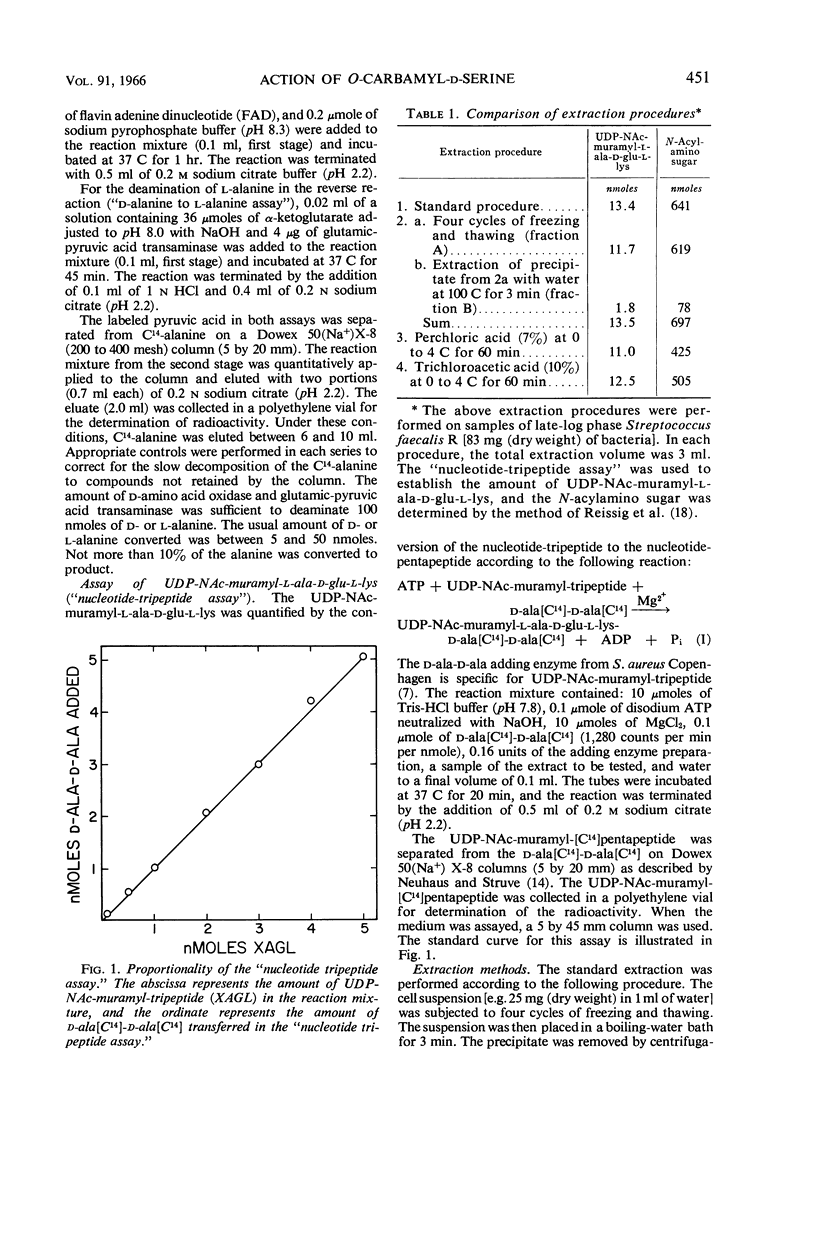
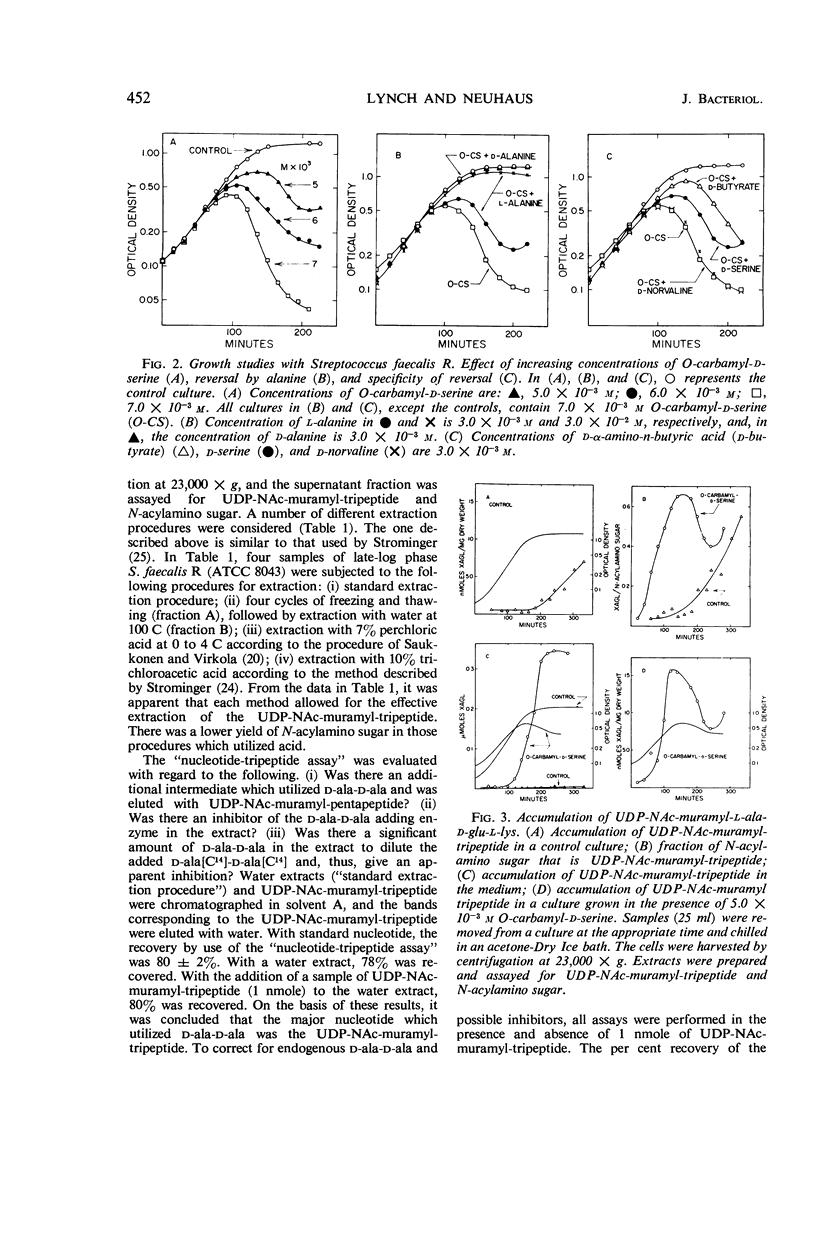
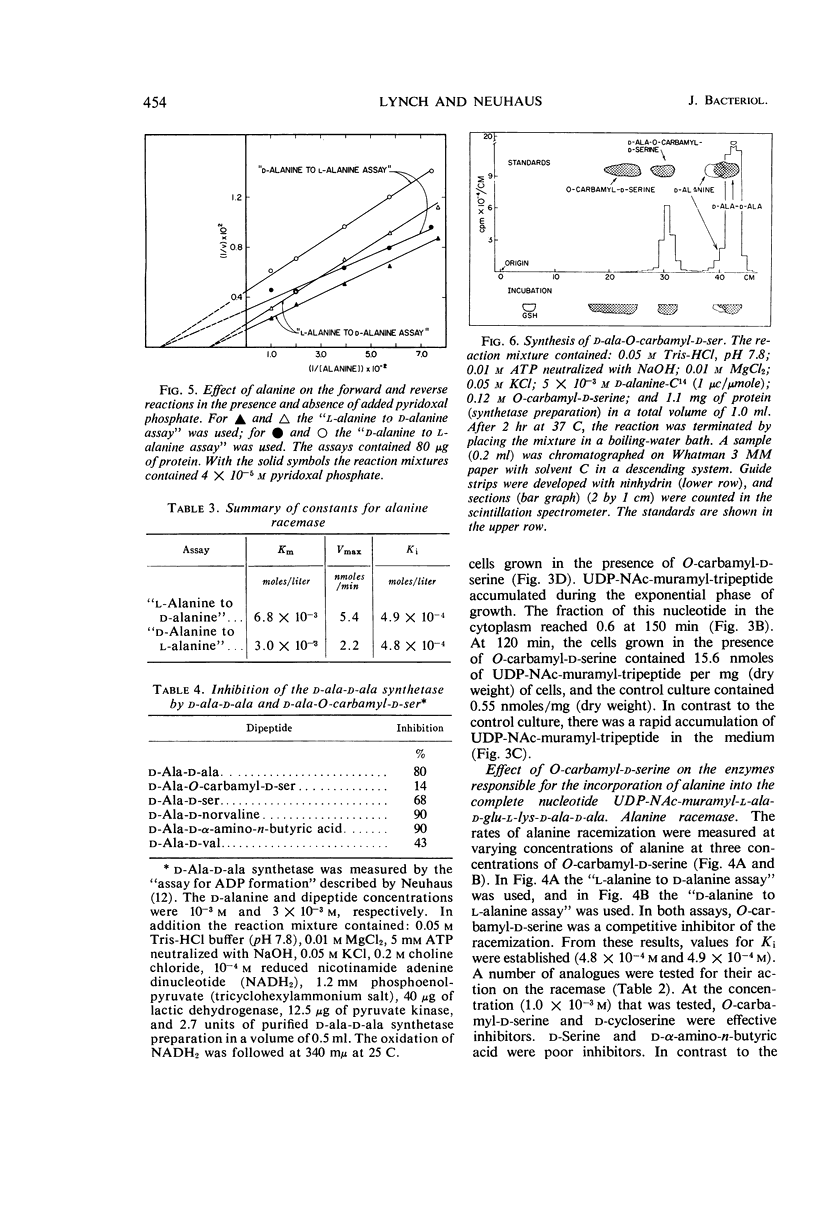
Abstract
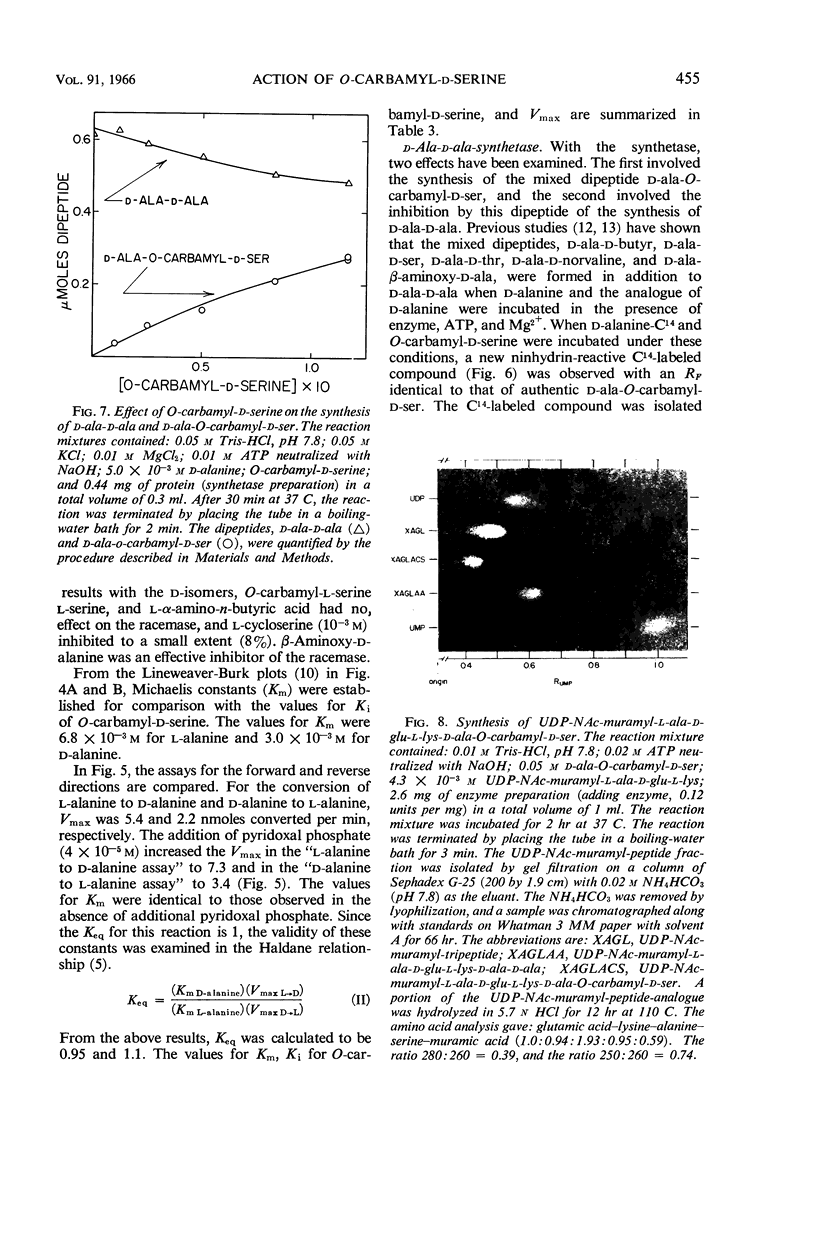
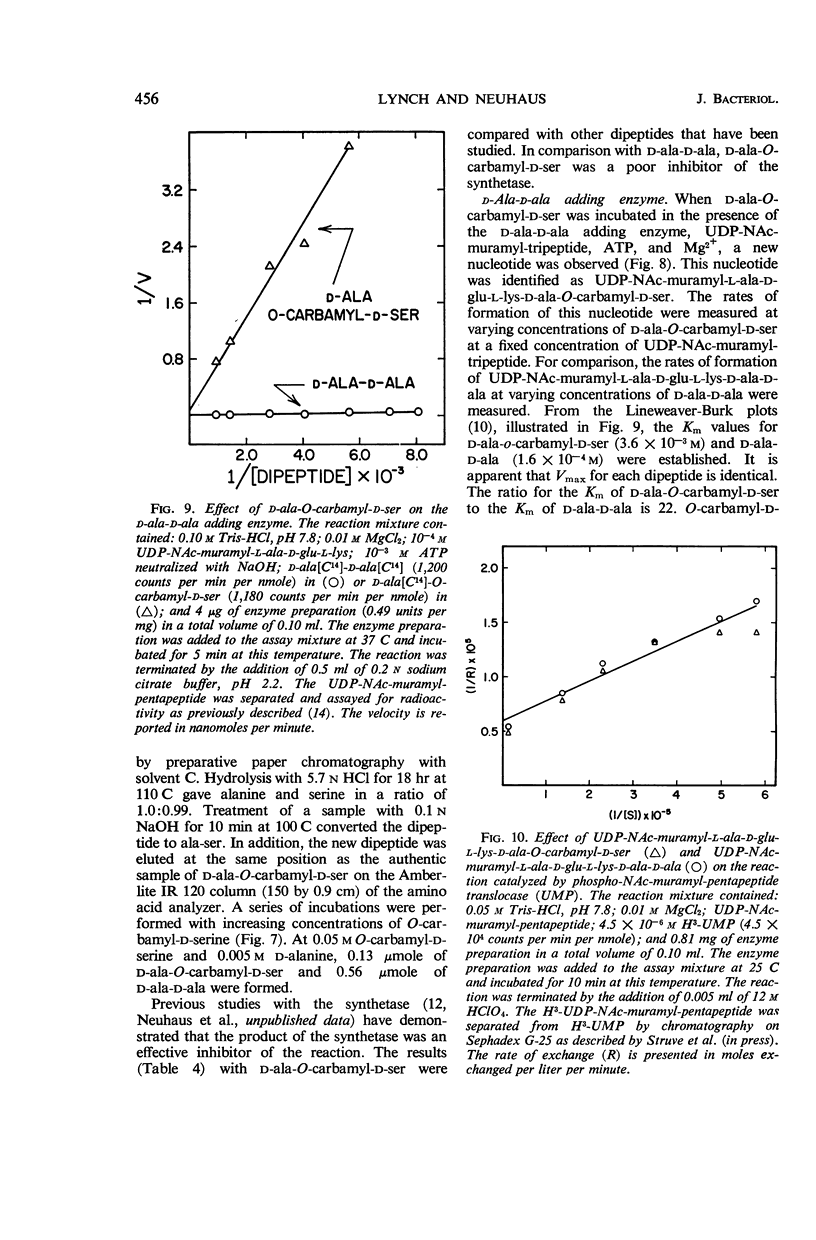
Lynch, Judith L. (Northwestern University, Evanston, Ill.), and Francis C. Neuhaus. On the mechanism of action of the antibiotic O-carbamyl-d-serine in Streptococcus faecalis. J. Bacteriol. 91:449–460. 1966.—The antibiotic O-carbamyl-d-serine, an analogue of d-alanine, is an inhibitor of bacterial cell-wall biosynthesis. Growth of Streptococcus faecalis R in the presence of O-carbamyl-d-serine resulted in the accumulation of the cell-wall precursor uridine diphosphate-NAc-muramyl-l-alanyl-d-glutamyl-l- lysine (UDP-NAc-muramyl-l-ala-d-glu-l-lys). The incorporation of d-alanine from l-alanine into peptidoglycan is catalyzed by the sequential action of the following enzymes: (i) alanine racemase; (ii) d-alanine: d-alanine ligase [adenosine diphosphate (ADP)]; (iii) UDP-NAc-muramyl-l-ala-d-glu-l-lys: d-ala-d-ala ligase (ADP); (iv) phospho-NAc-muramyl-pentapeptide translocase [uridine monophosphate (UMP)]. O-carbamyl-d-serine is an effective