Abstract
OBJECTIVE
Streptozotocin (STZ) is the most widely used diabetogenic agent in animal models of islet transplantation. However, the immunomodifying effects of STZ and the ensuing hyperglycemia on lymphocyte subsets, particularly on T regulatory cells (Tregs), remain poorly understood.
RESEARCH DESIGN AND METHODS
This study evaluated how STZ-induced diabetes affects adaptive immunity and the consequences thereof on allograft rejection in murine models of islet and skin transplantation. The respective toxicity of STZ and hyperglycemia on lymphocyte subsets was tested in vitro. The effect of hyperglycemia was assessed independently of STZ in vivo by the removal of transplanted syngeneic islets, using an insulin pump, and with rat insulin promoter diphtheria toxin receptor transgenic mice.
RESULTS
Early lymphopenia in both blood and spleen was demonstrated after STZ administration. Direct toxicity of STZ on lymphocytes, particularly on CD8+ cells and B cells, was shown in vitro. Hyperglycemia also correlated with blood and spleen lymphopenia in vivo but was not lymphotoxic in vitro. Independently of hyperglycemia, STZ led to a relative increase of Tregs in vivo, with the latter retaining their suppressive capacity in vitro. The higher frequency of Tregs was associated with Treg proliferation in the blood, but not in the spleen, and higher blood levels of transforming growth factor-β. Finally, STZ administration delayed islet and skin allograft rejection compared with naive mice.
CONCLUSIONS
These data highlight the direct and indirect immunosuppressive effects of STZ and acute hyperglycemia, respectively. Thus, these results have important implications for the future development of tolerance-based protocols and their translation from the laboratory to the clinic.
Many animal models of diabetes depend on the administration of diabetogenic drugs such as streptozotocin (STZ) or alloxan. These are toxic glucose analogs that target pancreatic β-cells via GLUT2 transporter uptake. Because of the simplicity and reproducibility of diabetes induction with STZ, a highly stable glucosamine-nitrosourea compound, it represents by far the most widely used model (1). Indeed, among 131 analyzed articles published in 2010 on murine islet transplantation, 100 (76.3%) used STZ to induce diabetes, whereas 21 (16%) were performed in models of spontaneous diabetes (mostly NOD mice), 3 (2.3%) involved alloxan, 5 (3.8%) relied on transplanted islets in nondiabetic mice, and 2 (1.5%) used alternative ways for diabetes induction. Whereas STZ is known to target β-cells via the transporter GLUT2, it is not specific, since the kidney and liver are also susceptible to STZ toxicity (1). Moreover, several groups have described immunomodifying effects of STZ both in vitro and in vivo, although the confounding impact of the induced hyperglycemia was not clearly distinguished (2–6). Luo et al. (7) reported that hyperglycemia, potentially via an increased level of corticosteroids, causes a rapid depletion in thymocytes and splenic T cells followed by homeostatic T-cell proliferation. However, the exact mechanisms leading to the observed immunosuppression after STZ administration, especially the effect on different lymphocyte subpopulations, including T regulatory cells (Tregs), remain elusive.
One of the major goals in islet transplantation (Tx) research is to find strategies to obviate the need for lifelong immunosuppression that is toxic to the β-cells and detrimental to the host (8). Indeed, using STZ-induced diabetes models, novel immunosuppressive and tolerance induction protocols for allogeneic as well as xenogeneic islet Tx have demonstrated long-term graft survival (9,10). However, many costimulation blockade–based Tx tolerance protocols that are successful in chemically induced diabetic mice failed to produce long-term graft tolerance in NOD mice or in other Tx models such as skin Tx, with the latter being considered as one of the most stringent models. The underlying autoimmunity directed against β-cells, the mode of Tx, and the skin-specific antigen–presenting cells all have been put forward to explain the observed resistance to Tx tolerance (10,11). Another possible explanation for these apparent differences is that immunosuppression related to STZ-induced diabetes could result in an overestimation of the efficacy of tolerance induction. Consistent with this notion, a protocol targeting costimulation that induced tolerance in STZ-induced diabetic recipients was unable to induce tolerance in nondiabetic recipients (7). Thus, STZ and/or the ensuing hyperglycemia seem to downregulate the adaptive immune response against islet grafts.
In the past few years, Tregs were reported to play a key role in long-term islet graft tolerance (12–15). However, none of these studies addressed the possible effect of STZ administration and the ensuing hyperglycemia on Tregs. Using a rat-to-mouse islet Tx model, we recently reported an increase of Tregs in lymphoid organs and within grafts of tolerant mice treated with rapamycin and anti-CD154 monoclonal antibody (mAb), suggesting a critical role for Tregs in the induction phase of tolerance (9,16). The aim of the current study was to analyze whether STZ-induced diabetes leads to changes in Treg numbers and function and whether this affects subsequent immune responses using models of islet and skin Tx.
RESEARCH DESIGN AND METHODS
C57BL/6 (H2b) mice, 6–10 weeks of age, were used as recipients (Centre d’Elevage R. Janvier, Le Genest-St-Isle, France). BALB/c (H2d) mice were used as islet donors and BL/6xDBA/2 F1 (H2bxH2d) as skin donors (Janvier). Rat insulin promoter diphtheria toxin receptor (RIP-DTR) transgenic mice were provided by Pedro Herrera (Geneva University Medical School, Geneva, Switzerland), and Foxp3 green fluorescent protein (GFP) knock-in mice were provided by David Scotts (Center for Vascular and Inflammatory Diseases, University of Maryland at Baltimore, Baltimore, MD). The Foxp3 knock-in mice have the GFP coding sequence inserted in the first exon of the Foxp3 gene and have been backcrossed on a C57BL/6 background. Animals were maintained in conventional housing facilities. Experiments involving animals were performed in compliance with the relevant laws according to the Geneva Medical Faculty’s Veterinary Authorities.
Islet and skin Tx.
C57BL/6 mice were rendered diabetic by a single intraperitoneal injection of STZ (220 mg/kg; Sigma-Aldrich, St. Louis, MO) and were transplanted 72 h later. Blood glucose levels were monitored three times weekly using a blood glucose meter (Precision Q.I.D, MediSense; Abbott Laboratories, Bedford, MA). Only mice with blood glucose levels >17 mmol/L were used for Tx. BALB/c pancreatic islets were isolated as previously described (9). A total of 400 islet equivalents were transplanted under the left kidney capsule. Blood glucose levels of <11 mmol/L on 2 consecutive days defined successful islet function. For skin Tx, full-thickness tail skin was grafted onto the flank of the recipients. Graft sites were protected under sterile gauze and plaster until day 10, observed daily afterward, and considered rejected when no viable skin remained.
Experimental design.
