Abstract
The emerging problem of antibiotic resistance, especially among Gram-negative bacteria (GNB), has become a serious threat to global public health. Very few new antibacterial classes with activity against antibiotic-resistant GNB have been brought to market. Renewed and growing attention to the development of novel compounds targeting antibiotic-resistant GNB, as well as a better understanding of strategies aimed at preventing the spread of resistant bacterial strains and preserving the efficacy of existing antibiotic agents, has occurred. The Gram-Negative Resistance Summit convened national opinion leaders for the purpose of analyzing current literature, epidemiologic trends, clinical trial data, therapeutic options, and treatment guidelines related to the management of antibiotic-resistant GNB infections. After an in-depth analysis, the Summit investigators were surveyed with regard to 4 clinical practice statements. The results then were compared with the same survey completed by 138 infectious disease and critical care physicians and are the basis of this article.
Antibiotic resistance among bacterial pathogens seems to be on an uninterrupted incline. Data from the Centers for Disease Control and Prevention show rapidly increasing rates of infection due to vancomycin-resistant Enterococcus faecium (VRE) and fluoroquinolone-resistant Pseudomonas aeruginosa [1]. This has resulted in the introduction of the term ESKAPE to describe the most common and problematic bacteria associated with increasing antimicrobial resistance (E. faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumanii, P. aeruginosa, and Enterobacter species) [2]. The increasing trend of antimicrobial resistance is most worrisome for Gram-negative bacteria (GNB) because there has been little successful development of new antibiotic agents targeting this class of pathogens [3]. Furthermore, we are now in the presence of GNB that have “extreme drug resistance,” indicating complete resistance of strains to first-line antibiotics used for the treatment of GNB infections (amikacin, tobramycin, cefepime, ceftazidime, imipenem, meropenem, piperacillin-tazobactam, ciprofloxacin, and levofloxacin) plus second-line drugs such as tigecycline and polymyxins [4]. Even more worrisome is the continued emergence of new mechanisms of multidrug resistance among GNB. Two recent examples of this include aminoglycoside 16S ribosomal RNA methylation and production of the New Delhi metallo-β-lactamase [5, 6].
Increasing antibiotic resistance of GNB has created a therapeutic challenge for clinicians treating patients with a known or suspected infection. Increasing rates of resistance lead many clinicians to treat patients empirically with multiple broad-spectrum antibiotics, which can perpetuate the cycle of increasing resistance and create an economic burden to society [7]. Conversely, inappropriate initial antimicrobial therapy (IIAT), defined as an antimicrobial regimen that lacks in vitro activity against the isolated organism(s) responsible for the infection, can lead to treatment failures and adverse patient outcomes [8]. IIAT is a potentially modifiable factor that has been associated with increased mortality in patients with serious infections [9–11]. Individuals with severe sepsis and septic shock seem to be at particularly high risk of excess mortality when IIAT is administered [12–15]. This therapeutic challenge recently has been addressed by the Surviving Sepsis Guidelines, which now recommend empirical combination therapy targeting GNB, particularly for patients with known or suspected Pseudomonas infections as a means to decrease the likelihood of administering IIAT [16]. However, the authors of this guideline readily acknowledge that “no study or meta-analysis has convincingly demonstrated that combination therapy produces a superior clinical outcome for individual pathogens in a particular patient group.” Nevertheless, the increasingly futile challenge of providing appropriate initial antimicrobial therapy to patients with serious infections has led to these types of empiric recommendations and the call for more rigorous trials of novel therapies for antibiotic-resistant infections. [17]
This supplement to Clinical Infectious Diseases represents the proceedings of a panel of clinical investigators whose goal was to assess the quality of evidence in support of various therapeutic approaches aimed at either attenuating the further emergence of antibiotic resistance or improving the initial delivery of appropriate antibiotic therapy. Four clinical practice statements were drafted by the chairperson and subsequently evaluated by the 4-member panel composed of leaders in infectious disease, pulmonary and critical care medicine, and pharmacology (Table 1). Before the Summit was convened, each participant was assigned a statement and instructed to systematically review and summarize the evidence supporting or refuting that statement. At the live meeting, each member presented the evidence for their statement to the full panel. When the data were presented, primary attention was given to the study methodology, the number of patients enrolled, and the outcome events. After the presentation of data for each statement, Summit members discussed the evidence, graded its strength, and voted on each statement by assigning a consensus numeric grade for the “Nature of Evidence,” using the grading scheme shown in Table 2 to record their individual level of support. In addition to defining the level of evidence in support of each statement, the Summit panelists also outlined additional data required to further refine the statements for future clinical use. One of the main intentions of this meeting was to provide a framework for future discussion and research in the area of antibiotic resistance among GNB. Before the Summit meeting, clinical perspectives of practicing physicians were measured via a web-based survey. Email polling was conducted to ascertain their level of support for the same 4 statements. The email invitation to participate was sent to 10 000 infectious disease and critical care physicians (all email addresses were active). Of those surveyed, 138 (1.38%) responded. The purpose of the electronic survey was to provide information that would allow for the comparison of data-driven responses from the content “experts” at the Summit with those of clinicians practicing in the field. The Summit participants and the surveyed physicians used the same grading scheme to rate the 4 statements (Table 2).
Table 1.
Gram-Negative Resistance Summit Statements for Evaluation
| 1. Empiric combination therapy using a carbapenem with other antibiotic classes should be used first-line in critically ill patients at risk for MDR GNB. (Author: Y. G.) |
| 2. Pharmacokinetic/pharmacodynamic optimization of antibiotics with Gram-negative activity can overcome resistance associated with MDR GNB. (Author: S. T. M.) |
| 3. Strategies to limit antibiotic exposure, such as shorter courses of antibiotics, attenuates the emergence of resistant GNB. (Author: A. F. S.) |
| 4. Active surveillance of MDR GNB with isolation should be an active component of infection control bundles to prevent the proliferation of MDR GNB. (Author: M. I. R.) |
NOTE. GNB, Gram-negative bacteria; MDR, multidrug-resistant.
Table 2.
Voting Schemes Used in the Gram-Negative Resistance Summit
| Nature of evidence | Level of support |
| I. Evidence obtained from ≥1 well-designed, randomized, controlled trial | 1. Accept statement completely |
| II. Evidence obtained from well-designed cohort or case-control studies | 2. Accept statement with some reservations |
| III. Evidence obtained from case series, case reports, or flawed clinical trials | 3. Accept statement with major reservations |
| IV. Opinions of respected authorities based on clinical experience, descriptive studies, or reports of expert committees | 4. Reject statement with reservations |
| V. Insufficient evidence to form an opinion | 5. Reject statement completely |
STATEMENT 1: EMPIRIC COMBINATION THERAPY USING A CARBAPENEM WITH OTHER ANTIBIOTIC CLASSES SHOULD BE USED FIRST-LINE IN CRITICALLY ILL PATIENTS AT RISK FOR MULTIDRUG-RESISTANT GRAM-NEGATIVE BACTERIA
Rationale and Definition of Statement
This systematic review aims to explore the evidence in support of the statement that combination therapy using a carbapenem with other antibiotic classes should be used first-line in critically ill patients at risk for multidrug-resistant (MDR) GNB. Critically ill patients were defined as patients admitted to the intensive care unit (ICU). This population was chosen because of its high risk of severe consequences of infection; thus, the initial choice of antibiotics could be a major determinant of the outcome. Theoretically, combining antibiotics with activity against antibiotic-resistant GNB can lead to a better outcome by one of several mechanisms. These include increased likelihood of pathogen coverage, improved pharmacodynamics (eg, synergism), improved pharmacokinetic parameters (eg, area under the curve), and reduced risk of emergence of antibiotic resistance. Because the goal of this review was to focus on patient outcomes (and not on antibiotic resistance), the following general questions were explored: For severe infections, does adequate empiric coverage of the causative pathogen lead to better outcomes? Is combination antibiotic therapy more likely to provide adequate empiric coverage? Is combining more than one antibiotic with activity against the causative pathogen better than using just one active antibiotic? Also explored was the more specific question of whether an empiric combination that includes a carbapenem will lead to better outcomes in patients with sepsis caused by an antibiotic-resistant GNB. Given the limited amount of specific data in support of the main statement, the review has been expanded to explore several clinically relevant permutations of the main statement, focusing on whether a combination of antibiotics should be used for the treatment of severe infections by antibiotic-resistant GNB in immunocompetent patients.
Literature Search
A search of the PubMed “1990–present” database was completed on 20 October 2010, to identify studies related to outcomes of treatments of GNB infections in ICU patients. The search was limited to English-language human studies. Studies that focus on in vitro activity, surveillance, or immunosuppressed populations were excluded. The search of the combined terms “Pseudomonas” OR “Klebsiella” OR “Acinetobacter” OR “gram negative” OR “gram negative resistance” AND “combination antibiotic therapy” OR “combined therapy” OR “combined antibiotics” produced 3986 articles. After the elimination of non–English-language studies, surveillance and animal studies, and studies in immunocompromised hosts, 16 relevant studies were identified and included in this review.
Evidence
For severe infections, does adequate empiric coverage of the causative pathogen lead to better outcomes? Is combination antibiotic therapy more likely to provide adequate empiric coverage? Several studies explored the relationship between mortality and adequacy of empiric antibiotics. Studies vary in their definition of adequate, severity of infection manifestation, and time of outcome assessment. Christoff et al analyzed the antibiotic susceptibility of >5000 isolates to evaluate the potential of combination therapy to increase the likelihood of activity against Gram-negative rods (GNRs) isolated in the ICU [18]. The addition of another GNR-active antibiotic to a backbone of piperacillin-tazobactam, ceftazidime, or imipenem significantly increased the likelihood of covering the pathogen (Table 3). However, the clinical relevance of the included isolates and the impact on clinical outcomes were not determined.
Table 3.
Antibiotic Susceptibility for Combination Therapy Versus Monotherapy
| Susceptibility for monotherapy,% | Susceptibility for combination therapy, % (P) |
||||
| Therapy | Gentamicin | Ciprofloxacin | Tobramycin | Amikacin | |
| Imipenem | 88.8 | 93.4 (.007) | 92.1 (.056) | 94.2 (.001) | 95.8 (<.001) |
| Ceftazidime | 69.2 | 84.4 (<.001) | 81.3 (<.001) | 84.6 (<.001) | 92.6 (<.001) |
| Piperacillin-tazobactam | 68.8 | 85.6 (<.001) | 81.4 (<.001) | 85.4 (<.001) | 91.6 (<.001) |
NOTE. Data from Christoff et al [18].
In a prospective cohort study that included 655 patients in ICUs treated for community- or hospital-acquired infections, the objective was to evaluate the relationship between inadequate antibiotic therapy for infections and hospital mortality [19]. Inadequate antibiotic therapy was defined as microbiologically documented infection that was not being effectively treated at the time of identification. The primary outcome was in-hospital mortality. Inadequate antibiotic therapy was associated with higher all-cause mortality (52.1% vs 23.5%; P < .001) and infection-related mortality (42% vs 17.7%; P < .001). In multivariate analysis, inadequate therapy was the strongest risk factor for mortality (adjusted odds ratio [OR], 4.3; 95% confidence interval [CI], 3.4–5.4).
More recently, Harbarth et al examined the effect of inadequate antibiotic therapy on all-cause 28-day mortality among 468 patients with severe sepsis and a documented bloodstream infection (BSI) [15]. Inadequate therapy was defined as no administration of an active antibiotic within the first 24 hours after the diagnosis of severe sepsis. Factors that were associated with inadequate therapy included admission to surgery, nosocomial infection, infection by an MDR organism, and a fungal or polymicrobial infection (all P < .05). Inadequate therapy was associated with higher mortality (39% vs 24%; P < .001) and remained so after careful adjustment for factors that were associated with mortality as well as adjustment for the propensity for inadequate therapy (OR, 1.8; 95% CI, 1.2–2.6). Similar results were reported by Ibrahim et al [11].
Assessing the impact of inadequate empiric antimicrobial therapy on mortality from septic shock, Kumar et al found that only 50% of patients with septic shock received adequate therapy within 6 hours of documented hypotension [20]. Mortality among patients with inadequate initial therapy was higher (adjusted OR, 1.12/hour of delay; 95% CI, 1.10–1.14).
Focusing on sepsis by GNB, several studies explored the effect of inadequate GNB coverage on mortality. In a cohort of 760 patients with severe sepsis or septic shock and Gram-negative bacteremia, Micek et al determined whether combination therapy is more likely to result in initial adequate pathogen coverage and whether adequate coverage is associated with decreased in-hospital mortality [21]. Combination therapy resulted in decreased likelihood of inadequate therapy (22.2% vs 36%; P < .001), particularly when an aminoglycoside was added to a β-lactam. Specifically, the addition of an aminoglycoside to a carbapenem would have increased appropriate initial therapy from 89.7% to 94.2% (Table 4). Inadequate therapy was associated with increased mortality in univariate analysis (51.7% vs 36.4%; P < .001) and multivariate analysis (adjusted OR, 2.3; 95% CI, 1.9–2.8). Mortality among those who received inadequate initial therapy was higher regardless of whether their infection was acquired in the community or the hospital.
Table 4.
Effectiveness of Various Antibiotic Combinations Against Gram-Negative Pathogens
| Susceptible to antibiotic, % |
|||
| Antibiotic | No addition | Plus ciprofloxacin | Plus gentamicin |
| Cefepime | 83.4 | 86.4 | 89.9 |
| Imipenem or meropenem | 89.7 | 92.4 | 94.2 |
| Piperacillin-tazobactam | 79.6 | 87.0 | 91.4 |
NOTE. Reprinted with permission from Micek et al [21].
Al-Hasan et al assessed the effect of β-lactam-fluoroquinolone combination vs β-lactam monotherapy on 28-day all-cause mortality among 702 patients with monomicrobial GNR bacteremia [22]. Although a slightly lower mortality was observed among non–critically ill patients (defined as a Pittsburgh bacteremia score of <4) who were treated with the combination (4.2% vs 8.8%; P = .044), mortality rates among more sick patients were similar in patients treated with the combination and those treated with monotherapy (25.6% vs 27.8%, respectively; P = .660). Similar results were observed when 14-day mortality was assessed, and they remained unchanged in multivariate analysis.
