Abstract
Background:
High myopia caused primarily due to abnormal emmetropization and excessive axial ocular elongation is associated with sight-threatening ocular pathology. Muscular dysfunction of ocular ciliary muscles due to altered intracellular calcium levels can result in defective mechanotransduction of the eye and retinal defocus. The vitamin D3 receptor (VDR; a intracellular hormone receptor) is known to mediate calcium homeostasis, influencing the development of myopia.
Materials and Methods:
In the present study, a total of 206 high myopia, 98 low myopia and 250 control samples were analyzed for VDR gene Fok1 (exon 2 start codon) polymorphism using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) technique.
Results:
High myopia patients revealed decrease in the frequency of ff homozygotes (8.3%) as compared to control group (14.0%), with a corresponding increase in frequency of FF homozygotes (68.9% in high myopia vs. 62.8% in controls). The frequency of f allele carriers (Ff and ff) was increased in females of high myopia (35.6%) and low myopia cases (45.4%). Elevated frequency of f allele was found only in early age at onset cases of high myopia (0.227) and later age at onset (10–20 years) cases of low myopia (0.273) as well as in low myopia cases with parental consanguinity (0.458) (P 0.035; χ2 = 6.692*).
Conclusion:
The results suggest that VDR gene might not be playing a direct role in the development of myopia, but might contribute indirectly to the risk conferred by mechanical stress factors or growth/development related factors through its role in calcium homeostasis and regulation of ciliary muscle function.
Keywords: Axial elongation, calcium homeostasis, high myopia, myopia development, polymorphism, VDR gene, vitreo-retinal degeneration
Introduction
Myopia (near-sightedness), a multifactorial ocular disorder, is a common cause of visual impairment in the world.[1] Low myopia (with refractive error <6 D) is primarily physiological, whereas high-grade or pathological myopia (with refractive error >6 D) is a progressive form that is often associated with vitreo-retinal degenerative changes and carries a significant risk for vision loss.[2] The global incidence of high myopia exhibits population variation, with higher prevalence rates in Asia (as high as 70-90%), particularly in countries like China, Japan, Hong Kong and Taiwan.[3,4]
Myopia development is considered to be influenced by multiple genes and environmental factors, and possibly their interactions.[5,6] Ample molecular genetic studies indicated high heritability (60–90%) and genetic heterogeneity for non-syndromic myopia.[6,7] Many studies have suggested a strong genetic background for high myopia.[8,9] So far, 24 chromosomal loci have been identified for myopia through linkage and association studies: Xq28 (MYP1), 18p11.31 (MYP2), 12q21-31 (MYP3), 7q36 (MYP4), 17q21-22 (MYP5), 22q37.1 (MYP6), 11p13 (MYP7), 3q26 (MYP8), 4q12 (MYP9), 8p23 (MYP10), 4q22-q27 (MYP11), 2q37.1 (MYP12), Xq23 (MYP13), 1p36 (MYP14), 10q21.2 (MYP15), 5p15.33-p15.2 (MYP16), 7p15 (MYP17), 14q22.1-q24.2 (MYP18), 15q12-13, 21q22.3, 12q24, 4q21, 9q34.11 and 2q37, which provide an array of positional candidate genes, all of which are linked in some way or the other to myopia development.[10] However, attempts to identify the causative gene through mutation analysis or expression studies were not fruitful.
