Abstract
T-bet was initially described as a T-box transcription factor with an essential role in orchestrating Th1 cell differentiation. Subsequently, it was determined that T-bet controls the expression of numerous cytokines and their receptors, adhesion molecules and chemokine receptors, and therefore determines the differentiation and development status of many types of immune cells. The critical role of T-bet in autoimmune diseases, particularly multiple sclerosis and its animal model experimental autoimmune encephalomyelitis, implicates it as a potential biomarker for pathogenic T cells as well as a therapeutic drug target.
Keywords: autoimmune disease, autoreactive CD4+ T cell, IFN-γ, T-bet, Th1 cell
T-bet is a key regulator of immunity & autoimmunity
For the past several decades, cytokines have been in the spotlight of immunology research and, as a result, we have gained important insights into the complex interactions between the various cell types of the immune system [1–3]. Despite this, the molecular mechanisms by which cytokines control the differentiation of T helper (Th) cells and mediate autoimmune diseases, such as multiple sclerosis (MS) and its animal model experimental autoimmune encephalomyelitis (EAE), remain poorly understood [4–9]. Recently, the transcription factor T-bet has emerged as a key regulator of Th1 cell differentiation and as a central player in autoimmune diseases [10–16].
T-bet (also known as Tbx-21) was first described by Szabo et al. in 2000 as a novel T-box transcription factor [14]. The gene for this transcription factor was cloned and named T-bet, for T-box expressed in T cells. T-bet is translated into a protein bearing a T-box DNA-binding domain. Importantly, it was found that T-bet expression was not only restricted to T lymphocytes, particularly Th1 cells, but also correlated with IFN-γ production in NK and B cells. Thus, it was shown that CD4+ T cells in T-bet−/− mice produced remarkably less IFN-γ and failed to differentiate into the Th1 lineage [17]. Furthermore, NK cells from T-bet−/− mice were impaired in IFN-γ production as well as in their cytotoxic effector functions. In addition, it was noted that T-bet deficiency in mice resulted in significantly reduced production of antigen-specific IgG2a by B cells. Since these initial reports, an increasing number of studies have revealed multiple roles for T-bet in the regulation of immunity and autoimmunity.
T-bet is a key regulator of Th1 differentiation in CD4+ T cells
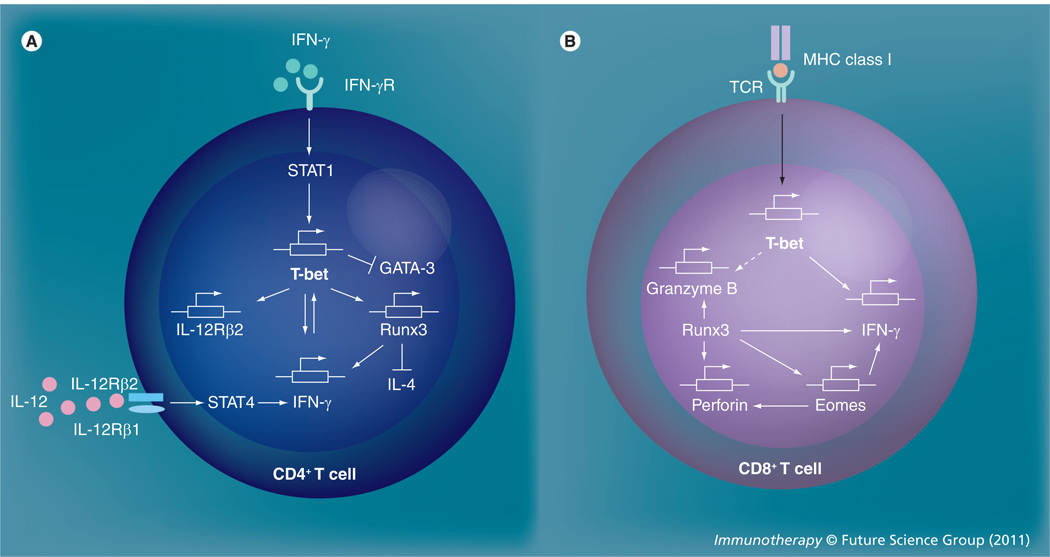
As previously mentioned, T-bet was initially described as a potent transactivator that can promote IFN-γ production by initiating Th1 lineage development from naive Th precursor cells, or even previously differentiated Th2 cells, following retroviral transduction [14]. Once induced, T-bet specifies Th1 cell fate by initiating chromatin/histone remodeling of IFN-γ gene alleles and by inducing IL-12 receptor β2 (IL-12Rβ2) expression, which takes place prior to IL-12-dependent lineage commitment [18]. T-bet promotes the transcription of the IFN-γ gene through dissociation of a corepressor (mSin3a) from the IFN-γ promoter without altering the methylation status at the promoter region [19], as well as by removal of Sin3A-histone deacetylase (HDAC) complexes, which leads to the accumulation of H4 acetylation marks at the Ifng locus [20]. Subsequently, the transcription factor Runx3, which is induced in a T-bet-dependent manner, maintains maximal production of IFN-γ and, together with T-bet, silences the IL-4 gene in Th1 cells [11,16]. The early induction of T-bet is dependent on signal transducer and activator of transcription (STAT)1, but not STAT4/IL-12, and can be enhanced rapidly and directly by its primary target IFN-γ in a positive-feedback loop [21,22]. After the initiation of Th1 differentiation, T-bet and STAT4 are both required to achieve complete IL-12-dependent Th1 development (Figure 1A) [23]. T-bet also promotes the Th1 phenotype by actively suppressing Th2 differentiation through tyrosine kinase ITK-mediated interactions