Abstract
Why both testosterone (T) suppression and cryptorchidism reverse the block in spermatogonial differentiation in adult mice homozygous for the juvenile spermatogonial depletion (jsd) mutation has been a conundrum. To resolve this conundrum, we analyzed interrelations between T suppression, testicular temperature, and spermatogonial differentiation and used in vitro techniques to separate the effects of the two treatments on the spermatogonial differentiation block in jsd mice. Temporal analysis revealed that surgical cryptorchidism rapidly stimulated spermatogonial differentiation whereas androgen ablation treatment produced a delayed and gradual differentiation. The androgen suppression caused scrotal shrinkage, significantly increasing the intrascrotal temperature. When serum T or intratesticular T (ITT) levels were modulated separately in GnRH antagonist-treated mice by exogenous delivery of T or LH, respectively, the inhibition of spermatogonial differentiation correlated with the serum T and not with ITT levels. Thus, the block must be caused by peripheral androgen action. When testicular explants from jsd mice were cultured in vitro at 32.5 C, spermatogonial differentiation was not observed, but at 37 C significant differentiation was evident. In contrast, addition of T to the culture medium did not block the stimulation of spermatogonial differentiation at 37 C, and androgen ablation with aminoglutethimide and hydroxyflutamide did not stimulate differentiation at 32.5 C, suggesting that T had no direct effect on spermatogonial differentiation in jsd mice. These data show that elevation of temperature directly overcomes the spermatogonial differentiation block in adult jsd mice and that T suppression acts indirectly in vivo by causing scrotal regression and thereby elevating the testicular temperature.
Juvenile spermatogonial depletion (jsd) mice carry a spontaneous mutation in the Utp14b gene (1–3). Whereas homozygous females are fertile, the adult male mice homozygous for Utp14bjsd mutation (hereafter mentioned as jsd in the text) are sterile, which is their only phenotypic defect. In these males, there is a progressive loss of germ cells because the spermatogonial differentiation becomes arrested after the first several waves of spermatogenesis (4). Thus undifferentiated type A spermatogonia are the only germ cells found in most seminiferous tubules of adult jsd mice. A constant pool of these undifferentiated type A spermatogonia is maintained in these mice through a continuous cycle of proliferation and apoptosis without differentiation (5). Spermatogonial transplantation experiments have shown that this arrest of spermatogonial differentiation is a result of an intrinsic defect in the spermatogonia (6, 7).
Previous studies (8, 9) have demonstrated that suppression of testosterone (T) by GnRH antagonists (GnRH-ant) reverses the block of spermatogonial differentiation, allowing recovery of spermatogenesis to the spermatocyte or early spermatid stage in jsd mice. Further studies (10, 11) showed that androgen action mediated by the androgen receptor (AR), but not the AR in the Sertoli cells (12), is required to maintain the block in spermatogonial differentiation. Subsequently, we made a surprising observation that the elevation of testicular temperature by cryptorchid surgery also restored differentiation of spermatogonia in these mutant mice (13) even in the presence of physiological serum and intratesticular T (ITT) levels.
Why two normally detrimental treatments have therapeutic effects on spermatogonial differentiation in jsd mice has been a conundrum. The conundrum is less profound than it appears at first, however. In normal mice, both low levels of T and elevation of testicular temperature primarily disrupt progression through meiosis and spermatid development (14, 15) but have little or no effect on the development of spermatogonia or their progression to the spermatocyte stage. Because the beneficial effects of these treatments in jsd mice occur at a different stage of spermatogenesis from their negative effects in normal mice, the two phenomena are independent and not really in contradiction. Indeed T suppression and elevated temperature do inhibit meiosis and spermiogenesis in jsd mice when the cells reach those stages (13, 16).
Still it is not clear whether the beneficial effects of these two factors observed during early spermatogenesis in jsd mice are related to each other. In the present study, we used both in vivo and in vitro approaches to determine whether there is an interrelationship between the suppression of androgen and elevation of testicular temperature in overcoming the mutation-induced defect in germ cells in jsd mice. Our results indicate that elevation of temperature directly restores spermatogonial differentiation, and the mechanism by which androgen suppression acts is primarily by shrinking the scrotum and thus elevating testicular temperature.
Materials and Methods
Animals
Prepubertal 6- to 7-d-old or adult 9- to 12-wk-old jsd mutant mice on a C3H-B6-129 hybrid background (designated HB129) were used (details in Supplemental Materials and Methods published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org. The mice were genotyped by PCR of tail DNA using the primers and HphI digestion of the PCR product, as previously described (11). All experimental procedures were approved by the MD Anderson Cancer Center Institutional Animal Care and Use Committee.
Cryptorchidism surgery
Jsd mutant mice were unilaterally cryptorchidized at 10–12 wk of age. The adipose tissue of the right caput epididymis was sutured to the lower part of the inner peritoneal wall at a position that placed the testis at, or slightly above, the level of the urinary bladder, as described earlier (13), without cutting the gubernacular cord. The left testis remained in the scrotum and was used as the control.
Androgen suppression and hormone treatment
T production was suppressed by treating 9–12 wk-old jsd mice with the GnRH-ant acyline (obtained from the Contraceptive Development Branch of National Institute of Child Health and Human Development, North Bethesda, MD). When used in combination with the AR antagonist flutamide (Sigma Aldrich, St. Louis, MO), the first dose of acyline was given as two injections (each 10 mg/kg) at separate sites, followed by one injection (10 mg/kg) every 2 wk (11). Where only T production was suppressed using GnRH-ant, a higher dose of acyline (acyline protocol II) was used in some cases. In such cases the initial dose was 20 mg/kg at one site plus 10 mg/kg at the second site, followed by injections of 10 mg/kg at two sites every 2 wk.
T (Sigma Aldrich) was administered in two ways. In experiments in which only one dose of T was used, 0.3-cm SILASTIC (Dow Corning Corp., Midland, MI) capsules were implanted as described earlier (10). To assess the dose-dependent effect of exogenous T at lower doses, T was initially mixed with cholesterol in different proportions, dissolved in absolute ethanol, and evaporated overnight at 37 C, after which the powdered residue was placed in 1-cm long SILASTIC capsules (17). Mixtures of T with cholesterol in proportions of 3:7, 1:9, and 0.3:9.7 were used to prepare 1-cm capsules, that would be equivalent in T delivery rate to T alone-filled SILASTIC capsules of 0.3, 0.1, and 0.03-cm lengths, respectively.
Highly purified human LH (NIDDK-hLH-B-SIAFP2) was obtained from the National Hormone and Pituitary Program (NHPP), Torrance, CA. It was dissolved in saline and given to mice sc every 2 d.
Histological evaluation
In the testes from in vivo studies, the tubule differentiation index (TDI) was determined in hematoxylin-stained sections of Bouin's fluid-fixed, glycol methacrylate-embedded testes by microscopic analysis, as described previously (10). The TDI represents the percentage of tubules that were clearly differentiating, which meant, unless otherwise stated, that they contained three or more germ cells at the type B spermatogonial stage or beyond.
