Abstract
OBJECTIVE
To test whether adding mobile application coaching and patient/provider web portals to community primary care compared with standard diabetes management would reduce glycated hemoglobin levels in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
A cluster-randomized clinical trial, the Mobile Diabetes Intervention Study, randomly assigned 26 primary care practices to one of three stepped treatment groups or a control group (usual care). A total of 163 patients were enrolled and included in analysis. The primary outcome was change in glycated hemoglobin levels over a 1-year treatment period. Secondary outcomes were changes in patient-reported diabetes symptoms, diabetes distress, depression, and other clinical (blood pressure) and laboratory (lipid) values. Maximal treatment was a mobile- and web-based self-management patient coaching system and provider decision support. Patients received automated, real-time educational and behavioral messaging in response to individually analyzed blood glucose values, diabetes medications, and lifestyle behaviors communicated by mobile phone. Providers received quarterly reports summarizing patient’s glycemic control, diabetes medication management, lifestyle behaviors, and evidence-based treatment options.
RESULTS
The mean declines in glycated hemoglobin were 1.9% in the maximal treatment group and 0.7% in the usual care group, a difference of 1.2% (P < 0.001) over 12 months. Appreciable differences were not observed between groups for patient-reported diabetes distress, depression, diabetes symptoms, or blood pressure and lipid levels (all P > 0.05).
CONCLUSIONS
The combination of behavioral mobile coaching with blood glucose data, lifestyle behaviors, and patient self-management data individually analyzed and presented with evidence-based guidelines to providers substantially reduced glycated hemoglobin levels over 1 year.
Diabetes affects 38 million people in the U.S.; 40% are undiagnosed, and another 87 million are considered prediabetic. Costs exceed $100 billion annually (1,2). Changes in lifestyle/self-care behaviors, complex medical regimens, use of glucose-testing devices, and frequent data assessment by patients and providers are required to improve blood glucose and subsequent outcomes. In clinical trials, better self-care/lifestyle resulted in better diabetes outcomes (3–5). However, these clinical trials improved outcomes for circumscribed patient populations (6–9). Patients with diabetes are diverse, treatment may involve multiple specialists, and care by primary care providers (PCPs) is limited to 15-min visits. Only 55% of individuals with type 2 diabetes receive diabetes education (10); 16% report adhering to recommended self-management activities (11). Concern that elevated blood glucose levels result in microvascular comorbidity motivates behavioral change and monitoring interventions to assist patients and PCPs (12–14). The Mobile Diabetes Intervention Study, reported here, evaluated a diabetes-coaching system, using mobile phones and patient/provider portals for patient-specific treatment and communication. The hypothesis tested was that mobile telephone feedback on self-management of blood glucose results and lifestyle and clinical management offered to patients with type 2 diabetes and their providers can reduce glycated hemoglobin levels over 1 year.
RESEARCH DESIGN AND METHODS
Eligibility and study design
The Mobile Diabetes Intervention Study was a cluster-randomized clinical trial conducted in primary care practices in four distinct Maryland areas. Eligible practices included groups of at least three physicians without academic affiliation who provided diabetes care to at least 10% of their patients and were identified from a list of primary care practices in the study geographic areas. A detailed description of the study design was reported previously (13). Group assignment was concealed until a practice agreed to participate in the study. Data were obtained by abstraction from patients’ medical charts and primary collection.
As shown in Fig. 1, 26 primary care practices were randomized to one of four study groups using a stepped intervention design for groups: group 1: control–usual care (UC), group 2: coach-only (CO), group 3: coach PCP portal (CPP), and group 4: coach PCP portal with decision-support (CPDS). A total of 2,602 patients were identified by these practices for screening; 2,103 were determined ineligible, 145 declined participation, 213 were enrolled, and 163 were included in analyses (UC, n = 56; CO, n = 23; CPP, n = 22; and CPDS, n = 62). We aimed to identify patients treated in community primary care settings who would benefit from an intensive diabetes intervention. Errors in consent form completion were found on audit after study enrollment was closed. Our Institutional Review Board asked us to repeat consent procedures to assure we obtained proper signatures from all parties. We completed repeat consent procedures for 163 patient participants and all 39 physician participants. We were unable to contact patients not reconsented; they did not significantly differ (P > 0.10) at baseline from included patients in age, sex, or baseline glycated hemoglobin. Participant data were analyzed according to physician practices’ original randomization treatment assignment (intention-to-treat analyses).
Figure 1.
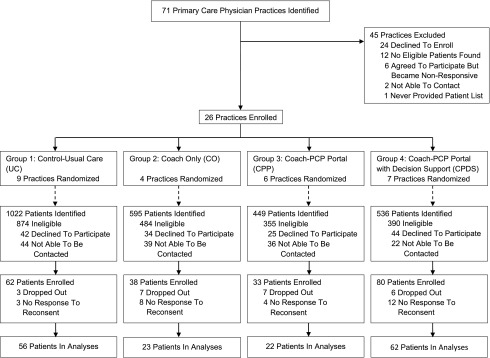
Flowchart of enrollment and patient status (n = 163).
