Abstract
Hyperinsulinemia is known to promote the progression/worsening of insulin resistance. Evidence reveals a hidden cost of hyperinsulinemia on plasma membrane (PM) phosphatidylinositol 4,5-bisphosphate (PIP2)-regulated filamentous actin (F-actin) structure, components critical to the normal operation of the insulin-regulated glucose transport system. Here we delineated whether increased glucose flux through the hexosamine biosynthesis pathway (HBP) causes PIP2/F-actin dysregulation and subsequent insulin resistance. Increased glycosylation events were detected in 3T3-L1 adipocytes cultured under conditions closely resembling physiological hyperinsulinemia (5 nm insulin; 12 h) and in cells in which HBP activity was amplified by 2 mm glucosamine (GlcN). Both the physiological hyperinsulinemia and experimental GlcN challenge induced comparable losses of PIP2 and F-actin. In addition to protecting against the insulin-induced membrane/cytoskeletal abnormality and insulin-resistant state, exogenous PIP2 corrected the GlcN-induced insult on these parameters. Moreover, in accordance with HBP flux directly weakening PIP2/F-actin structure, pharmacological inhibition of the rate-limiting HBP enzyme [glutamine-fructose-6-phosphate amidotransferase (GFAT)] restored PIP2-regulated F-actin structure and insulin responsiveness. Conversely, overexpression of GFAT was associated with a loss of detectable PM PIP2 and insulin sensitivity. Even less invasive challenges with glucose, in the absence of insulin, also led to PIP2/F-actin dysregulation. Mechanistically we found that increased HBP activity increased PM cholesterol, the removal of which normalized PIP2/F-actin levels. Accordingly, these data suggest that glucose transporter-4 functionality, dependent on PIP2 and/or F-actin status, can be critically compromised by inappropriate HBP activity. Furthermore, these data are consistent with the PM cholesterol accrual/toxicity as a mechanistic basis of the HBP-induced defects in PIP2/F-actin structure and impaired glucose transporter-4 regulation.
Decoding the harmful cellular basis of glucose-induced insulin resistance has been an important research initiative since the early 1980s. At that time, the concept of glucose toxicity emerged from human and animal observations showing that hyperglycemia impairs normal glucose uptake (1, 2). Since then, a concerted research effort has sought mechanistic insight into the desensitization of glucose transport into muscle and fat cells. In these cells exceedingly intricate assemblies of proteins regulate glucose transporter glucose transporter (GLUT)-4-mediated glucose transport (3). It is appreciated that insulin receptor activation propagates a signal(s) that mobilizes intracellular GLUT4-containing vesicles to the plasma membrane (PM) where subsequent membrane fusion increases PM GLUT4 content and glucose transport.
Marshall et al. (4), using a model system of primary adipocytes, first proposed that excess glucose flux through the hexosamine biosynthesis pathway (HBP) may play a role in the development of insulin resistance. Glucose entry into the HBP is catalyzed by the first and rate-limiting enzyme glutamine-fructose-6-phosphate amidotransferase (GFAT). This enzyme converts fructose-6-phosphate and glutamine into glucosamine 6-phosphate (GlcN-6-P) and glutamate. GlcN-6-P is further metabolized to uridine diphosphate-N-acetylglucosamine (UDP-GlcNAc), the major product of the pathway (4). UDP-GlcNAc and other amino sugars generated by the pathway provide building blocks of glycosyl side chains for proteins and lipids. UDP-GlcNAc is also the obligatory substrate of O-linked N-acetylglucosamine (O-GlcNAc) transferase (OGT), a cytosolic and nuclear enzyme that modifies Ser/Thr residues of certain proteins by attaching single N-acetylglucosamine (GlcNAc) moieties in O-linkage (5, 6).
Cell culture and rodent studies routinely model increased glucose flux through HBP by high glucose (25 mm) in the presence of insulin or, alternatively, treat with glucosamine (GlcN), which enters the HBP bypassing GFAT. In 1991, Garvey et al. (7) first demonstrated the synergistic effects of high glucose and insulin on the development of insulin resistance. During the continual dissection of insulin signaling pathways, several laboratories have used modified glucose/insulin and GlcN models to define a possible mode(s) of HBP-mediated alterations. However, a concern with most GlcN models is that they result in tremendous cellular accumulation of GlcN-6-PO4, whereas this compound is nearly undetectable in cells exposed to glucose alone (8).
Importantly, GlcN-6-PO4 accumulation depletes intracellular ATP, which was clearly demonstrated by Hresko et al. (9) in 3T3-L1 adipocytes incubated with high concentrations of GlcN, in the presence of insulin and no glucose. However, under milder conditions lacking insulin and/or provision of an energy source, ATP depletion due to GlcN treatment becomes trivial and insulin tyrosine phosphorylation-based signaling abnormalities do not occur, yet GlcN-induced insulin resistance persists (10). Interestingly, work has suggested a new molecular framework accounting for insulin-induced insulin resistance involving defects in PM phosphatidylinositol 4,5-bisphosphate (PIP2)-regulated cortical filamentous actin (F-actin) (11, 12). The basis for this insulin-induced defect causing insulin resistance is not known. Here we tested whether increased HBP activity coupled hyperinsulinemia to PIP2/F-actin defects and insulin resistance. Additional study assessed whether increased HBP activity and/or PIP2/F-actin defects resulted from membrane cholesterol accrual, recently observed in insulin-induced, insulin-resistant 3T3-L1 adipocytes (13).
Materials and Methods
Materials
Murine 3T3-L1 preadipocytes were purchased from American Type Culture Collection (Manassas, VA) and from Dr. Howard Green (Harvard Medical School, Boston, MA). DMEM was from Invitrogen (Grand Island, NY). Fetal bovine serum (FBS) and bovine calf serum were obtained from Hyclone Laboratories Inc. (Logan, UT). Phosphatidylinositol 4, 5-bisphosphate and histone carrier were obtained from Echelon (Salt Lake City, UT). Monoclonal mouse phosphatidylinositol 4, 5-bisphosphate antibody was purchased from Assay Designs Inc. (Ann Arbor, MI). Polyclonal rabbit GLUT4 antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). β-Actin-specific mouse IgM antihuman F-actin antibody was from Serotec (Oxford, UK). Rhodamine red-X-conjugated donkey antirabbit or goat antimouse antibodies were from Jackson ImmunoResearch Inc. (West Grove, PA). Fluorescein isothiocyanate (FITC)-conjugated phalloidin, insulin, 6-diazo-5-oxo-l-norleucine (DON) and all other chemicals were from Sigma (St. Louis, MO).
Cell culture
Preadipocytes were cultured in DMEM containing 25 mm glucose and 10% bovine calf serum at 37 C in an 8% CO2 atmosphere. Confluent cultures were induced to differentiate into adipocytes as previously described (11, 14, 15). All studies were performed on adipocytes that were between 8 and 12 d after differentiation.
Induction of insulin resistance
Adipocytes cultured/differentiated under the standard 25 mm glucose protocol were switched into DMEM/5.5 mm glucose medium containing 10% FBS for 2 d before inducing insulin resistance. Three experimental conditions were used routinely: 1) control, 2) 5 nm insulin, and 3) 2 mm GlcN. In each of these conditions, the culture medium for overnight induction of insulin resistance contained 1 mm pyruvate as an additional energy source. Overnight incubations were limited to 12 h to minimize complications due to effects of glucose deprivation on glucose transport (17). The three experimental conditions are listed below.
Control
Adipocytes were incubated for 12 h in serum-free DMEM/5.5 mm glucose medium in the absence of insulin. The concentration of glutamine in the incubation medium was 2 mm (hereafter referred to as control).
Insulin
Impairment of the glucose transport system was induced by 12 h treatment of cells in serum-free DMEM/5.5 mm glucose containing 5 nm insulin and 2 mm glutamine (hereafter referred to as 12 h Ins.).