The testicular pieces from culture were also fixed overnight in Bouin's fluid and embedded in glycol methacrylate, and 5-μm thick sections were stained with periodic acid Schiff-hematoxylin. In each of these testicular pieces the numbers of Sertoli cells and germ cells in the different developmental stages were counted using a ×100 objective, in systematic scans of the whole section area. In testicular pieces cultured from 6- to 7-d-old mice, the high density of the germ cells and Sertoli cells required us to count the germ cells in 20–25 60 × 60 μm frames per piece. The frames were selected by systematic random sampling using the Stereo Investigator (MicroBrightField, Colchester, VT) software package (18).
In addition, the abdominal and scrotal skin tissues from cremaster sac were fixed either by immersion or perfusion fixation in Bouin's fixative or 4% paraformaldehyde for histological and immunological staining (see Supplemental Materials and Methods).
Hormone measurement
The serum T, T levels in the culture medium, and ITT concentrations were measured using coated-tube RIA kits (DSL 4000; Diagnostics Systems Laboratories, Inc., Webster, TX; or TKTT1, Siemens Health Care Diagnostics, Deerfield, IL) (10). Both kits gave equivalent values. For serum T, the standards were prepared in rat or human serum from which steroids had been stripped. For ITT, the standards were prepared in PBS that contained 0.1% gelatin. The ITT was expressed as amount per gram of testis to reflect the concentrations of T to which the testicular cells were exposed. The intraassay and interassay coefficients of variation were 10% and 16%, respectively.
Temperature measurement by thermocouple probes
Temperature was measured within 8–25 sec after the mice were anesthetized with a 45- to 60-sec exposure to isoflurane. The mice were placed on an insulated pad under continuous exposure to anesthesia. A BAT-8 digital thermometer with the thermocouple microprobes was used (PhysiTemp, Clifton, NJ) A 29G needle was inserted through the skin into the scrotum adjacent to the testis. Because of the small testicular size of the mutant mice, the thermocouple probes could not be inserted into the testes, and the scrotal temperature was measured as a surrogate for testicular temperature. A separate 26G needle was inserted into the peritoneal cavity. The temperature was recorded as quickly as possible, because body temperature decreases during anesthesia. Intraperitoneal temperatures measured on 5 separate days were averaged, and intrascrotal temperatures measured on 2 consecutive days were averaged; the probe was inserted in different sides of the scrotum on each day.
Testes organ culture
The organ culture of testicular pieces of 12-wk-old jsd mice was based on previous reports (19, 20) with some modifications. Six-well culture plates (Becton Dickinson, Franklin Lakes, NJ) fitted with 24-mm Transwell permeable supports having a polyester membrane with a pore size of 0.4 μm (Corning, Inc., Corning, NY) were used. The basal medium, freshly prepared each time, contained powdered DMEM/F12 supplemented with BSA (0.5%), l-glutamine (5 mm) pyruvate (1 nm), nonessential amino acids (0.1 mm), gentamycin (10 μg/ml), insulin (10 μg/ml), retinyl acetate (100 ng/ml), retinoic acid (100 ng/ml), transferrin (10 μg/ml), NaHCO3 (1.2 mg/ml), sodium l-ascorbate (0.27 mm), and fungizone (2.5 μg/ml). The additional hormone supplements used were T (1000 ng/ml), hydroxyflutamide [HOF, 10 μm; dissolved in dimethylsulfoxide (DMSO)] and aminoglutethimide (AG, 250 μm; dissolved in DMSO). AG is an antisteroidogenic drug that reduces endogenous T production. Testosterone, prepared in absolute ethanol and stored at −80 C, was added each time to the culture medium in a volume ratio of 1:1000. Control wells contained the same amount of ethanol and/or DMSO without hormones. The culture medium DMEM/F12, l-glutamine, pyruvate, nonessential amino acids, and gentamycin were obtained from Invitrogen (Carlsbad, CA). Insulin, retinyl acetate, retinoic acid, BSA (fatty acid free), and AG were purchased from Sigma Aldrich (St Louis, MO). HOF was a product of Toronto Research Chemicals, Inc. (North York, Ontario, Canada).
In each experiment, adult mutant testes from a pool of four to six jsd mice were removed from the tunica albuginea and cut into 15–20 pieces and distributed into multiple Transwell membranes with different additional supplements. Testes from 6- to 7-d-old mice were cut into two pieces and cultured alone in one well because the culture was initiated before genotyping the mice. The filters bearing pieces of testes were placed above 2 ml culture medium and incubated at various temperatures from 32.5 to 38.5 C in a humidified atmosphere containing 95% air-5% CO2, for 3 or 9 d. The medium was changed every 48–72 h.
RNA preparation and quantitative real-time PCR
The RNA was extracted from the testis tissue samples at the end of the culture, and quantitative real-time PCR was performed using template cDNA generated from the RNA as previously described (21) (see Supplemental Materials and Methods).
Statistical analysis
The data for cell counts and TDI are presented as the arithmetic mean ± sem. The data on T measurements are the averages ± sem calculated from the log-transformed data. The significance of differences between genotypes, ages of mice, and treatments were evaluated by statistical tests indicated in the figure legends. A computer-assisted statistics program (SPSS version 11.5; SPSS, Inc., Chicago, IL) was used.
Results
Time course of stimulation of spermatogonial differentiation in vivo
Previously we reported that in adult jsd mice either androgen suppression for 4 wk (10) or elevation of testicular temperature by cryptorchidism for 8 wk (13) stimulated differentiation of spermatogonia, mostly to the spermatocyte stage. Examination of the time course of stimulation with GnRH-ant and flutamide treatment revealed no significant spermatogenic recovery after 2 wk of hormonal suppression but there was gradual recovery to a TDI value of 80% at 4 wk (Fig. 1A). Because ITT and serum T levels were rapidly suppressed to 5% and 15% of the pretreatment values, respectively, within 2 wk of treatment (Fig 1, B and C), and even within 4 d in a separate experiment (Fig 1, E and F), and flutamide should inhibit the action of residual T levels, the cause of the delay in recovery was not apparent.
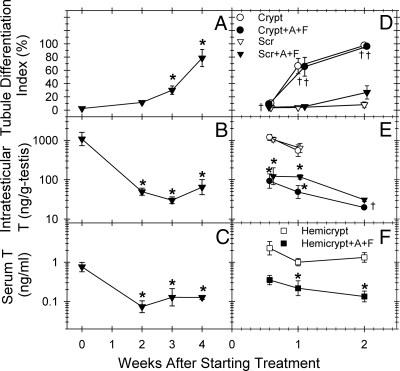
Fig. 1.