STZ was solubilized in citrate buffer and used in vitro at a concentration of 4.4 mmol/L. RIP-DTR transgenic mice were injected with 126 ng i.p. diphtheria toxin (DT) on days 0, 3, and 4 as previously described (17). Insulin pumps (Linbit; LinShin Canada, Inc., Toronto, Ontario, Canada) were implanted subcutaneously in STZ-induced diabetic mice 3 days after STZ administration and allowed a sustained release of insulin (0.1 units/day) for >30 days. Anti-CD154 mAb (MR1, hamster antimouse CD154 mAb [CD40 L]; Bio Express, West Lebanon, NH) diluted in PBS (Sigma-Aldrich) was administered intraperitoneally at 0.5 mg per mouse on days 0, 2, and 4 after Tx. The following experimental groups were performed:
STZ-induced diabetic C57BL/6 mice transplanted with syngeneic islets on day 3 post-STZ administration (+/−) nephrectomy on day 60 (n = 7)
DT-induced diabetic RIP-DTR mice (n = 4)
RIP-DTR control mice without DT treatment (n = 4)
STZ-induced diabetic C57BL/6 mice treated with an insulin pump starting on day 3 (n = 4)
Nondiabetic C57BL/6 mice transplanted with allogeneic BALB/c islets (n = 3)
STZ-induced diabetic C57BL/6 mice transplanted with allogeneic BALB/c islets (n = 3)
STZ-induced diabetic C57BL/6 mice treated with an insulin pump (day 3) and grafted with BL/6xDBA/2 F1 skin (day 3) (n = 7)
Nondiabetic C57BL/6 mice grafted with BL/6xDBA/2 F1 skin (day 3) (n = 8)
MR1-treated STZ-induced diabetic C57BL/6 mice treated with an insulin pump (day 3) and MR1 and grafted with BL/6xDBA/2 F1 skin (day 3) (n = 7)
MR1-treated nondiabetic C57BL/6 mice grafted with BL/6xDBA/2 F1 skin (day 3) (n = 11)
Blood leukocytes analysis.
Blood was harvested from the tail vein into heparinized tubes. White blood cell (WBC) counts and differentials were analyzed using a pocH-100i hematology analyzer (Sysmex, Mundelein, IL), which analyzed percent ratio of small WBCs (SCR, lymphocytes), middle WBCs (MCR, monocytes), and large WBCs (LCR, granulocytes). Alternatively, absolute numbers of WBCs were counted in a Neubauer chamber after red blood lysis using Zapoglobulin (Beckam Coulter, Fullerton, CA).
Flow cytometry.
Blood samples were analyzed on days 3, 7, and 12 after STZ administration, and splenocytes were harvested at day 3 or 13 after STZ administration. Cells were stained with anti-CD4 fluorescein isothiocyanate (FITC) or allophycocyanin (APC) (RM4–5; eBioscience, San Diego, CA), anti-CD25 APC (PC61.5; eBioscience), anti-Foxp3 phycoerythrin (PE) (FJK16s; eBioscience), anti-CD3 FITC (eBioscience), and anti-CD8 PE (eBioscience). All samples were stained, fixed, and permeabilized according to the eBioscience’s instructions described in the mouse regulatory T-cell staining kit. Isotype control antibodies were purchased from Becton Dickinson (Franklin Lakes, NJ) (rat IgG2a FITC, rat IgG2a PE, rat IgG1 APC, and rat IgG2b APC mAb). Proliferating cells were stained with PE Cy7 anti-CD4, PerCP anti-CD8, APC anti-CD25, and then fixed and permeabilized with Cytofix/Cytoperm and Perm/Wash buffers (eBioscience) and stained with Ki67/isotype control (Becton Dickinson, Franklin Lakes, NJ) and anti-Foxp3 (eBioscience). For determination of cell death/apoptosis, Annexin V and 7AAD were purchased from Becton Dickinson. Data were collected on a FACScanto (Becton Dickinson). All data were analyzed using Flowjo software (version 8.7.3; TreeStar, Inc., Ashland, OR).
In vitro proliferation and suppression assays.
For proliferation assays, 0.2 × 106 splenocytes were stimulated with concanavalin A (3 μg/mL), lipopolysaccharide (0.5 μg/mL), or anti-CD3e mAb (1 μg/mL, clone 145–2C11; eBioscience). For in vitro suppression assays, CD4+CD25+ (Treg phenotype) cells were isolated from spleens of naive (group 1) and diabetic mice 3 days (group 2) and 13 days (group 3) after STZ administration. CD4+CD25+ (Treg phenotype) and CD4+CD25– T cells (effector T cells) were isolated by CD4-negative selection followed by CD25-positive selection using the CD4+CD25+ regulatory T-cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). CD4+CD25+ and CD4+CD25– T-cell purities were >90%. The in vitro suppression assay was performed as followed: 5 × 104 purified CD4+CD25– effector T cells from naive mice were cocultured with 1 × 105 irradiated (3,500 rad) syngeneic splenocytes in the presence of anti-CD3e mAb (1 μg/mL). Thereafter, CD4+CD25+ Treg from group 1 (naive mice), group 2 (diabetic mice 3 days after STZ), and group 3 (diabetic mice 13 days after STZ) were added at different CD4+CD25+:CD4+CD25– ratios (1:1, 1:2, 1:4, 1:8). On day 3, cells were pulsed with 1 μCi [3H]thymidine for 18 h and harvested. Results are expressed as count per minutes, showing one representative experiment out of three.
RT-PCR for GLUT2 expression.
RNA of splenocytes, CD4+ cells, CD4+CD25+ cells, islets, and the liver was extracted using the Qiagen RNEasy Mini Extraction Kit (Qiagen, Hombrechtikon, Switzerland). Reverse transcription was performed using the ImProm-II Reverse Transcription System (Promega, Madison, WI), and cDNA was amplified with the GoTaq DNA polymerase (Promega). Primer sequences are listed in Supplementary Table 1.
Transforming growth factor-β measurement.
Plasma transforming growth factor (TGF)-β concentrations were assessed at 6 h and 1, 2, 3, and 7 days after STZ administration using the mouse/rat/porcine/canine TGF-β1 Quantikine ELISA kit (R&D Systems, Minneapolis, MN). Whole blood was sampled from the tail vein into heparinized tubes and centrifuged at 500g for 20 min at 20°C. Plasma was stored at −20°C.
Histopathology and immunohistology.
Nephrectomy (graftectomy) was performed 10 days after islet Tx. Kidneys were preserved in formol 10% and then embedded in paraffin and sectioned. Paraffin sections were used for hematoxylin and eosin staining. For insulin staining, paraffin sections were incubated using guinea pig antiporcine insulin antibody (DAKO A564; DAKO, Glostrup, Denmark) and subsequently with goat anti-guinea pig Alexa 488–conjugated antibodies (Invitrogen, Basel, Switzerland). For Foxp3 staining, paraffin sections were prepared as previously described using biotinylated anti-Foxp3 mAb (clone FJK-16s; eBioscience) and developed with streptavidin/HRP (DAKO PO397) (9). Slides were analyzed under an axiocam microscope (Zeiss; Axiophot, Göttingen, Germany).
