Abstract
Alzheimer’s disease (AD) is the most common form of neurodegenerative disease. The vast majority cases of AD are sporadic, without clear cause, and a combination of environmental and genetic factors have been implicated. The hypothesis that homocysteine (Hcy) is a risk factor for AD was initially prompted by the observation that patients with histologically confirmed AD had higher plasma levels of Hcy, also called hyperhomocysteinemia (HHcy), than age-matched controls. Most evidence accumulated so far implicates HHcy as a risk factor for AD onset, but conflicting results also exist. In this review, we summarize reports on the relationship between HHCy and AD from epidemiological investigations, including observational studies and randomized controlled clinical trials. We also examine recent in vivo and in vitro studies of potential mechanisms whereby HHcy may influence AD development. Finally, we discuss possible reasons for the existing conflicting data, and provide suggestions for future studies.
Homocysteine and Alzheimer’s disease
Alzheimer’s disease (AD) is the most common form of dementia and its pathological hallmarks in the brains are neuritic plaques composed mainly by amyloid-β (Aβ) peptides, and neurofibrillary tangles formed by hyperphosphorylated forms of tau protein1. Although AD is probably a multifactorial disease and the real cause remains unknown, various hypotheses have been proposed. For example, the amyloid hypothesis suggests that the accumulation of Aβs as the major cause of the disease. Aβs are the 40–42 amino acid peptides cleaved from the amyloid precursor protein (APP) by the subsequential action of the β-secretase-1 (BACE-1) and γ-secretase. By contrast, the tau hypothesis considers abnormally hyperphosphorylated tau as the major culprit of AD1.
Globally, more than 26 million people have been diagnosed with AD. As the population ages, prevalence of AD keeps rising and is projected to be over 100 million by 20502. More than just a devastating disease for the patients and their families, AD also puts a huge financial burden on the whole society3. Altough an effective treatment for AD is unavailable, interventions to control risk factors (e.g. lowering of high blood pressure and high cholesterol levels) can still reduce the number of cases and associated cost. Given the fact that this disease mainly targets people over 65 years old, a small 1 year delay in disease onset would result in 9.2 million fewer cases worldwide by 2050, and save billions in costs for the society2. An immense effort, therefore, has been spent on identifying risk factors for AD and developing treatments to reduce them.
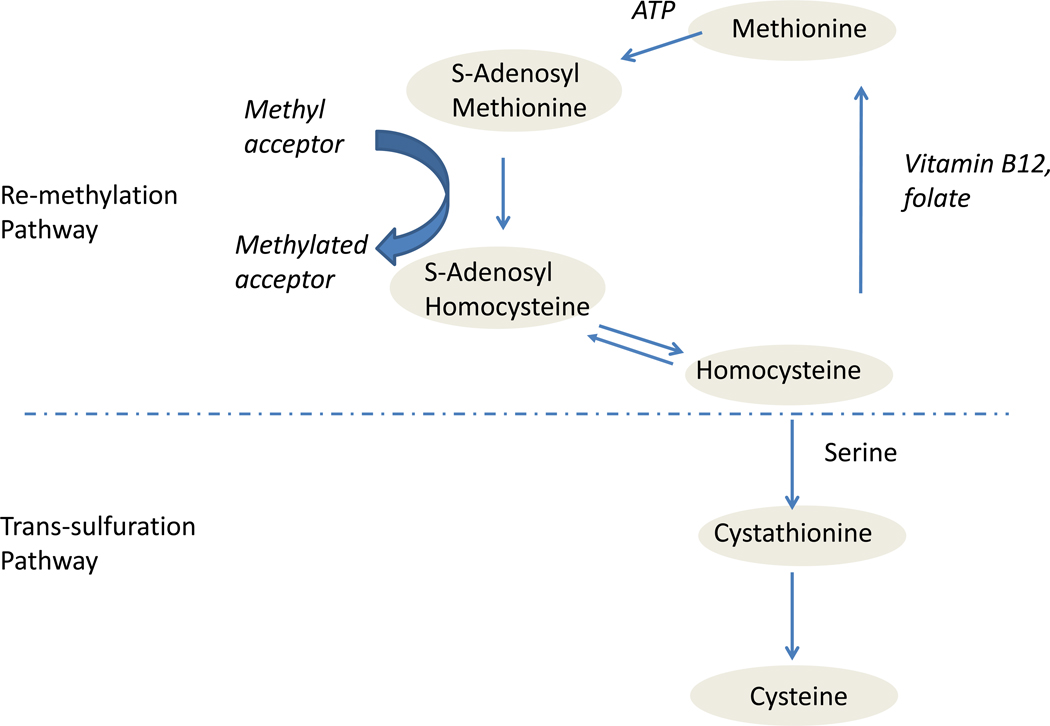
Hyperhomocysteinemia (HHcy), the abnormal elevation of blood levels of homocysteine (Hcy), has been proposed to be a modifiable risk factor for AD4. Hcy is a sulfur-containing, non-protein amino acid produced in the methionine cycle. Its metabolism is at the intersection of two main pathways: remethylation and trans-sulfuration (Figure 1). When the methionine level is low, Hcy is remethylated into methionine; a process which requires vitamin B12 and folic acid as cofactors. Methionine is then activated by ATP to form S-adenosyl-methionine (SAM), which serves as the major methyl group donor in the cell. After demethylation, SAM generates S-adenosyl-homocysteine (SAH) and eventually is hydrolyzed back to Hcy for a new cycle. When methionine levels are high, Hcy, through the trans-sulfuration pathway, condenses with serine to form cystathionine, and subsequently cysteine in an irreversible reaction. Therefore, elevated Hcy level, which is associated with low methylation potential, can be reduced by dietary intervention of folic acid and vitamin Bs.
Figure.
Homocysteine metabolism is at the intersection of two main pathways: remethylation and trans-sulfuration. When the methionine level is low, homocysteine is remethylated into methionine; a process which requires vitamin B12 and folic acid as cofactors. Methionine then forms S-adenosyl-methionine (SAM), which serves as the major methyl group donor in the cell. After demethylation, SAM generates S-adenosyl-homocysteine (SAH) and eventually is0 hydrolyzed back to homocysteine for a new cycle. When methionine levels are high, homocysteine, through the trans-sulfuration pathway, condenses with serine to form cystathionine, and subsequently cysteine in an irreversible reaction.
Since the first paper reporting the elevation of Hcy in AD patients in 19905, increasing numbers of studies have been conducted to explore the relationship between HHcy and the risk of AD. Evidence from human and animal studies has converged to suggest that moderate elevation of Hcy in aged population is a potential risk factor for AD6. With an Hcy level higher than 14 µM, the risk of AD almost doubles in people over 60 years old7. However, contradictory evidence also exists, and it is still controversial whether HHcy is an AD risk factor or merely a biomaker8. Several potential mechanisms have also been proposed to explain the connections between HHcy and AD, including oxidative stress9,10, demethylation11, cerebrovascular damage12, endoplasmic reticulum (ER) stress13, Aβ elevation11, 14, 15 and tau protein phosphorylation16.
This review summarizes the studies on the relationship between HHcy and AD, including observational clinical studies and randomized controlled trials. It also presents some of the mechanisms whereby HHcy may influence AD development by considering the latest results from in vitro and in vivo studies. Finally, several possible explanations for the existing conflicting results are discussed and suggestions for future studies provided. Although Vitamin B deficiency is also reported to be related with cognitive decline and AD, it will not be covered in this paper.
Clinical studies
Most of the direct evidence on the association between HHcy and AD comes from human studies. Regland and colleagues in 1990 first reported elevated Hcy levels in 22 primary degenerative dementia patients5. Since then, dozens of studies, both observational and interventional, have been conducted. Here, we summarize the findings from the studies with a minimal sample of 50 subjects.
Observational studies
Observational studies can be further grouped into retrospective or prospective categories.