Patients eligible for recruitment to the study met all inclusion criteria:
Physician diagnosis of type 2 diabetes for ≥6 months;
Glycated hemoglobin ≥7.5% within 3 months;
Age 18–64 years.
Patients were excluded for any of the following:
Medicare or Medicaid beneficiaries;
Uninsured;
Insulin pump users;
Not currently managed by study physicians;
Pregnant;
Active substance, alcohol, or drug abuser (sober <1 year);
Psychotic or schizophrenic under active care;
Severe hearing or visual impairment; or
No Internet or e-mail access.
The most common reasons for ineligibility were as follows: glycated hemoglobin <7.5% (72%); patient’s diabetes not currently managed by study physician (8%); not between the ages of 18 and 64 years (5%); uninsured or insured by Medicaid or Medicare (3%); not type 2 diabetes (2%); no Internet or e-mail access (2%); specified medical exclusion (2%); and psychiatric exclusion (1%). Patients were excluded if insured by Medicare or Medicaid or were uninsured because coverage of primary care services differs from patients commercially insured. These patients would be excluded from planned secondary analyses of claims data provided by a commercial insurer.
Patients covered by any commercial insurer were eligible. Patients on insulin pumps, pregnant, or not meeting other clinical criteria were excluded because their type 2 diabetes required different clinical management. Of the patients identified as eligible, 42% were enrolled (213) and 77% of those enrolled completed the study and were included in the analyses.
The intervention was a patient-coaching system and provider clinical decision support (13). The patient-coaching system included a mobile diabetes management software application and a web portal. The mobile software allowed patients to enter diabetes self-care data (blood glucose values, carbohydrate intake, medications, other diabetes management information) on a mobile phone and receive automated, real-time educational, behavioral, and motivational messaging specific to the entered data. The patient web portal augmented the mobile software application and consisted of a secure messaging center (for patient-provider communication), personal health record with additional diabetes information (e.g., laboratory values, eye examinations, foot screenings), learning library, and logbook to review historical data. The provider portal had different views of patient data on the basis of study group assignment. The data-only view (group 3, CPP) allowed providers to access unanalyzed patient data. Group 4 (CPDS) providers had access to analyzed patient data linked to standards of care and evidence-based guidelines.
Patients received a One Touch Ultra 2 (LifeScan, Milpitas, CA) glucose meter and supplies. Patients in the three active treatment groups received identical study materials: mobile phones, 1-year unlimited data and service plan, study mobile diabetes management software, and access to the web-based patient portal. The mobile diabetes management software incorporated over 1,000 automated self-management messages into a feedback algorithm. The algorithm displayed educational and motivational messages to patients after patients self-reported data into the mobile phone application (Supplementary Fig. 1). Diabetes educators were “virtual” case managers that intermittently reviewed patient data. Educators could supplement automated messages with electronic messages sent to the patient portal. Educator messages were based on longitudinal data trends. Patients in all three treatment groups were allowed to make telephone calls to educators but were encouraged to communicate electronically. On average, <50% of active patients made or received live phone calls, with an average of one phone call per month. Lastly, patients received an electronic action plan every 2.5 months to support improved diabetes self-management and to serve as previsit summaries for physician office visits. Providers were not informed of the level of communication to patients but knew whether patients were assigned to an intervention or to the UC group.
All providers received the most recent American Diabetes Association guidelines for diabetes care and were notified when patients enrolled in the study (7,8). Providers assigned to UC were asked to care for patients as usual. Active treatment providers were informed that their patients received a mobile and web-based patient–coaching system. Providers in the CO group received data from their patients if patients chose to share it. Providers in the CPP and CPDS groups were trained on accessing the provider Internet portal on office compatible computers (PCs), allowing visual access to patients’ unanalyzed data. Providers in the CPDS group were trained on accessing the provider Internet portal to view patient data on office PCs and also received quarterly reports (more often if needed) that summarized patients’ glycemic and metabolic control, adherence to medication, self-management skills, and relevant evidence-based guidelines. Reports were accessible by Internet portal or facsimile. Enrolled providers were reimbursed modestly for research effort ($250 per patient enrolled).
Primary outcome
The primary outcome of the study was change in glycated hemoglobin (%) comparing UC and maximal treatment (CPDS) at baseline versus 12 months. Medical chart reviews were used to ascertain patient data. For patients without a glycated hemoglobin within 4 months of the desired measurement, a glycated hemoglobin test was offered at no charge at baseline to determine eligibility and at 12 months. At baseline, glycated hemoglobin was measured using one device, the Bayer DCA 2000, by trained staff blinded to patient group assignment. At follow-up, if glycated hemoglobin was not ascertained within 14 days of the 12-month time point, reminders were provided to patients and physicians to complete the test. Glycated hemoglobin level at intermediate time points (3, 6, and 9 months) was collected from patients’ medical charts.
