Abstract
Mitochondria are present at high concentration at the site of sensory transduction in the peripheral terminals of nociceptors [14; 31]. Since nerve growth factor (NGF), which induces nociceptor sensitization by acting on the high affinity TrkA receptor [28], also produces local recruitment of mitochondria in DRG neurons [5; 6], we evaluated the role of mitochondria in NGF-induced mechanical hyperalgesia. Inhibition of three major mitochondrial functions—oxidation of nutrients, ATP production, and generation of reactive oxygen species—markedly attenuated NGF-induced mechanical hyperalgesia in the rat. Disruption of microtubules, which are required for the trafficking and subcellular localization of mitochondria, also attenuated NGF hyperalgesia. Our results suggest a contribution of mitochondrial localization and function to NGF-dependent pain syndromes.
Introduction
Nerve growth factor (NGF), which plays a critical role in the survival, growth, differentiation, and maintenance of sensory neurons, also acts on the peripheral terminals of nociceptors to induce hyperalgesia in animals and humans [24; 25; 47; 29; 34; 40]. Tissue injury and inflammation, such as that observed in cystitis and arthritis, result in elevated levels of NGF [3; 43] and antagonizing NGF has been proven effective in the treatment of osteoarthritis pain [23].
NGF sensitizes the peripheral terminal of the nociceptor by activating at the high-affinity receptor, tropomyosin receptor kinase A (TrkA), producing both thermal and mechanical hyperalgesia [24; 8; 28]. The underlying mechanism for NGF-induced thermal hypersensitivity has been attributed to increased membrane insertion of the heat-sensitive ion channel TRPV1 at peripheral terminals of nociceptors [48]. The mechanism by which NGF induces mechanical hyperalgesia, however, is less understood. Thus, while signaling pathways mediating NGF-induced mechanical hyperalgesia have been identified [28], the downstream targets and cellular processes that are involved have yet to be fully elucidated.
In addition to its role in nociceptor sensitization, NGF signaling is important for the trafficking and localization of mitochondria in neurons. Focal administration of NGF-coated beads along the growing axon of cultured dorsal root ganglion (DRG) neurons triggers local accumulation of mitochondria, presumably to fulfill higher demands for ATP at the growth cone [32; 5; 6]. NGF also upregulates the metabolic activity of mitochondria [46].
Mitochondria play an important role in several animal models of inflammatory and neuropathic pain [12; 17]. Moreover, the inhibition of two key mitochondrial functions, the generation of ATP and reactive oxygen species (ROS), attenuate protein kinase C epsilon (PKCε)-dependent forms of mechanical hyperalgesia [18]. Since NGF can produce mechanical hyperalgesia in vivo and recruit mitochondria to areas of local stimulation in DRG neurons as well as upregulate their metabolic activity in vitro [5; 6; 28; 46], we hypothesized that mitochondrial functions are important in NGF-dependent pain syndromes.
Materials and Methods
Animals
Experiments were performed on adult male Sprague Dawley rats (250–350 g; Charles River, Hollister, CA, USA). They were housed three per cage under a 12 hour light/dark cycle in a temperature and humidity controlled environment at the UCSF animal care facility. Food and water were available ad libitum. Experimental protocols were approved by the UCSF Committee on Animal Research and conformed to NIH Guidelines for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used.
Mechanical nociceptive threshold testing
Rats were trained before testing. Training sessions began on the day after their arrival at the UCSF animal care facility (day 1) and then again on days 3 and 5. Animals were brought to the laboratory and allowed to remain in their cages for 10 –15 min. They were allowed to crawl into individual Perspex cylinders and were lightly restrained there by closing both ends of the cylinder. The hind legs of the rats extended out of the cylinder through triangular openings on either side, which allowed access to the hind paws for nociceptive threshold testing. Each training session consisted of five rounds of testing and lasted about an hour. If they were quiet and gave consistent paw-withdrawal thresholds on day 5, the experiment was conducted on day 7. If not, additional training sessions were conducted every other day as needed. The nociceptive flexion reflex (Randall –Selitto paw-withdrawal test [37]) was quantified with a Basile Analgesymeter (Stoelting, Chicago, IL), which applies a linearly increasing mechanical force to the dorsum of the rat’s hind paw. The mechanical nociceptive threshold was defined as the force in grams at which the rat withdrew its paw. All data points, including the pre-injection baselines, were defined as the mean of three paw-withdrawal thresholds. Baseline paw-withdrawal thresholds varied between 100 and 120 grams of force. Hyperalgesia was defined as the percent decrease in paw-withdrawal thresholds from the baseline (i.e., paw-withdrawal threshold minus baseline paw-withdrawal threshold ÷ baseline paw-withdrawal threshold × 100).