that interfere with the binding of GATA-3 to its target DNA, which was initially believed to be the principal function of T-bet in promoting Th1 development [24,25]. T-bet is also involved in modulating the proliferation of T cells, as serine residue 508 (S508) on T-bet has been shown to be required for the optimal regulation of IL-2 production during development of Th1 cells [26]. In fact, not only the hallmark Th1 cytokine IFN-γ, but also CD122 (IL-2/IL-15 receptor β-chain) and CXCR3 are the direct gene targets of T-bet [27]. Furthermore, studies on Th cell migration showed that T-bet controls selectin ligand formation in Th1 cells, and is therefore critical for the binding of CD4+ T cells to P- and E-selectins on inflammatory endothelium [28,29]. Recent work has revealed an important role for T-bet in modulating the appropriate termination of proinflammatory Th1 immune responses by regulating the membrane protein Tim3, which is expressed late during development by IFN-γ-secreting CD4+ Th1 cells and dendritic cells (DCs) [30]. Thus, T-bet determines and sustains Th1 lineage commitment, as well as controlling Th1 cell trafficking into inflammatory sites.
Figure 1. Role of T-bet as a transcription factor in the differentiation of T cells.
(A) T-bet is a key regulator of the Th1 differentiation of CD4+ T cells. Once induced by the IFN-γ–STAT1 signaling pathway, T-bet promotes the transcription of IFN-γ and induces Runx3 and IL-12Rβ2 expression. IFN-γ can also enhance T-bet expression in a positive-feedback loop. Runx3 maintains the maximal production of IFN-γ and silences the IL-4-encoding gene. The completion of Th1 development requires IL-12–STAT4 signaling as well as the suppression of the Th2 differentiation-related transcription factor GATA-3 by T-bet. (B) T-bet is involved in the early induction of IFN-γ in the development of cytotoxic CD8+ effector T cells. Upon stimulation via the TCR, T-bet is induced and subsequently induces the transcription of IFN-γ. The transcription factor, Runx3, induces Eomes and then synergistically induces and maintains the production of IFN-γ, as well as the expression of granzyme B and perforin.
Eomes: Eomesodermin; IFN-γR: IFN-γ receptor; IL-12Rβ2: IL-12 receptor β2; TCR: T-cell receptor.
T-bet in immune cells other than CD4+ T cells
Although T-bet is considered the ‘master regulator’ of CD4+ Th1 cell differentiation and controls the production of IFN-γ by Th1 and NK cells, its requirement is less stringent in controlling the production of IFN-γ by cytotoxic effector CD8+ T cells [17]. A recent in vitro study has demonstrated that T-bet is induced quickly upon T cell receptor stimulation and is required for activated CD8+ T cells to begin early production of IFN-γ, whereas another T-box transcription factor, eomesodermin (Eomes), is induced later in order to sustain IFN-γ expression. During this process, Runx3, whose expression is T-bet independent in CD8+ T cells, cooperates with T-bet, Eomes and IL-2Rβ signals to promote the expression of granzyme B and perforin, two other mediators of cytotoxic T-cell effector function (Figure 1B) [31]. Similarly, T-bet and Eomes, together, were found to be important for maintaining effector function in long-term memory CD8+ T cells [32]. High or low expression levels of T-bet, respectively, can promote differentiation of activated CD8+ T cells into either transient cytotoxic effectors, or long-lived memory cells [33]. Furthermore, T-bet deficiency can impair the antigen-driven function of effector CD8+ T cells and result in reduced type 1 immune responses against certain viral infections [34]. T-bet is also essential for the optimal production of IFN-γ by DCs, and T-bet deficient DCs are impaired in their ability to activate antigen-specific Th1 cells in vivo [35]. For B cell function, T-bet is involved in the induction of IgG2a class switching mediated by IFN-γ [36,37]. Finally, T-bet is required for the control of IFN-γ production by NK cells, completion of terminal maturation, effector function and regulation of their homeostatic balance [17,38–40].
