Abstract
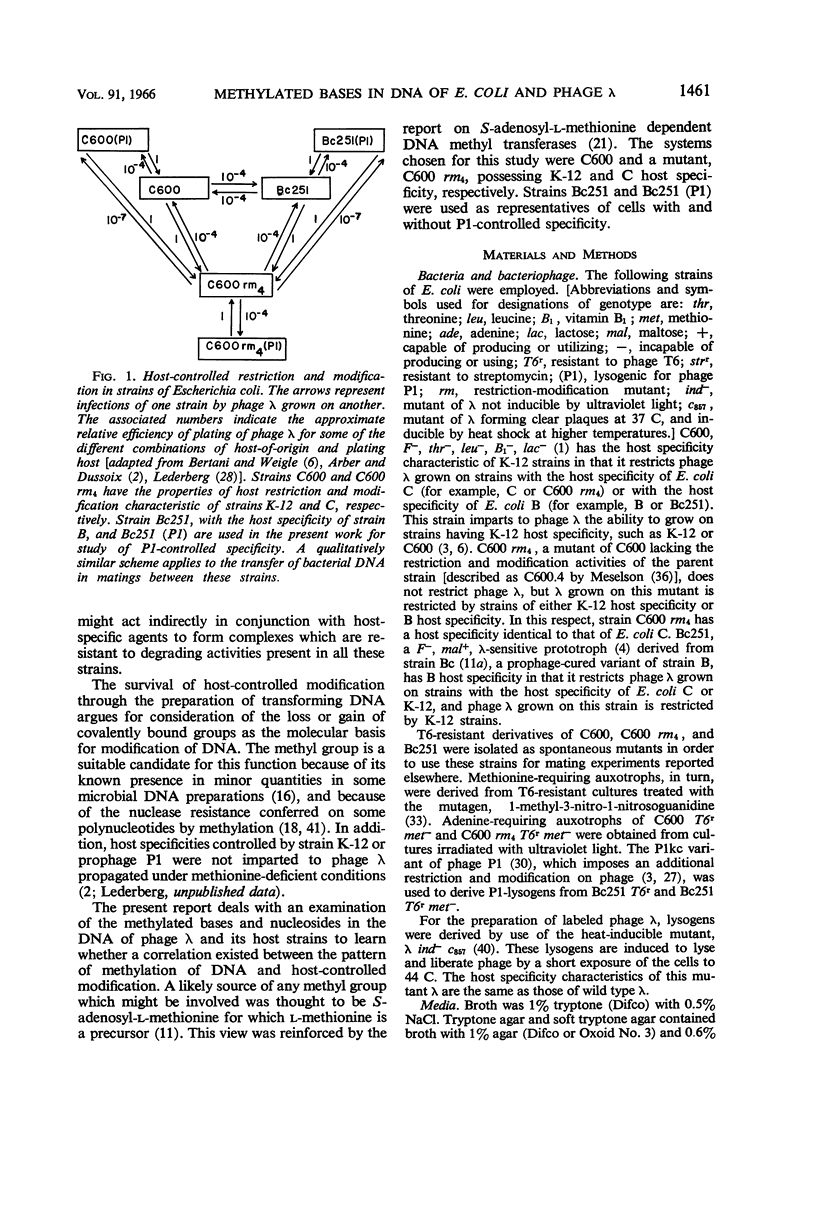
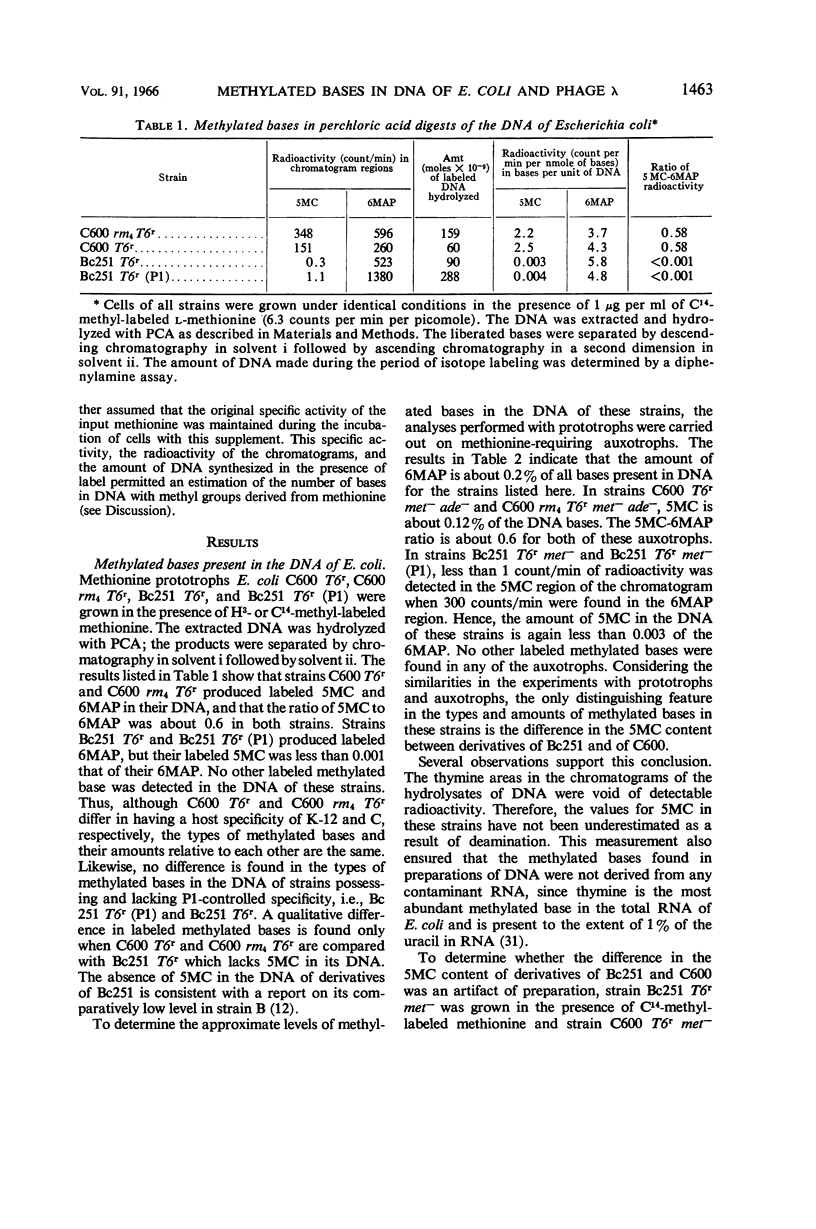
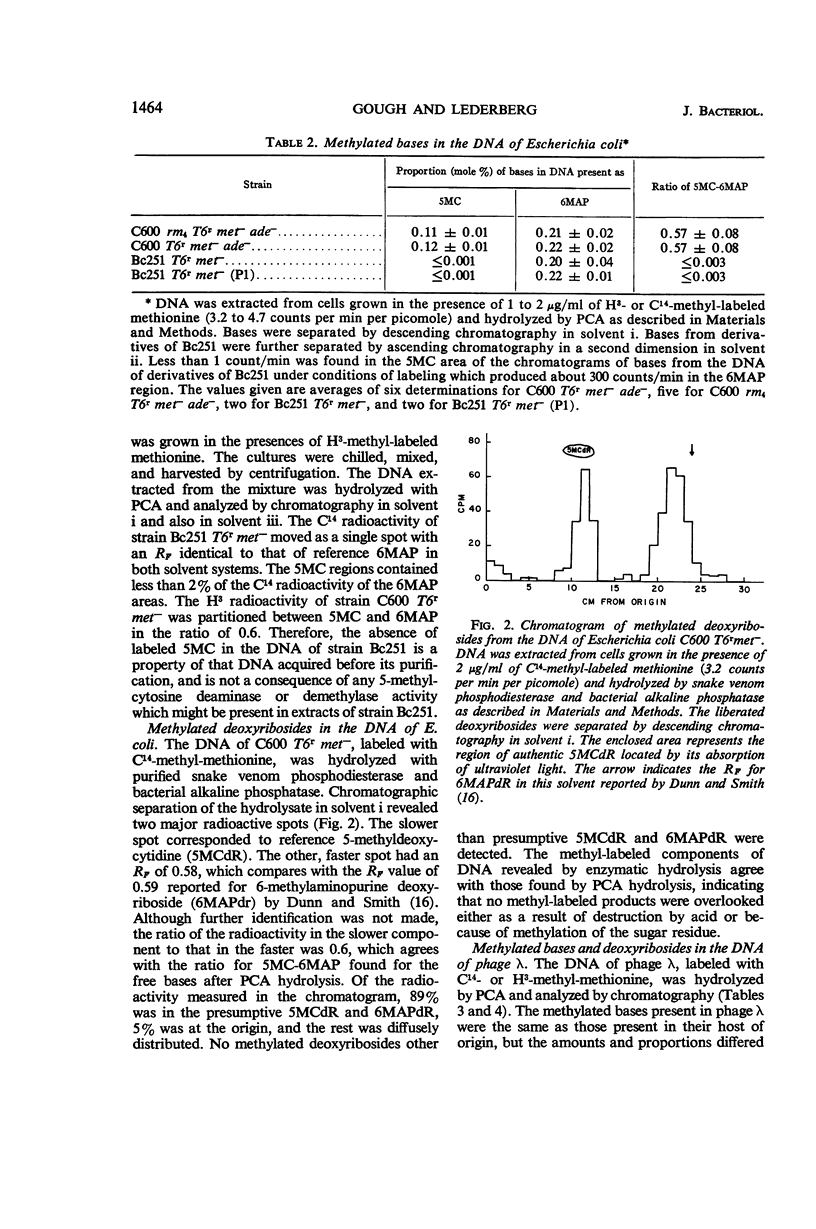
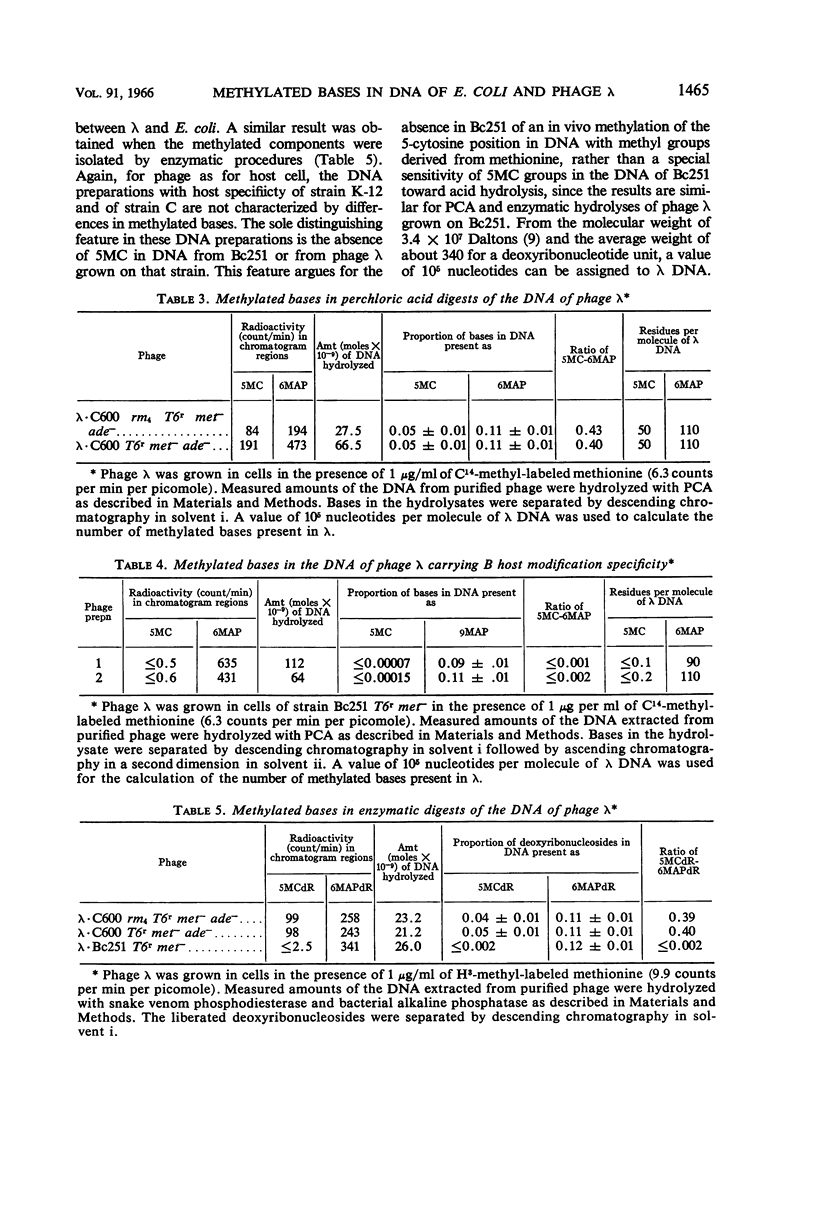
Gough, Michael (Brown University, Providence, R.I.), and Seymour Lederberg. Methylated bases in the host-modified deoxyribonucleic acid of Escherichia coli and bacteriophage λ. J. Bacteriol. 91:1460–1468. 1966.—The deoxyribonucleic acid (DNA) from strains of Escherichia coli and phage λ was examined to determine whether the types or amounts of methionine-derived methylated bases present correlated with the host-specific modification of that DNA. The DNA of strain C600 (which has K-12 modification specificity) and of a modificationless mutant of C600 are similar in their content of 5-methylcytosine and 6-methylaminopurine. Strains Bc251 and its P1-lysogen differ in P1-controlled specificity, but they have the same content of 6-methylaminopurine, and both lack 5-methylcytosine in their DNA. Phage λ contains the same methylated bases as its host of origin, but in reduced amounts and in different proportions. Although minor amounts of these methylated bases may have importance as a result of their location, the presence of the majority of these methylated bases is irrelevant to the specificity of host modification of DNA.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W., DUSSOIX D. Host specificity of DNA produced by Escherichia coli. I. Host controlled modification of bacteriophage lambda. J Mol Biol. 1962 Jul;5:18–36. doi: 10.1016/s0022-2836(62)80058-8. [DOI] [PubMed] [Google Scholar]