Several studies compared combination vs monotherapy in patients with hospital-acquired pneumonia. The outcomes of ICU patients with ventilator-associated pneumonia (VAP) receiving empiric meropenem plus ciprofloxacin therapy versus empiric meropenem alone were evaluated in a recent randomized unblinded trial [23]. Patients with known colonization by methicillin-resistant S. aureus (MRSA) or Pseudomonas were excluded. Combination therapy resulted in higher rates of adequate therapy (93.1% vs 85.1%; P = .01), but there were no differences in 28-day mortality, duration of ICU and hospital stay, or clinical and microbiologic treatment response. Despite a large cohort of 740 patients, relatively few were infected by hard-to-treat GNR. In the small subgroup of patients whose respiratory cultures grew Pseudomonas, Acinetobacter, or other MDR GNR, the use of combination therapy was associated with higher rates of adequate therapy and microbiologic eradication, but there were no differences in mortality or other clinical outcomes.
In another randomized trial, the combination of cefepime plus amikacin was compared with cefepime monotherapy in 200 patients with hospital-acquired pneumonia [24]. Combination therapy was associated with a higher treatment success rate (89% vs 71%; P < .05). However, this difference was driven by the subgroup of patients with isolation of Pseudomonas (success, 92% for combination therapy vs 46% for monotherapy; P < .05). In contrast, in a noncomparative study of 84 patients with trauma who were diagnosed with VAP and Pseudomonas isolation, monotherapy with cefepime resulted in microbiologic eradication in 94.1%, but rates of clinical cure and mortality were not provided [25].
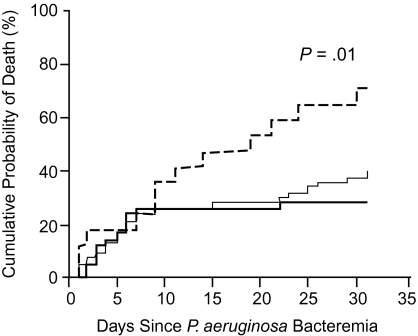
Two studies examined the relationship between combination therapy and mortality from Pseudomonas bacteremia. In 115 episodes from 1988 through 1998, in one-third of patients with neutropenia, Chamot et al found that inadequate empiric therapy was associated with higher 30-day mortality rates than patients given adequate combination therapy (72.1% vs 29.4%; P = .01) (Figure 1) [26]. However, patients were not stratified by severity of illness, and the number of patients with inadequate therapy was small (n = 17; 14.8%) and did not allow for adequate adjustment. In a larger, more recent study, Micek et al examined all-cause in-hospital mortality among 305 patients with Pseudomonas bacteremia from 1997 through 2002 [10]. Patients with inappropriate therapy were more likely to be treated with ciprofloxacin (25.3% vs 13.9%; P = .02) and less likely to be treated with ceftazidime or cefepime (see Table 5.). There were no significant differences between appropriate and inappropriate initial treatment among patients receiving imipenem therapy. Mortality was higher among those receiving inappropriate therapy (31% vs 18%; P = .02) and remained higher in multivariate analysis (adjusted OR, 2.04; 95% CI, 1.42–2.92; P = .048). However, the possibility of confounding by indication remained unexplored.
Figure 1.
Effectiveness of combination antibiotics for Pseudomonas aeruginosa bacteremia [26], displayed as cumulative risk of death for patients who received adequate empirical combination therapy (darker solid line), adequate empirical monotherapy (lighter solid line), or inadequate empirical therapy (dashed line). Reproduced with permission from Chamot et al [26].
Table 5.
Appropriateness of Initial Antibiotics
| Drug class | Appropriate, no. (%) | Inappropriate, no. (%) | P |
| (n = 230) | (n = 75) | ||
| Cefepime | 141 (61.3) | 33 (44.0) | .009 |
| Ceftazidime | 36 (15.7) | 3 (4.0) | .009 |
| Piperacillin-tazobactam | 5 (2.2) | 3 (4.0) | .686 |
| Imipenem | 31 (13.5) | 9 (12.0) | .742 |
| Aminoglycosidea | 102 (44.3) | 30 (40.0) | .509 |
| Ciprofloxacin | 32 (13.9) | 19 (25.3) | .021 |
| Aztreonam | 1 (0.4) | 0 (0.0) | >.999 |
| Other | 4 (1.7) | 2 (2.7) | .638 |
NOTE. Appropriate antimicrobial treatment was defined as in vitro susceptibility testing demonstrating a sensitive isolate of Pseudomonas aeruginosa to the antimicrobial agent prescribed. Reprinted with permission from Micek et al [10].
Includes gentamicin, tobramycin, and amikacin.
In a prospective, randomized trial of nonneutropenic patients with severe infections (pneumonia, peritonitis, or other sepsis), Cometta et al compared the efficacy of imipenem monotherapy with that of a combination of imipenem and netilmicin (an aminoglycoside) [27]. Of the 280 subjects evaluated, 204 were in the ICU, 136 were ventilated, and 177 had diagnoses of pneumonia. There were no significant differences in clinical cure rates, development of septic shock, or mortality from infection. Definite nephrotoxicity was higher among those receiving the combination (3.87% vs 0%; P = .01). Similarly, a smaller study comparing meropenem with the combination of ceftazidime and amikacin in 153 patients with sepsis found no differences in outcome [28].
In a comprehensive meta-analysis, Paul et al compared the effect on all-cause mortality of β-lactam monotherapy versus combination therapy with β-lactam and aminoglycoside in patients with sepsis enrolled in randomized and quasi-randomized studies [29]. In a subgroup analysis of patients with Gram-negative infections, of the studies that included the same β-lactam in both study arms, there were no differences between study groups with regard to all-cause mortality (3 studies: relative risk [RR], 0.56; 95% CI, .08–4.07) or clinical failure (10 studies: RR, 1.23; 95% CI, .90–1.68). In addition, in studies comparing monotherapy with a broader-spectrum β-lactam with a combination of a narrower-spectrum β-lactam plus an aminoglycoside, there were also no significant differences between study groups for both all-cause mortality (5 studies: RR 1.25; 95% CI, .80–1.95) and clinical failure (18 studies: RR, 0.85; 95% CI, .66–1.09).
Is combining more than 1 antibiotic with activity against the causative pathogen better than using just 1 active antibiotic? In a propensity-matched analysis, Kumar et al compared the rate of 28-day mortality among 2446 patients with septic shock who were adequately treated with combination therapy versus monotherapy [30]. Compared with mortality rates in those treated with monotherapy, rates were lower among those treated with combination therapy, regardless of whether therapy was initiated before the documentation of hypotension (combination mortality 29% vs monotherapy mortality 36.3%; P = .0002) or later (26.7% vs 37.6%; P = .007). The beneficial impact of combination therapy applied to both Gram-positive and Gram-negative infections was restricted to patients treated with β-lactams in combination with aminoglycosides, fluoroquinolones, macrolides, or clindamycin. In a much smaller study of patients with Pseudomonas bacteremia [26], the 30-day mortality rates were similar among those receiving adequate combination therapy and those receiving adequate monotherapy.
Grading of Evidence
Based on a review of the literature cited in this section, the 5 members of this Summit agreed that no top-level evidence was available to support or reject the statement that empiric combination therapy using a carbapenem with other antibiotic classes should be used as first-line treatment in critically ill patients at risk for MDR GNB. Two members of the Summit (40%) considered the evidence to support the statement to be from well-designed cohorts or case-controlled studies, and 2 members voted that the evidence was obtained from case series, case reports, or flawed clinical trials. One member (20%) voted there was insufficient evidence to form an opinion.
Level of Support
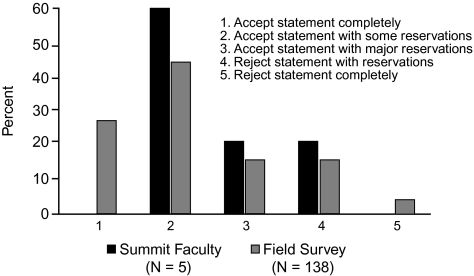
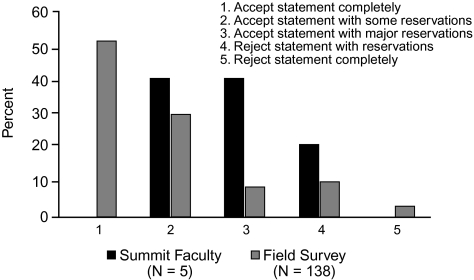
When voting on the support for statement 1 in the group at large, 60% (3) of the Summit participants accepted the statement to some extent, but none accepted it completely (Figure 2). Interestingly, despite the lack of top-level data, 26% (36) of the 138 infectious disease and critical care physicians who participated in the online survey accepted the statement completely, and almost none completely rejected the statement.
Figure 2.
Level of support for statement 1: Empiric combination therapy using a carbapenem with other antibiotic classes should be used first-line in critically ill patients at risk for multidrug-resistant Gram-negative bacteria.
Discussion
Available studies on this topic were found to be limited in several ways. Most studies were relatively small and lacked the power to show clinically meaningful differences between treatment groups. The majority of studies were retrospective in nature. As such, they all were vulnerable to bias by indication. Adjustment for such bias, using a propensity score model, was rarely attempted. The lack of robust data was reflected in the way the faculty voted. Neither of the two strongest recommendations to completely accept or reject the statement were chosen by the Summit faculty. Most (80%) believed there was enough circumstantial evidence in support of accepting the statement with minor or major reservations. Faculty agreed that the main source of support for statement 1 stemmed from studies that showed a higher likelihood of adequate empiric coverage when combination antibiotics were used, in addition to the assumption that adequate empiric therapy in ICU patients is associated with improved survival rates. Similar to the faculty, most (58%) of the participants in the field survey (n = 138) accepted statement 1 with some reservation. Notably, however, 26% accepted the statement completely. Given the lack of well-designed prospective trials supporting this statement, this pattern of voting may have been affected by personal experience in favor of using carbapenems in combination, practice or institutional guidelines that emphasize empiric combination therapy for health care–associated pneumonia, local patterns of antibiotic susceptibility, occurrence of MDR GNB infections (eg, a high rate of infections by GNRs producing extended-spectrum β-lactamase [ESBL]), the textbook notion that 2 drugs are needed to cover Pseudomonas, or a lack of familiarity with the literature.
Future Directions
The Summit participants acknowledge the fact that a decision regarding combination antimicrobial therapy will be affected by contemporary as well as geographic factors. Patterns of antimicrobial resistance change over time and are institution specific. Certain combinations, which work well in some hospitals at a specific point in time, may not work in other hospitals or at other times. In addition, the use of combination therapy can change the pattern of antimicrobial resistance in a way that will reduce its efficacy over time. Therefore, specific antimicrobial combinations, including those that contain a carbapenem, should be evaluated constantly to determine their value.
STATEMENT 2: PHARMACOKINETIC/PHARMACODYNAMIC OPTIMIZATION OF ANTIBIOTICS WITH GRAM-NEGATIVE ACTIVITY CAN OVERCOME RESISTANCE ASSOCIATED WITH MULTIDRUG-RESISTANT GRAM-NEGATIVE BACTERIA
Rationale and Definition of Statement
During the last decade, a number of evolving factors have made the administration of appropriate initial antimicrobial therapy more difficult to achieve in the clinical setting. Foremost has been the increase of antimicrobial resistance among bacterial and nonbacterial pathogens [7]. This is probably related to several factors, including the greater overall use of broad-spectrum antibiotics, much of which stems from the changing demographics of patients entering hospitals [31]. Patients are generally older, more likely to be immunosuppressed, and much more likely to have risk factors associated with health care exposure than patients a decade earlier [32, 33].
Despite increasing antimicrobial resistance from both Gram-positive bacteria and GNB, there are few novel antimicrobial agents in development. Perhaps of equal concern are the insidious increases in the minimum inhibitory concentration (MIC) of currently available antibiotics required to kill specific bacteria. Unfortunately, many clinical microbiology laboratories do not test for or report MIC routinely, and clinicians are often unaware of the relative insensitivity of the clinical isolate to the prescribed drug class. This is of great consequence because the treatment of Gram-negative infections with antibiotics demonstrating susceptible, yet elevated, MIC has been linked to treatment failures and greater mortality [34, 35].
Statement 2 explores whether optimization of pharmacokinetic/pharmacodynamic (PK/PD) end points in the use of antibiotics with Gram-negative activity can overcome insensitivity and/or resistance associated with MDR GNB. Optimization of PK/PD is based on 3 concepts: (1) the duration of time the drug concentration remains above the MIC (T>MIC); (2) the ratio of maximum drug concentration (peak concentration) to the MIC (Cmax/MIC); and (3) the 24-hour ratio of the area under the curve to the MIC. Antibiotic regimens with conventional dosing strategies (oral administration or intermittent [30–60 minutes] infusions) are likely to achieve the desired PK/PD end point in infections caused by pathogens for which the MIC is low. However, organisms for which the MIC is elevated jeopardize the attainment of PK/PD targets with such regimens; consequently, alternative means to achieve these end points must be prescribed. The concepts of extended duration infusions (antibiotic administered over a period of 3–4 hours) or continuous infusions and combination therapy regimens will be evaluated as means to optimize PK/PD parameters for the treatment of MDR Gram-negative infections.
Literature Search
A literature search using the PubMed database was performed on 29 October 2010. The search used the text words “gram negative infections” combined with the following: (1) “pharmacokinetics” and “pharmacodynamics” (378 articles); (2) “continuous infusion antibiotics” (62 articles); (3) “prolonged infusion antibiotics” (27 articles); and (4) “combination therapy” (2347 articles). The search results were narrowed to English-language publications and were further limited to include controlled trials, randomized controlled trials, or meta-analyses (410 articles). A review of the bibliographies from selected articles was also performed to identify references for statement justification, and 20 relevant articles were ultimately selected.
