Abstract
OBJECTIVE
Glucagon-like peptide 1 (GLP-1) is a gut-brain hormone that regulates food intake, energy metabolism, and cardiovascular functions. In the brain, through a currently unknown molecular mechanism, it simultaneously reduces femoral artery blood flow and muscle glucose uptake. By analogy to pancreatic β-cells where GLP-1 activates protein kinase C (PKC) to stimulate insulin secretion, we postulated that PKC enzymes would be molecular targets of brain GLP-1 signaling that regulate metabolic and vascular function.
RESEARCH DESIGN AND METHODS
We used both genetic and pharmacological approaches to investigate the role of PKC isoforms in brain GLP-1 signaling in the conscious, free-moving mouse simultaneous with metabolic and vascular measurements.
RESULTS
In normal wild-type (WT) mouse brain, the GLP-1 receptor (GLP-1R) agonist exendin-4 selectively promotes translocation of PKC-δ (but not -βII, -α, or -ε) to the plasma membrane. This translocation is blocked in Glp1r−/− mice and in WT mice infused in the brain with exendin-9, an antagonist of the GLP-1R. This mechanism coordinates both blood flow in the femoral artery and whole-body insulin sensitivity. Consequently, in hyperglycemic, high-fat diet–fed diabetic mice, hypothalamic PKC-δ activity was increased and its pharmacological inhibition improved both insulin-sensitive metabolic and vascular phenotypes.
CONCLUSIONS
Our studies show that brain GLP-1 signaling activates hypothalamic glucose-dependent PKC-δ to regulate femoral artery blood flow and insulin sensitivity. This mechanism is attenuated during the development of experimental hyperglycemia and may contribute to the pathophysiology of type 2 diabetes.
Glucagon-like peptide 1 (GLP-1) is secreted during a meal by intestinal L cells and directly enhances glucose-induced insulin secretion (1). Furthermore, GLP-1 is an enteric neuropeptide that triggers vagal signals from the intestine to the brain to activate the gut-brain-pancreatic axis (2–7). Through interaction between neural and direct receptor-dependent mechanisms, GLP-1 regulates multiple metabolic and cardiovascular functions (5,8). Moreover, GLP-1 is produced by neurons in the brainstem (9,10) and transported to several nuclei in the hypothalamus where its receptor has been localized (11–13). To unravel the metabolic importance of the central nervous system GLP-1 receptor (GLP-1R), we previously showed that activation of brain GLP-1 signaling simultaneously reduces femoral artery blood flow and muscle glucose utilization to favor hepatic glucose storage (5,8). This mechanism is impaired in high-fat diet (HFD)-induced diabetic mice (14). Although the corresponding molecular mechanisms regulating brain GLP-1 action remain unknown, a molecular hypothesis can be postulated by analogy with pancreatic β-cells. An obvious target is protein kinase A, a dominant molecular signaling mechanism of the GLP-1R (1). However, changes in protein kinase A activity have not been consistently associated with diabetes. GLP-1 also activates both the α and ε isoforms of protein kinase C (PKC) to stimulate insulin secretion (15). These proteins are translocated from a cytoplasmic location to the plasma membrane, where proteins such as ATP-sensitive K+ channels are phosphorylated on conserved threonine residues (T180) in the pore-forming subunit Kir6.2 by a calcium-dependent mechanism (16,17). It is important that brain PKCs have been implicated in the control of glucose metabolism in mice (18). The central injection of the specific PKC activator, 12-O-tetradecanoylphorbol-13-acetate (TPA), a phorbol ester, enhanced the hypoglycemic response to coadministered insulin (18). In healthy conscious rats, the coadministration of the hypothalamic PKC-δ inhibitor rottlerin with 1-oleoyl-2-acetyl-sn-glycerol (OAG), a synthetic diacylglycerol (DAG) analogue, prevented the ability of OAG to activate hypothalamic PKC-δ and to lower hepatic glucose production (19). It is noteworthy that hyperglycemia also triggers PKC activation, and in models of diabetes, these enzymes are excessively activated (20–23). Therefore, we postulated that brain GLP-1 could control metabolism or blood flow by a mechanism involving the activation of hypothalamic PKCs that might also be regulated by hyperglycemia and diabetes.
RESEARCH DESIGN AND METHODS
Experiments were carried out under protocols approved by the institutional animal care and use committee. Male C57BL/6J (Charles River Laboratories, Paris, France) and Glp1r−/− C57BL/6J mice (age 16 weeks) from our colony were studied. Groups of 4-week-old mice were fed a normal chow (NC) diet (SAFE, Epinay sur Orge, France; content: 12% fat, 28% protein, and 60% carbohydrate, low nitrate) or an HFD (SAFE; 72% fat [corn oil and lard], 28% protein, and <1% carbohydrate) for 12 weeks, which has been shown to induce diabetes and insulin resistance without overt body weight gain (7,14,24,25). Throughout the study period, mice were housed at 21–22°C with a normal daily cycle and ad libitum access to food and water.
Surgical procedures.
Femoral artery blood flow was continuously monitored during an intracerebroventricular infusion of pharmacological compounds as described previously (5,8,26). Briefly, a catheter (Charles River Laboratories) was inserted into the right lateral ventricle and secured on the top of the skull under anesthesia with isoflurane-oxygen. At 10 days after the cranial surgery, an intravenous catheter was introduced into the left femoral vein, sealed under the skin, and externalized at the back of the neck. The mice were allowed to recover for 3 days before an ultrasonic flow probe (Transonic Systems, Emka Technologies, Inc., Paris, France) was inserted around the right femoral artery. The probe wire was sealed under the skin at the back of the neck where it was secured using surgical thread. After surgery, the mice were returned to their cage for at least 4 days until their body weight recovered. At the end of the recovery period, mice that did not reach the presurgery weights were not studied further (i.e., 15% of the animals).
Infusions.
On the experimental day, the flow probe was connected to a transonic model T403 flowmeter (Transonic Systems, Emka Technologies, Inc.) to continuously record the blood flow (mL/min) of the artery and the heart rate bpm). The basal femoral artery blood flow was recorded for 30 min in overnight fasted, freely moving mice. Intracerebroventricular infusions were performed at a rate of 12 µL/h preceded by a 5-µL bolus with artificial cerebrospinal fluid (aCSF; pH 7.35, Na+ 144 mmol/L, Cl− 146 mmol/L, K+ 3 mmol/L, Mg2+ 1 mmol/L, Ca2+ 1.5 mmol/L, PO4− 1.2 mmol/L, vehicle/control group) or the test compounds (as described below) for 3 h. To validate that the probe was correctly recording the blood flow, at the end of the insulin infusion, mice were given a flash injection of a rapid nitric oxide donor (sodium nitroprusside, 10 mg/kg i.v., 25–40 µL, purchased from Sigma, Saint-Quentin Fallavier, France), which induced a 100% increase in blood flow and heart rate.
