Abstract
Leaf blight caused by Calonectria spp. is an important disease occurring on Eucalyptus trees grown in plantations of Southeast Asia. Symptoms of leaf blight caused by Calonectria spp. have recently been observed in commercial Eucalyptus plantations in FuJian Province in Southeast China. The aim of this study was to identify these Calonectria spp. employing morphological characteristics, DNA sequence comparisons for the β-tubulin, histone H3 and translation elongation factor-1α gene regions and sexual compatibility. Four Calonectria spp. were identified, including Ca. pauciramosa and three novel taxa described here as Ca. crousiana, Ca. fujianensis and Ca. pseudocolhounii. Inoculation tests showed that all four Calonectria spp. found in this study were pathogenic on two different E. urophylla × E. grandis hybrid clones, commercially utilised in eucalypt plantations in China.
Keywords: Cylindrocladium, Eucalyptus plantations, FuJian, pathogenicity
INTRODUCTION
Species of Calonectria (Ca.) (anamorph state: Cylindrocladium (Cy.)) are pathogenic to a wide range of plant hosts in tropical and subtropical areas of the world (Crous & Wingfield 1994, Crous 2002). Symptoms associated with infection by these fungi include stem cankers, leaf and shoot blight as well as root rot on many agronomic and forestry crop plants (Crous 2002, Old et al. 2003, Crous et al. 2004b, Lechat et al. 2010). Calonectria spp., particularly in their Cylindrocladium anamorph form, are especially well-known as pathogens of Eucalyptus trees in plantations where they cause the disease known as Cylindrocladium leaf blight (CLB) (Sharma & Mohanan 1991, 1992, Booth et al. 2000, Crous 2002, Old et al. 2003, Rodas et al. 2005). These fungi are also important causal agents of cutting rot and seedling blight in Eucalyptus nurseries (Sharma et al. 1984, Crous et al. 1991, Crous 2002, Old et al. 2003, Lombard et al. 2010c, d).
Symptoms of CLB on Eucalyptus include both leaf blotch and shoot blight, which develops upwards from the base of the trees and can result in tree mortality due to defoliation (Crous 2002, Old et al. 2003, Rodas et al. 2005). Symptoms begin as water-soaked lesions on young and mature leaves on the lower branches. These lesions coalesce and develop into extensive necrotic areas very rapidly. Under conditions of high humidity and frequent rainfall, the lesions can cover the entire leaf surface and infection of young shoot tips can result in dramatic blight. Defoliation typically moves upwards from the base and centres of affected trees and this can result in total defoliation of trees (Crous 2002, Old et al. 2003, Rodas et al. 2005). Severely affected trees can suffer reduction in growth vigour, with crowns and main stems becoming deformed (Booth et al. 2000, Old et al. 2003).
In South and Southeast Asia, CLB is one of the most prominent diseases associated with Eucalyptus trees grown in commercial plantations (Old et al. 2003). In these regions, CLB is caused by several Calonectria spp., including Ca. asiatica, Ca. brassicae, Ca. hurae, Ca. ilicicola, Ca. indusiata, Ca. kyotensis, Ca. multiseptata, Ca. pauciramosa, Ca. pteridis, Ca. reteaudii and Ca. sumatrensis (Sharma et al. 1984, Booth et al. 2000, Kang et al. 2001, Crous 2002, Old et al. 2003, Crous et al. 2004b). Of these Calonectria spp., Ca. reteaudii is regarded as the most important pathogen and it occurs primarily on Eucalyptus trees in tropical regions of Southeast Asia and India (Booth et al. 2000, Kang et al. 2001, Crous 2002, Old et al. 2003).
Commercial plantations of Eucalyptus are distributed over 19 provinces in Central and South China (Qi 2006). Approximately 2.6 M ha of Eucalyptus plantations have recently been established in GuangXi, GuangDong, HaiNan, FuJian and YunNan Provinces (Xie 2006, Iglesias-Trabad & Wilstermann 2008), to meet the high demand in pulp products in China. Similar to the situation in other countries (Wingfield et al. 2008), these trees are affected by pests and diseases, for which limited information is available in China (Zhou et al. 2008). Leaf and shoot blight caused by Calonectria spp. is regarded as one of the most serious threats to commercial Eucalyptus plantations and nurseries in this country (Wang 1992, Sun & Liu 2004, Zhou et al. 2008, Lombard et al. 2010d). Recent surveys of tree diseases in the FuJian Province in Southeast China revealed numerous examples of CLB on Eucalyptus spp. The aim of this study was to determine the identity of the Calonectria spp. collected from these trees. In addition, the pathogenicity of selected isolates was tested on various Eucalyptus clones commercially grown in China.
MATERIAL AND METHODS
Isolates
Eucalyptus leaves showing symptoms of CLB were collected from commercially propagated Eucalyptus trees in plantations in FuJian Province in 2007 (Table 1). Conidial masses were transferred directly from infected leaves to malt extract agar (2 % w/v; MEA: Biolab Diagnostic Ltd., Midrand, South Africa) and incubated at 25 °C under continuous near-ultraviolet light for 7 d. Isolates were transferred to MEA and further incubated at 25 °C for 7 d. Single conidial isolates were prepared and lodged in the culture collection (CMW) of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, South Africa (Table 1), and a duplicate set of isolates is maintained in a culture collection housed at the China Eucalypt Research Centre (CERC), Chinese Academy of Forestry (CAF), China. Representative isolates were also deposited with the Centraalbureau voor Schimmelcultures (CBS), Utrecht, The Netherlands (Table 1), and herbarium specimens in the National Collection of Fungi (PREM), Pretoria, South Africa.
Table 1.
Isolates used in the phylogenetic analyses and pathogenicity trials.
| Species | Isolate number 1 | β-tubulin 2 | Histone H3 2 | TEF-1α 2 | Host | Origin | Collector |
|---|---|---|---|---|---|---|---|
| Calonectria acicola | CBS 114812 | DQ190590 | DQ190692 | GQ267291 | Phoenix canariensis | New Zealand | H. Pearson |
| CBS 114813 T | DQ190591 | DQ190693 | GQ267292 | P. canariensis | New Zealand | H. Pearson | |
| Ca. brachiatica | CMW 25302 | FJ716708 | FJ716712 | GQ267295 | Pinus tecunumanii | Colombia | M.J. Wingfield |
| CBS 123700 (= CMW25298) T | FJ696388 | FJ696396 | GQ267296 | P. maximinoi | Colombia | M.J. Wingfield | |
| Ca. brassicae | CBS 111869 T | AF232857 | DQ190720 | FJ918568 | Argyreia sp. | Southeast Asia | |
| CBS 111478 | DQ190611 | DQ190719 | FJ918567 | Soil | Brazil | A.C. Alfenas | |
| Ca. cerciana | CBS 123639 (= CMW 25309) T | FJ918510 | FJ918528 | FJ918559 | Eucalyptus urophylla × E. grandis cutting | GuangDong, China | M.J. Wingfield & X.D. Zhou |
| CBS 123695 (= CMW 25290) | FJ918511 | FJ918529 | FJ918560 | E. urophylla × E. grandis cutting | GuangDong, China | M.J. Wingfield & X.D. Zhou | |
| Ca. chinensis | CBS 112744 | AY725618 | AY725660 | AY725709 | Soil | China | E.C.Y. Liew |
| Ca. colhounii | CBS 293.79 (= CMW 30999) T | DQ190564 | DQ190639 | GQ267301 | – | Indonesia | — |
| CBS 114704 | DQ190563 | DQ190638 | GQ267300 | Arachis pintoi | Australia | D. Hutton | |
| Ca. colombiana | CBS 115638 | FJ972422 | FJ972441 | FJ972491 | Soil | Colombia | M.J. Wingfield |
| CBS 115127 | FJ972423 | FJ972442 | FJ972492 | Soil | Colombia | M.J. Wingfield | |
| Ca. colombiensis | CBS 112221 | AY725620 | AY725663 | AY725712 | Soil | Colombia | M.J. Wingfield |
| Ca. crousiana | CMW 27249 A T (= CBS 127198) | HQ285794 | HQ285808 | HQ285822 | E. grandis | FuJian, China | M.J. Wingfield |
| CMW 27253 (= CBS 127199) | HQ285795 | HQ285809 | HQ285823 | E. grandis | FuJian, China | M.J. Wingfield | |
| CMW 27258 | HQ285796 | HQ285810 | HQ285824 | E. grandis | FuJian, China | M.J. Wingfield | |