GlcN
To induce insulin resistance, adipocytes were treated with DMEM containing 10% FBS and 2 mm GlcN for 12 h, in the absence of glucose, glutamine, and insulin (hereafter referred to as 12 h GlcN).
GFAT overexpression
To directly compare the effect of hexosamine metabolism on insulin-stimulated GLUT4 translocation and PIP2, we performed cotransfection experiments with either empty vector control or GFAT plasmid cDNA (kindly provided by Dr. Donald A. McClain, University of Utah, Salt Lake City, UT) with GLUT4-eGFP plasmid cDNA as we previously reported (14, 18, 19). Briefly, differentiated adipocytes were electroporated (0.16 kV and 960 μF). Experiments were performed with 50 μg GLUT4-eGFP plasmid DNA plus 200 μg of additional empty vector or GFAT plasmid DNA. We have previously documented that this approach of transfection at a ratio of 1:4 (i.e. 50 μg GLUT4-eGFP: 200 μg empty vector or GFAT plasmid) results in all GLUT4-eGFP expressing cells coexpressing the cotransfected plasmid (14, 18, 19). After electroporation, the adipocytes were replated on glass coverslips and allowed to recover for 16–18 h before use.
Treatments
To test whether inhibition of GFAT with DON protected against the insulin resistance displayed by the 12 h Ins. cells, 0 or 20 μm DON was included in the overnight incubation medium. DON was reconstituted using double-distilled water per the manufacturer suggestion, and dilutions were prepared using serum-free medium. To test whether the loss of insulin responsiveness in the 12 h GlcN cells was correctable with exogenous PIP2, cells were either left untreated or treated during the final hour of the overnight induction of insulin resistance with PIP2-histone complex, as we have previously established, produces reproducible PM PIP2 replenishment (11, 12, 20). To test whether the hyperinsulinemia-induced increase in PM cholesterol and loss of PIP2/F-actin were correctable by PM cholesterol depletion, cells were either left untreated or treated with 2.5 mm methyl-β-cyclodextrin (βCD) during the final 30 min of the overnight incubation of insulin resistance (21, 22). In all experiments, acute insulin stimulation was achieved by treating cells with 100 nm insulin during the last 30 min of the 12-h period. All groups were first washed with PBS before the acute insulin challenge. This step was important for the hyperinsulinemia experiments.
ATP measurement
Intracellular ATP content was measured using a luminescence ATP detection assay (ATPlite; PerkinElmer Inc., Waltham, MA). Briefly, after experimental treatments, 3T3-L1 adipocytes were lysed using a mammalian cell lysis buffer, which inactivates endogenous ATPase. Subsequently whole-cell lysates were incubated with substrate buffer, and luminescence was detected using the SpectraMax M2 (Molecular Devices, Sunnyvale, CA). Luminescence was converted to actual ATP content (nanomoles) by the use of an ATP standard curve. ATP concentrations were normalized for protein concentration determined by the Bradford method.
Membrane and actin analyses
Plasma membrane sheets were prepared as previously described (11, 23). Briefly, after treatments, cells were fixed by incubation for 20 min in a 2% paraformaldehyde solution containing PBS (GLUT4 analyses) or Tris-buffered saline (PIP2 analyses), and these membranes were used for immunofluorescence. For GLUT4 immunofluorescence, incubation with a 1:1000 dilution of GLUT4 antibody was followed by incubation with a 1:50 dilution of FITC-conjugated secondary antibody, both for 60 min at 25 C. For PIP2 immunofluorescence, incubation with a 1:50 dilution of PIP2 antibody was performed for 30 min at 37 C. Appropriate FITC-conjugated secondary antibody was added for 60 min at 25 C. For labeling of actin after fixation, cells were incubated with 5 mg/ml of FITC-conjugated phalloidin for 2 h at 25 C (confocal analyses) or blocked in fish serum (1 h at 25 C) and labeled with a 1:100 dilution of F-actin specific primary antibody (overnight at 4 C; LI-COR analyses, LI-COR, Lincoln, NE). All PM sheet and cell images were obtained using the Zeiss LSM 510 NLO confocal microscope (Carl Zeiss, Thornwood, NY), and all microscope settings were identical between groups. Images were quantitated with the LI-COR infrared imaging system as previously described (12, 23, 24). For cholesterol analysis, membrane fractions from cultured cells were prepared and assayed for protein and cholesterol content, via the Bradford assay and the Amplex Red cholesterol assay, respectively, as previously described (21).
Glucose transport assay
Cells were either untreated or stimulated with 100 nm insulin for 30 min and exposed to 50 μm 2-deoxy-D-glucose (2-DG) containing 0.5 μCi of 2-[3H]deoxyglucose in the absence or presence of 20 μm cytochalasin B. Glucose transport was determined as previously described (11).
Immunofluoresence quantitation
For GLUT4 quantitation, PM sheets were prepared and labeled as described above. The amount of glucose transporter on the PM was quantitated by digital image processing as described previously (21, 23, 25). To ensure that quantitation was performed in an unbiased manner, fields of sheets were selected based solely on their staining with rhodamine-conjugated wheat germ agglutinin (WGA). Additional population-based quantitation of PIP2 and F-actin was done using the LI-COR infrared imaging system as previously described (12, 23, 24).
Phosphatidylinositol 4, 5-bisphosphate antibody specificity analyses
These studies used a commercially available PIP2 antibody (Assay Designs, Ann Arbor, MI). Product specifications indicate that this antibody is highly specific for PI(4,5)P2, and cross-reactivity to phosphatidylcholine, phosphatidylserine, phosphatidylethanolamine, phosphatidylglycerol, cardiolipin, cholesterol, and diacylglycerol is less than 0.2%. The antibody does show greater (<5.0%) cross-reactivity to phosphatidic acid, yet, in agreement with these cross-reactivity levels being negligible, we found that the PI(4,5)P2 antibody signal was abolished in cells treated with LiCl (11, 12), widely recognized to decrease inositol phospholipids. With regard to antibody cross-reactivity to inositol phospholipids, product specifications indicate that the antibody binds to phosphatidylinositol 3,4,5-trisphosphate with approximately 30% of the affinity that it has for PI(4,5)P2 and does not bind to inositol 1,4,5-trisphosphate, phosphatidylinositol 3,4-bisphosphate, or phosphatidylinositol 3-phosphate and only weakly binds phosphatidylinositol 4-phosphate and phosphatidylinositol 5-phosphate. The PIP strip (Echelon, Salt Lake City, UT) studies that we performed with the antibody support the product specifications with the exception that we detected immunoreactivity to PI(3,4)P2. The basis for this is unclear, yet exogenous PI(4)P, PI(3,4)P2, and PI(3,4,5)P3 add-back studies that we have performed strongly support that the PI(4,5)P2 antibody has a clear specificity for PI(4,5)P2 when staining cells but not when using PIP strips. To further assure ourselves of PI(4,5)P2 antibody specificity, we expressed the phospholipase Cδ/pleckstrin homology domain as an enhanced green fluorescent protein fusion protein (PLCδ/PH-eGFP) that has a high affinity and specificity for PI(4,5)P2, and the PLCδ/PH-eGFP data mirrored the PI(4,5)P2 antibody data (23).
Statistical analyses
Values are presented as means ± se. The significance of differences between means was evaluated by ANOVA. Where differences among groups were indicated, the Newman-Keuls test was used for post hoc comparison between groups. Statistical comparisons of the percent change of ATP, PIP2, F-actin, and PM cholesterol from control were performed by two-tailed Student's t test analysis. GraphPad Prism 4 software (La Jolla, CA) was used for all analyses. P < 0.05 was considered significant.