Retrospective studies
Retrospective studies focus on the comparison between plasma Hcy levels in AD patients and their age-matched healthy controls in an attempt to establish a potential connection. At least 12 studies reported higher Hcy levels in AD patients than in age-matched healthy controls (Table 1). Plasma Hcy levels were ~ 11.5– 16.4 µM in control groups, whereas Hcy levels ranged from 16.3 µM to 23.52 µM in AD patients. By contrast, three other studies reported no difference in blood or cerebral spinal fluid (CSF) Hcy levels between AD patients and controls17–19. Overall, the majority of retrospective studies have observed an increase in Hcy in AD patients. However, they cannot establish whether this increase is the cause or the result of AD. To explore the temporal association between HHcy and AD, prospective studies are required.
Table 1.
Retrospective study of HHcy and AD/Cognitive decline
| Study | Average age of participants |
Sample size | Hcy level | |
|---|---|---|---|---|
| Hcy level elevated in AD | Joosten, Lesaffre et al. 199785 | 80 years | AD n=52 Hospitalized non-dementia n=50 Healthy control at home n=49 |
AD: 18.3 µM Hospitalized: 14.2µM At home Control:12.3 µM |
| Clarke, Smith et al. 199886 | 73 years | Histological AD n=76 Clinical AD n=164 Health control n=108 |
Histological AD: 16.3 µM Clinical AD: 15.3 µM Control: 13.2 µM |
|
| McCaddon, Davies et al. 199887 | 79 years | AD: n = 30 Control: n = 30 |
AD: 21.9 µM Control: 12.2 µM |
|
| Selley, Close et al. 200288 | 77.5 years | AD: 27 Control: 25 |
AD: 21.05 µM Control: 16.01 µM |
|
| Nagga, Rajani et al. 200389 | N/A | AD: n = 56 VD: n = 50 Control: n = 101 |
Plasma Hcy is significantly increased in dementia group | |
| Selley 200390 | 75 years | AD: n=25 Control: n=25 |
AD: 23.52 µM Control: 19.04 µM |
|
| Gallucci Zanardo et al. 200491 | 77 years | AD: n=137 VD: n = 40 Control: n = 42 |
AD: 21.4 µM VD: 24.4 µM Control: 15.5 µM |
|
| Genedani, Rasio et al. 200492 | 75 years | AD: n = 22 PD: n = 29 Control: n = 57 |
AD: 22.5 µM PD: 18 µM Control: 15.5 µM |
|
| Mizrahi, Bowirrat et al. 2004 93 | 78 years | AD: n=75 Control: n = 155 |
AD: 20.6 µM Control: 16.4 µM |
|
| Quadri, Fragiacomo et al. 2004 94 | 77 years | AD: n = 74 Control: n =55 |
AD: 16.8 µM Control: 14.6 µM |
|
| Guidi, Galimberti et al. 200595 | 72 years | AD: n =97 Control: n = 23 |
AD: 19.0 µM Control: 13.0 µM |
|
| Linnebank, Popp et al. 2010 96 | 73 years | AD: n = 60 Control: n = 60 |
Higher plasma Hcy is associated with AD | |
| Trojanowski, Vandeerstichele et al. 201097 | 75 years | AD: n = 200, MCI: n = 400 Control: n = 200 |
Both AD and MCI have significantly higher plasma Hcy than control | |
| No difference detected | Mizrahi, Jacobsen et al. 200317 | N/A | AD: n = 60 Control: n = 60 |
AD: 12.3 µM Control: 11.5 µM ns |
| Ariogul, Cankurtaran al. 200518 | 72 years | AD: n = 121 Non dementia patients: n = 795 |
AD: 17 µM Non dementia patients: 16.4 µM |
|
| Serot Barbe et al. 2005 19 | 75 years | AD: n = 38 Control: n =22 |
AD: 115 µM Control: 125 µM |
Prospective studies
A prospective study generally starts with a group of healthy subjects and follows them for a certain time (ranging from several months to decades), during which the incidence of AD /dementia is recorded, and the corresponding risk ratio calculated. The Hcy base levels from the subgroup of patients who develop AD and the rest of the original group are compared at the end of the study. Due to the age-dependent incidence of AD in normal population, it is quite challenging to get statistically sufficient number of AD-converting cases in most prospective studies. For this reason, in the current paper, we also include prospective studies assessing the relation between Hcy levels and cognitive decline (that does not meet the criteria for AD) in healthy elderly people. As shown in table 2, among 12 of such prospective studies, only 2 did not detect any association between HHcy and AD/cognitive decline. The other 10, with a maximum sample size of 1779 and longest follow-up time of 35 years, all found HHcy was a risk factor for AD or cognitive decline. For instance, one recently published study followed a group of women for 35 years and revealed that midlife high Hcy level is an independent risk factor for the development of late-stage dementia20. There are also prospective studies following the disease development in mild cognitive impairment (MCI) and AD patients, in which HHcy predicts the conversion of MCI to AD and further cognitive decline in AD, respectively21, 22. Most of these prospective studies reveal that elevation of Hcy levels precedes the cognitive decline or AD development in the elderly, supporting the hypothesis that HHcy is the culprit.
Table 2.
Prospective study of HHcy and AD/Cognitive decline
| Study | Average age of participants |
Sample size | Duration | Results | |
|---|---|---|---|---|---|
| HHcy as predictor for cognitive decline/AD | McCaddon, Hudson et al. 2001 98 | > 60 years | n= 32 | 5 years | Baseline Hcy level predict cognitive decline |
| Dufouil, Alperovitch et al. 2003 99 | 67 years | n= 1241 | 4 year | Cognitive decline 2.8 fold higher in people with Hcy >15 µM | |
| Seshadri, Beiser et al. 20027 | 76.6 years | n= 1092 | 8 years | Plasma Hcy > 14 µM nearly double the AD risk | |
| Ravaglia, Forti et al. 2005 100 | 74 years | N= 816 | 4 years | HHcy associated with AD risk | |
| Tucker, Qiao et al. 2005 101 | 67 years | n= 321 | 3 years | Hcy level predict the cognitive decline | |
| Annerbo, Wahlund et al. 200621 | > 60 year | n= 93 MCI patients |
6 years | Hcy level predict the AD development | |
| Haan Miller et al. 2006 102 | > 60 years | n= 1779 | 4.5 years | High hcy level is associated with dementia | |
| Annerbo, Kivipelto et al. 2009 103 | > 75 years | n= 200 | 6.7 year | High Hcy level is related with high AD risk | |
| Zylberstein, Lissner et al. 201120 | 38–60 years | n= 1368 | 35 years | Baseline Hcy level in midlife can predict the AD risk in later stage | |
| Oulhaj, Refsum et al.22 | > 50 years | n= 97 AD patients | 1.5–9.5 years | Raised hcy level predict the cognitive decline in Ad patients | |
| HHCy is not predictor of cognitive decline/AD | Kalmijn, Launer et al. 1999 101 | >55 years | n= 702 | 2.7 years | No association between HHcy and AD |
| Luchsinger, Tang et al. 2004 102 | >60 years | N= 679 | 5 years | HHcy is not associated with memory score |
Interventional studies
Despite the strong support offered by observational studies, the cause-effect relationship between HHcy and AD cannot be established without evidence obtained from interventional studies, particularly randomized controlled trials (RCTs). RCTs apply random allocation of different intervention to subjects, thus eliminating the potential selection bias, from either known or unknown prognostic factors. It is considered the gold standard for testing the efficacy of any therapeutic agent. The ideal design of a RCT should have Hcy-reducing treatment(s) or placebo in AD patients appropriately randomized, and should apply measures of cognitive changes after the treatment(s). Compared to observational studies, RCTs are more expensive and complex. Few RCTs have been carried out in AD patients so far, and none recruited AD patients with mild to elevated Hcy levels (Table 3)23–25. Among them, only one 6-month-long study found that Hcy-reducing treatment further improves the beneficial effect of cholinesterase inhibitors in AD patients23. Other RCTs, despite the observed reduction in the Hcy levels, failed to detect any cognitive benefit in AD patients. In particular, a recent RCT in 340 patients with mild to moderate AD followed for 3 years found that Hcy reducing treatment—high-dose supplement of folate, Vitamin B6 and B12—does not slow down cognitive decline24.
