Abstract
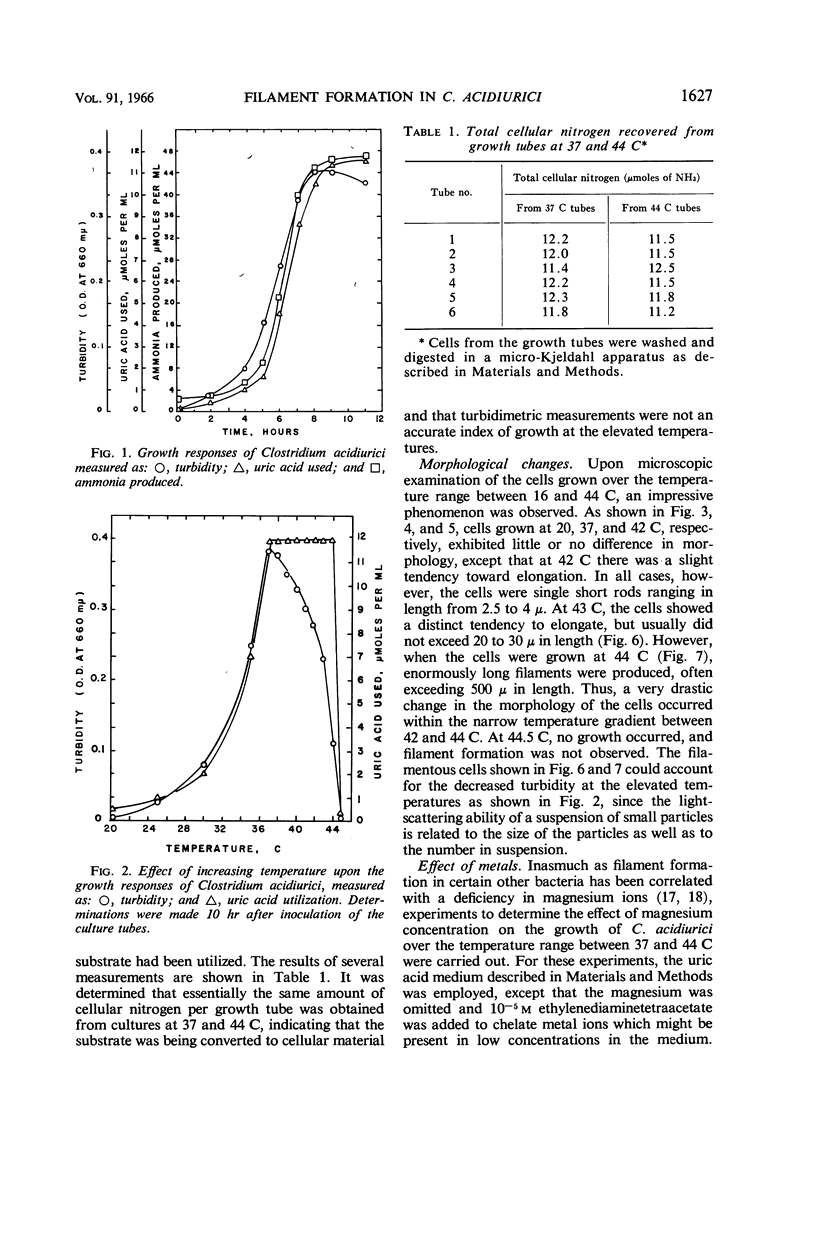
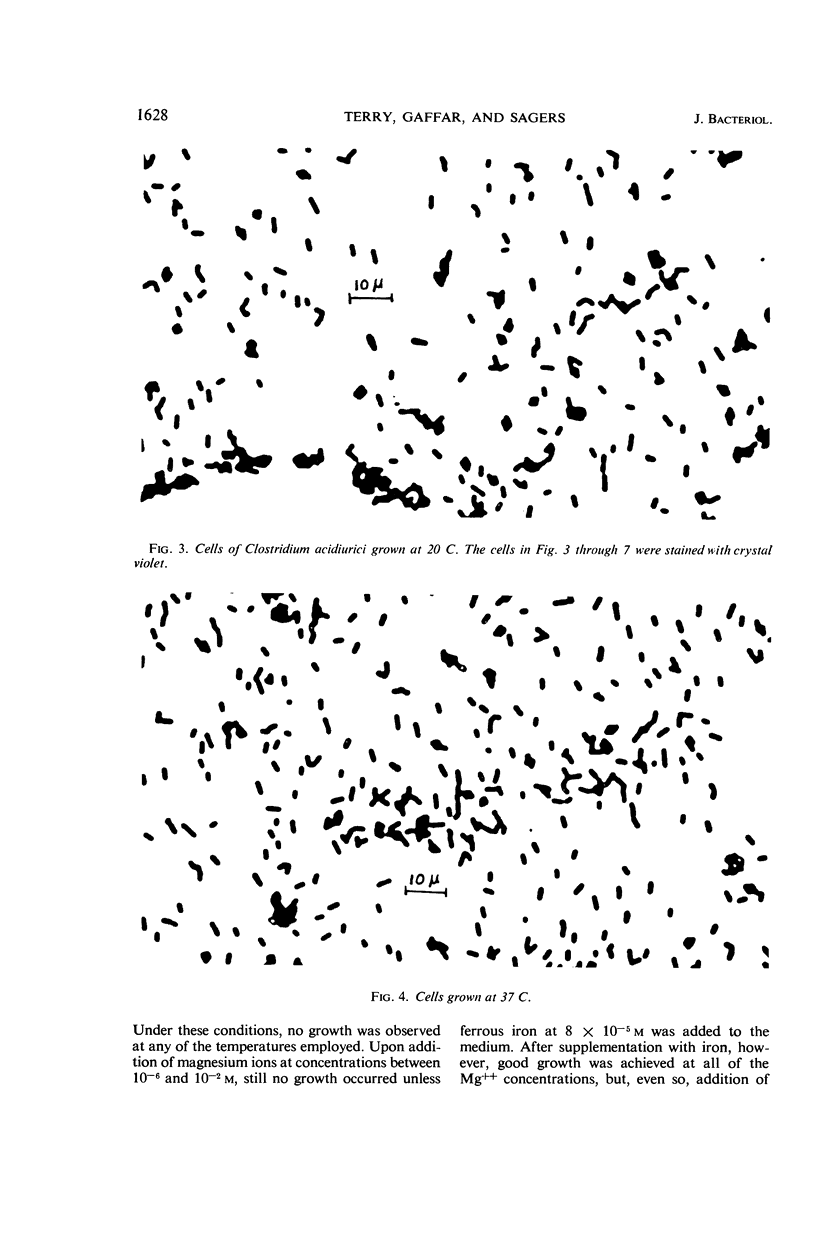
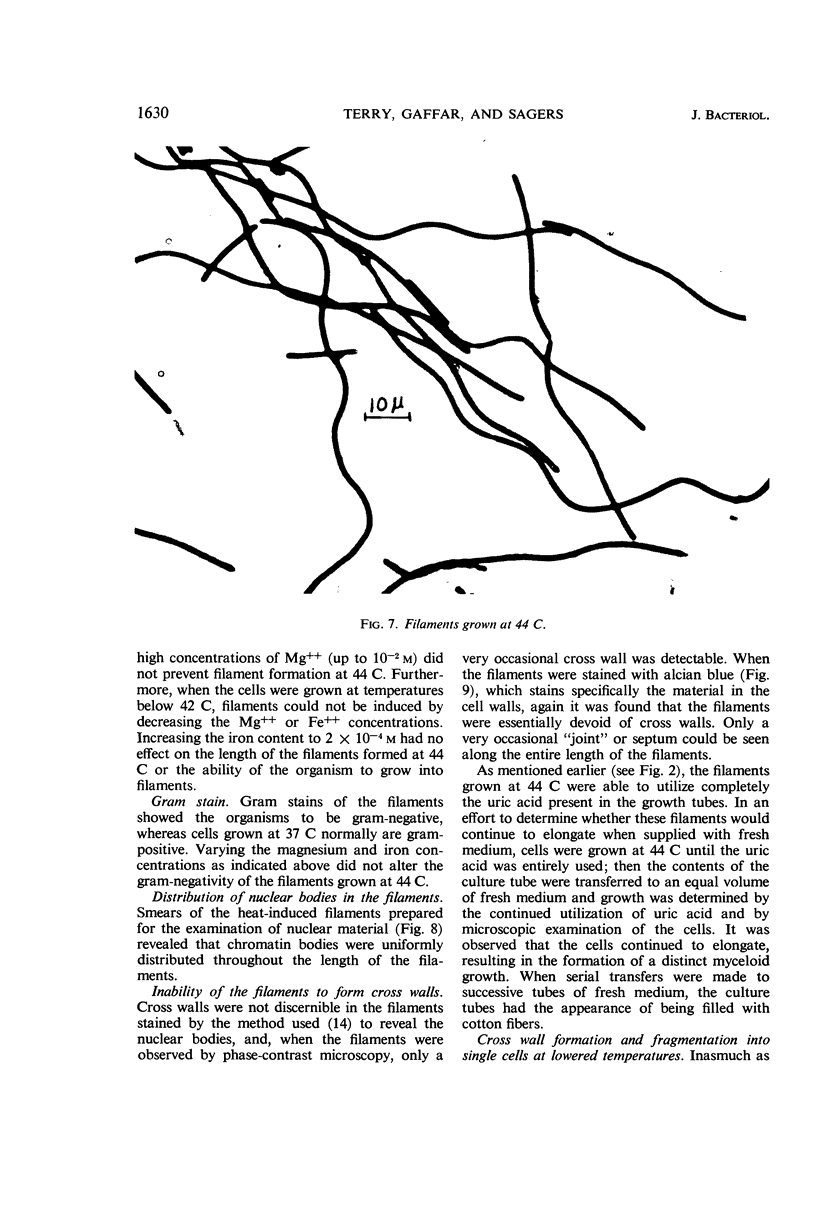
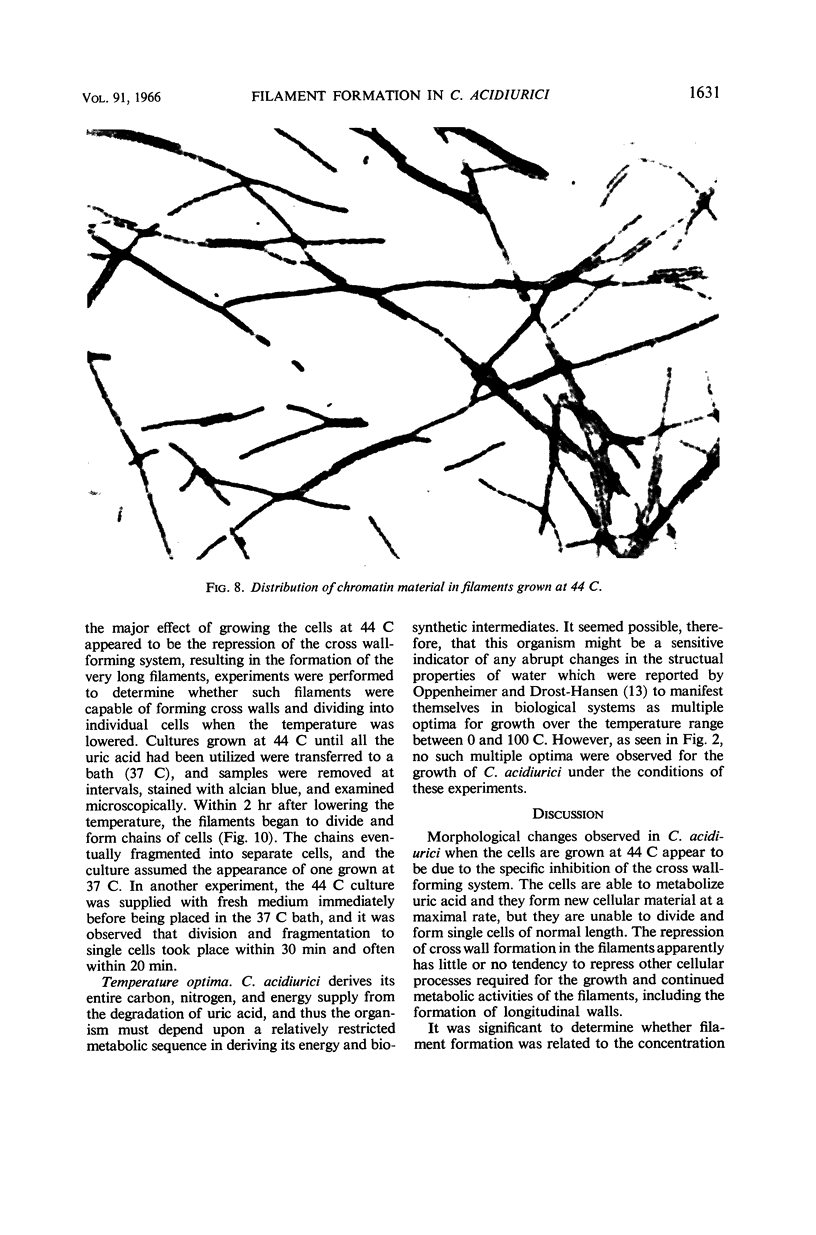
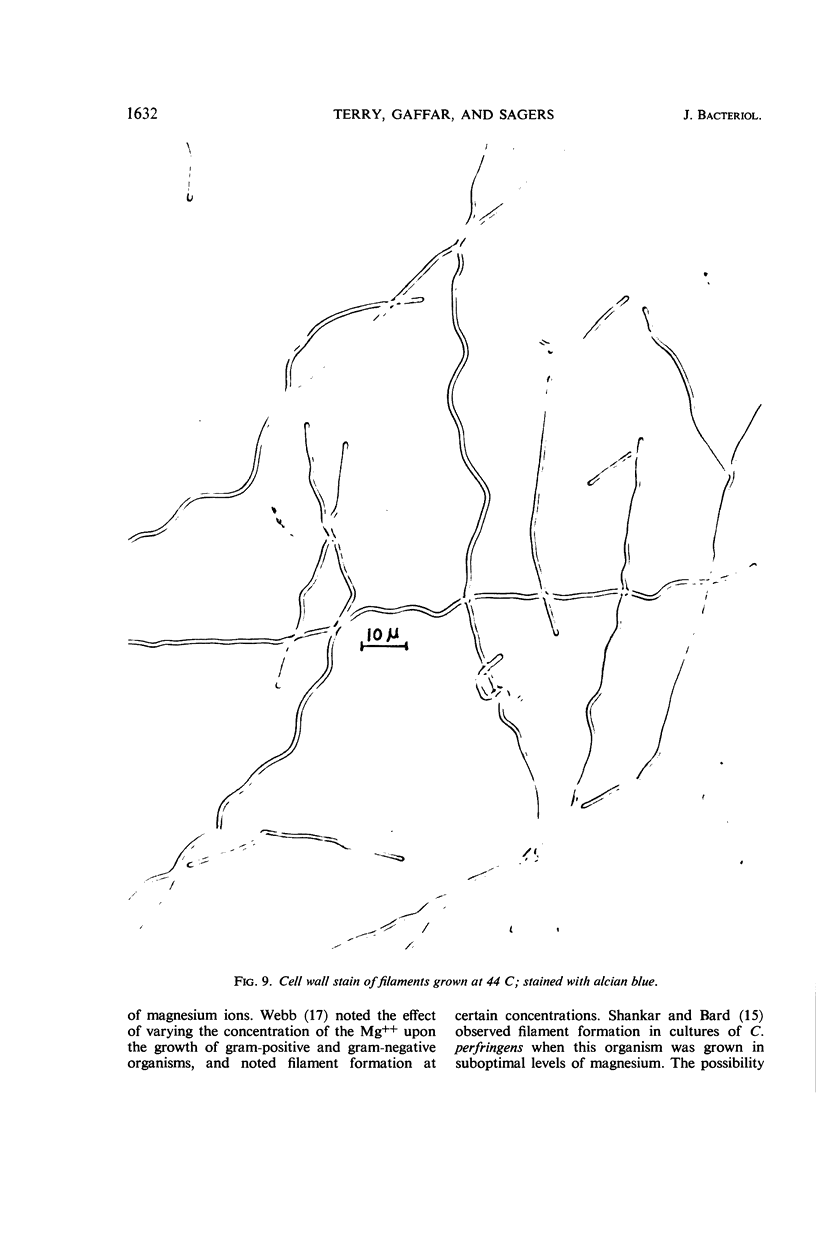
Terry, David R. (Brigham Young University, Provo, Utah), Abdul Gaffar, and Richard D. Sagers. Filament formation in Clostridium acidiurici under conditions of elevated temperatures. J. Bacteriol. 91:1625–1634. 1966.—Vegetative cells of Clostridium acidiurici, when grown at temperatures up to 42 C, are straight rods varying from 2.5 to 4 μ in length. When grown at 43 C, the cells show a definite tendency to elongate, and, when grown at 44 C, filaments are formed, often exceeding 500 μ in length. Only an occasional cross wall is apparent in the heat-induced long forms, but as the temperature is lowered they readily form cross walls and fragment into short, single cells. Chromatin material is distributed in evenly spaced clusters