Results
O-Linked glycosylation and insulin resistance
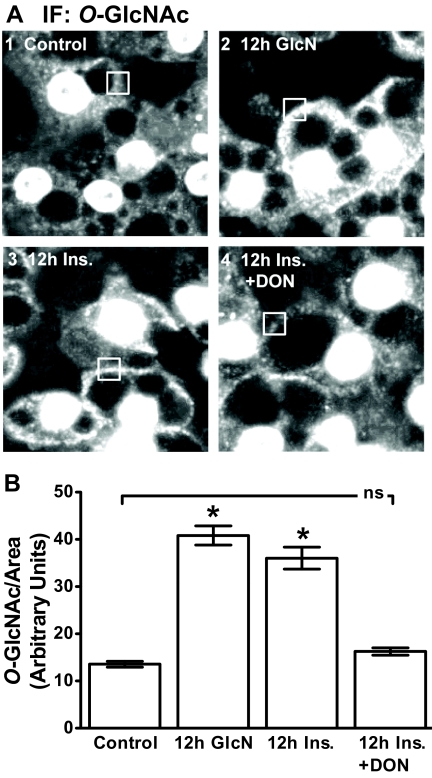
Previous study by our laboratory has examined insulin- (11, 12) and GlcN (14, 26)-induced insulin resistance. The former studies revealed novel PIP2/F-actin defects contributing to the insulin-induced insulin resistance. In addition, the later GlcN studies showed cell-surface O-linked glycosylation in the insulin-resistant cells (26). As a start to probing whether the insulin-induced PIP2/F-actin loss was coupled to inappropriate HBP activity, we visualized O-linked glycosylation to ensure replication of our earlier GlcN findings (26). Consistent with other reports, strong nuclear membrane labeling resulting from nuclear pore complex protein O-linked glycosylation was detected in all cells (26–29). Concomitant with this strong nuclear signal, 3T3-L1 adipocytes exposed to 2 mm GlcN for 12 h displayed a marked increase in cell surface membrane immunoreactivity to an O-GlcNAc-specific antibody (Fig. 1, compare panels 1 and 2). A qualitatively detectable increase in PM-localized O-linked glycosylation was also present in cells treated with 5 nm insulin for 12 h (Fig. 1, panel 3). Inhibition of GFAT with DON reduced the cell surface O-GlcNAc signal visualized in the cells exposed to the 12-h insulin treatment (Fig. 1A, compare panels 3 and 4), lending support to the involvement of the HBP in the insulin-induced increase in O-linked glycosylation. Figure 1B shows the quantitative results of digitally analyzing the O-GlcNAc signal in multiple boxed regions of the cell surface area in several images collected for each group from three to five independent experiments.
Fig. 1.
Insulin and GlcN induce similar changes in cellular O-linked glycosylation. After 36 h of incubation in DMEM containing 5.5 mm glucose, cells were left untreated (control) or treated overnight (12 h) with 5 nm insulin in the absence (12 h Ins.) or presence (12 h Ins. + DON) of DON. A subset of cells was treated with 2 mm glucosamine in the absence of insulin (12 h GlcN) for 12 h. Representative images of cells subjected to immunofluorescence (IF) microscopy with RL2 antibody (A) and quantitation (B) are shown. *, P < 0.05 vs. control; bar 1).
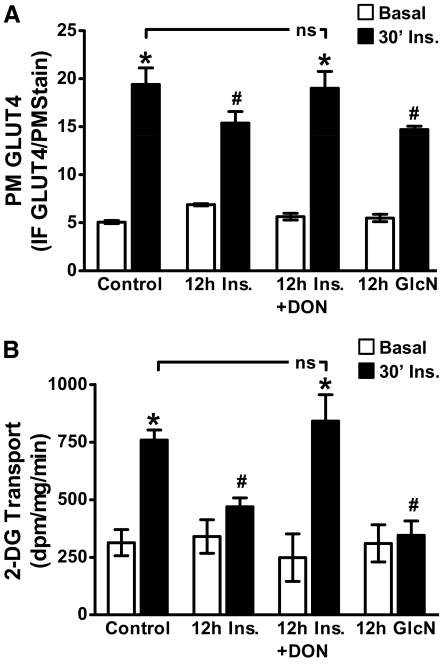
Consistent with the negative effect of increased HBP activity on insulin-regulated GLUT4 translocation (10, 11, 26), chronic insulin and GlcN treatments decreased the ability of an acute insulin challenge to stimulate GLUT4 translocation by 21 and 24%, respectively (Fig. 2A). Quantitation of these data entailed normalizing the GLUT4 signal to WGA labeling of the same PM sheets (see Supplemental Fig. 1). Consistent with these effects on GLUT4 translocation, reduction in glucose transport was also evident in these cells (Fig. 2B). Further confirming a role for HBP-induced insulin resistance, DON treatment completely restored both insulin-regulated GLUT4 translocation and glucose transport to control levels (Fig. 2, A and B). Basal and acute insulin-stimulated GLUT4 translocations and 2-DG uptakes in control cells were not affected by DON (data not shown).
Fig. 2.
Acute insulin responsiveness is impaired similarly by hyperinsulinemia and GlcN. Cells were treated exactly as described in Fig. 1. After treatments, cells were washed, either left untreated (basal) or acutely (30 min) challenged with 100 nm insulin (30′ Ins.), and GLUT4 translocation (B) and glucose transport (A) were determined. Means (±se) from three to six independent experiments are shown. IF, Immunofluorescence. *, P < 0.05 vs. control; #, P < 0.05 vs. control 30′ Ins.).
Plasma membrane and cytoskeletal defects
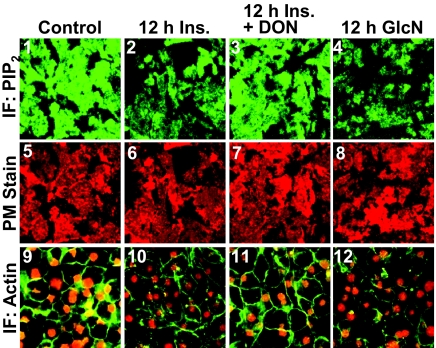
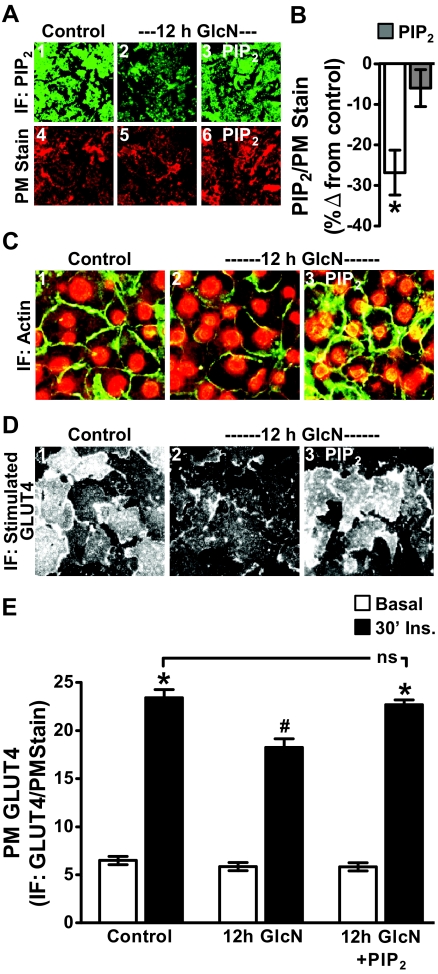
We recently documented that this failed insulin response induced by the 12-h Ins. treatment reflected a defect in PIP2-regulated F-actin (11, 12). To test whether the PIP2/F-actin disturbance resulted from increased glucose flux through HBP, we first examined whether inhibition of GFAT with DON protected against the insulin-induced decrease in detectable PM PIP2 and F-actin. For these studies we used a commercially available PIP2 antibody (Assay Designs) that we have previously documented to be specific for PI(4,5)P2 [(11, 12); see Materials and Methods for expanded detail]. PM sheets prepared from 12 h Ins. cells displayed a loss in detectable PM PIP2 compared with control cells (Fig. 3, compare panels 1 and 2). In contrast, DON pretreatment completely prevented this decrease (Fig. 3, panel 3). As visualized in panel 4, the overnight GlcN treatment was associated with a clear reduction in detectable PM PIP2, similar to that induced by the chronic insulin conditions. Shown are representative images captured from eight to 10 separate experiments, and again, to ensure that this qualitative measure was performed in an unbiased manner, fields of sheets were selected based solely on their staining with rhodamine-conjugated WGA (Fig. 3, panels 5–8). DON treatment did not appear to affect the control level of PM PIP2 or PM stain (data not shown).
