon 1 resulting in the missense mutation E329V; Zic2m1Nisw); a new allele of Shroom3 (a C-to-T change in Exon 9 resulting in the missense mutation R1663C; Shroom3m1Nisw), an allele of Frem2, Frem2my-F11 (11), an allele of Grainyhead-like2, Grhl2m1Nisw (12) and L3P for which the genetic component has been identified and will be described elsewhere (in preparation). The Sp2H mutation is a 32 base pair deletion in exon 5 of Pax3 (10). The ENU-induced lines were originally screened for NTDs on Tekland Rodent chow, which is not directly comparable with the FA diets used in this study due to differences in ingredients beyond the concentration of FA. On the Tekland diet, none of the heterozygotes showed NTDs. Zic2 homozygous mutants showed 100% penetrant spina bifida and ∼20% exencephaly. Grhl2 (12) and Shroom3 homozygous embryos exhibited 100% penetrant forebrain, midbrain and hindbrain exencephaly. Thirty percent of Frem2 mutants display midbrain exencephaly (11), and 30% of L3P mutants exhibited forebrain, midbrain and hindbrain exencephaly. Sp2H on the congenic background used here showed 100% penetrant spina bifida and 73% penetrant exencephaly.
Table 1.
Genes tested for responsiveness to FA diets
| Gene | Function in NT closure |
|---|---|
| Zic2 | Regulation of BMP antagonists dorsally and dorsolateral hinge point formation (16) |
| Frem2 | NT fusion, expressed in roof plate of diencephalon (11) |
| Shroom3 | Actin regulator (17) |
| Grhl2 | NT fusion, expressed in the non-neural ectoderm (12) |
| L3P | Neural patterning |
| Pax3 | Regulation of neural progenitor survival and differentiation (18,19) |
The studies here utilized two diets differing only in FA concentration: a 2 p.p.m. or 2 mg/kg of chow designated as control FA diet and a 10 p.p.m. or 10 mg/kg of chow designated as high FA diet (Fig. 1). The doses, routes and timing of administration of FA used in previous mouse studies vary greatly but the doses used here are in the range of other mouse FA supplementation studies (reviewed in 7). Total FA serum levels in all lines tested were 10.0 ng/ml (s.d. = 3.8) on control FA diet, increasing to 25.6 ng/ml (s.d. = 4.3) on high FA diet (Supplementary Material, Fig. S1). This level of serum folate is similar to that in humans, which ranges from 5.9 to 24.6 ng/ml, depending on the extent of fortification and supplementation (13).
To establish whether long-term high FA diet can lead to responsiveness and prevention of NTDs, we first evaluated Sp2H mutants, which have previously shown a decreased incidence of NTDs with short-term FA exposure by injection (10). Repeating these short-term FA injection experiments, we observed a partial rescue of NTDs (92% NTDs upon FA injection versus 100% with sham injection). Long-term high FA diet showed a similar NTD incidence (97%, Fig. 2; Supplementary Material, Fig. S2 documents the relative incidence of exencephaly and spina bifida). The level of NTD rescue was similar despite the large difference between serum folate levels (27 ng/ml on high FA diet versus 65 ng/ml by FA injection; Supplementary Material, Fig. S1). These results indicate that long-term multi-generational FA diet can be partially beneficial in Sp2H mutants.
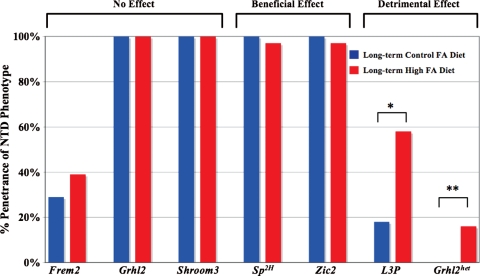
Figure 2.