| CMW 27267 A (= CBS 127203) | HQ285797 | HQ285811 | HQ285825 | E. grandis | FuJian, China | M.J. Wingfield | |
| Ca. eucadoriae | CBS 111394 | DQ190599 | DQ190704 | GQ267304 | Soil | Ecuador | M.J. Wingfield |
| CBS 111406 | DQ190600 | DQ190705 | GQ267303 | Soil | Ecuador | M.J. Wingfield | |
| Ca. eucalypti | CBS 125273 (= CMW 14890) | GQ267217 | GQ267266 | GQ267337 | E. grandis | Indonesia | M.J. Wingfield |
| CBS 125275 (= CMW 18444) T | GQ267218 | GQ267267 | GQ267338 | E. grandis | Indonesia | M.J. Wingfield | |
| Ca. fujianensis | CMW 27254 A (= CBS 127200) | HQ285791 | HQ285805 | HQ285819 | E. grandis | FuJian, China | M.J. Wingfield |
| CMW 27257 A T (= CBS 127201) | HQ285792 | HQ285806 | HQ285820 | E. grandis | FuJian, China | M.J. Wingfield | |
| CMW 27263 A (= CBS 127202) | HQ285793 | HQ285807 | HQ285821 | E. grandis | FuJian, China | M.J. Wingfield | |
| Ca. insulare | CBS 114558 | AF210861 | FJ918526 | FJ918556 | Soil | Madagascar | P.W. Crous |
| CBS 114559 | AF210862 | FJ918525 | FJ918555 | Soil | Madagascar | C. L. Schoch | |
| Ca. macroconidialis | CBS 114880 T | AF232855 | DQ190655 | GQ267313 | E. grandis | South Africa | P.W. Crous |
| Ca. madagascariensis | CBS 114572 (= CMW23686) T | DQ190572 | DQ190658 | GQ267314 | – | Madagascar | P.W. Crous |
| CBS 114571 (= CMW 30993) | DQ190571 | DQ190657 | GQ267315 | – | Madagascar | P.W. Crous | |
| Ca. morganii | CBS 110666 | FJ918509 | FJ918527 | FJ918557 | Rosa sp. | USA | N.E. Ell-Gholl |
| Ca. multiseptata | CBS 112682 T | DQ190573 | DQ190659 | FJ918535 | Eucalyptus sp. | Indonesia | M.J. Wingfield |
| Ca. pauciramosa | CMW 30823 | FJ918515 | FJ918532 | FJ918566 | E. grandis | South Africa | P.W. Crous |
| CMW 5683 | FJ918514 | FJ918531 | FJ918565 | Eucalyptus sp. | Brazil | A.C. Alfenas | |
| CMW 27199 A | HQ285784 | HQ285798 | HQ285812 | E. dunnii | FuJian, China | M.J. Wingfield | |
| CMW 27203 | HQ285785 | HQ285799 | HQ285813 | E. dunnii | FuJian, China | M.J. Wingfield | |
| CMW 27283 | HQ285786 | HQ285800 | HQ285814 | E. dunnii | FuJian, China | M.J. Wingfield | |
| CMW 27292 A | HQ285787 | HQ285801 | HQ285815 | E. dunnii | FuJian, China | M.J. Wingfield | |
| Ca. polizzii | CMW 7804 | FJ972417 | FJ972436 | FJ972486 | Callistemon citrinus | Italy | G. Polizzi |
| CMW 10151 | FJ972418 | FJ972437 | FJ972487 | Arbustus unedo | Italy | G. Polizzi | |
| Ca. pseudocolhounii | CMW 27209 A T (= CBS 127195) | HQ285788 | HQ285802 | HQ285816 | E. dunnii | FuJian, China | M.J. Wingfield |
| CMW 27213 A (= CBS 127196) | HQ285789 | HQ285803 | HQ285817 | E. dunnii | FuJian, China | M.J. Wingfield | |
| CMW 27214 A (= CBS 127197) | HQ285790 | HQ285804 | HQ285818 | E. dunnii | FuJian, China | M.J. Wingfield | |
| Ca. pseudoreteaudii | CBS 123694 (= CMW 25310) T | FJ918504 | FJ918519 | FJ918541 | E. urophylla × E. grandis cutting | GuangDong, China | M.J. Wingfield & X.D. Zhou |
| CBS 123696 (= CMW 25296) | FJ918505 | FJ918520 | FJ918542 | E. urophylla × E. grandis cutting | GuangDong, China | M.J. Wingfield & X.D. Zhou | |
| Ca. pteridis | CBS 111793 | DQ190578 | DQ190679 | FJ918563 | Arachnoides adiantiformis | USA | – |
| CBS 111871 | DQ190579 | DQ190680 | FJ918564 | Pinus sp. | Spain | T.L. Krugner | |
| Ca. queenslandica | CBS 112146 (= CMW30604) T | AF389835 | FJ918521 | FJ918543 | E. urophylla | Australia | B. Brown |
| CBS 112155 (= CMW30603) | AF389834 | DQ190667 | FJ918544 | E. pellita | Australia | K.M. Old | |
| Ca. reteaudii | CBS 112144 T | AF389833 | DQ190661 | FJ918537 | E. camaldulensis | Vietnam | M.J. Dudzinski |
| CBS 112143 | GQ240642 | DQ190660 | FJ918536 | E. camaldulensis | Vietnam | M.J. Dudzinski | |
| Ca. spathulata | CBS 112689 | AF308463 | FJ918524 | FJ918554 | E. viminalis | Brazil | N.E. Ell-Gholl |
| CBS 555.92 | GQ267215 | GQ267261 | GQ267331 | Araucaria angustifolia | Brazil | C. Hodges | |
| Ca. terraereginae | CBS 112151 (= CMW 30601) T | FJ918506 | FJ918522 | FJ918545 | E. urophylla | Australia | C. Hanwood |
| CBS 112634 (= CMW 30602) | FJ918507 | DQ190668 | FJ918546 | Xanthorrhoea australis | Australia | T. Baigent | |
| Ca. zuluensis | CMW 9188 T | FJ972414 | FJ972433 | FJ972483 | E. grandis × E. urophylla cutting | South Africa | L. Lombard |
| CMW 9896 | FJ972415 | FJ972434 | FJ972484 | E. grandis × E. urophylla cutting | South Africa | L. Lombard |
1 CBS: Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; CMW: culture collection of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa; isolate number in bold were sequenced in this study.
2 GenBank accession numbers.
A Isolates used for pathogenicity tests on Eucalyptus seedlings in China.
T Ex-type cultures.
DNA sequence comparisons
Single conidial cultures (Table 1) were grown on MEA for 7 d at 25 °C. Total genomic DNA was extracted using the method described by Smith et al. (2001). Three loci were amplified, using the primers T1 (O’Donnell & Cigelnik 1997) and Bt-2b (Glass & Donaldson 1995) to amplify a fragment of the β-tubulin (BT) gene region, part of the histone H3 (HIS3) gene region with primers H3-1a and H3-1b (Glass & Donaldson 1995), and primers EF1-728F and EF1-986R (Carbone & Kohn 1999) to amplify a fragment of the translation elongation factor-1 alpha (TEF-1α) gene region.
The PCR mixtures used to amplify the different loci consisted of 2.5 units Fast Start Taq polymerase (Roche Applied Science, USA), 1 × PCR buffer, 1–1.5 mM MgCl2, 0.25 mM of each dNTP, 0.5 μm of each primer and approximately 30 ng of fungal genomic DNA, made up to a total reaction volume of 25 μL with sterile de-ionised water. Amplified fragments were purified using High Pure PCR Product Purification Kit (Roche, USA) and sequenced in both directions with the same primers used for the DNA amplifications. For this purpose, the BigDye terminator sequencing kit v3.1 (Applied Biosystems, USA) and an ABI PRISMTM 3100 DNA sequencer (Applied Biosystems, USA) were used. All PCRs and sequencing reactions were performed on an Eppendorf Mastercycler Personal PCR (Eppendorf AG, Germany) with cycling conditions as described in Crous et al. (2004b, 2006) for all loci amplified.
Sequences generated were added to other sequences for Calonectria obtained from GenBank (http://www.ncbi.nlm.nih.gov) and were assembled and aligned using Sequence Navigator v1.0.1 (Applied Biosystems, USA) and MAFFT v5.11 (Katoh et al. 2002), respectively. The aligned sequences were then manually corrected where needed. Single nucleotide polymorphisms (SNPs) were determined for each gene region analysed using DnaSP v5.00.07 (Librado & Rozas 2009).
PAUP (Phylogenetic Analysis Using Parsimony, v4.0b10; Swofford 2002) was used to analyse the DNA sequence datasets. A partition homogeneity test (Farris et al. 1994) and a 70 % reciprocal bootstrap method (Mason-Gamer & Kellog 1996, Gueidan et al. 2007) were applied to evaluate the feasibility of combining the datasets. Phylogenetic relationships were estimated by heuristic searches based on 1 000 random addition sequences and tree bisection-reconnection, with the branch swapping option set on ‘best trees’ only.
All characters were weighed equally and alignment gaps were treated as missing data. Measures calculated for parsimony included tree length (TL), consistency index (CI), retention index (RI) and rescaled consistence index (RC). Bootstrap analyses (Hillis & Bull 1993) were based on 1 000 replications. The phylogenetic analyses included 57 partial gene sequences per gene, representing 27 Calonectria species (Table 1) closely related to the isolates studied. Calonectria colombiensis (CBS 112221) and Ca. chinensis (CBS 112744) were used as the outgroup taxa (Lombard et al. 2009, 2010d). All sequences were deposited in GenBank and the alignments in TreeBASE (http://www.treebase.org).