- ARBER W. HOST SPECIFICITY OF DNA PRODUCED BY ESCHERICHIA COLI V . THE ROLE OF METHIONINE IN THE PRODUCTION OF HOST SPECIFICITY. J Mol Biol. 1965 Feb;11:247–256. doi: 10.1016/s0022-2836(65)80055-9. [DOI] [PubMed] [Google Scholar]
- ARBER W., LATASTE-DOROLLE C. [Enlargement of the host area of bacteriophage lambda for Escherichia coli B]. Pathol Microbiol (Basel) 1961;24:1012–1018. [PubMed] [Google Scholar]
- ARBER W., MORSE M. L. HOST SPECIFICITY OF DNA PRODUCED BY ESCHERICHIA COLI. VI. EFFECTS ON BACTERIAL CONJUGATION. Genetics. 1965 Jan;51:137–148. doi: 10.1093/genetics/51.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTANI G., WEIGLE J. J. Host controlled variation in bacterial viruses. J Bacteriol. 1953 Feb;65(2):113–121. doi: 10.1128/jb.65.2.113-121.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOICE L. B., LURIA S. E. Behavior of prophage P1 in bacterial matings. I. Transfer of the defective prophage P1 dl. Virology. 1963 May;20:147–157. doi: 10.1016/0042-6822(63)90150-8. [DOI] [PubMed] [Google Scholar]
- BOYER H. GENETIC CONTROL OF RESTRICTION AND MODIFICATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Dec;88:1652–1660. doi: 10.1128/jb.88.6.1652-1660.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATONI G. L. S-Adenosylmethionine; a new intermediate formed enzymatically from L-methionine and adenosinetriphosphate. J Biol Chem. 1953 Sep;204(1):403–416. [PubMed] [Google Scholar]