Secondary outcomes
The Patient Health Questionnaire-9 (PHQ) was administered at baseline and at follow-up interviews to assess depressive symptoms (15). We used the 9-item version of the Self-Completion Patient Outcome Instrument to assess patient-reported symptoms associated with diabetes (16,17) and the 17-item Diabetes Distress Scale (18,19). Clinical measurement related to diabetes complications (blood pressure, lipid levels) was obtained from provider medical office records. Hypoglycemic events, hospitalization, and emergency room visits were ascertained through quarterly telephone calls to patients. Vital status was ascertained through review of physician charts if we could not contact patients. Study data for primary and secondary outcomes were collected by research staff separately from data transmitted through the device. A detailed description of the study design and rationale for primary and secondary outcomes has been reported previously (13).
Study oversight
The University of Maryland Baltimore institutional review board approved the study, and a Data and Safety Monitoring Board (DSMB) was appointed to review study procedures and adverse advents.
Statistical analysis
Practices were assigned to treatment groups according to a 1.5:1:1:1.5 (Group 1, UC:Group 2, CO:Group 3, CPP:Group 4, CPDS) ratio using a computer-generated list of random numbers. The ratios were higher in groups 1 and 4 for analyses of the main hypotheses. Sample size was determined on the basis of the primary outcome, change in glycated hemoglobin. The comparison of UC, which included 56 patients from nine practices, to CPDS, which included 62 patients from seven practices, had 80% power to detect a difference in mean glycated hemoglobin changes of 0.65 SD, corresponding to 1.0% if SD was 1.58%, using a two-sided test with 0.05 type I error after accounting for a within-cluster correlation of 0.10, similar to a previously reported study (20,21). Comparisons of the UC to CO (23 patients from four practices) and CPP (22 patients from six practices) had 80% power to detect a difference in mean outcome changes of 1.1% (0.7 SD difference) to 1.3% (0.8 SD difference) for glycated hemoglobin.
Linear mixed-effects models were used to compare mean changes in primary and secondary outcomes between UC and each active intervention. The primary analysis examined 12-month changes for glycated hemoglobin. Secondary analyses jointly compared 3-, 6-, 9-, and 12-month changes between groups. Random effects accounted for within-practice clustering and within-patient correlation. Model fixed effects were treatment group indicators, time indicators, and interactions between treatment group and time. Two secondary analyses of glycated hemoglobin were performed as follows: one analysis stratified by baseline glycated hemoglobin (≥9.0 vs. <9.0); the other (prespecified analysis) adjusted for baseline glycated hemoglobin as a covariate. We performed a sensitivity analysis using weighted estimating equations (WEE) to address any residual bias from missing data (22). Statistical significance was defined as P < 0.05 or 95% CI that excludes 0. Analyses were performed using SAS version 9.1 (SAS Institute, Inc. Cary, NC).
RESULTS
The 163 study patients had a mean baseline glycated hemoglobin of 9.4% (range 7.5–15.5) (Table 1). Mean age was 52.8 years, 50.3% were female, 39.3% were African American, and 31.3% were college educated. The mean duration of diabetes was 8.2 years. Most participants (76.1%) were obese (BMI ≥30 kg/m2). Participants had a mean PHQ-9 of 5.2 (minimal to mild depression scores). Most participants had hypertension (63.2%) and hypercholesterolemia (58.3%). CPDS patients had higher baseline glycated hemoglobin than UC (9.9 vs. 9.2%, P = 0.04). No other baseline patient variables differed significantly among the four study groups.
Table 1.