Tubule differentiation indices (A and D), intratesticular T (B and E), and serum T (C and F) levels in adult male jsd mice treated with GnRH-ant and flutamide without (A–C) or with hemicryptorchid surgery just before the initiation of androgen ablation treatment (D–F). The treatments were started either at 9 wk (A–C) or 12 wk (D–F) of age. Significance of the difference (Dunnett's test) from the respective values at the time of initiation of treatment in A–C: *, P < 0.01. Significance of the differences (t test, P < 0.05) of the values between the mice with and without androgen ablation (*), or between the cryptorchid and scrotal testis at specific times of treatment (†) in D–F. Crypt, Cryptorchid testis; Scr, scrotal testis; Hemicrypt, hemicryptorchid mouse; A, acyline (GnRH-ant); F, flutamide. Each group consisted of three to 10 mice.
Next, to compare the time course of stimulation of spermatogonial differentiation after androgen suppression with that observed after elevation of testicular temperature, adult jsd mice were hemicryptorchidized with no hormonal suppression or with GnRH-ant plus flutamide treatment (Fig. 1, D–F and Supplemental Fig. 1). ITT levels both in the cryptorchid and scrotal testis of GnRH-ant-treated mice, compared with the respectively positioned testis in untreated mice, were suppressed after 4 d; serum T levels were significantly suppressed after 1 wk of hormone-suppressive treatment (Fig. 1, E and F). Note that there were no or minimal changes in ITT (Fig 1E) and serum T (compare panels C and F in Fig. 1) resulting from the cryptorchidization procedure.
Cryptorchidism restored spermatogonial differentiation in jsd mice from a basal TDI value of 4–8% in the untreated scrotal testis (Fig. 1D and Supplemental Fig. 1) to 66% within 1 wk (Supplemental Fig. 1, C and D) and to 96% in 2 wk. In contrast, androgen suppression alone produced only a slight, not statistically significant, increase at 2 wk.
The combination of androgen suppression and cryptorchidization did not further increase the percentages of tubules showing differentiation compared with cryptorchidism alone (Fig. 1D). However, the differentiation process appeared to be slightly accelerated by the combination treatment, because percentage of tubules with spermatocytes, consisting mostly of preleptotenes, after 1 wk was 40% in the cryptorchid testis of hormone-suppressed mice compared with 6% with cryptorchidism alone; after 2 wk of treatment, most of the differentiated tubules in both the cryptorchidism-alone and cryptorchidism-with-androgen ablation groups had reached pachytene spermatocyte stage. Thus cryptorchidism had the major effect on spermatogenic recovery, and there was very little additive effect of androgen ablation.
Changes in scrotal morphology and temperature after androgen suppression
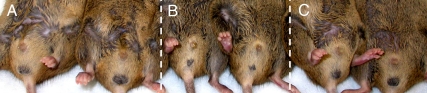
Suspecting a common mechanism of spermatogenic recovery after androgen suppression and cryptorchidism in jsd mutants, we carefully observed whether the stimulation of spermatogonial differentiation after androgen suppression was associated with any alterations that might affect testicular temperature, such as morphological changes in the pendulous scrotum observed in control mice (Fig. 2A). Indeed, androgen ablation gradually, but dramatically, reduced the hairless, pigmented bare area on the scrotum (Table 1) and scrotal size (Fig. 2B).
Fig. 2.
Scrotal morphology of the jsd mice that were untreated (A), totally androgen ablated with GnRH-ant + flutamide (B), or hormonally suppressed with GnRH-ant but supplemented with exogenous T during that time period (C). The treatments were initiated at 12 wk of age, and the mice were photographed after 4 wk of treatment. Note the shrinkage of the scrotum with reduced area of the pigmented scrotal skin with the GnRH ant + flutamide treatment. However, the scrotal morphology remained normal when the GnRH ant-treated mice were supplemented with exogenous T.
Table 1.
Area of hairless pigmented scrotal skin in normal and androgen-suppressed mice (mm2) (n = 3–11 mice per group)
| Treatment | Days of treatment |
|||
|---|---|---|---|---|
| 9 d | 14 d | 24 d | 29 d | |
| Control | 18 ± 2 | 20 ± 0 | 14 ± 3 | 16 ± 2 |
| GnRH-ant+flutamide | 11 ± 2 | 7 ± 1a | 4 ± 0a | 4 ± 1a |
Significantly different from control (P < 0.05, t test).
The muscles of the cremaster sac, enclosing the testes, are continuous with the internal abdominal striated muscles (Supplemental Fig. 2, A–D). A retraction of the cremaster sac was observed in the androgen-suppressed mice by gross morphology (Supplemental Fig. 2, A and C), and a saggittal section of the cremaster sac confirmed its shrinkage (Supplemental Fig. 2, B and D) and a marked reduction in scrotal size and free movement of the testes to the scrotum. Although we did not see differences in the position of the testis in the normal and androgen-suppressed jsd mice after perfusion fixation, this was possibly due to the retraction of the testes during euthanasia.
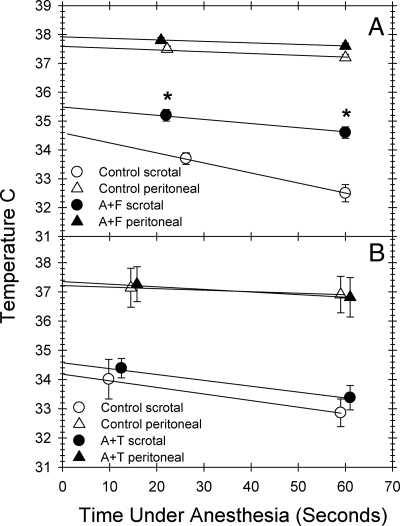
To determine whether morphological changes in the scrotum resulted in changes in testicular temperature, we measured intrascrotal temperatures in jsd mice after androgen suppression for 4 wk or after sham treatment. The scrotal temperature, as determined by back extrapolation of the time points (Fig. 3A), increased to 35.5 C in androgen-ablated mice compared with 34.6 C in the controls.
Fig. 3.
Scrotal and peritoneal temperatures in jsd mice after GnRH-ant plus flutamide treatment (A) and in those with GnRH-ant treatment (acyline protocol II) that were simultaneously administered exogenous T (B). The temperatures were recorded using thermocouple probes immediately at the earliest time point and at 1 min after they were under anesthesia, and the estimated temperatures were calculated by back extrapolation to zero along a straight line. *, Significance of the difference in temperatures between the mice with and without GnRH-ant + flutamide treatment, or between those with and without GnRH-ant + T-treatment at specific times of treatment (t test, P < 0.001). F, Flutamide; A, acyline.