A Markov Chain Monte Carlo (MCMC) algorithm was used to generate phylogenetic trees with Bayesian probabilities using MrBayes v3.1.1 (Ronquist & Huelsenbeck 2003) for the combined sequence datasets. Models of nucleotide substitution for each of the three genes were determined using MrModeltest (Nylander 2004) and included for each gene partition, which were used for the combined sequence analyses. Two independent runs of four MCMC chains were run simultaneously from random trees for 1 000 000 generations and sampled every 100 generations for the combined analysis of the gene partitions. Both runs converged on the same likelihood score and tree topology, and the first 1 000 trees were discarded as the burn-in phase of each analysis. Posterior probabilities were determined from the remaining 9 000 trees.
Sexual compatibility
Single conidial Calonectria isolates of unknown identity from China were crossed among themselves in all possible combinations. Crosses were made as described in Schoch et al. (1999) on minimal salt agar (MN) to which sterile tooth picks had been placed on the agar surface (Guerber & Correll 2001, Lombard et al. 2010a, b, d). Controls were of isolates crossed with themselves and it was thus also possible to distinguish between those species with heterothallic or homothallic mating systems. The plates were stacked in plastic containers and incubated at 20 °C for 6–8 wk. Crosses were regarded as successful when isolate combinations produced perithecia extruding viable ascospores.
Taxonomy
For morphological identification of Calonectria isolates, single conidial isolates were prepared on MEA and synthetic nutrient-poor agar (SNA) (Nirenburg 1981, Lombard et al. 2009, 2010a, b, d). Inoculated plates were incubated at room temperature and examined after 7 d. Gross morphological characteristics of the anamorph state were determined by mounting fungal structures in lactic acid and 30 measurements at ×1 000 magnification were made for each isolate. Teleomorph morphology was determined by mounting perithecia obtained from the sexual compatibility tests in Leica mountant (Setpoint Premier, Johannesburg, South Africa) and hand-sectioned with a Leica CM1100 cryostat (Setpoint Technologies) at −20 °C. The 12 μm sections were mounted in lactophenol and 3 % KOH. Gross morphological characteristics were determined as mentioned for the anamorph state. The 95 % confidence levels were determined and extremes of measurements are given in parentheses.
Optimal growth conditions for cultures were determined in the dark on MEA for each isolate, at temperatures ranging from 5–35 °C at 5 °C intervals. This was repeated three times for each isolate examined. Colony colours were determined after 7 d on MEA at 25 °C in the dark, using the colour charts of Rayner (1970). All descriptions, illustrations and nomenclatural data were deposited in MycoBank (www.mycobank.org; Crous et al. 2004a).
Pathogenicity tests
In order to test the pathogenicity of the Calonectria spp. collected in this study, 10 profusely sporulating isolates, representing different Calonectria species identified based on morphology and DNA sequence comparisons were selected for inoculation trails (Table 1). The isolates were transferred to MEA, and incubated for 10 d at 25 °C. A spore suspension was prepared for each isolate, by adding 2 mL of sterile water to the plates and dislodging conidia with a sterile glass rod. The spore suspension was strained through a layer of cheesecloth and the concentration adjusted to 3.3 × 105 conidia/mL. To ensure that conidia would adhere to the surface of the inoculated leaves, 2 mL Tween 80 (ChangJiang JingXi HuaGongChang, GuangZhou, China) was added to the suspension.
Two E. urophylla × E. grandis hybrid clones, CEPT-9 and CEPT-10 (height 30–40 cm), selected for inoculation, were acclimatised for 2 wk in a shade house subjected to natural climatic conditions (temperature 26–32 °C and humidity 60–90 %). For each of the 10 selected Calonectria isolates, nine plants of each clone were inoculated with the spore suspensions by spraying the leaves until run-off. The plants were covered with plastic bags for 48 h allowing sufficient humidity for infection. Control inoculations were done in a similar fashion with sterile water amended with 2 mL of Tween 80.
Pathogenicity tests were evaluated 14 d after inoculation. For every inoculated seedling, the percentage of the infected/diseased leaves was calculated. Results were analysed in SAS v8 using the PROC GLM (general linear model) (SAS Institute 1999). Analysis of variance (ANOVA) was used to determine the effects of fungal strain on lesion length. Prior to ANOVA, homogeneity of variance across treatments was verified. For significance tests amongst means, Fisher’s protected test was used. F values with P < 0.05 were considered significant. Isolations were made from lesions on the leaves of the test plants in each plot to ensure the presence of the inoculated fungi.
RESULTS
Isolates
A total of 97 isolates were collected from leaves in Eucalyptus plantations in the FuJian Province during the survey in 2007 (Table 1). Of these, 77 isolates were from diseased leaves on five E. dunnii trees, and an additional 20 isolates were obtained from diseased leaves on two E. grandis trees.
DNA sequence comparisons
Amplicons of approximately 500 bp were generated for the BT and TEF-1α gene regions and those for the HIS3 region were approximately 450 bp. Partition homogeneity tests for all possible combinations of the three gene regions used, consistently yielded a P-value of 0.001. The 70 % reciprocal bootstrap trees showed no conflict in tree topologies for the three gene regions. Based on the tree topologies and a P-value of 0.001 (Cunningham 1997, Dettman et al. 2003), the gene regions were combined. This resulted in a dataset consisting of 1 522 characters including gaps. Of these characters, 1 046 were constant and parsimony uninformative. The 476 parsimony informative characters included in the parsimony analyses yielded 54 equally most parsimonious trees (TL = 1110, CI = 0.722, RI = 0.879, RC = 0.634), of which the first tree is presented (Fig. 1). For Bayesian analyses, a HKY+I model was selected for BT, GTR+I+G model for HIS3 and a GTR+G model for TEF-1α and incorporated into the analyses. The consensus tree obtained for the Bayesian analyses confirmed the topology of the consensus tree obtained with the parsimony analysis (Fig. 1).
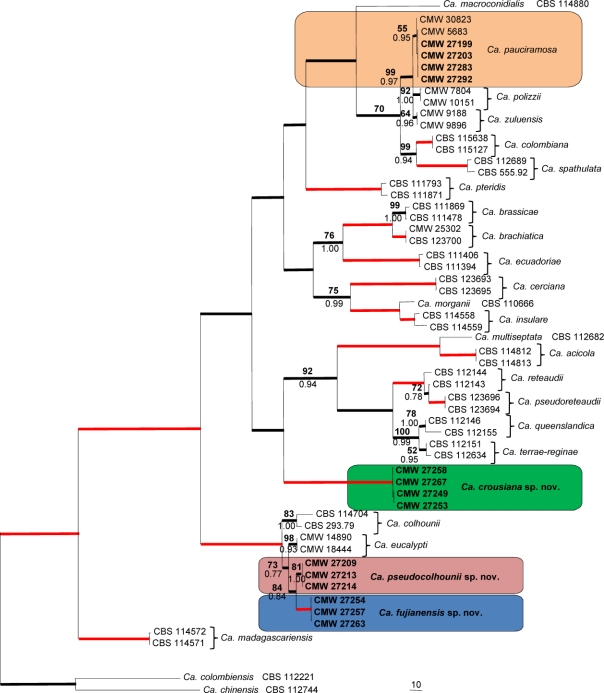
Fig. 1.
One of 54 most parsimonious trees obtained from a heuristic search with 1 000 random addition sequences of the combined sequences of β-tubulin, histone H3 and translation elongation factor 1α sequence alignments of the Ca. morganii complex and other closely related species. Scale bar shows 10 changes and bootstrap support values from 1 000 replicates are shown above the nodes in bold. Bayesian posterior probability values are indicated below the nodes. Red lines indicate bootstrap support values of 100 and posterior probability values of 1.00. Thickened lines indicate branches in the strict consensus tree and the consensus tree of the Bayesian analyses. The tree was rooted to Ca. colombiensis (CBS 112221) and Ca. chinensis (CBS 112744).
The phylogenetic tree showed a number of well-supported clades. Some isolates grouped in a clade representing Ca. pauciramosa with a bootstrap value (BP) of 55 and a Bayesian posterior probability (PP) value of 0.95. Other isolates grouped close to Ca. reteaudii, but in a distinct clade (BP = 100, PP = 1.00). Several isolates also clustered with Ca. colhounii and Ca. eucalypti, but separated from them to form a monophyletic group (BP = 84, PP = 0.84). These isolates also clustered into two well-supported clades (BP = 81, PP = 1.00 and BP = 100, PP = 1.00, respectively). SNP analyses for isolates CMW 27209, CMW 27213 and CMW 27214 showed that they shared two unique alleles, while isolates CMW 27254, CMW 27257 and CMW 27263 shared ten unique alleles for the three gene regions analysed, clearly distinguishing them from each other. Furthermore, these six Chinese isolates also shared three unique alleles, distinguishing them from Ca. colhounii and Ca. eucalypti (Table 2).
Table 2.