Long-term FA supplementation and the incidence of NTDs. Long-term FA supplementation did not reduce the incidence of NTDs in homozygous mutant embryos for Frem2 (39% NTD penetrance, 7/18 mutant embryos on the long-term high FA diet), Grhl2 (100%, 13/13) or Shroom3 (100%, 7/7) but did slightly decrease the incidence of NTDs in mutant embryos for Sp2H (97%, 29/30) and Zic2 (95%, 35/37 of which one lived postnatally). FA supplementation increased the rate of NTDs in L3P mutant embryos (58%, 14/24) and Grhl2 heterozygous embryos (16%, 6/37; listed as Grhl2het). *P= 0.012; **P= 0.009.
Next, we examined the effect of long-term FA diet on previously untested ENU alleles. Zic2 homozygous mutant embryos on control FA diet showed 100% penetrant NTDs (32/32; Fig. 2). On high FA diet, there was a slight beneficial response (95% NTDs) in that two Zic2 genotypic mutants had completely closed NTs, whereas the remaining 35 mutants had NTDs of similar severity and position as control FA diet (Fig. 2, Supplementary Material, Fig. S2). Moreover, on high FA, the expected 1:2:1 Mendelian ratio of embryos was observed but, on control FA diet, there was significant embryo loss as reflected by 25 and 43% fewer than expected heterozygous and homozygous mutant embryos observed after NT closure (P= 0.005, P= 0.003, respectively; Table 2). Thus, for the Zic2 mutation, long-term FA supplementation appears beneficial, protecting against both heterozygous and homozygous mutant embryo loss and providing a modest trend toward the rescue of NTDs.
Table 2.
Long-term FA supplementation can be either beneficial or detrimental in preserving observed embryo ratios
| Line/diet | Observed embryo ratio |
Resorptions | Litter size (number of litters) | % +/mut embryo loss | % mut/mut embryo loss | Effect of FA supplementation | ||
|---|---|---|---|---|---|---|---|---|
| Sp2H | +/+ | +/mut | mut/mut | |||||
| Long-term Control | 28 | 59 | 35 | 9 | 9.38 (13) | None | None | None |
| Long-term High | 31 | 59 | 30 | 10 | 7.50 (16) | None | None | |
| Zic2 | +/+ | +/mut | mut/mut | |||||
| Long-term Control | 56 | 84 | 32 | 14 | 6.14 (28) | 25a | 43b | Beneficial |
| Long-term High | 38 | 98 | 36 | 23 | 5.73 (30) | None | None | |
| Frem2 | +/+ | +/mut | mut/mut | |||||
| Long-term Control | 33 | 64 | 38 | 16 | 5.86 (23) | None | None | None |
| Long-term High | 21 | 50 | 18 | 9 | 6.36 (14) | None | None | |
| Shroom3 | +/+ | +/mut | mut/mut | |||||
| Long-term Control | 20 | 39 | 17 | 6 | 5.42 (14) | None | None | Detrimentald |
| Long-term High | 21 | 47 | 7 | 26 | 5.70 (13) | None | 67c | |
| L3P | +/+ | +/mut | mut/mut | |||||
| Long-term Control | 17 | 45 | 17 | 18 | 7.18 (11) | None | None | Detrimentalf |
| Long-term High | 42 | 98 | 24 | 13 | 6.07 (27) | None | 43e | |
| Grhl2 | +/+ | +/mut | mut/mut | |||||
| Long-term Control | 26 | 66 | 13 | 46 | 5.25 (20) | None | 50 | None |
| Long-term High | 28 | 40 | 13 | 19 | 4.76 (17) | None | 54 | |
| Grhl2 | +/+ | +/mut | mut/mut | |||||
| Short-term Control | 20 | 25 | 9 | 6 | 5.4 (10) | 38 | 55 | None |
| Short-term High | 16 | 23 | 4 | 7 | 6.14 (7) | 28 | 75 | |
| L3P | +/+ | +/mut | mut/mut | |||||
| Short-term Control | 28 | 40 | 11 | 15 | 7.18 (13) | None | 61g | Beneficialh |