To further test whether the changes in scrotal morphology, testicular temperature, and the progression of spermatogonial differentiation were associated with and were dependent on levels of T, mice hormonally suppressed with GnRH-ant were treated with exogenous T to restore serum T levels while keeping gonadotropins suppressed. Whereas a 4-wk GnRH-ant treatment produced differentiation in 60 ± 10% of the tubules in jsd mutants, the additional T treatment almost completely inhibited GnRH-ant-stimulated spermatogonial differentiation (Fig 4A). Furthermore, although GnRH-ant treatment produced significant shrinkage of the scrotum (data not shown) similar to that observed with GnRH ant + flutamide-treated mice, the additional T treatment prevented the scrotal shrinkage (Fig. 2C). The scrotal temperatures in the GnRH-ant+T-treated jsd mice (34.6 C) were not different from those in control sham-treated mice (34.2 C) (Fig. 3B), indicating that the alterations in testicular temperatures after GnRH-ant treatment were indeed due to androgen suppression.
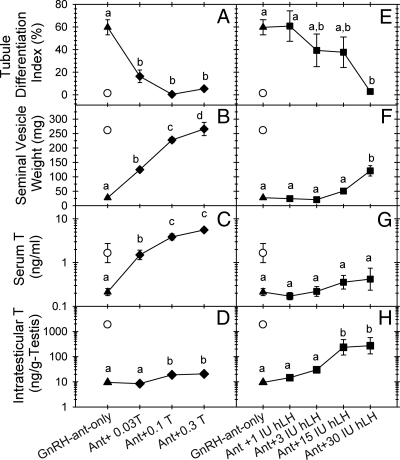
Fig. 4.
Tubule differentiation indices (A and E), seminal vesicle weight (B and F), serum T (C and G), and intratestisticular T (D and H) levels for control jsd mice (○), or jsd mice treated with GnRH-ant alone (▲), or combinations of either GnRH-ant plus increasing doses of T (♦) or GnRH antagonist plus increasing doses of human LH (■). Each group consisted of four to eight mice, except the untreated controls, which had three mice. Different letters indicate that these values are significantly different between groups (Student-Newman-Keuls test, P < 0.05).
These results show that the stimulation of spermatogonial differentiation by androgen suppression is, in fact, associated with changes in scrotal structure and testicular temperature. This conclusion suggests the hypothesis that androgen suppression only indirectly stimulates differentiation in jsd mice by elevating the testicular temperature, at least in part, by scrotal atrophy.
Site of action of T in suppression of differentiation
To test the above hypothesis, we further evaluated whether it was the serum levels of T, which would affect scrotal regression, or the ITT levels, which would directly act within the testis, that were responsible for the inhibition of differentiation. To independently manipulate the serum or ITT levels, we treated mice for 4 wk with GnRH-ant (acyline protocol II) and either with T or LH, respectively. In GnRH-ant-treated mice, T, even at the lowest dose, significantly increased serum T levels (Fig. 4C) and seminal vesicle weights (Fig. 4B), also an indicator of peripheral androgen activity. However, the ITT levels were only slightly increased (∼2-fold) and only at high doses of T (Fig. 4D). Grossly normal scrotal structure was maintained during GnRH-ant treatments with all doses of exogenous T (data not shown). In contrast, with the GnRH-ant and LH combination, modest increases in the seminal vesicle weights were observed only with the highest LH dose (30 IU), and serum T remained low (Fig. 4, F and G), but ITT levels increased by 30-fold at the two highest doses (Fig. 4H). The scrotum became shrunken in all these GnRH-ant + LH-treated mice, except those receiving the highest dose (data not shown).
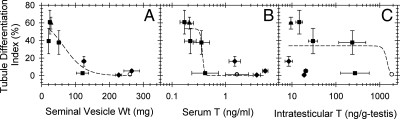
The GnRH-ant treatment alone stimulated differentiation in 60% of the tubules (Fig. 4, A and E). All doses of additional exogenous T drastically inhibited the GnRH-ant-induced TDI increase (Fig. 4A), whereas with the GnRH-ant and LH combination, a significant reduction of the TDI was observed only at the highest dose of LH (Fig. 4E). The analysis of the TDI produced by different treatments revealed that spermatogonial differentiation in these mice was inversely correlated with the increase in seminal vesicle weights (Fig. 5A) and serum T levels (Fig. 5B), but not with the ITT levels (Fig. 5C). These results demonstrate that peripheral androgen action, but not intratesticular action, inhibits spermatogonial differentiation in jsd mice, most likely indirectly by maintaining normal scrotal structure and the reduced testicular temperature.
Fig. 5.
Scatter plots to examine correlations between the tubule differentiation index vs. seminal vesicle weight (A) serum T (B), and intratesticular T levels (C). Points represent the data from untreated jsd mice (○), and jsd mice treated with GnRH-ant (▲); GnRH antagonist plus T (♦), and GnRH antagonist plus hLH (■). The curves were fitted using the three-parameter fits. Wt, Weight.
Differentiation of adult jsd spermatogonia in vitro
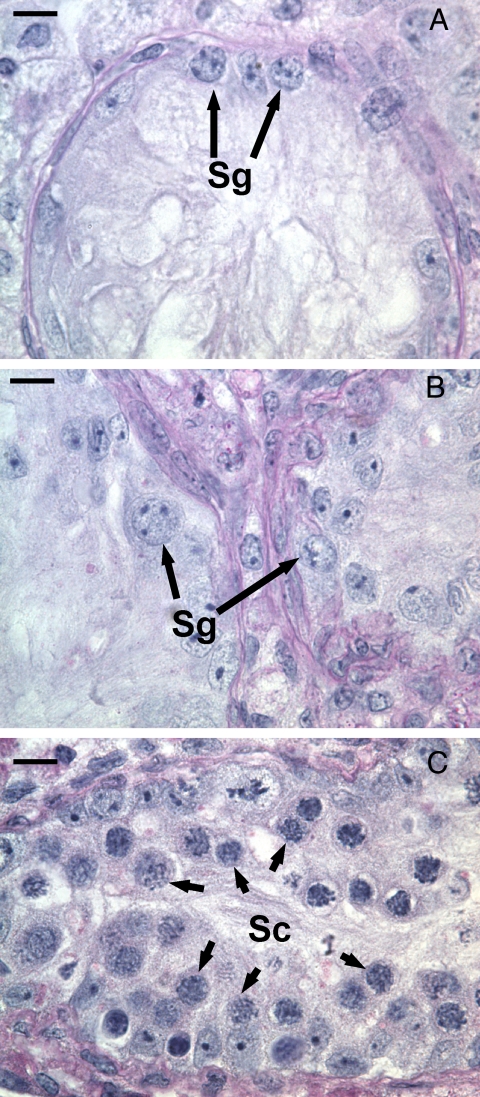
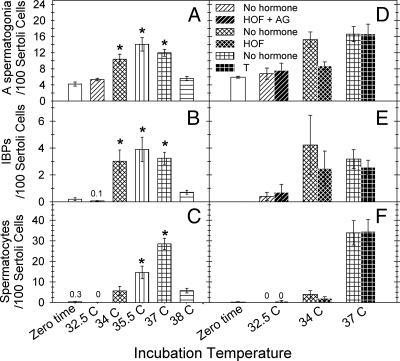
An in vitro system was used to test the effects of temperature and androgen levels on differentiation of defective spermatogonia in jsd testis more independently than can be done in vivo. Pieces of testicular tissue from adult jsd mice were cultured for 9 d. In initial studies using culture at 32.5 C, there was no differentiation either under the standard conditions or even after adding TNFα, FBS, or TGFα to the medium, or reducing oxygen concentration of the cultures. The type A spermatogonia remained in jsd testes but failed to differentiate to the spermatocyte stage (Fig. 6, A and B). Even under standard conditions, as the incubation temperature was increased, there were progressive increases in the numbers of type A spermatogonia (Fig. 7A) differentiated spermatogonia/preleptotene spermatocytes (Fig. 7B), and spermatocytes in meiotic prophase (Fig. 7C). A maximum yield of spermatocytes was obtained at an incubation temperature of 37 C (Figs. 6C and 7C). The morphological results showing enhancement of differentiation at 37 C were confirmed by two molecular markers, Sohlh1 (Supplemental Fig. 3A), which is preferentially expressed in differentiating spermatogonia (22), and Hist1h2ba (Supplemental Fig. 3B), which is weakly expressed in differentiated spermatogonia but strongly expressed in spermatocytes (23). We demonstrated that the expression of these genes markers increased when jsd testes were cultured at 37C, but not at 32.5–34 C.