Single nucleotide polymorphism comparisons between Calonectria colhounii, Ca. eucalypti, Ca. pseudocolhounii and Ca. fujianensis.
| Species | Isolate no. | β-tubulin |
Histone H3 |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 53 | 378 | 397 | 398 | 407 | 420 | 516 | 57 | 248 | 290 | 311 | 362 | 371 | 386 | 454 | 455 | ||
| Ca. colhounii | CBS 293.79 | C | C | C | T | A | C | C | A | T | A | T | C | T | C | A | C |
| CBS 114704 | C | C | G | T | A | T | T | A | T | A | T | C | T | C | A | C | |
| Ca. eucalypti | CBS 125237 | C | C | G | C | G | T | T | – | T | T | T | T | T | C | C | A |
| CBS 125275 | C | C | G | C | G | T | T | – | T | T | T | T | T | C | C | A | |
| Ca. pseudocolhounii | CMW 27209 | C | T | G | T | A | C | C | A | C | A | T | C | T | C | A | C |
| CMW 27213 | C | T | G | T | A | C | C | A | C | A | T | C | T | C | A | C | |
| CMW 27214 | C | T | G | T | A | C | C | A | C | A | T | C | T | C | A | C | |
| Ca. fujianensis | CMW 27254 | T | C | C | T | A | C | C | A | T | A | C | C | C | A | A | C |
| CMW 27257 | T | C | C | T | A | C | C | A | T | A | C | C | C | A | A | C | |
| CMW 27263 | T | C | C | T | A | C | C | A | T | A | C | C | C | A | A | C | |
| Species | Isolate no. | TEF-1α |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 31 | 92 | 93 | 94 | 95 | 96 | 123 | 127 | 181 | 182 | 183 | 184 | 261 | 454 | 458 | 469 | 474 | 500 | ||||
| Ca. colhounii | CBS 293.79 | G | C | C | A | C | A | A | – | A | – | – | – | – | G | C | C | C | C | C | ||
| CBS 114704 | G | C | C | A | C | A | A | – | A | – | – | – | – | G | C | C | C | C | C | |||
| Ca. eucalypti | CBS 125237 | C | A | – | – | – | – | – | T | A | – | – | – | – | A | T | T | T | T | C | ||
| CBS 125275 | C | A | – | – | – | – | – | T | A | – | – | – | – | A | T | T | T | T | C | |||
| Ca. pseudocolhounii | CMW 27209 | C | A | – | – | – | – | – | T | A | – | – | – | – | A | T | T | T | T | – | ||
| CMW 27213 | C | A | – | – | – | – | – | T | A | – | – | – | – | A | T | T | T | T | – | |||
| CMW 27214 | C | A | – | – | – | – | – | T | A | – | – | – | – | A | T | T | T | T | – | |||
| Ca. fujianensis | CMW 27254 | C | A | – | – | – | – | – | T | G | A | A | A | A | A | T | T | T | T | – | ||
| CMW 27257 | C | A | – | – | – | – | – | T | G | A | A | A | A | A | T | T | T | T | – | |||
| CMW 27263 | C | A | – | – | – | – | – | T | G | A | A | A | A | A | T | T | T | T | – | |||
1 Highlight and bold = unique polymorphisms; highlight = shared polymorphisms.
Sexual compatibility
Protoperithecia formed within 3 wk and mating tests produced perithecia within 6 wk on sterilised toothpicks on MN medium. Except for isolates of Ca. pauciramosa (CMW 27199, CMW 27203, CMW 27283, CMW 27292), all the control crosses of Calonectria isolates, included in this study, produced perithecia with viable ascospores. These results show that all the Calonectria isolates, except those of Ca. pauciramosa are self-fertile (homothallic).
Taxonomy
Based on morphology and DNA sequence comparisons (Fig. 1), Calonectria isolates from Eucalyptus trees in FuJian Province reside in four taxa that include Ca. pauciramosa and three previously undescribed species. Isolates CMW 27199, CMW 27203, CMW 27283 and CMW 27292 clearly represent Ca. pauciramosa, with obpyriform to ellipsoidal vesicles, and macroconidia being 40–65 × 3–5 μm (av. = 50 × 5 μm). The remaining isolates are described in the genus Calonectria as follows:
Calonectria crousiana S.F. Chen, L. Lombard, M.J. Wingf. & X.D. Zhou, sp. nov. — MycoBank MB518855; Fig. 2
Fig. 2.
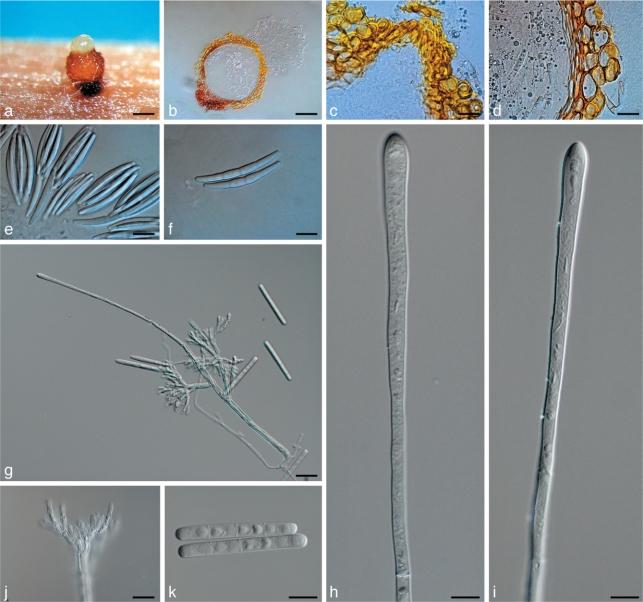
Calonectria crousiana. a–f. Teleomorph state; g–k. anamorph state. a. Perithecium; b. vertical section through a perithecium; c. cells around ostiolar region of perithecium; d. section through lateral perithecial wall; e. asci; f. ascospores; g. macroconidiophore; h, i. clavate vesicles; j. fertile branches; k. macroconidia. — Scale bars: a = 200 μm; b = 100 μm; c–e, g, j = 20 μm; f, k = 10 μm; h, i = 5 μm.
Teleomorpha Calonectriae indusiatae similis sed ascosporibus maioribus (56–)58–69(–76) × (5–)6.5–7.5(–8) μm, mediocriter 64 × 7 μm, differt. Anamorpha Cy. theae similis sed macroconidiis cylindricis utrinque rotundatis rectis (59–)61–67(–75) × (4–)4.5–5.5(–6) μm, mediocriter 64 × 5 μm, (semel vel) ter septatis, sine cicatrice abscissionis visibile, in fasciculis parallelis cylindricis muco contenitis, differt.
Etymology. This species is named for Prof. P.W. Crous recognising his monumental contributions to the taxonomy of Calonectria spanning more than two decades.
Perithecia solitary or in groups of up to five, orange, becoming red-brown with age; in section apex and body orange, base red-brown, subglobose to ovoid, (321–)352–499(–550) μm high, (260–)262–403(–465) μm diam, body turning dark orange to slightly red, and base dark red-brown in 3 % KOH; perithecial walls rough, consisting of two thick-walled layers: outside layer of textura globulosa, (32–)33–76(–90) μm wide, becoming more compressed towards inner layer of textura angularis, (10–)12–23(–30) μm wide, becoming thin-walled and hyaline towards the centre; outer cells (22–)26–38(–40) × (9–)16–29(–36) μm, inner cells (8–)9–15(–18) × (2.5–)3.5–6(–7) μm; perithecial base up to 241 μm wide, consisting of dark red, angular cells, merging with an erumpent stroma; cells of the outer wall layer continuing into the pseudoparenchymatous cells of the erumpent stroma. Asci 8-spored, clavate, (109–)120–175(–186) × (23–)24–25 μm, tapering to a long thin stalk. Ascospores aggregate in the upper third of the asci, hyaline, guttulate, fusoid with rounded ends, straight to slightly curved, (1–)3-septate, not or slightly constricted at the septum, (56–)58–69(–76) × (5–)6.5–7.5(–8) μm (av. = 64 × 7 μm). Macroconidiophores consisting of a stipe, a suite of penicillate arranged fertile branches, a stipe extension, and a terminal vesicle; stipe septate, hyaline, smooth (61–)63–160(–220) × (4.5–)6–8(–9.5) μm; stipe extensions septate, straight to flexuous (195–)225–404(–475) μm long, 3–6 μm wide at the apical septum, terminating in a clavate vesicle, (4–)4.5–5(–6) μm diam. Conidiogenous apparatus (63–)76–117(–138) μm long, (40–)53–98(–116) μm wide; primary branches aseptate to 1-septate, (19–)21–42(–70) × (4–)4.5–5.5(–6) μm; secondary branches aseptate, (13–)17–25(–28) × (3.5–)4–5 μm; tertiary branches aseptate, (11–)11.5–15(–18) × (3–)3.5–4(–4.5) μm; additional branches (–5), aseptate, (10–)10.5–14(–16) × (2.5–)3–4 μm; each terminal branch producing 1–4 phialides; phialides doliiform to allantoid, hyaline, aseptate, (9.5–)10.5–13.5(–15) × 34.5 μm, apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (59–)61–67(–75) × (4–)4.5–5.5(–6) μm (av. = 64 × 5 μm), (1–)3-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime.
Culture characteristics — Colonies reaching 64–80 mm diam after 7 d on MEA in the dark with optimal growth temperature at 25 °C. Colonies fast growing forming white to sienna aerial mycelium, with feathery, irregular margins. Surface and reverse with mikado-orange to sienna outer margin, and russet inner region, becoming argus-brown towards the centre. Chlamydospores arrange in chains, abundant throughout the medium, forming microsclerotia.
Substratum — Eucalyptus grandis.