Statistical analysis.
Prism software was used for statistical analysis (GraphPad Software, Inc., San Diego, CA). The nonparametric Kruskal-Wallis test (and Dunn’s method as a post-test) was used for analyzing in vivo data, whereas in vitro assays were analyzed using one-way ANOVA testing and Bonferroni’s multiple testing correction. Graft survivals between groups were compared using the log-rank test.
RESULTS
STZ-induced diabetic mice are lymphopenic.
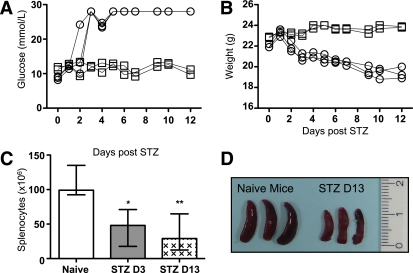
C57BL/6 mice became diabetic between 48 and 72 h after STZ injection (220 mg/kg) (Fig. 1A) and rapidly lost weight as a consequence of dehydration (Fig. 1B). Total splenocyte counts were assessed on days 3 and 13 after STZ administration, showing a rapid depletion (Fig. 1C). The size of the spleen was also significantly diminished after 13 days (Fig. 1D). Lymphoid organs revealed a similar reduction with respect to cell numbers and size (data not shown). The profile of peripheral blood leukocytes was further examined using an automated hematocytometer (Table 1). The number of small WBCs corresponding to the lymphocytes (SCR) was decreased as soon as 6 h after STZ administration, whereas the number of middle and large WBCs corresponding to monocytes (MCR) and granulocytes (LCR), respectively, remained stable over time. The same was true for erythrocytes and hemoglobin (Table 1). STZ is known to be hepatotoxic and nephrotoxic. Thus, a strong inflammatory response to STZ within these organs as well as in the pancreas, the peritoneum, or the heart could explain the observed lymphopenia. However, hematoxylin and eosin staining of these organs performed 3 days after STZ administration did not show any significant lymphocytic infiltration (Supplementary Fig. 1). In conclusion, STZ-induced diabetes led to a strong lymphopenia in the blood and in the spleen, which was not due to leukocyte redistribution and organ infiltration.
FIG. 1.
Diabetes induction with STZ diminishes the absolute numbers of splenocytes. A: Blood glucose levels (mmol/L). ○, STZ-induced diabetic mice; □, naive mice. B: Weight (g) of C57BL/6 mice after STZ administration. ○, STZ-induced diabetic mice; □, naive mice. C: Absolute numbers of splenocytes 3 (D3) and 13 (D13) days after STZ compared with naive mice (n = 5). The median and the range are shown and were analyzed with a nonparametric Kruskal-Wallis test. D: Three spleens of STZ-induced diabetic mice at day 13 compared with naive mice are shown. A P value inferior to 0.05 was considered statistically significant (*P < 0.05, **P < 0.01).
TABLE 1.
Blood repartition after STZ administration
| Peripheral blood | WBC (103/μL) | SCR (%) | MCR (%) | LCR (%) | RBC (106/μL) | Hemoglobin (g/dL) | n |
|---|---|---|---|---|---|---|---|
| Naive | 18.9 ± (10.5/23.6) | 93.2 ± (89.5/96.6) | 2.8 ± (2.1/5.4) | 3.7 ± (0.9/6) | 11.43 ± (10.4/12.4) | 15.9 ± (14.1/17) | 11 |
| Six h | 11.7 ± (10.4/13.7) | 80.9 ± (73.2/89.5) | 10 ± (7.4/16.7) | 8.7 ± (3.1/10.1) | 11.5 ± (10.2/12.7) | 15.6 ± (14.7/16.8) | 6 |
| Day 2 | 7.9 ± (6.8/10) | 83.9 ± (81.4/86.8) | 6.4 ± (4.3/7.8) | 9.7 ± (7.6/11.9) | 10 ± (9.5/10.8) | 14 ± (13.6/15) | 6 |
| Day 5 | 7.6 ± (5.1/10.6) | 84.2 ± (71.3/88.3) | 6.9 ± (5.3/12.5) | 8.9 ± (6.4/16.2) | 9.9 ± (9.3/10.6) | 14 ± (12.5/14.1) | 5 |
| Day 7 | 9.6 ± (5.5/11.2) | 80.2 ± (70.3/88.5) | 8.3 ± (5.5/11.8) | 11.1 ± (4.9/17.9) | 9.8 ± (9/10.7) | 13.7 ± (12.5/14.3) | 6 |
| Day 12 | 8.2 ± (7.1/10.3) | 88 ± (83.8/91.9) | 5.1 ± (3.2/8.4) | 6.9 ± (4.9/11.9) | 10.1 ± (9.2/11.8) | 13.9 ± (12.8/16) | 4 |
Absolute numbers of WBCs and erythrocytes (RBC); percent ratio of SCR (lymphocytes), MCR (monocytes), and LCR (granulocytes) WBCs; and hemoglobin were assessed at 6 h and 2, 5, 7, and 12 days.
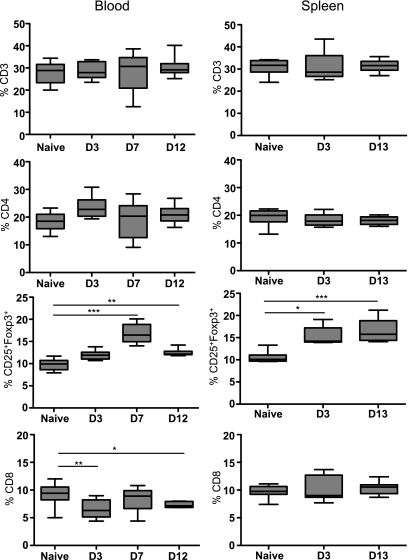
We further assessed by flow cytometry the relative distribution of the leukocyte subsets in circulation and in the spleen after STZ administration. Frequencies of CD3+, CD4+, Foxp3+CD25+ (gated within the CD4+ cells), and CD8+ cells were determined according to their respective isotype controls (Fig. 2 and Supplementary Fig. 2). Interestingly, the percentage of Foxp3+CD25+ Tregs significantly increased in both compartments, at day 3 in the spleen and at day 7 in the blood, although total numbers did not differ from naive mice. Similar results were found in lymph nodes (data not shown). Moreover, the proportion of CD8+ cells was diminished in the blood but not in the spleen 3 days after STZ administration (Fig. 2), whereas the B-cell levels remained unchanged (data not shown). Taken together, STZ-induced diabetes is associated with a higher frequency of Tregs in the blood and secondary lymphoid organs and reduced numbers of CD8+ T cells in the blood.