Baseline characteristics of patients and primary and secondary study outcomes
| Group 1: UC (n = 56) |
Group 2: CO (n = 23) |
Group 3: CPP (n = 22) |
Group 4: CPDS (n = 62) |
|||||
|---|---|---|---|---|---|---|---|---|
| n | % or mean ± SD* | N | % or mean ± SD* | n | % or mean ± SD* | n | % or mean ± SD* | |
| Baseline characteristics | ||||||||
| Glycated hemoglobin (%) | 56 | 9.2 ± 1.7 | 23 | 9.3 ± 1.8 | 22 | 9.0 ± 1.8 | 62 | 9.9 ± 2.1 |
| 7.5–8.9 | 35 | 62.5 | 13 | 56.5 | 13 | 59.1 | 28 | 45.2 |
| ≥9 | 21 | 37.5 | 10 | 43.5 | 9 | 40.9 | 34 | 54.8 |
| Age (years) | 56 | 53.2 ± 8.4 | 23 | 52.8 ± 8.0 | 22 | 53.7 ± 8.2 | 62 | 52 ± 8.0 |
| Sex | ||||||||
| Male | 28 | 50 | 12 | 52.2 | 10 | 45.5 | 31 | 50 |
| Female | 28 | 50 | 11 | 47.8 | 12 | 54.5 | 31 | 50 |
| Race | ||||||||
| Black (non-Hispanic) | 27 | 48.2 | 10 | 43.5 | 10 | 45.5 | 17 | 27.4 |
| White (non-Hispanic) | 26 | 46.4 | 12 | 52.2 | 9 | 40.9 | 39 | 62.9 |
| Other | 3 | 5.4 | 1 | 4.3 | 3 | 13.6 | 6 | 9.7 |
| Duration of diabetes diagnosis (years) | 56 | 9.0 ± 7.0 | 23 | 7.7 ± 5.6 | 22 | 6.8 ± 4.9 | 62 | 8.2 ± 5.3 |
| Smoking status | ||||||||
| Current smokers | 11 | 19.6 | 6 | 26.1 | 3 | 13.6 | 8 | 12.9 |
| Former smokers | 1 | 1.8 | 1 | 4.3 | 0 | 0 | 9 | 14.5 |
| Nonsmokers | 44 | 78.6 | 16 | 69.6 | 19 | 86.4 | 45 | 72.6 |
| Education | ||||||||
| High school/trade school or less | 14 | 25 | 7 | 30.4 | 9 | 40.9 | 19 | 30.6 |
| Some college or associates | 20 | 35.7 | 10 | 43.5 | 10 | 45.5 | 23 | 37.1 |
| Bachelors degree or higher | 22 | 39.3 | 6 | 26.1 | 3 | 13.6 | 20 | 32.3 |
| Depression (PHQ-9) | ||||||||
| Score | 56 | 4.7 ± 5.6 | 23 | 5.2 ± 4.8 | 22 | 5.5 ± 4.7 | 62 | 5.5 ± 5.4 |
| Minimal to mild (0–9) | 45 | 80.4 | 20 | 87 | 19 | 86.4 | 48 | 77.4 |
| Moderate (10–14) | 5 | 8.9 | 1 | 4.3 | 2 | 9.1 | 9 | 14.5 |
| Moderately severe (15–19) | 6 | 10.7 | 2 | 8.7 | 1 | 4.5 | 3 | 4.8 |
| Severe depression (20–27) | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3.2 |
| BMI | ||||||||
| BMI (kg/m2) | 56 | 34.3 ± 6.3 | 23 | 36.9 ± 7.5 | 22 | 35.5 ± 10.3 | 62 | 35.8 ± 7.1 |
| Underweight (16.5–18.4 kg/m2) | 1 | 1.8 | 0 | 0 | 1 | 4.5 | 0 | 0 |
| Normal (18.5–24.9 kg/m2) | 0 | 0 | 0 | 0 | 2 | 9.1 | 1 | 1.6 |
| Pre-obese (25–29.9 kg/m2) | 11 | 19.6 | 4 | 17.4 | 7 | 31.8 | 12 | 19.4 |
| Obese class 1 (30–34.9 kg/m2) | 22 | 39.3 | 6 | 26.1 | 1 | 4.5 | 18 | 29 |
| Obese class 2 (35–39.9 kg/m2) | 10 | 17.9 | 5 | 21.7 | 3 | 13.6 | 17 | 27.4 |
| Obese class 3 (≥40 kg/m2) | 12 | 21.4 | 8 | 34.8 | 8 | 36.4 | 14 | 22.6 |
| Comorbidities | ||||||||
| Hypertension | 29 | 51.8 | 18 | 78.3 | 13 | 59.1 | 43 | 69.4 |
| Hypercholesterolemia | 34 | 60.7 | 11 | 47.8 | 14 | 63.6 | 36 | 58.1 |
| Coronary artery disease | 5 | 8.9 | 2 | 8.7 | 0 | 0 | 5 | 8.1 |
| Microvascular complications, any | 8 | 14.3 | 1 | 4.3 | 2 | 9.1 | 6 | 9.7 |
| Primary outcome, glycated hemoglobin (%)† | ||||||||
| Baseline | 56 | 9.2 ± 1.7 | 23 | 9.3 ± 1.8 | 22 | 9.0 ± 1.8 | 62 | 9.9 ± 2.1 |
| 3 Months | 30 | 8.2 ± 1.2 | 13 | 7.6 ± 1.2 | 9 | 7.5 ± 0.6 | 41 | 7.8 ± 1.3 |
| 6 Months | 27 | 8.6 ± 2.0 | 15 | 7.6 ± 1.1 | 11 | 7.6 ± 0.7 | 30 | 7.5 ± 1.2 |