Glucose clamps.
Mice were clamped in hyperinsulinemic conditions to activate whole-body glucose utilization and induce vasodilation. Subgroups of mice were clamped in euglycemic (5.5 mmol/L) or hyperglycemic (20 mmol/L) conditions, as previously described (5,8). Briefly, insulin was infused through the intrafemoral catheter at a rate of 18 mU/kg/min for 3 h. Glycemia was clamped by adjusting an intrafemoral glucose infusion (30%). A control group was infused with NaCl 0.9% (saline) for 3 h at a rate that matched the rate of all infusions to ensure a similar liquid flow (8).
The intracerebroventricular infusions were started 30 min before the beginning of the clamp procedure and continued throughout the whole infusion period. Briefly, a 5 µL bolus was given followed by a continuous infusion with the aCSF or with the GLP-1R agonist exendin-4 (Ex4) or antagonist exendin-9 (Ex9) at a rate of 0.5 pmol/kg/min for 3 h, as described (5,8,13).
Phorbol myristic acid, calphostin C, and rottlerin infusions.
To evaluate the role of brain PKCs in the regulation of arterial blood flow and glucose turnover rates in response to brain GLP-1R activation, we examined the effects of a nonspecific PKC activator, the phorbol ester PMA (phorbol-12,13-myristic acid; Sigma), or inhibitors (calphostin C and rottlerin) infused for 3 h at a very low rate into the cerebroventricle (intracerebroventricularly). The PMA was diluted in aCSF and then infused at a rate of 0.1 nmol/µL, as previously described (27). In a separate set of mice, calphostin C (Sigma), a pan-specific inhibitor that specifically inhibits the binding of DAG to the regulatory domain of PKCs (28), was diluted in aCSF and then infused at a rate of 3 pmol/μL, as previously described (29). Rottlerin (Sigma), a specific PKC-δ inhibitor, was diluted in aCSF and then infused at a rate of 0.27 nmol/µL. Intracerebroventricular infusions were started 30 min before the beginning of the intravenous insulin/glucose infusions and continued for 3 h. All solutions used contained no more than 2% alcohol to dissolve the pharmacological agents.
Isolation of PKCs.
At the end of the infusions, cytosolic and membrane fractions were separated according to the procedure described below to evaluate the translocation of PKC. Briefly, when the clamp procedure ended, each hypothalamus was removed from the skull immediately after cervical dislocation and cooled down in a frozen brain frame (World Precision Instruments, Stevenage, U.K.) to stop all endogenous enzyme reactions and then frozen at −80°C in liquid nitrogen. A 3-mm coronal section corresponding to the hypothalamus was sliced out, and a triangle with a side length of 2 mm corresponding to the frozen hypothalamus was separated and kept frozen until studied, as previously described (26). Slices were then lysed in ice-cold 0.25 mol/L sucrose buffer (containing 25 mmol/L Tris-HCl, pH 7.4, 2 mmol/L EDTA, 1 mmol/L EGTA, 10 mmol/L β-mercaptoethanol, 1 μg/mL leupeptin, 1 μg/µL aprotinin, and 1 mmol/L phenylmethylsulfonyl fluoride) and centrifuged at 14,000g for 5 min at 4°C. The supernatant was then collected and centrifuged at 100,000g for 45 min at 4°C to separate the cytosolic fraction (supernatant) from the particulate fraction (pellet). The latter was resuspended in the same buffer, to which 0.1% Triton X-100 had been added and sonicated 12 times in ice before being recentrifuged at 100,000g for 30 min at 4°C. The supernatant was then removed. This last fraction is called the membrane fraction. In some preliminary studies, we validated the presence of the Na+-K+-ATPase pump, a component of the cytoplasmic membrane, in each membrane fraction. This membrane marker was totally absent from the cytosolic fraction.
PKC assay.
PKC activity was assayed in each fraction (cytosolic and membrane) using the PepTag nonradioactive PKC assay system (Promega Corporation, Madison, WI) as described (30). Briefly, 2 μg of each sample and purified PKC enzyme (internal control) were incubated at 30°C for 30 min with a fluorescent peptide substrate that is highly specific for PKCs and then loaded onto a 0.8% agarose gel in 50 mmol/L Tris-HCl, pH 8.0. Phosphorylation by PKCs alters the net charge of the peptide from 1 to −1. This change in the net charge of the substrate allows phosphorylated and nonphosphorylated substrates to be separated on an agarose gel. This assay can detect <10 ng of kinase. The intact agarose gel was then analyzed in a scanning densitometer using a wavelength of 570 nm and data interpreted qualitatively and quantitatively using ImageQuant 5.2 software. In each fraction, activity was quantified and normalized by the positive internal control to allow multiple comparisons between gels. Next, the PKC translocation was calculated for each hypothalamus by the corresponding membrane-to-cytosol ratio. The results of scanning densitometry expressed in arbitrary units (AUs) correspond to the upper band intensity divided by the sum of the upper and lower bands. The upper band corresponds to the phosphorylated and the lower band to the nonphosphorylated substrate.
Western blot analysis.
Western blots for specific PKCs expressed in the hypothalamus were performed on the same fractions used to assess the total PKC activity as described above. We used specific antibodies targeted against α (15), ε (15), βII (23), and δ (19) PKC isoforms. These isoforms are expressed in the brain (31–34) and activated by an increase in DAG in the cytosol or by a phorbol ester such as PMA (35). They are inhibited by calphostin C, a kinase inhibitor specific for PKC, which targets the DAG or phorbol ester binding site (36). After protein determination by the protein assay (Bio-Rad, Marnes-la-coquette, France), using BSA as a standard, samples were separated by 10% SDS-PAGE. For each hypothalamic sample, 20 μg of cytosolic or membrane fraction was loaded onto a 10% acrylamide SDS-PAGE. After separation, the proteins were transferred to a polyvinylidene difluoride membrane. Membranes were then blocked for 90 min with 3% BSA and incubated for 90 min at room temperature with a primary polyclonal antibody against PKC-α, -ε, -βII, or -δ (Santa Cruz Biotechnology, Inc., Santa Cruz, CA; references 937, 208, 214, and 210, respectively, were used with the following dilutions: anti-δ 1/1,000, anti-α 1/2,000, anti-βII 1/5,000, and anti-ε 1/8,000) (19). We used PKC-βII or PKC-ε as an internal control because they did not translocate under different experimental conditions. After washing three times, membranes were incubated with horseradish peroxidase–conjugated anti-rabbit IgG-antibody (Amersham Biosciences Europe, Orsay, France; dilution 1/8,000) for at least 60 min. Immunoreactivity was detected using an enhanced chemiluminescence detection kit (ECL system; Amersham) and exposure to X-ray film. Bands were quantified using ImageQuant software.