inhibitor of the alanine recemase (Ki= 4.8 × 10−4m, Km of l-alanine = 6.8 × 10−3m). In addition, d-ala-O-carbamyl-d-ser was formed when d-alanine and O-carbamyl-d-serine were incubated with d-alanine: d-alanine ligase (ADP). This dipeptide was utilized by the UDP-NAc-muramyl-l-ala-d-glu-l-lys: d-ala-d-ala ligase (ADP) with the formation of UDP-NAc-muramyl-l-ala-d-glu-l-lys-d-ala- O-carbamyl-d-ser. From a consideration of the following results, i.e., (i) accumulation of UDP-NAc-muramyl-l-ala-d-glu-l-lys; (ii) absence of d-ala-O-carbamyl-d-ser accumulation in bacterial cultures grown in the presence of O-carbamyl-d-serine; and (iii) effective inhibition of the racemase, it was concluded that the first enzyme, the racemase, is the primary site of antibiotic action.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON J. S., MATSUHASHI M., HASKIN M. A., STROMINGER J. L. LIPID-PHOSPHOACETYLMURAMYL-PENTAPEPTIDE AND LIPID-PHOSPHODISACCHARIDE-PENTAPEPTIDE: PRESUMED MEMBRANE TRANSPORT INTERMEDIATES IN CELL WALL SYNTHESIS. Proc Natl Acad Sci U S A. 1965 Apr;53:881–889. doi: 10.1073/pnas.53.