Abstract
A wealth of published studies indicate that a variety of chemokines are actively secreted by the prostatic microenvironment consequent to disruptions in normal tissue homeostasis due to the aging process or inflammatory responses. The accumulation of senescent stromal fibroblasts, and, possibly, epithelial cells, may serve as potential driving forces behind chemokine secretion in the aging and enlarged human prostate. Chronic prostatitis/ chronic pelvic pain syndrome (CP/CPPS) and histological inflammation may also potentially serve as rich sources of chemokine secretion in the prostate. Once bound to their cognate receptors, chemokines can stimulate powerful pro-proliferation signal transduction pathways and thus function as potent growth factors in the development and progression of Benign Prostatic Hyperplasia (BPH) and Lower Urinary Tract Symptoms (LUTS). These functions have been amply demonstrated experimentally and particularly point to robust Mitogen Activated Protein Kinase (MAPK) and Phosphoinositide 3-kinase (PI3K) signaling, as well as global transcriptional responses, which mediate chemokine-stimulated cellular proliferative responses. A small body of literature also suggests that chemokine-mediated angiogenesis may comprise a contributing factor to BPH/LUTS development and progression. Thus, the observed low-level secretion of multiple chemokines within the aging prostatic microenvironment may promote a concomitant low-level, but cumulative, over-proliferation of both stromal fibroblastic and epithelial cell types associated with increased prostatic volume. Though the accumulated evidence is far from complete and suffers from some rather extensive gaps in knowledge, it argues favorably for the conclusion that chemokines can, and likely do, promote prostatic enlargement and the associated lower urinary tract symptoms, and justifies further investigations examining chemokines as potential therapeutic targets to delay or ablate BPH/LUTS initiation and progression.
Keywords: prostate, aging, chemokine, BPH, LUTS
The Chemokine Family
Chemokines, or chemotactic cytokines, are soluble, small molecular weight (8–14 kD) proteins that bind to their cognate G-protein coupled receptors (GPCRs) to elicit cellular responses (Rot and von Andrian, 2004). Chemokines govern multiple aspects of host defense and inflammation such as hematopoiesis, leukocyte trafficking, and angiogenesis (Baggiolini, 1998). As recently reviewed by Charo and Ransohoff (2006), the approximately 50 members of the chemokine family can be divided into four groups based on the structural arrangement of intramolecular cysteine residues and bridges as well as molecular function. The largest of these groups, the CC chemokines, attract mononuclear cells (e.g., monocytes and lymphocytes) to sites of chronic inflammation. The CXC chemokines attract polymorphonuclear leukocytes (e.g., granulocytes, primarily neutrophils) to sites of acute inflammation. The smallest chemokine groups, the C and Cx3C groups, have just 2 and 1 members each, respectively. The Cx3C chemokine, fractaline, is the only chemokine that exists in a membrane-bound pro-form that must be cleaved to become soluble and active (Charo and Ransohoff, 2006; Koch, 2005).
Chemokine Secretion in BPH/LUTS Tissue
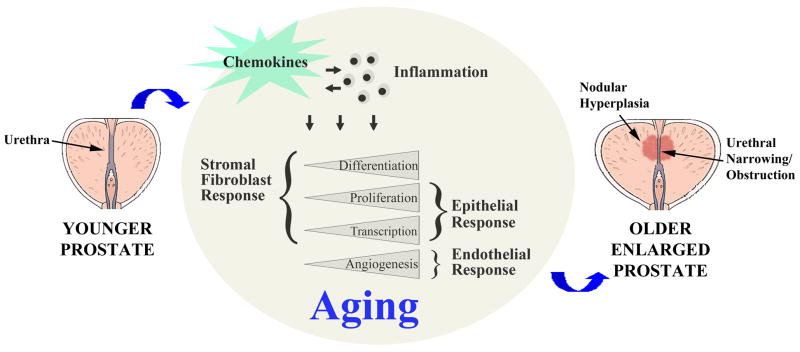
Chemokines can be secreted by multiple cell types within the tissue microenvironment, including endothelial cells, inflammatory cells, epithelial cells and fibroblastic cells (Gillitzer and Goebeler, 2001). Moreover, chemokine levels may increase consequent to disruptions in normal tissue homeostasis due to the aging process or due to inflammatory responses ensuing infection or trauma (Figure 1).
Figure 1. Chemokines Within the Aging Prostate Microenvironment Promote Benign Prostatic Hyperplasia (BPH) Development and Progression.
The diagram depicts a healthy prostate from a younger man (left) with competent urethral opening and no evidence of pathology. The middle circular diagram depicts the biological consequences of chemokine secretion consequent to the aging process. Continual and cumulative chemokine secretion by senescing prostate cells and inflammatory cells within the prostate microenvironment alters the gene transcription profiles of both stromal fibroblast and epithelial cells and thereby promotes cellular proliferation as well as fibroblast-myofibroblast differentiation. Chemokine-mediated endothelial chemotaxis promotes angiogenesis and vascularization. The net effect is an enlarged prostate with nodular hyperplasia and urethral narrowing with consequent obstructive lower urinary tract symptoms (LUTS).
Chemokine Secretion Consequent to Aging
BPH/LUTS is a disease of aging. In a survey of 1709 men without cancer recently reported by the Massachusetts Male Aging Study, the frequency of clinical BPH/LUTS (defined in terms of frequency/difficulty with urinating and evidence of an enlarged/swollen prostate) rose from 8.4% in men 38–49 years of age to 33.5% in men aged 60–70 years (p < 0.001) (Meigs et al., 2001). Using lower urinary tract symptoms as in indicator for BPH/LUTS, the Triumph project in the Netherlands reported a 2.7% prevalence rate for BPH/LUTS in men 45–49 years of age, which increased to 24% in men 80 years of age (Verhamme et al., 2002). Clearly, age is a profound risk factor associated with the development of BPH/LUTS.
BPH/LUTS can comprise expansion of both epithelial and stromal cell types within the prostate. Deering et al. (1994) performed a morphometric analysis of prostates from 30 patients diagnosed with BPH/LUTS and found that 50–75% of the total hyperplastic tissue consisted of non-muscular stroma. A recent study examining complete prostates obtained at autopsy from 281 men aged 20–84 years at the time of death reported that the average volume of both normal prostates and those with evidence of nodular hyperplasia increased with age, and that volumetric increases were largely attributable to increased stromal volume (Arenas et al., 2001). Other studies have reported higher proliferative and lower apoptotic rates for epithelial cells from hyperplastic compared to normal prostates, suggesting that some proportion of increased prostate volume with age is attributable to increased epithelial cell densities (Berges et al., 1995; Colombel et al., 1998). Taken together, these studies suggest that the proliferation of both fibroblastic stromal and epithelial cell types account for aging-associated prostatic enlargement, and with the development of hyperplasia in particular.