| Short-term High | 14 | 28 | 16 | 13 | 4.2 (10) | None | None | |
| Shroom3 | +/+ | +/mut | mut/mut | |||||
| Short-term Control | 15 | 21 | 16 | 11 | 6.25 (8) | None | None | Nonei |
| Short-term High | 29 | 56 | 22 | 5 | 7.62 (14) | None | None | |
The mutation for all lines is inherited according to Mendelian genetics such that ∼25% of the embryos in a litter will be wild-type (+/+), 50% heterozygous for the mutation (+/mut) and 25% will have both mutant alleles (mut/mut). The expected ratio was determined using the number of +/+ embryos, as diet is not expected to influence the number of wild-type embryos observed. Zic2 on long-term control FA diet shows a statistically significant reduction in number of heterozygous and mutant embryos (a112 expected, 84 observed, P= 0.005; b56 expected, 32 observed, P= 0.003, respectively), whereas embryo viability is rescued with high FA, indicating that FA supplementation is beneficial. Conversely, long-term FA supplementation is detrimentald,f for both Shroom3 and L3P, resulting in loss of mutant embryos (c21 expected, 7 observed, P= 0.004; e42 expected, 24 observed, P= 0.011; respectively). However, short-term high FA does not cause loss of mutant Shroom3 embryos seen with long-term high FAi,d. Short-term control FA causes loss of L3P mutant embryos (g28 expected, 11 observed, P= 0.002) which are rescued by short-term high FA and this beneficial effect of hshort high FA is directly opposite of the detrimental effect on flong-term high FA.
Long-term FA supplementation did not prevent NTDs in Frem2 or Shroom3 and had a surprising detrimental effect on L3P and Grhl2 genetic mutants. Frem2 had no differential response to control or high FA diets, showing similar midbrain exencephaly incidence (29 and 39%, P= 0.554) and expected ratios of mutant and heterozygous embryos on both diets (Fig. 2, Table 2). Shroom3 displayed 100% NTDs on both control and high FA diets (Fig. 2). In contrast, L3P and Grhl2 were adversely affected by FA supplementation in terms of NTD risk. First, L3P homozygous mutants showed an increased frequency of exencephaly with FA supplementation [18% NTDs (3/17) on control FA diet versus 58% (14/24) on high FA diet (P= 0.012; Fig. 2)]. Second, we observed NTDs in heterozygous Grhl2 embryos [16% NTDs (6/37) on high FA compared with 0% (0/55) on control FA diet (P= 0.009; Fig. 2)]. Thus, FA supplementation can have a significant detrimental effect on NT closure when combined with specific genetic factors, in this case, mutations in L3P and Grhl2.
Long-term high FA diet resulted in significant loss of Shroom3 and L3P homozygous mutant embryos (Table 2). For Shroom3, 67% (P= 0.004) of expected homozygous mutant embryos were not observed and the number of resorption sites (a reflection of failed embryonic development after implantation) corresponded to this increase in embryo loss (Table 2). For L3P, high FA diet resulted in 43% loss of homozygous mutants (P= 0.011; Table 2) but in those homozygotes that did survive, there was an increased frequency of NTDs (from 18 to 58%, Fig. 2). Grhl2 showed loss of homozygous mutant embryos that was similar on control and high FA diets (Table 2, 50 and 54%, respectively).