Distribution — FuJian Province, China.
Specimens examined. China, FuJian Province, on leaves of Eucalyptus grandis, Aug. 2007, M.J. Wingfield, Herb. PREM 60453, holotype of Ca. crousiana, culture ex-type CMW 27249 = CBS 127198; FuJian Province, on leaves of E. grandis, Aug. 2007, M.J. Wingfield, Herb. PREM 60454, culture CMW 27253 = CBS 127199; FuJian Province, on leaves of E. grandis, Aug. 2007, M.J. Wingfield, Herb. PREM 60455, culture CMW 27267 = CBS 127203; FuJian Province, on leaves of E. grandis, Aug. 2007, M.J. Wingfield, culture CMW 27258.
Notes — Calonectria crousiana is morphologically similar to Ca. indusiata, Ca. australiensis and species in the Ca. colhounii complex, that includes Ca. colhounii, Ca. eucalypti, Ca. macroconidialis and Ca. madagascariensis (Crous et al. 2006, Lombard et al. 2010b). With the exception of Ca. macroconidialis (macroconidia (1–)3(–6)-septate), all of these species produce clavate vesicles and (1–)3-septate macroconidia. Calonectria crousiana can be distinguished from species in the Ca. colhounii complex by its distinctly orange to red perithecia. This fungus can also be distinguished from Ca. indusiata and Ca. australiensis based on the dimensions of the macroconidia, with Ca. crousiana (av. = 64 × 5 μm) having shorter macroconidia than those of Ca. indusiata (av. = 81 × 6.0 μm) and narrower than those of Ca. australiensis (av. = 63 × 6.5 μm).
Calonectria pseudocolhounii S.F. Chen, L. Lombard, M.J. Wingf. & X.D. Zhou, sp. nov. — MycoBank MB518856; Fig. 3
Fig. 3.
Calonectria pseudocolhunii. a–h. Teleomorph state; i–m. anamorph state. a. Perithecium; b. vertical section through a perithecium; c. cells around ostiolar region of perithecium; d. section through lateral perithecial wall; e, f. asci; g, h. ascospores; i. macroconidiophore; j, k. clavate vesicles; l. fertile branches; m. macroconidia. — Scale bars: a = 200 μm; b = 100 μm; c = 40 μm; d–f, i–k = 20 μm; g, h, l, m = 10 μm.
Teleomorpha Calonectria colhounii similis sed ascosporis hyalinis guttulatis fusoidibus extremis rotundatis, rectis vel subcurvatis, (semel vel) ter septatis, in septo non vel leviter constrictis, (44–)50–62(–74) × (5–)6–7(–8) μm, mediocriter 56 × 6.5 μm, differt. Anamorpha Cy. colhounii similis sed macroconidiis cylindricis utrinque rotundatis rectis (49–)55–65(–74) × (3.5–)4–5(–5.5) μm, mediocriter 60 × 4.5 μm, (semel vel) ter septatis, sine cicatrice abscissionis visibile, in fasciculis parallelis cylindricis muco contenitis, differt.
Etymology. The name reflects the fact that this fungus is morphologically similar to Calonectria colhounii.
Perithecia solitary or in groups of up to four, bright yellow, becoming orange with age; in section apex and body yellow, base red-brown, subglobose to ovoid, (330–)350–453(–495) μm high, (227–)258–330(–390) μm diam, body turning dark yellow, and base dark red-brown in KOH+; perithecial walls rough consisting of two thick-walled layers: outside layer of textura globulosa, (26–)33–59(–65) μm wide, becoming more compressed towards inner layer of textura angularis, (10–)12–18(–22) μm wide, becoming thin-walled and hyaline towards the centre; outer cells (17–)21–34(–42) × (11–)12–21(–27) μm, inner cells (10–)11–14(–20) × (3–)5–6.5(–7) μm, perithecial base up to 180 μm wide, consisting of dark red, angular cells merging with an erumpent stroma, cells of the outer wall layer continuing into the pseudoparenchymatous cells of the erumpent stroma. Asci 4-spored, clavate, (130–)135–162(–167) × (16–)18–24(–30) μm, tapering to a long thin stalk. Ascospores aggregate in the upper third of the asci, hyaline, guttulate, fusoid with rounded ends, straight to slightly curved, (1–)3-septate, not or slightly constricted at the septum, (44–)50–62(–74) × (5–)6–7(–8) μm (av. = 56 × 6.5 μm). Macroconidiophores consisting of a stipe, a suite of penicillate arranged fertile branches, a stipe extension, and a terminal vesicle; stipe septate, hyaline, smooth (45–)53–192(–217) × (5.5–)6–7(–8) μm; stipe extensions septate, straight to flexuous (133–)168–252(–300) μm long, 3–6 μm wide at the apical septum, terminating in a clavate vesicle, (3.5–)4–5(–6) μm diam. Conidiogenous apparatus (41–)44–74(–91) μm long, (35–)38–65(–84) μm wide; primary branches aseptate to 1-septate, (13–)15–26(–33) × 3.5–4.5(–5) μm; secondary branches aseptate, (9–)11.5–20(–23) × 3–4(–4.5) μm; tertiary branches aseptate, 8.5–14(–17) × 3–4 μm; additional branches (–5), aseptate, (8–)8.5–13(–15) × 2.5–3(–3.5) μm; each terminal branch producing 2–4 phialides; phialides doliiform to reniform, hyaline, aseptate, (8–)9–12.5(–14) × 2.5–3(–3.5) μm, apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (49–)55–65(–74) × (3.5–)4–5(–5.5) μm (av. = 60 × 4.5 μm), (1–)3-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime.
Culture characteristics — Colonies reaching 38–44 mm diam after 7 d on MEA in the dark with optimal growth temperature at 25 °C. Colonies with white aerial mycelium in the centre, with feathery, irregular margins at the edges. Surface and reverse with white to buff-yellow outer margins, and russet inner region, becoming liver-brown towards the centre. Chlamydospores arrange in chains, abundant throughout the medium, forming microsclerotia.
Substratum — Eucalyptus dunnii.
Distribution — FuJian Province, China.
Specimens examined. China, FuJian Province, on leaves of Eucalyptus dunnii, Aug. 2007, M.J. Wingfield, Herb. PREM 60456, holotype of Ca. pseudocolhounii, culture ex-type CMW 27209 = CBS 127195; FuJian Province, on leaves of E. dunnii, Aug. 2007, M.J. Wingfield, Herb. PREM 60457, culture CMW 27213 = CBS 127196; FuJian Province, on leaves of E. dunnii, Aug. 2007, M.J. Wingfield, Herb. PREM 60458, culture CMW 27214 = CBS 127197.
Notes — Calonectria pseudocolhounii is similar to species in the Ca. colhounii complex that all have yellow perithecia, (1–)3-septate ascospores and clavate vesicles in the anamorph state. Calonectria pseudocolhounii is morphologically most similar to Ca. colhounii (macroconidia av. = 65 × 5 μm), but can be distinguished from this species by having smaller and narrower macroconidia (av. = 60 × 4.5 μm). The ascospores of Ca. pseudocolhounii (av. = 56 × 6.5 μm) are larger, while the macroconidia (av. = 60 × 4.5 μm) are smaller than those of Ca. eucalypti (ascospores av. = 33 × 6 μm; macroconidia av. = 72 × 6 μm).
Calonectria fujianensis S.F. Chen, L. Lombard, M.J. Wingf. & X.D. Zhou, sp. nov. — MycoBank MB518857; Fig. 4
Fig. 4.
Calonectria fujianensis. a–f. Teleomorph state; g–k. anamorph state. a. Perithecium; b. vertical section through a perithecium; c. cells around ostiolar region of perithecium; d. section through lateral perithecial wall; e. asci; f. ascospores; g. macroconidiophore; h, i. clavate vesicles; j. fertile branches; k. macroconidia. — Scale bars: a = 200 μm; b = 100 μm; c = 40 μm; d–g = 20 μm; h, i = 5 μm; j, k = 10 μm.
Teleomorpha Calonectria colhounii similis sed ascosporis hyalinis guttulatis fusoidibus extremis rotundatis, rectis vel subcurvatis, (semel vel) ter septatis, in septo non vel leviter constrictis, (38–)49–62(–72) × (5–)6–7.5(–8) μm, mediocriter 55.5 × 6.8 μm, differt. Anamorpha Cy. colhounii similis sed macroconidiis cylindricis utrinque rotundatis rectis (48–)50–55(–60) × (2.5–)3.5–4.5(–5) μm, mediocriter 52.5 × 4 μm, (semel vel) ter septatis, sine cicatrice abscissionis visibile, in fasciculis parallelis cylindricis muco contenitis, differt.
Etymology. Named after the FuJian Province of China where the fungus was first collected.