The vitamin D3 receptor (VDR) gene serves as a functional and positional candidate gene (located near MYP-3 locus) for myopia. Myopia is primarily the effect of abnormal emmetropization that may be caused by ciliary muscle dysfunction due to impaired calcium and mineral homeostasis or osteogenesis.[11,12] VDR (intracellular hormone receptor) is a ligand inducible transcriptional factor known to mediate these functions.[13] This gene encoding VDR is mapped to chromosome 12cen-q12, comprising at least five promoter regions, eight protein-coding exons and six untranslated exons, which are alternatively spliced.[14,15] Many association studies of VDR gene polymorphisms have described varied pleiotropic effects of VDR.[16] VDR gene variants are known to be associated with body mass, bone turnover and calcium absorption in several pathological conditions.[6] Of the several gene polymorphisms identified, Fok1 start codon polymorphism is the most important one that has been widely used as a genetic marker for several polygenic diseases related to calcium metabolism. It is located in the 5’ promoter region and can be considered an independent marker in the VDR gene since there is no linkage disequilibrium (LD) with any of the other VDR polymorphisms Bsm1, Apa1, Taq1, and the LD area surrounding this polymorphism seems to be very small.[17] It defines the presence of T > C transition polymorphism (ATG to ACG) in exon 2 of the VDR gene[18] that results in protein of different lengths[19] due to different translation initiation sites. The VDR gene Fok1 polymorphism, also known as start codon polymorphism, produces two different alleles (designated as f and F) distinguished on the basis of the presence or absence of Fok1 restriction site.[20] The allele f (with restriction site) initiates translation at first ATG site and encodes full-length VDR protein, while the F allele (with no restriction site) initiates translation at second ATG site and encodes shorter length protein that lacks three -NH2 terminal amino acids. The present study is an attempt to understand the association of VDR Fok1 polymorphism and its functional significance in myopia susceptibility.
Materials and Methods
Source and study design
The present case–control study was conducted during the period 2005–2008 after getting approval from the Institutional Ethical Committee. A total of 206 high myopia cases (>6 D) and around 98 low myopia (<6 D) cases (to serve as positive control group) were recruited from Maxivision Eye Hospital, Hyderabad, India, after explaining to them the purpose of the study and obtaining their informed consent. All the patients underwent a comprehensive ophthalmic examination which included cycloplegic refraction with cyclopentolate 1%, keratometry (measures central 3 mm), dilated fundoscopy and pachymetry. The mean age at onset for high myopia and low myopia cases was 24.9 ± 0.03 and 38.6 ± 0.48 years, respectively. The mean best corrected visual acuity was 20/125. Only patients who had myopia in both the eyes were included. Patients with ocular disease such as cataract or glaucoma, or a history of retinopathy or connective tissue disorders associated with myopia, such as Stickler or Marfan syndromes, were not included. Around 250 randomly selected age- and sex-matched normal healthy individuals without history of myopia and other ocular diseases were used as controls to compare with patient group. Detailed information on clinical, epidemiological and ophthalmic variables was recorded for every patient using a specified proforma. Genomic DNA from the blood samples collected from each participant in ethylenediaminetetraacetic acid (EDTA) vacutainers was isolated by using salting-out method[21] and used for polymorphic analysis using polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) technique.
Genotyping of Fok1 polymorphism
PCR amplification (Applied Biosystem-9800 Fast Thermal Cycler) of exon-2 of VDR gene was performed with 50 ng of genomic DNA, in 100 μl PCR reaction mixture containing 10 μl 10΄ PCR buffer [100 mM Tris-HCl (pH 8.8), 500 mM KCl, 15 mM MgCl2, 0.01% w/v gelatin]; 200 μM each of dATP, dGTP, dTTP, dCTP; 5.0 pmol of each primer and 2.5 U Taq polymerase, using the following set of primer sequences: forward F5’-AGCTGGCCCTGGCACTGACTCTGCTCT-3’ and reverse R5’-ATGGAAACACCTTGCTTCTTCTCCCTC-3’ to amplify 265 bp fragment.[22] The PCR cycling conditions included initial denatuaration at 94°C for 10 min, denaturation at 94°C for 45 s, annealing at 60°C for 45 s, and extension at 72°C for 45 s for 35 cycles with final extension at 72°C for 10 min. Amplified PCR products were checked on 2% agarose gel electrophoresis. The 265-bp PCR products derived from amplification of exon 2 constituting Fok1 restriction site were genotyped based on presence (f allele) or absence (F allele) of restriction site. Upon restriction digestion, F allele produces a single band of 265 bp while f allele produces 69 and 196 bp. Genotypes of the PCR products derived from individual subjects were presented as ff, Ff or FF. Five microliter aliquots of the PCR product were incubated at 37°C for 3 hours in a 10-μl reaction mixture containing reaction buffer (50 mmol/l NaCl, 10 mmol/l Tris-HCl (pH 7.5), 10 mmol/l MgCl2, 1 mmol/l 1,4-dithiothreitol) and 4 units of the restriction endonuclease Fok1 (New England Biolabs). Aliquots of the Fok1 digested products were electrophoresed for 30 min at 100 V on 3% agarose gel and genotyped based on the band sizes by comparing with standard 100 bp ladder [Figure 1].