Fig. 3.
Hyperinsulinemic and GlcN states induce a loss in PM PIP2 detection and cortical F-actin. Representative immunofluorescent (IF) images of PIP2 (panels 1–4) and WGA (panels 5–8) detected in PM sheets treated as described in the preceding figures are shown. Phalloidin stained F-actin and propidium iodide labeled nuclei in these cells (panels 9–12) are shown. Images are representative from eight to ten independent experiments.
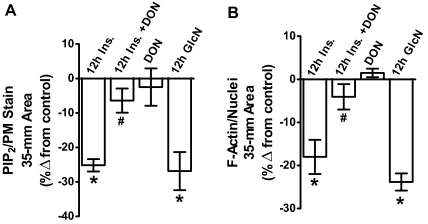
In addition to these qualitative measures of signal intensity from ×60 magnification fields, we also performed three separate population-based assays in which the PM PIP2 immunofluorescent signal was quantitated on an entire 35-mm cell culture plate well using the LI-COR Odyssey imaging system as we have previously described (12, 20, 23). As shown in Fig. 4A, PM PIP2 was reduced by 25% by both the chronic insulin exposure and the GlcN treatment (bars 1 and 4), and DON treatment prevented the insulin-induced PIP2 loss (bar 2). As we observed qualitatively, DON did not change control PM PIP2 (bar 3) and did not protect cells against PIP2 loss due to GlcN treatment (data not shown), consistent with GlcN entering the HBP distal to GFAT.
Fig. 4.
Population-based LI-COR Odyssey analyses quantitate a loss in PM PIP2 detection and cortical F-actin. For these analyses, PM sheets or cells on an entire 35-mm cell culture well were labeled as in Fig. 3 and the fluorescent signals in the entire well were quantitated using the LI-COR Odyssey system as described in Materials and Methods. PM PIP2 (A) and F-actin (B) means ± se from three independent experiments are shown. *, P < 0.05 vs. control; #, P < 0.05 vs. 12 h Ins.).
Concomitant changes in the F-actin immunofluorescent intensity were also detected by confocal imaging (Fig. 3, panels 9–12). In particular, microscopic analyses qualitatively revealed that cells treated overnight with 5 nm Ins. or 2 mm GlcN displayed a reduction in F-actin (Fig. 3, panels 10 and 12). Also, in the presence of DON, this insulin-induced visualized loss of F-actin appeared to be reversed. To ensure the imaging of a comparable number of cells, we costained nuclei with propidium iodine. As performed for the PIP2 analyses, we also conducted three separate population-based assays in which the F-actin immunofluorescent signal was quantitated on an entire 35-mm well area and normalized to nuclei. As shown in Fig. 4B, F-actin/nuclei detection was reduced by 18–24% by both overnight incubations (bars 1 and 4), and DON treatment prevented the insulin-induced F-actin loss (bar 2).
Both these microscopic field (Fig. 3) and whole-cell, population-based (Fig. 4) analyses suggest the analogous changes in O-linked glycosylation (Fig. 1), and impairments in GLUT4 translocation/2-DG uptake (Fig. 2) induced by either 5 nm Ins. or 2 mm GlcN result from a similar unappreciated HBP-coupled PIP2/F-actin mechanism. Nevertheless, work of Hresko et al. (9) underscores the importance of measuring intracellular ATP because the use of GlcN can, under certain conditions, result in intracellular ATP depletion greater than that induced by insulin and likely result in nonspecific defects.
Consistent with our deliberate choice of a low concentration of GlcN with the omission of insulin in the medium, we found the loss of intracellular ATP in the GlcN-treated cells (14.0 ± 5.6%; P = 0.042) to be similar to that induced by 5 nm Ins. (10.4 ± 3.4%; P = 0.019) (see Supplemental Fig. 2A, bars 1 and 2). Interestingly, although inosine prevented this loss of intracellular ATP (see Supplemental Fig. 2A, bars 3 and 4), it did not reverse the changes in O-linked glycosylation (data not shown), acute, insulin-stimulated PM GLUT4 (see Supplemental Fig. 2B), WGA PM stain (data not shown), PIP2/PM stain (data not shown), and F-actin/nuclei (data not shown) induced by these models.
Exogenous PIP2 protects against GlcN-induced loss of F-actin and insulin action
It is of interest that experimental replenishment of PIP2, as we previously reported (11, 12), or inhibition of GFAT as we report here (see Fig. 2) protects against insulin-induced insulin resistance. We reasoned that protection against GlcN-induced insulin resistance by PIP2 replenishment would lend credence to a coupling between increased HBP activity and PIP2/F-actin dysfunction. As we have established in other studies (11, 12, 20, 23), histone-mediated delivery of 1.25 μm exogenous PIP2 into cells displaying a 20–30% loss of immunodetectable PIP2 effectively restores antibody detection of this lipid's PM content to that witnessed in control cells (Figs. 5, A and B). This tactic concomitantly restored F-actin structure (Fig. 5C) and insulin-stimulated GLUT4 translocation (Fig. 5D) in GlcN-induced, insulin-resistant 3T3-L1 adipocytes. (Note: WGA labeling was performed but images are not shown.) As performed above, the GLUT4 signal was normalized to the WGA PM stain (Fig. 5E). Also, because we did note that the PIP2 antibody displayed some immunoreactivity to PI(3,4)P2 and PI(3,4,5)P3 on commercially available PIP strips (see Materials and Methods), we also tested whether delivery of exogenous PI(3,4)P2 and PI(3,4,5)P3, as well as PI(4)P mimicked the protective effect of PI(4,5)P2 and found that they did not [(20, 23), data not shown]. To the best of our knowledge, this is the first time that the negative effect of GlcN on insulin sensitivity has been experimentally eliminated.
Fig. 5.
PIP2 add-back restores the GlcN-induced PIP2 decrease and protects against GlcN-induced F-actin loss and insulin resistance. During the final 60 min of control or GlcN incubation, the medium was replaced with the same medium enriched with either histone H1 (A, panels 1, 2, 4, and 5; C and D, panels 1 and 2) or PIP2/histone H1 (A, panels 3 and 6; C and D, panels 3). Representative immunofluorescent (IF) images of PM PIP2 (A, panels 1–3) and WGA-stained PM (A, panels 4–6), and quantitation of PM PIP2 (B) are shown. Phalloidin-stained F-actin and propidium iodide-stained nuclei in these cells (C), insulin-stimulated PM GLUT4 images (D), and GLUT4 quantitation (E) are shown. All microscope and camera settings were identical between groups and images and means ± se are from three to five independent experiments. *, P < 0.05 vs. control; #, P < 0.008 vs. 30′ Ins.).
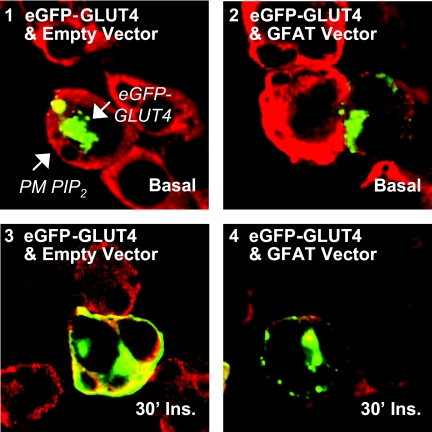
In line with these DON and GlcN data, GLUT4-eGFP expressing 3T3-L1 adipocytes cooverexpressing GFAT displayed a loss of PM PIP2 compared with nonexpressing neighboring cells (Fig. 6, panel 2). Coexpression of control empty vector had no effect on PM PIP2 (Fig. 6, panel 1). Also detected in these basal cells is the perinuclear localization of the eGFP-tagged transporter (Fig. 6, panels 1 and 2). Insulin-stimulated GLUT4-eGFP translocation to the PM was evident in empty vector expressing cells (Fig. 6, panel 3), but not in cells overexpressing GFAT (Fig. 6, panel 4). As previously reported (23), acute insulin stimulation in control cells was associated with a slight yet noticeable loss of PM PIP2, reflecting the phosphatidylinositol 3-kinase-induced conversion of that substrate to phosphatidylinositol 3,4,5-trisphosphate.