throughout the length of the filaments. The filaments grown at 44 C are gram-negative, whereas cells grown at 37 C are gram-positive. However, filament formation and gram-negativity apparently are not due to magnesium deficiency, since the gram-negative filaments are formed in concentrations of magnesium ranging from 10−6 to 10−2m. The rapid transition from filaments to single cells upon lowering the temperature from 44 to 37 C suggests that the temperature-related repression of the cross wall-forming system is a phenotypic response rather than the selection of specific mutants which produce the observed phenomena.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Hardigree A. A. Growth and Division of Filamentous Forms of Escherichia coli. J Bacteriol. 1965 Jul;90(1):223–226. doi: 10.1128/jb.90.1.223-226.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker H. A., Beck J. V. Clostridium acidi-uridi and Clostridium cylindrosporum, Organisms Fermenting Uric Acid and Some Other Purines. J Bacteriol. 1942 Mar;43(3):291–304. doi: 10.1128/jb.43.3.291-304.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLICK M. C., SALL T., ZILLIKEN F., MUDD S. Morphological changes of Lactobacillus bifidus var. pennsylvanicus produced by a cell-wall precursor. Biochim Biophys Acta. 1960 Jan 15;37:361–363. doi: 10.1016/0006-3002(60)90251-1. [DOI] [PubMed] [Google Scholar]
- GRULA E. A. Cell division in a species of Erwinia. I. Inhibition of division by D-amino acids. J Bacteriol. 1960 Sep;80:375–385. doi: 10.1128/jb.80.3.375-385.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRULA E. A., GRULA M. M. Cell division in a species of Erwinia. V. Effect of metabolic inhibitors on terminal division and composition of a "division" medium. J Bacteriol. 1962 Sep;84:492–499. doi: 10.1128/jb.84.3.492-499.