Blood sampling.
At the end of the infusions, blood was collected from the retro-orbital sinus into a tube, mixed with 1 µg/µL aprotinin per 0.1 mmol/L EDTA, and centrifuged at 8,000 rpm for 5 min at 4°C. Plasma was stored at −80°C until assay. Insulin level was measured using an ELISA kit (Mercodia, Uppsala, Sweden).
Data analysis and statistics.
Data are expressed as means ± SE. Data were analyzed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA) for statistical significance by applying, respectively, a Student t test and a one-way or a two-way ANOVA test for repeated measurements followed by a post hoc test (Bonferroni multiple comparison test) when appropriate. The level of significance was defined as P < 0.05.
RESULTS
Brain GLP-1R signaling activates plasma membrane translocation of PKC in the hypothalamus under hyperinsulinemic and hyperglycemic conditions.
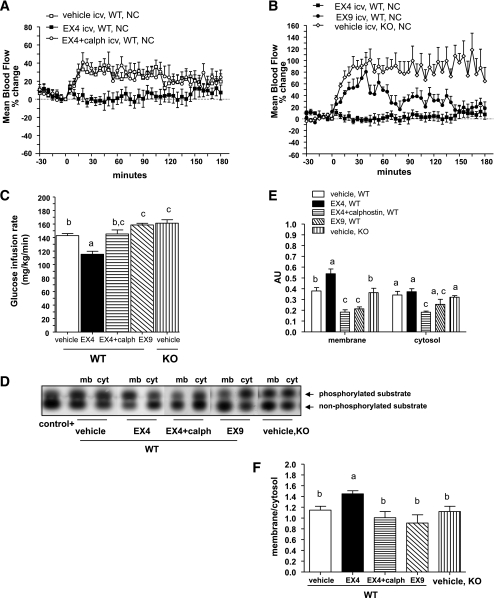
To determine whether brain GLP-1 signaling regulates hypothalamic PKC activity and vascular/metabolic functions, we studied mice under hyperglycemic and hyperinsulinemic conditions during a 3-h clamp procedure. Under these conditions, the Ex4 infusion decreased both femoral artery blood flow and whole-body glucose infusion rates (GIRs) compared with the control group receiving the vehicle into the lateral ventricle of the brain (Fig. 1A and C). Brain Ex4 infusion enhanced PKC translocation to the plasma membrane and increased the corresponding membrane-to-cytosol ratio (Fig. 1D–F). The PKC translocation was higher in response to brain Ex4 infusion than in vehicle-treated mice. Basal femoral artery blood flow and GIR were relatively increased in Glp1r−/− mice and in wild-type (WT) mice infused with Ex9, showing that control of blood flow and GIR were dependent on GLP-1R signaling (Fig. 1B and C). In a similar manner, the Ex4-dependent stimulation of membrane PKC translocation was abolished in mice with pharmacological blockade or GLP-1R genetic disruption (Fig. 1D–F).
FIG. 1.
Brain GLP-1 signaling recruits PKCs to control femoral artery blood flow and whole-body GIR in WT mice during hyperinsulinemic-hyperglycemic clamp studies. A and B: Mean arterial blood flow in percentage changes over baseline during a hyperinsulinemic-hyperglycemic clamp where vehicle, Ex4, Ex9, or Ex + calphostin (calph) were infused into the brain of WT (A and B) or Glp1r−/− (knock-out [KO]) (B) mice. C: Whole-body GIRs simultaneously recorded in the same mice. Data are means ± SE, n = 5–8 mice per group. D: Gels showing the phosphorylated and nonphosphorylated PKC substrates in the membrane (mb) and cytosolic (cyt) fractions isolated from the hypothalamus of WT or Glp1r−/− mice clamped under similar conditions. E and F: Quantification of PKC activity in AUs and in the membrane-to-cytosol ratio. Differences between groups for the activities assessed in the membrane or cytosolic fractions were analyzed separately. Data with different superscript letters are significantly different (P < 0.05) according to the two-way ANOVA test. Differences between GIR were analyzed according to the one-way ANOVA test. icv, intracerebroventricularly.
To analyze the effect of PKC inactivation (Fig. 1D–F), a nonspecific inhibitor, calphostin C, was infused into the brain under similar experimental conditions. Coinfusion of calphostin C with Ex4 reversed the reductions in femoral artery blood flow and GIR produced by Ex4 alone (Fig. 1A and C). The blood flow and GIR in mice infused with calphostin C were similar to that observed in the control group (Fig. 1A and C).
To activate PKC activity, we infused PMA, a nonspecific activator of PKC, into the brain (Fig. 2A–C) and clamped the mice under hyperglycemic conditions. PMA brain infusion decreased femoral artery blood flow and GIR compared with the vehicle-infused control group (Fig. 2D and E).
FIG. 2.
Pharmacological activation of brain PKCs regulates femoral artery blood flow and whole-body GIR in Glp1r−/− mice during hyperinsulinemic-hyperglycemic clamp studies. A: Gels showing the phosphorylated and nonphosphorylated PKC substrates in each hypothalamic fraction isolated from Glp1r−/− (knock-out [KO]) mice infused into the brain with vehicle or PMA and clamped under hyperinsulinemic-hyperglycemic conditions. B and C: Quantification of PKC activity in AUs and in the membrane-to-cytosol ratio. D and E: Corresponding mean arterial blood flow in percentage changes over baseline and whole-body GIRs. Data are means ± SE, n = 7–8 mice per group. Differences between groups, for the activities assessed in the membrane (mb) or cytosolic (cyt) fractions, were analyzed separately. Data with different superscript letters are significantly different (P < 0.05) according to the two-way ANOVA test. Differences between GIR were analyzed with the Student t test. icv, intracerebroventricularly.