| 9 Months | 43 | 8.5 ± 1.4 | 16 | 7.6 ± 0.9 | 15 | 7.9 ± 1.4 | 48 | 7.9 ± 2.0 |
| 12 Months | 51 | 8.5 ± 1.8 | 21 | 7.7 ± 1.0 | 21 | 7.9 ± 1.4 | 56 | 7.9 ± 1.7 |
| Change from baseline to 12 months (mean)‡ | −0.7 | −1.6 | −1.2 | −1.9 | ||||
| Change from baseline to 12 months (95% CI)‡ | −1.1 to −0.3 | −2.3 to −1.0 | −1.8 to −0.5 | −2.3 to −1.5 | ||||
| Secondary outcomes, patient-reported outcomes | ||||||||
| Diabetes Distress Scale | ||||||||
| Baseline | 56 | 2.4 ± 0.9 | 22 | 2.7 ± 0.9 | 21 | 2.8 ± 0.7 | 58 | 2.6 ± 0.9 |
| 12 Months | 46 | 2.3 ± 0.9 | 20 | 2.6 ± 0.9 | 21 | 2.4 ± 0.8 | 61 | 2.3 ± 0.8 |
| Change from baseline to 12 months (mean)‡ | −0.1 | −0.1 | −0.3 | −0.3 | ||||
| Change from baseline to 12 months (95% CI)‡ | −0.4 to 0.1 | −0.4 to 0.3 | −0.7 to 0.0 | −0.5 to 0.0 | ||||
| Diabetes symptom inventory | ||||||||
| Baseline | 56 | 15.6 ± 5.6 | 22 | 16.4 ± 5.7 | 22 | 18.1 ± 6.4 | 62 | 17 ± 5.6 |
| 12 Months | 46 | 14.6 ± 4.8 | 21 | 15.5.0 ± 4.5 | 21 | 16.2 ± 5.8 | 62 | 16.7 ± 5.2 |
| Change from baseline to 12 months (mean)‡ | −2.3 | −2.8 | −4.3 | −1 | ||||
| Change from baseline to 12 months (95% CI)‡ | −5.5 to 0.9 | −7.7 to 2.0 | −9.0 to 0.4 | −3.8 to 1.8 | ||||
| Depression (PHQ-9) | ||||||||
| Baseline | 56 | 4.7 ± 5.6 | 23 | 5.2 ± 4.8 | 22 | 5.5 ± 4.7 | 62 | 5.5 ± 5.4 |
| 12 Months | 44 | 3.6 ± 4.1 | 21 | 4.6 ± 5.0 | 21 | 3.9 ± 5.3 | 62 | 4.8 ± 4.8 |
| Change from baseline to 12 months (mean)‡ | −1.1 | −0.6 | −1.2 | −0.7 | ||||
| Change from baseline to 12 months (95% CI)‡ | −3.2 to 3.0 | −2.7 to 1.4 | −3.3 to 0.8 | −1.9 to 0.5 | ||||
| Secondary outcomes, laboratory values | ||||||||
| Systolic blood pressure (mmHg) | ||||||||
| Baseline | 56 | 130 ± 22 | 23 | 130 ± 18 | 22 | 133 ± 14 | 62 | 130 ± 14 |
| 12 Months | 45 | 133 ± 20 | 21 | 134 ± 25 | 20 | 134 ± 16 | 51 | 128 ± 19 |
| Change from baseline to 12 months (mean)‡ | +2 | +4 | 2 | −2 | ||||
| Change from baseline to 12 months (95% CI)‡ | −3 to 7 | −4 to 11 | −6 to 10 | −6 to 3 | ||||
| Diastolic blood pressure (mmHg) | ||||||||
| Baseline | 56 | 78 ± 12 | 23 | 79 ± 11 | 22 | 79 ± 9 | 62 | 79 ± 9 |
| 12 Months | 45 | 79 ± 13 | 21 | 82 ± 11 | 20 | 78 ± 9 | 51 | 78 ± 10 |
| Change from baseline to 12 months (mean)‡ | +1 | +2 | −2 | −1 | ||||
| Change from baseline to 12 months (95% CI)‡ | −2 to 4 | −2 to 7 | −6 to 3 | −4 to 2 | ||||
| LDL (mg/dL) | ||||||||
| Baseline | 51 | 102 ± 36 | 23 | 103 ± 29 | 22 | 103 ± 33 | 55 | 106 ± 33 |
| 12 Months | 42 | 91 ± 34 | 19 | 94 ± 32 | 15 | 94 ± 47 | 45 | 102 ± 32 |
| Change from baseline to 12 months (mean)‡ | −6 | −8 | −14 | −5 | ||||
| Change baseline to 12 months (95% CI)‡ | −15 to 3 | −21 to 5 | −29 to 0 | −13 to 4 | ||||
| HDL (mg/dL) | ||||||||
| Baseline | 56 | 44 ± 11 | 23 | 44 ± 11 | 22 | 43 ± 11 | 59 | 43 ± 11 |
| 12 Months | 44 | 45 ± 12 | 16 | 42 ± 9 | 15 | 44 ± 11 | 48 | 45 ± 10 |
| Change from baseline to 12 months (mean)‡ | +1 | 0 | 0 | +2 | ||||
| Change from baseline to 12 months (95% CI)‡ | −1 to 3 | −4 to 3 | −3 to 4 | 0 to 3 | ||||
| Triglycerides (mg/dL) | ||||||||
| Baseline | 56 | 185 ± 167 | 23 | 172 ± 100 | 22 | 164 ± 105 | 59 | 187 ± 145 |