- COHEN D. A variant of phage P2 originating in Escherichia coli, strain B. Virology. 1959 Jan;7(1):112–126. doi: 10.1016/0042-6822(59)90180-1. [DOI] [PubMed] [Google Scholar]
- DOSKOCIL J., SORMO'VA Z. THE OCCURRENCE OF 5-METHYLCYTOSINE IN BACTERIAL DEOXYRIBONUCLEIC ACIDS. Biochim Biophys Acta. 1965 Mar 15;95:513–515. [PubMed] [Google Scholar]
- DUNN D. B., SMITH J. D. The occurrence of 6-methylaminopurine in deoxyribonucleic acids. Biochem J. 1958 Apr;68(4):627–636. doi: 10.1042/bj0680627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUSSOIX D., ARBER W. HOST SPECIFICITY OF DNA PRODUCED BY ESCHERICHIA COLI. IV. HOST SPECIFICITY OF INFECTIOUS DNA FROM BACTERIOPHAGE LAMBDA. J Mol Biol. 1965 Feb;11:238–246. doi: 10.1016/s0022-2836(65)80054-7. [DOI] [PubMed] [Google Scholar]
- Doskocil J., Sormová Z. The sequences of 5-methylcytosine in the DNA of Escherichia coli. Biochem Biophys Res Commun. 1965 Jul 26;20(3):334–339. doi: 10.1016/0006-291x(65)90369-4. [DOI] [PubMed] [Google Scholar]
- Duerre J. A., Bowden P. M. Utilization of ribosylhomocysteine by various microorganisms. Biochem Biophys Res Commun. 1964 Jun 1;16(2):150–155. doi: 10.1016/0006-291x(64)90353-5. [DOI] [PubMed] [Google Scholar]
- EGAMI F., ISHIHARA H., SHIMOMURA M. Uber die methylierte Heferibonucleinsäure. Hoppe Seylers Z Physiol Chem. 1953;295:349–354. doi: 10.1515/bchm2.1953.295.1.349. [DOI] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- HATCH F. T., LARRABEE A. R., CATHOU R. E., BUCHANAN J. M. Enzymatic synthesis of the methyl group of methionine. I. Identification of the enzymes and cofactors involved in the system isolated from Escherichia coli. J Biol Chem. 1961 Apr;236:1095–1101. [PubMed] [Google Scholar]