Brain GLP-1R signaling stimulates plasma membrane translocation of PKC-δ isoform.
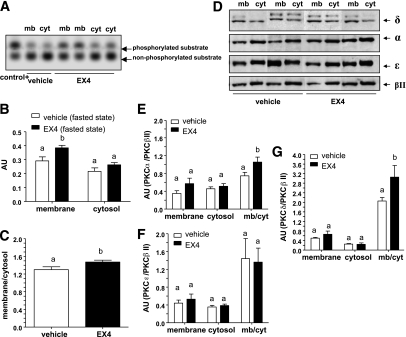
In the basal fasted state, the Ex4 brain infusion increased plasma membrane activity of PKC (Fig. 3A–C), which corresponded to increased levels of PKC-α (Fig. 3D and E) and -δ (Fig. 3D and G) but not -ε and -βII (Fig. 3D and F). During experimental hyperinsulinemia and hyperglycemia, the Ex4 brain infusion activated the translocation of PKC-δ (Fig. 4A and B) but not -α, -ε, or -βII to the plasma membrane. Under hyperinsulinemia and hyperglycemia, levels of PKC-δ were higher in response to Ex4 when compared with vehicle-treated mice. However, this set of data does not demonstrate that control of PKC-δ is strictly glucose dependent.
FIG. 3.
Brain GLP-1R signaling stimulates the translocation of PKC-α/δ in the hypothalamus of fasted WT mice. A: Gels illustrate the phosphorylated and nonphosphorylated PKC substrates in each hypothalamic fraction isolated from WT mice in the fasted state and after infusion into the brain of vehicle or Ex4. B and C: Quantification of PKC activity in AUs and in the membrane-to-cytosol ratio. D–G: Representative Western blot analyses of PKC isoforms and the quantification in AUs. Normalization is performed with PKC-βII. Data are means ± SE, n = 5–6 mice per group. Differences between groups, for the activities assessed in the membrane (mb) or cytosolic (cyt) fractions, were analyzed separately. Data with different superscript letters are significantly different (P < 0.05) according to the two-way ANOVA test.
FIG. 4.
Brain GLP-1R signaling stimulates PKC-δ translocation in the hypothalamus during hyperinsulinemic-hyperglycemic clamp studies. A: Representative Western blot analysis of PKC isoforms in each hypothalamic fraction of WT or Glp1r−/− (knock-out [KO]) mice clamped in hyperinsulinemic-hyperglycemic conditions and infused into the brain with vehicle, Ex4, or Ex9. B: The quantification in AUs. Normalization is performed with PKC-βII. Differences between groups, for the activities assessed in the membrane (mb) or cytosolic (cyt) fractions, were analyzed separately. Data are means ± SE, n = 5–6 mice per group. Data with different superscript letters are significantly different (P < 0.05) according to the two-way ANOVA test.
This effect was prevented by the concomitant infusion of Ex9 into the brain of WT mice, and PKC localization in Ex9-treated mice was similar to the pattern detected in Glp1r−/− mice (Fig. 4A and B). We did not observe translocation to the membrane fraction of other PKC isoforms (ε, α, and βII) (Fig. 4A). The PKC-δ activation by Ex4 was totally blunted in Glp1r−/−mice (Supplementary Fig. 1). The performance of both the euglycemic-hyperinsulinemic and the hyperglycemic-hyperinsulinemic clamps are provided in Supplementary Table 1. We illustrate the steady-state glycemia obtained during the clamps during the last hours of the infusion, which shows the quality of the experiments.
Specific brain PKC-δ blockade prevents the vascular and metabolic effects of Ex4 infusion into the brain.
Rottlerin, a specific PKC-δ inhibitor, was concomitantly infused into the brain with Ex4 under hyperglycemic-hyperinsulinemic conditions. Rottlerin totally prevented the reduction of femoral artery blood flow and GIR produced by Ex4 (Fig. 5A and B) and inhibited Ex4-induced PKC-δ translocation (Fig. 5C and D). Under these experimental conditions, levels of PKC-βII and the ratio of PKC-ε to PKC-βII and PKC-α to PKC-βII remained unchanged (not shown).
FIG. 5.
Pharmacological inhibition of hypothalamic PKC-δ by rottlerin improves femoral artery blood flow and whole-body GIR in WT mice during hyperinsulinemic-hyperglycemic clamp studies. A and B: Mean arterial blood flow in percentage changes over baseline and whole-body GIRs during a hyperinsulinemic-hyperglycemic clamp where WT mice were infused into the brain with vehicle, Ex4, or rottlerin (Rott). C and D: The corresponding representative Western blot analysis of PKC isoforms in each hypothalamic fraction and the quantification in AUs. Normalization was performed with PKC-βII. Differences between groups, for the activities assessed in the membrane (mb) or cytosolic (cyt) fractions, were analyzed separately. Data are means ± SE, n = 6–8 mice per group. Data with different superscript letters are significantly different (P < 0.05) according to the two-way ANOVA test. icv, intracerebroventricularly.
Increased GLP-1R–dependent hypothalamic PKC activity in diabetic mice.
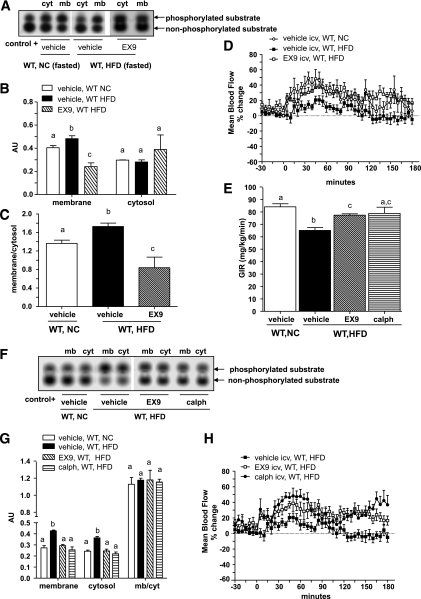
Diabetes is frequently associated with insulin resistance and vasoconstriction; hence, we analyzed whether an increase in brain GLP-1 signaling was associated with this phenotype under fasted and insulin-stimulated conditions. In the fasted state, plasma membrane PKC activity was increased in the hypothalamus of fasted HFD-fed diabetic mice and reduced by Ex9 (Fig. 6A–C).