Table 3.
Randomized Controlled Trials on HHcy and AD
| Treatment effect |
Study | Age | Sample size |
Subjects | Duration | Medication per day |
Results |
|---|---|---|---|---|---|---|---|
| Yes | Connelly, Prentice et al. 200823 | 76.2 years | N =57 | AD without HHcy | 6 months | Folate: 1mg | Hcy reducing treatment improves the effect of anti-cholinesterase drugs on cognitive decline |
| No | Sun, Lu et al. 200725 | 75.3 years | N = 89 | Mild AD patient without HHcy | 26 weeks | Folate: 1mg Vitamin B12: 0.5mg |
Although Hcy levels decrease after treatment, no difference found in cognition |
| Aisen, Schneider et al. 2008 24 | 76 years | N =340 | Mild to moderate AD without HHcy | 3 years | Folate: 5 mg Vitamin B6: 25mg Vitamin B12: 1mg |
No sign of slowing AD development after treatment |
Potential Mechanisms
The biological association between HHcy and AD revealed in human studies has stimulated a large research effort into the investigation of its underlying mechanism(s). A better understanding of these mechanisms could provide valuable insights for future human studies and ultimately therapeutic opportunities against HHcy-dependent AD development and/or progression.
By using in vitro and in vivo models diverse possible mechanistic connections between HHcy and AD pathogenesis have been revealed. Below we list some of the most investigated mechanisms.
Oxidative stress
As a thiol group, Hcy can undergo auto-oxidation to generate hydrogen peroxide and other reactive oxygen species, leading to oxidative stress26. It can also promote oxidative stress by reducing activity of antioxidants such as glutathione, probably through increasing SAH level27. Hcy binding to proteins has also been recently reported to be able to induce oxidative stress under physiological conditions28. Experiments in cell lines, primary neuronal culture and rodent hippocampus slices, have all shown that high amounts of Hcy induce oxidative stress10, 29. Hcy can also augment the toxicity of Aβ and metal ions by exacerbating their pro-oxidant activity9, 30, whereas antioxidants such as vitamin E can rescue these Hcy-induced deficits9.
Considering that the central nervous system (CNS) is extremely vulnerable to oxidative imbalance, owing to its high oxygen consumption, high iron and lipid concentration, and the relatively low activity of antioxidant defense, the oxidative stress induced by high concentration of Hcy is likely to impair important neural functions. This impairment probably leads to cognitive problems in AD.
Demethylation
The S-adenosyl-homocystein (SAH) hydrolase reaction, which forms Hcy from SAH, is reversible, with the equilibrium actually favoring the condensation of Hcy and adenosine to form SAH31 (Figure 1). For this reason, HHcy is usually associated with elevation of intracellular SAH, a competitive universal inhibitor for methyltransferase, and depletion of S-adenosyl-methionine (SAM), the major methyl group donor, resulting in an overall decrease of cellular methylation32. HHcy-dependent demethylation of DNA/histone or proteins can modulate both gene expression and enzyme activities involved in AD pathogenesis. In both cell culture and AD transgenic mouse models, HHcy demethylates the promoters of BACE-1 and Presenilin 1(a major component of the γ-secretase complex), resulting in an increase in their protein levels and Aβ formation11, 33–35. At the protein level, demethylation of protein phosphatase 2A (PP2A) can alter its substrate specificity and enzyme activity36. PP2A is a major brain Ser/Thr phosphatase and mediates tau protein phosphorylation37. Its expression levels have been found significantly reduced in brains of patients with AD38. Although kinases such as glycogen synthase kinase 3β (GSK3β) and cyclin-dependent kinase 5 (CDK5) are also involved in tau phosphorylation, previous study suggested that the decrease of PP2A activity, rather than the increase of these kinases activity, is crucial for tau hyperphosphorylation associated with neurofibrillary tangles formation39. Interestingly, in vitro and in vivo data also show that high doses of Hcy or SAH, through PP2A demethylation, increases phosphorylation of tau and APP, alters APP processing and promotes Aβ production16, 40.
Cerebrovascular damage
For more than four decades, HHcy has also been recognized as a risk factor for cardiovascular disease (CVD)41. Its deleterious effects on the vascular system of the brain could also partially explain the connection between HHcy and AD. For example, HHcy-induced cerebrovascular impairments correlate with the cognitive deficits in mice12.
Furthermore, HHcy impairs the integrity of blood-brain barrier (BBB), both in AD patients and AD transgenic mice42, 43, and causes leakage of detrimental molecules into the tightly-controlled CNS environment, leading to cell damage and cognitive decline44. HHcy-dependent BBB alteration45 could be also responsible for abnormal GABA signaling45, with subsequent damage to endothelial cells46 and astrocytes47. Interestingly, a high-folate diet treatment in MCI patients with HHcy is effective in restoring the BBB integrity, providing further support for this mechanism48. However, whether HHcy can directly cause vascular damage is still under debate, and conflicting data also exist, showing that HHcy is only associated with AD and not with vascular dementia49. In summary, the contribution of HHcy-dependent vascular damage to AD pathogenesis is still very much controversial.
Excitatory damage
Hcy and its acidic derivatives such as homocysteic acid are analogs of glutamate and N-methyl-D-aspartate (NMDA), and act as agonists on the NMDA receptors50. These derivatives are also metabolic glutamate receptor agonists51, and activators of their downstream signaling pathways, which can result in brain excitotoxicity. Thus, in vitro studies have provided evidence that glutamate receptor antagonists (including MK801and MSOP) could attenuate the damage to neurons that are exposed to high concentrations of Hcy52.
Aβ elevation and tau phosphorylation
Recent studies provide evidence that HHcy could directly affect Aβ and tau metabolism. Direct injection of Hcy into rat brain increases Aβ level and tau phosphorylation53, 54. Both diet- and genetic-induced HHcy result in an elevation of Aβ levels and deposition in the brain of transgenic mouse models of AD-like amyloidosis14, 15, 55. GSK3 phosphorylation levels were found reduced in AD transgenic mice with HHcy, suggesting that this signaling pathway may be involved in HHcy-induced Aβ elevation, as GSK3 activation (low phosphorylation levels) can modulate the Aβ production through the γ-secretase pathway14, 56. Moreover, HHcy is found to bind to Aβ40 and favor its β sheet structure formation both in vitro and in vivo, thus facilitating its deposition57. Hcy thiolactone, an intramolecular thioester of Hcy, may also react with Aβ and cause its precipitation58. Another derivative of Hcy, homocysteic acid, increases intracellular Aβ42 in vitro59, and intracranially injected antibody against this metabolite to 3xTg mice results in Aβ reduction and cognitive preservation60.
ER stress
The ER is the principal site for protein synthesis and maturation in the cell. Its physiological functions include regulation of protein production, folding, modification and targeting. ER stress can be induced when some proteins become unfolded or misfolded and tend to accumulate and/or aggregate. If not resolved, this phenomenon slows down protein synthesis, activates protein degradation pathways and eventually triggers apoptosis61. HHcy can induce ER stress by disrupting disulphide bonds and causing misfolding of proteins traversing the ER. In vitro Hcy-induced ER stress can trigger neuronal apoptosis, and synergize the toxic effect of Aβ62.
Several ER stress response proteins, including GRP78, GRP94, X-box-binding protein 1 and HHcy-induced ER response protein (Herp), can also be increased by HHcy63–65. The Herp protein has been shown in vitro to increase Aβ level through the γ-secretase pathway13. Even though its levels are elevated in an AD transgenic mouse overexpressing human APP Swedish mutation, i.e. Tg257666, with diet-induced HHcy67, they are unaltered in a genetic HHcy model mouse, i.e., CBS+/−. By contrast, incubation of cells with high Hcy concentration induces an increase in Aβ formation, but not significant changes in Herp protein or mRNA levels67.