Drugs and solutions
Nerve growth factor (NGF) was purchased from R&D Systems (Minneapolis, MN, USA). Rotenone, a selective inhibitor of complex I of the mitochondrial electron transport chain (mETC), the anti-oxidants N-acetyl-L-cysteine (NAC) and glutathione, and epinephrine were purchased from Sigma Aldrich (St Louis, MO, USA). Oligomycin, a selective ATP synthase inhibitor; Latrunculin B, an actin cytoskeleton disruptor; and nocodazole, a microtubule disruptor, were purchased from EMD biosciences (La Jolla, CA, USA). A stock solution of NGF (1 μg/μL in phosphate buffered saline containing 0.5% bovine serum albumin) was further diluted to 0.2 μg/μL at the time of injection [28]. Rotenone, oligomycin, nocodazole, and Latrunculin B were dissolved in an aqueous solution of 10 % DMSO and administered by intradermal injection 30 minutes before NGF administration, at the site of nociceptive testing. Glutathione and NAC were dissolved in 0.9% NaCl. The doses of rotenone (1 μg) and oligomycin (1 μg), as well as the effects of the drugs alone, were shown in our previous study [17], and the doses of nocodazole (1 μg) and Latrunculin B (1 μg), as well as the effects of the drugs alone, were shown in another study [11]. Doses of NAC (10 μg) and glutathione (5 μg) were based on a dose response curve conducted as part of the present study.
A stock solution of epinephrine (1 μg/μL in 0.9% NaCl) was mixed with an equal volume of ascorbic acid (1 μg/μL in 0.9% NaCl) and further diluted to 20 ng/uL at the time of injection. Except for glutathione and NAc, all intradermal injections were delivered in a volume of 5 μL via a 30-gauge hypodermic needle into the dorsal surface of the hind paw at the site of nociceptive testing. Glutathione and NAC were injected using the hypotonic shock method, which consisted of 2.5 μL H2O followed by 1 μL air followed by 2.5 μL antagonist. The testing protocol in all experiments was as follows: After baseline thresholds were recorded the antagonist or its vehicle was injected. Thirty minutes later NGF was injected. Testing then began thirty minutes after the NGF injection, as depicted in the figures. A separate NGF control group was tested simultaneously with each antagonist. Each drug/condition was tested in a naive group of rats. No group was used for more than one experiment. Drug and control groups (i.e., vehicle/NGF and antagonist/NGF groups) were tested during the same testing session. As in previous studies employing simple reflex behaviors, for example, Taiwo, 1989 [44], Khasar 1995, 1999[22; 21], Aley, 2000 [2], Alessandri-Haber, 2004 [1], Dina 2005, 2009 [11; 10], and Joseph, 2010 [18], the current experiments were not performed blinded, which can be difficult to maintain, particularly when substances injected into the paw produce visible changes in the skin.
Statistical Analysis
To determine if there were significant differences between experimental groups, we employed, as appropriate, either a one-way ANOVA or two-way repeated measures ANOVA with one within-subjects factor (time) and one between-subjects factor (group). Percent change scores as shown in the figures were used for these analyses. If there was a significant group × time interaction, indicating that the groups in the analysis demonstrated significantly different time courses, multivariate analyses (i.e., one-way ANOVAs with Scheffé post hoc analyses) were performed for all time points in order to determine which points accounted for the interaction. In these cases, a Bonferroni correction was applied in order to account for multiple comparisons. For within-subjects effects the Mauchly criterion was used to determine if the assumption of sphericity was met; if not, Greenhouse-Geiser p-values are presented. If the main effect of group was significant and there were more than two groups, Scheffé post hoc analyses were used to determine the basis of the difference.