FIG. 2.
Flow cytometry analysis of T cells in STZ-induced diabetic mice. CD3+, CD4+, CD25+Foxp3+ (gated in the CD4+ population), and CD8+ cell percentages were analyzed in the blood at day 3 (D3), 7 (D7), and 12 (D12) and in the spleen at days 3 (D3) and 13 (D13). Blood from 15 naive and 8 STZ-induced diabetic mice was analyzed. Spleens of eight naive and five STZ-induced diabetic mice were analyzed. Median and the range are shown. A nonparametric Kruskal-Wallis test was performed. *P < 0.05, **P < 0.01, ***P < 0.001.
Distinct effect of STZ and acute hyperglycemia on leukocytes.
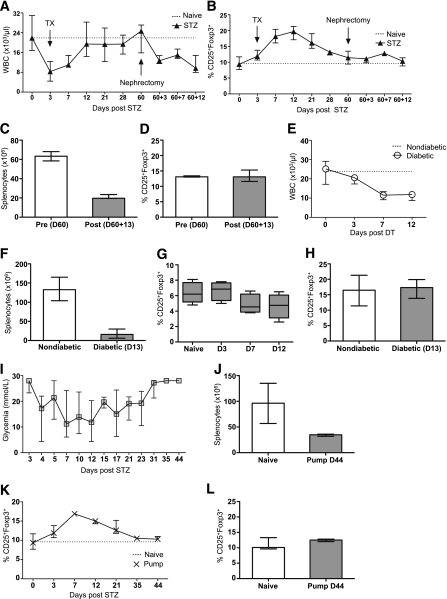
To understand the respective roles of STZ and acute hyperglycemia in the observed induction of lymphopenia and increase in Treg frequencies, three different models were established. First, diabetic mice were transplanted with syngeneic islets under the kidney capsule 3 days after STZ, which reinstalled normoglycemia in these animals (data not shown). After 60 days, the kidney bearing the islets was removed to induce diabetes without STZ. Total leukocyte number and Treg frequencies were measured in the blood at day 60 and day 60 + 3, +7, and +12 after nephrectomy (Fig. 3A and B) and in the spleen before nephrectomy (day 60) and after nephrectomy (day 60 + 13) (Fig. 3C and D). Treg frequencies increased only after STZ administration, although both STZ and hyperglycemia induced lymphopenia. Second, RIP-DTR transgenic mice, which express the coding sequence of the DT receptor under the control of an insulin promoter, were made diabetic by DT administration (18). Total leukocyte numbers were decreased in the blood (Fig. 3E) and in the spleen (Fig. 3F), although the frequency of Tregs remained unaffected in both compartments (Fig. 3G and H), indicating that diabetes induction only is sufficient to deplete leukocytes. Third, the effect of long-term hyperglycemia was tested in STZ-induced diabetic mice. Three days after STZ administration, an insulin pump was implanted subcutaneously. This procedure allowed us to control the glycemia for approximately 20 days (Fig. 3I). Thereafter, the insulin pump was ineffective and the mice became diabetic again. Consistent with the two previous models, the number of leukocytes was decreased in the spleen at day 44 compared with naive mice (Fig. 3J). However, the initial increase of the Treg frequency was not maintained in the blood and in the spleen in these mice, suggesting a critical role of STZ in modulating the Treg compartment (Fig. 3K and L). Thus, the increase in Treg percentage correlated with STZ administration, whereas the hyperglycemia alone also induced leukopenia.
FIG. 3.
The effects of STZ and acute hyperglycemia. The respective effects of STZ and hyperglycemia were analyzed in three different models. First (A–D), C57BL/6 mice were transplanted 3 days after STZ administration with 400 islets equivalent under the left kidney capsule. Sixty days after Tx, a nephrectomy (graftectomy) was performed to induce diabetes without STZ. Absolute numbers of white blood cells (A) and percentage of CD25+Foxp3+ Tregs gated in the CD4+ population (B) were assessed at different time points in the blood (days 0, 3, 7, 12, 21, 28, and 60 after STZ administration and 60 + 3, 60 + 7, and 60 + 12 after nephrectomy). Absolute numbers of splenocytes (C) and percentage of CD25+Foxp3+ Tregs (D) were assessed in the spleen before nephrectomy (day 60 [D60]) and after nephrectomy (day 60 + 13 [D60+13]). Second (E–H), RIP-DTR transgenic mice were made diabetic by administration of DT. Absolute numbers of leukocytes were assessed in the blood at days 3, 7, and 12 (E) and in the spleen at day 13 (D13) after DT administration (F). Percentage of Tregs in RIP-DTR transgenic mice was assessed at the same time points in the blood (G) and in the spleen (H). Third (I–L), 3 days after STZ administration, an insulin pump was implanted subcutaneously to control the glycemia for ∼20 days. Thereafter, the insulin pump was ineffective, and the mice became diabetic again (I). Absolute numbers of splenocytes were assessed at day 44 (D44) after STZ (J). Treg percentage was calculated in the blood at days 3, 7, 12, 21, 35, and 44 (K) and in the spleen at day 44 (D44) (L). The median and the range are shown.
STZ is toxic for leukocytes in vitro.