Other mechanisms
Although less investigated, many other mechanisms have been proposed to explain the association between HHcy and AD. For example, DNA repair mechanisms could be impaired by HHcy and lead to apoptosis and hypersensitivity to other types of damages68, 69. Higher Hcy levels are associated with hippocampal or cortical atrophy in healthy elderly adults70, 71 and white matter changes in AD patients72. Other mechanisms that have also been suggested include inhibition of adult neurogenesis73, immune activation74, and blockage of the nitric oxide (NO) signaling pathway75.
Reconciling the differential findings on HHcy and AD
So far, we have summarized the clinical data regarding the relationship between HHcy and AD and introduced some of the potential mechanisms accounting for it. The controversial data from the human studies are hard to interpret. Even though most observational studies suggest that HHcy is a risk factor for AD, RCTs conducted so far cannot demonstrate a beneficial effect of HHcy reducing treatment in AD patients. As the RCT design is still relatively new in the neurodegenerative disease field and most of these trials show negative results, it could suggest that there might be some intrinsic methodological problems3. We believe that more careful consideration and better designed studies are required before jumping to the conclusion that there is no cause-effect relationship between HHcy and AD based on the failure of these trials. Here we will discuss several possibilities to explain the discrepancy in the existing data and provide some suggestions for future research.
Recruitment of AD patients with actual HHcy
One common aspect of the published RCTs is that they recruited subjects with Hcy levels in the normal range for the particular age of the population, rather than subjects with actual HHcy. Based on observational studies, only mildly elevated Hcy level (>14µM), rather than normal Hcy (<14µM) per se, is a risk factor for AD. Thus, providing folate and B vitamins to patients with Hcy within the normal range level is very unlikely to result in any benefit. By contrast, the same treatment in AD patients with mild/high HHcy may provide significant and meaningful benefits.
Recent studies have also shown that reducing HHcy is beneficial for cognition in animal and human trials using subjects with elevated Hcy levels. Two months of Hcy-reducing treatment in Tg2576 mice with HHcy decreases brain amyloid levels and improve their cognitive impairments76. In humans, the FACIT study, a folate acid treatment in 818 seniors between 50–70 years old with HHcy, showed benefit in cognition after 3 years of treatment77. In another study, 5 year folate and vitamin B supplement in older women (n=5540) with CVD showed significant cognitive improvement only in subjects with low folate/vitamin B levels at baseline before treatment78. These studies further support the idea that subjects with circulating Hcy levels above 14µM are the ones who best benefit from Hcy-lowering strategies. Another study using folate treatment in subjects over 65 years old with HHcy failed to detect cognitive improvement. However, compared to the studies just mentioned, it had a smaller sample size (n= 246) and shorter treatment period (2 years)79.
Taken together, these data suggest that future prevention studies with Hcy reducing strategies should recruit subjects with actual HHcy and treat them for an appropriate length of time.
Appropriate cognitive tests
The average Hcy reducing treatment period of most of the published studies is relatively short compared to the years or decades of accumulated HHcy-dependent deleterious effects on cognition. Given this and the fact that the treatment generally occurs in patients at their late stage of life, the beneficial effect of treatment is very likely to be subtle and probably needs appropriate and very sensitive cognitive assessments to detect it. Global cognitive measures such as MMSE are unlikely to be sensitive enough for small and domain-specific changes after Hcy-reducing treatment3. The FACIT study, which detected cognitive benefit after a 3-year folate diet treatment in healthy people, would have missed the changes in memory and information processing speed in the treated group, if they had only implemented MMSE rather than domain-specific tests such as the sensorimotor speed test77. Appropriate cognitive tests, particularly tests focusing on memory and information processing speed, should be included in future HHcy-lowering study in AD patients.
Population difference
Another possible explanation for the discrepancy between the observational studies and RCT is that these studies usually include different patient populations. It takes considerable effort to be part of a RCT, because it requires initial screening, blood sample collection, taking daily medication and participating in different kinds of cognitive tests during the follow-up period. Thus, the subject group is more limited to certain type of patients who are easier to recruit for long-term treatment. These subjects are usually more educated, more concerned about their own health and more likely to take care of themselves. By contrast, observational studies require less effort (if any) from the patients. Therefore, samples collected by observational study are very likely from a population which is larger and more diverse than the one who participates in a RCT. Hcy-reducing treatment(s) may yield better results if a larger and more diverse population is recruited in the trials.
Influence of other factors: lessons from cardiovascular studies
Because HHcy was originally recognized as a risk factor for CVD, many more studies has been conducted in this area than in the AD field. Thus, some lessons learned from those studies could be useful to better understand the connection between HHcy and AD. One thing particularly intriguing is that, similar to what we described in AD, only observational studies, but not RCTs, provide evidence supporting HHcy as a risk factor for CVD. Most folate and vitamin B12 treatment in CVD patients failed to show any improvement80. Are there some common factors that could explain the failure of Hcy-reducing treatment(s) both in CVD and AD?
Interestingly, the AtheroGene study found that the pathogenic effect of HHcy is significantly influenced by the overall cardiovascular redox state81. This study followed 643 subjects with coronary artery disease for 7.1 years and found that both HHcy and GPx-1, one of the most important antioxidant enzymes, were the strongest predictors for future cardiovascular events. Further analysis revealed that in subjects with high GPx-1 activity, HHcy did not predict future cardiovascular events. In the subjects with low GPx-1 activity, plasma Hcy level above the median, however, increased the cardiovascular risk 3.2 fold. This finding suggests that other associated factors, such as oxidative stress, could influence the effect of HHcy on CVD. This would partly explain the lack of benefit secondary to Hcy reduction alone in CVD. It should be interesting to see if a similar effect can also be found in AD patients.
Hcy, folate or vitamin B
Although many studies have examined the connection between Hcy and AD, there is hardly any clinical study that can clearly distinguish a difference in the effect of Hcy, folate or vitamin B12 on cognition and/or AD pathogenesis. None of the current trials can tell if the observed cognitive decline is mediated by HHcy or vitamin Bs. This is probably due to the complexity of Hcy metabolism which closely connects Hcy with folate and B vitamins. Given that almost all the Hcy reducing treatment in RCTs use diet supplement of folate or vitamin B12 or both, the failure of these RCT on slowing AD development cannot rule out the potential of HHcy-reducing treatment using alternative non-vitamin approaches. These non-vitamin-related means, such as the use of N-acetylcysteine, are also imperative to determine the actual effect of HHcy in AD development82. Thus, as shown by several studies, long-term folate treatment could induce adverse effects through mechanisms such as NO signaling inhibition24, 83. These adverse effects may offset the benefits of HHcy reducing treatment in AD and provide an important rationale to test a non-vitamin reducing therapeutic approach.
The SAH / SAM hypothesis
A novel and emerging hypothesis is that SAH is potentially the pathologic mediator of HHcy-dependent effect on AD pathogenesis. It postulates that HHcy is basically a marker of an altered cellular methylation potential, which by affecting the transcription of genes in neuronal cells, modulates molecular events of functional importance in the neurobiology of AD. In particular, HHcy results in high intracellular SAH levels. Because SAH is a competitive inhibitor with SAM, elevated SAH will result in reduced levels of methyltransferase activity, which will then cause reduced levels of methylation of DNA, histone and transcription factors ultimately altering gene expression. Importantly, because SAH and SAM are not readily diffusible through mammalian membranes, they are not at equilibrium between cells and plasma. To remove excessive intracellular SAH, the cell must first convert it to Hcy, which can then be secreted. This hypothesis could also provide a biochemically explanation for the negative results of the vitamin lowering Hcy strategies. Thus, despite the fact that folic acid reduces plasma Hcy levels, it does not influence SAH intracellular concentration and actually, by facilitating methionine synthesis indirectly even increases SAH production, which further aggravates hypomethylation. If this hypothesis holds true, the real target in a condition of HHcy should be SAH and not Hcy itself. However, at the moment, there is no available therapeutic strategy which would specifically target intracellular SAH. Studies targeting SAH by gene therapy approach in animal models of HHcy and AD are warranted.