Figure 1.
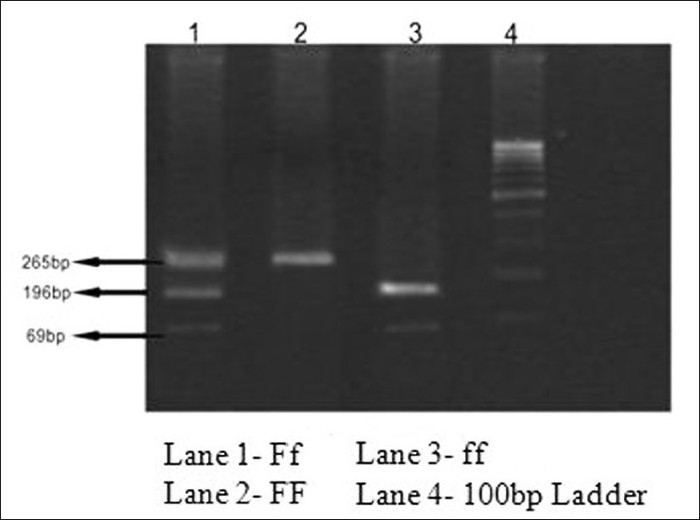
Gel picture of vitamin D3 receptor Fok1 genotypes
Statistical analysis
Statistical analyses were performed with Statistical Package for the Social Sciences (SPSS) 15.0. Allele and genotype frequencies were calculated and Chi-square test was used to test the significance of genotype distribution with myopia development. All the P values were two-sided and the level of significance was taken as P < 0.05.
Results
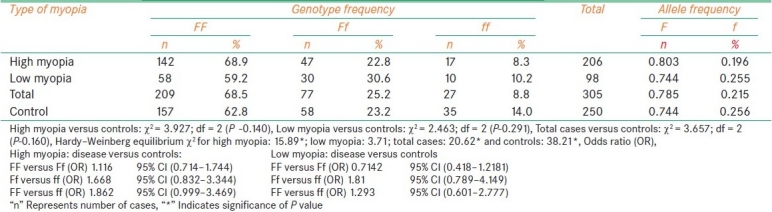
In the present study, the genotype distribution of Fok1 polymorphism of VDR gene in high myopia patients revealed considerable decrease in the frequency of ff homozygotes (8.3%) as compared to control group (14.0%), with a corresponding increase in frequency of FF homozygotes (68.9% in high myopia vs. 62.8% in controls) without much variation in heterozygote frequencies. When the comparison was made between high and low myopia cases (positive controls), the frequency of Ff and ff genotypes showed considerable decrease in the former compared to latter. Both high myopia cases and control cases deviated from Hardy-Weinberg equilibrium (χ2 = 15.89 for high myopia and χ2 = 38.21 for control group), while low myopia cases did not exhibit deviation from Hardy–Weinberg equilibrium. The allelic distribution revealed reduction in the f allele frequency in high myopia cases (0.196) as compared to that of control group (0.256). However, χ2 values for the distribution were insignificant for high (3.927), low (2.463) and pooled cases (3.657) [Table 1].To understand further the functional significance of this polymorphism, the genotype data were stratified with respect to epidemiological variables such as sex of the proband, age at onset, diet, presence of family history and parental consanguinity, and ophthalmic variables such as axial length (AL), anterior chamber depth (ACD), corneal thickness (CT) and corneal curvature (CC).