| 12 Months | 44 | 169 ± 124 | 16 | 113 ± 42 | 15 | 151 ± 74 | 48 | 139 ± 91 |
| Change baseline to 12 months (mean)‡ | −23 | −53 | −12 | −31 | ||||
| Change baseline to 12 months (95% CI)‡ | −58 to 12 | −110 to 4 | −71 to 47 | −65 to 3 | ||||
| Total cholesterol (mg/dL) | ||||||||
| Baseline | 56 | 182 ± 51 | 23 | 181 ± 35 | 22 | 177 ± 42 | 59 | 184 ± 41 |
| 12 Months | 44 | 168 ± 40 | 16 | 151 ± 34 | 15 | 168 ± 52 | 48 | 174 ± 42 |
| Change baseline to 12 months (mean)‡ | −11 | −24 | −14 | −9 | ||||
| Change baseline to 12 months (95% CI)‡ | −22 to 1 | −43 to −5 | −35 to 5 | −21 to 2 | ||||
n = 163.
*Unless otherwise indicated.
†Primary outcome, glycated hemoglobin change over 12 months; group 4 (P < 0.001) and group 2 (P = 0.003) have significantly larger changes than group 1. No other outcomes are significant.
‡Mean change and CI values are from the mixed-effects model.
Table 1 shows primary and secondary outcome measures. CPDS mean glycated hemoglobin decreased 1.9% (95% CI 1.5–2.3) over 12 months. UC mean glycated hemoglobin decreased 0.7% (0.3–1.1). Table 1 and Fig. 2 show that the mean 12-month decrease in CPDS glycated hemoglobin was 1.2% more than UC (95% CI 0.6–1.8%; P < 0.001). Furthermore, the CPDS patients had a significantly greater decrease in mean glycated hemoglobin than the UC patients when compared at all follow-up time points (P < 0.001).
Figure 2.
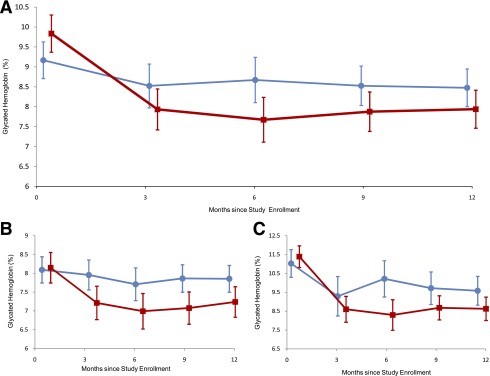
Primary study outcome and baseline A1C stratified analyses.
CO and CPP mean glycated hemoglobin levels also decreased over 12 months. Both had greater 12-month glycated hemoglobin reductions than UC (CO, P = 0.02; CPP, P = 0.45). CO and CPP were similar to CPDS over all follow-up time points (P > 0.05 for both comparisons).
In a stratified analysis, a greater decline was found with CPDS than UC for the stratum with baseline glycated hemoglobin <9.0% (difference in decrease 0.7%, 95% CI 0.2–1.1, P = 0.003) and the stratum with baseline glycated hemoglobin at least 9.0% (difference in decrease 1.3%, 0.2–2.4, P = 0.01) (shown in Fig. 2B and C). The test of interaction was not significant (P = 0.49) for baseline glycated hemoglobin stratum and treatment group over time. We obtained the same conclusion whether or not we analyzed the baseline to 12-month changes with intermediate glycated hemoglobin measures.
Glycated hemoglobin results were unchanged after adjusting for baseline glycated hemoglobin and after performing the WEE analysis. Although there were mean declines across all groups in lipid values and blood pressure readings, Diabetes Distress, Diabetes Symptoms, and PHQ-9 Depression, none of the 12-month changes comparing the UC to any of the active interventions were significantly different (P > 0.05).
Hypoglycemic events, hospitalizations, and emergency-room visits were infrequent in all groups. One patient in group 4 (CPDS) was hospitalized twice for reasons not reported to the study. The DSMB determined that there were no direct study-related adverse events found. No patients died during the 12 months of this study.
