Abstract
Gartner, T. K. (University of California, Santa Barbara), and E. Orias. Effects of mutations to streptomycin resistance on the rate of translation of mutant genetic information. J. Bacteriol. 91:1021–1028. 1966.—The effects of mutations to streptomycin resistance of independent origin upon the translation of suppressible mutant information were studied in an isogenic series of strains of Escherichia coli. The group of suppressible mutants included 1 mutation in the z gene of the lac operon of E. coli (O02 allele), 12 mutations distributed among the two rII cistrons of T4, and 13 mutations distributed among at least five cistrons of phage T7. It was concluded that the mutations to streptomycin resistance cause a significant decrease in the rate of translation of the suppressible codons, and that this effect is limited to a few types of codons.
Full text
PDF


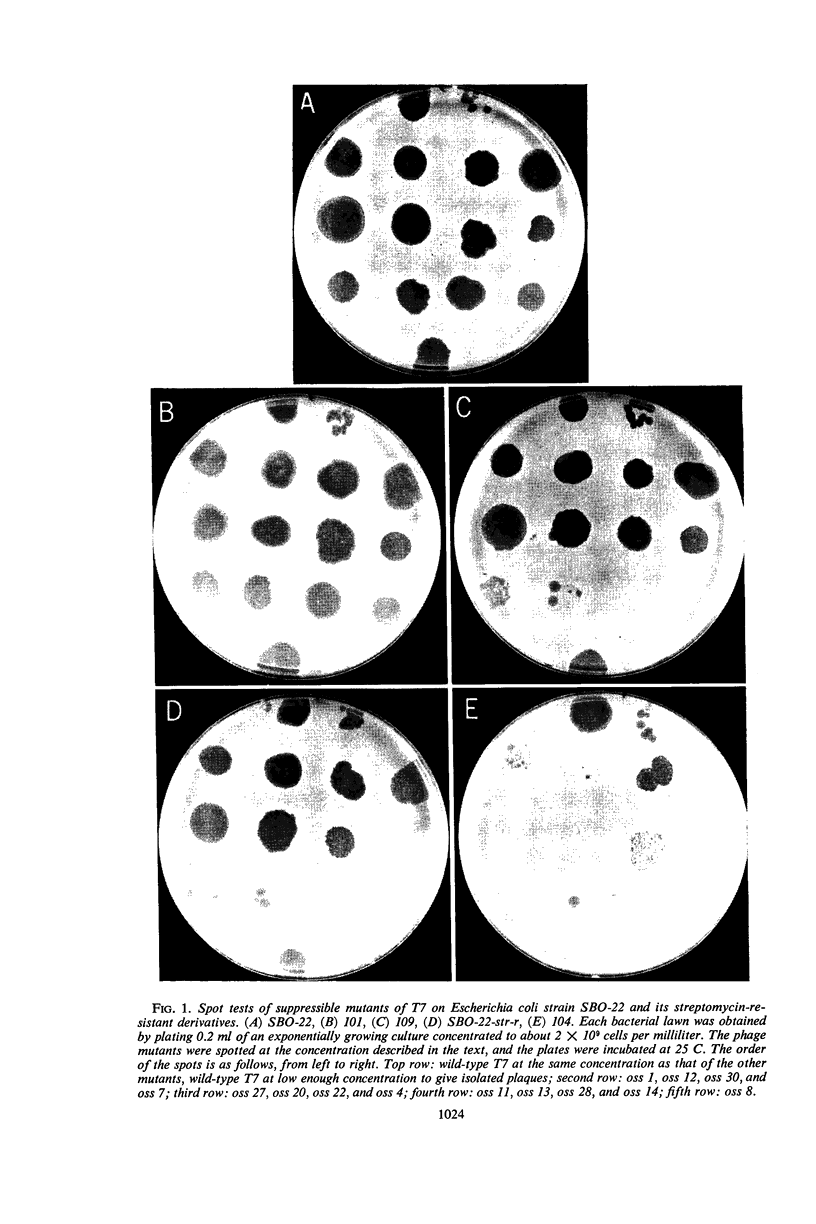
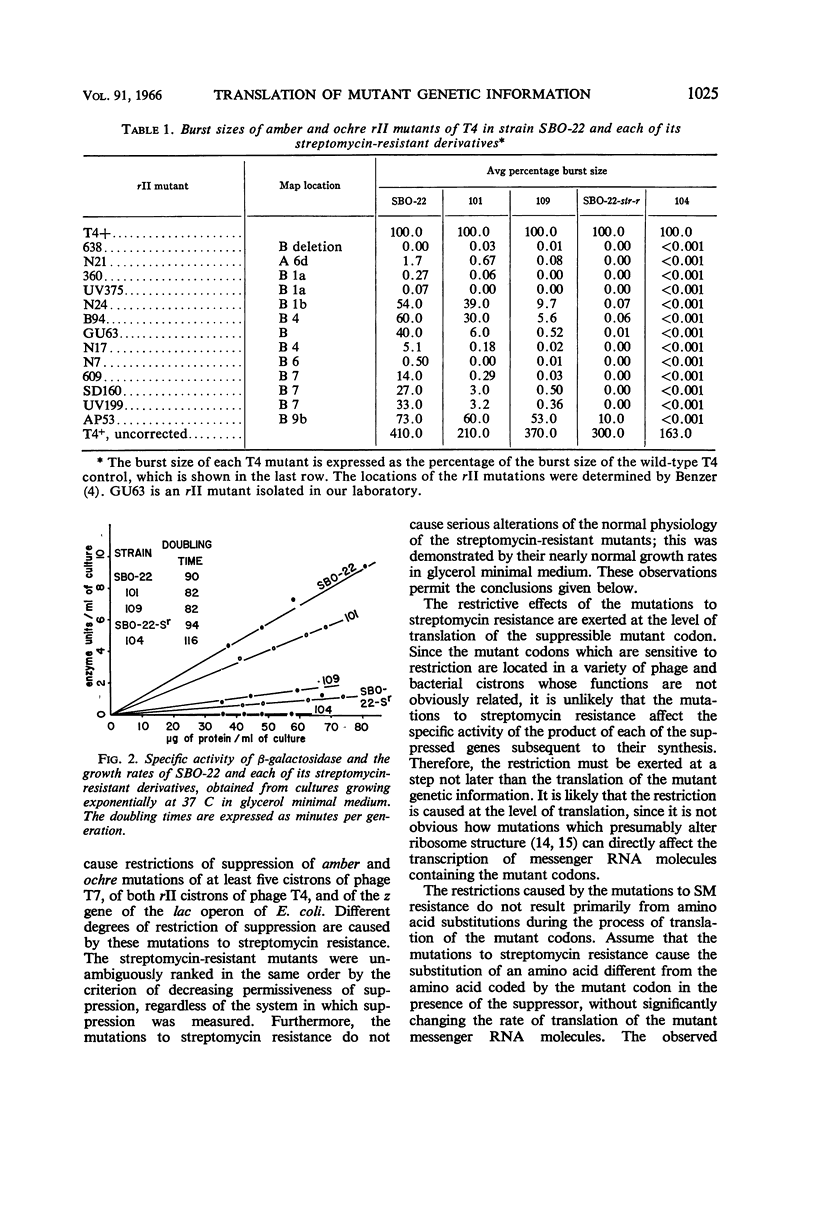
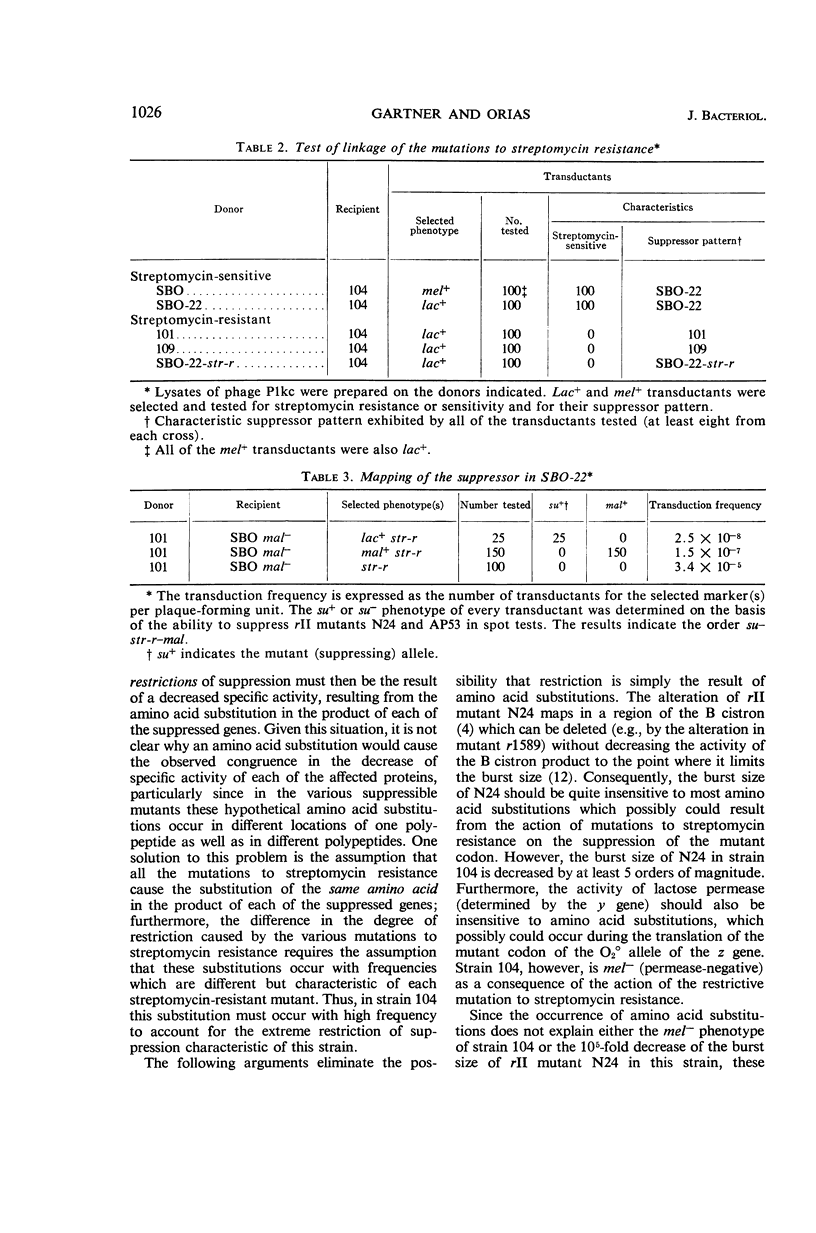


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKWITH J. RESTORATION OF OPERON ACTIVITY BY SUPPRESSORS. Biochim Biophys Acta. 1963 Sep 17;76:162–164. [PubMed] [Google Scholar]
- BENZER S., CHAMPE S. P. A change from nonsense to sense in the genetic code. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1114–1121. doi: 10.1073/pnas.48.7.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer S., Champe S. P. AMBIVALENT rII MUTANTS OF PHAGE T4. Proc Natl Acad Sci U S A. 1961 Jul;47(7):1025–1038. doi: 10.1073/pnas.47.7.