Table 1.
Genotype distribution of VDR gene (Fok1) polymorphism with respect to the type of myopia
The frequency of f allele carriers (Ff and ff) was found to be increased in females with high myopia (35.6%) and low myopia cases (45.4%) when compared to male probands (26.7% with high myopia, 34.9% of low myopia) which reflected in the increase of f allele frequency in female patients among high myopia (0.227) and low myopia (0.29). In comparison to low myopia females, the frequency of f allele carriers was found to be reduced in high myopia females [Table 2]. The frequency of ff genotype was found to be elevated in both high and low myopia patients with early age at onset (10.6% in high myopia and 14.3% in low) as compared to patients with age at onset >10 years (7.14%: 10/140 in high myopia and 9.52%: 8/84 in low myopia). However, the allelic distribution revealed higher frequency of f allele only in early age at onset cases of high myopia (0.227) and late age at onset (10-20 years) cases of low myopia (0.273) [Table 3]. The frequency of carriers was found to be increased in vegetarian group (40.3% high myopia, 44.5% low myopia) as compared to non-vegetarian group (27.4% high myopia, 38.3% low myopia) irrespective of the type of myopia [Table 4]. When parental consanguinity and familial incidence of myopia were considered, the frequency of ff genotype did not show variation with respect to familial incidence among high and low myopia groups [Table 5], but showed an increase in consanguineous (16.7%) group compared to non-consanguineous (9.3%) group in low myopia. The heterozygote (Ff) genotype frequency was also increased in consanguineous group of low myopia, reflecting a significant increase of f allele frequency (0.458) in low myopia cases with parental consanguinity compared to cases with no consanguinity (0.226). The P value (0.035) was also found to be significant (χ 2 value = 6.692) [Table 6]. However, high myopia cases did not show much varaition in the f allele frequency with respect to consanguinity. The mean distribution of ophthalmic variables with respect to Fok1 polymorphism in high and low myopia indicated elevated mean axial length and reduced ACD in low myopia patients with ff genotype.
Table 2.
Fok1 polymorphism and sex of the proband
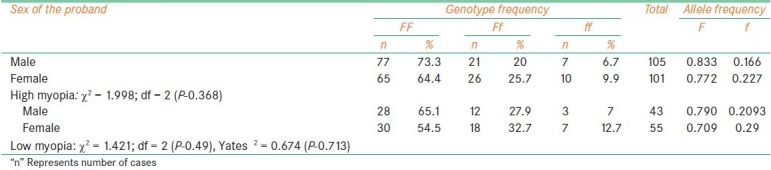
Table 3.
Fok1 polymorphism and age at onset
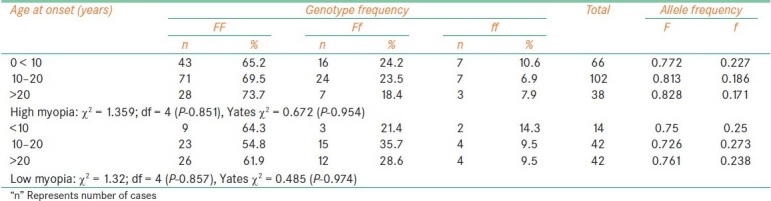
Table 4.
Fok1 polymorphism and diet
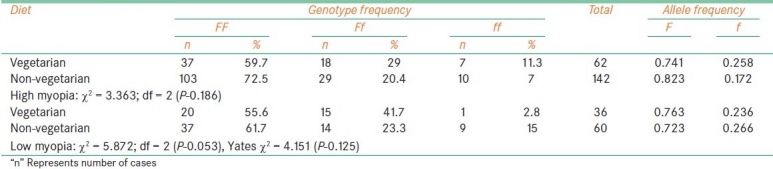
Table 5.