Animal studies
Recent animal studies, especially the ones using AD-like transgenic mouse models, provide further insights into the potential mechanisms by which HHcy might influence AD development. The availability of genetically induced HHcy has been used to test the hypothesis of its influence on the AD-like phenotype of these mouse models. Crossing heterozygous cystathionine-beta-synthase mutant mice, which spontaneously develop HHcy, with a transgenic mouse model AD-like amyloidosis resulted in higher brain Aβ levels15. In that study the author reported no apparent changes in APP metabolism. Other studies have implemented a diet-induced HHcy models approach to test the same hypothesis. However, caution needs to be taken before comparing the results from different HHcy inducing approaches (e.g. high methionine diet vs. low folate diet), as these different approaches could trigger different downstream mechanisms. Moreover, data accumulated so far indicate that the diet-induced HHcy is also affected by the strain difference. As shown in Table 4, although 6 weeks of a low-folate diet increases plasma Hcy to over 300 µM in the TgCRND8 mice at the age of 3 months11, 7 months feeding of a similar diet can only elevate plasma Hcy to 35µM in Tg2576 mice55. The difference in the transgene between the Tg2576 and TgCRND8 mice (APP Swedish mutation vs. APP Swedish and Indiana mutation) as well as their genetic background (C57BL6 vs. Hybrid C3H/He-C57BL/6) could provide some explanations for the discrepancy in the obtained Hcy levels by dietary intervention. Interestingly the C57BL6 mice per se also show small elevation in plasma Hcy after low-folate diet feeding12. By contrast, studies in human and animal models have also shown that extreme HHcy (Hcy levels over 100 µM) as caused by rare genetic mutations or severe nutrition deficits is not associated with significant elevation of Aβ7, 84. Therefore, since extreme HHcy rarely occurs in the normal aging population it is unlikely that is involved in AD pathogenesis.
Table 4.
Diet-induced HHcy studies in AD transgenic mouse models
| Mouse strain |
Diet | Starting age |
Duration | Hcy level | Effect | study |
|---|---|---|---|---|---|---|
| APP695 Line C3-3 | No folate 4.5g/kg D,L-homocysteine |
7 months old | 3 months | Increased from 3 µm to 27 µm | Hippocampus neuronal death No change in Aβ levels |
Kruman, Kumaravel et al. 2002 69 |
| Tg2576 | No folate No methionine 4.5g/kg D,L-homocysteine |
16–18 months old | 6 months | Increased from 100 µm to 200 µm | Impaired water maze performance No change in Thioflavin-S-staining of amyloid aggregate |
Bernardo McCord et al. 2007 106 |
| Methionine 7.7g/kg | 8 months old | 7 months | Increased from 6 µm to 35 µm | Increase Aβ levels and deposition GSK3 signaling pathway altered Impaired cognitive tests |
Zhuo, Portugal et al. 2010 14 | |
| No folate, vitamin B6 or B12 | 8 months old | 7 months | Increased from 6 µm to 28 µm | Increase Aβ level and deposition | Zhuo and Pratico 2010 55 | |
| No folate, vitamin B6 or B12; Methionine 7.7g/kg |
8 months old | 7 months | Increased from 6 µm to over 150 µm | No change in Aβ levels | Zhuo and Pratico 2010 84 | |
| Methionine 7.7g/kg | 9 months old | 5 months | Increased from 3 µm to 15 µm | 2 months normal diet feeding restores the Hcy levels to normal, and slow Aβ deposition | Zhuo and Pratico 2010 76 | |
| TgCRND8 | No folate, vitamin B6 or B12 | 15 days | 45 days or 60 days | Increased from 0.2 µm to over 200 µm (45 days) or over 400 µm (60 days) | Increase Aβ level and deposition Increase PS1 and BACE1 mRNA and protein level expression |
Fuso, Nicolia et al. 2008 11 |
| PSNE1 gene is regulated by HHcy | Fuso, Nicolia et al. 2009 107 | |||||
| SAM treatment restores the methylation and oxidative system damaged by HHcy | Cavallaro, Fuso et al. 2010 108 |
Concluding remarks
In its sporadic form, AD is a chronic neurodegenerative disease resulting from a complex interplay between exogenous and endogenous factors. Although our knowledge about risk factors is still incomplete, consistent evidence suggests that there are some modifiable factors which play a role in AD pathogenesis. In its original description, HHcy was considered an AD risk factor, but later on it has become evident that it could only be just a marker of the disease. Some recent negative RTCs do not support either definition, and have even seriously questioned the validity of the therapeutic approaches to lower Hcy in AD. However, based on the discussion provided in this article, we believe that the evidence in support of this recent hypothesis should be considered inconclusive and cannot be used to deny a role for HHcy in AD.
In summary, the hypothesis that HHcy is a risk factor for AD onset is still very much alive and viable, but a great deal of work needs to be done. This will involve both basic science research as well as clinical studies. In particular, we lack good animal models of HHcy which more closely reflect the human condition, and clinical trials with better study design are urgently needed. If, in the near future, these two issues are addressed, then we will be able to establish or deny a mechanistic link between AD and HHcy in a conclusive manner.
Acknowledgements
The work from the laboratory of the authors described in the present review article was supported in part by grants from the National Institute of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mudher A, Lovestone S. Alzheimer's disease-do tauists and baptists finally shake hands? Trends Neurosci. 2002;25:22–26. doi: 10.1016/s0166-2236(00)02031-2. [DOI] [PubMed] [Google Scholar]
- 2.Brookmeyer R, et al. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 3.Andrieu S, et al. Methodological issues in primary prevention trials for neurodegenerative dementia. J Alzheimers Dis. 2009;16:235–270. doi: 10.3233/JAD-2009-0971. [DOI] [PubMed] [Google Scholar]
- 4.Morris MS. Homocysteine and Alzheimer's disease. Lancet Neurol. 2003;2:425–428. doi: 10.1016/s1474-4422(03)00438-1. [DOI] [PubMed] [Google Scholar]
- 5.Regland B, et al. Vitamin B12 Analogues, Homocysteine, Methylmalonic Acid, and Transcobalamins in the Study of Vitamin B12 Deficiency in Primary Degenerative Dementia. Dement Geriatr Cogn Disord. 1990;1:272–277. [Google Scholar]
- 6.Van Dam F, Van Gool WA. Hyperhomocysteinemia and Alzheimer's disease: A systematic review. Arch Gerontol Geriatr. 2009;48:425–430. doi: 10.1016/j.archger.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Seshadri S, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer's disease. N Engl J Med. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 8.Seshadri S. Elevated plasma homocysteine levels: risk factor or risk marker for the development of dementia and Alzheimer's disease? J Alzheimers Dis. 2006;9:393–398. doi: 10.3233/jad-2006-9404. [DOI] [PubMed] [Google Scholar]
- 9.Ho PI, et al. Homocysteine potentiates beta-amyloid neurotoxicity: role of oxidative stress. J Neurochem. 2001;78:249–253. doi: 10.1046/j.1471-4159.2001.00384.x. [DOI] [PubMed] [Google Scholar]
- 10.Streck EL, et al. In vitro effect of homocysteine on some parameters of oxidative stress in rat hippocampus. Metab Brain Dis. 2003;18:147–154. doi: 10.1023/a:1023815119931. [DOI] [PubMed] [Google Scholar]