Fig. 6.
Overexpression of GFAT reduces detectable cell-surface PIP2 concomitant with a reduction in insulin-stimulated GLUT4-eGFP translocation. Differentiated 3T3-L1 adipocytes were electroporated with 50 μg GLUT4-eGFP cDNA and 200 μg GFAT cDNA empty vector or GFAT cDNA. The cells were allowed to recover for 16 h. Cells were subsequently incubated in serum-free medium for 2 h and then left untreated (basal, panels 1 and 2) or treated (30′ Ins., panels 3 and 4) with 100 nm insulin for 30 min. Cells were fixed, labeled for PIP2, and subjected to confocal fluorescence microscopy. Representative images from three independent experiments are shown.
Glucose challenge in the absence of insulin negatively impacts PM PIP2/F-actin structure
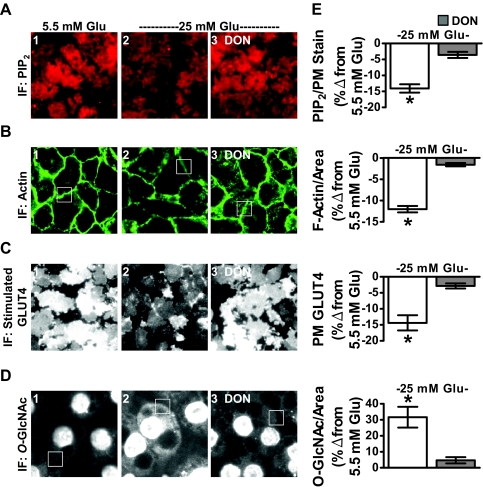
Adipocyte studies presented up to this point used the conventional 3T3-L1 adipocyte differentiation protocol. This protocol calls for 25 mm glucose to be present at all stages of differentiation and culturing. As described in Materials and Methods, our 12 h Ins. treatment took place during the last 12 h of a 48-h low glucose (5.5 mm) incubation. Work by Lin et al. (16) demonstrated that it is possible to propagate and differentiate 3T3-L1 cells at 5.5 mm glucose. An advantage to be understood further is that these euglycemic cultured cells were more insulin responsive (16). In accord, we observed that these cells displayed a robust labeling of PM PIP2 that was decreased with exposure to 25 mm glucose medium for 16 h alone (Fig. 7A, compare panels 1 and 2). The same was true for F-actin (Fig. 7B, compare panels 1 and 2). Also, we found that the 25-mm glucose challenge decreased insulin-regulated GLUT4 translocation and increased cytoplasmic and PM O-GlcNAc immunofluoresence (Figs. 7, C and D, compare panels 1 and 2). The effects of high glucose on PIP2, F-actin, GLUT4, and O-GlcNAc were all inhibited by DON (Figs. 7, A-D, panel 3), supporting the negative contribution of flux through the HBP. These data were normalized to WGA PM stain (PIP2 and GLUT4) or area (F-actin and O-GlcNAc) and quantitated (Fig. 7E).
Fig. 7.
High glucose alone induces PIP2/F-actin loss and insulin resistance in 3T3-L1 adipocytes that were cultured and differentiated in 5.5 mm glucose. Murine 3T3-L1 preadipocytes cultured and differentiated in DMEM containing 5.5 mm glucose were left untreated (5.5 mm Glu, panel 1) or treated overnight (16 h) with 25 mm glucose (25 mm Glu, panels 2 and 3) in the absence or presence of DON (panel 3). PM PIP2 (A), F-actin (B), insulin-stimulated PM GLUT4 (C), and O-linked glycosylation (D) were determined and quantitated (E) as described in preceding figures. All microscope and camera settings were identical between groups, and representative images from three independent experiments are shown. Values are means ± se from three independent experiments. IF, Immunofluorescence. *, P < 0.05 vs. control).
Membrane cholesterol accrual as a basis of HBP-induced membrane/cytoskeletal insulin resistance
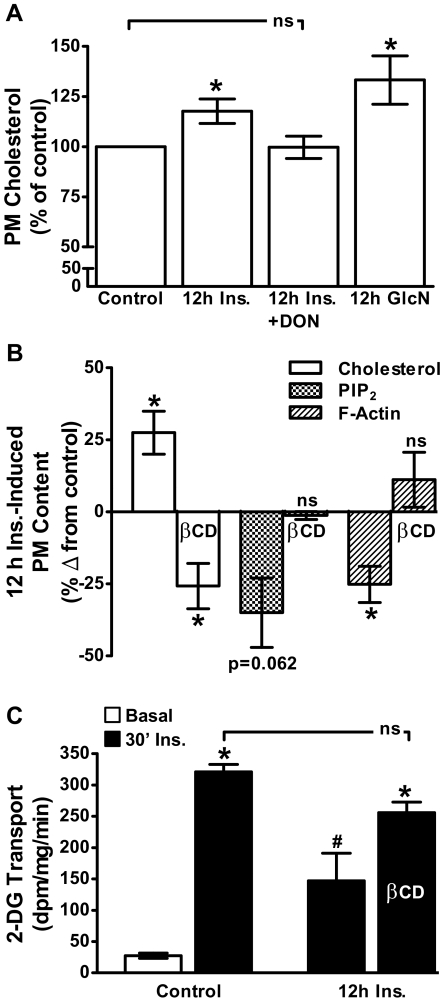
Study has suggested that PM PIP2 and F-actin are intimately linked to cholesterol-enriched PM microdomains (3). Interestingly, we have found that adipocytes cultured in 5.5 mm glucose for 16 h have less PM cholesterol than that observed in adipocytes cultured in 25 mm glucose (30). These results lead us to speculate that PM cholesterol accrual could be a negative consequence of increased HBP activity and the basis of the cytoskeletal dysfunction. Therefore, we tested whether the 12 h Ins. or 12 h GlcN treatments increased PM cholesterol. Figure 8A shows that the chronic insulin and GlcN exposures increased PM cholesterol by 18 and 33%, respectively. In addition, we observed that PM cholesterol is elevated 33% in cells cultured/differentiated in 25 mm glucose compared with that in cells cultured/differentiated in 5.5 mm glucose (see Supplemental Fig. 3A). Note in this experiment we compared PM cholesterol content in cells cultured/differentiated in 25 mm vs. 5.5 mm glucose conditions. This was unlike the experiment presented in Fig. 7 in which we challenged cells cultured/differentiated in 5.5 mm glucose with 25 mm glucose. A similar reduction in PM PIP2 and F-actin was observed in cells cultured in 25 mm glucose vs. 5.5 mm glucose (see Supplemental Fig. 3B). Consistent with increased HBP activity contributing to PM cholesterol accrual, DON treatment prevented the increase in PM cholesterol induced by hyperinsulinemia (Fig. 8A, bar 3). To test whether the loss of PM PIP2, F-actin, and insulin responsiveness resulted from PM cholesterol accrual, we used 2.5 mm methyl-βCD to lower PM cholesterol. As we have previously reported (21, 22), this treatment lowered PM cholesterol in control cells by 40% (data not shown). It also significantly lowered PM cholesterol by 56% in 12 h Ins. cells to a level 28% less than that seen in control cells (Fig. 8B, compare bars 1 and 2). Strikingly, this lowering of PM cholesterol normalized PM PIP2 and F-actin in 12 h Ins. cells to levels seen in control cells (Fig. 8B, compare bars 3–6). Finally, as shown in Fig. 8C, these βCD-induced corrections in membrane/cytoskeletal changes increased insulin-stimulated glucose transport in 12 h Ins. cells to a level that was not significantly different from that observed in control cells.