Previous testing of FA responsiveness in mouse lines generally involved a short exposure to FA, whereas our study involves a longer, multi-generational exposure. Thus, to address the question of whether the developmental outcome depends on the length of exposure to FA, we tested lines that showed a detrimental response on long-term high FA diet. Grhl2, Shroom3 and L3P heterozygous females were given a short exposure to control or high FA diet from fertilization until the time of dissection. Total serum FA levels increased from 13.0 ng/ml (s.d. = 4.1) on the control FA diet to 25.0 ng/ml (s.d. = 2.8) on the high FA diet, similar to long-term FA diets (Supplementary Material, Fig. S1). Grhl2 heterozygous embryos on short-term high FA diet showed a detrimental response (18% NTDs, 4/23, P= 0.046) similar to long-term high FA diet (16%), versus 0% NTDs on short-term (0/16) or long-term control FA diet (Fig. 3 compared with Fig. 2). Thus, for Grhl2, the length of FA exposure does not alter NTD risk. However, for Shroom3 and L3P, the length of FA exposure did influence NTD risk and embryo loss. For Shroom3, short-term high FA diet prevented embryo loss (29 expected, 22 observed homozygous mutant embryos, P= 0.37, Table 2) that was seen with long-term high FA (67% loss). Moreover, short-term high FA exposure prevented NTDs of Shroom3 homozygous embryos, reducing the NTD incidence to 72% (16/22 mutant embryos, P= 0.02; Fig. 3), a striking result as NTD penetrance was 100% on long-term FA diet. Thus, for Shroom3, short-term FA was beneficial but long-term FA was detrimental. For L3P, short-term high FA diet prevented embryo loss seen on short-term control FA (61% loss, P= 0.002; Table 2). This outcome is the opposite of that seen with long-term FA diets for L3P. Additionally, for L3P, short-term FA exposure resulted in no statistically significant change in NTD rates between control (27%, 3/11 mutant embryos) and high FA (31%, 5/16 mutant embryos; Fig. 3) diets. This is in contrast to the increased NTD risk observed on long-term high FA diet, indicating that chronic and perhaps generational exposure to FA is necessary to increase the NTD rate in L3P. These results indicate that the length of FA exposure can alter risks for NTDs and embryo loss in mouse genetic models of NTDs.
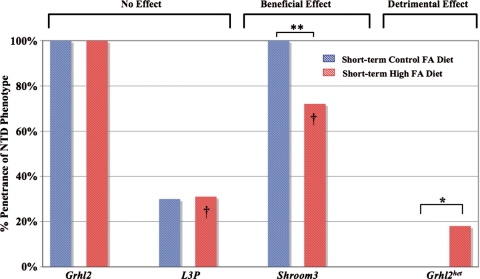
Figure 3.
Short-term FA supplementation and incidence of NTDs. Short-term high FA did not affect the incidence of NTDs in homozygous mutant embryos for Grhl2 (100% NTD penetrance, 4/4 mutant embryos) or L3P (31%, 5/16). Short-term high FA decreased the NTD rate in Shroom3 mutant embryos (72%, 16/22), but increased the NTD rate in Grhl2 heterozygous embryos (18%, 4/23; listed as Grhl2het, similar to long-term high FA). The beneficial (Shroom3) or no effect (L3P) of short-term high FA differed from no effect (Shroom3) or detrimental (L3P) response upon long-term high FA (highlighted by dagger). *P = 0.046; **P = 0.02.
DISCUSSION
FA fortification and supplementation in humans has been clearly shown to decrease the incidence of NTDs. However, our findings in a panel of mouse NTD lines indicate a more diverse range of responses to FA supplementation in the diet, from partially positive outcomes to profoundly negative gene–environment interactions. First, we observed beneficial or detrimental changes in NTD risk, depending on the gene mutation. Surprisingly, we also found that FA supplementation resulted in NTDs in heterozygous Grhl2 mice, whereas heterozygotes on lower FA complete NT closure. Second, viability of homozygous mutant embryos was differentially affected by FA diet dependent on the gene mutation. Long-term high FA prevented Zic2 embryo loss but induced embryo loss for L3P and Shroom3. One implication of induced embryo loss with FA supplementation is that if the genotype is unknown, fewer embryos with NTDs could be interpreted as NTD prevention. This would have been the case for Shroom3 if genotyping data were not available, as only seven mutants survived to the time of NT closure due to the high frequency of earlier lethality. Conversely, although almost half of L3P homozygotes were lost due to earlier lethality, those that survived showed an increased risk for NTDs relative to control FA diet. If these results are relevant to humans, depending on the genetic background, both biological responses (prolonged survival of phenotypic embryos or exacerbation of the developmental defects resulting in earlier embryonic loss) could be occurring in addition to true NTD prevention. Finally, the observation that the length of FA exposure could cause a genetic mutant to show different phenotypic responses highlights the need for further study of the potentially differing effects of short-term and long-term FA exposure.