Fok1 polymorphism and familial incidence
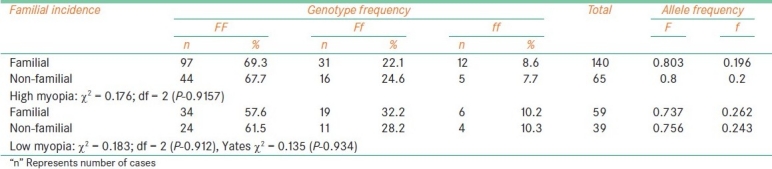
Table 6.
Fok1 polymorphism and parental consanguinity
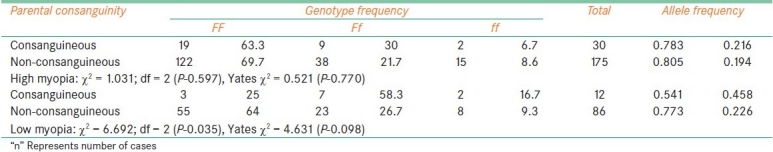
Discussion
Multiple well-coordinated biological functions operate with great precision to effect visual function, and therefore, the disturbances in any of the interrelated function would disturb the normal emmetropization mechanism of the eye, contributing to ocular refraction in myopia. The ciliary muscle is one of the most important extralenticular components of the eye involved in accommodation because its force deforms the crystalline lens to produce clear retinal imagery. It was suggested that a deficit in the sympathetic inputs to the ciliary muscle may be associated with a propensity for myopia development.[20,23] Excessive stress on the muscular function of ciliary muscles that can result due to mechanical factors, variations of ocular refractive components, defects in calcium homeostasis, bone mineralization, etc. may lead to defective accommodation and retinal defocus.[24,25] Certain interactive genes of ocular signal transduction pathway involved in these functions, such as VDR, appear to trigger myopia progression.
Few studies reported association between VDR mediated effects and myopia development. Recent studies have revealed the functional significance of VDR gene Fok1 polymorphism and its association with lower bone mineral density (BMD) and altered calcium metabolism,[26] but there are no previous studies on the association of VDR gene Fok1 polymorphism with myopia development till date.
The results of our study on Fok1 polymorphism could not indicate any genotype with specific risk for both high and low myopia, suggesting that VDR gene might not have a direct role in the development of myopia. However, observations with respect to sex distribution indicated that f allele might show sex-specific risk to develop myopia in females, especially low myopia. Age at onset is the most important risk factor in the development of high myopia. The mean age at onset among high myopia cases in our data was found to be lower (15.1 ± 0.672 years) as compared to low myopia cases who had mean age at onset of 19.75 ± 10.78 years. The frequency of f allele carriers (Ff and ff) was found to be increased in age at onset group 10-20 years in low myopia, indicating that the presence of f allele might confound the risk conferred by growth related factors involved in low myopia development during adolescence. With regard to diet, our observations suggest that f allele might trigger myopia development in individuals who are on vegetarian diet. The presence of f allele might have an interactive role with certain dietary components, and diet taken by these individuals might have exerted cumulative risk to develop myopia. Fok1 genotype distribution in high myopia cases did not show any association with respect to familial incidence, parental consanguinity and genetically influenced ophthalmic variables including AL and ACD, indicating clearly that VDR gene may not be a direct interacting factor for myopia. Another interesting observation is the significant association of f allele with low myopia cases showing parental consanguinity, and of ff genotype with mean ACD and mean AL in low myopia only. Since the development of low myopia is more related to mechanical stress factors, it is understood that the presence of f allele might contribute further to the risk in myopia causation and progression.