The molecular mechanisms responsible for increased stromal and epithelial cell proliferation in hyperplastic human prostates are not well described. Work accomplished using human-rat tissue recombinants suggest that paracrine interactions between human prostate epithelial cells and rat urogenital sinus mesenchyme act homeostatically to maintain both cell types in a non-proliferative, differentiated state (Cunha et al., 1996; Hayward et al., 1998). More recent work has shown that these paracrine interactions likely become dysfunctional consequent to aging. A study from this author’s laboratory showed that stromal fibroblast cells cultured from the prostates of older men (aged 63 – 81 at the time of prostatectomy) were less able to suppress the proliferation of non-malignant prostate epithelial cells than those cultured from the prostates of younger men (aged 40–52 years) (Begley et al, 2005). Moreover, these studies showed that the transcriptome of aging prostate stroma is characterized by the up-regulation of several genes that encode secreted inflammatory mediators, including CXC-type chemokines (CXCL1, CXCL2, CXCL5, CXCL6, CXCL12), interleukins (IL11, IL33), and transcripts with cytokine homology (CYTL1) (Begley et al., 2005, 2008). Fibroblastic cells cultured from the prostates of older men secreted higher levels of CXCL1, CXCL5, CXCL6 and CXCL12 protein than those cultured from the prostates of younger men (Begley et al, 2005, 2008). Subsequent studies have confirmed the secretion of CXCL5 and CXCL12 (McDowell et al., 2010) and CXCL8 (Penna et al., 2009; McDowell et al, 2010) by human prostate stromal fibroblastic cells and have also demonstrated secretion of CXCL10 and IL-6 (Penna et al., 2009). Fujita et al. (2010) demonstrated >2-fold higher levels of IL-1β, IL-7, CCL2, and IL-6 in the extraprostatic secretions (EPS) of large (>60g) compared to small (<40g) prostates, and showed that the source of CCL2 secretion was prostate stromal fibroblastic (but not epithelial) cells. High levels of CCL2 secretion by prostate stromal fibroblast cells was also demonstrated by McDowell et al. (2010). Together, these studies suggest that a diverse and robust chemokine ‘secretome’ is expressed by stromal fibroblast cells in the human aging and enlarged prostate.
Chemokine secretion has also been documented for BPH/LUTS-associated prostate epithelial cells. Kramer et al (2002) showed that prostatic epithelial cells cultured from transurethral resection-derived prostate (TURP) tissues expressed transcripts for IFN-γ, IL-2, and IL-4. Similarly, Steiner et al (2003) demonstrated expression of IL-17 transcript by prostate epithelial cells cultured from BPH/LUTS tissues. Several non-malignant and malignant prostate epithelial cell lines have been characterized as secreting CXCL5, CXCL8, CXCL12 and CCL2 (McDowell et al, 2010).
Mechanisms that Promote Aging-Associated Chemokine Secretion in the Prostate
What possible mechanisms might account for the secretion of chemokines in the aging prostate microenvironment? Clues that may help answer this question are provided by work accomplished in the Campisi laboratory which has investigated the role of cellular senescence in aging-associated pathobiology. With the exception of those cell types that comprise continually renewing tissues originating from particular types of stem cells, many types of mammalian cells become growth-arrested, or senescent, over time. By definition, senescent cells are non-replicative. Cells may become senescent because they have reached their Hayflick limit, i.e., their chromosomal telomeres are too short to permit further DNA synthesis and cell division. Such cells have effectively reached replicative exhaustion, and have entered replicative senescence. Cells may also become senescent because they have become stressed, often resulting in DNA damage and growth-arrest. Although these cells have not reached their Hayflick limit, they are, nevertheless, non-replicative, and have entered cellular Senescence. Many studies have shown that senescent cells accumulate with age in vivo (Dimri et al., 1995; Nishimura et al., 1997; Hjelmeland et al, 1999; Kajstura et al., 2000; Chkhotua et al, 2002). Senescence is essentially controlled by tumor suppressor genes, including p16, Arf, p53, and RB1, that serve as checkpoints to prevent the proliferation of cells at risk for neoplastic transformation (Krishnamurthy et al., 2004; Campis, 2005).
Two key publications support the idea that senescent fibroblastic-type cells secrete a medley of proteins, including chemokines, which promote cellular proliferation. In the first study, Bavik et al. (2006) induced normal human prostate stromal fibroblasts to undergo senescence after achieving replicative exhaustion or after exposure to agents that caused oxidative stress or DNA damage. Gene expression profiling of senescent compared to non-senescent prostatic fibroblasts demonstrated significant up-regulation of transcripts encoding several cytokines, including the chemokines CXCL1, CXCL8, CXCL12, CCL2, CCL7, CCL11, CCL13 and CCL20, in RNA isolated from senescent cells. Similar senescence-associated chemokine expression profiles were observed independent of the mechanism through which senescence was induced, indicating that cells examined in this study expressed a common ‘senescence phenotype’ regardless of the actual “road” taken to senescence.
In the second study, Coppe et al. (2008) from the Campisi laboratory studied five human fibroblast cell cultures: two derived from embryonic lung (WI-3S and IMR-90), two derived from neonatal foreskin (BJ, HCA2), and one derived from adult breast (hBF184). The cells were permitted to grow to quiescence (>80% confluent) or were induced to undergo senescence by repeatedly passaging the cells to replicative exhaustion or by exposing the cells to a relatively high dose (10Gy) of ionizing radiation. Antibody arrays identified several proteins preferentially secreted by senescent compared to quiescent cells, including the interleukins IL-1β, IL-6, IL-7, IL-11, IL-13 and IL-15, the CC-type chemokines CCL2, CCL3, CCL8, CCL13, CCL16, CCL20, and CCL26 and the CXC-type chemokines CXCL1, CXCL2, CXCL3 and CXCL8. Although the fibroblasts used in these studies were not prostatic in origin, their senescence-associated secretory profiles (SASPs) were remarkably similar to each other as well as to those previously identified for senescent prostate stromal fibroblasts and to those isolated from aging and/or enlarged human prostates (Begley et al, 2005, 2008; Penna et al., 2009; Fujita et al., 2010). Remarkably, normal human prostate epithelial cells induced to undergo senescence subsequent to ionizing radiation demonstrated a senescence-associated secretome that was very similar to that exhibited by senescent fibroblasts (Coppe et al., 2008). Similar to the study by Bavik et al., (2006), similar senescence-associated chemokine expression profiles were observed independent of the mechanism through which senescence was induced.
Fibroblasts are not the only cell type observed to undergo senescence in the human prostate. A study by Castro et al. (2003) demonstrated increasing levels of β-galactosidase activity, a biological marker for senescence, in prostate extracts concomitant with patient age, prostate weight, and prostate specific antigen (PSA) expression levels. Combined with the study by Coppe et al. (2008), these results suggest that senescent epithelial cells may also serve as sources of chemokine secretion in the prostate.
The results reported in the studies cited above are consistent with the accumulation of senescent stromal fibroblasts, and, possibly, epithelial cells, as a potential driving force behind chemokine secretion in the aging and enlarged human prostate.
Chemokine Secretion Consequent To Inflammatory Responses
1. Prostatitis
As recently summarized by Habermacher et al. (2006), acute or chronic bacterial infection of the prostate (Category I and II by the National Institutes of Health classification system) are relatively rare, and together comprise only 4–10% of diagnoses of prostatitis. Chronic nonbacterial prostatitis/chronic pelvic pain syndrome (CP/CPPS; Category III) is much more prevalent and accounts for 90-95% of cases. CP/CPPS is further described as inflammatory or noninflammatory based on the presence or absence of leukocytes in the prostatic fluid. The incidental observation of leukocytes in the prostatic secretions of non-symptomatic men is classified as Category IV prostatitis but is not subject to clinical intervention. Overall, prostatitis is considered a common condition, and accounts for nearly 2 million ambulatory care encounters annually in the United States (Habermacher et al., 2006).