T-bet in autoimmune disease models
T cells are believed to be important mediators of many autoimmune diseases and T-bet may have a dichotomous role in either promoting or downregulating disease (Table 1). T-bet is a key regulator for Th1 CD4+ cells, but has also been shown to be involved in the regulation of CD8+ T cells; therefore, its role in autoimmune diseases has been under investigation since its discovery. For example, in autoimmune experimental colitis, a model for Crohn’s disease, T-bet was shown to be a key regulator of mucosal Th1 activation via IFN-γ production and cell surface IL-12Rβ2 augmentation [13,41]. T-bet deficiency has a protective role in Th1-mediated adoptive transfer-induced colitis in severe combined immunodeficiency (SCID) mice. However, it results in increased susceptibility in a Th2-mediated oxazolone-induced inflammatory bowel disease model [13]. Furthermore, in a mouse model for autoimmune Type 1 diabetes (T1D), T-bet intrinsically controlled the generation of autoreactive CD8+ T cells during disease development. In the absence of T-bet the disease was ameliorated, which correlated with a reduced number of autoaggressive CD8+ T cells and decreased production of IFN-γ [42]. Since T-bet is also required for IgG2a antibody isotype switching of B cells [36,37], T-bet-deficient mice were found to be less susceptible in an experimental model of systemic lupus erythematosus [36] as well as in experimental autoimmune myasthenia gravis [43]. In addition, in an animal model for human rheumatoid arthritis, T-bet expression in DCs was found to be important for mediating autoimmune inflammatory responses [44]. By contrast, T-bet has been shown to negatively regulate experimental autoimmune myocarditis, an experimental model for human acute myocarditis, by limiting tissue-specific IL-17 production in the inflamed heart [45]. Overall, the absence of T-bet in most animal models reduced the susceptibility to Th1 type autoimmune diseases, whereas it promoted Th2- or Th17-mediated autoimmune responses.
Table 1.
Effect of T-bet deficiency in different autoimmune disease models.
| Autoimmune disease/experimental model | Effect of T-bet deficiency |
|---|---|
| Autoimmune myasthenia gravis/EAMG | Decreased susceptibility |
| Autoimmune myocarditis/EAM | Exacerbation |
| Inflammatory bowel disease/AT-SCID/oxazolone-induced | Protection and increased susceptibility |
| Multiple sclerosis/EAE | Protection |
| Rheumatoid arthritis/CIA | Disease reduction |
| Type 1 diabetes/LCMV/NOD | Disease reduction and protection |
AT-SCID: Adoptive transfer into SCID recipients (a mouse model for inflammatory bowel disease induced by adoptive transfer of CD4+CD62L+ cells to SCID mice); CIA: Collagen-induced arthritis model; EAE: Experimental autoimmune encephalomyelitis; EAM: Experimental autoimmune myocarditis; EAMG: Experimental autoimmune myasthenia gravis; LCMV: Lymphocytic choriomeningitis virus (a transgenic mouse model for virally induced Type 1 diabetes); NOD: Non-obese diabetic mouse model; SCID: Severe combined immunodeficiency.
In EAE, the identification of pathogenic subsets of autoreactive CD4+ T cells has been hindered by conflicting results showing that IFN-γ-secreting Th1 cells and IL-17-secreting Th17 cells are both involved in the pathogenesis of disease, yet neither have been shown to be indispensable for the initiation and/or progression of pathology [46–52]. Clearly, no single ‘pathogenic’ cytokine, such as IFN-γ, IL-17 or IL-23 can take full responsibility for determining the pathogenesis of EAE [53–55]. However, studies of T-bet in the context of MS and EAE have suggested that it is indispensible for the pathogenesis of the disease. In particular, T-bet−/− mice are resistant to the development of disease, while STAT1−/− mice are highly susceptible to EAE, even though T-bet is regulated downstream of STAT1 [10]. These results are consistent with other studies that have used T-bet-specific antisense oligonucleotides and siRNAs to demonstrate inhibition of EAE in mice by silencing T-bet expression and subsequent downregulation of IFN-γ production, as well as IL-23 receptor and IL-17 expression in the CNS [56,57]. Similarly, EAE induced via adoptive transfer of encephalitogenic T cells in mice showed that T-bet plays a critical role in maintaining CD4+ T-cell pathogenicity during disease progression [58]. Work by Yang and colleagues demonstrated that the expression of T-bet is the key factor for the ability of encephalitogenic Th1 or Th17 cells to induce EAE, regardless of Th1 or Th17 cytokine expression [15]. Our own studies showed that T-bet was profoundly inhibited in neuroantigen-reactive T cells by the glucocorticoid (GC) dexamethasone [Ji, N & Forsthuber, TG: Unpublished Data], which correlated with the severity of disease, providing additional support for the view that T-bet is critical for determining the pathogenicity of autoreactive T cells.