Results
Electron transport complex 1 inhibitor attenuates NGF-hyperalgesia
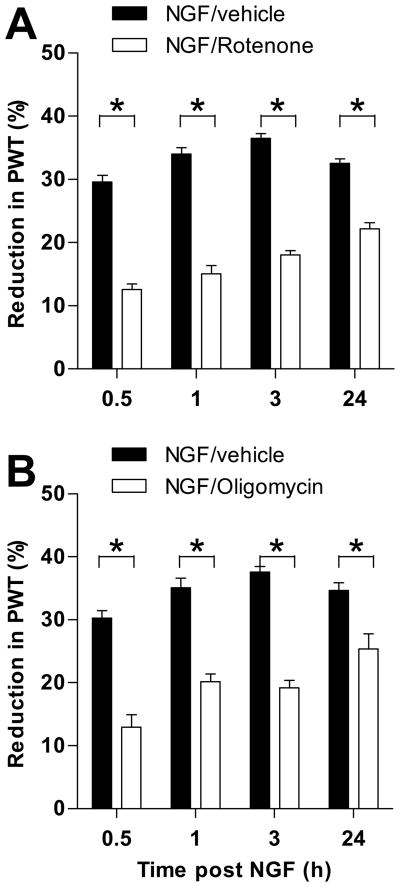
NGF (1 μg), delivered intradermally on the dorsum of the rat’s hind paw, produces mechanical hyperalgesia that is detectable by 10 minutes after injection, peaks at 3 hours, and lasts between 96 and 120 hours [28]. To evaluate the functional role of the electron transport chain in NGF-hyperalgesia, we pretreated animals with rotenone (1 μg), a specific inhibitor of NADH-dehydrogenase, at a dose previously demonstrated effective in models of other pain syndromes [17]. As shown in Figure 1A, pre-administration of rotenone significantly attenuated NGF-hyperalgesia at all time points tested, while animals injected with vehicle showed hyperalgesia consistent with previous NGF time course studies, peaking at 3 hours and lasting over 24 hours [28]. Two-way ANOVA showed a significant time x group interaction (F3,30 = 17.280; p<0.001); multivariate analyses showed significant differences between the groups at all time points (p<0.001 at each time point). There was also a main effect of group (F1,10 = 244.323; p<0.001). Note that, despite the similarity in the responses of the NGF/vehicle control groups in this study, each experiment was performed with its own control group tested simultaneously with the experimental group.
Figure 1.
Effect of electron transport inhibitors on NGF hyperalgesia. A. Rotenone, a mETC complex I inhibitor, attenuated NGF hyperalgesia at all time points. B. Oligomycin, a mETC complex V (ATP synthase) inhibitor, attenuated NGF hyperalgesia at all time points. N = 6 for all groups. Data are plotted as mean ± SEM. Significant differences between the groups are indicated by asterisks (*) plotted above the time points where they apply. Abbreviation: PWT = paw-withdrawal threshold.
Electron transport complex 5 inhibitor attenuates NGF-hyperalgesia
Since the oxidation of nutrients is coupled with the generation of ATP (oxidative phosphorylation of ADP), we determined if pretreatment with oligomycin (1 μg), a selective inhibitor of the ATP-synthase complex, could prevent NGF-hyperalgesia. As shown in Figure 1B, pre-administration of oligomycin, 30 min before NGF, significantly attenuated NGF-hyperalgesia at all time points tested whereas vehicle alone had no effect. The two-way ANOVA showed a significant time x group interaction (F3,30 = 6.138; p=0.004); multivariate analyses showed significant differences between the groups at all time points (p<0.001 at 30 min., 1 hour, and 3 hours, and p=0.007 at 24 hours). There was also a main effect of group (F1,10 = 78.412; p<0.001).
Antioxidants attenuate NGF-hyperalgesia
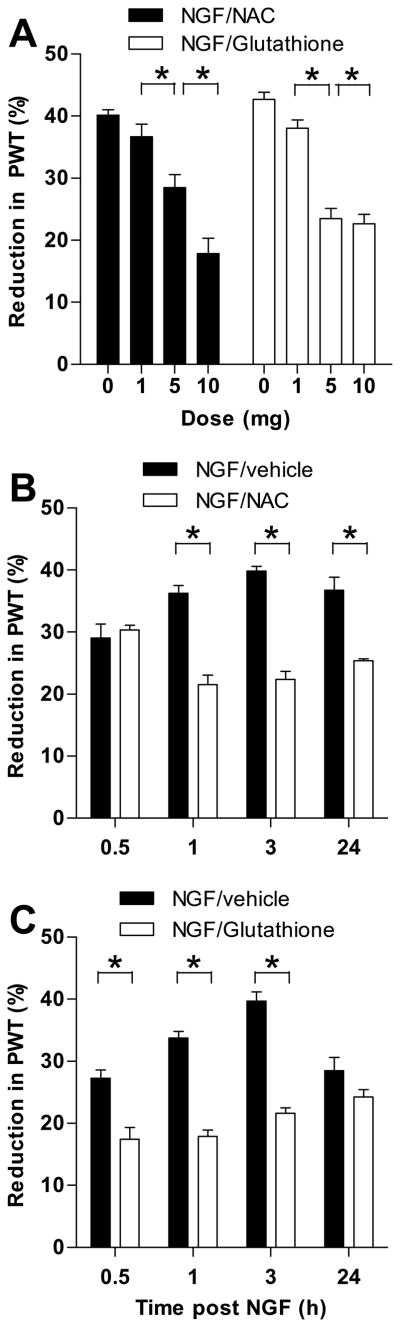
Reactive oxygen species (ROS) are a byproduct of oxidative phosphorylation. The production of ROS is known to be upregulated under inflammatory conditions and can induce nociceptor sensitization [45; 7; 19]. To analyze whether the production of ROS contributes to NGF-hyperalgesia, we tested the effect of two antioxidants, N-acetyl-L-cysteine (NAC) and glutathione, on NGF-induced hyperalgesia. Dose response studies were conducted for both NAC and glutathione in order to determine an appropriate dose to follow over time (Fig. 2A). Three doses of each of these agents (1 μg, 5 μg, and 10 μg) were tested. Separate groups of animals received vehicle as a control. The one-way ANOVA for NAC showed a significant main effect of group (F3,20 = 25.714; p<0.001); Scheffé post hoc analysis showed that the two highest doses differed significantly from each other (p=0.010), and the 5 μg dose differed significantly from the vehicle group (p=0.005), but the 1 μg dose group did not differ significantly from the vehicle group (p=0.668). Because the effect of the 10 μg dose was significantly greater than the 5 μg dose, the 10 μg dose was chosen for testing.