Chemokine secretion has been evaluated in both semen and expressed prostatic secretions (EPS) from men with clinically diagnosed prostatitis. A study by Alexander et al. (1998) reported elevated levels of TNF-α and IL-1β in semen of men with CP/CPPS and that these levels were not associated with white blood cells (WBC) counts in these samples. Nadler et al. (2000) then reported that TNF-α and IL-1β levels were elevated in EPS from men with acute inflammatory Category II CP/PPS and asymptomatic inflammatory Category IV CP/PPS compared to healthy men or those diagnosed with BPH/LUTS. Like the study by Alexander et al. (1998), Nadler et al. (2000) found no correlation between chemokine levels and WBC counts. A later study by this same group examined chemokine levels in EPS from healthy men as well as men diagnosed with Category I or II CP/CPPS bacterial prostatitis, inflammatory or non-inflammatory Category III CP/CPPS, asymptomatic Category IV CP/CPPS, or BPH/LUTS. These studies found that both the CXCL5 and CXCL8 chemokines were elevated in the EPS from men with bacterial prostatitis, chronic inflammatory Category II CP/PPS, and asymptomatic inflammatory Category IV CP/PPS, and that chemokine secretion was associated with the number of white blood cells present in the EPS (Hochreiter et al.,2000). The results of this study suggested that at least 2 chemokines, CXCL5 and CXCL8, that are both highly angiogenic and leukoattractant, are elevated in the context of actual bacterial infection or inflammatory CP/CPPS in the prostate. Lastly, a recent study by Liu et al. (2009) found that the mean level of CXCL8 was significantly elevated in EPS from men with BPH/LUTS and chronic prostatitis than in ‘simple’ BPH/LUTS (e.g., BPH/LUTS without evidence of concurrent chronic prostatitis) and was associated with higher WBC counts.
Whereas prostatitis is the most common presenting diagnosis for men less than 50 years of age in outpatient urology clinics (Collins et al., 1998), BPH associated LUTS are clearly diseases of aging which, conservatively, affect 25–35% of men aged 60 or older and result in a tremendous negative impact on quality of life (Meigs et al., 2001; Verhamme et al., 2002). This apparent chronology associated with the incidence and prevalence of CP/CPPS and BPH/LUTS, as well as some evidence just described that particular chemokines may be commonly elevated in CP/CPPS and BPH/LUTS, has led some investigators to question whether this chronology actually reflects disease development, e.g., whether CP/CPPS precedes, and is causal to, BPH/LUTS. Sutcliffe et al. reported that data derived from the Health Professionals Follow-up Study showed that a history of gonorrheal infection or young-onset prostatitis were significantly associated with the later development of LUTS (Sutcliffe et al., 2005). Data derived from the Olmsted County Men’s Health Study showed that men with physician-diagnosed prostatitis were significantly (p<.0001) more likely to later have a medical record report of prostatism, BPH/LUTS, or enlarged prostate compared to those without a physician diagnosis of prostatitis, and were also significantly more likely to later receive treatment for BPH/LUTS (p<.0001) and to develop acute urinary retention (p=.01). Moreover, even after adjusting for age and number of baseline physician visits, men with physician-diagnosed prostatitis were also more likely to later receive treatment for BPH/LUTS (St. Sauver et al., 2008). Finally, a comparison of data reported by five studies surveying 10,617 men suggested that men reporting a history of prostatitis symptoms had substantially increased rates of BPH/LUTS, LUTS, and prostate cancer (Krieger et al., 2008).
2. Histological Inflammation (Inflammatory Infiltrate)
Immunohistochemical studies examining the histopathology of BPH have reported the presence of pervasive inflammatory infiltrate comprising leukocytes associated with acute inflammation, chronic inflammation, or both. Inflammatory cells comprising neutrophillic or lymphocytic infiltrates were identified in 90% of transurethral resections of the prostate (TURP) specimens from 80 patients diagnosed with BPH/LUTS but no history of prostatitis or prostatic infection (Nickel et al., 1999). Chronic inflammatory infiltrate was also detected in 30–60% of 1197 randomly selected men with BPH/LUTS as part of the Medical Therapy of Prostatic Symptoms (MTOPS) study. Patients with chronic inflammatory infiltrate had larger prostate volumes and demonstrated significantly more clinical progression and acute urinary retention than those with no evidence of inflammation (Roehrborn et al., 2005; Robert et al., 2009). A study that prospectively analyzed 167 autopsied prostates identified 93 glands harboring BPH/LUTS, and 75% of these demonstrated inflammatory infiltrate (predominantly chronic inflammation) compared to 50% of those without BPH/LUTS and 55% of those with evidence of cancer (Delongchamps et al., 2008).
As originally reported by Theyer et al. (1992) and Steiner et al. (2003) and recently summarized by Kramer et al. (2006), inflammatory infiltrates are very commonly observed in BPH/LUTS specimens and comprise 70% T lymphocytes, 15% B cells, and 15% macrophages as well as mast cells. The T lymphocyte population in particular expands enormously in BPH/LUTS, increasing from a mean of 7 cells/mm2 in the normal prostate to a mean of 195 cells/mm2 in fully developed BPH/LUTS (Kramer et al., 2007). Moreover, infiltrating CD3+ lymphocytes isolated from BPH/LUTS nodules express high levels of IFN-γ, IL-10, IL-1α, and TNF-α (Elsasser-Beile, 2000). Kramer et al. (2002) identified IFN-γ, IL-2 and IL-4 transcripts, while Steiner et al. further identified abundant IL-17 transcript, expressed by infiltrating T-lymphocytes in BPH/LUTS tissues. It is clear from these studies that resident T-lymphocyte populations in prostate tissues actively secrete a diverse array of chemokines into the surrounding microenvironment.
In summary, the studies cited above point to CP/CPPS and histological inflammation as potentially rich sources of chemokine secretion in the prostate (Figure 1). It is not clear, however, whether chemokine secretion in the context of CP/CPPS or histological inflammation contributes to the actual development of BPH/LUTS. There is some suggestion of chronology, e.g., CP/CPPS more frequently affects men <50 years of age, whereas BPH/LUTS is more common among older men. However, this apparent chronology does not establish a causal connection between CP/CPPS and BPH/LUTS.
Chemokines as Leukoattractants in BPH/LUTS
Chemokines as Stromally-Secreted Leukoattractants
It is entirely possible that secretion of chemokines by prostatic stroma may serve to attract leukocytes associated with infection and/or wound healing to the prostate. It has been postulated that fibroblasts within diverse tissues dictate and regulate site-specific leukocyte accumulation within those tissues (Parsonage et al., 2005). Perhaps aging prostate stromal fibroblasts similarly advertise a ‘stromal address code’ that attracts leukocytes to the prostate tissue microenvironment. If so, chemokine secretion in the aging and enlarged prostate may initially be due to senescing fibroblasts but may then be augmented by the attraction of leukocytes to the prostate and the addition of leukocyte-secreted chemokines to the aging prostate tissue microenvironment (Figure 1).