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer S. ON THE TOPOGRAPHY OF THE GENETIC FINE STRUCTURE. Proc Natl Acad Sci U S A. 1961 Mar;47(3):403–415. doi: 10.1073/pnas.47.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer S. ON THE TOPOLOGY OF THE GENETIC FINE STRUCTURE. Proc Natl Acad Sci U S A. 1959 Nov;45(11):1607–1620. doi: 10.1073/pnas.45.11.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., Stretton A. O., Kaplan S. Genetic code: the 'nonsense' triplets for chain termination and their suppression. Nature. 1965 Jun 5;206(988):994–998. doi: 10.1038/206994a0. [DOI] [PubMed] [Google Scholar]
- CHAMPE S. P., BENZER S. An active cistron fragment. J Mol Biol. 1962 Apr;4:288–292. doi: 10.1016/s0022-2836(62)80006-0. [DOI] [PubMed] [Google Scholar]
- CLARK A. J. Genetic analysis of a "double male" strain of Escherichia coli K-12. Genetics. 1963 Jan;48:105–120. doi: 10.1093/genetics/48.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COX E. C., WHITE J. R., FLAKS J. G. STREPTOMYCIN ACTION AND THE RIBOSOME. Proc Natl Acad Sci U S A. 1964 Apr;51:703–709. doi: 10.1073/pnas.51.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R., Gussin G. N. Suppression in vitro: Identification of a Serine-sRNA as a "Nonsense" Suppressor. Science. 1965 Jul 23;149(3682):417–422. doi: 10.1126/science.149.3682.417. [DOI] [PubMed] [Google Scholar]