Taken together, a mounting body of evidence supports a critical role for T-bet in the differentiation and pathogenesis of autoreactive T cells beyond its role in regulating cytokine production, but the exact mechanisms remain to be elucidated.
T-bet as a biomarker for autoimmune disease & potential target for therapy
In addition to evidence from animal models, studies in human patients have suggested that T-bet is associated with episodes of disease and relapse in various autoimmune disease conditions, and therefore could potentially be an important target for new therapies as well as a biomarker to monitor disease. For example, investigation of T-bet polymorphisms in T1D found an association with the Gln+ genotype of T-bet [59]. In addition, polymorphisms in the gene encoding either T-bet or its promoter have been reported to differentially contribute to the development of asthma in humans [60–62]. Thus, the Gln+ genotype of T-bet has been associated with improved asthma symptoms in patients treated with inhaled GCs [62]. Interestingly, several epidemiological studies have shown a significant correlation between idiopathic dilated cardiomyopathy and a history of asthma, and therefore T-bet might have a common molecular mechanism in these pathologies [63,64]. In systemic lupus erythematosus, a 4-year follow-up study on the urinary mRNA expression of T-bet in patients showed that high levels of T-bet correlated with a significantly higher risk of disease flare-ups, indicating that urinary T-bet could serve as an independent predictor of lupus relapses [65]. Additional studies showed that T-bet was one of the transcription factors upregulated in peripheral blood mononuclear cells (PBMCs) of relapsing–remitting MS patients during the relapsing phase compared with patients in remission or healthy subjects [12,66]. Another study focused on the analysis of T-bet levels in PBMCs from MS patients after 1 year of IFN-β treatment and showed that patients who responded well to IFN-β treatment also exhibited a decrease in baseline T-bet mRNA in PBMCs [67].
Thus, evidence from human studies also implicates T-bet as a strong candidate for a ‘biomarker’ of autoimmune disease activity, as well as a predictor for treatment responses and as a potential drug target. The latter view is supported by literature showing that inhibition of T-bet could be one of the mechanisms underlying the immunosuppressive properties of GCs [68,69]. Accordingly, activated GC receptors can inhibit T-bet by either direct physical interaction, resulting in reduced T-bet binding to DNA and inhibition of transcriptional activity of T-bet at the IFN-γ promoter, or interference at the mRNA and protein expression level, for which the first zinc-finger region of the GC receptor is required [69]. Furthermore, high-dose intravenous administration of GCs has been shown to greatly reduce T-bet and pSTAT1 expression in circulating lymphocytes as well as decrease IFN-γ production by PBMCs [68]. Our own studies have demonstrated that the cytokine macrophage migration inhibitory factor (MIF) promotes resistance to GC treatment in murine EAE, and that the suppressive effects of GCs on disease are substantially enhanced in MIF−/− mice, and are accompanied by a dramatic downregulation of T-bet expression and decreased pathogenicity of neuroantigen-reactive T cells [Ji N & Forsthuber TG, Unpublished Data]. A potentially important role for MIF in the regulation of T-bet is further supported by data indicating that MIF deficiency can result in the reduction of T-bet and IFN-γ expression in an infectious disease model [70]. Conceivably, targeting molecules such as MIF may therefore provide an alternative pathway for modulating the expression of T-bet, thereby reducing the pathogenicity of autoreactive T cells.
Alternative therapeutic candidates that could provide T-bet inhibition, such as the COX-2 inhibitors rofecoxib and lumiracoxib, have been shown to significantly reduce the incidence and severity of EAE in mice by suppressing T-bet expression [71]. In fact, the transcription factors that control T-bet could also be considered potential candidates for therapeutic targets in autoimmune diseases. For example, Sp1 is a positive transcriptional regulator of human T-bet that enhances T-bet expression, and its inhibition can diminish T-bet expression [72]. Another upstream regulator, early growth response 1, was recently reported as an essential transcription factor of human T-bet [73]. Strategically, the inhibition or interference of any of these upstream regulators may be more feasible and potent than direct targeting of T-bet in suppressing autoimmune disease in human patients.