Some evidence that prostate stromal fibroblasts attract and even activate chemokine-secreting leukocytes is provided in the study by Penna et al. (2008). This study showed that cultured primary prostate stromal fibroblasts constitutively expressed toll-like receptors (TLRs) which, when stimulated by various agonists, induced these cells to secrete higher levels of CXCL8, CXCL10 and IL-6 than un-stimulated cells. Moreover, chemokine-secreting prostate fibroblasts were then capable of activating alloantigen-specific CD4+ T lymphocytes to secrete IFN-γ and IL-17, and these chemokines stimulated further CXCL8 and IL-6 secretion by stromal fibroblasts. Another study by McDowell et al. (2010) demonstrated that peripheral blood mononuclear cells largely comprising T-lymphocytes migrated readily towards serum-free media conditioned by primary prostate stromal fibroblasts cultured ex vivo from the prostates of four different men. Thus, prostate stromal fibroblasts and leukocytes can clearly participate in an interactive, mutually stimulatory chemokine-dependent relationship.
Several studies previously cited in this chapter suggest that chemokine secretion in the aging and enlarged prostate may attract leukocytes associated with both acute and chronic inflammation. Particular CXC-type chemokines, including CXCL1, CXCL5, and CXCL6, secreted by aging prostate stromal fibroblasts and senescent fibroblasts, are strongly neutrophillic and may produce a persistent, but weak, neutrophillic chemotaxic gradient. Activated neutrophils within prostatic tissues may then release chemokines and cytokines, e.g., TNF-alpha and IL-1, which are chemotaxic for activated macrophages (Feghali, 1997; Gillitzer and Goebeler, 2001). Both activated neutrophils and macrophages may then secrete cytokines, such as IL-1, IL-6, and CXCL8 that are chemotaxic for various types of lymphocytes involved in the chronic inflammatory response (Feghali, 1997; Takata et al., 2004). Activated macrophages also secrete TGF-β, which is chemotaxic for various types of lymphocytes, is pro-angiogenic like CXCL1, CXCL5, CXCL6, and CXCL12, and has been shown to promote both the development of reactive stroma and the proliferation of prostate epithelial cells [Feghali, 1997; Singh et al., 2004). Moreover, all of these different types of activated leukocytes secrete cytokines that are growth-stimulatory for diverse cell types. For example, T-lymphocytes isolated from BPH/LUTS tissues have been shown to express high amounts of IFN-γ and IL-2 that promoted the proliferation of prostate stromal cells (Kramer et al., 2002). Taken together, these studies suggest the possibility that many of these immune-mediated responses could act synergistically with aging prostate stroma to promote the low-level, but cumulative, proliferation of stromal fibroblasts/myofibroblasts and epithelial cells associated with the development and progression of BPH/LUTS.
Chemokines as Cytokines in BPH/LUTS
Chemokine-Mediated Mechanisms that Promote Proliferation
Chemokines transmit intracellular signals through binding interactions with the extracellular amino-terminal domain (and, depending upon the receptor, one or more extracellular “loop” domains) of one more members of a large family of 7-transmembrane domain G-protein coupled receptors (GPCRs). Chemokine receptors can both homo- and heterodimerize with each other and with other GPCRs, interactions which clearly modulate their responses to ligand (Kramp et al., 2010). Upon binding ligand, chemokine receptors undergo conformational changes that are transmitted to the intracellular carboxy domain and to the associated Gαβγ heterotrimeric protein. In response to this stimulus, GDP bound to the α subunit of the heterotrimeric G protein is exchanged for GTP, and the heterotrimeric G protein dissociates into discrete Gα and Gβγ subunits. GTP-bound Gα and Gβγ subunits then transduce the activated receptor signals intracellularly (Johnston and Siderovski, 2007; Kobilka, 2007; Oldham et al., 2007). The 18 isoforms of the Gα-type subunits can be divided into four homology classes - Gs, Gi, Gq, and G12 – which activate signaling through cAMP, PLCβ, or RhoGEFs (Simon et al., 1991; Ye, 2001; Neves et al., 2002). The Gβγ subunits, of which 5 Gβ and 12 Gγ isoforms have been identified to-date (Smrcka, 2008), engage a multitude of effector molecules through direct protein: protein interactions. Although the Gβγ subunits have no enzymatic activities, they nevertheless effectively facilitate signal transduction events by binding to cytosolic proteins and recruiting them to the plasma membrane, or by binding to and inducing functional conformation changes in transmembrane-localized proteins.
Upon dissociation, some Gα as well as Gβγ subunits can activate isoforms of protein lipase C (PLC), which then catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) to produce two second messengers: inositol 1,4,5-trisphosphate (Ins (1,4,5) P3) and diacylglycerol (DAG). Ins (1,4,5) P3 binds to intracellular receptors in the endoplasmic reticulum (ER), and triggers the mobilization of Ca2+ from ER stores leading to a rapid increase in the intracellular concentration of Ca2+ (Rozengurt, 2007). Elevation of intracellular calcium, in turn, regulates diverse cellular events, including cell-cell adhesion, cellular motility, gene expression, and cellular proliferation (Kurosaki et al., 2010). Both (Ins (1,4,5) P3)-mediated Ca2+ influx and DAG can activate various protein kinase C (PKC) isoforms. As recently reviewed by Reyland (2010), activation of PKC-alpha promotes cellular survival likely through up-regulation of the p21 cell cycle protein. Activation of PKC-epsilon promotes cellular proliferation and also suppresses apoptosis through diverse mechanisms. Both PKC-lambda/iota and PKC-zeta promote cell survival through activation of NFkappaB signaling, whereas PKC-delta promotes apoptosis through phosphorylation-dependent nuclear-localized mechanisms.
Once dissociated from their Gα partners, activated Gβγ subunits can partner with diverse effector molecules to mediate intracellular signaling. Some of these effectors overlap with those activated by Gα, e.g., PLCβ, some forms of adenyl cyclase and Src family kinases, but others are unique to Gβγ activation, notably PI3K signaling pathway activation and Ras-dependent MAPK signaling pathways (Crespo et al., 1994a,b; Koch et al., 1994; Gutkind, 1998; Ye, 2001; Rozengurt, 2007; for comprehensive tables of effector molecules, see Milligan and Kostenis, 2006; Smrcka, 2008).
Evidence that Chemokines Can Promote Prostate Cell Proliferation
The fact that powerful pro-proliferation signal transduction pathways (e.g., MAPK, PI3K) can be activated by chemokine GPCRs is consistent with a role for chemokines functioning as potent growth factors in BPH/LUTS development and progression. BPH/LUTS is pathologically characterized by the proliferation of fibroblast/myofibroblast and epithelial cell types within the periurethral, or transitional zone, region of the prostate gland (Bierhoff et al., 1996; Meigs et al., 2001; Verhamme et al., 2002). Previous studies have shown that BPH/LUTS develops consequent to a gradual increase in prostatic volume that occurs over decades of life through a process of low-level, but cumulative, cellular proliferation that increases post-pubertal prostatic volume by approximately 0.8–1.6%, equivalent to only 0.2–0.4 ml, per year (Jacobsen et al., 2001; Jakobsen et al., 1988). Therefore, the observed low-level secretion of multiple chemokines by prostatic stroma and resident inflammatory cells may promote the concomitant low-level, but cumulative, over-proliferation of both stromal fibroblastic and epithelial cell types associated with increased prostate volume in aging men.