Figure 2.
The effect of the anti-oxidants N-acetyl-L-cysteine (NAC) and glutathione on NGF-hyperalgesia. A. Various doses of both NAC and glutathione were administered thirty minutes after NGF in order to determine the doses to be used for testing. For NAC the highest dose (10 μg) was chosen because it attenuated NGF hyperalgesia significantly more than any of the lower doses. For glutathione the effect of the 10 μg dose was not significantly greater than that of the 5 μg dose, therefore the 5 μg dose was chosen for testing. B. NAC (10 μg) attenuated NGF hyperalgesia detectable at 1 hour and lasting up to 24 hours. C. Glutathione (5 μg) attenuated NGF hyperalgesia detectable at 30 minutes, but the effect was no longer significant by 24 hours. N = 6 for all groups. Data are plotted as mean ± SEM. Significant difference between the groups are indicated by asterisks (*) plotted above the time points where they apply. Abbreviation: PWT = paw-withdrawal threshold.
The one-way ANOVA for glutathione showed a significant main effect of group (F3,20 = 49.434; p<0.001); Scheffé post hoc analysis showed that the two highest doses did not differ significantly (p=0.981), but the 5 μg dose differed significantly from both the 1 μg dose group (p<0.001) and the vehicle group (p<0.001). Because the effect of the 10 μg dose was not significantly greater than the 5 μg dose, the 5 μg dose was chosen for testing.
As shown in Figure 2B, NAC, significantly attenuated NGF-induced mechanical hyperalgesia at 1, 3 and 24 hours post-injection whereas the administration of vehicle had no effect.
The two-way ANOVA showed a significant time x group interaction (F3,30 = 16.222; p<0.001); multivariate analyses showed that the NGF group differed significantly from the NAC group at 1 hour (p<0.001), 3 hours (p<0.001) and 24 hours (p<0.03), but not at 30 minutes (p<0.586). There was also a main effect of group (F1,10 = 109.876; p<0.001).
Glutathione (Fig. 2C) also significantly attenuated NGF-induced hyperalgesia; however this effect began at 30 minutes, and lasted through 3 hours.
The two-way ANOVA showed a significant time x group interaction (F3,30 = 8.217; p=0.001); multivariate analyses showed that the NGF group differed significantly from the GSH group at 30 minutes (p=0.002), 1 hour (p<0.001), 3 hours (p<0.001), but not at 24 hours (p<0.112). There was also a main effect of group (F1,10 = 241.135; p<0.001).
Taken together, our results suggest that NGF-hyperalgesia is dependent on three major mitochondrial functions: oxidation of nutrients, ATP synthesis, and generation of reactive oxygen species.