Conclusion & future perspective
T-bet has emerged as a key regulator of T-cell differentiation and pathogenicity of autoimmune T cells and therefore offers an attractive therapeutic target to control inflammation during innate and adaptive immune responses. A growing number of evidence supports the notion that most models of inflammatory autoimmune diseases are, in one form or another, dependent on T-bet, such as in asthma [60,62], atherosclerosis [74], Crohn’s disease [13,41], myasthenia gravis [43], myocarditis [45], rheumatoid arthritis [44], systemic lupus erythematosus [65] and T1D [42,59]. Thus, monitoring of T-bet expression could serve as a biomarker for the disease process in a number of autoimmune disorders, including MS. Studies examining T-bet’s role in the pathogenesis of EAE, as well as in MS patients, strongly correlated T-bet expression with disease severity [10,12,15,56–58,66,67]. Since targeting of transcription factors directly with small molecule inhibitors had little success in the past, modulating upstream regulators of T-bet may provide a better alternative. For example, inhibition of MIF could prove successful in light of a recent study showing that MIF inhibition resulted in similar reduction of T-bet expression as inhibiting T-bet directly [70]. In support of this view, our as yet unpublished work showed that inhibition of MIF greatly increased the efficacy of GC treatment in EAE and substantially downregulated the expression of T-bet.
In conclusion, modulation of T-bet has been shown to attenuate clinical disease and to reduce inflammatory infiltrates in a number of T-cell-mediated autoimmune diseases. Despite unresolved questions surrounding its precise mechanism of action it therefore appears likely that T-bet will be useful as a biomarker as well as a therapeutic target in autoimmune disease and other inflammatory conditions.
Executive summary.
-
▪
A critical need exists for useful biomarkers for the monitoring of clinical activity of autoimmune diseases and treatment efficacy of immunomodulatory drugs.
-
▪
T-bet expression correlates well with disease severity in experimental models of autoimmune diseases and in human autoimmune diseases (e.g., multiple sclerosis).
-
▪
T-bet may be a more stringent marker for pathogenic T cells compared with cytokines such as IFN-γ or IL-17.
-
▪
T-bet is downregulated upon treatment with glucocorticoids.
-
▪
T-bet may therefore be a valid biomarker of disease activity and treatment efficacy.
-
▪
T-bet itself or its upstream regulators may be valid therapeutic targets.
Acknowledgments
This work was supported by grant NS-52177 and 2G12RR013646-11 from the NIH and grants RG3499 and RG3701 from the National Multiple Sclerosis Society (to Thomas G Forsthuber).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Commins SP, Borish L, Steinke JW. Immunologic messenger molecules: cytokines, interferons, and chemokines. J. Allergy Clin. Immunol. 2010;125:S53–S72. doi: 10.1016/j.jaci.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Kunz M, Ibrahim SM. Cytokines and cytokine profiles in human autoimmune diseases and animal models of autoimmunity. Mediators Inflamm. 2009 doi: 10.1155/2009/979258. Abstract 979258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 4.Axtell RC, de Jong BA, Boniface K, et al. T helper Type 1 and 17 cells determine efficacy of interferon-β in multiple sclerosis and experimental encephalomyelitis. Nat. Med. 2010;16:406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon γ-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J. Exp. Med. 2000;192:123–128. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Das J, Ren GW, Zhang LY, et al. Transforming growth factor β is dispensable for the molecular orchestration of Th17 cell differentiation. J. Exp. Med. 2009;206:2407–2416. doi: 10.1084/jem.20082286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleinschek MA, Owyang AM, Joyce-Shaikh B, et al. IL-25 regulates Th17 function in autoimmune inflammation. J. Exp. Med. 2007;204:161–170. doi: 10.1084/jem.20061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang XXO, Chang SH, Park H, et al. Regulation of inflammatory responses by IL-17F. Exp. Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher H, Kuchroo VK. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J. Exp. Med. 2004;200:79–87. doi: 10.1084/jem.20031819.. ▪ Demonstrates the distinctive effect of T-bet versus STAT1 deficiency in experimental autoimmune encephalolmyelitis (EAE) models, despite the fact that STAT1 is the inducer of T-bet in Th1 differentiation.
- 11.Djuretic IM, Levanon D, Negreanu V, Groner Y, Rao A, Ansel KM. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper Type 1 cells. Nat. Immunol. 2007;8:145–153. doi: 10.1038/ni1424. [DOI] [PubMed] [Google Scholar]
- 12.Frisullo G, Angelucci F, Caggiula M, et al. pSTAT1, pSTAT3, and T-bet expression in peripheral blood mononuclear cells from relapsing-remitting multiple sclerosis patients correlates with disease activity. J. Neurosci. Res. 2006;84:1027–1036. doi: 10.1002/jnr.20995. [DOI] [PubMed] [Google Scholar]
- 13.Neurath MF, Weigmann B, Finotto S, et al. The transcription factor T-bet regulates mucosal T cell activation in experimental colitis and Crohn’s disease. J. Exp. Med. 2002;195:1129–1143. doi: 10.1084/jem.20011956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Szabo SJ, Kim ST, Costa GL, Zhang XK, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3.. ▪▪ First study to report isolating and identifying T-bet as a novel transcription factor whose expression correlates with IFN-γ expression in Th1 and NK cells, as well as an initiator and promoter of Th1 differentiation.