Evidence
The significance of reduced antibiotic susceptibility (eg, elevated MIC) often observed after years of clinical use, coupled with the advent of new resistance mechanisms, has been demonstrated in 2 recent retrospective analyses of Gram-negative infections. Bhat et al observed that patients with Gram-negative bacteremia treated with cefepime at an MIC of ≥8 μg/mL had a significantly greater 28-day mortality than those treated with the same antibiotic at an MIC of <8 μg/mL (54.8% vs 24.1%; P = .001) [34]. Similarly, Tam and colleagues found an association between elevated piperacillin-tazobactam MIC (32 μg/mL or 64 μg/mL) and increased 30-day mortality in patients with P. aeruginosa bacteremia [35]. The authors of both studies postulated that the difference in outcomes was due in part to the lack of PK/PD target attainment in the isolates for which MICs are elevated. Support for this assertion was demonstrated in a retrospective study that observed significantly higher rates of clinical cure (82% vs 33%; P = .002) and bacteriologic eradication (97% vs 44%; P < .001) in patients in whom the PK/PD target was attained (T>MIC, 100%) with cefepime or ceftazidime than in patients in whom this target was not attained (T>MIC, <100%) in the treatment of serious bacterial infections [36].
The most logical means to attain antibiotic PK/PD targets is through a daily dosage increase or a lowering of the MIC breakpoint. The latter has recently been done for select cephalosporin, carbapenem, and aztreonam breakpoints for Enterobacteriaceae, to better represent the effect these agents should have when treating infections caused by contemporary isolates [37, 38]. Unfortunately, this change in breakpoint does not offer a solution to the pathogen for which the MICs for these antibiotics are elevated. The option of increasing doses above what is recommended as a means to achieve PK/PD targets, however, is not an acceptable alternative owing to the probability of eliciting unacceptable toxic effects.
One concept of considerable appeal given this era of increasing MICs is not novel but continues to be of interest. This is the concept of using extended or continuous infusions of β-lactam antibiotics (off-label use) at the recommended daily dose in an effort to optimize T>MIC. Monte Carlo dosing simulations (used to assess the probability of target attainment) suggest that extended or continuous infusions will be of greatest benefit in patients who are infected with a pathogen for which MICs are elevated, who maintain normal functioning kidneys [39, 40], and/or who have altered volumes of drug distribution [41].
Retrospective studies comparing patients who were given extended or continuous infusions with historical controls who were given bolus doses of β-lactam antibiotics have provided some insight into the clinical impact of this method of PK/PD optimization. Lorente and colleagues published a series of reports describing their experience with continuous infusions of meropenem, ceftazidime, or piperacillin-tazobactam in combination with tobramycin for the treatment of VAP [42–44]. Collectively, for each antibiotic evaluated, continuous infusion significantly improved clinical cure rates compared with the cure rates seen in patients given intermittent infusions. Potential patient selection bias significantly limits the ability to generalize from these studies, however, because the method of antibiotic administration was at the discretion of the treating physician.
The administration of piperacillin-tazobactam (3.375 g intravenously every 8 hours) infused over a 4-hour period has been evaluated in 2 retrospective analyses. Lodise et al reported a significant reduction in 14-day mortality (12.2% vs 31.6%; P = .04) and median length of ICU stay (21 vs 38 days; P = .02) in a subgroup of patients with P. aeruginosa infections and Acute Physiological and Chronic Health Evaluation (APACHE) II scores of ≥17 when patients given 4-hour infusion were compared with historical controls who received bolus administration of piperacillin-tazobactam [45]. However, the same benefit was not observed when the entire cohort was considered. In a retrospective review by Patel et al of patients with various Gram-negative infections from 2 institutions (n = 129), no difference was observed in 30-day mortality rates between patients who had piperacillin-tazobactam infused over a 4-hour period and historical controls who received intermittent infusions [46].
Nicasio and colleagues studied the effect of a pharmacodynamic-based clinical pathway on mortality and clinical outcomes. The pharmacodynamic-based clinical pathway used extended infusions (3 hours) of cefepime or meropenem in combination with tobramycin and vancomycin for the empiric treatment of VAP [47]. Compared with a historical control group given intermittent infusions of the same antibiotics, the group treated via the VAP clinical pathway, which utilized extended infusion regimens, had significantly reduced infection-related mortality (8.5% vs 21.6%; P = .029) and infection-related length of stay (11.7 vs 26.1 days; P < .001). In addition, clinical success was observed in 8 of 9 patients who were infected with P. aeruginosa isolates at levels near or above the respective breakpoint who were managed via the extended infusion pathway.
A systematic review of 9 randomized, controlled trials investigated the clinical benefits of various β-lactam antibiotics administered via extended administration or continuous infusions compared with standard bolus doses [48]. Cumulatively, neither a survival benefit (OR, 1.00; 95% CI, .48–2.06; P = 1.0) nor improvement in clinical cure (OR, 1.04; 95% CI, .74–1.46; P = .83) was observed with the administration of continuous infusion or extended administration of a β-lactam.
A contemporary prospective, randomized trial that included extended infusion administration as part of the intervention strategy was a phase III study (n = 531) that compared doripenem (500 mg/8 hours via a 4-hour infusion) with imipenem (500 mg/6 hours via a 30-min infusion or 1000 mg/8 hours via a 60-min infusion), both with the option of administering amikacin in combination for the treatment of VAP [49]. The primary efficacy end point of clinical cure in the clinical modified intent-to-treat (59.0% vs 57.8%) and clinically evaluable (68.3% vs 64.2%) populations found that doripenem was not inferior to imipenem. A secondary subgroup analysis in the trial found that the doripenem group was associated with a reduction in the development of resistance in P. aeruginosa isolates, defined as ≥4-fold increase in MIC. Among the explanations for this finding could be improved PK/PD target attainment with the doripenem extended infusion.
Finally, evidence surrounding combination therapy regimens as a means to optimize PK/PD parameters was evaluated. Several treatment guidelines recommend an empiric combination therapy regimen (≥2 antibiotics) if patients are likely to be infected with MDR Gram-negative pathogens [16, 50]. A positive association between appropriate initial therapy and treatment using combination therapy has been demonstrated, as was an association between appropriate therapy and improved survival. However, direct comparisons of combination therapy with monotherapy generally demonstrate insignificant differences with respect to mortality in populations with widely varying severity of illness [23, 51, 52]. A plausible explanation for this finding is that comparative studies have excluded patients and/or have not controlled for patient risk for infection caused by antibiotic-resistant pathogens. Two recent publications comparing combination and monotherapy in patients with septic shock suggest a survival benefit for combination therapy [30, 53].
In theory, combination therapy should improve the probability of meeting the desired PK/PD end point via additive pharmacodynamic interactions. For antibiotics given at dosing intervals within 3–4 half-lives (as with many β-lactams and fluoroquinolones), there is general concordance with attainment of PK/PD targets, particularly the ratio of the area under the inhibitory curve to the MIC. Thus, in cases in which the infecting organism demonstrates an elevated MIC for a single antibiotic, such that the PK/PD end point would not be met, the addition of a second agent would aid in overcoming the deficit (Table 6) [54].
Table 6.
Additive Pharmacodynamic Interactions
| [53] | MIC, μg/mL |
AUIC24 |
|||
| Organism | Ciprofloxacin | Piperacillin | Ciprofloxacin | Piperacillin | Both |
| Pseudomonas aeruginosa | |||||
| Strain 1 | 0.5 | 6 | 70 | 226 | 296 |
| Strain A | 0.25 | 12 | 140 | 113 | 253 |
| Enterobacter cloacae, strain 1 | 0.25 | 48 | 140 | 28 | 168 |
NOTE. AUIC24, area under the inhibitory curve over 24 hours; MIC, minimum inhibitory concentration.
Grading of Evidence and Level of Support
Based on a review of the literature cited above, 4 members (80%) of the Summit workshop considered the evidence to support statement 2 to be from well-designed cohort or case-controlled studies. One member (20%) voted that the evidence was obtained from case series, case reports, or flawed clinical trials. All members of the workshop voted to accept statement 2, 40% (2 of 5) with some reservations, 60% (3 of 5) with major reservations. In comparison, of the 138 infectious disease and critical care physicians who participated in the email survey, 69% voted to accept statement 2 (completely [10%]), with some reservations [37%], or with major reservations [22%]). In contrast to the Summit members, 30% of survey voters rejected the statement either with reservations (23%) or completely (7%) (Figure 3).
Figure 3.
Level of support for statement 2: Pharmacokinetic/pharmacodynamic optimization of antibiotics with Gram-negative activity can overcome resistance associated with multidrug-resistant Gram-negative bacteria.
Discussion
The clinical impact of PK/PD optimization for the treatment of Gram-negative infections using extended or continuous infusions of β-lactams or combination therapy with agents having Gram-negative activity has yet to be fully realized. This is probably not because of failure of the science but because of the lack of study in patients in whom these dosing strategies will be of greatest benefit. For example, in the prospective, randomized trial by Chastre et al (previously reviewed) that compared extended-infusion doripenem with imipenem, the overwhelming majority of isolates (94%) had low MICs [49]. The retrospective, multisite comparison by Patel et al evaluating 4-hour piperacillin-tazobactam infusions versus traditional bolus doses included only a small sample of patients overall and an even smaller group infected with a Gram-negative pathogen for which the MIC was elevated [46]. In addition, the majority of patients in the trial had some degree of renal dysfunction.
In contrast, the evaluations revealing the potential benefits of PK/PD optimization may have included patients with the right mix of ideal characteristics. The retrospective evaluations of continuous β-lactam infusions conducted by Lorente et al included only patients with estimated creatinine clearance values of ≥60 mL/min [42–44]. In addition, it is possible that the patients in the continuous infusion group were more likely to be at risk for infection with an antibiotic-resistant pathogen, because the method of infusion was left to the discretion of the treating physician. Likewise, the benefit of combination therapy in the recent studies by Kumar et al transcend the concept of improved probability of administering initially appropriate therapy because this was controlled for in the analysis and may point to other factors, such as PK/PD optimization in patients with septic shock [30, 53].
There was a difference between the Summit members and the survey participants with regard to acceptance of statement 2 because the latter group had a faction of voters who rejected the statement. Certainly, the literature supporting improved clinical outcomes with PK/PD optimization for the treatment of antibiotic-resistant Gram-negative infections is in its infancy and perhaps not as well known to a diverse survey population. In addition, the statement may have been interpreted through the myopic lens of individual practice sites; it is probably different for each clinician based on the resistant pathogens frequently encountered in his or her own practice. In general, the literature in this field has focused on P. aeruginosa infections and has limited evaluation for the treatment of other MDR pathogens, including Acinetobacter species, Enterobacter species, and K. pneumoniae.
Future Directions
Currently, many clinical practices have implemented strategies designed to optimize PK/PD end points as standards of care for the treatment of antibiotic-resistant Gram-negative infections. To fully recognize the theorized benefit, studies should be conducted in patients in whom these dosing strategies are likely to be of greatest benefit, including those infected with pathogens for which MICs are elevated, those with preserved renal function, and/or those with altered volumes of drug distribution.
STATEMENT 3: STRATEGIES TO LIMIT ANTIBIOTIC EXPOSURE, SUCH AS SHORTER COURSES OF ANTIBIOTICS, ATTENUATES THE EMERGENCE OF RESISTANT GRAM-NEGATIVE BACTERIA
Rationale and Definition of Statement
As other authors in this supplement have noted, antibiotic resistance, particularly among GNB, continues to present a serious challenge. Beyond the historic issues related to both P. aeruginosa and A. baumannii, we now must clinically combat ESBL organisms. Furthermore, recent studies have described the evolution and spread of carbapenamase-producing bacteria [55, 56]. Such bacteria are essentially resistant to all common antimicrobials. Because resistance among GNB is now routine and because these organisms cause not only nosocomial infections but also health care–associated infections, the potential morbidity and mortality related to infections caused by these pathogens may be substantial. In any event, a higher prevalence of resistance increases the potential for physicians to prescribe inappropriate initial therapy, increasing the risk for worse outcomes.
Many options exist to help stem the tide of resistance. Limiting the spread and impact of antimicrobial resistance, irrespective of pathogen type, requires a multifaceted approach. The need to contain the spread of antimicrobial resistance is even more pressing because few new antibiotics for these pathogens are likely to become commercially available [2]. Although both prevention and traditional measures of infection control (IC) are crucial, unique aspects of antimicrobial stewardship programs are likely to be equally important. For example, one key component focuses on limiting the duration of antibiotic treatment [57]. Historically, physicians prescribed antibiotics for a range of infections for durations up to and even exceeding 14 days. This unnecessarily added to the tonnage of antibiotics administered in hospitals. As a result, many came to believe that shortening the duration of antibiotic treatment may lead to less resistance.
Dennesen and colleagues conducted one of the initial analyses connecting the duration of treatment with resistance [58]. In a prospective report describing 27 patients with bronchoscopically confirmed VAP, these investigators monitored the time to the resolution of signs of infection. Subjects received a median of 13 days of antibiotic therapy. In 6 subjects (22%), a new infection developed, and in half of these patients, the resulting pathogen (P. aeruginosa in all cases) was resistant to all antibiotics previously received. The rate of emergence of resistance and superinfection did not begin to climb until approximately day 6. Beyond day 6, new GNB resistance increased substantially. Although limited because of its single-center design, this study is unique in that it clearly showed how resistance evolved in patients being actively treated for infection as a function of ongoing antibiotic exposure. The authors specifically concluded that “tracheal colonization with resistant pathogens frequently occurs during the second week of therapy” [58]. They speculated that a shorter duration of antimicrobial therapy may “reduce the risks of colonization and subsequent infection with more resistant pathogens” [58].
The proposed statement linking antimicrobial treatment duration with resistance reveals several important points. First, resistance can evolve in a patient receiving therapy, and the term can be used to describe the spread of resistance to other patients. Resistance developing in a patient that results in a new infection (ie, a superinfection) often leads to devastating consequences. The spread of resistance from one subject to another can similarly result in adverse outcomes. For this statement, both aspects of the spread of resistance were explored.
Second, the extension of resistance to other persons beyond the index subject can promote resistance disproportionately in different settings within the hospital. Often, resistance rates and antibiograms are developed to reflect patterns seen either in selected areas such as the ICU or for the entire institution. Therefore, the statement requires one to look at the impact of shorter courses of therapy on resistance both in and outside the ICU. Conversely, the inability to detect a difference in resistance rates across a hospital does not preclude the possibility that such differences do emerge and exist only in the ICU.