Fig. 8.
Insulin- and glucosamine-treated cells display an increase in PM cholesterol, removal of which with βCD protects against PIP2/F-actin loss and impaired insulin-stimulated glucose transport. PM cholesterol was determined in cells after 12 h Ins. and 12 h GlcN incubations in the absence or presence of DON as described in preceding figure legends (A). Cells were also exposed for 30 min to 2.5 mm βCD before PM cholesterol, PIP2, and F-actin (B) or 2-DG transport (C) determinations. Values are means ± se from three to six independent experiments. *, P < 0.05 vs. control; #, P < 0.05 vs. 30′ Ins.-control).
Discussion
In this study, we demonstrated that increased HBP activity and impaired insulin action could be coupled to adverse changes in PM PIP2 and F-actin structure. Recently we observed that this membrane/cytoskeletal defect occurred in 3T3-L1 adipocytes (11) and L6 myotubes (12) exposed to 5 nm insulin for 12 h. The prevention of insulin-induced PIP2/F-actin loss and insulin resistance by GFAT inhibition implicates increased HBP activity as a basis for the membrane/cytoskeletal-associated insulin resistance. To further probe this phenomenon, we used a GlcN treatment demonstrated to mimic the effects of insulin-induced increased glucose flux (10). The experimental condition entailed a low dose of GlcN (2 mm) without insulin in the medium. Many studies testing the effects of GlcN use medium containing insulin to accelerate GlcN transport into the cell (9, 31). A caveat to this approach is that chronic insulin treatment per se can induce insulin resistance and render data analysis complex (32). As reported by the late Stuart Ross and his colleagues (10), we observed exposure of the cells to 2 mm GlcN overnight induced an insulin-resistant phenotype very similar to that provoked by sustained insulin treatment. In particular, both experimental conditions induced analogous increases in O-linked glycosylation and decreases in GLUT4, 2-DG uptake, PM PIP2, and F-actin. Additional studies conducted found that the insulin, GlcN, and glucose treatments all uniformly increased PM cholesterol, the reversal of which restored PM PIP2/F-actin structure and insulin sensitivity. Moreover, DON treatment completely prevented the PM cholesterol accrual in cells chronically exposed to insulin. Together these findings suggest a scenario that increased HBP activity induces PM cholesterol accrual that compromises PM PIP2/F-actin structure and insulin sensitivity.
In terms of human health, we know that insulin resistance is a progressive syndrome with many associated pathologies appearing as insulin sensitivity steadily worsens. Many research efforts have clearly documented various signal transduction abnormalities as key contributors of insulin resistance (33–35). We speculate that findings presented herein support another critical feature of the insulin-resistant state that entails a weakening of membrane and cytoskeletal systems important in GLUT4-mediated glucose transport.
Although careful longitudinal study is required to test whether these membrane/cytoskeletal defects precede documented signaling defects in vivo, several cross-sectional studies demonstrate a clear association between excess glucose and membrane/cytoskeletal disorganization. For example, isolated neutrophils from patients with type 2 diabetes display decreased actin polymerization compared with neutrophils from nondiabetic control subjects (36, 37). This impairment is associated with persistent expression of the endothelial adhering β2-integrin CD11b/CD18, potentially exacerbating vascular dysfunction in diabetic patients. Also, diabetic retinal endothelial cells have a prominent reduction in F-actin integrity, a finding closely linked to vascular leakage (38). Loss of rat mesangial cell F-actin has also been reported after exposure to early-diabetic state conditions (39, 40), possibly causing diabetic hyperfiltration. Further human support has been derived from collected erythrocytes from overweight insulin-resistant individuals showing marked changes in the PM phospholipid composition (41, 42), some of which may directly affect the actin cytoskeleton. Current animal studies are underway to test these findings in vivo. In direct support of our findings, we have observed that epitrochlearis skeletal muscle from insulin-resistant, hyperinsulinemic Zucker fatty rats display a loss of F-actin structure compared with that in muscle from lean insulin-sensitive littermates (12). Together these human and animal observations highlight that F-actin integrity is potentially influenced by the glycemic state.
A caveat to the PIP2 analyses is that antibody detection does not determine whether there is an actual loss of this lipid from the PM after the 12-h insulin exposure. Careful biochemical study to distill this information is currently underway. We believe that a likely event may entail recruitment of one or more PIP2 binding proteins that preclude antibody detection of the affected PIP2. This possibility would also provide a basis for the loss of PIP2-regulated F-actin polymerization. Certainly other possibilities exist because PM PIP2 pools are under numerous forms of regulation (reviewed in Ref. 43). For example, one alternate possibility could be that a localized PIP2 hydrolysis by PLC occurs because PLC has been reported to localize and turn over PIP2 in caveolin-enriched membrane domains (44).
The fact that PM PIP2 detection (and its regulation of GLUT4/glucose transport) is lost is an important observation, considering it is specifically reversible with addition of exogenous PIP2 and not several other phosphatidylinositols tested. Data suggesting that the HBP induces PM cholesterol accrual are interesting in this regard. As highlighted next, studies underway in our laboratory suggest that key lipogenic transcription factors directly involved in the expression of genes dedicated to the synthesis and uptake of cholesterol, fatty acids, triglycerides, and phospholipids are activated by hyperinsulinemia and inhibited by blocking the HBP. Thus, a preliminary prediction we have is that PIP2 levels may actually be elevated, supporting the idea that antibody detection of PM PIP2 is hindered. Although future studies precisely defining whether the decrease in PIP2 antibody immunoreactivity results from a physical blockade (e.g. chronic insulin induced protein and/or lipid interaction) and/or an actual decrease in PIP2 are warranted, the clear HBP-induced PM PIP2 visual loss and insulin resistance seen here highlight an important PIP2-based aspect of HBP-induced dysregulation of GLUT4.
Regardless of the exact mechanism for the decreased detection of PM PIP2, a possibility we favor is that the accrual of excess PM cholesterol results from increased HBP flux via the induction of the transactivating capacity of specificity protein 1 (45, 46), a transcription factor shown to promote the maximal transcriptional activation of sterol regulatory element-binding protein (SREBP)-1c (47, 48). SREBP-1c is encoded from the SREBP-1 gene that also encodes an almost identical protein designated SREBP-1a. Interestingly, expression of SREBP-1 is enhanced by insulin in liver, fat, and skeletal muscle (49–52). Similarly, levels of SREBP-1 are increased in the presence of hyperinsulinemia (53–55). Because SREBP-1 plays an active role in regulating the transcription of genes involved in fatty acid synthesis and, albeit to a lesser extent, those involved in cholesterol synthesis (56), perhaps an unappreciated downfall of increased SREBP-1 regulation via HBP-modified specificity protein 1 activity is increased cholesterol synthesis resulting in PM cholesterol accrual that perturbs PIP2/F-actin status.
In line with this reasoning, Yang et al. (57) observed that OGT overexpression increased hepatic cholesterol content. It is important to note that those studies show that OGT has a phosphoinositide-binding domain, and after insulin stimulation phosphatidylinositol 3,4,5-trisphosphate recruits OGT from the nucleus to the PM, in which this enzyme catalyzes dynamic modification of the insulin signaling pathway by O-GlcNAc (57). These signaling events target specific residues on the insulin receptor substrate-1 and Akt proteins (e.g. phosphorylation of Akt on threonine 308, but not serine 473, is decreased). Although we have not witnessed changes in signaling, we have not specifically monitored phosphorylation of threonine 308. In another article Dentin et al. (58) reported that the transcriptional regulatory protein called transducer of regulated cAMP response element-binding protein 2 (58) is a target of O-linked glycosylation. The modification of cAMP response element-binding protein 2 activates glucose production in the liver and is an example of a mechanism by which glucose serves a signaling function in controlling a process, in this case metabolism in the liver. In closing, in vitro and in vivo studies, we are currently conducting are addressing the possibility that increased HBP activity negatively impacts PIP2/F-actin structure essential for insulin-regulated GLUT4 translocation and glucose transport via increasing cellular cholesterol synthesis and PM cholesterol accrual.