Microtubule disruptors attenuate NGF-hyperalgesia
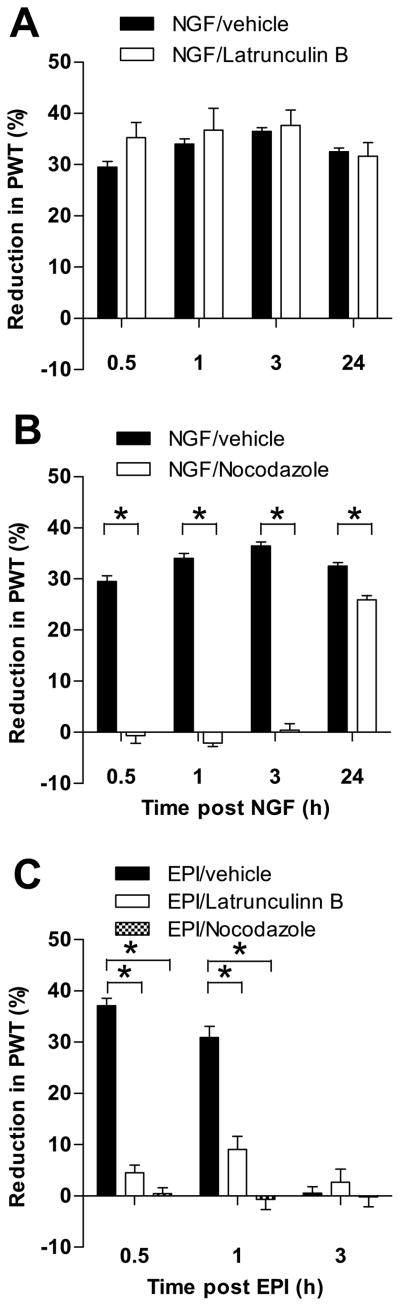
The transport and subcellular localization of mitochondria depends on the microtubule and actin cytoskeleton [6; 38; 15]. To evaluate whether an intact cytoskeleton is necessary for NGF-hyperalgesia, we tested the effect of nocodazole (1 μg, Fig. 3A), a well-characterized microtubule disruptor [11; 13] and Latrunculin B (1 μg, Fig. 3B), a selective actin cytoskeleton disruptor [11; 6] on NGF-induced mechanical hyperalgesia. As epinephrine-induced mechanical hyperalgesia depends on an intact microtubule and actin cytoskeleton [11], we administered epinephrine (100 ng) to a separate group of rats that were either pre-treated with nocodazole (1 μg) or Latrunculin B (1 μg, Fig. 3C), as a positive control.
Figure 3.
Effect of cytoskeletal inhibitors on NGF hyperalgesia. A. Latrunculin B, an actin disruptor had no significant effect on NGF hyperalgesia and any time point. B. Nocodazole, a microtubule disruptor, attenuated hyperalgesia up to 24 hours after NGF administration of hyperalgesic agent (n=6). C. Both Latrunculin B and nocodazole significantly attenuated epinephrine-induced hyperalgesia as demonstrated previously (Dina et al., 2003) (shown here as a positive control). Note that this experiment was not carried out to 24 hours because epinephrine-induced hyperalgesia lasts less than three hours. N = 6 for all groups. Data are plotted as mean ± SEM. Significant difference between the groups are indicated by asterisks (*) plotted above the time points where they apply. Abbreviations: PWT = paw-withdrawal threshold; EPI = epinephrine.
Nocodazole administration significantly attenuated NGF-hyperalgesia at all time-points, indicating a functional role for microtubules in NGF-hyperalgesia. ANOVA showed a significant group x time interaction (F3,30 = 159.494; p<0.001) and a significant main effect of group (F1,10 = 669.667; p<0.001). Multivariate analysis showed a significant difference at all time points (p<0.001).
Latrunculin B administration had no effect on NGF-hyperalgesia. The ANOVA showed that neither the time x group interaction (F3,30 = 2.264; p=0.119) nor the main effect of group (F1,10 = 0.532; p=0.486) were significant.
Both nocodazole and Latrunculin B significantly attenuated epinephrine-induced hyperalgesia at the 30 minute and one hour time points. The ANOVA showed a significant main effect of group (F2,15= 56.951; p<0.001) as well as a significant group × time interaction (F4,30= 75.486; p<0.001). Multivariate analysis showed significant differences between the effect of epinephrine alone and the effect of epinephrine in combination with either nocodazole and Latrunclin B at the 30 minute (p<0.001 for both) and the one hour (p< 0.001 for both) time points. There were no significant differences at the three hour time point because the effect of epinephrine alone had waned by that time.
Discussion
NGF induces mechanical hyperalgesia by direct action on the peripheral terminals of the primary afferent nociceptor [47; 30; 27; 28]. Although some of the signaling pathways mediating NGF-induced mechanical hyperalgesia have been identified [28; 9], our knowledge of the downstream targets and cellular processes of NGF signaling that explain nociceptor sensitization remain incomplete [34].
Because mitochondria in neurons are known to accumulate in the presence of NGF [5; 6], the present study was designed to investigate whether mitochondria play a role in NGF-induced hyperalgesia. We examined this question in two ways: 1) by blocking mitochondrial function (i.e., ATP synthesis and ROS generation) and 2) by blocking mitochondrial accumulation (i.e., by disrupting mitochondrial axonal transport). Both approaches elevated paw-withdrawal thresholds, demonstrating that mitochondria play an important role in NGF-induced hyperalgesia.