- 15. Yang YH, Weiner J, Liu Y, et al. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. J. Exp. Med. 2009;206:1549–1564. doi: 10.1084/jem.20082584.. ▪▪ Challenges the importance of Th1 and Th17 cytokines in determining the pathogenicity of autoreactive T cells by providing new evidence of T-bet-dependent encephalitogenicity in the EAE model.
- 16.Zhuang YH, Huang Z, Nishida J, Brown M, Zhang L, Huang H. A continuous T-bet expression is required to silence the interleukin-4-producing potential in T helper type 1 cells. Immunology. 2009;128:34–42. doi: 10.1111/j.1365-2567.2009.03049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in Th1 lineage commitment and IFN-γ production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543.. ▪ First study to provide evidence of T-bet’s role in the regulation of Th1 lineage development as well as its effect on IFN-γ production in CD4+, CD8+ T cells and NK cells using T-bet-deficient mice.
- 18.Mullen AC, High FA, Hutchins AS, et al. Role of T-bet in commitment of Th1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 19.Tong YK, Aune T, Boothby M. T-bet antagonizes mSin3a recruitment and transactivates a fully methylated IFN-γ promoter via a conserved T-box half-site. Proc. Natl Acad. Sci. USA. 2005;102:2034–2039. doi: 10.1073/pnas.0409510102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang SJ, Collins PL, Aune TM. T-bet dependent removal of Sin3A-histone deacetylase complexes at the Ifng locus drives Th1 differentiation. J. Immunol. 2008;181:8372–8381. doi: 10.4049/jimmunol.181.12.8372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lighvani AA, Frucht DM, Jankovic D, et al. T-bet is rapidly induced by interferon-γ in lymphoid and myeloid cells. Proc. Natl Acad. Sci. USA. 2001;98:15137–15142. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Afkarian M, Sedy JR, Yang J, et al. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat. Immunol. 2002;3:549. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 23.Thieu VT, Yu Q, Chang HC, et al. Signal transducer and activator of transcription 4 is required for the transcription factor T-bet to promote T helper 1 cell-fate determination. Immunity. 2008;29:679–690. doi: 10.1016/j.immuni.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005;307:430–433. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- 25.Usui T, Preiss JC, Kanno Y, et al. T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. J. Exp. Med. 2006;203:755–766. doi: 10.1084/jem.20052165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang ES, Hong JH, Glimcher LH. IL-2 production in developing Th1 cells is regulated by heterodimerization of RelA and T-bet and requires T-bet serine residue 508. J. Exp. Med. 2005;202:1289–1300. doi: 10.1084/jem.20051044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuda JL, George TC, Hagman J, Gapin L. Temporal dissection of T-bet functions. J. Immunol. 2007;178:3457–3465. doi: 10.4049/jimmunol.178.6.3457. [DOI] [PubMed] [Google Scholar]
- 28.Underhill GH, Zisoulis DG, Kolli KP, Ellies LG, Marth JD, Kansas GS. A crucial role for T-bet in selectin ligand expression in T helper 1 (Th1) cells. Blood. 2005;106:3867–3873. doi: 10.1182/blood-2005-03-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lord GM, Rao RM, Choe H, et al. T-bet is required for optimal proinflammatory CD4+ T-cell trafficking. Blood. 2005;106:3432–3439. doi: 10.1182/blood-2005-04-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson AC, Lord GM, Dardalhon V, et al. T-bet, a Th1 transcription factor regulates the expression of Tim-3. Eur. J. Immunol. 2010;40:859–866. doi: 10.1002/eji.200939842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cruz-Guilloty F, Pipkin ME, Djuretic IM, et al. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J. Exp. Med. 2009;206:51–59. doi: 10.1084/jem.20081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Intlekofer AM, Takemoto N, Wherry EJ, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 33.Joshi NS, Cui W, Chandele A, et al. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc. Natl Acad. Sci. USA. 2003;100:15818–15823. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lugo-Villarino G, Maldonado-Lopez R, Possemato R, Penaranda C, Glimcher LH. T-bet is required for optimal production of IFN-γ and antigen-specific T cell activation by dendritic cells. Proc. Natl Acad. Sci. USA. 2003;100:7749–7754. doi: 10.1073/pnas.1332767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng SL, Szabo SJ, Glimcher LH. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc. Natl Acad. Sci. USA. 2002;99:5545–5550. doi: 10.1073/pnas.082114899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerth AJ, Lin L, Peng SL. T-bet regulates T-independent IgG2a class switching. Int. Immunol. 2003;15:937–944. doi: 10.1093/intimm/dxg093. [DOI] [PubMed] [Google Scholar]