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMAN H., FRANK M. E. TEMPERATURE LIMITS, GENEALOGICAL ORIGIN, DEVELOPMENTAL COURSE, AND ULTIMATE FATE OF HEAT-INDUCED FILAMENTS IN ESCHERICHIA COLI MICROCULTURES. J Bacteriol. 1963 Jun;85:1221–1234. doi: 10.1128/jb.85.6.1221-1234.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACLEAN F. I., MUNSON R. J. Some environmental factors affecting the length of Escherichia coli organisms in continuous cultures. J Gen Microbiol. 1961 May;25:17–27. doi: 10.1099/00221287-25-1-17. [DOI] [PubMed] [Google Scholar]
- OPPENHEIMER C. H., DROST-HANSEN W. A relationship between multiple temperature optima for biological systems and the properties of water. J Bacteriol. 1960 Jul;80:21–24. doi: 10.1128/jb.80.1.21-24.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHANKAR K., BARD R. C. The effect of metallic ions on the growth and morphology of Clostridium perfringens. J Bacteriol. 1952 Feb;63(2):279–290. doi: 10.1128/jb.63.2.279-290.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMCSIK J., GRACE J. B. Bacterial cells walls as revealed by the specific cell-wall reaction and by direct staining with alcian blue. J Gen Microbiol. 1955 Aug;13(1):105–110. doi: 10.1099/00221287-13-1-105. [DOI] [PubMed] [Google Scholar]
- WEBB M. The influence of magnesium on cell division. VI. The action of certain hydrolytic enzymes on the filamentous and chain forms of gram-positive rod-shaped organisms. J Gen Microbiol. 1951 Aug;5(3):496–501. doi: 10.1099/00221287-5-3-496. [DOI] [PubMed] [Google Scholar]
- ZAMENHOF S., DE GIOVANNI R., RICH K. Escherichia coli containing unnatural pyrimidines in its deoxyribonucleic acid. J Bacteriol. 1956 Jan;71(1):60–69. doi: 10.1128/jb.71.1.60-69.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOBELL C. E., COBET A. B. FILAMENT FORMATION BY ESCHERICHIA COLI AT INCREASED HYDROSTATIC PRESSURES. J Bacteriol. 1964 Mar;87:710–719. doi: 10.1128/jb.87.3.710-719.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]