- DAVIES J. E. STUDIES ON THE RIBOSOMES OF STREPTOMYCIN-SENSITIVE AND RESISTANT STRAINS OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Apr;51:659–664. doi: 10.1073/pnas.51.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., SIDDIQI O. Suppression of mutations in the alkaline phosphatase structural cistron of E. coli. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1121–1127. doi: 10.1073/pnas.48.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARTNER T. K., ORIAS E. PLEIOTROPIC EFFECTS OF SUPPRESSORS OF A LAC-"OPERATOR NEGATIVE" MUTATION IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1965 Jan;53:62–68. doi: 10.1073/pnas.53.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., KATAJA E. PHENOTYPIC REPAIR BY STREPTOMYCIN OF DEFECTIVE GENOTYPES IN E. COLI. Proc Natl Acad Sci U S A. 1964 Mar;51:487–493. doi: 10.1073/pnas.51.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. Streptomycin Resistance in Escherichia Coli Analyzed by Transduction. Genetics. 1960 Jan;45(1):49–62. doi: 10.1093/genetics/45.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERBERG E. M., CAVALLI-SFORZA L., LEDERBERG J. INTERACTION OF STREPTOMYCIN AND A SUPPRESSOR FOR GALACTOSE FERMENTATION IN E. COLI K-12. Proc Natl Acad Sci U S A. 1964 Apr;51:678–682. doi: 10.1073/pnas.51.4.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDERBERG J. Streptomycin resistance; a genetically recessive mutation. J Bacteriol. 1951 May;61(5):549–550. doi: 10.1128/jb.61.5.549-550.