- KAISER A. D., HOGNESS D. S. The transformation of Escherichia coli with deoxyribonucleic acid isolated from bacteriophage lambda-dg. J Mol Biol. 1960 Dec;2:392–415. doi: 10.1016/s0022-2836(60)80050-2. [DOI] [PubMed] [Google Scholar]
- KLEIN A., SAUERBIER W. ROLE OF METHYLATION IN HOST CONTROLLED MODIFICATION OF PHAGE T1. Biochem Biophys Res Commun. 1965 Feb 3;18:440–445. doi: 10.1016/0006-291x(65)90728-x. [DOI] [PubMed] [Google Scholar]
- LEDERBERG S. Suppression of the multiplication of heterologous bacteriophages in lysogenic bacteria. Virology. 1957 Jun;3(3):496–513. doi: 10.1016/0042-6822(57)90006-5. [DOI] [PubMed] [Google Scholar]
- LEDINKO N. OCCURRENCE OF 5-METHYLDEOXYCYTIDYLATE IN THE DNA OF PHAGE LAMBDA. J Mol Biol. 1964 Sep;9:834–835. doi: 10.1016/s0022-2836(64)80191-1. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W., DUNN D. B. The occurrence and distribution of thymine and three methylated-adenine bases in ribonucleic acids from several sources. Biochem J. 1958 Dec;70(4):642–651. doi: 10.1042/bj0700642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LURIA S. E. Host-induced modifications of viruses. Cold Spring Harb Symp Quant Biol. 1953;18:237–244. doi: 10.1101/sqb.1953.018.01.034. [DOI] [PubMed] [Google Scholar]