- 38.Townsend MJ, Weinmann AS, Matsuda JL, et al. T-bet regulates the terminal maturation and homeostasis of NK and Vα14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 39.Robbins SH, Tessmer MS, Van Kaer L, Brossay L. Direct effects of T-bet and MHC class I expression, but not STAT1, on peripheral NK cell maturation. Eur. J. Immunol. 2005;35:757–765. doi: 10.1002/eji.200425797. [DOI] [PubMed] [Google Scholar]
- 40.Jenne CN, Enders A, Rivera R, et al. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J. Exp. Med. 2009;206:2469–2481. doi: 10.1084/jem.20090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsuoka K, Inoue N, Sato T, et al. T-bet upregulation and subsequent interleukin 12 stimulation are essential for induction of Th1 mediated immunopathology in Crohn’s disease. Gut. 2004;53:1303–1308. doi: 10.1136/gut.2003.024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Juedes AE, Rodrigo E, Togher L, Glimcher LH, von Herrath MG. T-bet controls autoaggressive CD8 lymphocyte responses in Type I diabetes. J. Exp. Med. 2004;199:1153–1162. doi: 10.1084/jem.20031873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu RL, Hao JW, Dayao CS, Shi FD, Campagnolo DI. T-bet deficiency decreases susceptibility to experimental myasthenia gravis. Exp. Neurol. 2009;220:366–373. doi: 10.1016/j.expneurol.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 44.Wang JS, Fathman JW, Lugo-Villarino G, et al. Transcription factor T-bet regulates inflammatory arthritis through its function in dendritic cells. J. Clin. Invest. 2006;116:414–421. doi: 10.1172/JCI26631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rangachari M, Mauermann N, Marty RR, et al. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J. Exp. Med. 2006;203:2009–2019. doi: 10.1084/jem.20052222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferber IA, Brocke S, TaylorEdwards C, et al. Mice with a disrupted IFN-α gene are susceptible to the induction of experimental autoimmune encephalolmyelitis (EAE) J. Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 47.Haak S, Croxford AL, Kreymborg K, et al. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J. Clin. Invest. 2009;119:61–69. doi: 10.1172/JCI35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heremans H, Dillen C, Groenen M, Martens E, Billiau A. Chronic relapsing experimental autoimmune encephalomyelitis (CREAE) in mice: enhancement by monoclonal antibodies against interferon-γ. Eur. J. Immunol. 1996;26:2393–2398. doi: 10.1002/eji.1830261019. [DOI] [PubMed] [Google Scholar]
- 49.Komiyama Y, Nakae S, Matsuki T, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 50.Voskuhl RR, Martin R, Bergman C, Dalal M, Ruddle NH, Mcfarland HF. T-helper-1 (Th1) functional phenotype of human myelin basic protein-specific T-lymphocytes. Autoimmunity. 1993;15:137–143. doi: 10.3109/08916939309043888. [DOI] [PubMed] [Google Scholar]
- 51.Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-γ plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J. Immunol. 1996;157:3223–4227. [PubMed] [Google Scholar]
- 52.Lublin FD, Knobler RL, Kalman B, et al. Monoclonal anti-γ interferon antibodies enhance experimental allergic encephalomyelitis. Autoimmunity. 1993;16:267–274. doi: 10.3109/08916939309014645. [DOI] [PubMed] [Google Scholar]
- 53.Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J. Exp. Med. 2008;205:1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sutton C, Brereton C, Keogh B, Mills KHG, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFγ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 56. Lovett-Racke AE, Rocchini AE, Choy J, et al. Silencing T-bet defines a critical role in the differentiation of autoreactive T lymphocytes. Immunity. 2004;21:719–731. doi: 10.1016/j.immuni.2004.09.010.. ▪▪ First study to demonstrate T-bet as a potential therapeutic target in T helper cell-mediated autoimmune diseases using antisense oligonucleotides and siRNA.
- 57. Gocke AR, Cravens PD, Ben LH, et al. T-bet regulates the fate of Th1 and Th17 lymphocytes in autoimmunity. J. Immunol. 2007;178:1341–1348. doi: 10.4049/jimmunol.178.3.1341.. ▪ Gives new insights into T-bet’s role in the pathogenicity of Th17 cells via the regulation of IL-23 receptor expression.