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHATTERJEE A. N., PARK J. T. BIOSYNTHESIS OF CELL WALL MUCOPEPTIDE BY A PARTICULATE FRACTION FROM STAPHYLOCOCCUS AUREUS. Proc Natl Acad Sci U S A. 1964 Jan;51:9–16. doi: 10.1073/pnas.51.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMB D. G. The enzymatic addition of D-alanyl-D-alanine to a uridine nucleotide-peptide. J Biol Chem. 1962 May;237:1601–1604. [PubMed] [Google Scholar]
- LARK C., BRADLEY D., LARK K. G. FURTHER STUDIES ON THE INCORPORATION OF D-METHIONINE INTO THE BACTERIAL CELL WALL; ITS INCORPORATION INTO THE R-LAYER AND THE STRUCTURAL CONSEQUENCES. Biochim Biophys Acta. 1963 Oct 29;78:278–288. doi: 10.1016/0006-3002(63)91638-x. [DOI] [PubMed] [Google Scholar]
- LARK C., LARK K. G. Studies on the mechanism by which D-amino acids block cell wall synthesis. Biochim Biophys Acta. 1961 May 13;49:308–322. doi: 10.1016/0006-3002(61)90130-5. [DOI] [PubMed] [Google Scholar]
- NEUHAUS F. C., LYNCH J. L. THE ENZYMATIC SYNTHESIS OF D-ALANYL-D-ALANINE. 3. ON THE INHIBITION OF D-ALANYL-D-ALANINE SYNTHETASE BY THE ANTIBIOTIC D-CYCLOSERINE. Biochemistry. 1964 Apr;3:471–480. doi: 10.1021/bi00892a001. [DOI] [PubMed] [Google Scholar]