FIG. 6.
Inhibition of brain GLP-1R signaling and hypothalamic PKC activity improve femoral artery blood flow and whole-body GIRs in diabetic mice during hyperinsulinemic-euglycemic clamp studies. A–C: Blots depict the phosphorylated and nonphosphorylated PKC substrates, the quantification in each fraction isolated from NC diet or HFD WT mice at the fasted state and infused into the brain with vehicle or Ex9, and the membrane-to-cytosol ratio. D and E: Mean arterial blood flow in percentage changes over baseline and the GIRs in NC or HFD WT mice clamped under hyperinsulinemic-euglycemic conditions with either vehicle or Ex9 brain infusions. Data are means ± SE, n = 5–8 mice per group. F and G: Same representation described in A with hypothalamic fractions isolated from HFD WT mice clamped and infused into the brain with Ex9 and calphostin (calph) and the quantification in AUs. H: The corresponding mean arterial blood flow in percentage changes over baseline. Data are means ± SE, n = 5–7 mice per group. Differences between groups, for the activities assessed in the membrane (mb) or cytosolic (cyt) fractions, were analyzed separately. Data with different superscript letters are significantly different (P < 0.05) according to the two-way ANOVA test. icv, intracerebroventricularly.
To assess the effect of chronic hyperglycemia because of the HFD and not simply the effect of acute hyperglycemia as a result of the infusion, as in the first sets of experiments, we clamped both NC and diabetic (HFD) mice under euglycemic conditions. During the euglycemic and hyperinsulinemic clamp procedures, diabetic mice exhibited reduced insulin-induced vascular blood flow and GIR when compared with normal mice. The Ex9 brain infusion improved the GIR and normalized blood flow (Fig. 6D and E). In a similar manner, the inhibition of PKC activity by calphostin C (Fig. 6F and G) had the same effect as Ex9 on blood flow and GIR (Fig. 6E and H). The translocation of PKC-α and -δ (but not of -βII and -ε) was increased in the hypothalamus of diabetic mice (Fig. 7A–F). It is important to note that we normalized data relative to PKC-ε levels but not PKC-βII since PKC-βII is modulated in experimental diabetes (23).
FIG. 7.
PKC-δ controls femoral artery blood flow and whole-body insulin sensitivity in diabetic mice during hyperinsulinemic-euglycemic clamp studies. A–C: A representative Western blot analysis of PKC isoforms in each fraction isolated from WT NC diet and HFD mice clamped in hyperinsulinemic-euglycemic conditions and infused into the brain with vehicle (veh) and the quantification in AUs. D–F: Western blot analysis from hypothalamic fractions isolated from HFD mice (WT and Glp1r −/− [knock-out {KO}]) clamped and infused into the brain with Ex9 or vehicle and the quantification in AU. Data are means ± SE, n = 4–5 mice per group. G: Western blot representation of PKC isoforms in hypothalami from NC or HFD WT mice clamped and infused into the brain with vehicle or rottlerin (Rott). H–K: Quantification, corresponding mean femoral artery blood flow in percentage changes over baseline, and whole-body GIRs. Data are means ± SE, n = 7–8 mice per group. Differences between groups, for the activities assessed in the membrane (mb) or cytosolic (cyt) fractions, were analyzed separately. Normalization is performed with PKC-ε. Data with different superscript letters are significantly different (P < 0.05) according to the two-way ANOVA test. icv, intracerebroventricularly.
It is interesting that the Ex9 brain infusion could prevent membrane translocation of PKC-δ (Fig. 7E) but not of PKC-α (Fig. 7F). The other isoforms (βII and ε) remained unaffected (not shown). To ascertain the role of PKC-δ in the control of femoral artery blood flow and glucose metabolism, rottlerin was infused into the brain of diabetic mice to specifically inhibit PKC-δ activity (Fig. 7G and H). Rottlerin increased both femoral artery blood flow (Fig. 7J) and GIR (Fig. 7K), rendering values for these two parameters similar to that observed in control WT mice. PKC-δ translocation (and not PKC-α) was concomitantly reduced by the inhibitor (Fig. 7G–I), whereas PKC-ε remained unchanged (Fig. 7G).
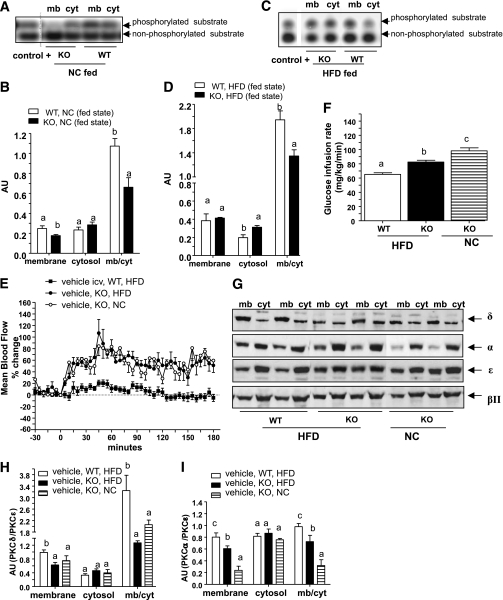
Also of interest is that in the fed state and in Glp1r−/− mice, PKC translocation to the membrane fraction remained lower than in WT mice in NC-fed (Fig. 8A and B) and in HFD-fed groups (Fig. 8C and D). Diabetic Glp1r−/− mice were then clamped under hyperinsulinemic conditions. Femoral artery blood flow was similar in NC and HFD Glp1r−/− mice. They were therefore unaffected by the dietary treatment (Fig. 8E), whereas the GIR remained slightly increased, although more than in the diabetic WT mice (Fig. 8F). In Glp1r−/− mice, PKC-δ translocation to the plasma membrane remained similar between NC and HFD mice; therefore, the dietary treatment did not increase PKC-δ translocation to the plasma membrane, whereas levels of PKC-α were still increased (Fig. 8G–I) but not levels of PKC-βII (not shown). These findings demonstrate a relationship between PKC-δ, blood flow, and insulin resistance.
FIG. 8.