Functional studies suggest that the ff genotype results in a longer protein that is a less potent transcriptional activator.[18,27] The Fok1 site is located at N terminal region in exon 2 of the gene, and residues at N terminal of the first DNA binding zinc finger domain of VDR are vital for establishing contact with general transcription factor IIB. The encoded protein of F allele, which is shorter by three aminoacids, can form complex TFIIB more effectively so that elevated transcriptional regulation could be achieved compared to f allele.[28] This impaired or inefficient regulation by f allele might bring in alterations in signaling cascade affected by VDR protein. Further, it was reported that ff genotype individuals had lowered BMD[29] and are susceptible to certain diseases like breast cancer.[30] The relation between BMD and mechanical stress in ocular growth has been well documented.[31]
VDR is known to play significant role in the alteration of intracellular calcium levels which might result in impaired contraction and relaxation of ciliary muscles of the eye and defective neuromuscular transduction during myopia development. Apart from calcium homeostasis, it plays a role in bone formation due to altered mineral homeostasis that might influence ocular growth and scleral fibril architecture. It was suggested that VDR might suppress osteoblast differentiating factor, Runx2, to control osteogenesis.[32] In addition, VDR forms a heterodimer with Retinoid X receptor (RXR) which is involved in retinoid functions of the eye like accommodation, pupil responses, and aqueous humor production.[33] Hence, VDR is expected to have an indirect and functional role in the ocular growth and myopia development.
Conclusion
Although significant association was not observed between Fok1 polymorphism of VDR gene and high myopia, the increased frequency of f allele carriers observed among female probands (both high and low myopia), high myopia cases with early age at onset, low myopia cases with late age at onset and vegetarian group of both high and low myopia suggests that VDR gene might not be playing a direct role in myopia development but might contribute indirectly to the risk conferred by mechanical stress factors or growth/development related factors through its role in calcium homeostasis and regulation of ciliary muscle function. Large-scale studies, functional studies and studies of other VDR polymorphisms are necessary to understand the interaction of VDR and other ocular pathway proteins in the development of myopia.[34]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Wojciechowski R. Nature and nurture: The complex genetics of myopia and refractive error. Clin Genet. 2011;79:301–20. doi: 10.1111/j.1399-0004.2010.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young TL. Molecular Genetics of Human Myopia: An Update. Optom Vis Sci. 2009;86:E8–22. doi: 10.1097/OPX.0b013e3181940655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saw SM. A synopsis of the prevalence rates and environmental risk factors for myopia. Clin Exp Optom. 2003;86:289–94. doi: 10.1111/j.1444-0938.2003.tb03124.x. [DOI] [PubMed] [Google Scholar]
- 4.Xu L, Li J, Cui T, Hu A, Fan G, Zhang R, et al. Refractive error in urban and rural adult Chinese in Beijing. Ophthalmology. 2005;112:1676–83. doi: 10.1016/j.ophtha.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Saw SM, Chua WH, Wu HM, Yap E, Chia KS, Stone RA. Myopia: Gene-environment interaction. Ann Acad Med Singapore. 2000;29:290–7. [PubMed] [Google Scholar]