- NEUHAUS F. C., STRUVE W. G. ENZYMATIC SYNTHESIS OF ANALOGS OF THE CELL-WALL PRECURSOR. I. KINETICS AND SPECIFICITY OF URIDINE DIPHOSPHO-N-ACETYLMURAMYL-L-ALANYL-D-GLUTAMYL-L-LYSINE:D-ALANYL-D-ALANINE LIGASE (ADENOSINE DIPHOSPHATE) FROM STREPTOCOCCUS FAECALIS R. Biochemistry. 1965 Jan;4:120–131. doi: 10.1021/bi00877a020. [DOI] [PubMed] [Google Scholar]
- NEUHAUS F. C. The enzymatic synthesis of D-alanyl-D-alanine. I. Purification and properties of D-alanyl-D-alanine synthetase. J Biol Chem. 1962 Mar;237:778–786. [PubMed] [Google Scholar]
- PARK J. T. Uridine-5'-pyrophosphate derivatives. II. A structure common to three derivatives. J Biol Chem. 1952 Feb;194(2):885–895. [PubMed] [Google Scholar]
- PARK J. T. Uridine-5'-pyrophosphate derivatives. II. Isolation from Staphylococcus aureus. J Biol Chem. 1952 Feb;194(2):877–884. [PubMed] [Google Scholar]
- PARK J. T. Uridine-5'-pyrophosphate derivatives. III. Amino acid-containing derivatives. J Biol Chem. 1952 Feb;194(2):897–904. [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- SAITO M., ISHIMOTO N., ITO E. URIDINE DIPHOSPHATE N-ACETYLAMINO SUGAR DERIVATIVES IN PENICILLIN-TREATED STAPHYLOCOCCUS AUREUS. J Biochem. 1963 Sep;54:273–278. doi: 10.1093/oxfordjournals.jbchem.a127783. [DOI] [PubMed] [Google Scholar]