We recently reported that some PKCε-dependent forms of mechanical hyperalgesia can be attenuated by inhibitors of the mitochondrial electron transport chain or the administration of antioxidants [18].Mitochondria are the primary source for the generation of ROS [33; 36]. Cellular targets of ROS include TRPV1, TRPA1 and PKCε [16; 45; 7; 19], which are known to be present in the peripheral terminals of nociceptors [35; 42; 20; 39]. Oxidation of these receptors has been shown to induce nociceptor sensitization to thermal and mechanical stimulati [16; 7; 19]. Given that under physiological conditions the half-life of ROS is in the nanosecond to millisecond range (t1/2 (OH; O2−) = 10−9s) [41; 33], and the presence of mitochondria as well as relevant receptors in nociceptor terminals, the action of NGF in this study was highly likely to be local to the site of injection. In addition, of particular interest is the finding of Ibi and colleagues [16] that the NGF-induced upregulation of ROS in cultured dorsal root ganglion neurons is accompanied by the activation and translocation of PKCε. Since NGF-induced hyperalgesia can be alleviated by a selective PKCε translocation inhibitor [21] and PKCε-induced mechanical hyperalgesia can be attenuated by the same class of drugs used in the present study to attenuate NGF-hyperalgesia, it is tempting to speculate that NGF-induced hyperalgesia is mediated by a PKCε-dependent mechanism [18].
The structural and functional asymmetry of neurons requires that the subcellular localization of mitochondria be tightly regulated. Mitochondria are transported along and anchored to cytoskeletal “tracks” made of microtubules or F-actin [26; 6]. To analyze whether an intact cytoskeleton is a prerequisite for mitochondrial function and whether there are any differences in the importance of distinct cytoskeletal components for mitochondrial function, we tested the effect of two well-characterized cytoskeleton disruptors, nocodazole and Latrunculin B, on NGF-induced hyperalgesia. As epinephrine induced mechanical hyperalgesia is dependent on an intact cytoskeleton [11] we used epinephrine as a positive control (Fig. 2C). Nocodazole, a selective inhibitor of microtubule formation, effectively blocked NGF-induced as well as epinephrine-induced hyperalgesia (Fig. 2B,C). In contrast, Latrunculin B, a selective inhibitor of actin polymerization, blocked epinephrine hyperalgesia but not NGF hyperalgesia (Fig. 2A, C).
Mitochondria are not randomly distributed throughout the sensory neuron, but rather, accumulate at regions of increased energy consumption, for example, at the nodes of Ranvier or at sites of sensory transduction in the peripheral terminals [31; 4]. Mitochondria are synthesized in the cell body and actively transported to axons and dendrites. As most of the anterograde mitochondrial transport is microtubule-dependent [26; 15], it is not surprising that NGF-hyperalgesia is blocked by disruption of the microtubule cytoskeleton. Although Latrunculin B failed to block NGF-induced hyperalgesia in the present study, Hollenbeck and colleagues showed that NGF-signaling promotes the docking of mitochondria to the actin-cytoskeleton [5; 6]. Whether these results suggest that mitochondrial docking to actin is not requisite for hyperalgesia or are explained by an as yet unknown mechanism is not known. It may be relevant that our results are based on in vivo experiments on adult rats, while the findings of Chada and Hollenbeck are based on observations in cultured embryonic DRG neurons. How these differences are reflected in neuronal function remains to be explored.
In summary, we have explored the role of mitochondria in NGF-induced mechanical hyperalgesia. Multiple mitochondrial mechanisms as well as a functioning microtubule skeleton were shown to be required. Given the key role that NGF signaling plays in the nociceptor growth cone, further analysis of the relationship of axon sprouting and pain should focus on shared demand for energy metabolism.
Acknowledgments
This work was supported by the National Institutes of Health. Carissa Chu was supported by a Dean’s Summer Research Fellowship from the UCSF Office of Student Research.