- 6.Lyhne N, Sjolie AK, Kyvik KO, Green A. The importance of genes and environment for ocular refraction and its determiners: A population based study among 20-45 year-old twins. Br J Ophthalmol. 2001;85:1470–6. doi: 10.1136/bjo.85.12.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammond CJ, Snieder H, Gilbert CE, Spector TD. Genes and environment in refractive error: The twin eye study. Invest Ophthalmol Vis Sci. 2001;42:1232–6. [PubMed] [Google Scholar]
- 8.Mutti DO, Semina E, Marazita M, Cooper M, Murray JC, Zadnik K. Genetic loci for pathological myopia are not associated with juvenile myopia. Am J Med Genet. 2002;112:355–60. doi: 10.1002/ajmg.10683. [DOI] [PubMed] [Google Scholar]
- 9.Ibay G, Doan B, Reider L, Dana D, Schlifka M, Hu H, et al. Candidate high myopia loci on chromosomes 18p and 12q do not play a major role in susceptibility to common myopia. BMC Med Genet. 2004;5:20. doi: 10.1186/1471-2350-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng TK, Lam CY, Lam DS, Chiang SW, Tam PO, Wang DY, et al. AC and AG dinucleotide repeats in the PAX6 P1 promoter are associated with high myopia. Mol Vis. 2009;15:2239–48. [PMC free article] [PubMed] [Google Scholar]
- 11.Dulhunty AF, Beard NA, Pouliquin P, Kimura T. Novel regulators of RyR Ca2+ release channels: Insight into molecular changes in genetically-linked myopathies. J Muscle Res Cell Motil. 2006;27:351–65. doi: 10.1007/s10974-006-9086-1. [DOI] [PubMed] [Google Scholar]
- 12.Lorenzon NM, Beam KG. Voltage-Gated Calcium Channels: Molecular Biology Intelligence Unit. In: Zamponi G, editor. Calcium Channelopathies. New York: KluwerAcademic/PlenumPublishers; 2005. pp. 240–61. [Google Scholar]
- 13.Kato S. The function of vitamin D receptor in vitamin D action. J Biochem. 2000;127:717–22. doi: 10.1093/oxfordjournals.jbchem.a022662. [DOI] [PubMed] [Google Scholar]
- 14.Crofts LA, Hancock MS, Morrison NA, Eisman JA. Multiple promoters the tissue- specific expression of novel N-terminal variant human vitamin D receptor gene transcripts. Proc Natl Acad Sci USA. 1998;95:10529–34. doi: 10.1073/pnas.95.18.10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyamoto K, Kesterson RA, Yamamoto H, Taketani Y, Nishikawa E, Tatsumi S, et al. Structural organization of the human vitamin D receptor chromosomal gene and its promoter. Mol Endocrinol. 1997;11:1165–79. doi: 10.1210/mend.11.8.9951. [DOI] [PubMed] [Google Scholar]
- 16.Zmuda JM, Cauley JA, Ferrell RE. Molecular epidemiology of Vitamin D receptor variants. Epidemiol Rev. 2000;22:203–17. doi: 10.1093/oxfordjournals.epirev.a018033. [DOI] [PubMed] [Google Scholar]
- 17.Kostner K, Denzer N, Muller CS, Klein R, Tilgen W, Reichrath J. The relevance of vitamin D receptor (VDR) gene polymorphisms for cancer: A review of the literature. Anticancer Res. 2009;29:3511–36. [PubMed] [Google Scholar]
- 18.Arai H, Miyamoto K, Taketani Y, Yamamoto H, Iemori Y, Morita K, et al. A vitamin D receptor gene polymorphism in the translation initiation codon: Effect on protein activity and relation to bone mineral density in Japanese women. J Bone Miner Res. 1997;12:915–21. doi: 10.1359/jbmr.1997.12.6.915. [DOI] [PubMed] [Google Scholar]
- 19.Baker AR, McDonnell DP, Hughes M, Crisp TM, Mangelsdorf DJ, Haussler MR, et al. Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc Natl Acad Sci USA. 1988;85:3294–8. doi: 10.1073/pnas.85.10.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen JC, Schmid KL, Brown B. The autonomic control of accommodation and implications for human myopia development: A review. Ophthalmic Physiol Opt. 2003;23:401–22. doi: 10.1046/j.1475-1313.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 21.Lahiri DK, Nurnberger JI., Jr A rapid non-enzymatic method for the preparation of HMW DNA from blood RFLP studies. Nucleic Acids Res. 1991;19:5444. doi: 10.1093/nar/19.19.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris SS, Eccleshall TR, Gross C, Dawson-Hughes B, Feldman D. The vitamin D receptorstart codon polymorphism (Fok1) and bone mineral density in premenopausal American Black and White women. J Bone Miner Res. 1997;12:1043–8. doi: 10.1359/jbmr.1997.12.7.1043. [DOI] [PubMed] [Google Scholar]