- SAUKKONEN J. J., VIRKOLA P. Acid-soluble nucleotides of Staphylococcus aureus. II. Methods of preparation. Ann Med Exp Biol Fenn. 1963;41:220–227. [PubMed] [Google Scholar]
- STROMINGER J. L. Accumulation of uridine and cytidine nucleotides in Staphylococcus aureus inhibited by gentian violet. J Biol Chem. 1959 Jun;234(6):1520–1524. [PubMed] [Google Scholar]
- STROMINGER J. L., PARK J. T., THOMPSON R. E. Composition of the cell wall of Staphylococcus aureus: its relation to the mechanism of action of penicillin. J Biol Chem. 1959 Dec;234:3263–3268. [PubMed] [Google Scholar]
- STRUVE W. G., NEUHAUS F. C. EVIDENCE FOR AN INITIAL ACCEPTOR OF UDP-NAC-MURAMYL-PENTAPEPTIDE IN THE SYNTHESIS OF BACTERIAL MUCOPEPTIDE. Biochem Biophys Res Commun. 1965 Jan 4;18:6–12. doi: 10.1016/0006-291x(65)90873-9. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Conover M. J., Kolb J. J., Riley L. S., Toennies G. NUTRITIONAL REQUIREMENTS FOR BACTERIAL CELL WALL SYNTHESIS. J Bacteriol. 1961 Jan;81(1):44–50. doi: 10.1128/jb.81.1.44-50.