Fig. 6.
Photomicrographs of the sections of testicular explants of jsd testes showing representative tubules of starting material collected before culture (A), and tissue fragments cultured for 9 d at 32.5 C (B), or at 37 C (C). Note the dramatic differentiation of spermatogonia (Sg) to spermatocytes (Sc) when the temperature was increased to 37 C. Bar, 10 μm.
Fig. 7.
The relative numbers of type A spermatogonia (A and D), differentiated early spermatogenic cells [IBPs: intermediate spermatogonia (type B), spermatogonia, and preleptotene spermatocytes] (B and E) and later spermatocytes (C and F), counted in sections of 12 wk-old jsd testes explants before culture and after culture under standard conditions for 9 d at different temperatures (A–C); before and after culture at either 32.5 C or 34 C with or without the addition of HOF + AG or HOF alone; or at 37 C with or without the addition of T (D–F). In panels A–C the values for each temperature are averages from seven to 31 transwells and in panels D–F the values represent averages from three to eight transwells. *, Significance of the difference (Dunnett's test, P < 0.01) from the respective germ cell numbers in testicular explants before culture in panels A–C. There were no significant differences in the relative numbers of differentiated germ cells between cultures without hormone addition and those treated with HOF or T in vitro. HOF, Hydroxyflutamide; AG, aminoglutethimide.
To determine the possible direct inhibitory action of T on spermatogonial differentiation, we modulated T levels and action during the culture of jsd testes. Without any additives to the culture medium, endogenous production caused T levels to rise to 370 ng/ml during the 0- to 2-d period and to 46 ng/ml during the 6- to 9-d period (Supplemental Fig. 4). Inhibition of T action by addition of 10 μm HOF to the media failed to enhance the ability of type A spermatogonia to differentiate to the B spermatogonial or spermatocyte stages in the testis tissues during culture under nonpermissive temperatures of 32.5 (data not shown) and 34 C (Fig. 7, D–F). In addition, use of a combination of HOF and AG, to ensure a complete blockade of androgen action at the 10 μm concentration of HOF (Fig. 7, D–F, and Supplemental Fig. 4) failed to induce differentiation at 32.5 C, whereas culture at 37 C with HOF plus AG stimulated differentiation of spermatogonia to a similar level as that observed without any additives (data not shown), demonstrating that HOF and AG do not have any unexpected inhibitory effects on spermatogonial differentiation. This result also confirmed that androgen suppression did not further enhance the differentiation of spermatogonia stimulated by elevated temperature. Because T levels were reduced to zero after media changes and declined with time in culture, T was added to 37 C cultures to stabilize its level at 1000 ng/ml, which is close to the intratesticular levels in jsd mice (Supplemental Fig. 4). Even at such high levels, T failed to inhibit differentiation of spermatogonia in jsd mutant testis tissue cultured at 37 C (Fig. 7, D–F).
To demonstrate that the doses of HOF and T used in the culture medium indeed exerted biological activities, the mRNA levels of the AR-regulated gene, Rhox5, were determined by real-time RT-PCR. Addition of HOF to the medium decreased Rhox5 mRNA levels in cultured jsd testes to 15% of those cultured in the control medium at 32.5 C (Supplemental Fig. 5A). To examine the effectiveness of T, we first had to reduce endogenous T production with AG, which did suppress T levels to 3% of control levels after 2 d of culture (Supplemental Fig. 4) and reduced Rhox5 expression to 31%. Subsequent addition of T at 1000 ng/ml significantly increased Rhox5 levels, to 53% of the control levels (Supplemental Fig. 5B). Thus, the failure of biologically active levels of HOF or T to alter spermatogonial differentiation in vitro further supports the in vivo data that the direct mechanism for overcoming the differentiation block in jsd spermatogonia is the elevation of temperature and that T suppression acts only indirectly in vivo by elevating testicular temperature.
Differentiation of neonatal jsd spermatogonia in vitro
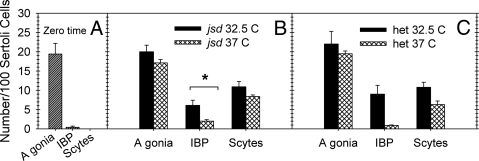
Because spermatogonial differentiation proceeds in jsd mice during the first 4 wk of age, we used the in vitro system to test whether that was simply due to the elevated testicular temperature before testicular descent. The results showed that in 6- to 7-d-old jsd mutant testes differentiation proceeded during 9 d in culture at both 32.5 C and 37 C (Fig. 8, A and B). Furthermore, in contrast to results with jsd adult mice (Fig. 7, A–C), differentiation proceeded at least as well at 32.5 C as at 37 C, suggesting that the spermatogenic cells in the neonatal jsd testes have different properties than those in mature mice. In addition, we cultured testes from phenotypically normal 6- to 7-d-old jsd heterozygotes at 32.5 C and 37 C (Fig. 8, A and C). There was no difference between the normal and jsd testes in the extent of differentiation at either temperature, confirming the lack of phenotype of the jsd mutation on spermatogenesis in mice of this age.
Fig. 8.