Footnotes
The authors declare that no conflict of interest exists in connection with this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alessandri-Haber N, Dina OA, Yeh JJ, Parada CA, Reichling DB, Levine JD. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J Neurosci. 2004;24(18):4444–4452. doi: 10.1523/JNEUROSCI.0242-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20(12):4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aloe L, Tuveri MA. Nerve growth factor and autoimmune rheumatic diseases. Clin Exp Rheumatol. 1997;15(4):433–438. [PubMed] [Google Scholar]
- 4.Baloh RH. Mitochondrial dynamics and peripheral neuropathy. Neuroscientist. 2008;14(1):12–18. doi: 10.1177/1073858407307354. [DOI] [PubMed] [Google Scholar]
- 5.Chada SR, Hollenbeck PJ. Mitochondrial movement and positioning in axons: the role of growth factor signaling. J Exp Biol. 2003;206(Pt 12):1985–1992. doi: 10.1242/jeb.00263. [DOI] [PubMed] [Google Scholar]
- 6.Chada SR, Hollenbeck PJ. Nerve growth factor signaling regulates motility and docking of axonal mitochondria. Curr Biol. 2004;14(14):1272–1276. doi: 10.1016/j.cub.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 7.Chuang H-h, Lin S. Oxidative challenges sensitize the capsaicin receptor by covalent cysteine modification. Proc Natl Acad Sci U S A. 2009;106(47):20097–20102. doi: 10.1073/pnas.0902675106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411(6840):957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- 9.Di Castro A, Drew LJ, Wood JN, Cesare P. Modulation of sensory neuron mechanotransduction by PKC- and nerve growth factor-dependent pathways. Proc Natl Acad Sci U S A. 2006;103(12):4699–4704. doi: 10.1073/pnas.0508005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dina OA, Khasar SG, Gear RW, Levine JD. Activation of Gi induces mechanical hyperalgesia poststress or inflammation. Neuroscience. 2009;160(2):501–507. doi: 10.1016/j.neuroscience.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dina OA, McCarter GC, de Coupade C, Levine JD. Role of the sensory neuron cytoskeleton in second messenger signaling for inflammatory pain. Neuron. 2003;39(4):613–624. doi: 10.1016/s0896-6273(03)00473-2. [DOI] [PubMed] [Google Scholar]
- 12.Flatters SJL, Bennett GJ. Studies of peripheral sensory nerves in paclitaxel-induced painful peripheral neuropathy: evidence for mitochondrial dysfunction. Pain. 2006;122(3):245–257. doi: 10.1016/j.pain.2006.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goswami C, Dreger M, Jahnel R, Bogen O, Gillen C, Hucho F. Identification and characterization of a Ca2+-sensitive interaction of the vanilloid receptor TRPV1 with tubulin. J Neurochem. 2004;91(5):1092–1103. doi: 10.1111/j.1471-4159.2004.02795.x. [DOI] [PubMed] [Google Scholar]
- 14.Hensel H, Andres KH, von Düring M. Structure and function of cold receptors. Pflugers Arch. 1974;352(1):1–10. doi: 10.1007/BF01061945. [DOI] [PubMed] [Google Scholar]
- 15.Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118(Pt 23):5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibi M, Matsuno K, Shiba D, Katsuyama M, Iwata K, Kakehi T, Nakagawa T, Sango K, Shirai Y, Yokoyama T, Kaneko S, Saito N, Yabe-Nishimura C. Reactive oxygen species derived from NOX1/NADPH oxidase enhance inflammatory pain. J Neurosci. 2008;28(38):9486–9494. doi: 10.1523/JNEUROSCI.1857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joseph EK, Levine JD. Mitochondrial electron transport in models of neuropathic and inflammatory pain. Pain. 2006;121(1–2):105–114. doi: 10.1016/j.pain.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Joseph EK, Levine JD. Multiple PKCε-dependent mechanisms mediating mechanical hyperalgesia. Pain. 2010;150(1):17–21. doi: 10.1016/j.pain.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keeble JE, Bodkin JV, Liang L, Wodarski R, Davies M, Fernandes ES, Coelho CdF, Russell F, Graepel R, Muscara MN, Malcangio M, Brain SD. Hydrogen peroxide is a novel mediator of inflammatory hyperalgesia, acting via transient receptor potential vanilloid 1-dependent and independent mechanisms. Pain. 2009;141(1–2):135–142. doi: 10.1016/j.pain.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Kerstein PC, del Camino D, Moran MM, Stucky CL. Pharmacological blockade of TRPA1 inhibits mechanical firing in nociceptors. Mol Pain. 2009;5:19. doi: 10.1186/1744-8069-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron. 1999;24(1):253–260. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PubMed] [Google Scholar]
- 22.Khasar SG, Miao FJ, Levine JD. Inflammation modulates the contribution of receptor-subtypes to bradykinin-induced hyperalgesia in the rat. Neuroscience. 1995;69(2):685–690. doi: 10.1016/0306-4522(95)00280-v. [DOI] [PubMed] [Google Scholar]
- 23.Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD, Brown MT. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. 2010;363(16):1521–1531. doi: 10.1056/NEJMoa0901510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewin GR, Mendell LM. Nerve growth factor and nociception. Trends Neurosci. 1993;16(9):353–359. doi: 10.1016/0166-2236(93)90092-z. [DOI] [PubMed] [Google Scholar]