Third, many factors affect antimicrobial resistance rates. Because many potential confounding factors are at play, it may be hard to assess whether and how shorter courses of antibiotic treatment alter resistance. The baseline endemicity of resistance, compliance with IC practices, and patient case mix all contribute to one’s understanding of resistance and its evolution among GNB. Therefore, it seems likely no single intervention, such as using shorter courses of therapy, will dramatically alter resistance rates. Furthermore, resistance patterns develop and change over time (measured in years), while most studies and trials of antibiotic treatment durations occur over a much shorter time frame. This fact, again, makes assessing the nexus between duration of therapy and global resistance patterns difficult. One must be cautious not to commit the logical misstep of concluding that shorter courses of antibiotic treatment do not lead to less resistance if the data do not conclusively demonstrate this. It may be that the correlation is not yet as firmly established as we may have hoped.
Literature Search
A PubMed database search was conducted on 4 September 2010 to identify studies relating to duration of antibiotic therapy and resistance to GNB. A search using text words “antibiotic duration” AND “resistance” yielded 1803 citations. The results of this search were then combined using the word “AND” with the text word “gram negative” (206 articles). The vast majority of these articles represented case reports and case series. Three articles from this search were deemed relevant to the statement. The text words “short course,” “antibiotics,” and “resistance” also were combined in a search. Of the 329 articles noted, 19 remained after restricting results with text word “gram negative.” After review, none of the resulting articles were deemed relevant to the Summit statement. In addition, “antibiotic stewardship” as a search term resulted in 187 articles, but unfortunately, none of these studies bore directly on the statement after reviewing for GNB. Of note, there were no well-conducted randomized trials of “antibiotic stewardship” to include in the Summit discussion. “Procalcitonin” and “resistance” as text words resulted in 24 articles. Eight studies were deemed relevant to the statement and are included in the Summit discussion.
Evidence
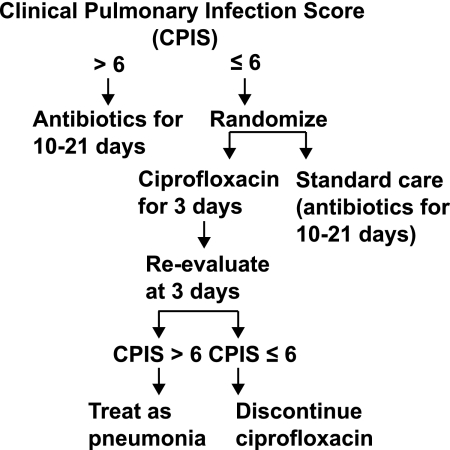
Singh et al conducted the first randomized trial to test the hypothesis that shorter durations of antibiotic treatment can result in less GNB resistance [59]. These investigators employed a complicated design in a group of patients at low risk for VAP (see Figure 4). They enrolled 81 subjects who were being treated for VAP in the neurosurgical ICU and had clinical pulmonary infection scores (CPISs) of ≤6. In the control arm, patients were treated with antibiotics for 10–21 days, based on physician preference. In the intervention arm, subjects initially received 3 days of ciprofloxacin. If by day 3 the CPIS remained ≤6, treatment was stopped. If the CPIS increased, the patient received a longer duration of treatment. There was a trend toward a decrease in mortality with the short-course approach. Antibiotic resistance and superinfection rates fell substantially in the intervention group. More than one-third of persons in the control arm had a resistant infection or superinfection, compared with only 14% in the intervention cohort. The impact of the short-course treatment strategy reduced both Gram-positive and Gram-negative resistance (Table 7).
Figure 4.
Flow chart representing study design. Reproduced with permission from Singh et al [59].
Table 7.
Antimicrobial Resistance and Superinfections in the Experimental and Standard Therapy Groups
| Variable | Experimental therapy, % (No.) | Standard therapy, % (No.) |
| Antimicrobial resistance and/or superinfectionsa | 14 (5/37) | 38 (14/37) |
| Microorganismsb | ||
| Pseudomonas aeruginosa | 8 (3/37) | 16 (6/37) |
| Enterobacter cloacae | — | 5 (2/37) |
| MRSA | 5 (2/37) | 14 (5/37) |
| Pseudomonas cepacia | 3 (1/37) | — |
| Citrobacter freundii | — | 3 (1/37) |
| Pseudomonas stutzeri | — | 3 (1/37) |
| Enterococcus species | 3 (1/37) | 11 (4/37) |
| E. faecalis | 3 (1/37) | 8 (3/37) |
| Vancomycin-resistant E. faecium | 0 (0/37) | 3 (1/37) |
| Candida species | 8 (3/37) | 14 (5/37) |
| C. albicans | 8 (3/37) | 8 (3/37) |
| C. glabrata | 0 (0/37) | 5 (2/37) |
NOTE. Table 7 represents the percentage and number of patients (n/N) with documented antimicrobial resistance and/or superinfections, broken down by the associated microorganism in the experimental versus standard therapy groups. Reprinted with permission from Singh et al [59]. MRSA, methicillin-resistant Staphylococcus aureus.
Patients who died <7 days after study entry were excluded from this analysis. P = .0177 for comparison of therapy groups.
Patients may have >1 microorganism.
It is important to note the many limitations of this study that constrain its helpfulness for addressing the statement. First, the study was not blinded. The authors note that during the course of the trial the average duration of antibiotic treatment for VAP in the ICU from which patients were enrolled fell. The practice started to change either directly or indirectly as a result of the intervention protocol. This confounds the ability to conclude that the study protocol per se led to the results described. As noted earlier, many variables affect resistance rates, and one cannot be sure that other antibiotic and IC practices were not being altered concurrent with the conduct of this study.
Second, it is unclear whether the authors truly investigated VAP. Although the CPIS has drawbacks as a diagnostic test for VAP, a CPIS of ≤6 almost certainly precludes a diagnosis of VAP [60]. In other words, many of the patients enrolled simply did not require treatment at all and probably would have done well with serial observation. Therefore, it would be more appropriate to consider this an analysis of means to contain “unindicated” antibiotic exposure rather than a true study of short-course therapy in a documented infection.
Third, the control arm in the trial received a rather long duration of therapy. Allowing up to 21 days of treatment certainly artificially increases the likelihood that resistance will develop. A more proper comparison would have limited the control arm to ≤14 days of therapy. Finally, the focus of the study was resistance in the study subject. No data were provided regarding how this protocol affected resistance rates generally in the ICU or the spread of nosocomial infections with resistant GNB to nonstudy patients.
Although theirs was not a randomized trial, Ibrahim et al conducted a prospective observational study of short-course treatment for VAP [61]. The primary objective of this analysis was to determine whether an approach implementing a deescalation strategy for antibiotic prescribing would help increase rates of initially appropriate antibiotic therapy while simultaneously allowing more limited durations of treatment. The cohort comprised 102 patients with microbiologically confirmed VAP. In the preprotocol period, nearly half of the subjects received initially inappropriate therapy, and the median duration of treatment approached 14 days. After implementation of the protocol, nearly all were treated appropriately, and the average antibiotic exposure was cut by nearly half. Despite fewer days of treatment, there was no significant difference in mortality. Episodes of superinfection, however, were reduced from 24% in the before period to 7.7% in the after period (P = .03). Given the general microbiology of VAP, one would presume this meant fewer new infections with highly resistant GNB. Unfortunately, the authors presented no specific data to confirm this belief.
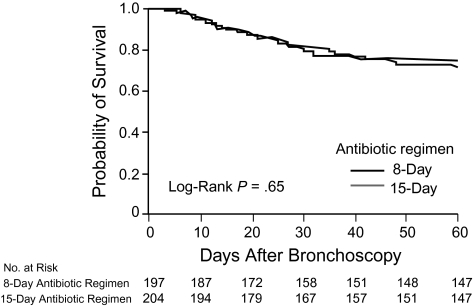
Multiple additional studies have addressed shorter courses of therapy. None, however, have reported data specifically on the issue of subsequent antibiotic resistance with respect to either superinfection in the same patient or general patterns of antimicrobial resistance in the hospitals and ICUs evaluated. Chastre et al, for example, completed a landmark multicenter, partially blinded, randomized trial of 8 versus 15 days of antibiotic treatment for VAP [62]. Among 401 patients with VAP, crude mortality and relapse rates were similar for persons randomized to 8 days of antibiotics and those allocated to the 15-day treatment arm (Figure 5). There were more recurrences in the subgroup of patients with non–lactose fermenting GNB, but no excess mortality in these patients. Emergence of resistance was not monitored as an end point.
Figure 5.
Kaplan-Meier estimates of the probability of survival for 8 versus 15 days of antibiotic therapy. The probability of survival is for the 60 days after ventilator-associated pneumonia onset as a function of the duration of antibiotic administration. Reproduced with permission from Chastre et al [62].
One novel approach for shortening and individualizing the duration of antibiotic treatment employs serial procalcitonin (PCT) monitoring. As a precursor to calcitonin, PCT is up-regulated in severe sepsis and bacterial infections, making it a possible biomarker for guiding therapy [63]. Recently, several trials have shown that antibiotic prescribing protocols directed by PCT levels result in shorter durations of antibiotic exposure [63, 64]. Some of these studies have enrolled a broad array of patients, including persons with community-acquired pneumonia or bronchitis, whereas others have been more limited to those who are critically ill. Bouadma and colleagues completed a multicenter, randomized trial in ICU patients to determine whether a PCT-guided algorithm resulted in shorter courses of antibiotic therapy [64]. For those whose antibiotic course was not guided by PCT levels, the physician determined the course of treatment. The treating physician for the non-PCT arm was encouraged to follow published guidelines to set the course of antibiotics. Among 621 subjects, 60-day mortality rates were similar for the 2 approaches. With PCT, antibiotic treatment duration was reduced by approximately 3 days. Unlike Ibrahim et al, these investigators specifically sought evidence of emergence of resistance. Nonetheless, they could not demonstrate that a shorter course of treatment resulted in fewer cases of infection with resistant pathogens. The authors acknowledge this fact and suggest that this failure to detect a difference may indicate that screening fornew resistant organisms was somewhat inconsistent across sites. They also note a 3-day reduction in antibiotic exposure may not be an extreme enough decline to affect rates of resistance.
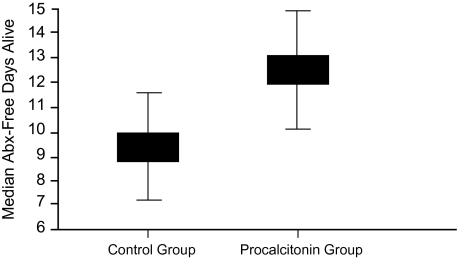
Stoltz et al further provided confirmatory evidence of PCT as an antibiotic containment tool [63]. Unlike Bouadma and colleagues, these researchers limited their study of PCT to patients with VAP. Again, patients were randomized to have their antibiotic therapy determined based on either serial PCT measurement or physician discretion. In the PCT group, antibiotics were withheld if the initial PCT level was low and were terminated when the PCT value had fallen substantially from its initial measure. In the control arm, antibiotics were to be stopped as recommended by the current Infectious Disease Society of America/American Thoracic Society guideline. The final study included 101 patients. The number of antibiotic-free days alive (ie, in patients alive within 28 days of inclusion in the study, the number of days without antibiotics) represented the primary end point rather than a simple comparison of durations of treatment. Given the high mortality rate in VAP, it was necessary to adjust for how the timing of death would alter the duration of antibiotic exposure. In the end, the PCT paradigm resulted in more antibiotic-free days (Figure 6).
Figure 6.
Number of antibiotic-free days alive for procalcitonin vs control groups, shown as medians 28 days after onset of ventilator-associated pneumonia (P = .049). Reproduced with permission from Stolz et al [62].
The magnitude of difference in overall median antibiotic treatment duration was similar to that reported by Bouadma et al [64]. Strikingly, even though physicians in the control arm were to follow guideline recommendations for treatment duration, the median duration of antibiotic treatment in this group was much longer than the suggested 8–10 days. This relatively long duration of treatment in the control group was also noted in the study by Bouadma and colleagues. One interpretation of these analyses of PCT-guided therapy is that physicians simply fail to follow guideline recommendations, even though the PCT measurement within itself results directly in less exposure to antibiotics. Irrespective of the explanation, Stolz et al failed to report information on resistance patterns either in the enrolled patients who developed new infections or in the ICUs that participated in the trial [63]. Hence, this analysis and similar reports of PCT-guided treatment document shorter courses of therapy are achievable without compromising patient safety. They do not, however, provide direct evidence that limiting antibiotic exposure in this way leads to fewer cases of infections with resistant bacteria.
A recent systematic review and meta-analysis confirms that few studies of PCT utilization have explored resistance and superinfection as end points. Kopterides et al identified 7 randomized trials exploring PCT utilization in the ICU. The population included 1131 subjects and encompassed medical and surgical patients [65]. As was seen in the reports by Stolz et al and Bouadma et al, patients whose treatment was based on a PCT approach received ∼3 fewer days of antibiotics than those treated conventionally. There was no difference in mortality between the PCT and control cohorts. Additionally, PCT-based approaches were not associated with fewer days of mechanical ventilation, more rapid discharge from the ICU, or shorter hospital stays. In summary, the only benefit with respect to resource use appeared to be related to fewer antibiotic treatment days. The key potential benefit of needing fewer days of antibiotic therapy—namely fewer superinfections or relapsed infections—was systematically reported in only 3 of the 7 studies in the meta-analysis [65]. PCT did not alter rates of either of these events, suggesting that even in a larger population the theoretical benefit of PCT for containing antimicrobial resistance may be quite small.
The limited data demonstrating the nexus between antibiotic duration of therapy and subsequent emergence of resistance indicate that this entire concept may be either incorrect or much more complicated than we currently understand. Donaldson and colleagues provide some indirect evidence questioning the connection between the duration of a course of treatment and resistance [66]. These authors prospectively evaluated 415 critically ill subjects, all treated with a carbapenem (either imipenem-cilastatin or meropenem). The development of a nosocomial BSI with a resistant GNB pathogen or MRSA represented the primary end point. A new nosocomial BSI occurred in 7.5% of the cohort. Age, ventilation >96 hours, hospital length of stay, and a diagnosis of cancer correlated with an increased risk for a nosocomial BSI with a resistant organism (using a Cox proportional hazards model). The duration of carbapenem exposure, however, was not related. The investigators concluded that factors other than duration of treatment are probably more important in driving rates of antimicrobial resistance. Reinforcing this hypothesis was the link observed between overall duration of hospitalization and BSIs. It may be that general exposures in the hospital (eg, baseline density of resistance, IC or lack thereof) are more significant variables that alter resistance rates.