The relative numbers of type A spermatogonia (A gonia), differentiated early spermatogenic cells (IBPs: intermediate spermatogonia, type B spermatogonia and preleptotene spermatocytes), and spermatocytes (Scytes), in testicular explants of 6- to 7-d-old jsd (B) or phenotypically normal jsd/+ (C) mice cultured for 9 d at 32.5 C or 37 C. The values before the culture for both genotypes, which were not significantly different, were averaged (A). The values are averages from two to six wells at each temperature for each genotype. *, Significant difference between the different incubation temperatures (t test, P < 0.05). The germ cell numbers are not significantly different between the jsd and jsd/+ mice either at 32.5 C or 37 C. het, Heterozygous jsd.
There was a question of whether the more rapid cell kinetics at elevated temperatures (24) was significantly responsible for the stimulation of differentiation at 37 C in the adult testes by allowing the cells to reach the spermatocyte stage more rapidly at the higher temperatures. However, the results on prepubertal mice, showing slightly greater differentiation to the spermatocyte stage at 32.5 C than at 37 C (Fig. 8), ruled out increased cell kinetics as the reason for the increased differentiation in jsd testis at elevated temperatures.
Discussion
The results from the current in vivo and in vitro studies show conclusively that androgen suppression restores differentiation of spermatogonia in jsd mice by elevating the testicular temperature. In vivo, the timing of the restoration of spermatogonial differentiation showed that differentiation was gradual and correlated with the scrotal regression and consequent increase in the testicular temperature in androgen-suppressed jsd mice, whereas it was rapidly initiated by direct elevation of testicular temperature by cryptorchidism, without changes in T levels. The in vitro study showed that stimulation of spermatogonial differentiation occurred at abdominal temperature, but not at scrotal temperature, and was unaltered by modulations in the androgen levels and action. Thus the actual stimulus for the differentiation of jsd spermatogonia induced either by cryptorchidism (13) or androgen suppression (9, 10) must therefore be the elevation of testicular temperature close to the general body temperature.
Our observations indicate that there are several mechanisms by which T suppression produces the elevation of scrotal temperature. First, it induces anatomical changes in the scrotum including a smaller region of pigmented scrotal skin, which lacks hair and has a thin subdermal fat layer, whereas the nonpigmented regions of scrotal skin have a thick fat layer (Supplemental Fig. 7). The increases in fat layer and hair covering reduce the normal cooling through efficient heat transfer to the environment. We have also demonstrated the presence of AR in several cell types of this tissue, such as the hair follicles and epidermis of the scrotum and the muscle cells of the cremaster sac (Supplemental Fig. 6, A and B), providing a mechanism through which T can act.
Second, androgen suppression can influence whether the testis descends from a low abdominal to a scrotal position, which would improve its cooling efficiency. Observations on live untreated mice showed that temporarily retracted testes (either in anesthetized or unanesthetized mice) can be easily manually descended in to the scrotum, whereas in androgen-suppressed mice this was not possible. The inability to descend could be attributed to the shrinkage of the cremaster sac and outer scrotal skin (Fig. 2 and Supplemental Fig. 2), coupled with possible effects of reduced abdominal pressure (25).
In addition, blood flow to and from the testis through the pampiniform plexus (26) also helps maintain the low testicular temperature by using the cooler venous blood returning from the testis to somewhat cool the arterial blood reaching the testis. The arteries and veins of the pampiniform plexus contain AR (unpublished data, Meistrich, M.L., W. Zhou, and G. Shetty), raising the possibility that there might also be changes in the efficiency of the pampiniform plexus on cooling the incoming blood during androgen suppression.
Our finding that the inhibition of spermatogonial differentiation in jsd testis is correlated with increases in serum T levels or peripheral androgen actions rather than the ITT levels supports the conclusion that the above alterations in scrotal morphology, rather than intratesticular mechanisms, are responsible for the regulation of spermatogonial differentiation in these mice. The lack of involvement of a testicular mechanism is also consistent with the failure of stimulation of spermatogonial differentiation when AR of Sertoli cells was selectively eliminated in jsd mice (12). Thus, bridging the pieces together, it is apparent that peripheral androgen action causes morphological changes in the scrotum and in testicular position and environment, maintaining low testicular temperatures, which in turn blocks the differentiation of spermatogonia in jsd testes.
Our demonstration that decreasing T levels in adult mice results in elevated testicular temperatures has implications in the interpretation of the results of previous publications on androgen suppression of normal mice (14). Because both androgen suppression and elevation of temperature (15) produce similar detrimental effects on normal spermatogenesis, some of the effects of androgen suppression could have also been due to temperature elevation. Only studies involving certain cell type-specific knockouts of AR that show normal scrotal structure clearly dissociate the effects of androgen from temperature alterations (27).
The in vitro studies with immature mice also provided additional information on spermatogonial differentiation. We had hypothesized that the reason spermatogonial differentiation occurred in vivo in immature jsd mice was because of the elevated testicular temperature before testicular descent and expected a block in the differentiation of spermatogonia in 6- to 7-d-old jsd testis when cultured at 32.5 C. However, the differentiation of spermatogonia from 6- to 7-d-old jsd testis progressed during the 9-d culture at 32.5 C; one explanation could be a difference between the phenotype of the cells in juveniles (during the first wave) and adults (28). For example, because the first wave of differentiating spermatogonia are derived from gonocytes, bypassing multiple divisions of the type A spermatogonia, the stores of molecules produced in the gonocytes may be sufficient for spermatocyte differentiation at the lower temperatures, whereas in the adult those in the stem spermatogonia get diluted by additional divisions. An alternative reason for the difference is that molecules necessary to overcome the block in differentiation may have accumulated at the higher temperatures in vivo in the immature mice and had not yet undergone depletion during the culture at lower temperatures, whereas the testes from adult jsd mice were already at scrotal temperatures in vivo.
The macromolecular process that is deficient in the Utp14bjsd mutants appears to be the formation of the 18S ribosomes. Utp14b and its paralog Utp14a are homologous with the yeast UTP14 gene, which forms a complex with U3 small nucleolar RNA that is essential for 18S ribosome formation (3, 29). A recent report (30) confirms that, in mammalian cells, UTP14A protein and the complex formed with U3 small nucleolar RNA is necessary for the processing of 18S rRNA. The unique requirement for Utp14b during mouse spermatogenesis results from the silencing of Utp14a in pachytene spermatocytes and the expression of Utp14b, which is almost exclusively in germ cells, to compensate for Utp14a's repression (31). We also have data showing that Utp14b is involved in 18S rRNA processing in mouse male germ cells (Zhao, M., and M. L. Meistrich, unpublished data). We hypothesize that the elevated temperature can overcome the defect by enabling 18S rRNA processing in the absence of UTP14 proteins, by facilitating RNA conformational changes to overcoming abnormal folding of U3 RNA and the rRNA precursor that could occur at low temperatures. Elevation of temperature can provide activation energy for structural transitions to assist in the assembly of protein-rRNA complexes (32, 33) and may overcome the defect in rRNA processing caused by the absence of UTP14 proteins.