- 25.Lewin GR, Rueff A, Mendell LM. Peripheral and central mechanisms of NGF-induced hyperalgesia. Eur J Neurosci. 1994;6(12):1903–1912. doi: 10.1111/j.1460-9568.1994.tb00581.x. [DOI] [PubMed] [Google Scholar]
- 26.Ligon LA, Steward O. Role of microtubules and actin filaments in the movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. J Comp Neurol. 2000;427(3):351–361. doi: 10.1002/1096-9861(20001120)427:3<351::aid-cne3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 27.Lu J, Zhou XF, Rush RA. Small primary sensory neurons innervating epidermis and viscera display differential phenotype in the adult rat. Neurosci Res. 2001;41(4):355–363. doi: 10.1016/s0168-0102(01)00293-0. [DOI] [PubMed] [Google Scholar]
- 28.Malik-Hall M, Dina OA, Levine JD. Primary afferent nociceptor mechanisms mediating NGF-induced mechanical hyperalgesia. Eur J Neurosci. 2005;21(12):3387–3394. doi: 10.1111/j.1460-9568.2005.04173.x. [DOI] [PubMed] [Google Scholar]
- 29.McMahon SB. NGF as a mediator of inflammatory pain. Philos Trans R Soc Lond B Biol Sci. 1996;351(1338):431–440. doi: 10.1098/rstb.1996.0039. [DOI] [PubMed] [Google Scholar]
- 30.McMahon SB, Bennett DL, Priestley JV, Shelton DL. The biological effects of endogenous nerve growth factor on adult sensory neurons revealed by a trkA-IgG fusion molecule. Nat Med. 1995;1(8):774–780. doi: 10.1038/nm0895-774. [DOI] [PubMed] [Google Scholar]
- 31.Messlinger K. Functional morphology of nociceptive and other fine sensory endings (free nerve endings) in different tissues. Prog Brain Res. 1996;113:273–298. doi: 10.1016/s0079-6123(08)61094-8. [DOI] [PubMed] [Google Scholar]
- 32.Morris RL, Hollenbeck PJ. Axonal transport of mitochondria along microtubules and F-actin in living vertebrate neurons. J Cell Biol. 1995;131(5):1315–1326. doi: 10.1083/jcb.131.5.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicol GD, Vasko MR. Unraveling the story of NGF-mediated sensitization of nociceptive sensory neurons: ON or OFF the Trks? Mol Interv. 2007;7(1):26–41. doi: 10.1124/mi.7.1.6. [DOI] [PubMed] [Google Scholar]
- 35.Parada CA, Yeh JJ, Reichling DB, Levine JD. Transient attenuation of protein kinase Cepsilon can terminate a chronic hyperalgesic state in the rat. Neuroscience. 2003;120(1):219–226. doi: 10.1016/s0306-4522(03)00267-7. [DOI] [PubMed] [Google Scholar]
- 36.Poyton RO, Ball KA, Castello PR. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol Metab. 2009;20(7):332–340. doi: 10.1016/j.tem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther. 1957;111(4):409–419. [PubMed] [Google Scholar]
- 38.Reynolds IJ, Rintoul GL. Mitochondrial stop and go: signals that regulate organelle movement. Sci STKE. 2004;2004(251):PE46. doi: 10.1126/stke.2512004pe46. [DOI] [PubMed] [Google Scholar]
- 39.Ro JY, Lee J-S, Zhang Y. Activation of TRPV1 and TRPA1 leads to muscle nociception and mechanical hyperalgesia. Pain. 2009;144(3):270–277. doi: 10.1016/j.pain.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rukwied R, Mayer A, Kluschina O, Obreja O, Schley M, Schmelz M. NGF induces non-inflammatory localized and lasting mechanical and thermal hypersensitivity in human skin. Pain. 2010;148(3):407–413. doi: 10.1016/j.pain.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 41.Sies H. Strategies of antioxidant defense. Eur J Biochem. 1993;215(2):213–219. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- 42.Ständer S, Moormann C, Schumacher M, Buddenkotte J, Artuc M, Shpacovitch V, Brzoska T, Lippert U, Henz BM, Luger TA, Metze D, Steinhoff M. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp Dermatol. 2004;13(3):129–139. doi: 10.1111/j.0906-6705.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- 43.Steers WD, Tuttle JB. Mechanisms of Disease: the role of nerve growth factor in the pathophysiology of bladder disorders. Nat Clin Pract Urol. 2006;3(2):101–110. doi: 10.1038/ncpuro0408. [DOI] [PubMed] [Google Scholar]
- 44.Taiwo YO, Bjerknes LK, Goetzl EJ, Levine JD. Mediation of primary afferent peripheral hyperalgesia by the cAMP second messenger system. Neuroscience. 1989;32(3):577–580. doi: 10.1016/0306-4522(89)90280-7. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi N, Mizuno Y, Kozai D, Yamamoto S, Kiyonaka S, Shibata T, Uchida K, Mori Y. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels (Austin) 2008;2(4):287–298. doi: 10.4161/chan.2.4.6745. [DOI] [PubMed] [Google Scholar]
- 46.Verburg J, Hollenbeck PJ. Mitochondrial membrane potential in axons increases with local nerve growth factor or semaphorin signaling. J Neurosci. 2008;28(33):8306–8315. doi: 10.1523/JNEUROSCI.2614-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62(2):327–331. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]
- 48.Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24(24):4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]