Grading of Evidence
Based on a review of the studies cited above, 1 Summit faculty member (20%) considered the evidence available to support statement 3 to be category I (evidence obtained from at least 1 well-designed, randomized, controlled trial) and 20% considered it to be category II (evidence obtained from well-designed cohort or case-controlled studies). Three (60%) faculty members considered the evidence to be category III (evidence obtained from case series, case reports, or flawed clinical trials) and none considered it to be category IV (opinions of respected authorities based on clinical experience of descriptive studies or reports of expert committees) or V (insufficient evidence to form an opinion).
Level of Support
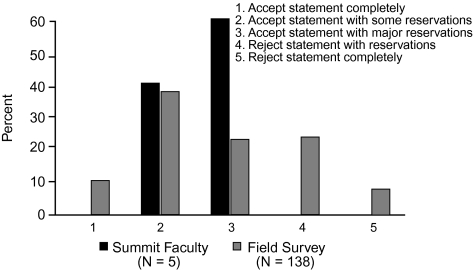
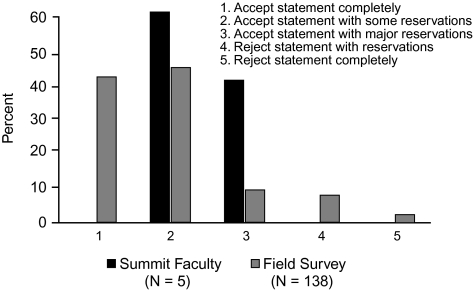
When voting on the support for statement 3, none of the Summit participants voted to accept the statement completely, 60% voted to accept it with some reservations, 40% voted to accept it with major reservations, and none voted to reject it. In comparison, of the 138 infectious disease and critical care physicians who participated in the online survey, 41% voted to accept the statement completely, 44% voted to accept it with some reservations, 8% voted to accept it with major reservations, 6% voted to reject the statement with reservations, and 1% voted to reject it completely (Figure 7).
Figure 7.
Level of support for statement 3: Strategies to limit antibiotic exposure, such as shorter courses of antibiotics, attenuates the emergence of resistant Gram-negative bacteria.
Discussion
The general trend of support seems to be that participants in the field survey are more skeptical of the statement than those participating in the Summit. This probably reflects a general belief in the statement; however, after reviewing the data more closely, Summit participants became more doubtful. Summit participants came to appreciate the limits of the literature better after more careful consideration. Nonetheless, general reaction to the statement by Summit faculty and field survey respondents was positive.
Clearly, multiple studies documenting shorter durations of antibiotic therapy are appropriate and achievable, even in the most severely ill patients. Antibiotic (mis)use can be successfully contained through reliance on clinical scoring tools, adherence to recommendations from evidence-based national and international treatment guidelines, and/or PCT monitoring. Without question, we as clinicians have been overexposing patients to antibiotics. Moreover, shorter courses of treatment do not compromise important clinical outcomes, such as mortality and length of stay. It remains less certain, however, that limiting antibiotic treatment duration represents an important tool in the continuing battle against antimicrobial resistance. Although not a focus of this review, the association between shorter courses of treatment and the prevention of Clostridium difficile colitis is more evident [67]. In that sense, continuing to stress the use of shorter courses of antibiotics remains an important endeavor. Similarly, antibiotics disproportionately contribute to hospital pharmacy costs. In turn, curtailing durations of therapy can help save resources.
Clinical trials and observational studies attempt to minimize the effect of various potential confounders via randomization or statistical modeling. The point is to link an intervention causally with a specific outcome. However, when one is dealing with issues of antimicrobial resistance, many variables tend to come into play that either (1) cannot be measured directly at all or (2) are difficult, if not impossible, to control.
Similarly, factors outside the conduct of any single study likely contribute to antimicrobial resistance. For example, the rate of resistant infections presenting to the emergency department from either the community or nursing homes certainly affects the prevalence rates of resistance while concurrently representing a reservoir for potential pathogens that might spread to other patients. No effort to shorten the duration of antibiotic exposure in the hospital will change this fact, nor will it modify it if resistance is already endemic in an institution. Likewise, limiting the duration of treatment with antibiotics will not balance or prevent the deleterious effects of IC failure. The association between antibiotic exposure and resistance in the hospital, furthermore, may be driven more by inappropriate administration of prophylactic antibiotics or by failure to address preventive options.
Prevention of infection has the added benefit of possibly eliminating an entire course of treatment and thus limiting colonization pressure within the patient, which in turn can limit both documented infections and the spread of infection to others. No single clinical study can hope to control for each of these major confounders. Underscoring the potential complexity in appreciating drivers of resistance within hospitals, D’Agata and colleagues developed a mathematical model to try to capture this dynamic process [68]. Input data were required for >15 different variables, along with complicated differential equations. Only one of these input factors was the duration of antimicrobial treatment. Therefore, the expectation that conventional approaches to clinical trials will help us to appreciate the connection between antibiotic duration of treatment and resistance may be misplaced. This is discussed in more detail in the next section (Future Directions).
In summary, there seems to be scant to limited evidence that shorter courses of antibiotic therapy contain or decrease the spread of resistant pathogens. There are many reasons to adopt treatment strategies relying on limited treatment durations. However, one should not expect this approach in isolation to substantially alter resistance patterns currently noted in hospitals.
Future Directions
The reasons why studies have failed to note differences in rates of antimicrobial resistance to shorter courses of antibiotics can be used to suggest changes in research methods in this area. First, few reports have systematically examined emergence of resistance as an end point. In the same manner, when resistance has been recorded as an end point in studies, the emphasis has been in terms of superinfection and not overall resistance rates in the participating hospital. Perhaps, shorter courses of therapy contain or help contain resistance, especially among GNB; we just have not looked deep enough to conclusively answer the question. The “absence of proof” does not constitute “proof of an absence,” and further analyses will be required to either demonstrate or refute a link between antimicrobial treatment durations and resistance.
Second, the true impact of shorter courses of therapy may take time to become apparent. Shortening the duration of treatment represents a relatively novel concept. For it to alter rates of resistance, this change in practice pattern must be in place perhaps for years. This point is particularly valid given that many investigations of shorter treatment courses have followed up subjects for only 90 days at most. Third, the absolute reduction in days of therapy achieved when shorter courses of antibiotics are administered may be insufficient to alter resistance patterns. With either arbitrary cutoff points after a week of therapy or PCT-guided alternatives, one reduces antibiotic exposure anywhere from 3 to 7 days. It may be more important to eliminate any antibiotic exposure rather than shorten the course of treatment in order to produce a measurable impact on resistance. Many of the randomized trials of more limited treatment durations have not specified the antibiotic regimen, thereby leaving the decision to the treating clinician.
Different antimicrobials may drive resistance in different ways. For example, some have concluded that quinolone utilization relative to that of other anti-infectives alters rates of MRSA [69]. Thus, the central issue may not be about administering antimicrobials for fewer days but rather may revolve around the use of specific antibiotics or classes of antibiotics for fewer days. The assumption in many trials of shorter courses of treatment has been that all anti-infectives exert a similar impact on resistance. This belief is probably false, and future research in this area should reflect this.
STATEMENT 4: ACTIVE SURVEILLANCE OF MDR GNB WITH ISOLATION SHOULD BE AN ACTIVE COMPONENT OF INFECTION CONTROL BUNDLES TO PREVENT THE PROLIFERATION OF MULTIDRUG-RESISTANT GRAM-NEGATIVE BACTERIA
Rationale and Definition of Statement
Antimicrobial resistance has emerged as an important determinant of outcome for patients in the ICU [70, 71]. Certain groups of pathogens have an increased risk of developing antimicrobial resistance. The emergence of antimicrobial-resistant pathogens makes the treatment of infections more difficult and in some instances even impossible. The frequency of antibiotic-resistant health care–associated infections has increased during the past 3 decades, but some pathogens are highly recognized as MDR.
A recent multinational study known as Extended Prevalence of Infection in Intensive Care (EPIC II) included 13796 adult patients from 1265 ICUs in 75 countries and found that half of the patients were infected while in the ICU, and 71% were receiving antimicrobial agents [72]. The most common pathogens causing infection in ICU patients were the Gram-negative bacilli. Some of these are highlighted as the most important MDR pathogens, including microorganisms such as P. aeruginosa, Acinetobacter spp, and ESBL-producing Escherichia coli and Klebsiella spp [50]. In addition, the MDR GNB causing severe infections (eg, hospital-acquired infections, including pneumonia, BSIs, and sepsis) are associated with increased morbidity, prolonged length of hospitalization, increased health care costs, and increased mortality [9, 10, 12, 19, 56, 61, 73–77]. This is why it has been suggested that surveillance and isolation are important measures for controlling the proliferation of MDR GNB. Therefore, the focus of this statement includes relevant reports of studies that have performed surveillance or in which patients were placed in contact isolation as part of the IC bundle as a preventive package for MDR GNB.
Literature Search
A PubMed database search to locate studies related to GNB likely to be MDR and the need for surveillance and isolation was completed on 17 October 2010. The use of a combined strategy using the word “OR” to include highly resistant GNB manuscripts included the following terms: “gram-negative bacteria,” “Enterobacteriaceae,” “E. coli,” “gram-negative bacilli,” “multidrug-resistant,” “Pseudomonas infections,” “A. baumannii,” “Acinetobacter infections,” “Enterobacteriaceae infections,” “Klebsiella infections,” “beta-lactamases,” and “ESBL” yielded 726,062 articles on MDR GNB. The result of this search was then combined using the word “AND” with the following text words: “surveillance” (6982 articles) and “isolation” (371 articles), and limited to studies in English involving humans (271 articles). Abstracts were reviewed for 271 articles, and 37 articles were reviewed in full, with a final selection of 11 articles relevant to the statement [78–88].
Evidence
Based on prior literature with MRSA and VRE, stratification based on the most common MDR GNB identified the 3 most important groups of GNB to focus on for review related to statement 4: P. aeruginosa, Acinetobacter spp, and ESBL-producing bacteria.
Pseudomonas Infections
Only one article on Pseudomonas infections was identified; this study focused on a nosocomial outbreak by alginate-producing panantibiotic-resistant P. aeruginosa, using a matched case-control study design over a 7-month period (November 2004 through May 2005). Yakupogullari et al identified 35 panantibiotic-resistant P. aeruginosa isolates in 28 patients and 7 from environmental surveillance [78]. A control group was obtained from a retrospective review matching for age, sex, mean length of hospital stay, APACHE II scores, and risks/comorbidity variables. The interventions introduced during the outbreak included contact precautions, hand disinfection, use of gloves and gowns, contact isolation, masks, and eye protection. Two months after the intervention was introduced in response to the outbreak, there was a decreased incidence in the number of cases after the initiation of strict contact precautions. However, this study was limited by the description and implementation of the intervention to only 1 site location, thereby decreasing a measurable association of response.
Acinetobacter Infections
Four studies were identified that involved Acinetobacter infections. Rodriguez-Bano et al [79] assessed a multifaceted intervention for long-term control of hospital-wide endemic MDR A. baumannii. The study used a quasi-experimental design in a 950-bed, tertiary acute care hospital. The authors compared 3 periods: preintervention (2 years, 1994–1995), immediate postintervention (2 years, 1996–1997), and late postintervention (6 years, 1998–2003). The intervention included an IC bundle consisting of staff education, promotion of hand hygiene, contact and isolation precautions, and environmental cleaning. In addition, targeted surveillance was performed if the subjects were in the ICU for >2 days, with weekly cultures during periods of transmission (new cases). Before the bundle was implemented, the rate of colonization/infection was 0.82 cases/100 admissions. However, the colonization/infection rates in the immediate postintervention and late postintervention periods showed a sustainable decrease to 0.46 and 0.21/100 admissions, respectively (P < .001). Coincident with the institution of the IC program, the rate of bacteremia decreased 6-fold during the 8-year observation period.
Mastoraki and colleagues [80] assessed a prevention strategy for MDR A. baumanii susceptible only to colistin. During an outbreak, a 16-month prospective observational cohort study was performed in a surgical ICU. The 2-scale program included scale 1 (entire 16 months), with standard IC (eg, contact and droplet isolation, antibiotic policy, patient transfers from other hospitals cultured for MDR GNB), and scale 2 (2 periods of 3 weeks), with intermittent isolation and surveillance. Among 151 of 1935 infected patients, 20 were colonized or infected with strains of MDR A. baumannii susceptible only to colistin. Intermittent eradication of MDR A. baumannii was achieved during the intervention period after introduction of the IC measures (ICMs).
A study by Marchaim et al [81] focused on surveillance cultures and duration of carriage of MDR A. baumannii by assessing prospectively a surveillance sensitivity study in a 1200-bed hospital. The authors used ICMs and identified previous (≥6 months) and new (≤10 days) carriers. The sites cultured included nostrils, pharynx, skin, rectum, wounds, and endotracheal aspirates. The study identified 12 of 22 new carriers with ≥1 positive culture, with a sensitivity of 55% when 6 body sites were cultured. In addition, screening cultures were positive in 5 of 30 patients who were previous carriers (17%), with mean duration of 17.5 months from the last culture. Single-site sensitivity ranged from 13.5% to 29.0%, and previous carriers had positive screening cultures from the skin or pharynx but not from the nose, rectum, wounds, or endotracheal aspirates. Therefore, current methods to detect MDR A. baumannii carriage are suboptimal, and persistent carriage occurs in a substantial proportion of patients.
Finally, one of the best study designs in this review was reported by Apisarnthanarak et al from Thailand. This study assessed the value of MDR Enterobacteriaceae surveillance as an effective IC strategy in the absence of an outbreak, focusing on reducing pandrug-resistant A. baumannii colonization [82]. This study was a 3-year prospective, controlled, quasi-experimental study conducted in the medical, surgical, and coronary ICUs for a 1-year period before intervention (period 1), after intervention (period 2), and for a 1-year follow-up period (period 3). The interventions included contact isolation, appropriate hand hygiene, active surveillance (cultured on admission to ICUs), cohorting of colonized patients, and change of environmental cleaning solutions. The multifaceted intervention featuring active surveillance and environmental cleaning resulted in sustained reductions in the rates of pandrug-resistant A. baumannii colonization and infection from 3.6 cases/1000 patient days to 1.2 (period 2) and 0.85 (period 3) cases/1000 patient days (P < .001), respectively (Figure 8). In addition, the monthly hospital antibiotic cost of treating pandrug-resistant A. baumannii colonization and/or infection and the hospitalization cost for each patient in the intervention units also were reduced by 36%–42% (P < .001) and 25%–36% (P < .001) during periods 2 and 3, respectively.