Perithecia solitary or in groups of up to four, bright yellow, becoming orange with age; in section apex and body yellow, base red-brown, subglobose to ovoid, (310–)351–465(–492) μm high, (206–)226–329(–382) μm diam, body turning dark yellow, and base dark red-brown in KOH+; perithecial walls rough consisting of two thick-walled layers: outside layer of textura globulosa, (26–)35–58(–61) μm wide, becoming more compressed towards inner layer of textura angularis, (10–)12–21(–24) μm wide, becoming thin-walled and hyaline towards the centre; outer cells (15–)17–35(–41) × (8–)11–20(–24) μm, inner cells (9–)10–20(–26) × (2.5–)3.5–6.0(–6.5) μm; perithecial base up to 180 μm wide, consisting of dark red, angular cells, merging with an erumpent stroma, cells of the outer wall layer continuing into the pseudoparenchymatous cells of the erumpent stroma. Asci 4-spored, clavate, (118–) 132–152(–155) × (14–)16–23(–29) μm, tapering to a long thin stalk. Ascospores aggregate in the upper third of the ascus, hyaline, guttulate, fusoid with rounded ends, straight to slightly curved, (1–)3-septate, not or slightly constricted at the septum, (38–)49–62(–72) × (5–)6–7.5(–8) μm (av = 55.5 × 6.8 μm). Macroconidiophores consisting of a stipe, a suite of penicillate arranged fertile branches, a stipe extension, and a terminal vesicle; stipe septate, hyaline, smooth, (33–)36–152(–210) × (3.5–)4.5–8(–8.5) μm; stipe extensions septate, straight to flexuous (147–)167–248(–261) μm long, 3–5 μm wide at the apical septum, terminating in a clavate vesicle, (3–)3.5–4.5(–5) μm diam. Conidiogenous apparatus (36–)43–72(–89) μm long, (21–)31–61(–65) μm wide; primary branches aseptate to 1-septate, (11–)12–28(–32) × (3–)3.5–4.5 μm; secondary branches aseptate, 8–20(–26) × 3–4(–4.5) μm; tertiary branches aseptate, (8–)10–12(–12.5) × 2.5–3(–4) μm; additional branches (–5), aseptate, (8–)9–10 × 2.5–3(–3.5) μm; each terminal branch producing 2–4 phialides; phialides doliiform to reniform, hyaline, aseptate, (6.5–)8–11 × (2–)2.5–3 μm, apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (48–)50–55(–60) × (2.5–)3.5–4.5(–5) μm (av. = 52.5 × 4 μm), (1–)3-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime.
Culture characteristics — Colonies reaching 44–52 mm diam after 7 d on MEA in the dark with optimal growth temperature at 25 °C. Colonies with white to cream-coloured aerial mycelium in the centre, with feathery, irregular margins at the edges. Surface and reverse with cream coloured to white outer margins, and russet inner region, becoming argus-brown towards the centre. Chlamydospores arranged in chains, abundant throughout the medium, forming microsclerotia.
Substratum — Eucalyptus grandis.
Distribution — FuJian Province, China.
Specimens examined. China, FuJian Province, on leaves of Eucalyptus grandis, Aug. 2007, M.J. Wingfield, Herb. PREM 60460, holotype of Ca. fujianensis, culture ex-type CMW 27257 = CBS 127201; FuJian Province, on leaves of E. grandis, Aug. 2007, M.J. Wingfield, Herb. PREM 60461, culture CMW 27263 = CBS 127202; FuJian Province, on leaves of E. grandis, Aug. 2007, M.J. Wingfield, Herb. PREM 60459, culture CMW 27254 = CBS 127200.
Notes — Calonectria fujianensis is morphologically distinguishable from Ca. colhounii and Ca. pseudocolhounii having smaller macroconidia (av. = 52.5 × 4 μm) than Ca. colhounii (av. = 65 × 5 μm) and Ca. pseudocolhounii (av. = 60 × 4.5 μm). The ascospores of Ca. fujianensis (av. = 55.5 × 6.8 μm), Ca. pseudocolhounii (av. = 56 × 6.5 μm) and Ca. colhounii (av. = 55 ×6 μm), are larger than those of Ca. eucalypti (av. = 33 × 6 μm), while the macroconidia of the former three species are smaller than those of Ca. eucalypti (av. = 72 × 6 μm).
Pathogenicity tests
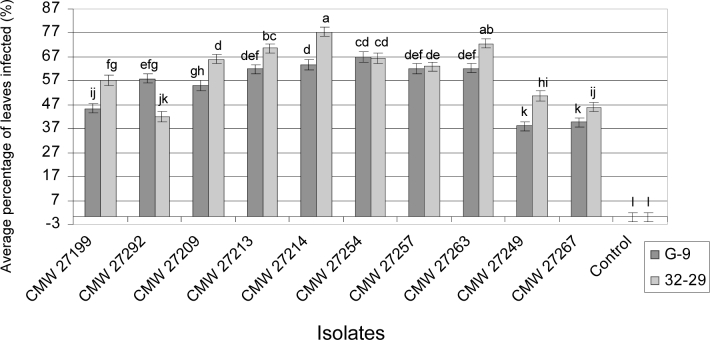
All plants representing the two Eucalyptus clones inoculated with Calonectria spp. (Ca. crousiana, Ca. fujianensis, Ca. pauciramosa, Ca. pseudocolhounii) in this study, developed leaf spot symptoms whereas no disease was observed on the leaves of the control plants (Fig. 5). The inoculated fungi were successfully re-isolated from the leaf spots and no Calonectria spp. was isolated from the control plants. The average percentage of leaf surface affected by the test isolates showed no significant differences between the two experimental plots (P = 0.0578), and the interactions between the two experiments and two clones were not significantly different (P = 0.0535). Subsequently, the data for the two plots were combined and analysed collectively. The combined results showed significant isolate × clone interaction (P < 0.05), indicating that not all Calonectria isolates reacted similarly to the two Eucalyptus clones tested. The percentage of infected leaves arising from inoculation with Ca. pauciramosa (CMW 27199, CMW 27192), Ca. pseudocolhounii (CMW 27209, CMW 27213, CMW 27214), Ca. crousiana (CMW 27249, CMW 27267) and Ca. fujianensis (CMW 27263) were significantly different (P < 0.05) on the two clones tested. In contrast, there was no significant difference (P > 0.05) in the percentage of infected leaves for the two clones inoculated with isolates (CMW 27254, CMW 27257) of Ca. fujianensis (Fig. 5).
Fig. 5.
Histogramme indicating the average percent leaves infected (%) resulting from inoculation trials of two Eucalyptus urophylla × E. grandis clones inoculated with isolates of Calonectria pauciramosa (CMW 27199, CMW 27192), Ca. pseudocolhounii (CMW 27209, CMW 27213, CMW 27214), Ca. fujianensis (CMW 27254, CMW 27257, CMW 27263), Ca. crousiana (CMW 27249, CMW 27267) and the controls. Bars represent 95 % confidence limits for each treatment. Different letters above the bars indicate treatments that were statistically significantly different (P = 0.05).
The Eucalyptus clone CEPT-10 displayed a significantly (P < 0.05) higher percentage of infected leaves when inoculated with Ca. pauciramosa (CMW 27199, CMW 27292), Ca. pseudocolhounii (CMW 27209, CMW 27213, CMW 27214) and Ca. fujianensis (CMW 27254, CMW 27257, CMW 27263) than with Ca. crousiana (CMW 27249, CMW 27267) (Fig. 5). Isolate CMW 27254 (Ca. fujianensis) displayed the highest average percentage of leaf surface infected on clone CEPT-10 (Fig. 5).
The Eucalyptus clone CEPT-9 showed a significantly (P < 0.05) higher percentage of infected leaves caused by isolates of Ca. pseudocolhounii (CMW 27209, CMW 27213, CMW 27214) and Ca. fujianensis (CMW 27254, CMW 27257, CMW 27263) than with those of Ca. pauciramosa (CMW 27199, CMW 27292) and Ca. crousiana (CMW 27249, CMW 27267) (Fig. 5). Isolate CMW 27214 (Ca. pseudocolhounii) resulted in the highest average percentage of leaves infected for CEPT-9 (Fig. 5).
DISCUSSION
In this study, four Calonectria spp. were identified from leaves collected on diseased Eucalyptus trees grown in commercial plantations of FuJian Province in Southeast China. These included Ca. pauciramosa and three previously undescribed species for which the names Ca. crousiana, Ca. fujianensis and Ca. pseudocolhounii are provided. The identification of these fungi was supported by DNA sequence comparisons as well as by morphological characteristics. Based on phylogenetic inference, Ca. crousiana is closely related to taxa in the Ca. reteaudii species complex, whereas Ca. pseudocolhounii and Ca. fujianensis reside in the Ca. colhounii complex. Pathogenicity tests showed that all four species are capable of causing leaf infections on two of the most widely planted E. urophylla × E. grandis clones in South China.
Calonectria pauciramosa resides in the Ca. scoparia species complex (Crous et al. 1993, Schoch et al. 1999, Lombard et al. 2010b, d) and was recently found killing plants in a commercial Eucalyptus nursery in the GuangDong Province of China (Lombard et al. 2010d). This study represents the first report of this pathogen infecting leaves of Eucalyptus trees growing in plantations. In the past, Ca. pauciramosa has been associated with nursery diseases in Australia, Italy, South Africa, Spain and USA (Koike et al. 1999, Polizzi & Crous 1999, Schoch et al. 1999, 2001, Koike & Crous 2001, Polizzi et al. 2006, 2009, Perez-Sierra et al. 2007). This fungus has also been isolated from tropical areas of GuangDong Province (Lombard et al. 2010d) and in this study was found in an area that has a sub-tropical climate. The climatic conditions of these regions differ significantly, supporting the view that Ca. pauciramosa can tolerate a wide range of temperature conditions.