Several studies have demonstrated that both epithelial and stromal fibroblast cells proliferate in response to low levels of chemokine similar to levels secreted within the aging, inflammatory prostatic microenvironment. Work from this author’s research group first demonstrated that stromal fibroblasts cultured from the prostates of older men promote the proliferation of non-malignant prostate epithelial cells. Moreover, these cells secrete low levels of several CXC-type chemokines, notably CXCL12 (10–100 pg/ml; 1–10pM), CXCL1, CXCL5 and CXCL6 (100–1000 pg/ml; 10–100pM) that were sufficient to promote modest but reproducible proliferative responses from both prostate stromal fibroblasts and non-transformed prostate epithelial cells (Begley et al., 2005, 2008; McDowell et al., 2010) in vitro. These studies suggest that chemokine secretion in the prostate consequent to the aging process may account for the low-level, but cumulative, proliferation of both epithelial and fibroblastic/myofibroblastic cell types that characterizes the aging-associated development of BPH.
Low (1–10pM) levels of CXCL12 were also sufficient to promote robust intracellular signaling, particularly pro-proliferative MAPK (MEK/ERK) and pro-survival NFkappaB signaling, and to stimulate a previously undefined and complex global transcriptional response in prostate epithelial cells (Begley et al, 2005, 2007). This CXCL12/CXCR4-mediated transcriptional response was heavily MEK/ERK-dependent and directly stimulated the expression of genes encoding proteins involved in the promotion of cellular proliferation and progression through the cell cycle (EGR1, EGFR, CD44) and down-regulated the expression of genes associated with resistance to apoptosis or cell cycle arrest (TP53, CDK2, CDK9) (Begley et al, 2007). Taken together, these studies are consistent with the involvement of CXC-type chemokines, particularly CXCL12, in the etiology of benign proliferative disease in the context of an aging tissue microenvironment.
Bavik et al. (2006) demonstrated that non-malignant prostate epithelial cells responded proliferatively when co-cultured with senescent prostate stromal fibroblasts. Gene expression profiling of senescent compared to non-senescent fibroblasts demonstrated significant up-regulation of transcripts encoding several cytokines, including the chemokines CXCL1, CXCL8, CXCL12, CCL2, CCL7, CCL11, CCL13 and CCL20, in RNA isolated from senescent cells, a profile with some similarity to that observed by Begley et al. (2005, 2008) for stromal fibroblasts cultured from the prostates of older men. The study by Bavik et al. (2006) also identified amphiregulin (AREG), a ligand of the Epidermal Growth Factor Receptor (EGFR), as a major soluble cytokine secreted by senescent prostatic stromal cells, and showed that low (0.1–1nm) levels of AREG were sufficient to significantly promote the proliferation of both transformed and non-transformed prostate epithelial cells. AREG was also identified as transcriptionally up-regulated in stromal fibroblasts cultured from the prostates of older men in the study by Begley et al. (2005). Moreover, this group later showed that sub-nanomolar levels of CXCL12 stimulated AREG shedding by non-malignant prostate epithelial cells (Kasina et al., 2009). Taken together, these studies suggest that high levels of the pro-proliferative EGFR ligand, AREG, may arise from stromal shedding by aging/senescent prostate fibroblasts, as well as epithelial shedding mediated by stromally-secreted CXCL12, and may thereby play dual roles in BPH/LUTS development and/or progression.
As noted above, elevated levels of CXCL8 have been observed in association with prostatitis, BPH/LUTS, and both epithelial and fibroblast senescence. The Rowley laboratory has also demonstrated high levels of CXCL8 expression by human prostatic epithelium within BPH/LUTS nodules, and have shown that adjacent stromal fibroblasts exhibit a myofibroblastic ‘reactive stroma’ phenotype (Schauer et al., 2008). Additional work accomplished by this group has shown that transgenic mice engineered to over-express keratinocyte-derived chemokine (KC), the functional murine homolog of CXCL8, exhibit hyperplastic prostatic epithelium, characterized by age-associated acinar infolding and significant increases in acinar diameter. Moreover, overexpression of KC was associated with a prototypical reactive stromal phenotype characterized by overexpression of tenascin-C and pro-collagen I. These results were recapitulated in both subcutaneous and orthotopic xenograft implants composed of human prostate epithelial cells engineered to over-express CXCL8 mixed with human prostate stromal cells. CXCL8 over-expression by the epithelial component of these xenografts was associated with increased proliferation of both epithelial and stromal cell types, altered nodule-like morphology, and increased survival (Schauer et al., 2009).
CC-type, as well as CXC-type, chemokines have been noted in association with cellular senescence and as promoters of cellular proliferation in the context of BPH/LUTS. High levels of CCL2 secretion by prostate stromal fibroblast cells was recently demonstrated by McDowell et al. (2010), and Fujita et al. (2010) demonstrated high levels of CCL2 in the extraprostatic secretions (EPS) of large (>60g) prostates, and showed that the source of CCL2 secretion was prostate stromal fibroblastic (but not epithelial) cells. Moreover, CCL2 selectively stimulated the proliferation of non-transformed prostate epithelial, but not fibroblastic, cells, and these proliferative responses could be abrogated using a small molecule inhibitor or anti-CCL2 monoclonal antibody.
Taken together, these studies suggest that both CXC- and CC-type chemokines are secreted at higher levels in the context of BPH/LUTS than in the context of normal prostate. Although the majority of studies demonstrate chemokine secretion by aging and/or senescent prostate stromal fibroblasts, some studies also point to epithelial secretion as a possible source for these chemokines. And, as detailed above, it is highly likely that infiltrating leukocytes contribute significantly to the overall chemokine ‘load’ within the aging prostate microenvironment. In any event, the conclusion that can be drawn from these diverse studies is clear: chemokines are part of the aging prostate microenvironment, are capable of promoting prostate cellular differentiation, proliferation, and gene transcription (Figure 1), and thereby likely promote BPH/LUTS development and progression.
Chemokines as Angiogenic Agents that Support Prostatic Enlargement
Some members of the CXC-type chemokine family possess the highly conserved Glutamine-Leucine-Arginine (ELR) motif as part of their amino acid sequence. These chemokines, which include CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7, and CXCL8, are potent inducers of angiogenic activity and powerful chemotactic factors for endothelial cells. Conversely, CXC-type chemokines that lack the ELR motif, e.g., CXCL4, CXCL9, and CXCL10, and CXCL11, are actually largely angiostatic (Streiter et al., 1995). The exception to this ‘rule’ is CXCL12, which, despite lacking the ELR motif, is highly angiogenic (Salcedo et al, 1999). Three members of the CC-type chemokine family, CCL2, CCL11, and CCL16, have also been shown to demonstrate angiogenic activity (recently reviewed by Keeley et al., 2008). As noted above, many of these chemokines are up-regulated in the aging prostate and especially in the context of BPH/LUTS. These observations suggest that chemokines may promote vascularization within BPH/LUTS tissues, and that this serves as another function whereby chemokines promote hyperplastic proliferation in the aging prostate. A small number of studies have compared vascularization in normal prostate, BPH/LUTS, and cancer specimens. These studies have demonstrated increased microvessel density (MVD) in BPH/LUTS compared to normal prostate tissue (Deering et al., 1995) and even in BPH/LUTS compared to malignant tissue (Shih et al., 2003). These studies provide some rationale for exploring chemokine-mediated angiogenesis as a contributing factor to BPH/LUTS development and progression (Figure 1).