Acknowledgments
We are grateful to Dr. Whitney Sealls for helpful discussion and review of the manuscript.
This work was supported by National Institutes of Health Grant AT001846 (to J.S.E.) funded by the National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements; National Institutes of Health Grants DK082773 (to J.S.E.) and DK082773–01S1 (to J.S.E.) funded by the National Institute of Diabetes and Digestive and Kidney Diseases; a DeVault Fellowship (to K.M.H.) funded by the Indiana University Diabetes and Obesity Research Training Program; and Predoctoral Fellowship 0615574Z (to A.M.M.) funded by the American Heart Association Midwest Affiliation.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- βCD
- β-Cyclodextrin
- 2-DG
- 2-deoxy-D-glucose
- DON
- 6-diazo-5-oxo-l-norleucine
- eGFP
- enhanced green fluorescent protein fusion protein
- F-actin
- filamentous actin
- FBS
- fetal bovine serum
- FITC
- fluorescein isothiocyanate
- GFAT
- glutamine-fructose-6-phosphate amidotransferase
- GlcN
- glucosamine
- GlcNAc
- N-acetylglucosamine
- GlcN-6-P
- glucosamine 6-phosphate
- GLUT
- insulin-responsive glucose transporter
- HBP
- hexosamine biosynthesis pathway
- O-GlcNAc
- O-linked N-acetylglucosamine
- OGT
- O-GlcNAc transferase
- PIP2
- phosphatidylinositol 4,5-bisphosphate
- PLC
- phospholipase C
- PM
- plasma membrane
- SREBP
- sterol regulatory element-binding protein
- UDP-GlcNAc
- uridine diphosphate-GlcNAc
- WGA
- wheat germ agglutinin.
References
- 1. Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. 1987. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest 79:1510–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yki-Järvinen H, Helve E, Koivisto VA. 1987. Hyperglycemia decreases glucose uptake in type I diabetes. Diabetes 36:892–896 [DOI] [PubMed] [Google Scholar]
- 3. Hoffman NJ, Elmendorf JS. 2011. Signaling, cytoskeletal and membrane mechanisms regulating GLUT4 exocytosis. Trends Endocrinol Metab 22:110–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marshall S, Bacote V, Traxinger RR. 1991. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem 266:4706–4712 [PubMed] [Google Scholar]
- 5. Kreppel LK, Blomberg MA, Hart GW. 1997. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem 272:9308–9315 [DOI] [PubMed] [Google Scholar]
- 6. Lubas WA, Frank DW, Krause M, Hanover JA. 1997. O-linked GlcNAc transferase is a conserved nucleocytoplasmic protein containing tetratricopeptide repeats. J Biol Chem 272:9316–9324 [DOI] [PubMed] [Google Scholar]
- 7. Garvey WT, Maianu L, Huecksteadt TP, Birnbaum MJ, Molina JM, Ciaraldi TP. 1991. Pretranslational suppression of a glucose transporter protein causes insulin resistance in adipocytes from patients with non-insulin-dependent diabetes mellitus and obesity. J Clin Invest 87:1072–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buse MG. 2006. Hexosamines, insulin resistance, and the complications of diabetes: current status. Am J Physiol Endocrinol Metab 290:E1–E8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hresko RC, Heimberg H, Chi MM, Mueckler M. 1998. Glucosamine-induced insulin resistance in 3T3-L1 adipocytes is caused by depletion of intracellular ATP. J Biol Chem 273:20658–20668 [DOI] [PubMed] [Google Scholar]
- 10. Ross SA, Chen X, Hope HR, Sun S, McMahon EG, Broschat K, Gulve EA. 2000. Development and comparison of two 3T3-L1 adipocyte models of insulin resistance: increased glucose flux vs glucosamine treatment. Biochem Biophys Res Commun 273:1033–1041 [DOI] [PubMed] [Google Scholar]
- 11. Chen G, Raman P, Bhonagiri P, Strawbridge AB, Pattar GR, Elmendorf JS. 2004. Protective effect of phosphatidylinositol 4,5-bisphosphate against cortical filamentous actin loss and insulin resistance induced by sustained exposure of 3T3-L1 adipocytes to insulin. J Biol Chem 279:39705–39709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCarthy AM, Spisak KO, Brozinick JT, Elmendorf JS. 2006. Loss of cortical actin filaments in insulin-resistant skeletal muscle cells impairs GLUT4 vesicle trafficking and glucose transport. Am J Physiol Cell Physiol 291:C860–C868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sealls W, Penque BA, Elmendorf JS. 2011. Evidence that chromium modulates cellular cholesterol homeostasis and ABCA1 functionality impaired by hyperinsulinemia—brief report. Arterioscler Thromb Vasc Biol 31:1139–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kralik SF, Liu P, Leffler BJ, Elmendorf JS. 2002. Ceramide and glucosamine antagonism of alternate signaling pathways regulating insulin- and osmotic shock-induced glucose transporter 4 translocation. Endocrinology 143:37–46 [DOI] [PubMed] [Google Scholar]
- 15. Green H, Meuth M. 1974. An established pre-adipose cell line and its differentiation in culture. Cell 3:127–133 [DOI] [PubMed] [Google Scholar]
- 16. Lin Y, Berg AH, Iyengar P, Lam TK, Giacca A, Combs TP, Rajala MW, Du X, Rollman B, Li W, Hawkins M, Barzilai N, Rhodes CJ, Fantus IG, Brownlee M, Scherer PE. 2005. The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. J Biol Chem 280:4617–4626 [DOI] [PubMed] [Google Scholar]
- 17. van Putten JP, Krans HM. 1985. Glucose as a regulator of insulin-sensitive hexose uptake in 3T3 adipocytes. J Biol Chem 260:7996–8001 [PubMed] [Google Scholar]
- 18. Min J, Okada S, Kanzaki M, Elmendorf JS, Coker KJ, Ceresa BP, Syu LJ, Noda Y, Saltiel AR, Pessin JE. 1999. Synip: a novel insulin-regulated syntaxin 4-binding protein mediating GLUT4 translocation in adipocytes. Mol Cell 3:751–760 [DOI] [PubMed] [Google Scholar]
- 19. Thurmond DC, Ceresa BP, Okada S, Elmendorf JS, Coker K, Pessin JE. 1998. Regulation of insulin-stimulated GLUT4 translocation by Munc18c in 3T3L1 adipocytes. J Biol Chem 273:33876–33883 [DOI] [PubMed] [Google Scholar]
- 20. Strawbridge AB, Elmendorf JS. 2006. Endothelin-1 impairs glucose transporter trafficking via a membrane-based mechanism. J Cell Biochem 97:849–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen G, Liu P, Pattar GR, Tackett L, Bhonagiri P, Strawbridge AB, Elmendorf JS. 2006. Chromium activates glucose transporter 4 trafficking and enhances insulin-stimulated glucose transport in 3T3-L1 adipocytes via a cholesterol-dependent mechanism. Mol Endocrinol 20:857–870 [DOI] [PubMed] [Google Scholar]
- 22. Liu P, Leffler BJ, Weeks LK, Chen G, Bouchard CM, Strawbridge AB, Elmendorf JS. 2004. Sphingomyelinase activates GLUT4 translocation via a cholesterol-dependent mechanism. Am J Physiol Cell Physiol 286:C317–C329 [DOI] [PubMed] [Google Scholar]
- 23. Strawbridge AB, Elmendorf JS. 2005. Phosphatidylinositol 4,5-bisphosphate reverses endothelin-1-induced insulin resistance via an actin-dependent mechanism. Diabetes 54:1698–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Razidlo GL, Kortum RL, Haferbier JL, Lewis RE. 2004. Phosphorylation regulates KSR1 stability, ERK activation, and cell proliferation. J Biol Chem 279:47808–47814 [DOI] [PubMed] [Google Scholar]
- 25. Hausdorff SF, Frangioni JV, Birnbaum MJ. 1994. Role of p21ras in insulin-stimulated glucose transport in 3T3-L1 adipocytes. J Biol Chem 269:21391–21394 [PubMed] [Google Scholar]