- Lederberg S. Host-controlled restriction and modification of deoxyribonucleic acid in Escherichia coli. Virology. 1965 Nov;27(3):378–387. doi: 10.1016/0042-6822(65)90117-0. [DOI] [PubMed] [Google Scholar]
- MANDELL J. D., GREENBERG J. A new chemical mutagen for bacteria, 1-methyl-3-nitro-1-nitrosoguanidine. Biochem Biophys Res Commun. 1960 Dec;3:575–577. doi: 10.1016/0006-291x(60)90064-4. [DOI] [PubMed] [Google Scholar]
- MARKHAM R., SMITH J. D. Chromatographic studies of nucleic acids; a technique for the identification and estimation of purine and pyrimidine bases, nucleosides and related substances. Biochem J. 1949;45(3):294–298. doi: 10.1042/bj0450294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSHAK A., VOGEL H. J. Microdetermination of purines and pyrimidines in biological materials. J Biol Chem. 1951 Apr;189(2):597–605. [PubMed] [Google Scholar]
- MESELSON M. ON THE MECHANISM OF GENETIC RECOMBINATION BETWEEN DNA MOLECULES. J Mol Biol. 1964 Sep;9:734–745. doi: 10.1016/s0022-2836(64)80178-9. [DOI] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- SUSSMAN R., JACOB F. [On a thermosensitive repression system in the Escherichia coli lambda bacteriophage]. C R Hebd Seances Acad Sci. 1962 Feb 19;254:1517–1519. [PubMed] [Google Scholar]
- SZER W., SHUGAR D. N-Methylation of uridylic acid and preparation of oligonucleotides of 3-methyluridylic acid. Acta Biochim Pol. 1960;7:491–504. [PubMed] [Google Scholar]
- WILLIAMS E. J., SUNG S. C., LASKOWSKI M., Sr Action of venom phosphodiesterase on deoxyribonucleic acid. J Biol Chem. 1961 Apr;236:1130–1134. [PubMed] [Google Scholar]