Based on phylogenetic inference, Ca. crousiana is closely related to Calonectria spp. in the Ca. reteaudii complex. Similar to Ca. reteaudii, Ca. crousiana also produces orange to red perithecia and has a Cylindrocladium state with clavate vesicles. However, septation of the macroconidia is distinct in these species with Ca. crousiana having (1–)3-septate macroconidia that distinguish it from the other species in the Ca. reteaudii complex, including Ca. reteaudii ((1–)5(–6)-septate), Ca. pseudoreteaudii (1(–3)-septate), Ca. queenslandica ((1–)3(–6)-septate) and Ca. terraereginae ((1–)3(–6)-septate) (Crous 2002, Lombard et al. 2010b). Morphological comparisons showed that Ca. crousiana is very similar to Ca. indusiata and Ca. australiensis, which have clavate vesicles and (1–)3-septate macroconidia (Crous 2002, Crous et al. 2006, Lombard et al. 2010b).
Previous studies have shown that Ca. indusiata and species in the Ca. reteaudii species complex are pathogens causing leaf blight and cutting rot on Eucalyptus trees and seedlings in Australia, South America and Southeast Asia (Pikethley 1976, Bolland et al. 1985, Sharma & Mohanan 1991, 1992, Booth et al. 2000, Crous & Kang 2001, Crous 2002, Rodas et al. 2005, Lombard et al. 2010d). In this study, Ca. crousiana was isolated from diseased leaves on E. grandis trees in FuJian Province. Based on the results of pathogenicity tests on two Eucalyptus hybrid clones, Ca. crousiana should be regarded as an important pathogen of Eucalyptus in China.
Past studies have shown that Ca. colhounii is closely related to Ca. madagascariensis and Ca. macroconidialis (Crous et al. 1999). Recently, a newly described species, Ca. eucalypti, was also identified in this complex (Lombard et al. 2010b). This Calonectria complex is characterised by having unique yellow perithecia, (1–)3-septate ascospores and clavate vesicles (Crous et al. 1999, Crous 2002, Lombard et al. 2010b). In the present study, Ca. pseudocolhounii and Ca. fujianensis were described as new species with both species sharing unique morphological characteristics with the other species in the complex. There are, however, a number of morphological differences distinguishing Ca. pseudocolhounii and Ca. fujianensis from the other species in this complex. All species other than Ca. madagascariensis (8-spore asci) produce asci with four ascospores. Macroconidia of Ca. fujianensis (av. = 52.5 × 4 μm) are smaller than those of Ca. pseudocolhounii (av. = 60 × 4.5 μm), while these structures in both species are smaller than those of Ca. colhounii (av. = 65 × 5 μm) and Ca. eucalypti (av. = 72 × 6 μm). Species in the Ca. colhounii complex have been isolated from the Eucalyptus trees or soil under these trees in Africa, America and Southeast Asia (Crous 2002, Lombard et al. 2010d). Pathogenicity tests in this study showed that Ca. pseudocolhounii and Ca. fujianensis are both aggressive pathogens on the Eucalyptus clones tested.
Pathogenicity tests in this study showed that all four species of Calonectria found in FuJian Province are important pathogens of Eucalyptus. Calonectria pseudocolhounii and Ca. fujianensis were more pathogenic than Ca. pauciramosa and Ca. crousiana, while Ca. pauciramosa was more pathogenic than Ca. crousiana. These results also showed that the tolerance of the two tested Eucalyptus hybrid clones are significantly different for some of the isolates tested. This implies that it might be possible to select disease tolerant planting stock based on nursery screening.
Leaf and shoot blight associated with Calonectria spp. is one of the most serious threats to commercial Eucalyptus plantations and nurseries in China (Wang 1992, Sun & Liu 2004, Zhou et al. 2008, Lombard et al. 2010d). Although Ca. reteaudii has been regarded as the dominant pathogen responsible for CLB in South America and Southeast Asia (Pikethley 1976, Bolland et al. 1985, Sharma & Mohanan 1991, 1992, Booth et al. 2000, Crous & Kang 2001, Crous 2002, Rodas et al. 2005), no isolates of this fungus were obtained during this study. This could be due to the cooler climatic conditions of the region surveyed, as Ca. reteaudii has been only reported from tropical regions (Booth et al. 2000, Crous 2002).
This study has added considerably to the base of knowledge of the species of Calonectria and their Cylindrocladium anamorphs in China. The discovery of three new species was surprising and this suggests that additional species await discovery in that country. Calonectria spp. are well-known to have wide host ranges. Results of this study add substance to the view that those species occurring in the soil below Eucalyptus spp., are likely to infect the leaves of these trees, assuming that climatic conditions are favourable for infection. Very little is known regarding the host specificity of these important pathogens but inoculation tests in this study show clearly that different clones respond differently to inoculation by different species of Calonectria. This could provide opportunities to tailor planting to avoid damage due to CLB. However, given the large number of Calonectria spp. that are now known to occur in China, such complex deployment of clones may not be financially feasible.
Calonectria spp. are important Eucalyptus pathogens (Crous 2002, Old et al. 2003, Rodas et al. 2005, Lombard 2010d). The fact that they are soil-borne also contributes to the ease with which they might be moved globally. In this regard, very little is known regarding their origins. Some species with wide global distributions in agricultural and forestry environments, such as Ca. pauciramosa, seem very likely to have been moved to new environments. It is difficult to predict how these fungi might respond to new host encounters. However, they add to the growing threats that pathogens pose to Eucalyptus plantation forestry (Wingfield et al. 2008) and every effort should be made to avoid their movement.
This study represents an important contribution to the taxonomy of species of Calonectria, and highlights the distribution of these pathogens in Eucalyptus plantations in China. The first pathogenicity tests using these fungi on Eucalyptus clones in this country were also conducted. These results will offer valuable information on the management of Calonectria pathogens in Eucalyptus plantations, and will advance breeding strategies aimed at developing resistant Eucalyptus clones in China.
Acknowledgments
This study was initiated through the bilateral agreement between South Africa and China, and we are grateful for funding via projects 2007DFA31190, 30771732 and 2008B050100014. We also appreciate the financial support of the members of Tree Protection Co-operative Programme (TPCP) and the associated THRIP Initiative of the Department of Trade and Industry (South Africa). We are grateful to Prof. Hennie Groeneveld and Dr Mike van der Linde for assistance with the statistical analyses, and Dr H. Glen, South African National Botanical Institute (SANBI), for providing the Latin descriptions and valuable suggestions for names of the new species. The first author further acknowledges his colleagues of LeiZhou Forestry Bureau, XinTao Mou and GuiXiang Zhao for their valuable assistance in conducting field work.
REFERENCES
- Bolland L, Tierney JW, Tierney BJ.1985. Studies on leaf spot and shoot blight of Eucalyptus caused by Cylindrocladium quinqueseptatum. European Journal of Forest Pathology 15: 385 – 397 [Google Scholar]
- Booth TH, Jovanovic T, Old KM, Dudzinski MJ.2000. Climatic mapping to identify high-risk areas for Cylindrocladium quinqueseptatum leaf blight on eucalypts in main land South East Asia and around the world. Environmental Pollution 108: 365 – 372 [DOI] [PubMed] [Google Scholar]
- Carbone I, Kohn LM.1999. A method for designing primer sets for the speciation studies in filamentous ascomycetes. Mycologia 91: 553 – 556 [Google Scholar]
- Crous PW.2002. Taxonomy and pathology of Cylindrocladium (Calonectria) and allied genera. APS Press, St. Paul, Minnesota, USA: [Google Scholar]
- Crous PW, Alfenas AC, Wingfield MJ.1993. Calonectria scoparia and Calonectria morganii sp. nov., and variations among isolates of their Cylindrocladium anamorphs. Mycological Research 97: 701 – 708 [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G.2004a. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19 – 22 [Google Scholar]
- Crous PW, Groenewald JZ, Risède J-M, Simoneau P, Hyde KD.2006. Calonectria species and their Cylindrocladium anamorphs: species with clavate vesicles. Studies in Mycology 55: 213 – 226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Groenewald JZ, Risède J-M, Simoneau P, Hywel-Jones NL.2004b. Calonectria species and their Cylindrocladium anamorphs: species with sphaeropedunculate vesicles. Studies in Mycology 50: 415 – 430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Kang J-C.2001. Phylogenetic confirmation of Calonectria spathulata and Cylindrocladium leucothoes based on morphology, and sequence data of the β-tubulin and ITS rRNA genes. Mycoscience 42: 51 – 57 [Google Scholar]
- Crous PW, Kang J-C, Schoch CL, Machau GRA.1999. Phylogenetic relationship of Cylindrocladium pseudogracile and Cylindrocladium rumohrae with morphologically similar taxa, based on morphology and DNA sequence of internal transcribed spacers and β-tubulin. Canadian Journal of Botany 77: 1813 – 1820 [Google Scholar]
- Crous PW, Phillips AJL, Wingfield MJ.1991. The genera Cylindrocladium and Cylindrocladiella in South Africa, with special reference to forest nurseries. South African Forestry Journal 157: 69 – 85 [Google Scholar]
- Crous PW, Wingfield MJ.1994. A monograph of Cylindrocladium, including anamorphs of Calonectria. Mycotaxon 51: 341 – 435 [Google Scholar]
- Cunningham CW.1997. Can three incongruency tests predict when data should be combined? Molecular Biology and Evolution 14: 733 – 740 [DOI] [PubMed] [Google Scholar]
- Dettman JR, Jacobson DJ, Taylor JW.2003. A multilocus genealogical approach to phylogenetic species recognition in the model eukaryote Neurospora. Evolution 57: 2703 – 2720 [DOI] [PubMed] [Google Scholar]
- Farris JS, Källersjö M, Kluge AG, Bult C.1994. Testing significance of incongruence. Cladistics 10: 315 – 320 [Google Scholar]
- Glass NL, Donaldson GC.1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous Ascomycetes. Applied and Environment Microbiology 61: 1323 – 1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueidan C, Roux C, Lutzoni F.2007. Using multigene phylogeny analysis to assess generic delineation and character evolution in Verrucariaceae (Verrucariales, Ascomycota). Mycological Research 111: 1145 – 1168 [DOI] [PubMed] [Google Scholar]
- Guerber JC, Correll JC.2001. Characterization of Glomerella acutata, the teleomorph of Colletotrichum acutatum. Mycologia 93: 216 – 229 [Google Scholar]
- Hillis DM, Bull JJ.1993. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology 42: 182 – 192 [Google Scholar]
- Iglesias-Trabad G, Wilstermann D.2008. Eucalyptus universalis. Global cultivated eucalypt forest map 2008 Version 1.0.1 in GIT Forestry Consulting’s EUCALYPTOLOGICS: Information resources on Eucalyptus cultivation worldwide. Retrieved from http://www.git-forestry.com (29 March 2009).