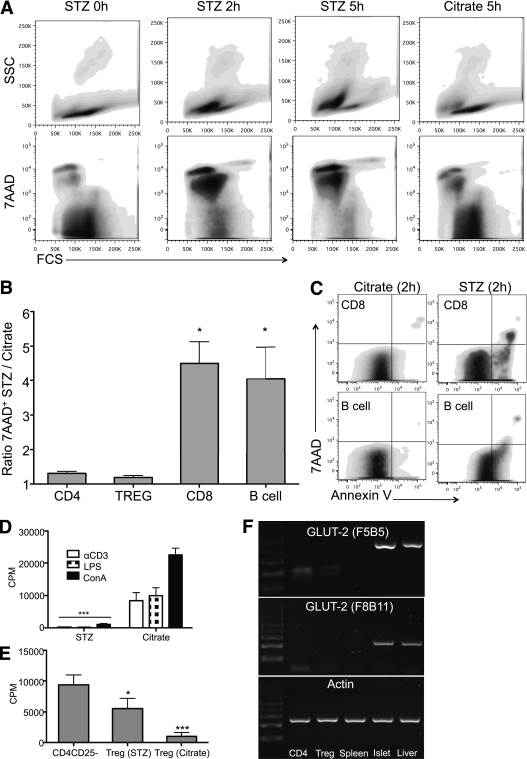
The effect of STZ was further tested on splenocytes in vitro. As a positive control, we first exposed C57BL/6 islets with 4.4 mmol/L STZ in 10% FCS medium or citrate buffer control medium for 1 h. This concentration was based on previous work evaluating the effect of STZ on islets in vitro (18). Thereafter, islets were washed and maintained in culture for 24 h in 10% FCS medium. STZ conditioned islets showed a typical necrotic aspect with loss of the integrity of the collagen capsule. In contrast, the morphology of islets cultured for 1 h in citrate buffer remained intact after 24 h (Supplementary Fig. 3A). These finding were confirmed by 7AAD staining of disrupted islet cells (Supplementary Fig. 3B). Splenocytes were harvested from Foxp3 knock-in mice and cultured for 1 h in STZ or citrate buffer–conditioned medium. Thereafter, the cells were washed and analyzed for 7AAD 0, 2, and 5 h after STZ/citrate exposition. STZ-conditioned splenocytes first shifted toward apoptosis at 2 h and then mainly toward death at 5 h (Fig. 4A). These results were confirmed by trypan blue staining (data not shown). Percentage of 7AAD+ cells in CD4+, Foxp3+, CD8+, and B cells were further analyzed at time point 2 h after STZ or citrate exposition to determine which cell subtypes were more sensitive to STZ. The ratio of 7AAD+ CD8 and B cells after STZ exposure compared with the citrate buffer control was 4.5 and 4.1. In contrast, this ratio was only 1.3 and 1.2 for CD4+ and Treg, respectively (Fig. 4B). Moreover, CD8 and B cells stained positive for Annexin V 2 h after exposure to STZ, suggesting that cell death was mediated through apoptotic pathways (Fig. 4C). Functional tests were also performed. The proliferation of splenocytes stimulated with concanavalin A, lipopolysaccharide, or anti-CD3e mAb was abolished if exposed for 1 h to STZ (Fig. 4D). The susceptibility of Treg to STZ was further analyzed in vitro. Purified CD4+CD25+ T cells were incubated for 1 h with STZ or citrate buffer. Thereafter, an in vitro suppression assay was performed. CD4+CD25+ T cells still suppressed, although not completely, anti–CD3e-stimulated proliferation of CD4+CD25– T cells (Fig. 4E). Furthermore, we analyzed GLUT2 mRNA in the subsets of lymphocytes, since it is one of the main pathways for uptake of STZ (1). Neither splenocytes nor purified CD4+CD25– nor CD4+CD25+ cells were positive for GLUT2 mRNA (Fig. 4F).
FIG. 4.
Effect of STZ on leukocytes in vitro. A: Splenocytes were harvested from Foxp3 GFP knock-in mice and cultured for 1 h in 4.4 mmol/L STZ or control citrate-conditioned medium (10% FCS). 7AAD+ cells were analyzed by flow cytometry 0, 2, and 5 h after STZ exposition. One representative experiment out of four is shown. B: Percentage of 7AAD+ cells in CD4+, Foxp3+, CD8+, and B cells were further analyzed 2 h after STZ or citrate exposition to determine which cell subtypes were more sensitive to STZ. The results were calculated as followed: % of 7AAD+ cells (STZ condition) divided by percent of 7AAD+ cells (citrate condition). The mean and SD of four separate experiments pooled together are shown and analyzed by one-way ANOVA. C: CD8+ and B220+ cells were analyzed for Annexin V expression 2 h after STZ or citrate exposition. One representative experiment out of four is shown. D: After exposition to STZ or citrate-conditioned medium, splenocytes were stimulated with concanavalin A, lipopolysaccharide, or anti-CD3e for 3 days, and their proliferation rate was assessed by [3H]thymidine incorporation. The mean and SD of one representative experiment (performed in triplicate) out of three is shown and analyzed by one-way ANOVA. E: Purified CD4+CD25+ cells were cultured for 1 h in 4.4 mmol/L STZ or citrate-conditioned medium (10% FCS). Thereafter, an in vitro suppression assay was performed. CD4+CD25+ T cells from naive or STZ-induced diabetic mice (day 3 [D3] or 13 [D13]) were added at different CD4+CD25+:CD4+CD25– ratios (1:1, 1:2, 1:4, 1:8). The mean and SD of one representative experiment (performed in triplicate) out of three is shown and analyzed by one-way ANOVA. F: GLUT2 mRNA was analyzed in splenocytes, purified CD4+ cells, and Tregs. Purified islets and liver tissue were used as the control. Two separate experiments were performed. *P < 0.05, ***P < 0.001.
To test whether high levels of glycemia directly affect their viability and proliferation, splenocytes harvested from naive mice were incubated with increasing amounts of glucose (11, 22, and 33 mmol/L) for 24 h. Hyperglycemia failed to induce significant toxicity, as shown by 7AAD staining (Supplementary Fig. 3C). Moreover, high levels of glucose in the medium did not affect the proliferation of splenocytes (Supplementary Fig. 3D). Altogether, these results showed a high susceptibility of CD8 and B cells to STZ, whereas Tregs were partially resistant. These effects were not mediated via the GLUT2 transporter, suggesting that STZ enters into lymphocytes by other pathways. Finally, the observed lymphopenia in response to hyperglycemia in vivo was not explained by a direct lymphotoxic effect of glucose in vitro.
Tregs of STZ-induced diabetic mice are suppressive in vitro and proliferate in vivo.
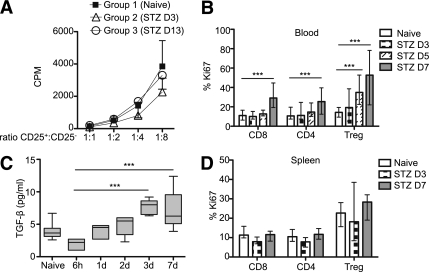
The suppressive function of CD4+CD25+ (Treg phenotype) purified from naive (group 1) or diabetic mice 3 (group 2) and 13 (group 3) days after STZ administration was then tested in vitro. The purity of CD4+CD25+ T cells from STZ-induced diabetic mice was similar to those isolated from naive mice (data not shown). No difference was found with respect to the in vitro suppressive capacity of CD4+CD25+ Tregs, neither at day 3 nor at day 13 after STZ administration in comparison with Tregs of naive mice. These results suggest that Tregs in diabetic mice retain their suppressive function in vivo (Fig. 5A). The proliferation rate of Tregs was further analyzed in the blood and in the spleen. Ki67-positive cells were gated in CD8+, total CD4+, and CD4+CD25+Foxp3+ T cells. Treg proliferation increased at days 5 and 7 in the blood (Fig. 5B). Moreover, TGF-β measured in the plasma of STZ mice was also enhanced at day 7 (Fig. 5C). In the spleen, no difference was detected in Treg proliferation between days 3 and 7, although their basal proliferation rate was higher than that of total CD4+ cells (Fig. 5D). In addition, Treg proliferation accounted for up to 50% of the total CD4+ cell proliferation (data not shown). Thus, these results demonstrate that the proliferative response of Tregs to STZ in vivo also contributes to the increase of their frequency in the blood but not in the spleen.