- 26. Chen G, Liu P, Thurmond DC, Elmendorf JS. 2003. Glucosamine-induced insulin resistance is coupled to O-linked glycosylation of Munc18c. FEBS Lett 534:54–60 [DOI] [PubMed] [Google Scholar]
- 27. Holt GD, Snow CM, Senior A, Haltiwanger RS, Gerace L, Hart GW. 1987. Nuclear pore complex glycoproteins contain cytoplasmically disposed O-linked N-acetylglucosamine. J Cell Biol 104:1157–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Snow CM, Senior A, Gerace L. 1987. Monoclonal antibodies identify a group of nuclear pore complex glycoproteins. J Cell Biol 104:1143–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sterne-Marr R, Blevitt JM, Gerace L. 1992. O-linked glycoproteins of the nuclear pore complex interact with a cytosolic factor required for nuclear protein import. J Cell Biol 116:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pattar GR, Tackett L, Liu P, Elmendorf JS. 2006. Chromium picolinate positively influences the glucose transporter system via affecting cholesterol homeostasis in adipocytes cultured under hyperglycemic diabetic conditions. Mutat Res 610:93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nelson BA, Robinson KA, Buse MG. 2000. High glucose and glucosamine induce insulin resistance via different mechanisms in 3T3-L1 adipocytes. Diabetes 49:981–991 [DOI] [PubMed] [Google Scholar]
- 32. Heart E, Choi WS, Sung CK. 2000. Glucosamine-induced insulin resistance in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab 278:E103–E112 [DOI] [PubMed] [Google Scholar]
- 33. Birnbaum MJ. 2001. Turning down insulin signaling. J Clin Invest 108:655–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chakraborty C. 2006. Biochemical and molecular basis of insulin resistance. Curr Protein Pept Sci 7:113–121 [DOI] [PubMed] [Google Scholar]
- 35. Saltiel AR. 2001. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell 104:517–529 [DOI] [PubMed] [Google Scholar]
- 36. Advani A, Marshall SM, Thomas TH. 2004. Increasing neutrophil F-actin corrects CD11b exposure in type 2 diabetes. Eur J Clin Invest 34:358–364 [DOI] [PubMed] [Google Scholar]
- 37. Advani A, Marshall SM, Thomas TH. 2002. Impaired neutrophil actin assembly causes persistent CD11b expression and reduced primary granule exocytosis in type II diabetes. Diabetologia 45:719–727 [DOI] [PubMed] [Google Scholar]
- 38. Yu PK, Yu DY, Cringle SJ, Su EN. 2005. Endothelial F-actin cytoskeleton in the retinal vasculature of normal and diabetic rats. Curr Eye Res 30:279–290 [DOI] [PubMed] [Google Scholar]
- 39. Zhou X, Hurst RD, Templeton D, Whiteside CI. 1995. High glucose alters actin assembly in glomerular mesangial and epithelial cells. Lab Invest 73:372–383 [PubMed] [Google Scholar]
- 40. Cortes P, Méndez M, Riser BL, Guérin CJ, Rodríguez-Barbero A, Hassett C, Yee J. 2000. F-actin fiber distribution in glomerular cells: structural and functional implications. Kidney Int 58:2452–2461 [DOI] [PubMed] [Google Scholar]
- 41. Candiloros H, Zeghari N, Ziegler O, Donner M, Drouin P. 1996. Hyperinsulinemia is related to erythrocyte phospholipid composition and membrane fluidity changes in obese nondiabetic women. J Clin Endocrinol Metab 81:2912–2918 [DOI] [PubMed] [Google Scholar]
- 42. Younsi M, Quilliot D, Al-Makdissy N, Delbachian I, Drouin P, Donner M, Ziegler O. 2002. Erythrocyte membrane phospholipid composition is related to hyperinsulinemia in obese nondiabetic women: effects of weight loss. Metabolism 51:1261–1268 [DOI] [PubMed] [Google Scholar]
- 43. Santarius M, Lee CH, Anderson RA. 2006. Supervised membrane swimming: small G-protein lifeguards regulate PIPK signalling and monitor intracellular PtdIns(4,5)P2 pools. Biochem J 398:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pike LJ, Casey L. 1996. Localization and turnover of phosphatidylinositol 4,5-bisphosphate in caveolin-enriched membrane domains. J Biol Chem 271:26453–26456 [DOI] [PubMed] [Google Scholar]
- 45. Comer FI, Hart GW. 1999. O-GlcNAc and the control of gene expression. Biochim Biophys Acta 1473:161–171 [DOI] [PubMed] [Google Scholar]
- 46. Du XL, Edelstein D, Rossetti L, Fantus IG, Goldberg H, Ziyadeh F, Wu J, Brownlee M. 2000. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci USA 97:12222–12226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cagen LM, Deng X, Wilcox HG, Park EA, Raghow R, Elam MB. 2005. Insulin activates the rat sterol-regulatory-element-binding protein 1c (SREBP-1c) promoter through the combinatorial actions of SREBP, LXR, Sp-1 and NF-Y cis-acting elements. Biochem J 385:207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Deng X, Yellaturu C, Cagen L, Wilcox HG, Park EA, Raghow R, Elam MB. 2007. Expression of the rat sterol regulatory element-binding protein-1c gene in response to insulin is mediated by increased transactivating capacity of specificity protein 1 (Sp1). J Biol Chem 282:17517–17529 [DOI] [PubMed] [Google Scholar]
- 49. Foretz M, Guichard C, Ferré P, Foufelle F. 1999. Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc Natl Acad Sci USA 96:12737–12742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Guillet-Deniau I, Mieulet V, Le Lay S, Achouri Y, Carré D, Girard J, Foufelle F, Ferré P. 2002. Sterol regulatory element binding protein-1c expression and action in rat muscles: insulin-like effects on the control of glycolytic and lipogenic enzymes and UCP3 gene expression. Diabetes 51:1722–1728 [DOI] [PubMed] [Google Scholar]
- 51. Kim JB, Sarraf P, Wright M, Yao KM, Mueller E, Solanes G, Lowell BB, Spiegelman BM. 1998. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J Clin Invest 101:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sewter C, Berger D, Considine RV, Medina G, Rochford J, Ciaraldi T, Henry R, Dohm L, Flier JS, O'Rahilly S, Vidal-Puig AJ. 2002. Human obesity and type 2 diabetes are associated with alterations in SREBP1 isoform expression that are reproduced ex vivo by tumor necrosis factor-α. Diabetes 51:1035–1041 [DOI] [PubMed] [Google Scholar]
- 53. Boizard M, Le Liepvre X, Lemarchand P, Foufelle F, Ferré P, Dugail I. 1998. Obesity-related overexpression of fatty-acid synthase gene in adipose tissue involves sterol regulatory element-binding protein transcription factors. J Biol Chem 273:29164–29171 [DOI] [PubMed] [Google Scholar]
- 54. Kakuma T, Lee Y, Higa M, Wang Z, Pan W, Shimomura I, Unger RH. 2000. Leptin, troglitazone, and the expression of sterol regulatory element binding proteins in liver and pancreatic islets. Proc Natl Acad Sci USA 97:8536–8541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shimomura I, Bashmakov Y, Horton JD. 1999. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem 274:30028–30032 [DOI] [PubMed] [Google Scholar]
- 56. Brown MS, Goldstein JL. 1997. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89:331–340 [DOI] [PubMed] [Google Scholar]
- 57. Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ, Evans RM. 2008. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature 451:964–969 [DOI] [PubMed] [Google Scholar]
- 58. Dentin R, Hedrick S, Xie J, Yates J, 3rd, Montminy M. 2008. Hepatic glucose sensing via the CREB coactivator CRTC2. Science 319:1402–1405 [DOI] [PubMed] [Google Scholar]