The recent demonstration of a new mechanism of action for UTP14 proteins, in that they promote the degradation of p53, either directly or by minimizing the nucleolar stress signaling from defective rRNA processing that stabilizes p53, and thereby minimize apoptosis (30), provides further insight regarding the block in spermatogenesis in jsd mice. We previously showed that the block in spermatogonial differentiation in jsd mice was p53 dependent but that the block in spermatocyte differentiation was p53 independent (34). It is likely that an accumulation of p53 in jsd spermatogonia causes apoptosis and the loss of these cells. It remains to be elucidated whether the elevation of temperature facilitates p53 turnover by enhancing rRNA processing as described above or whether it acts directly on p53 turnover or downstream actions. The p53-independent block in survival of spermatocytes in jsd mice may due to a deficiency in protein synthesis resulting from the observed defect in 18S rRNA processing and may be overcome in part by enhanced efficiency of protein and RNA synthesis at elevated temperatures (35, 36).
The knowledge of the factors that stimulate spermatogenesis in Utp14bjsd mice may have some application to human infertility. In humans, a mutation in the testis-expressed UTP14C gene is associated with male infertility (37). Restoration of production of fertile sperm in jsd mice was demonstrated previously by a transient suppression of T, allowing the production of spermatocytes, which then went on to produce sperm after discontinuing the suppressive therapy (16). However, since we have shown that androgen suppression acts in mice exclusively by raising testicular temperature, it is not likely to cause similar scrotal regression and temperature elevation in humans, because they do not have an open inguinal canal as do mice. We have preliminary data showing that transient elevation of temperature by cryptorchidism and reversal by orchiopexy stimulates the production of testicular sperm in jsd mice (Shetty, G., and M. L. Meistrich, unpublished observations). As in the jsd mouse model, mild and transient heating of the testis (38) could possibly stimulate production of spermatocytes, which can develop into sperm, in men who are infertile due to a similar genetic defect.
Acknowledgments
We thank Dr. Anna Steinberger (University of Texas Medical School, Houston, Texas) for her advice during the initial setting up of the in vitro culture of testicular explants and Mr. Kuriakose Abraham and Nalini Patel (MD Anderson Cancer Center, Houston, Texas) for the histological preparations. We also thank Mr. Walter Pagel (MD Anderson Cancer Center, Houston, Texas) for editorial advice and Ms. Lai Yi Liang (MD Anderson Cancer Center, Houston, Texas) for maintenance and genotyping of jsd mice and assistance with the culture experiments. In addition, we thank Drs. R.P. Blye, Hyun K. Kim, June Lee, and Min S. Lee from National Institute of Child Health and Human Development for providing the Acyline.
This work was supported by research grants HD 40397 from National Institutes of Health / National Institute of Child Health and Human Development, Cancer Center Support Grant CA 16672 from the National Institutes of Health.
Disclosure Summary: The authors have no conflicts of interest to declare.
Footnotes
- AG
- Aminoglutethimide
- AR
- androgen receptor
- DMSO
- dimethylsulfoxide
- GnRH-ant
- GnRH antagonist
- HOF
- hydroxyflutamide
- ITT
- intratesticular T
- T
- testosterone
- TDI
- tubule differentiation index.
References
- 1. Beamer WG, Cunliffe-Beamer TL, Shultz KL, Langley SH, Roderick TH. 1988. Juvenile spermatogonial depletion (jsd): A genetic defect of germ cell proliferations of male mice. Biol Reprod 38:899–908 [DOI] [PubMed] [Google Scholar]
- 2. Bradley J, Baltus A, Skaletsky H, Royce-Tolland M, Dewar K, Page DC. 2004. An X-to-autosome retrogene is required for spermatogenesis in mice. Nat Genet 36:872–876 [DOI] [PubMed] [Google Scholar]
- 3. Rohozinski J, Bishop CE. 2004. The mouse juvenile spermatogonial depletion (jsd) phenotype is due to a mutation in the X-derived retrogene, mUtp14b. Proc Natl Acad Sci USA 101:11695–11700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kojima Y, Kominami K, Dohmae K, Nonomura N, Miki T, Okuyama A, Nishimune Y, Okabe M. 1997. Cessation of spermatogenesis in juvenile spermatogonial depletion (jsd/jsd) mice. Int J Urol 4:500–507 [DOI] [PubMed] [Google Scholar]
- 5. de Rooij DG, Okabe M, Nishimune Y. 1999. Arrest of spermatogonial differentiation in jsd/jsd, Sl17H/Sl17H, and cryptorchid mice. Biol Reprod 61:842–847 [DOI] [PubMed] [Google Scholar]
- 6. Boettger-Tong HL, Johnston DS, Russell LD, Griswold MD, Bishop CE. 2000. Juvenile spermatogonial depletion (jsd) mutant seminiferous tubules are capable of supporting transplanted spermatogenesis. Biol Reprod 63:1185–1191 [DOI] [PubMed] [Google Scholar]
- 7. Ohta H, Yomogida K, Tadokoro Y, Tohda A, Dohmae K, Nishimune Y. 2001. Defect in germ cells, not in supporting cells, is the cause of male infertility in the jsd mutant mouse: proliferation of spermatogonial stem cells without differentiation. Int J Androl 24:15–23 [DOI] [PubMed] [Google Scholar]
- 8. Matsumiya K, Meistrich ML, Shetty G, Dohmae K, Tohda A, Okuyama A, Nishimune Y. 1999. Stimulation of spermatogonial differentiation in juvenile spermatogonial depletion (jsd) mutant mice by gonadotropin-releasing hormone antagonist treatment. Endocrinology 140:4912–4915 [DOI] [PubMed] [Google Scholar]
- 9. Tohda A, Matsumiya K, Tadokoro Y, Yomogida K, Miyagawa Y, Dohmae K, Okuyama A, Nishimune Y. 2001. Testosterone suppresses spermatogenesis in juvenile spermatogonial depletion (jsd) mice. Biol Reprod 65:532–537 [DOI] [PubMed] [Google Scholar]
- 10. Shetty G, Wilson G, Huhtaniemi I, Boettger-Tong H, Meistrich ML. 2001. Testosterone inhibits spermatogonial differentiation in juvenile spermatogonial depletion mice. Endocrinology 142:2789–2795 [DOI] [PubMed] [Google Scholar]
- 11. Shetty G, Weng CC, Porter KL, Zhang Z, Pakarinen P, Kumar TR, Meistrich ML. 2006. Spermatogonial differentiation in juvenile spermatogonial depletion (jsd) mice with androgen receptor or follicle stimulating hormone mutations. Endocrinology 147:3563–3570 [DOI] [PubMed] [Google Scholar]