Reduced hypothalamic PKC-δ activity improved femoral artery blood flow and insulin sensitivity in HFD-fed Glp1r−/− mice during hyperinsulinemic-euglycemic clamp studies. A and C: Gels representing phosphorylated and nonphosphorylated PKC substrates in each fraction isolated from the NC diet or HFD WT or Glp1r−/− (knock-out [KO]) mice in the fed state. B and D: The corresponding PKC activity expressed in AUs. E and F: Mean femoral artery blood flow in percentage changes over baseline and corresponding whole-body GIRs in WT or Glp1r−/− mice fed with an NC diet or HFD and clamped in euglycemic hyperinsulinemic conditions while infused into the brain with vehicle. Data are means ± SE, n = 6–7 mice per group. G–I: The corresponding Western blot analysis of PKC isoforms in the membrane (mb) and cytosolic (cyt) fractions isolated from the hypothalamus of these mice at the end of the clamps and quantification in AUs. Normalization is performed against PKC-ε. Data are means ± SE, n = 4–5 mice per group. Differences between groups, for the activities assessed in the membrane or cytosolic fractions, were analyzed separately. Data with different superscript letters are significantly different (P < 0.05) according to the two-way ANOVA test. icv, intracerebroventricularly.
DISCUSSION
In this study, we demonstrated that hypothalamic PKC-δ constitutes a GLP-1–sensitive molecular mechanism required for the coordinated control of femoral artery blood flow and whole-body insulin sensitivity. This mechanism is strictly brain GLP-1– and glucose-dependent and overtly activated in chronic hyperglycemic conditions, which contribute to the development of insulin resistance and vasoconstriction during diabetes.
During the last decade, we and others have shown that the regulation of vascular blood flow and muscle glucose utilization is tightly coordinated. One of the key corresponding mechanisms was the activation by insulin of endothelial nitric oxide synthase, an identified molecular target that linked both the metabolic and vascular phenotypes in the WT mouse (14,37–39). In the quest for physiological endocrine regulators of peripheral glucose homeostasis, we identified a role for brain GLP-1 signaling (4,5,7,8). Hence, it was important to determine the molecular mechanisms activated by GLP-1 in the brain that coordinated the regulation of both vascular and metabolic functions.
Here, we have shown that brain GLP-1 signaling acutely triggers the translocation of cytoplasmic PKC to the plasma membrane of cells in the hypothalamus. The activation of PKC by GLP-1 has been previously reported in β-cells (15), which by analogy supports the hypothesis that PKCs could be part of molecular mechanisms mediating brain GLP-1 signaling. PKCs are serine/threonine kinases that function as second messengers responsible for the phosphorylation of numerous substrates. In the brain, PKCs regulate the synthesis and release of neurotransmitters and electrical activation of neuronal cells (34). In previous studies, the molecular signaling mechanisms associated with GLP-1–induced insulin secretion were via the α and ε PKC isoforms (15). To identify the precise isoforms in the hypothalamus responsible for the regulation of vascular and metabolic homeostasis, we focused our attention on PKC-ε/α/δ and -βII, which are all expressed in the brain (31–34). These isoforms are sensitive to glucose and can be activated by glucoregulatory hormones (15,18,19,23). We showed that hypothalamic PKC-δ, but neither -α, -ε, nor -βII, was activated when GLP-1 was directly infused into the brain. Therefore, our present data strongly support the hypothesis that brain PKC activation could mediate neuronal activity (40), leading to the regulation of vascular and metabolic functions.
To test this hypothesis, we first blocked GLP-1 signaling using an Ex9 infusion into the brain. GLP-1R blockade reduced the plasma membrane translocation of PKC activity by Ex4 under conditions of hyperinsulinemic hyperglycemia and increased insulin-induced vascular blood flow and whole-body glucose utilization. Similar data implicating a role for the hypothalamic GLP-1R system were obtained in studies with Glp1r−/− mice. In a second set of experiments, the nonspecific PKC inhibitor calphostin prevented the effect of GLP-1, clearly indicating a role for hypothalamic PKCs in brain GLP-1 activity. Furthermore, in a third set of experiments, we activated brain PKCs using the DAG activator, PMA, which reproduced the findings observed with Ex4. Taken together, these data demonstrate that brain GLP-1 signaling regulates PKC activity in the hypothalamus to control vascular and metabolic homeostasis. This mechanism most likely requires PKC-δ since a specific inhibitor, rottlerin, abrogated these effects. It is noteworthy that levels of PKC-α, -βII, and -ε were unchanged by the infusion of rottlerin, further underscoring the specific activation of PKC-δ by GLP-1.
Insulin resistance and vasoconstriction are major features of diabetes, and several lines of evidence link PKC overactivity to chronic hyperglycemia (41). This overactivity has also been suggested to encompass nitric oxide synthase activity in brain cells (42–44), perhaps linked to impairment of the control of femoral artery blood flow and muscle glucose utilization in response to central insulin (14,39,45). However, whether brain GLP-1–induced PKC-δ plays a role in the pathophysiological consequences stemming from chronic hyperglycemia is unknown.
Our data show that total PKC activity was increased in diabetic WT mice both under basal and during hyperinsulinemic euglycemic clamp conditions. This was associated with an increased translocation of PKC-δ and -α to the plasma membrane. It is interesting that only PKC-δ translocation could be inhibited by the GLP-1R antagonist Ex9. In addition, brain infusion of Ex9 and calphostin C normalized the impaired insulin sensitivity and vascular function observed during experimental diabetes. These data demonstrate that excessive hypothalamic PKC activity is deleterious for both metabolic and vascular functions in response to activation of the brain GLP-1R. Experimental studies on diabetic rodents showed that brain GLP-1 signaling is stimulated during diabetes and may perturb the overall control of glycemia. This last observation is supported by previous data from our group and others demonstrating that the expression of the proglucagon gene encoding GLP-1 was increased in the brainstem of diabetic HFD-fed mice (7) and obese Zucker rats (46), which contains cell bodies of GLP-1 neurons projecting to the hypothalamus (9–13). The functional disruption of the GLP-1R in Glp1r−/− mice prevents PKC activation to the membrane and PKC-δ translocation.