Summary and Conclusions
This manuscript has endeavored to review the literature regarding the potential role of chemokines in BPH/LUTS development and progression. There is a wealth of published studies indicating that a variety of chemokines are actively secreted within the prostatic microenvironment consequent to disruptions in normal tissue homeostasis due to the aging process and/or due to inflammatory responses. Several studies cited in this chapter are consistent with the identification of accumulating senescent stromal fibroblasts, and, possibly, epithelial cells, as potential driving forces behind chemokine secretion in the aging and enlarged human prostate. Multiple studies summarized in this chapter also point to CP/CPPS and histological inflammation as potentially rich sources of chemokine secretion in the prostate. Once bound to their cognate GPCR-type receptors, chemokines can stimulate powerful pro-proliferation signal transduction pathways and thus function as potent growth factors in BPH/LUTS development and progression. These functions have been amply demonstrated experimentally and particularly point to robust MAPK and PI3K signaling, as well as global transcriptional responses, as mediating chemokine-stimulated cellular proliferative responses. Lastly, a small body of literature suggest that chemokine-mediated angiogenesis may comprise a contributing factor to BPH/LUTS development and progression (see Figure 1).
Taken together, the studies cited above suggest that the observed low-level secretion of multiple chemokines by prostatic stroma and resident inflammatory cells may promote the concomitant low-level, but cumulative, over-proliferation of both stromal fibroblastic and epithelial cell types associated with increased prostatic volume in aging men. Though the accumulated evidence is far from complete and suffers from some rather extensive gaps in knowledge, it argues favorably for the conclusion that chemokines can, and likely do, promote prostatic enlargement and the associated lower urinary tract symptoms. Future studies would do well to explore chemokines as potential therapeutic targets to delay or ablate BPH/LUTS initiation and progression.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Alexander RB, Ponniah S, Hasday J, Hebel JR. Elevated levels of proinflammatory cytokines in the semen of patients with chronic prostatitis/chronic pelvic pain syndrome. Urology. 1998 Nov;52(5):744–9. doi: 10.1016/s0090-4295(98)00390-2. [DOI] [PubMed] [Google Scholar]
- Arenas MI, Romo E, Royuela M, Ruiz A, Fraile B, Sanchez-Chapado M, Paniagua R. Morphometric evaluation of the human prostate. Int J of Andrology. 2001;24:37–47. doi: 10.1046/j.1365-2605.2001.00267.x. [DOI] [PubMed] [Google Scholar]
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998 Apr;392(6676):565–8. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- Bavik C, Coleman I, Dean JP, Knudsen B, Plymate S, Nelson PS. The gene expression program of prostate fibroblast senescence modulates neoplastic epithelial cell proliferation through paracrine mechanisms. Cancer Res. 2006 Jan 15;66(2):794–802. doi: 10.1158/0008-5472.CAN-05-1716. [DOI] [PubMed] [Google Scholar]
- Begley L, Monteleon C, Shah RB, Macdonald JW, Macoska JA. CXCL12 overexpression and secretion by aging fibroblasts enhance human prostate epithelial proliferation in vitro. Aging Cell. 2005 Dec;4(6):291–8. doi: 10.1111/j.1474-9726.2005.00173.x. [DOI] [PubMed] [Google Scholar]
- Begley LA, Kasina S, MacDonald J, Macoska JA. The inflammatory microenvironment of the aging prostate facilitates cellular proliferation and hypertrophy. Cytokine. 2008 Aug;43(2):194–9. doi: 10.1016/j.cyto.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley LA, MacDonald JW, Day ML, Macoska JA. CXCL12 activates a robust transcriptional response in human prostate epithelial cells. J Biol Chem. 2007 Sep 14;282(37):26767–74. doi: 10.1074/jbc.M700440200. [DOI] [PubMed] [Google Scholar]
- Berges RR, Vukanovic J, Epstein JI, CarMichel M, Cisek L, Johnson DE, Veltri RW, Walsh PC, Isaacs JT. Implication of cell kinetic changes during the progression of human prostatic cancer. Clin Cancer Res. 1995;1:473–480. [PMC free article] [PubMed] [Google Scholar]
- Bierhoff E, Vogel J, Benz M, Giefer T, Wernert N, Pfeifer U. Stromal nodules in benign prostatic hyperplasia. Eur Urol. 1996;29:345–54. doi: 10.1159/000473774. [DOI] [PubMed] [Google Scholar]
- Campisi J. Suppressing cancer: the importance of being senescent. Science. 2005 Aug 5;309(5736):886–7. doi: 10.1126/science.1116801. [DOI] [PubMed] [Google Scholar]
- Castro P, Giri D, Lamb D, Ittmann M. Cellular senescence in the pathogenesis of benign prostatic hyperplasia. Prostate. 2003 Apr 1;55(1):30–8. doi: 10.1002/pros.10204. [DOI] [PubMed] [Google Scholar]
- Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006 Feb 9;354(6):610–21. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- Chkhotua A, Shohat M, Tobar A, Magal N, Kaganovski E, Shapira Z, Yussim A. Replicative senescence in organ transplantation-mechanisms and significance. Transpl Immunol. 2002 May;9(2–4):165–71. doi: 10.1016/s0966-3274(02)00003-5. [DOI] [PubMed] [Google Scholar]
- Collins MM, Stafford RS, O’Leary MP, Barry MJ. How common is prostatitis? A national survey of physician visits. J Urol. 1998 Apr;159(4):1224–8. [PubMed] [Google Scholar]
- Colombel M, Vacherot F, Diez SG, Fontaine E, Buttyan R, Chopin D. Zonal variation of apoptosis and proliferation in the normal prostate and in benign prostatic hyperplasia. Br J Urol. 1998;82:380–385. doi: 10.1046/j.1464-410x.1998.00752.x. [DOI] [PubMed] [Google Scholar]
- Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008 Dec 2;6(12):2853–68. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo P, Xu N, Daniotti JL, Troppmair J, Rapp UR, Gutkind JS. Signaling through transforming G protein-coupled receptors in NIH 3T3 cells involves c-Raf activation. Evidence for a protein kinase C-independent pathway. J Biol Chem. 1994b Aug 19;269(33):21103–9. [PubMed] [Google Scholar]
- Crespo P, Xu N, Simonds WF, Gutkind JS. Ras-dependent activation of MAP kinase pathway mediated by G-protein beta gamma subunits. Nature. 1994a Jun 2;369(6479):418–20. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Hayward SW, Dahiya R, Foster BA. Smooth muscle-epithelial interactions in normal and neoplastic prostatic development. Acta Anat. 1996;155:63–72. doi: 10.1159/000147791. [DOI] [PubMed] [Google Scholar]
- Deering RE, Bigler SA, Brown M, Brawer MK. Microvascularity in benign prostatic hyperplasia. Prostate. 1995 Mar;26(3):111–5. doi: 10.1002/pros.2990260302. [DOI] [PubMed] [Google Scholar]