It should be noted that the genes studied here have no apparent roles in FA metabolism or other processes related to this pathway. FA metabolism plays an extremely important role in the cell by providing the building blocks for RNA and DNA biosynthesis (purines and thymidylate), as well as the methyl donors utilized in methylation reactions. Thus, FA can potentially enhance cell proliferation as seen in wild-type embryos (9). Additionally, FA can also conceivably affect the epigenetic regulation of gene expression through histone and DNA methylation. FA-induced epigenetic changes may occur during development, thus altering gene expression. Moreover, these epigenetic changes may be inherited, as recently suggested by increased epigenetic variability following long-term FA diet in wild-type mice (14). Therefore, FA likely acts through diverse mechanisms to influence NT closure and this appears to be reflected in the diverse responses we observed, indicating that FA does not act through a single common pathway. In the future, it will be of interest to determine the mechanistic basis of the specific gene–environment interactions that together influence NT closure.
The benefits of FA in terms of global decrease in NTD risk are clear. However, although no detrimental effects of FA at current intakes have been proven, the biologic plausibility for a negative impact on individuals has been raised (15). FA fortification was implemented with the specific intent to reduce NTD risk or, at the very least, do no harm. Our unexpected findings highlight the need to understand how FA may influence NT closure and the mechanisms and genetics underlying the response to FA to expand the scientific discussion of best health policy practices.
MATERIALS AND METHODS
The ENU-induced mutations were generated on a C57/Bl6 background and then outcrossed to 129S1/SvlmJ for more than 10 generations. The Sp2H mutation is on C3H/HeN background. As schematized in Figure 1, males and females maintained on Tekland Rodent Diet (2018S) were timed-mated and upon discovery of a mating plug indicating fertilization, the female was placed on either 2 p.p.m. (called control) or 10 p.p.m. (called high) FA diet; all other diet components are the same (Research Diets, Inc.: 2 p.p.m. is D05072702; 10 p.p.m. is D05072701; p.p.m. is equivalent to mg/kg of chow and is not based on body weight). Based on the amount of food consumed daily by our mice (2.78 g, s.d. = 0.56), the daily dose of FA on the control diet ranges between 4 and 6 µg, and the dose of FA on the high diet is between 22 and 33 µg. Males and females were maintained on diet for two generations (indicated as Rounds 1 and 2 on Fig. 1). Embryos from these timed-pregnant females were obtained between embryonic day (E) 10.5 and E12.5 for Grhl2m1Nisw and Shroom3m1Nisw; E12.5 for L3P, Zic2m1Nisw and Frem2my-F11; E10.5 for Sp2H. Embryos were scored for NTD phenotypes characteristic of the line. For short-term FA exposure, heterozygous males and females were maintained on Tekland Rodent Diet, timed-mated and, upon discovery of the mating plug, the females were placed on control or high FA diet until dissection as indicated above. FA injection (10 mg/kg of mouse body weight/day by IP injection over days E7.5, 8.5, 9.5) was performed on Sp2H heterozygous females as described (10). The FA dose administered daily between E7.5 and 9.5, based on the average weight of the heterozygous females (∼25 g), was ∼250 µg. All embryos were genotyped utilizing appropriate primers (sequences available upon request). In addition, all embryos for Zic2m1Nisw, Shroom3m1Nisw, Grhl2m1Nisw and Frem2my-F11 that were genotyped as homozygous, as well as any phenotypic embryos that were discordant with the expected genotype, were sequenced to score for the single nucleotide change representative of the mutant line (primer sequences available upon request).