- 11.Fuso A, et al. B-vitamin deprivation induces hyperhomocysteinemia and brain S-adenosylhomocysteine, depletes brain S-adenosylmethionine, and enhances PS1 and BACE expression and amyloid-beta deposition in mice. Mol Cell Neurosci. 2008;37:731–746. doi: 10.1016/j.mcn.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Troen AM, et al. B-vitamin deficiency causes hyperhomocysteinemia and vascular cognitive impairment in mice. Proc Natl Acad Sci U S A. 2008;105:12474–12479. doi: 10.1073/pnas.0805350105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sai X, et al. Endoplasmic reticulum stress-inducible protein, Herp, enhances presenilin-mediated generation of amyloid beta-protein. J Biol Chem. 2002;277:12915–12920. doi: 10.1074/jbc.M112372200. [DOI] [PubMed] [Google Scholar]
- 14.Zhuo JM, et al. Diet-induced hyperhomocysteinemia increases amyloid-beta formation and deposition in a mouse model of Alzheimer's disease. Curr Alzheimer Res. 2010;7:140–149. doi: 10.2174/156720510790691326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pacheco-Quinto J, et al. Hyperhomocysteinemic Alzheimer's mouse model of amyloidosis shows increased brain amyloid beta peptide levels. Neurobiol Dis. 2006;22:651–656. doi: 10.1016/j.nbd.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Sontag E, et al. Protein phosphatase 2A methyltransferase links homocysteine metabolism with tau and amyloid precursor protein regulation. J Neurosci. 2007;27:2751–2759. doi: 10.1523/JNEUROSCI.3316-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizrahi EH, et al. Plasma total homocysteine levels, dietary vitamin B6 and folate intake in AD and healthy aging. J Nutr Health Aging. 2003;7:160–165. [PubMed] [Google Scholar]
- 18.Ariogul S, et al. Vitamin B12, folate, homocysteine and dementia: are they really related? Arch Gerontol Geriatr. 2005;40:139–146. doi: 10.1016/j.archger.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Serot JM, et al. Homocysteine and methylmalonic acid concentrations in cerebrospinal fluid: relation with age and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2005;76:1585–1587. doi: 10.1136/jnnp.2004.056119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zylberstein DE, et al. Midlife homocysteine and late-life dementia in women. A prospective population study. Neurobiol Aging. 2011;32:380–386. doi: 10.1016/j.neurobiolaging.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 21.Annerbo S, et al. The significance of thyroid-stimulating hormone and homocysteine in the development of Alzheimer's disease in mild cognitive impairment: a 6-year follow-up study. Am J Alzheimers Dis Other Demen. 2006;21:182–188. doi: 10.1177/1533317506289282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oulhaj A, et al. Homocysteine as a predictor of cognitive decline in Alzheimer's disease. Int J Geriatr Psychiatry. 2010;25:82–90. doi: 10.1002/gps.2303. [DOI] [PubMed] [Google Scholar]
- 23.Connelly PJ, et al. A randomised double-blind placebo-controlled trial of folic acid supplementation of cholinesterase inhibitors in Alzheimer's disease. Int J Geriatr Psychiatry. 2008;23:155–160. doi: 10.1002/gps.1856. [DOI] [PubMed] [Google Scholar]
- 24.Aisen PS, et al. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. Jama. 2008;300:1774–1783. doi: 10.1001/jama.300.15.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Y, et al. Efficacy of multivitamin supplementation containing vitamins B6 and B12 and folic acid as adjunctive treatment with a cholinesterase inhibitor in Alzheimer's disease: a 26-week, randomized, double-blind, placebo-controlled study in Taiwanese patients. Clin Ther. 2007;29:2204–2214. doi: 10.1016/j.clinthera.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Perna AF, et al. Homocysteine and oxidative stress. Amino Acids. 2003;25:409–417. doi: 10.1007/s00726-003-0026-8. [DOI] [PubMed] [Google Scholar]
- 27.Tchantchou F, et al. S-adenosylmethionine mediates glutathione efficacy by increasing glutathione S-transferase activity: implications for S-adenosyl methionine as a neuroprotective dietary supplement. J Alzheimers Dis. 2008;14:323–328. doi: 10.3233/jad-2008-14306. [DOI] [PubMed] [Google Scholar]
- 28.Sibrian-Vazquez M, et al. Homocystamides promote free-radical and oxidative damage to proteins. Proc Natl Acad Sci U S A. 2010;107:551–554. doi: 10.1073/pnas.0909737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho PI, et al. Folate deprivation induces neurodegeneration: roles of oxidative stress and increased homocysteine. Neurobiol Dis. 2003;14:32–42. doi: 10.1016/s0969-9961(03)00070-6. [DOI] [PubMed] [Google Scholar]
- 30.White AR, et al. Homocysteine potentiates copper- and amyloid beta peptide-mediated toxicity in primary neuronal cultures: possible risk factors in the Alzheimer's-type neurodegenerative pathways. J Neurochem. 2001;76:1509–1520. doi: 10.1046/j.1471-4159.2001.00178.x. [DOI] [PubMed] [Google Scholar]
- 31.De La Haba G, Cantoni GL. The enzymatic synthesis of S-adenosyl-L-homocysteine from adenosine and homocysteine. J Biol Chem. 1959;234:603–608. [PubMed] [Google Scholar]
- 32.Caudill MA, et al. Intracellular S-adenosylhomocysteine concentrations predict global DNA hypomethylation in tissues of methyl-deficient cystathionine beta-synthase heterozygous mice. J Nutr. 2001;131:2811–2818. doi: 10.1093/jn/131.11.2811. [DOI] [PubMed] [Google Scholar]
- 33.Fuso A, et al. gamma-Secretase is differentially modulated by alterations of homocysteine cycle in neuroblastoma and glioblastoma cells. J Alzheimers Dis. 2007;11:275–290. doi: 10.3233/jad-2007-11303. [DOI] [PubMed] [Google Scholar]
- 34.Lin HC, et al. S-Adenosylhomocysteine increases beta-amyloid formation in BV-2 microglial cells by increased expressions of beta-amyloid precursor protein and presenilin 1 and by hypomethylation of these gene promoters. Neurotoxicology. 2009;30:622–627. doi: 10.1016/j.neuro.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Fuso A, et al. S-adenosylmethionine/homocysteine cycle alterations modify DNA methylation status with consequent deregulation of PS1 and BACE and beta-amyloid production. Mol Cell Neurosci. 2005;28:195–204. doi: 10.1016/j.mcn.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Tolstykh T, et al. Carboxyl methylation regulates phosphoprotein phosphatase 2A by controlling the association of regulatory B subunits. Embo J. 2000;19:5682–5691. doi: 10.1093/emboj/19.21.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goedert M, et al. p42 MAP kinase phosphorylation sites in microtubule-associated protein tau are dephosphorylated by protein phosphatase 2A1. Implications for Alzheimer's disease [corrected] FEBS Lett. 1992;312:95–99. doi: 10.1016/0014-5793(92)81418-l. [DOI] [PubMed] [Google Scholar]
- 38.Vogelsberg-Ragaglia V, et al. PP2A mRNA expression is quantitatively decreased in Alzheimer's disease hippocampus. Exp Neurol. 2001;168:402–412. doi: 10.1006/exnr.2001.7630. [DOI] [PubMed] [Google Scholar]
- 39.Planel E, et al. Inhibition of protein phosphatase 2A overrides tau protein kinase I/glycogen synthase kinase 3 beta and cyclin-dependent kinase 5 inhibition and results in tau hyperphosphorylation in the hippocampus of starved mouse. J Biol Chem. 2001;276:34298–34306. doi: 10.1074/jbc.M102780200. [DOI] [PubMed] [Google Scholar]