Figure 8.
Interventions to reduce pandrug-resistant Acinetobacter baumannii colonization. Rates of pandrug-resistant A. baumanii infection and colonization are shown for 3 intensive care units. Period 1 was the baseline period (1 January 2005 through 31 December 2005), period 2 the intervention period (1 January through 31 December 2006), and period 3 the follow-up period (1 January through 31 December 2007). Reproduced with permission from Apisarnthanarak et al [82].
ESBL Infections
Kochar et al [83] assessed the effect of enhanced ICMs in a medical/surgical ICU, specifically screening for gastrointestinal colonization and limiting the spread of carbapenem-resistant K. pneumoniae. In the 2-year retrospective observational study, the interventions included routine rectal surveillance, contact isolation, and decontamination of hands and environmental surfaces. If patients were colonized or infected with K. pneumoniae carbapenemase (KPC), VRE, or MRSA, they were placed on contact isolation and placed in a cohort at one end of the unit. The authors found a reduction in the mean number of new patients with cultures for carbapenem-resistant K. pneumoniae, but no change was observed in MRSA, VRE, carbapenem-resistant A. baumannii, or P. aeruginosa after the intervention. In addition, there was no association between antibiotic usage patterns and carbapenem-resistant K. pneumoniae rates.
A similar study by Souweine et al [84] assessed the role of ICMs in limiting morbidity associated with MDR organisms in critically ill patients. In this retrospective comparative study, colonization and infections due to MRSA, ESBLs, and MDR Enterobacter aerogenes were observed in ICU patients during 2 consecutive 1-year periods (before and after intervention). The IC bundle included the following: ICMs, surveillance cultures (admission, weekly during ICU stay, and at discharge), isolation, disinfectants, contact precautions, and education. ICMs were able to control infections and colonizations due to MRSA and K. pneumoniae ESBLs but not those with MDR Enterobacter aerogenes.
A 5-year surveillance (1996–2000) study in Northern France [85] attempted to determine the incidence and trends in an annual evaluation of MRSA and Enterobacteriaceae-producing ESBLs at 1182 hospitals after the introduction of national guidelines distributed in 1999. The control measures implemented identified reservoirs by detection, early notification, labeling carriers, and notification of carriage when patients transferred to another unit or hospital. Additional control measures included barriers to prevent colonization (antiseptic hand washing, gloves and gowns) and a systematic detection of carriers in high-risk areas such as the ICU, contamination, and control of antibiotic use. During the 5-year surveillance period, the overall incidence rates of clinical specimens positive for ESBLs K. pneumoniae and E. aerogenes were 0.05 and 0.12/1000 hospital days, respectively. In the 23 hospitals that participated in the survey every year, the incidence of ESBL bacteria decreased to 0.13/1000 hospital days (P = .06), whereas the MRSA incidence increased. Therefore, a positive effect was achieved with the introduction of isolation precautions only for the ESBL bacteria and not for MRSA, despite an initial decline in the incidence rate before the IC guidelines were initiated. This suggests that the intervention did not change the trend in the incidence of ESBL bacteria, and perhaps other analyses, including time series analyses, will more accurately support trend differences.
Munoz-Price et al [86] showed in a quasi-experimental design a successful control of KPC-producing K. pneumoniae at a long-term acute care hospital (70 beds) over a 1-year period (January through December 2008) after the identification of an outbreak. The IC bundle used to control the outbreak included daily 2% chlorhexidine gluconate baths for patients, enhanced environmental cleaning, surveillance cultures at admission, serial point prevalence surveillance, isolation precautions, and training of personnel. After implementation of the IC bundle in June 2008, monthly surveillance was performed 5 times, showing prevalences of colonization with KPC-producing isolates of 12%, 5%, 3%, 0%, and 0% (P < .001), a reduction from the baseline prevalence of 21%. The authors concluded that a bundled intervention that included ICMs was successful in preventing horizontal spread of KPC-producing GNB, despite ongoing admission of patients colonized with KPC producers.
Another study by Kola et al [87] quantified the rate of nosocomial infections and the extent of clonal transmission caused by ESBL-producing GNB after implementation of ICMs. The design was a 3-year observational study (1400-bed hospital), in which a surveillance program was implemented and positive samples of ESBL-producing K. pneumoniae, E. coli, or P. mirabilis were subsequently tagged and targeted. For subjects colonized or infected with ESBL-positive bacteria, barrier precautions were implemented, including the use of gloves and gowns and patient cohorts in private rooms, and screening was completed with perirectal cultures. Contact isolation precautions were carried out for 80% of the cases, with a median duration of contact isolation precautions of 14 days. The overall incidence of ESBL-producing GNB was 0.12/1000 patient days; 61.2% of ESBL-producing strains proved to be colonization, with only 7 patient-to-patient transmissions noted. In only 6.8% of cases (10 cases) was colonization cleared with ICMs.
Gardam et al [88] assessed the impact of routine surveillance for MDR Enterobacteriaceae on colonization/infection rates in solid organ transplant recipients. An 18-month, prospective cohort study was performed in 287 transplant recipients (in a single nursing unit) tracked for colonization/infection. The assessment of MDR Enterobacteriaceae resistance to third-generation cephalosporins was screened at admission, weekly for the first month, then every second week, and finally at discharge. The colonization status was not made known, no additional education or enforcement of IC policies was instituted, and colonized patients were not isolated. Standard precautions were used for open and draining wounds. Of 287 transplant recipients, 69 (24%) were colonized during the study period, 30 (43%) developed infections with drug-susceptible Enterobacteriaceae, and only 6 (9%) had MDR Enterobacteriaceae infections. However, the annual cost of a surveillance program was calculated at $1130184.44 (Canadian). Therefore, the authors concluded that routine and costly use of MDR Enterobacteriaceae surveillance and isolation precautions is not warranted in the absence of a clonal outbreak, even in this high-risk population of new organ transplant recipients.
Grading of Evidence
Based on a review of the studies cited above, 2 of the 5 Summit faculty members (40%) considered the evidence available to support statement 4 to be category II (evidence obtained from well-designed cohort or case-controlled studies). Three (60%) considered the evidence to be category III (evidence obtained from case series, case reports, or flawed clinical trials), and none considered it to be category IV (opinions of respected authorities based on clinical experience of descriptive studies or reports of expert committees) or category V (insufficient evidence to form an opinion).
Level of Support
When voting on the support for statement 4, none of the Summit participants voted to accept the statement completely, 40% voted to accept it with some reservations, 40% voted to accept it with major reservations, 20% voted to reject it with reservations, and none voted to reject it completely. In comparison, of the 138 infectious disease and critical care physicians who participated in the online survey, 51% voted to accept statement 4 completely, 29% voted to accept it with some reservations, 8% voted to accept it with major reservations, 9% voted to reject it with reservations, and 2% voted to reject it completely (Figure 9).
Figure 9.
Level of support for statement 4: Active surveillance of multidrug-resistant (MDR) Gram-negative bacteria (GNB) with isolation should be an active component of infection control bundles to prevent the proliferation of MDR GNB.
Discussion
As an initial step to examine this complex statement, it was discussed whether there is evidence that active surveillance of MDR GNB managed with contact isolation should be an important component of IC bundles to prevent the proliferation of MDR GNB. Two main questions should be discussed based on the studies reviewed. First, does active surveillance provide appropriate identification of MDR GNB, and, if so, which groups should be targeted? Second, does this identification require isolation to control the spread of MDR GNB?
Most, but not all, of the studies reviewed suggested that active surveillance was prompted by the presence of an outbreak. Active surveillance may identify patients colonized with MDR GNB who could potentially infect other patients. The problem is that a large number of patients colonized with MDR GNB are identified not by clinical cultures but rather by surveillance cultures. This issue has important implications. MDR GNB represents a diverse group of microorganisms with individual specific identification strategies and susceptibility patterns that are time consuming and without immediate results. Therefore, rapid testing could assist not only in the identification of highly resistant bacteria but also in the decision to isolate patients in a more timely manner than with standard culture techniques. In addition, the lack of sensitivity observed for the different body sites in which MDR GNB could be detected makes the situation even more complex [81].
Surveillance for MDR GNB may be effective in clonal outbreaks; however, it is unclear whether there is a role in the setting of endemic MDR GNB colonization, in which infection may result from endogenous flora rather than transmission from another patient. Active surveillance in the setting of endemic MDR GNB may provide little assistance in the control of MDR GNB and may prompt potentially unnecessary and costly contact precautions [87, 88]. Low incidence rates below 1/1000 patient days may not be enough to start an active surveillance program owing to the lack of a possible beneficial effect, particularly when the effects are not similar for all MDR GNB [79, 83–85, 87]. Another important issue regarding surveillance is the heterogeneity of the studied populations: ICU, long-term care, and hospitalized patients may not have the same risk factors for MDR GNB, and the implementation of contact isolation may vary from use in standard practice for all patients to use only in specific high-risk patients. Many patients may come from the ward service already colonized with MDR GNB and may begin to have clinical findings and positive culture isolates at the time of ICU admission.
Does the identification of MDR GNB require contact isolation to control the spread of MDR GNB? Results of several studies suggested that IC bundles including contact isolation may effectively reduce MDR GNB, but the results of these studies are conflicting. Furthermore, it is unclear which measures were effective. The benefit of the IC bundle (including contact isolation) in some studies is unclear; it is possible that hand hygiene compliance, for example, may have been the most relevant intervention. Therefore, these data do not fully support that contact isolation may improve outcomes or prevent infection with MDR GNB. A consistent association between increasing contact isolation precautions and an effective reduction in MDR GNB is lacking, based on the reviewed studies. In addition, similar analyses showed that the beneficial effects of IC bundles were not universal for all tested MDR GNB. Additionally, strategies using universal screening and standard IC strategies including contact isolation found contradictory results regarding MRSA infections [89, 90].
These intervention studies using ICMs, including contact isolation, are limited by the lack of randomized control trials; therefore, other unmeasured factors that may influence the results may be present at the time of the intervention. Other limitations include the lack of documentation of compliance with the IC bundle strategies and how frequently contact isolation was used. A possible publication bias in which only positive studies tend to be published cannot be excluded. It also is difficult to extract from these data the possibility that non-MDR GNB may become MDR during a patient’s hospitalization or treatment in clinical cases. Therefore, improved clinical outcomes and costs associated with the use of IC strategies may be difficult to measure.
Future Directions
For research in this field to advance, a better understanding is needed of how screening patients with new active surveillance methods (beyond obtaining cultures from several body sites) may help determine whether to add preventive strategies to the decision-making process. Current surveillance methods, driven by standardized bacterial culture identification and susceptibilities for diverse GNB, threaten to overwhelm and divert resources from microbiology laboratories, IC programs, and inpatient units. In addition, cost-effectiveness studies are needed in this area.
Preventive strategies including isolation as part of the bundle of preventive measures may have variable results, depending on the different clinical settings and patient populations, compliance issues, and resources. Nevertheless, several results deserve attention. When there is an established MDR GNB outbreak, screening is probably useful in high-risk units to identify the reservoir and to initiate contact precautions. However, whether screening should be more extensive and from multiple sites remains a matter of debate. Standard and contact precautions, as well as hand hygiene, are crucial to increasing compliance by health care workers and to successful control of MDR GNB. Finally, prospective cohort studies examining the benefit of surveillance for patients at risk outside of an outbreak event may lead to the implementation of novel and targeted preventive strategies, including contact isolation, which ultimately will improve outcomes for patients with or at risk for MDR GNB.
CONCLUSIONS
The ultimate goal for clinicians treating patients with serious infections is to provide effective, timely, and adequately administered antibiotic therapy to the patient while minimizing further emergence of antibiotic resistance. Unfortunately, the escalating development of antibiotic resistance among GNB decreases the likelihood that appropriate therapy will be initially delivered. This development also promotes the widespread empiric use of broad-spectrum antibiotics. Because IIAT is associated with increased mortality and overuse of antibiotics leads to increased antibiotic resistance, the current environment is something of a “perfect storm,” where antibiotic therapy is expected to both fail clinically and lead to further resistance. The ultimate fear is that we may be entering a preantibiotic era where therapeutic nihilism is increasingly common and accepted. The emergence of the New Delhi metallo-β-lactamase with its potential for widespread global dissemination within Enterobacteriaceae is a real threat for the occurrence of such a scenario [91].
The goal of the Gram-Negative Resistance Summit was to critically appraise the existing literature in order to assess the relative strengths and limitations of our current knowledge in this area. The review was particularly challenging given the changing landscape of antibiotic resistance among GNB and the multitude of bacteria and resistance mechanisms involved in this process. Overall, it is very clear that much is still unknown regarding every aspect of the 4 statements examined during the Summit. Additionally, the rapidly evolving playing field of GNB resistance suggests that many of the conclusions drawn by the Summit panel will require updating as new scientific information becomes available.
Several important issues related to the topic of GNB resistance were simply beyond the scope of this Summit, yet they deserve critical attention. These include the global problem of antibiotic use in agriculture, the widespread availability of generic antibiotics without adequate quality control, and the lack of penetration of antibiotic stewardship principles in many developing countries.
A recurring theme, regardless of which practice statement was being discussed, was the paucity of high-quality scientific data in support of or refuting the clinical statement. Agreement was reached that antimicrobial resistance is one of the world's most pressing public health problems. The worldwide use and misuse of antimicrobials in medicine and agriculture have resulted in the selection of bacteria resistant to the microbiologic activity of these agents. These resistant bacteria fail to respond to treatment, resulting in prolonged hospitalizations, increased costs, and greater risk of death. However, agreement on strategies for the optimal prevention and treatment of resistant GNB was more difficult to achieve. Given that extreme antibiotic resistance is a relatively new problem, there is room for debate regarding how to best define this problem. Factors to be considered include the prevalence of various antibiotic-resistant pathogens and their relative impact on patient and health care system outcomes, strategies for limiting the spread of such bacteria locally as well as globally, frameworks for optimizing the use of existing antimicrobial agents, and ways to promote the development of novel therapies aimed at these emerging pathogens.