- 58.Nath N, Prasad R, Giri S, Singh AK, Singh I. T-bet is essential for the progression of experimental autoimmune encephalomyelitis. Immunology. 2006;118:384–391. doi: 10.1111/j.1365-2567.2006.02385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sasaki Y, Ihara K, Matsuura N, et al. Identification of a novel Type 1 diabetes susceptibility gene, T-bet. Hum. Genet. 2004;115:177–184. doi: 10.1007/s00439-004-1146-2. [DOI] [PubMed] [Google Scholar]
- 60.Akahoshi M, Obara K, Hirota T, et al. Functional promoter polymorphism in the TBX21 gene associated with aspirin-induced asthma. Hum. Genet. 2005;117:16–26. doi: 10.1007/s00439-005-1285-0. [DOI] [PubMed] [Google Scholar]
- 61.Raby BA, Hwang ES, Van Steen K, et al. T-bet polymorphisms are associated with asthma and airway hyperresponsiveness. Am. J. Resp. Crit. Care Med. 2006;173:64–70. doi: 10.1164/rccm.200503-505OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tantisira KG, Hwang ES, Raby BA, et al. TBX21: a functional variant predicts improvement in asthma with the use of inhaled corticosteroids. Proc. Natl Acad. Sci. USA. 2004;101:18099–180104. doi: 10.1073/pnas.0408532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coughlin SS, Szklo M, Baughman K, Pearson TA. Idiopathic dilated cardiomyopathy and atopic disease – epidemiologic evidence for an association with asthma. Am. Heart J. 1989;118:768–774. doi: 10.1016/0002-8703(89)90591-7. [DOI] [PubMed] [Google Scholar]
- 64.Sengstock DM, Obeidat O, Pasnoori V, Mehra P, Sandberg KR, McCullough PA. Asthma, β-agonists, and development of congestive heart failure: results of the ABCHF study. J. Card. Fail. 2002;8:232–238. doi: 10.1054/jcaf.2002.127771. [DOI] [PubMed] [Google Scholar]
- 65.Chan RWY, Lai FM, Li EKM, et al. Expression of T-bet, a Type 1 T-helper cell transcription factor, in the urinary sediment of lupus patients predicts disease flare. Rheumatology. 2007;46:44–48. doi: 10.1093/rheumatology/kel192. [DOI] [PubMed] [Google Scholar]
- 66.Iorio R, Frisullo G, Nociti V, et al. T-bet, pSTAT1 and pSTAT3 expression in peripheral blood mononuclear cells during pregnancy correlates with post-partum activation of multiple sclerosis. Clin. Immunol. 2009;131:70–83. doi: 10.1016/j.clim.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 67.Drulovic J, Savic E, Pekmezovic T, et al. Expression of Th1 and Th17 cytokines and transcription factors in multiple sclerosis patients: does baseline T-bet mRNA predict the response to interferon-β treatment? J. Neuroimmunol. 2009;215:90–95. doi: 10.1016/j.jneuroim.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 68.Frisullo G, Nociti V, Lorio R, et al. Glucocorticoid treatment reduces T-bet and pSTAT1 expression in mononuclear cells from relapsing remitting multiple sclerosis patients. Clin. Immunol. 2007;124:284–293. doi: 10.1016/j.clim.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 69.Liberman AC, Refojo D, Druker J, et al. The activated glucocorticoid receptor inhibits the transcription factor T-bet by direct protein–protein interaction. FASEB J. 2007;21:1177–1188. doi: 10.1096/fj.06-7452com. [DOI] [PubMed] [Google Scholar]
- 70.Wong BL, Zhu SL, Huang XR, et al. Essential role for macrophage migration inhibitory factor in gastritis induced by Helicobacter pylori. Am. J. Pathol. 2009;174:1319–1328. doi: 10.2353/ajpath.2009.080708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ni J, Shu YY, Zhu YN, et al. COX-2 inhibitors ameliorate experimental autoimmune encephalomyelitis through modulating IFN-β and IL-10 production by inhibiting T-bet expression. J. Neuroimmunol. 2007;186:94–103. doi: 10.1016/j.jneuroim.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 72.Yu JH, Wei M, Boyd Z, et al. Transcriptional control of human T-bet expression: the role of Sp1. Eur. J. Immunol. 2007;37:2549–2561. doi: 10.1002/eji.200737088. [DOI] [PubMed] [Google Scholar]
- 73.Shin HJ, Lee JB, Park SH, Chang J, Lee CW. T-bet expression is regulated by EGR1-mediated signaling in activated T cells. Clin. Immunol. 2009;131:385–394. doi: 10.1016/j.clim.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 74.Buono C, Binder CJ, Stavrakis G, Witztum JL, Glimcher LH, Lichtman AH. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc. Natl Acad. Sci. USA. 2005;102:1596–1601. doi: 10.1073/pnas.0409015102. [DOI] [PMC free article] [PubMed] [Google Scholar]