- 40.Kuszczyk M, et al. Homocysteine-induced acute excitotoxicity in cerebellar granule cells in vitro is accompanied by PP2A-mediated dephosphorylation of tau. Neurochem Int. 2009;55:174–180. doi: 10.1016/j.neuint.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 41.Huang T, et al. Cardiovascular pathogenesis in hyperhomocysteinemia. Asia Pac J Clin Nutr. 2008;17:8–16. [PubMed] [Google Scholar]
- 42.Bowman GL, et al. Blood-brain barrier impairment in Alzheimer disease: stability and functional significance. Neurology. 2007;68:1809–1814. doi: 10.1212/01.wnl.0000262031.18018.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ujiie M, et al. Blood-brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation. 2003;10:463–470. doi: 10.1038/sj.mn.7800212. [DOI] [PubMed] [Google Scholar]
- 44.Arshavsky YI. Why Alzheimer's disease starts with a memory impairment: neurophysiological insight. J Alzheimers Dis. 2010;20:5–16. doi: 10.3233/JAD-2009-1339. [DOI] [PubMed] [Google Scholar]
- 45.Kumar M, et al. GABAA receptor agonist mitigates homocysteine-induced cerebrovascular remodeling in knockout mice. Brain Res. 2008;1221:147–153. doi: 10.1016/j.brainres.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hohsfield LA, Humpel C. Homocysteine Enhances Transmigration of Rat Monocytes Through a Brain Capillary Endothelial Cell Monolayer via ICAM-1. Curr Neurovasc Res. 2010 doi: 10.2174/156720210792231787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin Y, Brennan L. Effects of homocysteine on metabolic pathways in cultured astrocytes. Neurochem Int. 2008;52:1410–1415. doi: 10.1016/j.neuint.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Lehmann M, et al. Vitamin B12-B6-folate treatment improves blood-brain barrier function in patients with hyperhomocysteinaemia and mild cognitive impairment. Dement Geriatr Cogn Disord. 2003;16:145–150. doi: 10.1159/000071002. [DOI] [PubMed] [Google Scholar]
- 49.Ravaglia G, et al. Blood inflammatory markers and risk of dementia: The Conselice Study of Brain Aging. Neurobiol Aging. 2007;28:1810–1820. doi: 10.1016/j.neurobiolaging.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Lipton SA, et al. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 1997;94:5923–5928. doi: 10.1073/pnas.94.11.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi Q, et al. L-homocysteine sulfinic acid and other acidic homocysteine derivatives are potent and selective metabotropic glutamate receptor agonists. J Pharmacol Exp Ther. 2003;305:131–142. doi: 10.1124/jpet.102.047092. [DOI] [PubMed] [Google Scholar]
- 52.Boldyrev AA, Johnson P. Homocysteine and its derivatives as possible modulators of neuronal and non-neuronal cell glutamate receptors in Alzheimer's disease. J Alzheimers Dis. 2007;11:219–228. doi: 10.3233/jad-2007-11209. [DOI] [PubMed] [Google Scholar]
- 53.Luo Y, et al. Homocysteine induces tau hyperphosphorylation in rats. Neuroreport. 2007;18:2005–2008. doi: 10.1097/WNR.0b013e3282f29100. [DOI] [PubMed] [Google Scholar]
- 54.Zhang CE, et al. Hyperhomocysteinemia increases beta-amyloid by enhancing expression of gamma-secretase and phosphorylation of amyloid precursor protein in rat brain. Am J Pathol. 2009;174:1481–1491. doi: 10.2353/ajpath.2009.081036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhuo JM, Pratico D. Acceleration of brain amyloidosis in an Alzheimer's disease mouse model by a folate, vitamin B6 and B12-deficient diet. Exp Gerontol. 2010;45:195–201. doi: 10.1016/j.exger.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Phiel CJ, et al. GSK-3alpha regulates production of Alzheimer's disease amyloidbeta peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 57.Agnati LF, et al. Abeta peptides as one of the crucial volume transmission signals in the trophic units and their interactions with homocysteine. Physiological implications and relevance for Alzheimer's disease. J Neural Transm. 2007;114:21–31. doi: 10.1007/s00702-006-0564-9. [DOI] [PubMed] [Google Scholar]
- 58.Toohey JI. Mercaptopropionaldehyde from homocysteine: implications for Alzheimer's disease. J Alzheimers Dis. 2007;12:241–243. doi: 10.3233/jad-2007-12305. [DOI] [PubMed] [Google Scholar]
- 59.Hasegawa T, et al. Homocysteic acid induces intraneuronal accumulation of neurotoxic Abeta42: implications for the pathogenesis of Alzheimer's disease. J Neurosci Res. 2005;80:869–876. doi: 10.1002/jnr.20514. [DOI] [PubMed] [Google Scholar]
- 60.Hasegawa T, et al. Treatment of Alzheimer's disease with anti-homocysteic acid antibody in 3xTg-AD male mice. PLoS One. 2010;5:e8593. doi: 10.1371/journal.pone.0008593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schroder M. Endoplasmic reticulum stress responses. Cell Mol Life Sci. 2008;65:862–894. doi: 10.1007/s00018-007-7383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim HJ, et al. Synergistic induction of ER stress by homocysteine and betaamyloid in SH-SY5Y cells. J Nutr Biochem. 2008;19:754–761. doi: 10.1016/j.jnutbio.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 63.Ji C, Kaplowitz N. Hyperhomocysteinemia, endoplasmic reticulum stress, and alcoholic liver injury. World J Gastroenterol. 2004;10:1699–1708. doi: 10.3748/wjg.v10.i12.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kokame K, et al. Herp, a new ubiquitin-like membrane protein induced by endoplasmic reticulum stress. J Biol Chem. 2000;275:32846–32853. doi: 10.1074/jbc.M002063200. [DOI] [PubMed] [Google Scholar]
- 65.Hosoi T, et al. Homocysteine induces X-box-binding protein 1 splicing in the mice brain. Neurochem Int. 2010;56:216–220. doi: 10.1016/j.neuint.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 66.Hsiao K, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 67.Zhuo JM, et al. The Herp protein pathway is not involved in the pro-amyloidogenic effect of hyperhomocysteinemia. J Alzheimers Dis. 2010;20:569–576. doi: 10.3233/JAD-2010-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kruman II, et al. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J Neurosci. 2000;20:6920–6926. doi: 10.1523/JNEUROSCI.20-18-06920.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kruman II, et al. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer's disease. J Neurosci. 2002;22:1752–1762. doi: 10.1523/JNEUROSCI.22-05-01752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.den Heijer T, et al. Homocysteine and brain atrophy on MRI of non-demented elderly. Brain. 2003;126:170–175. doi: 10.1093/brain/awg006. [DOI] [PubMed] [Google Scholar]
- 71.Williams JH, et al. Minimal hippocampal width relates to plasma homocysteine in community-dwelling older people. Age Ageing. 2002;31:440–444. doi: 10.1093/ageing/31.6.440. [DOI] [PubMed] [Google Scholar]
- 72.Kim SR, et al. Plasma Total Homocysteine Levels are not Associated with Medial Temporal Lobe Atrophy, but with White Matter Changes in Alzheimer's Disease. J Clin Neurol. 2009;5:85–90. doi: 10.3988/jcn.2009.5.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kruman II, et al. Folate deficiency inhibits proliferation of adult hippocampal progenitors. Neuroreport. 2005;16:1055–1059. doi: 10.1097/00001756-200507130-00005. [DOI] [PubMed] [Google Scholar]
- 74.Schroecksnadel K, et al. Association of hyperhomocysteinemia in Alzheimer disease with elevated neopterin levels. Alzheimer Dis Assoc Disord. 2004;18:129–133. doi: 10.1097/01.wad.0000127443.23312.31. [DOI] [PubMed] [Google Scholar]
- 75.Selley ML. Homocysteine increases the production of asymmetric dimethylarginine in cultured neurons. J Neurosci Res. 2004;77:90–93. doi: 10.1002/jnr.20070. [DOI] [PubMed] [Google Scholar]