Serum FA levels were measured following the protocol outlined for the Vitamin B9 (Folic Acid) Microbiological Test Kit (ALPCO Immunoassays). Pregnant heterozygote females were removed from food sources 2h prior to blood collection via retro-orbital bleeds and then the embryos were removed for analysis. Total FA serum levels in the tested lines are shown in Supplementary Material, Figure S1.
Fisher's exact test was used to evaluate differences in the rate of NTDs observed in mutant or heterozygous embryos. Chi square goodness-of-fit test was used to assess significance between expected and observed embryonic Mendelian ratios and heterozygous/mutant embryo loss.
AUTHOR CONTRIBUTIONS
A.M. initiated the FA diet experiments and provided data on L3P, Shroom3, Grhl2, Frem2 and Zic2. A.G. provided data on Sp2H and Shroom3; Y.Z. identified the Zic2 mutation and provided diet data on Zic2; A.M. and L.N. contributed to the experimental design; all authors helped in manuscript preparation.
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by the National Institutes of Health (grant number NS058979).
Supplementary Material
ACKNOWLEDGEMENTS
We gratefully acknowledge L. Bulwith for help with mouse breeding, C. Pyrgaki for the identification of the Shroom3 and Grhl2 alleles, L. Fenton for the illustration of Figure 1, M. Ross and J. Nadeau and their laboratories for advice during this project and for statistical advice, and our laboratory colleagues for stimulating discussions. We thank B. Appel, J. DeGregori and R. Johnston for comments on the manuscript. L.N. is an investigator of the Howard Hughes Medical Institute.
Conflict of Interest statement. The authors state there is no conflict of interest.
REFERENCES
- 1.Mitchell L.E. Epidemiology of neural tube defects. Am. J. Med. Genet. C Semin. Med. Genet. 2005;135C:88–94. doi: 10.1002/ajmg.c.30057. doi:10.1002/ajmg.c.30057. [DOI] [PubMed] [Google Scholar]
- 2.Detrait E.R., George T.M., Etchevers H.C., Gilbert J.R., Vekemans M., Speer M.C. Human neural tube defects: developmental biology, epidemiology, and genetics. Neurotoxicol. Teratol. 2005;27:515–524. doi: 10.1016/j.ntt.2004.12.007. doi:10.1016/j.ntt.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–137. doi:10.1016/0140-6736(91)90133-A. [PubMed] [Google Scholar]
- 4.De Wals P., Tairou F., Van Allen M.I., Uh S.H., Lowry R.B., Sibbald B., Evans J.A., Van den Hof M.C., Zimmer P., Crowley M., et al. Reduction in neural-tube defects after folic acid fortification in Canada. N. Engl. J. Med. 2007;357:135–142. doi: 10.1056/NEJMoa067103. doi:10.1056/NEJMoa067103. [DOI] [PubMed] [Google Scholar]
- 5.Houk V.N., Oakley G.P., Jr., Erickson D., Mulinare J., James L.M. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Recomm. Rep. 1992;41(RR-14):1–7. [PubMed] [Google Scholar]
- 6.Harris M.J., Juriloff D.M. An update to the list of mouse mutants with neural tube closure defects and advances toward a complete genetic perspective of neural tube closure. Birth Defects Res. A Clin. Mol. Teratol. 2010;88:653–669. doi: 10.1002/bdra.20676. doi:10.1002/bdra.20676. [DOI] [PubMed] [Google Scholar]
- 7.Harris M.J. Insights into prevention of human neural tube defects by folic acid arising from consideration of mouse mutants. Birth Defects Res. A Clin. Mol. Teratol. 2009;85:331–339. doi: 10.1002/bdra.20552. doi:10.1002/bdra.20552. [DOI] [PubMed] [Google Scholar]