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell E. E., Guirard B. M. Some Interrelationships of Pyridoxine, Alanine and Glycine in Their Effect on Certain Lactic Acid Bacteria. Proc Natl Acad Sci U S A. 1943 Feb;29(2):66–73. doi: 10.1073/pnas.29.2.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANAKA N., SASHIKATA K., WADA T., SUGAWARA S., UMEZAWA H. MECHANISM OF ACTION OF O-CARBAMYL-D-SERINE. J Antibiot (Tokyo) 1963 Nov;16:217–221. [PubMed] [Google Scholar]
- TANAKA N., UMEZAWA H. SYNERGISM OF D-4-AMINO-3-ISOXAZOLIDONE AND O-CARBAMYL-D-SERINE. J Antibiot (Tokyo) 1964 Jan;17:8–10. [PubMed] [Google Scholar]
- TOENNIES G., SHOCKMAN G. D., KOLB J. J. Differential effects of amino acid deficiencies on bacterial cytochemistry. Biochemistry. 1963 Mar-Apr;2:294–296. doi: 10.1021/bi00902a017. [DOI] [PubMed] [Google Scholar]
- WOOD W. A., GUNSALUS I. C. D-Alanine formation; a racemase in Streptococcus faecalis. J Biol Chem. 1951 May;190(1):403–416. [PubMed] [Google Scholar]
- Whitney J. G., Grula E. A. Incorporation of D-serine into the cell wall mucopeptide of Micrococcus lysodeikticus. Biochem Biophys Res Commun. 1964;14:375–381. doi: 10.1016/s0006-291x(64)80013-9. [DOI] [PubMed] [Google Scholar]