Our data further show that in the fed state, the gut-to-brain axis is activated through a GLP-1–dependent mechanism, as previously described (4). We also previously demonstrated that brain GLP-1 signaling allows hepatic glucose repletion upon fasting by preventing excessive muscle glucose utilization (5) that was associated with reduced muscle blood flow (8). The hypothalamic arcuate nucleus is likely to comprise the central site responsible for this mechanism because direct administration of GLP-1 into this nucleus decreases peripheral glucose uptake and production (47). We attributed this metabolic response to a feedback loop that might arise pursuant to excessive insulin-stimulated glucose utilization, operating to prevent hypoglycemia and favor hepatic glucose storage. We further showed that during diabetes, this mechanism is exacerbated and associated with increased brain GLP-1 signaling (7). We now demonstrate that signals emanating from the hypothalamic GLP-1R require PKC-δ activation, a mechanism continuously stimulated during HFD-induced insulin resistance and vasoconstriction. However, we cannot rule out that the GLP-1R could also mediate part of its effect through a ligand-independent activity since in Ex9-treated mice, blood flow was increased by the treatment. Previous data support this interpretation because in GLP-1R–expressing cell lines, the intracellular cAMP concentration was reduced by Ex9 even in the absence of GLP-1 (48). Hence, our data suggest that hypothalamic PKC-δ is activated in response to brain GLP-1R stimulation, perhaps contributing to the central control of insulin resistance and vasoconstriction. Therefore, current therapeutic treatments of type 2 diabetes using GLP-1R agonists delivered systematically may also target brain GLP-1R signaling with implications for regulation of peripheral blood flow and insulin sensitivity (49,50).
Supplementary Material
ACKNOWLEDGMENTS
C.C. has received grants from the European Association for the Study of Diabetes and the Amylin Company and from the Société Francophone d’Etude du Diabète and is a member of the European Club for the Study of GLP-1 (EuCSGLP-1; http://www.glp1.eu). R.B. has received subsidies from INSERM and is a member of EuCSGLP-1. D.J.D. was supported in part by Heart and Stroke Foundation of Ontario Grant NA6997 and the Canada Research Chairs program. No other potential conflicts of interest relevant to this article were reported.
C.C. performed experiments and wrote the manuscript. C.V. performed experiments. G.C. and D.J.D. contributed to discussion and reviewed the manuscript. R.B. wrote the manuscript.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-0464/-/DC1.
REFERENCES
- 1.Thorens B, Waeber G. Glucagon-like peptide-I and the control of insulin secretion in the normal state and in NIDDM. Diabetes 1993;42:1219–1225 [DOI] [PubMed] [Google Scholar]
- 2.Nakabayashi H, Nishizawa M, Nakagawa A, Takeda R, Niijima A. Vagal hepatopancreatic reflex effect evoked by intraportal appearance of tGLP-1. Am J Physiol 1996;271:E808–E813 [DOI] [PubMed] [Google Scholar]
- 3.Nishizawa M, Nakabayashi H, Uchida K, Nakagawa A, Niijima A. The hepatic vagal nerve is receptive to incretin hormone glucagon-like peptide-1, but not to glucose-dependent insulinotropic polypeptide, in the portal vein. J Auton Nerv Syst 1996;61:149–154 [DOI] [PubMed] [Google Scholar]
- 4.Knauf C, Cani PD, Kim DH, et al. Role of central nervous system glucagon-like peptide-1 receptors in enteric glucose sensing. Diabetes 2008;57:2603–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knauf C, Cani PD, Perrin C, et al. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Invest 2005;115:3554–3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burcelin R, Da Costa A, Drucker D, Thorens B. Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes 2001;50:1720–1728 [DOI] [PubMed] [Google Scholar]
- 7.Knauf C, Cani PD, Ait-Belgnaoui A, et al. Brain glucagon-like peptide 1 signaling controls the onset of high-fat diet-induced insulin resistance and reduces energy expenditure. Endocrinology 2008;149:4768–4777 [DOI] [PubMed] [Google Scholar]
- 8.Cabou C, Campistron G, Marsollier N, et al. Brain glucagon-like peptide-1 regulates arterial blood flow, heart rate, and insulin sensitivity. Diabetes 2008;57:2577–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 1997;77:257–270 [DOI] [PubMed] [Google Scholar]
- 10.Vrang N, Hansen M, Larsen PJ, Tang-Christensen M. Characterization of brainstem preproglucagon projections to the paraventricular and dorsomedial hypothalamic nuclei. Brain Res 2007;1149:118–126 [DOI] [PubMed] [Google Scholar]
- 11.Larsen PJ, Tang-Christensen M, Jessop DS. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology 1997;138:4445–4455 [DOI] [PubMed] [Google Scholar]
- 12.Navarro M, Rodriquez de Fonseca F, Alvarez E, et al. Colocalization of glucagon-like peptide-1 (GLP-1) receptors, glucose transporter GLUT-2, and glucokinase mRNAs in rat hypothalamic cells: evidence for a role of GLP-1 receptor agonists as an inhibitory signal for food and water intake. J Neurochem 1996;67:1982–1991 [DOI] [PubMed] [Google Scholar]
- 13.Turton MD, O’Shea D, Gunn I, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996;379:69–72 [DOI] [PubMed] [Google Scholar]
- 14.Cabou C, Cani PD, Campistron G, et al. Central insulin regulates heart rate and arterial blood flow: an endothelial nitric oxide synthase-dependent mechanism altered during diabetes. Diabetes 2007;56:2872–2877 [DOI] [PubMed] [Google Scholar]
- 15.Suzuki Y, Zhang H, Saito N, Kojima I, Urano T, Mogami H. Glucagon-like peptide 1 activates protein kinase C through Ca2+-dependent activation of phospholipase C in insulin-secreting cells. J Biol Chem 2006;281:28499–28507 [DOI] [PubMed] [Google Scholar]
- 16.McClenaghan NH, Flatt PR, Ball AJ. Actions of glucagon-like peptide-1 on KATP channel-dependent and -independent effects of glucose, sulphonylureas and nateglinide. J Endocrinol 2006;190:889–896 [DOI] [PubMed] [Google Scholar]
- 17.Light PE, Bladen C, Winkfein RJ, Walsh MP, French RJ. Molecular basis of protein kinase C-induced activation of ATP-sensitive potassium channels. Proc Natl Acad Sci USA 2000;97:9058–9063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amir S, Shechter Y. Apparent involvement of protein kinase C in the central glucoregulatory action of insulin. Brain Res 1988;450:272–279 [DOI] [PubMed] [Google Scholar]