- 76.Zhuo JM, Pratico D. Normalization of hyperhomocysteinemia improves cognitive deficits and ameliorates brain amyloidosis of a transgenic mouse model of Alzheimer's disease. Faseb J. 2010;24:3895–3902. doi: 10.1096/fj.10-161828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Durga J, et al. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet. 2007;369:208–216. doi: 10.1016/S0140-6736(07)60109-3. [DOI] [PubMed] [Google Scholar]
- 78.Kang JH, et al. A trial of B vitamins and cognitive function among women at high risk of cardiovascular disease. Am J Clin Nutr. 2008;88:1602–1610. doi: 10.3945/ajcn.2008.26404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McMahon JA, et al. A controlled trial of homocysteine lowering and cognitive performance. N Engl J Med. 2006;354:2764–2772. doi: 10.1056/NEJMoa054025. [DOI] [PubMed] [Google Scholar]
- 80.Smulders YM, Blom HJ. The homocysteine controversy. J Inherit Metab Dis. 2010 doi: 10.1007/s10545-010-9151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schnabel R, et al. Glutathione peroxidase-1 and homocysteine for cardiovascular risk prediction: results from the AtheroGene study. J Am Coll Cardiol. 2005;45:1631–1637. doi: 10.1016/j.jacc.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 82.Roes EM, et al. Effects of oral N-acetylcysteine on plasma homocysteine and whole blood glutathione levels in healthy, non-pregnant women. Clin Chem Lab Med. 2002;40:496–498. doi: 10.1515/CCLM.2002.086. [DOI] [PubMed] [Google Scholar]
- 83.Joseph J, et al. Quo vadis: whither homocysteine research? Cardiovasc Toxicol. 2009;9:53–63. doi: 10.1007/s12012-009-9042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhuo JM, Pratico D. Severe In vivo hyper-homocysteinemia is not associatedwith elevation of amyloid-beta peptides in the Tg2576 mice. J Alzheimers Dis. 2010;21:133–140. doi: 10.3233/JAD-2010-100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Joosten E, et al. Is metabolic evidence for vitamin B-12 and folate deficiency more frequent in elderly patients with Alzheimer's disease? J Gerontol A Biol Sci Med Sci. 1997;52:M76–M79. doi: 10.1093/gerona/52a.2.m76. [DOI] [PubMed] [Google Scholar]
- 86.Clarke R, et al. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol. 1998;55:1449–1455. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- 87.McCaddon A, et al. Total serum homocysteine in senile dementia of Alzheimer type. Int J Geriatr Psychiatry. 1998;13:235–239. doi: 10.1002/(sici)1099-1166(199804)13:4<235::aid-gps761>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 88.Selley ML, et al. The effect of increased concentrations of homocysteine on the concentration of (E)-4-hydroxy-2-nonenal in the plasma and cerebrospinal fluid of patients with Alzheimer's disease. Neurobiol Aging. 2002;23:383–388. doi: 10.1016/s0197-4580(01)00327-x. [DOI] [PubMed] [Google Scholar]
- 89.Nagga K, et al. Cobalamin, folate, methylmalonic acid, homocysteine, and gastritis markers in dementia. Dement Geriatr Cogn Disord. 2003;16:269–275. doi: 10.1159/000072812. [DOI] [PubMed] [Google Scholar]
- 90.Selley ML. Increased concentrations of homocysteine and asymmetric dimethylarginine and decreased concentrations of nitric oxide in the plasma of patients with Alzheimer's disease. Neurobiol Aging. 2003;24:903–907. doi: 10.1016/s0197-4580(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 91.Gallucci M, et al. Homocysteine in Alzheimer disease and vascular dementia. Arch Gerontol Geriatr Suppl. 2004:195–200. doi: 10.1016/j.archger.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 92.Genedani S, et al. Studies on homocysteine and dehydroepiandrosterone sulphate plasma levels in Alzheimer's disease patients and in Parkinson's disease patients. Neurotox Res. 2004;6:327–332. doi: 10.1007/BF03033443. [DOI] [PubMed] [Google Scholar]
- 93.Mizrahi EH, et al. Plasma homocysteine, vitamin B12 and folate in Alzheimer's patients and healthy Arabs in Israel. J Neurol Sci. 2004;227:109–113. doi: 10.1016/j.jns.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 94.Quadri P, et al. Homocysteine, folate, and vitamin B-12 in mild cognitive impairment, Alzheimer disease, and vascular dementia. Am J Clin Nutr. 2004;80:114–122. doi: 10.1093/ajcn/80.1.114. [DOI] [PubMed] [Google Scholar]
- 95.Guidi I, et al. Influence of the Glu298Asp polymorphism of NOS3 on age at onset and homocysteine levels in AD patients. Neurobiol Aging. 2005;26:789–794. doi: 10.1016/j.neurobiolaging.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 96.Linnebank M, et al. S-Adenosylmethionine Is Decreased in the Cerebrospinal Fluid of Patients with Alzheimer's Disease. Neurodegener Dis. 2010;7:373–378. doi: 10.1159/000309657. [DOI] [PubMed] [Google Scholar]
- 97.Trojanowski JQ, et al. Update on the biomarker core of the Alzheimer's Disease Neuroimaging Initiative subjects. Alzheimers Dement. 2010;6:230–238. doi: 10.1016/j.jalz.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McCaddon A, et al. Homocysteine and cognitive decline in healthy elderly. Dement Geriatr Cogn Disord. 2001;12:309–313. doi: 10.1159/000051275. [DOI] [PubMed] [Google Scholar]
- 99.Dufouil C, et al. Homocysteine, white matter hyperintensities, and cognition in healthy elderly people. Ann Neurol. 2003;53:214–221. doi: 10.1002/ana.10440. [DOI] [PubMed] [Google Scholar]
- 100.Ravaglia G, et al. Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am J Clin Nutr. 2005;82:636–643. doi: 10.1093/ajcn.82.3.636. [DOI] [PubMed] [Google Scholar]
- 101.Tucker KL, et al. High homocysteine and low B vitamins predict cognitive decline in aging men: the Veterans Affairs Normative Aging Study. Am J Clin Nutr. 2005;82:627–635. doi: 10.1093/ajcn.82.3.627. [DOI] [PubMed] [Google Scholar]
- 102.Haan MN, et al. Homocysteine, B vitamins, and the incidence of dementia and cognitive impairment: results from the Sacramento Area Latino Study on Aging. Am J Clin Nutr. 2007;85:511–517. doi: 10.1093/ajcn/85.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Annerbo S, et al. A prospective study on the development of Alzheimer's disease with regard to thyroid-stimulating hormone and homocysteine. Dement Geriatr Cogn Disord. 2009;28:275–280. doi: 10.1159/000242439. [DOI] [PubMed] [Google Scholar]
- 104.Kalmijn S, et al. Total homocysteine and cognitive decline in a community-based sample of elderly subjects: the Rotterdam Study. Am J Epidemiol. 1999;150:283–289. doi: 10.1093/oxfordjournals.aje.a010000. [DOI] [PubMed] [Google Scholar]
- 105.Luchsinger JA, et al. Plasma homocysteine levels and risk of Alzheimer disease. Neurology. 2004;62:1972–1976. doi: 10.1212/01.wnl.0000129504.60409.88. [DOI] [PubMed] [Google Scholar]
- 106.Bernardo A, et al. Impaired spatial memory in APP-overexpressing mice on a homocysteinemia-inducing diet. Neurobiol Aging. 2007;28:1195–1205. doi: 10.1016/j.neurobiolaging.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 107.Fuso A, et al. Changes in Presenilin 1 gene methylation pattern in diet-induced B vitamin deficiency. Neurobiol Aging. 2011;32:187–199. doi: 10.1016/j.neurobiolaging.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 108.Cavallaro RA, et al. S-adenosylmethionine prevents oxidative stress and modulates glutathione metabolism in TgCRND8 mice fed a B-vitamin deficient diet. J Alzheimers Dis. 2010;20:997–1002. doi: 10.3233/JAD-2010-091666. [DOI] [PubMed] [Google Scholar]