CONCLUSIONS
To our knowledge, this is the first cluster-randomized study of a mobile diabetes-coaching intervention conducted in a community setting over a 1-year treatment period. Few previous studies of electronic or mobile communication interventions for diabetes were randomized, included a control group, or covered 1 year (13). Our study included minority study participants and found clinically meaningful differences and few adverse events, none of which were related to the study or treatment. Our study evaluated the intervention for commercially insured patients in primary care settings, where the majority of diabetes care is provided. Enrolling and treating study participants according to random assignment of physician practices (clusters) reduced the risk of bias in treatment application. We found that a mobile phone–based treatment/behavioral coaching intervention improved glycated hemoglobin by 1.9%, compared with 0.7% for UC, a difference of 1.2% (P < 0.001) over 12 months. This result pertained to people with poorly controlled glycated hemoglobin (≥9.0%) and people with less severe abnormal initial glycated hemoglobin values (7.5–8.9%).
The results stratified on baseline glycated hemoglobin (Fig. 2) demonstrate three key features. First, since CPDS and UC had similar mean baseline glycated hemoglobin within strata of baseline A1C (<9 vs. ≥9.0%), and the treatment effect is similar in each of the strata, our findings provide evidence of true 12-month treatment differences in glycated hemoglobin, rather than regression to the mean. This stratified analysis is important, showing large changes in A1C by adjusting for baseline A1C. Second, the treatment effect in the higher glycated hemoglobin stratum shows this intervention to be suitable to obtain the goals of the more conservative ACCORD approach (23). Neither ACCORD nor this study collected person-specific data on dietary, physical activity, and pharmacological management adjustments made for individual patients. Because of the personalized quality of the mobile phone technology, we expect to be able to make those distinctions in future investigations now that its observed effects on glycated hemoglobin justify their study. Third, mobile phone management is efficacious in patients whose glycated hemoglobin levels are clearly above the desired levels as well as patients whose glycated hemoglobin levels are less egregiously elevated. Our finding is consistent with the Cochrane Collaboration review, suggesting the benefit of individual education on glycemic control (24). However, we did not see convincing improvements in patient-reported diabetes symptoms, diabetes distress, depression, or other clinical (e.g., blood pressure) or laboratory (e.g., lipid) values.
We advise caution in generalizing our findings. The interventions took place through community physician practices and were implemented through electronic communications. Physicians in the community have different experiences with and access to resources, including access to specialists, clinical practice guidelines, and experience or use of electronic communication. We attempted to address these differences by enrolling multiple community physicians to participate in the study and randomization at the practice level. The patient population in the study may also be distinctive because private health care insurance coverage and access to the Internet (either at work or home) were required. Although not all participants provided data at all planned study visits, we addressed missing data in this study in two ways. First, the primary analysis used mixed-effects models, which have the effect of implicitly imputing missing observations (25). Second, we performed the WEE sensitivity analysis that used baseline characteristic data to upweight observations from participants who were most similar to participants with missing data (22). As a measure of long-term blood glucose control, change in glycated hemoglobin is an important, commonly used outcome. Although low glycated hemoglobin does not imply that diabetes is being well managed, well managed diabetes is characterized by glycated hemoglobin at normal or near-normal levels (13). We screened >2,600 patients; 72% were ineligible because glycated hemoglobin was lower than eligibility criterion; many physicians referred patients they thought were not adequately managing their diabetes because of poor control relevant to everyday life, such as blurred vision or pain, self-assessed control of diabetes, or depression (13). In this study, we did not observe convincing changes in these indicators. Communications as specific for these indicators as ours were for glycated hemoglobin may be able to make a larger difference in future studies. Future studies should also consider how mobile communication changes behavior related to blood glucose: medication adherence, treatment intensification, increased physical activity, and number and quality of communications between providers and patients. These may be important mechanisms to explain change in glycated hemoglobin but were not primary or secondary analyses planned for this study. Future studies of mobile health should address more specific characterization of patient and provider behaviors that support change in clinical health parameters.
Mobile phones are ubiquitous—more than 2.7 billion people own mobile phones worldwide. In the United States alone, users have increased from 34 million in 1995 to 290 million in 2010. Mobile phone and Internet users are increasingly diverse in age and race. The widespread distribution of mobile phones and electronic communication, across socioeconomic, sex, and age-groups, combined with the ability to process and communicate data in real time, make these modalities ideal platforms to create simple, effective, diabetes management programs (14). We found mobile phone and web portal communications for diabetes to have a consequential treatment effect when used by patients and their PCPs.
Supplementary Material
Acknowledgments
This research project is funded through a contract between the University of Maryland Baltimore and WellDoc in addition to contributions by WellDoc, CareFirst Blue Cross/Blue Shield of Maryland, LifeScan, and Sprint. Additional funding was provided by the Maryland Industrial Partnerships program through the University of Maryland, an initiative of the A. James Clark School of Engineering’s Maryland Technology Enterprise Institute. No other potential conflicts of interest relevant to this article were reported.
C.C.Q. was Principal Investigator for these studies. C.C.Q., M.D.S., M.L.T., and A.L.G.-B. were responsible for the design, data analyses, writing, and review of the manuscript. E.A.B. was responsible for the data analyses and manuscript review. S.H.B. contributed to the writing and review of the manuscript.