- Deering RE, Bigler SA, King J, Choongkittaworn M, Aramburu E, Brawer MK. Morphometric quantitation of stroma in human benign prostatic hyperplasia. Urology. 1994;44:64–70. doi: 10.1016/s0090-4295(94)80011-1. [DOI] [PubMed] [Google Scholar]
- Delongchamps NB, de la Roza G, Chandan V, Jones R, Sunheimer R, Threatte G, Jumbelic M, Haas GP. Evaluation of prostatitis in autopsied prostates--is chronic inflammation more associated with benign prostatic hyperplasia or cancer? J Urol. 2008 May;179(5):1736–40. doi: 10.1016/j.juro.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995 Sep 26;92(20):9363–7. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsässer-Beile U, Przytulski B, Gierschner D, Grussenmeyer T, Katzenwadel A, Leiber C, Deckart A, Wetterauer U. Comparison of the activation status of tumor infiltrating and peripheral lymphocytes of patients with adenocarcinomas and benign hyperplasia of the prostate. Prostate. 2000 Sep 15;45(1):1–7. doi: 10.1002/1097-0045(20000915)45:1<1::aid-pros1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Front Biosci. 1997 Jan 1;2:d12–26. doi: 10.2741/a171. Review. [DOI] [PubMed] [Google Scholar]
- Fujita K, Ewing CM, Getzenberg RH, Parsons JK, Isaacs WB, Pavlovich CP. Monocyte chemotactic protein-1 (MCP-1/CCL2) is associated with prostatic growth dysregulation and benign prostatic hyperplasia. Prostate. 2010 Apr 1;70(5):473–81. doi: 10.1002/pros.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol. 2001 Apr;69(4):513–21. [PubMed] [Google Scholar]
- Gutkind JS. The pathways connecting G protein-coupled receptors to the nucleus through divergent mitogen-activated protein kinase cascades. J Biol Chem. 1998 Jan 23;273(4):1839–42. doi: 10.1074/jbc.273.4.1839. [DOI] [PubMed] [Google Scholar]
- Habermacher GM, Chason JT, Schaeffer AJ. Prostatitis/chronic pelvic pain syndrome. Annual Rev Med. 2006;57:195–206. doi: 10.1146/annurev.med.57.011205.135654. Review. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Haughney PC, Rosen MA, Greulich KM, Weier HU, Dahiya R, Cunha GR. Interactions between adult human prostatic epithelium and rat urogenital sinus mesenchyme in a tissue recombination model. Differentiation. 1998;63:131–140. doi: 10.1046/j.1432-0436.1998.6330131.x. [DOI] [PubMed] [Google Scholar]
- Hjelmeland LM, Cristofolo VJ, Funk W, Rakoczy E, Katz ML. Senescence of the retinal pigment epithelium. Mol Vis. 1999 Nov 3;5:33. [PubMed] [Google Scholar]
- Hochreiter WW, Nadler RB, Koch AE, Campbell PL, Ludwig M, Weidner W, Schaeffer AJ. Evaluation of the cytokines interleukin 8 and epithelial neutrophil activating peptide 78 as indicators of inflammation in prostatic secretions. Urology. 2000 Dec 20;56(6):1025–9. doi: 10.1016/s0090-4295(00)00844-x. [DOI] [PubMed] [Google Scholar]
- Jacobsen SJ, Girman CJ, Lieber MM. Natural history of benign prostatic hyperplasia. Urology. 2001 Dec;58(6 Suppl 1):5–16. doi: 10.1016/s0090-4295(01)01298-5. discussion 16. [DOI] [PubMed] [Google Scholar]
- Jakobsen H, Torp-Pedersen S, Juul N. Ultrasonic evaluation of age-related human prostatic growth and development of benign prostatic hyperplasia. Scand J Urol Nephrol Suppl. 1988;107:26–31. [PubMed] [Google Scholar]
- Johnston CA, Siderovski DP. Receptor-mediated activation of heterotrimeric G-proteins: current structural insights. Mol Pharmacol. 2007 Aug;72(2):219–30. doi: 10.1124/mol.107.034348. [DOI] [PubMed] [Google Scholar]
- Kajstura J, Pertoldi B, Leri A, Beltrami CA, Deptala A, Darzynkiewicz Z, Anversa P. Telomere shortening is an in vivo marker of myocyte replication and aging. Am J Pathol. 2000 Mar;156(3):813–9. doi: 10.1016/S0002-9440(10)64949-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasina S, Scherle PA, Hall CL, Macoska JA. ADAM-mediated amphiregulin shedding and EGFR transactivation. Cell Prolif. 2009 Dec;42(6):799–812. doi: 10.1111/j.1365-2184.2009.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of neovascularization. Arterioscler Thromb Vasc Biol. 2008 Nov;28(11):1928–36. doi: 10.1161/ATVBAHA.108.162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilka BK. G protein coupled receptor structure and activation. Biochim Biophys Acta. 2007 Apr;1768(4):794–807. doi: 10.1016/j.bbamem.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch AE. Chemokines and their receptors in rheumatoid arthritis: future targets? Arthritis Rheum. 2005 Mar;52(3):710–21. doi: 10.1002/art.20932. [DOI] [PubMed] [Google Scholar]
- Koch WJ, Hawes BE, Allen LF, Lefkowitz RJ. Direct evidence that Gi-coupled receptor stimulation of mitogen-activated protein kinase is mediated by G beta gamma activation of p21ras. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12706–10. doi: 10.1073/pnas.91.26.12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer G, Mitteregger D, Marberger M. Is benign prostatic hyperplasia (BPH) an immune inflammatory disease? Eur Urol. 2007 May;51(5):1202–16. doi: 10.1016/j.eururo.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Kramer G, Steiner GE, Handisurya A, Stix U, Haitel A, Knerer B, Gessl A, Lee C, Marberger M. Increased expression of lymphocyte-derived cytokines in benign hyperplastic prostate tissue, identification of the producing cell types, and effect of differentially expressed cytokines on stromal cell proliferation. Prostate. 2002 Jun 1;52(1):43–58. doi: 10.1002/pros.10084. [DOI] [PubMed] [Google Scholar]
- Kramp BK, Sarabi A, Koenen RR, Weber C. Heterophilic chemokine receptor interactions in chemokine signaling and biology. Exp Cell Res. 2010 Dec 10; doi: 10.1016/j.yexcr.2010.11.014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Krieger JN, Lee SW, Jeon J, Cheah PY, Liong ML, Riley DE. Epidemiology of prostatitis. Int J Antimicrob Agents. 2008 Feb;31(Suppl 1):S85–90. doi: 10.1016/j.ijantimicag.2007.08.028. Epub 2007 Dec 31. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004 Nov;114(9):1299–307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Li Q, Han P, Li X, Zeng H, Zhu Y, Wei Q. Evaluation of interleukin-8 in expressed prostatic secretion as a reliable biomarker of inflammation in benign prostatic hyperplasia. Urology. 2009 Aug;74(2):340–4. doi: 10.1016/j.urology.2009.02.064. [DOI] [PubMed] [Google Scholar]
- McDowell KL, Begley LA, Mor-Vaknin N, Markovitz DM, Macoska JA. Leukocytic promotion of prostate cellular proliferation. Prostate. 2010 Mar 1;70(4):377–89. doi: 10.1002/pros.21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meigs JB, Mohr B, Barry MJ, Collins MM, McKinlay JB. Risk factors for clinical benign prostatic hyperplasia in a community-based population of healthy ageing men. J Clin Epidemiol. 2001;54:935–944. doi: 10.1016/s0895-4356(01)00351-1. [DOI] [PubMed] [Google Scholar]