- Kang J-C, Crous PW, Old KM, Dudzinski MJ.2001. Non-conspecificity of Cylindrocladium quinqueseptatum and Calonectria quinqueseptata based on a β-tubulin gene phylogeny and morphology. Canadian Journal of Botany 79: 1241 – 1247 [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T.2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research 30: 3059 – 3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike ST, Crous PW.2001. First report of a root and crown rot disease of myrtle in California caused by Cylindrocladium pauciramosum. Plant Disease 85: 44 [DOI] [PubMed] [Google Scholar]
- Koike ST, Henderson DM, Crous PW, Schoch CL, Tjosvold SA.1999. A new root and crown rot disease of heath in California caused by Cylindrocladium pauciramosum. Plant Disease 83: 589 [DOI] [PubMed] [Google Scholar]
- Lechat C, Crous PW, Groenewald JZ.2010. The enigma of Calonectria species occurring on leaves of Ilex aquifolium in Europe. IMA Fungus 1: 101 – 108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Librado P, Rozas J.2009. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25: 1451 – 1452 [DOI] [PubMed] [Google Scholar]
- Lombard L, Crous PW, Wingfield BD, Wingfield MJ.2010a. Multigene phylogeny and mating tests reveal three cryptic species related to Calonectria pauciramosa. Studies in Mycology 66: 15 – 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, Crous PW, Wingfield BD, Wingfield MJ.2010b. Phylogeny and systematics of the genus Calonectria. Studies in Mycology 66: 31 – 69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, Crous PW, Wingfield BD, Wingfield MJ.2010c. Species concepts in Calonectria (Cylindrocladium). Studies in Mycology 66: 1 – 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, Rodas CA, Crous PW, Wingfield BD, Wingfield MJ.2009. Calonectria (Cylindrocladium) species associated with dying Pinus cuttings. Persoonia 23: 41 – 47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, Zhou XD, Crous PW, Wingfield BD, Wingfield MJ.2010d. Calonectria species associated with cutting rot of Eucalyptus. Persoonia 24: 1 – 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason-Gamer R, Kellogg E.1996. Testing for phylogenetic conflict among molecular datasets in the tribe Triticeae (Graminae). Systematic Biology 45: 524 – 545 [Google Scholar]
- Nirenburg HI.1981. A simplified method for identifying Fusarium spp. occurring on wheat. Canadian Journal of Botany 59: 1599 – 1609 [Google Scholar]
- Nylander JAA.2004. MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University; [Google Scholar]
- O’Donnell K, Cigelnik E.1997. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103 – 116 [DOI] [PubMed] [Google Scholar]
- Old KM, Wingfield MJ, Yuan ZQ.2003. A manual of diseases of eucalypts in South-East Asia. Centre for International Forestry Research, Indonesia: [Google Scholar]
- Perez-Sierra A, Alvarez LA, Leon M, Abad-Campos P, Armengol J, Garcia-Jimenez J.2007. First report of leaf spot, blight, and stem lesions caused by Cylindrocladium pauciramosum on Callistemon in Spain. Plant Disease 91: 1057 [DOI] [PubMed] [Google Scholar]
- Pikethley RN.1976. Cylindrocladium quinqueseptatum on myrtaceous tree seedlings. Australian Plant Pathology Newsletter 5: 57 [Google Scholar]
- Polizzi G, Crous PW.1999. Root and collar rot of milkwort caused by Cylindrocladium pauciramosum, a new record for Europe. European Journal of Plant Pathology 105: 407 – 411 [Google Scholar]
- Polizzi G, Vitale A, Aiello D, Castello I, Guarnaccia V, Parlavecchio G.2009. First report of crown rot caused by Cylindrocladium pauciramosum on brush cherry in Italy. Plant Disease 93: 547 [DOI] [PubMed] [Google Scholar]
- Polizzi G, Vitale A, Aiello D, Parlavecchio G.2006. First record of crown and root rot caused by Cylindrocladium pauciramosum on California lilac in Italy. Plant Disease 90: 1459 [DOI] [PubMed] [Google Scholar]
- Qi SX.2006. The introduction and development situation of Eucalyptus in China. Guangxi Forestry Science 35: 250 – 252 (In Chinese.) [Google Scholar]
- Rayner RW.1970. A mycological colour chart. British Mycological Society, Commonwealth Mycological Institute, Kew, Surry: [Google Scholar]
- Rodas CA, Lombard L, Gryzenhout M, Slippers B, Wingfield MJ.2005. Cylindrocladium blight of Eucalyptus grandis in Colombia. Australasian Plant Pathology 34: 143 – 149 [Google Scholar]
- Ronquist F, Huelsenbeck JP.2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572 – 1574 [DOI] [PubMed] [Google Scholar]
- SAS Institute 1999. SAS/STAT users guide, Version 8. Cary NC, SAS Institute Inc; [Google Scholar]
- Schoch CL, Crous PW, Polizzi G, Koike ST.2001. Female fertility and single nucleotide polymorphism comparisons in Cylindrocladium pauciramosum. Plant Disease 85: 941 – 946 [DOI] [PubMed] [Google Scholar]
- Schoch CL, Crous PW, Wingfield BD, Wingfield MJ.1999. The Cylindrocladium candelabrum species complex includes four distinct mating populations. Mycologia 91: 286 – 298 [Google Scholar]
- Sharma JK, Mohanan C.1991. Pathogenic variation in Cylindrocladium quinqueseptatum causing leaf blight of Eucalyptus. European Journal of Forest Pathology 21: 210 – 217 [Google Scholar]
- Sharma JK, Mohanan C.1992. Relative susceptibility of Eucalyptus provenances to Cylindrocladium leaf blight in Kerala, India. European Journal of Forest Pathology 22: 257 – 265 [Google Scholar]
- Sharma JK, Mohanan C, Florence EJM.1984. Nursery diseases of Eucalyptus in Kerala. European Journal of Forest Pathology 14: 77 – 89 [Google Scholar]
- Smith H, Crous PW, Wingfield MJ, Coutinho TA, Wingfield BD.2001. Botryosphaeria eucalyptorum sp. nov., a new species in the B. dothidea-complex on Eucalyptus in South Africa. Mycologia 93: 277 – 285 [Google Scholar]
- Sun YX, Liu JF.2004. Current situation and control of diseases and insect pests in eucalypts. Forest Pest and Disease 4: 36 – 38 (In Chinese.) [Google Scholar]
- Swofford DL.2002. PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods). 4.0b10. Computer program. Sinauer Associates, Sunderland, Massachusetts, USA: [Google Scholar]
- Wang WY.1992. Survey of Eucalyptus diseases in Taiwan. Bulletin Taiwan Forest Research Institute 7: 179 – 194 (In Chinese.) [Google Scholar]
- Wingfield MJ, Slippers B, Hurley BP, Coutinho TA, Wingfield BD, Roux J.2008. Eucalypt pests and diseases: growing threats to plantation productivity. Southern Forests 70: 139 – 144 [Google Scholar]
- Xie YJ.2006. Eucalypt research at the beginning of 21st century. China Forestry House Press, Beijing, China: (In Chinese.) [Google Scholar]
- Zhou XD, Xie YJ, Chen SF, Wingfield MJ.2008. Diseases of eucalypt plantations in China: challenges and opportunities. Fungal Diversity 32: 1 – 7 [Google Scholar]