FIG. 5.
Treg suppression in vitro and proliferation in vivo. A: CD4+CD25+ cells were purified from naive (group 1) and STZ-induced diabetic mice at day 3 (group 2) (D3) and day 13 (group 3) (D13). The results of an in vitro suppression assay, as described in research design and methods, are shown as mean and SDs. One representative experiment out of three is shown. B: Ki67 expression was analyzed in the blood in CD4+ (total population), CD8+, and Foxp3+ T cells at day 3 (D3), 5 (D5), and 7 (D7) after STZ administration (n = 8–20 per group/time point). C: Plasma TGF-β concentrations were assessed at 6 h and 1, 2, 3, and 7 days (1d, 2d, 3d, 7d) after STZ administration (n = 6). D: Ki67 expression was analyzed in the spleen at days 3 and 7 after STZ administration (n = 6). The median and the range are shown in B, C, and D and were analyzed with the Kruskal-Wallis test. ***P < 0.001.
STZ-induced diabetic mice are immunosuppressed.
We further investigated the effect of STZ-induced lymphopenia and increased percentage of Tregs on allograft rejection. First, STZ-induced diabetic and naive C57BL/6 mice were transplanted with BALB/c pancreatic islets. Ten days after Tx, the mice were killed and grafts were analyzed by immunohistology. Islets were completely destroyed in naive mice (Fig. 6D and E), whereas in STZ-induced diabetic mice, the islets were stained positive for insulin (Fig. 6A and B). The median graft survival (MGS) in STZ-induced diabetic mice was 17.5 days (data not shown). Moreover, higher numbers of Foxp3+ cells were found within the graft of diabetic mice compared with naive mice (Fig. 6C–F). To confirm these results in a more stringent Tx model, STZ-induced diabetic and naive C57BL/6 (H2b) mice were grafted with BL/6xDBA/2 F1 (H2bxH2d) skin 3 days after STZ administration. At the same time, an insulin pump was implanted subcutaneously in STZ-induced diabetic mice to correct the blood glucose, allowing the mice to survive. Skin grafts were rejected approximately 1 week later in STZ-induced diabetic mice (MGS 20 days) compared with untreated mice (MGS, 14 days) (Fig. 6G and H). These results demonstrate that STZ treatment delays allogeneic graft rejection and that this immunosuppressive effect is not specific to islet Tx. To further confirm the immunosuppressive effect of STZ-induced diabetes, BL/6xDBA/2 F1 (H2bxH2d) skin grafts were transplanted to naive and STZ-induced diabetic mice (+ insulin pump) treated with anti-CD154 mAb (MR1). MR1 alone had a similar effect than STZ with an MGS of 18 days. MR1 therapy significantly improved the skin graft survival in STZ-induced diabetic mice (MGS 38 days, P < 0.0001), demonstrating that diabetes induction with STZ potentiates the immunosuppressive effect of the MR1 (Fig. 6G). Thus, these results suggest that many results obtained in STZ-induced diabetic mice with respect to long-term graft tolerance may be overestimated.
FIG. 6.
Islet and skin allografts in STZ-treated mice. C57BL/6 STZ-induced diabetic (A–C) and naive (D–F) mice were transplanted with BALB/c islets. Islet grafts were analyzed by histology 10 days after Tx. Hematoxylin and eosin (A and D), insulin immunofluorescence (Alexa Fluor 488–conjugated) (B and E), and Foxp3 immunohistochemistry (streptavidin/HRP, black arrows) (C and F) were performed. Magnification is ×200. A–F: Survival curve of BL/6xDBA/2 F1 (H2bxH2d) skin grafts in naive and STZ-induced diabetic mice treated with an implanted insulin pump with or without MR1 treatment. G: Graft survival between groups was compared using the log-rank test. H: Representative skin graft at day 14 after Tx in STZ compared with the naive group. (A high-quality digital representation of this figure is available in the online issue.)
DISCUSSION
The goal of this study was to analyze the effect of STZ on the immune system and the outcome of allotransplantation with respect to graft rejection and tolerance. Our results demonstrate that STZ-induced diabetic mice are immunosuppressed compared with naive mice, leading to prolonged islet and skin allograft survival. Two mechanisms are put forward to explain the observed immunosuppression associated with STZ treatment: the induction of lymphopenia and a shift toward Tregs.
Consistent with previous reports, STZ was directly toxic for lymphocytes, inducing apoptosis in vitro, and was responsible for early depletion of blood and spleen lymphocytes in vivo. A study published over 25 years ago (2) had shown that incubation of splenocytes with STZ in vitro abrogated their capacity to proliferate in response to various stimuli. However, these data were not further investigated since then with respect to the characterization of the different susceptibility of lymphocytes subset to STZ. It is noteworthy that, in our study, CD8+ T cells and B cells were particularly sensitive to STZ toxicity in vitro. This observation is in line with the decrease in CD8+ T-cell numbers in the peripheral blood of STZ-treated mice. Because CD8+ T cells are known to play a critical role in β-cell autoimmunity and rejection, direct CD8+ depletion induced by STZ administration may contribute to the better islet engraftment described after Tx. Indeed, NOD mice treated with anti-CD8 mAb or small doses of STZ in early life are protected from spontaneous diabetes (5,19,20). In addition, our results demonstrate that the uptake of STZ into lymphocytes occurs independently of the transporter GLUT2, which is known to be the main pathway for STZ uptake into β-cells (21). Because STZ is a glucose analog and lymphocytes depend on glucose (22), several other active and passive glucose transporters such as GLUT1 could be implicated. Thus, further investigation is warranted to determine the receptors responsible for the transport of STZ into lymphocytes and its toxic effects that finally lead to their death.
Importantly, STZ is not the only factor leading to T-cell depletion in STZ-induced diabetic mice. In agreement with similar observations in various animal models of diabetes (7,23), we demonstrate here that acute hyperglycemia induces lymphopenia. Three different models of diabetes were used, and all induced lymphopenia independently of STZ: 1) new-onset (diabetes induction in RIP-DTR mice), 2) recurrent (nephrectomy of transplanted syngeneic islets), and 3) continuous hyperglycemia (STZ-induced diabetic mice treated with an insulin pump). However, the mechanisms leading to hyperglycemia-induced lymphopenia remain poorly defined. Our results support the notion that indirect pathways are responsible for the observed lymphopenia in vivo because exposure of lymphocytes to high levels of glucose in vitro did not significantly affect their viability or their capacity to proliferate. Diabetes onset is known to be associated with high serum levels of glucocorticoids, which are lymphotoxic (24). A potential role of glucocorticoids in hyperglycemia-induced lymphopenia is further supported by the fact that adrenalectomy before diabetes induction hampered the increase of glucocorticoids and the associated lymphopenia (7,25). Others showed an adrenal hypersensitivity preceding the blood hypercorticism (26). Thus, it is likely that dehydration in diabetic mice induces antidiuretic hormone release, which in turn directly sensitizes the adrenal glands to produce glucocorticoids (27). Nevertheless, this hypothesis still remains to be confirmed in STZ-induced diabetic mice.