- Milligan G, Kostenis E. Heterotrimeric G-proteins: a short history. Br J Pharmacol. 2006 Jan;147(Suppl 1):S46–55. doi: 10.1038/sj.bjp.0706405. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler RB, Koch AE, Calhoun EA, Campbell PL, Pruden DL, Bennett CL, Yarnold PR, Schaeffer AJ. IL-1beta and TNF-alpha in prostatic secretions are indicators in the evaluation of men with chronic prostatitis. J Urol. 2000 Jul;164(1):214–8. [PubMed] [Google Scholar]
- Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002 May 31;296(5573):1636–9. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]
- Nickel JC, Downey J, Young I, Boag S. Asymptomatic inflammation and/or infection in benign prostatic enlargement. BJU Int. 1999;84:976–81. doi: 10.1046/j.1464-410x.1999.00352.x. [DOI] [PubMed] [Google Scholar]
- Nishimura F, Terranova VP, Braithwaite M, Orman R, Ohyama H, Mineshiba J, Chou HH, Takashiba S, Murayama Y. Comparison of in vitro proliferative capacity of human periodontal ligament cells in juvenile and aged donors. Oral Dis. 1997 Sep;3(3):162–6. doi: 10.1111/j.1601-0825.1997.tb00029.x. [DOI] [PubMed] [Google Scholar]
- Oldham WM, Hamm HE. How do receptors activate G proteins? Adv Protein Chem. 2007;74:67–93. doi: 10.1016/S0065-3233(07)74002-0. Review. [DOI] [PubMed] [Google Scholar]
- Parsonage G, Filer AD, Haworth O, Nash GB, Rainger GE, Salmon M, Buckley CD. A stromal address code defined by fibroblasts. Trends Immunol. 2005 Mar;26(3):150–6. doi: 10.1016/j.it.2004.11.014. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna G, Fibbi B, Amuchastegui S, Cossetti C, Aquilano F, Laverny G, Gacci M, Crescioli C, Maggi M, Adorini L. Human benign prostatic hyperplasia stromal cells as inducers and targets of chronic immuno-mediated inflammation. J Immunol. 2009 Apr 1;182(7):4056–64. doi: 10.4049/jimmunol.0801875. [DOI] [PubMed] [Google Scholar]
- Reyland ME. Protein kinase C isoforms: Multi-functional regulators of cell life and death. Front Biosci. 2009 Jan 1;14:2386–99. doi: 10.2741/3385. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert G, Descazeaud A, Nicolaïew N, Terry S, Sirab N, Vacherot F, Maillé P, Allory Y, de la Taille A. Inflammation in benign prostatic hyperplasia: a 282 patients’ immunohistochemical analysis. Prostate. 2009 Dec 1;69(16):1774–80. doi: 10.1002/pros.21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roehrborn CG, Kaplan SA, Noble WD, Lucia MS, Slawin KM, McVary KT, Kusek JW, Nyberg LM. The impact of acute or chronic inflammation in baseline biopsy on the risk of clinical progression of BPE: Results from the MTOPS study. AUA Meeting; 2005. Abstract #1277. [Google Scholar]
- Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. Review. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol. 2007 Dec;213(3):589–602. doi: 10.1002/jcp.21246. [DOI] [PubMed] [Google Scholar]
- Salcedo R, Wasserman K, Young HA, Grimm MC, Howard OM, Anver MR, Kleinman HK, Murphy WJ, Oppenheim JJ. Vascular endothelial growth factor and basic fibroblast growth factor induce expression of CXCR4 on human endothelial cells: In vivo neovascularization induced by stromal-derived factor-1alpha. Am J Pathol. 1999 Apr;154(4):1125–35. doi: 10.1016/s0002-9440(10)65365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer IG, Ressler SJ, Rowley DR. Keratinocyte-derived chemokine induces prostate epithelial hyperplasia and reactive stroma in a novel transgenic mouse model. Prostate. 2009 Mar 1;69(4):373–84. doi: 10.1002/pros.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer IG, Ressler SJ, Tuxhorn JA, Dang TD, Rowley DR. Elevated epithelial expression of interleukin-8 correlates with myofibroblast reactive stroma in benign prostatic hyperplasia. Urology. 2008 Jul;72(1):205–13. doi: 10.1016/j.urology.2007.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih SJ, Dall’Era MA, Westphal JR, Yang J, Sweep CG, Gandour-Edwards R, Evans CP. Elements regulating angiogenesis and correlative microvessel density in benign hyperplastic and malignant prostate tissue. Prostate Cancer Prostatic Dis. 2003;6(2):131–7. doi: 10.1038/sj.pcan.4500637. [DOI] [PubMed] [Google Scholar]
- Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science. 1991 May 10;252(5007):802–8. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- Singh H, Dang TD, Ayala GE, Rowley DR. Transforming growth factor-beta1 induced myofibroblasts regulate LNCaP cell death. J Urol. 2004 Dec;172(6 Pt 1):2421–5. doi: 10.1097/01.ju.0000138082.68045.48. [DOI] [PubMed] [Google Scholar]
- Smrcka AV. G protein betagamma subunits: central mediators of G protein-coupled receptor signaling. Cell Mol Life Sci. 2008 Jul;65(14):2191–214. doi: 10.1007/s00018-008-8006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Sauver JL, Jacobson DJ, McGree ME, Girman CJ, Lieber MM, Jacobsen SJ. Longitudinal association between prostatitis and development of benign prostatic hyperplasia. Urology. 2008 Mar;71(3):475–9. doi: 10.1016/j.urology.2007.11.155. discussion 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner GE, Stix U, Handisurya A, Willheim M, Haitel A, Reithmayr F, Paikl D, Ecker RC, Hrachowitz K, Kramer G, Lee C, Marberger M. Cytokine expression pattern in benign prostatic hyperplasia infiltrating T cells and impact of lymphocytic infiltration on cytokine mRNA profile in prostatic tissue. Lab Invest. 2003 Aug;83(8):1131–46. doi: 10.1097/01.lab.0000081388.40145.65. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, Dzuiba J, Van Damme J, Walz A, Marriott D, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995 Nov 10;270(45):27348–57. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- Sutcliffe S, Giovannucci E, De Marzo AM, Willett WC, Platz EA. Sexually transmitted infections, prostatitis, ejaculation frequency, and the odds of lower urinary tract symptoms. Am J Epidemiol. 2005 Nov 1;162(9):898–906. doi: 10.1093/aje/kwi299. [DOI] [PubMed] [Google Scholar]
- Takata H, Tomiyama H, Fujiwara M, Kobayashi N, Takiguchi M. Cutting edge: expression of chemokine receptor CXCR1 on human effector CD8+ T cells. J Immunol. 2004 Aug 15;173(4):2231–5. doi: 10.4049/jimmunol.173.4.2231. [DOI] [PubMed] [Google Scholar]
- Theyer G, Kramer G, Assmann I, Sherwood E, Preinfalk W, Marberger M, Zechner O, Steiner GE. Phenotypic characterization of infiltrating leukocytes in benign prostatic hyperplasia. Lab Invest. 1992 Jan;66(1):96–107. [PubMed] [Google Scholar]
- Verhamme K, Dieleman J, Bleumink G, van der Lei J, Sturkenboom M. Incidence and prevalence of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in primary care-the Triumph project. Eur Urol. 2002;42:323–328. doi: 10.1016/s0302-2838(02)00354-8. [DOI] [PubMed] [Google Scholar]
- Ye RD. Regulation of nuclear factor kappaB activation by G-protein-coupled receptors. J Leukoc Biol. 2001 Dec;70(6):839–48. [PubMed] [Google Scholar]