The funders of this study did not play a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data, or in the preparation of the manuscript. WellDoc did not have veto power over or have say about changing any manuscript text other than the description of the software coaching system they provided. Dr. Ram Miller served as the DSMB.
The authors thank Dr. Nanette Steinle, Assistant Professor, Division of Endocrinology, Diabetes and Nutrition, University of Maryland, and Interim Chief, Endocrine Section, Baltimore Veterans Medical Center, for advice on the manuscript and clinical laboratory data.
Footnotes
Clinical trial reg. no. NCT01107015, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc11-0366/-/DC1.
References
- 1.Huang ES, Basu A, O’Grady M, Capretta JC. Projecting the future diabetes population size and related costs for the U.S. Diabetes Care 2009;32:2225–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988-1994 and 2005-2006. Diabetes Care 2009;32:287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaede PH, Jepsen PV, Larsen JN, Jensen GV, Parving HH, Pedersen OB. The Steno-2 study. Intensive multifactorial intervention reduces the occurrence of cardiovascular disease in patients with type 2 diabetes. Ugeskr Laeger 2003;165:2658–2661 [PubMed] [Google Scholar]
- 4.Solomon CG. Reducing cardiovascular risk in type 2 diabetes. N Engl J Med 2003;348:457–459 [DOI] [PubMed] [Google Scholar]
- 5.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleland JG, Ekman I. Enlisting the help of the largest health care workforce: patients. JAMA 2010;304:1383–1384 [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association. Standards of medical care in diabetes: 2008. Diabetes Care 2008;31(Suppl. 1):S12–S54 [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Standards of medical care in diabetes: 2009. Diabetes Care 2009;32(Suppl. 1):S13–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saaddine JB, Cadwell B, Gregg EW, et al. Improvements in diabetes processes of care and intermediate outcomes: United States, 1988–2002. Ann Intern Med 2006;144:465–474 [DOI] [PubMed] [Google Scholar]
- 10.Peyrot M, Rubin RR, Lauritzen T, Snoek FJ, Matthews DR, Skovlund SE. Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diabet Med 2005;22:1379–1385 [DOI] [PubMed] [Google Scholar]
- 11.Renders CM, Valk GD, Griffin S, Wagner EH, Eijk JT, Assendelft WJ. Interventions to improve the management of diabetes mellitus in primary care, outpatient and community settings. Cochrane Database Syst Rev 2001:CD001481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin S, Kinmonth AL. Diabetes care: the effectiveness of systems for routine surveillance for people with diabetes. Cochrane Database Syst Rev 2000:CD000541. [DOI] [PubMed] [Google Scholar]
- 13.Quinn CC, Gruber-Baldini AL, Shardell M, et al. Mobile diabetes intervention study: testing a personalized treatment/behavioral communication intervention for blood glucose control. Contemp Clin Trials 2009;30:334–346 [DOI] [PubMed] [Google Scholar]
- 14.Quinn CC, Clough SS, Minor JM, Lender D, Okafor MC, Gruber-Baldini A. WellDoc mobile diabetes management randomized controlled trial: change in clinical and behavioral outcomes and patient and physician satisfaction. Diabetes Technol Ther 2008;10:160–168 [DOI] [PubMed] [Google Scholar]
- 15.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitty P, Steen N, Eccles M, et al. A new self-completion outcome measure for diabetes: is it responsive to change? Qual Life Res 1997;6:407–413 [DOI] [PubMed] [Google Scholar]
- 17.McColl E, Steen IN, Meadows KA, et al. Developing outcome measures for ambulatory care: an application to asthma and diabetes. Soc Sci Med 1995;41:1339–1348 [DOI] [PubMed] [Google Scholar]
- 18.Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care 2005;28:626–631 [DOI] [PubMed] [Google Scholar]
- 19.Fisher L, Glasgow RE, Mullan JT, Skaff MM, Polonsky WH. Development of a brief diabetes distress screening instrument. Ann Fam Med 2008;6:246–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shea S, Weinstock RS, Starren J, et al. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus. J Am Med Inform Assoc 2006;13:40–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayes RJ, Bennett S. Simple sample size calculation for cluster-randomized trials. Int J Epidemiol 1999;28:319–326 [DOI] [PubMed] [Google Scholar]
- 22.Robins J, Rotnitzky A, Zhao L. Analysis of semiparametric regression models for repeated outcomes under the presence of missing data. J Am Stat Assoc 1995;90:106–121 [Google Scholar]
- 23.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duke SA, Colagiuri S, Colagiuri R. Individual patient education for people with type 2 diabetes mellitus. Cochrane Database Syst Rev 2009:CD005268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verbeke G, Lesaffre E. Repeated Measurements. Wiley: New York, 2007 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