The second important observation in STZ-induced diabetic mice was the increase of Treg frequencies in the spleen, the peripheral blood, and also in lymph nodes (data not shown). In addition, Tregs were detected in islet allografts after STZ administration, with longer graft survival in STZ-induced diabetic mice compared with control mice. On the basis of these observations and similar reports, we and others suggested a role of Tregs in the control of immune responses after allo- and xenogeneic islet Tx (9,13) or solid organ Tx (28,29). It is not necessarily an increase of the absolute numbers of Tregs but rather a favorable shift of the Treg/T effector cell ratio that is “tolerogenic” (30,31). Importantly, the relative increase of Tregs correlated specifically with the administration of STZ and was not a consequence of hyperglycemia, since neither early (DT administration to RIP-DTR mice) nor late (removal of islet grafts long after STZ diabetes induction) hyperglycemia affected the percentage of Tregs. The relative increase of Tregs was also associated with a higher level of Treg proliferation in the blood. Finally, TGF-β, which has been shown to convert naive CD4+CD25– T cells into CD4+CD25+ Tregs in vitro, was increased in the peripheral blood of mice 3 and 7 days after STZ injection (32). Parallel findings were reported after lymphocyte depletion using monoclonal antibodies (either anti-CD3 antibody therapy [33,34] or Campath [anti-CD52] [35,36]). Thus, it is likely that a profound depletion of T cells mediated by STZ, anti-CD3, or Campath may lead to an increased proliferation of Tregs. More recently, a predominant role for the production of systemic TGF-β was attributed to macrophages and dendritic cells (37). Furthermore, the association between apoptotic cell phagocytosis and TGF-β−mediated Treg expansion has led to the development of experimental therapies consisting in intravenous apoptotic cell infusion (38,39). Taken together, one might speculate that Treg expansion in STZ-induced diabetic mice is mediated by TGF-β in an apoptosis-prone lymphopenic environment, but this hypothesis warrants further testing.
Overall, this study emphasizes the immunomodulation that occurs after STZ administration. Indeed, diabetic mice display a severe lymphopenia combined with a relative increase of Tregs in the blood, spleen, and lymph nodes. These are important changes to consider and to discuss in future studies using STZ-induced diabetic mice, in particular, in the setting of tolerogenic protocols for islet Tx. Because our work also shows that acute hyperglycemia is sufficient to induce lymphopenia, it is likely that any model that is based on diabetes induction is biased toward immunosuppression. In conclusion, the results of previous studies of Tx tolerance induction and immunosuppressive protocols might be overestimated using STZ-induced diabetes models and need to be reinterpreted in light of the present findings.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Swiss National Research Fund grants 3200BO-102134 (to P.M. and L.H.B.) and 32323B-111370/32003B-111371 (to D.G.) and the Hans Wilsdorf (to J.D.S.), the Insuleman (to L.H.B.), and E. & L. Schmidheiny Foundations (to Y.D.M.). D.G. was also supported by the Faculty of Biology and Medicine of Lausanne University and Foundation Medi-CAL Futur.
No potential conflicts of interest relevant to this article were reported.
Y.D.M., D.G., D.E., L.H.B., and J.D.S. conceived and designed the experiments. Y.D.M., D.G., D.E., J.C.W., L.G., R.M., V.S.-B., and G.P.Y. performed the experiments. Y.D.M., D.G., L.H.B., and J.D.S. analyzed the data. D.G., P.M., L.H.B., and J.D.S. contributed reagents/materials/analysis tools. Y.D.M., L.H.B., and J.D.S. wrote the manuscript.
We thank Christian Toso and Géraldine Parnaud (from the Surgical Research Unit, Department of Surgery, University Hospital Geneva, Geneva, Switzerland) and Anne-Laurence Blanc (from Institut Pasteur, Paris, France) for critical reading of the manuscript; Isabelle Avril (from the Department of Genetic Medicine and Development, University of Geneva Medical School, Geneva, Switzerland) and Domenico Bosco (from the Surgical Research Unit, Department of Surgery, University Hospital Geneva, Geneva, Switzerland) for helpful discussions; and Corinne Sinigaglia, Nadine Pernin, David Matthey-Doret, Solange Masson, and Caroline Rouget (all from the Surgical Research Unit, Department of Surgery, University Hospital Geneva, Geneva, Switzerland) for technical assistance.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0159/-/DC1.
L.H.B. and J.D.S. share co-senior authorship in this article.