- 23.Gilmartin B, Bullimore MA. Adaptation of tonic accommodation to sustained visual tasks in emmetropia and late-onset myopia. Optom Vis Sci. 1991;68:22–6. doi: 10.1097/00006324-199101000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Wiederholt M, Thieme H, Stumpff F. The regulation of trabecular meshwork and ciliary muscle contractility. Prog Retin Eye Res. 2000;19:271–95. doi: 10.1016/s1350-9462(99)00015-4. [DOI] [PubMed] [Google Scholar]
- 25.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–68. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Gross C, Eccleshall TR, Malloy PJ, Villa ML, Marcus R, Feldman D. The presence of a polymorphism at the translation initiation site of the vitamin D receptor gene is associated with low bone mineral density in postmenopausal Mexican- American women. J Bone Miner Res. 1996;11:1850–5. doi: 10.1002/jbmr.5650111204. [DOI] [PubMed] [Google Scholar]
- 27.Colin EM, Weel AE, Uitterlinden AG, Buurman CJ, Birkenhager JC, Pols HA, et al. Consequences of vitamin D receptor gene polymorphisms for growth inhibition of cultured human peripheral blood mononuclear cells by 1,25-dihydroxyvitamin D3. Clin Endocrinol (Oxf) 2000;52:211–6. doi: 10.1046/j.1365-2265.2000.00909.x. [DOI] [PubMed] [Google Scholar]
- 28.Jurutka PW, Whitfield GK, Hsieh JC, Thompson PD, Haussler CA, Haussler MR. Molecular nature of the vitamin D receptor and its role in regulation of gene expression. Rev Endocr Metab Disord. 2001;2:203–16. doi: 10.1023/a:1010062929140. [DOI] [PubMed] [Google Scholar]
- 29.Kubota M, Yoshida S, IKieda M, Okada Y, Arai H, Miyamoto K, et al. Association between two types of vitamin D receptor gene polymorphism and bone status in premenopausal Japanese women. Calcif Tissue Int. 2001;68:16–22. doi: 10.1007/BF02684998. [DOI] [PubMed] [Google Scholar]
- 30.Chen WY, Bertone-Johnson ER, Hunter DJ, Willett WC, Hankinson SE. Associations between polymorphisms in the vitamin D receptor and breast cancer risk. Cancer Epidemiol Biomarkers prev. 2005;14:2335–9. doi: 10.1158/1055-9965.EPI-05-0283. [DOI] [PubMed] [Google Scholar]
- 31.Sakai A, Oshige T, Zenke Y, Yamanaka Y, Nagaishi H, Nakamura T. Unipedal standing exercise and hip bone mineral density in postmenopausal women: A randomized controlled trial. J Bone Miner Metab. 2009;28:42–8. doi: 10.1007/s00774-009-0100-8. [DOI] [PubMed] [Google Scholar]
- 32.Marcellini S, Bruna C, Henríquez JP, Albistur M, Reyes AE, Barriga EH, et al. Evolution of the interaction between Runx2 and VDR, two transcription factors involved in osteoblastogenesis. BMC Evol Biol. 2010;10:78–89. doi: 10.1186/1471-2148-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori M, Ghyselinck NB, Chambon P, Mark M. Systematic immunolocalization of retinoid receptors in developing and adult mouse eyes. Invest Ophthalmol Vis Sci. 2001;42:1312–8. [PubMed] [Google Scholar]
- 34.Horst-Sikorska W, Kalak R, Wawrzyniak A, Marcinkowska M, Celczynska-Bajew L, Slomski R. Association analysis of the polymorphisms of the VDR gene with bone mineral density and the occurrence of fractures. J Bone Miner Metab. 2007;25:310–9. doi: 10.1007/s00774-007-0769-5. [DOI] [PubMed] [Google Scholar]