In spite of this, there was unanimous agreement that implementation of optimal practices aimed at curtailing the emergence and spread of antibiotic-resistant bacteria should be put into action as soon as possible, consistently and globally. Enhancing antimicrobial stewardship (ie, appropriate selection, dosing, route, and duration of therapy) should continue to be a goal of all institutions [92].
The complexity of the problem of antibiotic resistance in GNB was evident throughout the Summit. These complex theoretical concerns translated into discrepant opinions regarding empirical therapeutic approaches, the duration of treatment, optimal prevention strategies for the further prevention of resistance, and the direction of future research. Nevertheless, there was broad agreement on the need to promote more sound scientific investigation in the area of antimicrobial resistance in order to slow the tide of escalating resistance and provide more effective therapies.
The disparities between Summit participants’ opinions regarding the clinical practice statements and those of a large pool of practicing infectious disease and critical care clinicians are interesting. Generally speaking, practicing specialists were much more likely to accept the statements than were the Summit participants. These disparities are probably attributable to differences in interpretation of the statements and in familiarity with the evidence in their support.
At the conclusion of the Summit, participants identified several areas of research that merit priority to refine the care of patients with serious GNB infections:
large-scale, multicenter, multinational observational cohort studies with rigorous microbiologic data to better define the precise problem of resistance in GNB and better delineate risk factors for the acquisition and spread of specific pathogens,
studies investigating the impact of antibiotic-resistant GNB on the outcomes in patients with infection attributed to these pathogens as well as the economic consequences of these infections,
the development of novel compounds aimed at the treatment of antibiotic-resistant GNB, and
identification of optimal antibiotic practices aimed at providing appropriate treatment while minimizing the emergence of resistance (eg, specific studies on the use of combination antibiotic therapy, optimal PK/PD targets, and the most favorable duration of antibiotic therapy).
Acknowledgments
Financial support. This work was supported by Consensus Medical Communication (consulting fees and travel support to M. H. K., Y. G., S. T. M., M. I. R.; payment for the development of educational presentations to S. T. M.; payment for writing manuscript to all authors); Merck (payment for lectures to M. H. K. and Y. G., manuscript preparation to M. H. K., and consultancy to Y. G.; grant support to Y. G.); Pfizer (Wyeth) (payment for lectures to M. H. K., Y. G., and M. I. R.; payment for manuscript preparation to M. H. K.; payment for consultancies to Y. G. and M. I. R.; grant support to Y. G. and S. T. M.); Astellas (payment for lectures and manuscript preparation to M. H. K., grant support to Y. G.); Sanofi Pasteur and Astrazeneca (payment for lectures and manuscript preparation to M. H. K.); Cubist (grant support to Y. G. and S. T. M.; payment for lectures to Y. G.); Vernco MedEd (payment for the development of educational presentations to S. T. M.); Ortho-McNeil-Janssen (Johnson & Johnson) (payment for board membership and consultancy to M. I. R.; grants to Y. G. and S. T. M.); Therafan (payment for board membership and consultancy to M. I. R.); Forest Laboratories and Novartis (payment for board membership to M. I. R.); TRIUS (payment for consultancy to M. I. R.); Covidien and BARD (payment for lectures to M. I. R.). M. I. R. is supported and his time partially protected by a grant from the National Heart, Lung, and Blood Institute (award no. K23HL096054).
Supplement sponsorship. This article was published as part of a supplement entitled “Appraising Contemporary Strategies to Combat Multidrug Resistant Gram-Negative Bacterial Infections–Proceedings and Data From the Gram-Negative Resistance Summit,” jointly sponsored by the University of Colorado School of Medicine and Consensus Medical Communications and is supported by an educational grant from Ortho-McNeil Janssen Scientific Affairs, LLC.
Potential conflicts of interest. Yoav Golan, MD: is on the speakers' bureau for Merck, Pfizer, and Cubist; received grants and/or research support from Merck, Cubist, Pfizer, Astellas, and Johnson & Johnson; is a consultant for Merck, Pfizer, and Cubist. Marin Kollef, MD: is on the speakers' bureau for Merck, Pfizer, Astellas, Sanofi Pasteur, and AstraZeneca. Scott Micek, PharmD: no reported conflicts or financial disclosures. Marcos Restrepo, MD: is on the speakers' bureau for Covidien; received grant and/or research support from the National Heart, Lung, and Blood Institute; is also a consultant for Ortho-McNeil-Janssen (Johnson & Johnson), Forest Laboratories, Novartis, Theravance, and Pfizer (Wyeth). Andrew Shorr, MD: is on the speakers' bureau for Astellas, AstraZeneca, Boehringer Ingleheim, Covidien, and Pfizer; received grants and/or research support from Astellas, GlaxoSmithKline, and Pfizer; is a consultant for Astellas, Bayer, Bard, Cadence Pharmaceuticals, Canyon Pharmaceuticals, Johnson & Johnson, Eli Lilly and Company, Eisai Pharmaceuticals, Forest Laboratories, MedCo, and Theravance.
References
- 1.National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–85. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 2.Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 3.Talbot GH, Bradley J, Edwards JE, Jr, Gilbert D, Scheld M, Bartlett JG. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis. 2006;42:657–68. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 4.Paterson DL, Doi Y. A step closer to extreme drug resistance (XDR) in gram-negative bacilli. Clin Infect Dis. 2007;45:1179–81. doi: 10.1086/522287. [DOI] [PubMed] [Google Scholar]
- 5.Doi Y, Arakawa Y. 16S ribosomal RNA methylation: emerging resistance mechanism against aminoglycosides. Clin Infect Dis. 2007;45:88–94. doi: 10.1086/518605. [DOI] [PubMed] [Google Scholar]
- 6.Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arias CA, Murray BE. Antibiotic-resistant bugs in the 21st century: a clinical super-challenge. N Engl J Med. 2009;360:439–43. doi: 10.1056/NEJMp0804651. [DOI] [PubMed] [Google Scholar]
- 8.Kollef MH. Broad-spectrum antimicrobials and the treatment of serious bacterial infections: getting it right up front. Clin Infect Dis. 2008;47(Suppl 1):S3–13. doi: 10.1086/590061. [DOI] [PubMed] [Google Scholar]
- 9.Kollef MH. Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin Infect Dis. 2000;31(Suppl 4):S131–8. doi: 10.1086/314079. [DOI] [PubMed] [Google Scholar]
- 10.Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chemother. 2005;49:1306–11. doi: 10.1128/AAC.49.4.1306-1311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118:146–55. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 12.Garnacho-Montero J, Garcia-Garmendia JL, Barrero-Almodovar A, Jimenez-Jimenez FJ, Perez-Paredes C, Ortiz-Leyba C. Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Crit Care Med. 2003;31:2742–51. doi: 10.1097/01.CCM.0000098031.24329.10. [DOI] [PubMed] [Google Scholar]
- 13.Dhainaut JF, Laterre PF, LaRosa SP, et al. The clinical evaluation committee in a large multicenter phase 3 trial of drotrecogin alfa (activated) in patients with severe sepsis (PROWESS): role, methodology, and results. Crit Care Med. 2003;31:2291–301. doi: 10.1097/01.CCM.0000085089.88077.AF. [DOI] [PubMed] [Google Scholar]
- 14.Ferrer R, Artigas A, Suarez D, et al. Effectiveness of treatments for severe sepsis: a prospective, multicenter, observational study. Am J Respir Crit Care Med. 2009;180:861–6. doi: 10.1164/rccm.200812-1912OC. [DOI] [PubMed] [Google Scholar]
- 15.Harbarth S, Garbino J, Pugin J, Romand JA, Lew D, Pittet D. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am J Med. 2003;115:529–35. doi: 10.1016/j.amjmed.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 17.Paterson DL, Rogers BA. How soon is now? The urgent need for randomized, controlled trials evaluating treatment of multidrug-resistant bacterial infection. Clin Infect Dis. 2010;51:1245–7. doi: 10.1086/657243. [DOI] [PubMed] [Google Scholar]
- 18.Christoff J, Tolentino J, Mawdsley E, Matushek S, Pitrak D, Weber SG. Optimizing empirical antimicrobial therapy for infection due to gram-negative pathogens in the intensive care unit: utility of a combination antibiogram. Infect Control Hosp Epidemiol. 2010;31:256–61. doi: 10.1086/650446. [DOI] [PubMed] [Google Scholar]
- 19.Kollef MH, Sherman G, Ward S, Fraser VJ. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115:462–74. doi: 10.1378/chest.115.2.462. [DOI] [PubMed] [Google Scholar]
- 20.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–96. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 21.Micek ST, Welch EC, Khan J, et al. Empiric combination antibiotic therapy is associated with improved outcome against sepsis due to gram-negative bacteria: a retrospective analysis. Antimicrob Agents Chemother. 2010;54:1742–8. doi: 10.1128/AAC.01365-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Hasan MN, Wilson JW, Lahr BD, et al. Beta-lactam and fluoroquinolone combination antibiotic therapy for bacteremia caused by gram-negative bacilli. Antimicrob Agents Chemother. 2009;53:1386–94. doi: 10.1128/AAC.01231-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heyland DK, Dodek P, Muscedere J, Day A, Cook D. Randomized trial of combination versus monotherapy for the empiric treatment of suspected ventilator-associated pneumonia. Crit Care Med. 2008;36:737–44. doi: 10.1097/01.CCM.0B013E31816203D6. [DOI] [PubMed] [Google Scholar]
- 24.Chaudhary M, Shrivastava SM, Varughese L, Sehgal R. Efficacy and safety evaluation of fixed dose combination of cefepime and amikacin in comparison with cefepime alone in treatment of nosocomial pneumonia patients. Curr Clin Pharmacol. 2008;3:118–22. doi: 10.2174/157488408784293660. [DOI] [PubMed] [Google Scholar]
- 25.Magnotti LJ, Schroeppel TJ, Clement LP, et al. Efficacy of monotherapy in the treatment of Pseudomonas ventilator-associated pneumonia in patients with trauma. J Trauma. 2009;66:1052–8. doi: 10.1097/TA.0b013e31819a06e0. discussion 1058–59. [DOI] [PubMed] [Google Scholar]
- 26.Chamot E, Boffi El Amari E, Rohner P, Van Delden C. Effectiveness of combination antimicrobial therapy for Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother. 2003;47:2756–64. doi: 10.1128/AAC.47.9.2756-2764.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cometta A, Baumgartner JD, Lew D, et al. Prospective randomized comparison of imipenem monotherapy with imipenem plus netilmicin for treatment of severe infections in nonneutropenic patients. Antimicrob Agents Chemother. 1994;38:1309–13. doi: 10.1128/aac.38.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solberg CO, Sjursen H. Safety and efficacy of meropenem in patients with septicaemia: a randomised comparison with ceftazidime, alone or combined with amikacin. J Antimicrob Chemother. 1995;36(Suppl A):157–66. doi: 10.1093/jac/36.suppl_a.157. [DOI] [PubMed] [Google Scholar]
- 29.Paul M, Silbiger I, Grozinsky S, Soares-Weiser K, Leibovici L. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev. 2006;(1):CD003344. doi: 10.1002/14651858.CD003344.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Kumar A, Zarychanski R, Light B, et al. Early combination antibiotic therapy yields improved survival compared with monotherapy in septic shock: a propensity-matched analysis. Crit Care Med. 2010;38:1773–85. doi: 10.1097/CCM.0b013e3181eb3ccd. [DOI] [PubMed] [Google Scholar]
- 31.Pakyz AL, MacDougall C, Oinonen M, Polk RE. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med. 2008;168:2254–60. doi: 10.1001/archinte.168.20.2254. [DOI] [PubMed] [Google Scholar]
- 32.Kollef MH, Napolitano LM, Solomkin JS, et al. Health care-associated infection (HAI): a critical appraisal of the emerging threat-proceedings of the HAI Summit. Clin Infect Dis. 2008;47(Suppl 2):S55–99. doi: 10.1086/590937. ; quiz S100–1. [DOI] [PubMed] [Google Scholar]
- 33.Kollef MH, Morrow LE, Baughman RP, et al. Health care-associated pneumonia (HCAP): a critical appraisal to improve identification, management, and outcomes: proceedings of the HCAP Summit. Clin Infect Dis. 2008;46(Suppl 4):S296–334. doi: 10.1086/526355. ; quiz 335–8. [DOI] [PubMed] [Google Scholar]
- 34.Bhat SV, Peleg AY, Lodise TP, Jr, et al. Failure of current cefepime breakpoints to predict clinical outcomes of bacteremia caused by gram-negative organisms. Antimicrob Agents Chemother. 2007;51:4390–5. doi: 10.1128/AAC.01487-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tam VH, Gamez EA, Weston JS, et al. Outcomes of bacteremia due to Pseudomonas aeruginosa with reduced susceptibility to piperacillin-tazobactam: implications on the appropriateness of the resistance breakpoint. Clin Infect Dis. 2008;46:862–7. doi: 10.1086/528712. [DOI] [PubMed] [Google Scholar]
- 36.McKinnon PS, Paladino JA, Schentag JJ. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents. 2008;31:345–51. doi: 10.1016/j.ijantimicag.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 37. CLSI M100-S20 (2010) cephalosporin and aztreonam breakpoint revisions fact sheet. Clinical and Laboratory Standards Institute. Available at: http://www.clsi.org/Content/NavigationMenu/Committees/Microbiology/AST/CephalosporinandAztreonamBreakpointRevisionFactSheet/CephalosporinAztreonamBreakpointFactSheet.pdf. Accessed 26 July 2011. [Google Scholar]
- 38.Cockerill III FR, Wikler MA, Bush K. Performance standards for antimicrobial susceptibility testing: twentieth informational supplement (June 2010 update). Clinical and Laboratory Standards Institute. Available at: http://www.clsi.org/source/orders/free/m100-s20-u.pdf. Published 1 June 2010. Accessed 26 July 2011. [Google Scholar]