- 8.Zohn I.E., Anderson K.V., Niswander L. Using genomewide mutagenesis screens to identify the genes required for neural tube closure in the mouse. Birth Defects Res. A Clin. Mol. Teratol. 2005;73:583–590. doi: 10.1002/bdra.20164. doi:10.1002/bdra.20164. [DOI] [PubMed] [Google Scholar]
- 9.Gray J.D., Nakouzi G., Slowinska-Castaldo B., Dazard J.E., Rao J.S., Nadeau J.H., Ross M.E. Functional interactions between the LRP6 WNT co-receptor and folate supplementation. Hum. Mol. Genet. 2010;19:4560–4572. doi: 10.1093/hmg/ddq384. doi:10.1093/hmg/ddq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burren K.A., Savery D., Massa V., Kok R.M., Scott J.M., Blom H.J., Copp A.J., Greene N.D. Gene–environment interactions in the causation of neural tube defects: folate deficiency increases susceptibility conferred by loss of Pax3 function. Hum. Mol. Genet. 2008;17:3675–3685. doi: 10.1093/hmg/ddn262. doi:10.1093/hmg/ddn262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Timmer J.R., Mak T.W., Manova K., Anderson K.V., Niswander L. Tissue morphogenesis and vascular stability require the Frem2 protein, product of the mouse myelencephalic blebs gene. Proc. Natl Acad. Sci. USA. 2005;102:11746–11750. doi: 10.1073/pnas.0505404102. doi:10.1073/pnas.0505404102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pyrgaki C., Liu A., Niswander L. Grainyhead-like 2 regulates neural tube closure and adhesion molecule expression during neural fold fusion. Dev. Biol. 2011;353:38–49. doi: 10.1016/j.ydbio.2011.02.027. doi:10.1016/j.ydbio.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeung L., Yang Q., Berry R.J. Contributions of total daily intake of folic acid to serum folate concentrations. JAMA. 2008;300:2486–2487. doi: 10.1001/jama.2008.742. doi:10.1001/jama.2008.742. [DOI] [PubMed] [Google Scholar]
- 14.Li C.C., Cropley J.E., Cowley M.J., Preiss T., Martin D.I., Suter C.M. A sustained dietary change increases epigenetic variation in isogenic mice. PLoS Genet. 2011;7:e1001380. doi: 10.1371/journal.pgen.1001380. doi:10.1371/journal.pgen.1001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cordero A., Mulinare J., Berry R.J., Boyle C., Dietz W., Johnston R., Jr., Leighton J., Popovic T. CDC Grand Rounds: additional opportunities to prevent neural tube defects with folic acid fortification. Morb. Mortal Wkly Rep. 2010;59:980–984. [PubMed] [Google Scholar]
- 16.Nagai T., Aruga J., Minowa O., Sugimoto T., Ohno Y., Noda T., Mikoshiba K. Zic2 regulates the kinetics of neurulation. Proc. Natl Acad. Sci. USA. 2000;97:1618–1623. doi: 10.1073/pnas.97.4.1618. doi:10.1073/pnas.97.4.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hildebrand J.D., Soriano P. Shroom, a PDZ domain-containing actin-binding protein, is required for neural tube morphogenesis in mice. Cell. 1999;99:485–497. doi: 10.1016/s0092-8674(00)81537-8. doi:10.1016/S0092-8674(00)81537-8. [DOI] [PubMed] [Google Scholar]
- 18.Greene N.D., Massa V., Copp A.J. Understanding the causes and prevention of neural tube defects: insights from the splotch mouse model. Birth Defects Res. A Clin. Mol. Teratol. 2009;85:322–330. doi: 10.1002/bdra.20539. doi:10.1002/bdra.20539. [DOI] [PubMed] [Google Scholar]
- 19.Pani L., Horal M., Loeken M.R. Rescue of neural tube defects in Pax-3-deficient embryos by p53 loss of function: implications for Pax-3-dependent development and tumorigenesis. Genes Dev. 2002;16:676–680. doi: 10.1101/gad.969302. doi:10.1101/gad.969302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.