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOOMIS W. F., Jr, MAGASANIK B. THE RELATION OF CATABOLITE REPRESSION TO THE INDUCTION SYSTEM FOR BETA-GALACTOSIDASE IN ESCHERICHIA COLI. J Mol Biol. 1964 Mar;8:417–426. doi: 10.1016/s0022-2836(64)80205-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- NEWCOMBE H. B., NYHOLM M. H. The inheritance of streptomycin resistance and dependence in crosses of Escherichia coli. Genetics. 1950 Nov;35(6):603–611. doi: 10.1093/genetics/35.6.603b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORIAS E., GARTNER T. K. SUPPRESSION OF A CLASS OF RII MUTANTS OF T4 BY A SUPPRESSOR OF A LAC -"OPERATOR NEGATIVE" MUTATION. Proc Natl Acad Sci U S A. 1964 Sep;52:859–864. doi: 10.1073/pnas.52.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARDEE A. B. An inducible mechanism for accumulation of melibiose in Escherichia coli. J Bacteriol. 1957 Mar;73(3):376–385. doi: 10.1128/jb.73.3.376-385.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REVEL H. R., LURIA S. E., ROTMAN B. Biosynthesis of B-D-galactosidase controlled by phage-carried genes. I. Induced beta-D-galactosidase biosynthesis after transduction of gene z-plus by phage. Proc Natl Acad Sci U S A. 1961 Dec 15;47:1956–1967. doi: 10.1073/pnas.47.12.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid P. J., Orias E., Gartner T. K. Ribosomal protein differences in a strain of E. coli carrying a suppressor of an ochre mutation. Biochem Biophys Res Commun. 1965 Oct 8;21(1):66–71. doi: 10.1016/0006-291x(65)90427-4. [DOI] [PubMed] [Google Scholar]