- 39.Nicasio AM, Ariano RE, Zelenitsky SA, et al. Population pharmacokinetics of high-dose, prolonged-infusion cefepime in adult critically ill patients with ventilator-associated pneumonia. Antimicrob Agents Chemother. 2009;53:1476–81. doi: 10.1128/AAC.01141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Wart SA, Andes DR, Ambrose PG, Bhavnani SM. Pharmacokinetic-pharmacodynamic modeling to support doripenem dose regimen optimization for critically ill patients. Diagn Microbiol Infect Dis. 2009;63:409–14. doi: 10.1016/j.diagmicrobio.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 41.Roberts JA, Kirkpatrick CM, Roberts MS, Dalley AJ, Lipman J. First-dose and steady-state population pharmacokinetics and pharmacodynamics of piperacillin by continuous or intermittent dosing in critically ill patients with sepsis. Int J Antimicrob Agents. 2010;35:156–63. doi: 10.1016/j.ijantimicag.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Lorente L, Lorenzo L, Martin MM, Jimenez A, Mora ML. Meropenem by continuous versus intermittent infusion in ventilator-associated pneumonia due to gram-negative bacilli. Ann Pharmacother. 2006;40:219–23. doi: 10.1345/aph.1G467. [DOI] [PubMed] [Google Scholar]
- 43.Lorente L, Jimenez A, Palmero S, et al. Comparison of clinical cure rates in adults with ventilator-associated pneumonia treated with intravenous ceftazidime administered by continuous or intermittent infusion: a retrospective, nonrandomized, open-label, historical chart review. Clin Ther. 2007;29:2433–9. doi: 10.1016/j.clinthera.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 44.Lorente L, Jimenez A, Martin MM, Iribarren JL, Jimenez JJ, Mora ML. Clinical cure of ventilator-associated pneumonia treated with piperacillin/tazobactam administered by continuous or intermittent infusion. Int J Antimicrob Agents. 2009;33:464–8. doi: 10.1016/j.ijantimicag.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 45.Lodise TP, Jr, Lomaestro B, Drusano GL. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended-infusion dosing strategy. Clin Infect Dis. 2007;44:357–63. doi: 10.1086/510590. [DOI] [PubMed] [Google Scholar]
- 46.Patel GW, Patel N, Lat A, et al. Outcomes of extended infusion piperacillin/tazobactam for documented gram-negative infections. Diagn Microbiol Infect Dis. 2009;64:236–40. doi: 10.1016/j.diagmicrobio.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Nicasio AM, Eagye KJ, Nicolau DP, et al. Pharmacodynamic-based clinical pathway for empiric antibiotic choice in patients with ventilator-associated pneumonia. J Crit Care. 2010;25:69–77. doi: 10.1016/j.jcrc.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 48.Roberts JA, Webb S, Paterson D, Ho KM, Lipman J. A systematic review on clinical benefits of continuous administration of beta-lactam antibiotics. Crit Care Med. 2009;37:2071–8. doi: 10.1097/CCM.0b013e3181a0054d. [DOI] [PubMed] [Google Scholar]
- 49.Chastre J, Wunderink R, Prokocimer P, Lee M, Kaniga K, Friedland I. Efficacy and safety of intravenous infusion of doripenem versus imipenem in ventilator-associated pneumonia: a multicenter, randomized study. Crit Care Med. 2008;36:1089–96. doi: 10.1097/CCM.0b013e3181691b99. [DOI] [PubMed] [Google Scholar]
- 50.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aarts MA, Hancock JN, Heyland D, McLeod RS, Marshall JC. Empiric antibiotic therapy for suspected ventilator-associated pneumonia: a systematic review and meta-analysis of randomized trials. Crit Care Med. 2008;36:108–17. doi: 10.1097/01.CCM.0000297956.27474.9D. [DOI] [PubMed] [Google Scholar]
- 52.Paul M, Benuri-Silbiger I, Soares-Weiser K, Leibovici L. Beta lactam monotherapy versus beta lactam-aminoglycoside combination therapy for sepsis in immunocompetent patients: systematic review and meta-analysis of randomised trials. BMJ. 2004;328:668. doi: 10.1136/bmj.38028.520995.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar A, Safdar N, Kethireddy S, Chateau D. A survival benefit of combination antibiotic therapy for serious infections associated with sepsis and septic shock is contingent only on the risk of death: a meta-analytic/meta-regression study. Crit Care Med. 2010;38:1651–64. doi: 10.1097/CCM.0b013e3181e96b91. [DOI] [PubMed] [Google Scholar]
- 54.Schentag JJ, Strenkoski-Nix LC, Nix DE, Forrest A. Pharmacodynamic interactions of antibiotics alone and in combination. Clin Infect Dis. 1998;27:40–6. doi: 10.1086/514621. [DOI] [PubMed] [Google Scholar]
- 55.Maragakis LL. Recognition and prevention of multidrug-resistant gram-negative bacteria in the intensive care unit. Crit Care Med. 2010;38(Suppl 8):S345–51. doi: 10.1097/CCM.0b013e3181e6cbc5. [DOI] [PubMed] [Google Scholar]
- 56.Carmeli Y, Troillet N, Karchmer AW, Samore MH. Health and economic outcomes of antibiotic resistance in Pseudomonas aeruginosa. Arch Intern Med. 1999;159:1127–32. doi: 10.1001/archinte.159.10.1127. [DOI] [PubMed] [Google Scholar]
- 57.George P, Morris AM. Pro/con debate: should antimicrobial stewardship programs be adopted universally in the intensive care unit? Crit Care. 2010;14:205. doi: 10.1186/cc8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dennesen PJ, van der Ven AJ, Kessels AG, Ramsay G, Bonten MJ. Resolution of infectious parameters after antimicrobial therapy in patients with ventilator-associated pneumonia. Am J Respir Crit Care Med. 2001;163:1371–5. doi: 10.1164/ajrccm.163.6.2007020. [DOI] [PubMed] [Google Scholar]
- 59.Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit: a proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162:505–11. doi: 10.1164/ajrccm.162.2.9909095. [DOI] [PubMed] [Google Scholar]
- 60.Zilberberg MD, Shorr AF. Ventilator-associated pneumonia: the clinical pulmonary infection score as a surrogate for diagnostics and outcome. Clin Infect Dis. 2010;51(Suppl 1):S131–5. doi: 10.1086/653062. [DOI] [PubMed] [Google Scholar]
- 61.Ibrahim EH, Ward S, Sherman G, Schaiff R, Fraser VJ, Kollef MH. Experience with a clinical guideline for the treatment of ventilator-associated pneumonia. Crit Care Med. 2001;29:1109–15. doi: 10.1097/00003246-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 62.Chastre J, Wolff M, Fagon JY, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290:2588–98. doi: 10.1001/jama.290.19.2588. [DOI] [PubMed] [Google Scholar]
- 63.Stolz D, Smyrnios N, Eggimann P, et al. Procalcitonin for reduced antibiotic exposure in ventilator-associated pneumonia: a randomised study. Eur Respir J. 2009;34:1364–75. doi: 10.1183/09031936.00053209. [DOI] [PubMed] [Google Scholar]
- 64.Bouadma L, Luyt CE, Tubach F, et al. Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375:463–74. doi: 10.1016/S0140-6736(09)61879-1. [DOI] [PubMed] [Google Scholar]
- 65.Kopterides P, Siempos, Tsangaris I, Tsantes A, Armaganidis A. Procalcitonin-guided algorithms of antibiotic therapy in the intensive care unit: a systematic review and meta-analysis of randomized controlled trials. Crit Care Med. 2010;38:1–13. doi: 10.1097/CCM.0b013e3181f17bf9. [DOI] [PubMed] [Google Scholar]
- 66.Donaldson AD, Razak L, Liang LJ, Fisher DA, Tambyah PA. Carbapenems and subsequent multiresistant bloodstream infection: does treatment duration matter? Int J Antimicrob Agents. 2009;34:246–51. doi: 10.1016/j.ijantimicag.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 67.Freeman J, Bauer MP, Baines SD, et al. The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev. 2010;23:529–49. doi: 10.1128/CMR.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.D'Agata EM, Magal P, Olivier D, Ruan S, Webb GF. Modeling antibiotic resistance in hospitals: the impact of minimizing treatment duration. J Theor Biol. 2007;249:487–99. doi: 10.1016/j.jtbi.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dancer SJ. The effect of antibiotics on methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2008;61:246–53. doi: 10.1093/jac/dkm465. [DOI] [PubMed] [Google Scholar]
- 70.Lawrence KL, Kollef MH. Antimicrobial stewardship in the intensive care unit: advances and obstacles. Am J Respir Crit Care Med. 2009;179:434–8. doi: 10.1164/rccm.200809-1394CP. [DOI] [PubMed] [Google Scholar]
- 71.Kollef MH, Fraser VJ. Antibiotic resistance in the intensive care unit. Ann Intern Med. 2001;134:298–314. doi: 10.7326/0003-4819-134-4-200102200-00014. [DOI] [PubMed] [Google Scholar]
- 72.Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323–9. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 73.Nseir S, Grailles G, Soury-Lavergne A, Minacori F, Alves I, Durocher A. Accuracy of American Thoracic Society/Infectious Diseases Society of America criteria in predicting infection or colonization with multidrug resistant bacteria at intensive-care unit admission. Clin Microbiol Infect. 2010;16:902–8. doi: 10.1111/j.1469-0691.2009.03027.x. [DOI] [PubMed] [Google Scholar]
- 74.Rello J, Ausina V, Ricart M, Castella J, Prats G. Impact of previous antimicrobial therapy on the etiology and outcome of ventilator-associated pneumonia. Chest. 1993;104:1230–5. doi: 10.1378/chest.104.4.1230. [DOI] [PubMed] [Google Scholar]
- 75.Bukholm G, Tannaes T, Kjelsberg AB, Smith-Erichsen N. An outbreak of multidrug-resistant Pseudomonas aeruginosa associated with increased risk of patient death in an intensive care unit. Infect Control Hosp Epidemiol. 2002;23:441–6. doi: 10.1086/502082. [DOI] [PubMed] [Google Scholar]
- 76.Heyland DK, Cook DJ, Griffith L, Keenan SP, Brun-Buisson C. The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. The Canadian Critical Trials Group. Am J Respir Crit Care Med. 1999;159:1249–56. doi: 10.1164/ajrccm.159.4.9807050. [DOI] [PubMed] [Google Scholar]
- 77.Kollef MH, Ward S. The influence of mini-BAL cultures on patient outcomes: implications for the antibiotic management of ventilator-associated pneumonia. Chest. 1998;113:412–20. doi: 10.1378/chest.113.2.412. [DOI] [PubMed] [Google Scholar]
- 78.Yakupogullari Y, Otlu B, Dogukan M, et al. Investigation of a nosocomial outbreak by alginate-producing pan-antibiotic-resistant Pseudomonas aeruginosa. Am J Infect Control. 2008;36:e13–8. doi: 10.1016/j.ajic.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 79.Rodriguez-Bano J, Garcia L, Ramirez E, et al. Long-term control of hospital-wide, endemic multidrug-resistant Acinetobacter baumannii through a comprehensive “bundle” approach. Am J Infect Control. 2009;37:715–22. doi: 10.1016/j.ajic.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mastoraki A, Douka E, Kriaras I, Stravopodis G, Saroglou G, Geroulanos S. Preventing strategy of multidrug-resistant Acinetobacter baumanii susceptible only to colistin in cardiac surgical intensive care units. Eur J Cardiothorac Surg. 2008;33:1086–90. doi: 10.1016/j.ejcts.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 81.Marchaim D, Navon-Venezia S, Schwartz D, et al. Surveillance cultures and duration of carriage of multidrug-resistant Acinetobacter baumannii. J Clin Microbiol. 2007;45:1551–5. doi: 10.1128/JCM.02424-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Apisarnthanarak A, Pinitchai U, Thongphubeth K, Yuekyen C, Warren DK, Fraser VJ. A multifaceted intervention to reduce pandrug-resistant Acinetobacter baumannii colonization and infection in 3 intensive care units in a Thai tertiary care center: a 3-year study. Clin Infect Dis. 2008;47:760–7. doi: 10.1086/591134. [DOI] [PubMed] [Google Scholar]
- 83.Kochar S, Sheard T, Sharma R, et al. Success of an infection control program to reduce the spread of carbapenem-resistant Klebsiella pneumoniae. Infect Control Hosp Epidemiol. 2009;30:447–52. doi: 10.1086/596734. [DOI] [PubMed] [Google Scholar]
- 84.Souweine B, Traore O, Aublet-Cuvelier B, et al. Role of infection control measures in limiting morbidity associated with multi-resistant organisms in critically ill patients. J Hosp Infect. 2000;45:107–16. doi: 10.1053/jhin.2000.0734. [DOI] [PubMed] [Google Scholar]
- 85.Albertini MT, Benoit C, Berardi L, et al. Surveillance of methicillin-resistant Staphylococcus aureus (MRSA) and Enterobacteriaceae producing extended-spectrum beta-lactamase (ESBLE) in Northern France: a five-year multicentre incidence study. J Hosp Infect. 2002;52:107–13. doi: 10.1053/jhin.2002.1286. [DOI] [PubMed] [Google Scholar]
- 86.Munoz-Price LS, Hayden MK, Lolans K, et al. Successful control of an outbreak of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae at a long-term acute care hospital. Infect Control Hosp Epidemiol. 2010;31:341–7. doi: 10.1086/651097. [DOI] [PubMed] [Google Scholar]
- 87.Kola A, Holst M, Chaberny IF, Ziesing S, Suerbaum S, Gastmeier P. Surveillance of extended-spectrum beta-lactamase-producing bacteria and routine use of contact isolation: experience from a three-year period. J Hosp Infect. 2007;66:46–51. doi: 10.1016/j.jhin.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 88.Gardam MA, Burrows LL, Kus JV, et al. Is surveillance for multidrug-resistant Enterobacteriaceae an effective infection control strategy in the absence of an outbreak? J Infect Dis. 2002;186:1754–60. doi: 10.1086/345921. [DOI] [PubMed] [Google Scholar]
- 89.Harbarth S, Fankhauser C, Schrenzel J, et al. Universal screening for methicillin-resistant Staphylococcus aureus at hospital admission and nosocomial infection in surgical patients. JAMA. 2008;299:1149–57. doi: 10.1001/jama.299.10.1149. [DOI] [PubMed] [Google Scholar]
- 90.Robicsek A, Beaumont JL, Paule SM, et al. Universal surveillance for methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Ann Intern Med. 2008;148:409–18. doi: 10.7326/0003-4819-148-6-200803180-00003. [DOI] [PubMed] [Google Scholar]
- 91.Detection of Enterobacteriaceae isolates carrying metallo-beta-lactamase: United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:750. [PubMed] [Google Scholar]
- 92.Dellit TH, Owens RC, McGowan JE, Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–77. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]