- 12. Wang G, Weng CC, Shao SH, Zhou W, de Gendt K, Braun RE, Verhoeven G, Meistrich ML. 2009. Androgen receptor in Sertoli cells is not required for testosterone-induced suppression of spermatogenesis, but contributes to Sertoli cell organization in UTP14bisd mice. J Androl 30:338–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shetty G, Weng CCY. 2004. Cryptorchidism rescues spermatogonial differentiation in juvenile spermatogonial depletion (jsd) mice. Endocrinology 145:126–133 [DOI] [PubMed] [Google Scholar]
- 14. Kangasniemi M, Dodge K, Pemberton AE, Huhtaniemi I, Meistrich ML. 1996. Suppression of mouse spermatogenesis by a gonadotropin-releasing hormone antagonist and antiandrogen: failure to protect against radiation-induced gonadal damage. Endocrinology 137:949–955 [DOI] [PubMed] [Google Scholar]
- 15. Kon Y, Endoh D. 2001. Heat-shock resistance in experimental cryptorchid testis of mice. Mol Reprod Dev 58:216–222 [DOI] [PubMed] [Google Scholar]
- 16. Tohda A, Okuno T, Matsumiya K, Okabe M, Kishikawa H, Dohmae K, Okuyama A, Nishimune Y. 2002. Restoration of spermatogenesis and fertility in azoospermic mutant mice by suppression and reelevation of testosterone followed by intracytoplasmic sperm injection. Biol Reprod 66:85–90 [DOI] [PubMed] [Google Scholar]
- 17. Lim P, Allan CM, Notini AJ, Axell AM, Spaliviero J, Jimenez M, Davey R, McManus J, MacLean HE, Zajac JD, Handelsman DJ. 2008. Oestradiol-induced spermatogenesis requires a functional androgen receptor. Reprod Fertil Dev 20:861–870 [DOI] [PubMed] [Google Scholar]
- 18. Shetty G, Weng CC, Meachem SJ, Bolden-Tiller OU, Zhang Z, Pakarinen P, Huhtaniemi I, Meistrich ML. 2006. Both testosterone and FSH independently inhibit spermatogonial differentiation in irradiated rats. Endocrinology 147:472–482 [DOI] [PubMed] [Google Scholar]
- 19. Steinberger A. 1975. In vitro techniques for the study of spermatogenesis. Methods Enzymol 39:283–296 [DOI] [PubMed] [Google Scholar]
- 20. Haneji T, Maekawa M, Nishimune Y. 1983. In vitro differentiation of type A spermatogonia from mouse cryptorchid testes in serum-free media. Biol Reprod 28:1217–1223 [DOI] [PubMed] [Google Scholar]
- 21. Zhou W, Bolden-Tiller OU, Shetty G, Shao SH, Weng CC, Pakarinen P, Liu Z, Stivers DN, Meistrich ML. 2010. Changes in gene expression in somatic cells of rat testes resulting from hormonal modulation and radiation-induced germ cell depletion. Biol Reprod 82:54–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ballow D, Meistrich ML, Matzuk M, Rajkovic A. 2006. Sohlh1 is essential for spermatogonial differentiation. Dev Biol 294:161–167 [DOI] [PubMed] [Google Scholar]
- 23. Meistrich ML, Bucci LR, Trostle-Weige PK, Brock WA. 1985. Histone variants in rat spermatogonia and primary spermatocytes. Dev Biol 112:230–240 [DOI] [PubMed] [Google Scholar]
- 24. Meistrich ML, Eng VW, Loir M. 1973. Temperature effects on the kinetics of spermatogenesis in the mouse. Cell Tissue Kinet 6:379–393 [DOI] [PubMed] [Google Scholar]
- 25. Frey HL, Peng S, Rajfer J. 1983. Synergy of abdominal pressure and androgens in testicular descent. Biol Reprod 29:1233–1239 [DOI] [PubMed] [Google Scholar]
- 26. Setchell B, Maddocks S, Brooks D. 1994. Anatomy, vasculature, innervation, and fluids of the male reproductive tract. In: Knobil E, Neill JD. eds. The physiology of reproduction. 2nd ed New York: Raven Press; 1063–1175 [Google Scholar]
- 27. De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lécureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G. 2004. A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA 101:1327–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoshida S, Sukeno M, Nakagawa T, Ohbo K, Nagamatsu G, Suda T, Nabeshima Y. 2006. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development 133:1495–1505 [DOI] [PubMed] [Google Scholar]
- 29. Dragon F, Gallagher JE, Compagnone-Post PA, Mitchell BM, Porwancher KA, Wehner KA, Wormsley S, Settlage RE, Shabanowitz J, Osheim Y, Beyer AL, Hunt DF, Baserga SJ. 2002. A large nucleolar U3 ribonucleoprotein required for 18S ribosomal RNA biogenesis. Nature 417:967–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hu L, Wang J, Liu Y, Zhang Y, Zhang L, Kong R, Zheng Z, Du X, Ke Y. 2011. A small ribosomal subunit (SSU) processome component, the human U3 protein 14A (hUTP14A) binds p53 and promotes p53 degradation. J Biol Chem 286:3119–3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao M, Rohozinski J, Sharma M, Ju J, Braun RE, Bishop CE, Meistrich ML. 2007. Utp14b: a unique retrogene within a gene that has acquired multiple promoters and a specific function in spermatogenesis. Dev Biol 304:848–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Talkington MW, Siuzdak G, Williamson JR. 2005. An assembly landscape for the 30S ribosomal subunit. Nature 438:628–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mangat CS, Brown ED. 2008. Ribosome biogenesis; the KsgA protein throws a methyl-mediated switch in ribosome assembly. Mol Microbiol 70:1051–1053 [DOI] [PubMed] [Google Scholar]
- 34. Shetty G, Shao SH, Weng CC. 2008. p53-dependent apoptosis in the inhibition of spermatogonial differentiation in juvenile spermatogonial depletion (Utp14bjsd) mice. Endocrinology 149:2773–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nakamura M, Namiki M, Okuyama A, Koh E, Kondoh N, Takeyama M, Fujioka H, Nishimune Y, Matsumoto K, Matsuda M. 1988. Optimal temperature for synthesis of DNA, RNA, and protein by human testis in vitro. Arch Androl 20:41–44 [DOI] [PubMed] [Google Scholar]
- 36. Dresios J, Aschrafi A, Owens GC, Vanderklish PW, Edelman GM, Mauro VP. 2005. Cold stress-induced protein Rbm3 binds 60S ribosomal subunits, alters microRNA levels, and enhances global protein synthesis. Proc Natl Acad Sci USA 102:1865–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rohozinski J, Lamb DJ, Bishop CE. 2006. UTP14c Is a recently acquired retrogene associated with spermatogenesis and fertility in man. Biol Reprod 74:644–651 [DOI] [PubMed] [Google Scholar]
- 38. Mieusset R, Bujan L. 1994. The potential of mild testicular heating as a safe, effective and reversible contraceptive method for men. Int J Androl 17:186–191 [DOI] [PubMed] [Google Scholar]