REFERENCES
- 1.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008;51:216–226 [DOI] [PubMed] [Google Scholar]
- 2.Gaulton GN, Schwartz JL, Eardley DD. Assessment of the diabetogenic drugs alloxan and streptozotocin as models for the study of immune defects in diabetic mice. Diabetologia 1985;28:769–775 [DOI] [PubMed] [Google Scholar]
- 3.Koulmanda M, Qipo A, Auchincloss HJ, Jr, Smith RN. Effects of streptozotocin on autoimmune diabetes in NOD mice. Clin Exp Immunol 2003;134:210–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nichols WK, Spellman JB, Vann LL, Daynes RA. Immune responses of diabetic animals: direct immunosuppressant effects of streptozotocin in mice. Diabetologia 1979;16:51–57 [DOI] [PubMed] [Google Scholar]
- 5.Takayama Y, Ichikawa T, Maki T. Effect of STZ administration on islet isograft and allograft survival in NOD mice. Diabetes 1993;42:324–329 [DOI] [PubMed] [Google Scholar]
- 6.Hugues S, Mougneau E, Ferlin W, et al. Tolerance to islet antigens and prevention from diabetes induced by limited apoptosis of pancreatic beta cells. Immunity 2002;16:169–181 [DOI] [PubMed] [Google Scholar]
- 7.Luo B, Chan WF, Lord SJ, et al. Diabetes induces rapid suppression of adaptive immunity followed by homeostatic T-cell proliferation. Scand J Immunol 2007;65:22–31 [DOI] [PubMed] [Google Scholar]
- 8.Niclauss N, Bosco D, Morel P, Giovannoni L, Berney T, Parnaud G. Rapamycin impairs proliferation of transplanted islet β cells. Transplantation 2011;91:714–722 [DOI] [PubMed] [Google Scholar]
- 9.Muller YD, Mai G, Morel P, et al. Anti-CD154 mAb and rapamycin induce T regulatory cell mediated tolerance in rat-to-mouse islet transplantation. PLoS ONE 2010;5:e10352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearson T, Markees TG, Serreze DV, et al. Islet cell autoimmunity and transplantation tolerance: two distinct mechanisms? Ann N Y Acad Sci 2003;1005:148–156 [DOI] [PubMed] [Google Scholar]
- 11.Horner BM, Randolph MA, Huang CA, Butler PE. Skin tolerance: in search of the Holy Grail. Transpl Int 2008;21:101–112 [DOI] [PubMed] [Google Scholar]
- 12.Arefanian H, Tredget EB, Rajotte RV, Gill RG, Korbutt GS, Rayat GR. Short-term administrations of a combination of anti-LFA-1 and anti-CD154 monoclonal antibodies induces tolerance to neonatal porcine islet xenografts in mice. Diabetes 2010;59:958–966 [DOI] [PMC free article] [PubMed]
- 13.Webster KE, Walters S, Kohler RE, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med 2009;206:751–760 [DOI] [PMC free article] [PubMed]
- 14.Luo X, Pothoven KL, McCarthy D, et al. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc Natl Acad Sci U S A 2008;105:14527–14532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tao R, de Zoeten EF, Ozkaynak E, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med 2007;13:1299–1307 [DOI] [PubMed] [Google Scholar]
- 16.Muller YD, Golshayan D, Ehirchiou D, Wekerle T, Seebach JD, Bühler LH. T regulatory cells in xenotransplantation. Xenotransplantation 2009;16:121–128 [DOI] [PubMed] [Google Scholar]
- 17.Thorel F, Nepote V, Avril I, et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 2010;464:1149-1154 [DOI] [PMC free article] [PubMed]
- 18.Strandell E, Eizirik DL, Korsgren O, Sandler S. Functional characteristics of cultured mouse pancreatic islets following exposure to different streptozotocin concentrations. Mol Cell Endocrinol 1988;59:83–91 [DOI] [PubMed] [Google Scholar]
- 19.Knip M, Siljander H. Autoimmune mechanisms in type 1 diabetes. Autoimmun Rev 2008;7:550–557 [DOI] [PubMed] [Google Scholar]
- 20.Thivolet C, Bendelac A, Bedossa P, Bach JF, Carnaud C. CD8+ T cell homing to the pancreas in the nonobese diabetic mouse is CD4+ T cell-dependent. J Immunol 1991;146:85–88 [PubMed] [Google Scholar]
- 21.Hosokawa M, Dolci W, Thorens B. Differential sensitivity of GLUT1- and GLUT2-expressing beta cells to streptozotocin. Biochem Biophys Res Commun 2001;289:1114–1117 [DOI] [PubMed] [Google Scholar]
- 22.Maciver NJ, Jacobs SR, Wieman HL, Wofford JA, Coloff JL, Rathmell JC. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J Leukoc Biol 2008;84:949–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otton R, Soriano FG, Verlengia R, Curi R. Diabetes induces apoptosis in lymphocytes. J Endocrinol 2004;182:145–156 [DOI] [PubMed] [Google Scholar]
- 24.De Nicola AF, Fridman O, Del Castillo EJ, Foglia VG. Abnormal regulation of adrenal function in rats with streptozotocin diabetes. Horm Metab Res 1977;9:469–473 [DOI] [PubMed] [Google Scholar]
- 25.Han F, Ozawa H, Matsuda KI, Lu H, De Kloet ER, Kawata M. Changes in the expression of corticotrophin-releasing hormone, mineralocorticoid receptor and glucocorticoid receptor mRNAs in the hypothalamic paraventricular nucleus induced by fornix transection and adrenalectomy. J Neuroendocrinol 2007;19:229–238 [DOI] [PubMed] [Google Scholar]
- 26.Revsin Y, van Wijk D, Saravia FE, Oitzl MS, De Nicola AF, de Kloet ER. Adrenal hypersensitivity precedes chronic hypercorticism in streptozotocin-induced diabetes mice. Endocrinology 2008;149:3531–3539 [DOI] [PubMed] [Google Scholar]
- 27.Roberts EM, Pope GR, Newson MJ, Lolait SJ, O’Carroll AM. The vasopressin V1b receptor modulates plasma corticosterone responses to dehydration-induced stress. J Neuroendocrinol 2011;23:12–19 [DOI] [PubMed]
- 28.Golshayan D, Jiang S, Tsang J, Garin MI, Mottet C, Lechler RI. In vitro-expanded donor alloantigen-specific CD4+CD25+ regulatory T cells promote experimental transplantation tolerance. Blood 2007;109:827–835 [DOI] [PubMed] [Google Scholar]
- 29.Joffre O, Santolaria T, Calise D, et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med 2008;14:88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mansour H, Homs S, Desvaux D, et al. Intragraft levels of Foxp3 mRNA predict progression in renal transplants with borderline change. J Am Soc Nephrol 2008;19:2277–2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 2008;358:353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25– naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 2003;198:1875–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belghith M, Bluestone JA, Barriot S, Mégret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med 2003;9:1202–1208 [DOI] [PubMed] [Google Scholar]
- 34.Ochi H, Abraham M, Ishikawa H, et al. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+ CD25- LAP+ T cells. Nat Med 2006;12:627–635 [DOI] [PubMed] [Google Scholar]
- 35.Noris M, Casiraghi F, Todeschini M, et al. Regulatory T cells and T cell depletion: role of immunosuppressive drugs. J Am Soc Nephrol 2007;18:1007–1018 [DOI] [PubMed] [Google Scholar]
- 36.Bloom DD, Chang Z, Fechner JH, et al. CD4+ CD25+ FOXP3+ regulatory T cells increase de novo in kidney transplant patients after immunodepletion with Campath-1H. Am J Transplant 2008;8:793–802 [DOI] [PubMed] [Google Scholar]
- 37.Perruche S, Zhang P, Liu Y, Saas P, Bluestone JA, Chen W. CD3-specific antibody-induced immune tolerance involves transforming growth factor-beta from phagocytes digesting apoptotic T cells. Nat Med 2008;14:528–535 [DOI] [PubMed] [Google Scholar]
- 38.Maeda A, Schwarz A, Kernebeck K, et al. Intravenous infusion of syngeneic apoptotic cells by photopheresis induces antigen-specific regulatory T cells. J Immunol 2005;174:5968–5976 [DOI] [PubMed] [Google Scholar]
- 39.Kleinclauss F, Perruche S, Masson E, et al. Intravenous apoptotic spleen cell infusion induces a TGF-beta-dependent regulatory T-cell expansion. Cell Death Differ 2006;13:41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.