- 19.Ross R, Wang PY, Chari M, et al. Hypothalamic protein kinase C regulates glucose production. Diabetes 2008;57:2061–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramakrishnan R, Sheeladevi R, Suthanthirarajan N, Namasivayam A. An acute hyperglycemia or acidosis-induced changes of indolamines level correlates with PKC-alpha expression in rat brain. Brain Res Bull 2005;67:46–52 [DOI] [PubMed] [Google Scholar]
- 21.Ramakrishnan R, Sheeladevi R, Suthanthirarajan N. PKC-alpha mediated alterations of indoleamine contents in diabetic rat brain. Brain Res Bull 2004;64:189–194 [DOI] [PubMed] [Google Scholar]
- 22.Lee TS, Saltsman KA, Ohashi H, King GL. Activation of protein kinase C by elevation of glucose concentration: proposal for a mechanism in the development of diabetic vascular complications. Proc Natl Acad Sci USA 1989;86:5141–5145 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 23.Naruse K, Rask-Madsen C, Takahara N, et al. Activation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes 2006;55:691–698 [DOI] [PubMed] [Google Scholar]
- 24.Burcelin R, Crivelli V, Dacosta A, Roy-Tirelli A, Thorens B. Heterogeneous metabolic adaptation of C57BL/6J mice to high-fat diet. Am J Physiol Endocrinol Metab 2002;282:E834–E842 [DOI] [PubMed] [Google Scholar]
- 25.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007;56:1761–1772 [DOI] [PubMed] [Google Scholar]
- 26.Perrin C, Knauf C, Burcelin R. Intracerebroventricular infusion of glucose, insulin, and the adenosine monophosphate-activated kinase activator, 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside, controls muscle glycogen synthesis. Endocrinology 2004;145:4025–4033 [DOI] [PubMed] [Google Scholar]
- 27.Young EA, Duchemin AM, Neff NH, Hadjiconstantinou M. Parallel modulation of striatal dopamine synthetic enzymes by second messenger pathways. Eur J Pharmacol 1998;357:15–23 [DOI] [PubMed] [Google Scholar]
- 28.Redman C, Lefevre J, MacDonald ML. Inhibition of diacylglycerol kinase by the antitumor agent calphostin C. Evidence for similarity between the active site of diacylglycerol kinase and the regulatory site of protein kinase C. Biochem Pharmacol 1995;50:235–241 [DOI] [PubMed] [Google Scholar]
- 29.Ohsawa M, Kamei J. Modification of the expression of naloxone-precipitated withdrawal signs in morphine-dependent mice by diabetes: possible involvement of protein kinase C. Jpn J Pharmacol 1999;79:303–311 [DOI] [PubMed] [Google Scholar]
- 30.Quagliaro L, Piconi L, Assaloni R, Martinelli L, Motz E, Ceriello A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes 2003;52:2795–2804 [DOI] [PubMed] [Google Scholar]
- 31.Borghini I, Ania-Lahuerta A, Regazzi R, et al. Alpha, beta I, beta II, delta, and epsilon protein kinase C isoforms and compound activity in the sciatic nerve of normal and diabetic rats. J Neurochem 1994;62:686–696 [PubMed] [Google Scholar]
- 32.Roberts RE, McLean WG. Protein kinase C isozyme expression in sciatic nerves and spinal cords of experimentally diabetic rats. Brain Res 1997;754:147–156 [DOI] [PubMed] [Google Scholar]
- 33.Irani BG, Donato J, Jr, Olson DP, et al. Distribution and neurochemical characterization of protein kinase C-theta and -delta in the rodent hypothalamus. Neuroscience 2010;170:1065–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signaling. Annu Rev Neurosci 1994;17:551–567 [DOI] [PubMed] [Google Scholar]
- 35.Steinberg SF. Structural basis of protein kinase C isoform function. Physiol Rev 2008;88:1341–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azzi A, Boscoboinik D, Hensey C. The protein kinase C family. Eur J Biochem 1992;208:547–557 [DOI] [PubMed] [Google Scholar]
- 37.Baron AD, Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G. Insulin-mediated skeletal muscle vasodilation contributes to both insulin sensitivity and responsiveness in lean humans. J Clin Invest 1995;96:786–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest 1994;94:1172–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shankar R, Zhu JS, Ladd B, Henry D, Shen HQ, Baron AD. Central nervous system nitric oxide synthase activity regulates insulin secretion and insulin action. J Clin Invest 1998;102:1403–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaughan PF, Walker JH, Peers C. The regulation of neurotransmitter secretion by protein kinase C. Mol Neurobiol 1998;18:125–155 [DOI] [PubMed] [Google Scholar]
- 41.Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res 2010;106:1319–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirata K, Kuroda R, Sakoda T, et al. Inhibition of endothelial nitric oxide synthase activity by protein kinase C. Hypertension 1995;25:180–185 [DOI] [PubMed] [Google Scholar]
- 43.Bredt DS, Ferris CD, Snyder SH. Nitric oxide synthase regulatory sites. Phosphorylation by cyclic AMP-dependent protein kinase, protein kinase C, and calcium/calmodulin protein kinase; identification of flavin and calmodulin binding sites. J Biol Chem 1992;267:10976–10981 [PubMed] [Google Scholar]
- 44.Kim H, Sasaki T, Maeda K, Koya D, Kashiwagi A, Yasuda H. Protein kinase Cbeta selective inhibitor LY333531 attenuates diabetic hyperalgesia through ameliorating cGMP level of dorsal root ganglion neurons. Diabetes 2003;52:2102–2109 [DOI] [PubMed] [Google Scholar]
- 45.Shankar RR, Wu Y, Shen HQ, Zhu JS, Baron AD. Mice with gene disruption of both endothelial and neuronal nitric oxide synthase exhibit insulin resistance. Diabetes 2000;49:684–687 [DOI] [PubMed] [Google Scholar]
- 46.Vrang N, Larsen PJ, Jensen PB, et al. Upregulation of the brainstem preproglucagon system in the obese Zucker rat. Brain Res 2008;1187:116–124 [DOI] [PubMed] [Google Scholar]
- 47.Sandoval DA, Bagnol D, Woods SC, D’Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes 2008;57:2046–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serre V, Dolci W, Schaerer E, et al. Exendin-(9-39) is an inverse agonist of the murine glucagon-like peptide-1 receptor: implications for basal intracellular cyclic adenosine 3′,5′-monophosphate levels and beta-cell glucose competence. Endocrinology 1998;139:4448–4454 [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto H, Lee CE, Marcus JN, et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest 2002;110:43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Isacson R, Nielsen E, Dannaeus K, et al. The glucagon-like peptide 1 receptor agonist exendin-4 improves reference memory performance and decreases immobility in the forced swim test. Eur J Pharmacol 2011;650:249–255 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.