Abstract
Broad-scale studies of climate change effects on freshwater species have focused mainly on temperature, ignoring critical drivers such as flow regime and biotic interactions. We use downscaled outputs from general circulation models coupled with a hydrologic model to forecast the effects of altered flows and increased temperatures on four interacting species of trout across the interior western United States (1.01 million km2), based on empirical statistical models built from fish surveys at 9,890 sites. Projections under the 2080s A1B emissions scenario forecast a mean 47% decline in total suitable habitat for all trout, a group of fishes of major socioeconomic and ecological significance. We project that native cutthroat trout Oncorhynchus clarkii, already excluded from much of its potential range by nonnative species, will lose a further 58% of habitat due to an increase in temperatures beyond the species’ physiological optima and continued negative biotic interactions. Habitat for nonnative brook trout Salvelinus fontinalis and brown trout Salmo trutta is predicted to decline by 77% and 48%, respectively, driven by increases in temperature and winter flood frequency caused by warmer, rainier winters. Habitat for rainbow trout, Oncorhynchus mykiss, is projected to decline the least (35%) because negative temperature effects are partly offset by flow regime shifts that benefit the species. These results illustrate how drivers other than temperature influence species response to climate change. Despite some uncertainty, large declines in trout habitat are likely, but our findings point to opportunities for strategic targeting of mitigation efforts to appropriate stressors and locations.
Keywords: global change, hydrology, invasive species, niche model, distribution modeling
Nearly all broad-scale analyses of climate effects on freshwater species have focused on temperature shifts, to the exclusion of other climate-driven drivers. Although temperature is a critical determinant of metabolic and physical processes (1), important ecosystem effects on streams and rivers may also be mediated by flow regime and biotic interactions. Flow regime has been described as a “master variable” (2) that controls or influences many aspects of the physical aquatic environment, as well as the timing of reproduction and migration of many organisms (3). Biotic interactions are increasingly recognized as important components of climate-species relationships (4, 5), but are rarely included in projections of species distributions under future climates, with some notable exceptions (6). Competitive interactions in particular are not commonly modeled (but see ref. 7), despite interest in invasive-native species interaction under climate change (8). It is likely that all three factors—temperature, flow regime, and biotic interactions—will play important roles in future aquatic species distributional shifts (8–11).
Trout serve as excellent model organisms for examining how these mechanisms could alter population dynamics and species distributions, for three reasons (“trout” include fishes in the genera Oncorhynchus, Salmo, and Salvelinus). First, although all trout are coldwater specialists, their temperature sensitivities and preferences vary by species (12), implying different responses to warming. Second, trout are also likely to be differentially affected by flow regime changes. Trout are sensitive to high flows after spawning because such flows can scour eggs from gravel nests or wash away newly emerged fry; thus, fall-spawning trout are sensitive to winter floods and spring-spawning trout are sensitive to summer floods (13–15). In some regions, winter floods are projected to increase with warming due to precipitation shifts from snow to a snow-rain mix (16). Third, many native trout populations are threatened by invasions of introduced trout species. For example, brook trout Salvelinus fontinalis have displaced brown trout Salmo trutta in Scandinavia (17), and brown trout have displaced native trout in North America (18). Strong competitive interactions such as these set the stage for cascading effects of climate change, whereby climate-driven population changes to one species drive population changes in other species (7, 19).
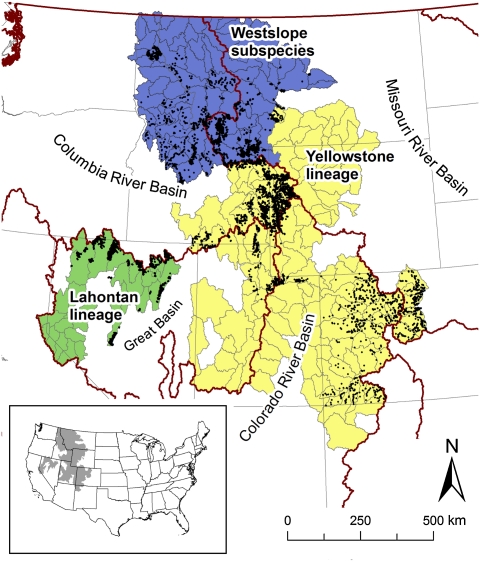
We assessed the effects of temperature, flow regime (particularly flood seasonality), and biotic interactions, as well as topographic and land use variables, on distributions of four trout species: native cutthroat trout Oncorhynchus clarkii, and nonnative brook trout, brown trout, and rainbow trout Oncorhynchus mykiss (native to 6% of the region and introduced elsewhere). Our domain was the historical range of cutthroat trout in the inland western United States (1.01 million km2; Fig. 1), where the species is represented by three main lineages (westslope, Lahontan, and Yellowstone groups) and numerous subspecies, all of conservation concern (20). Candidate variables predicting the distribution of each species under current conditions were tested using multilevel generalized linear models parameterized with fish surveys from 9,890 sites. We used multimodel averaging to combine well supported alternative models into a composite model for each species (21). We then forecasted species suitable habitat under climate change using estimated future temperature and flow metrics from general circulation models (GCMs) simulating conditions in the 2040s and 2080s under the A1B emissions scenario (22), accounting for biotic interactions. We bracketed variability in climate warming predictions by using outputs from one GCM that predicted high warming (MIROC3.2), one that predicted low warming (PCM1), and a composite of 10 GCMs (23). Finally, we used a sensitivity analysis to evaluate the relative importance of temperature, flow, and biotic interactions in determining distributional changes of each trout species under future climates.
Fig. 1.
Study domain and fish collection sites (black dots; n = 9890). Boundaries of major river basins (and Canadian border) are shown in red; state boundaries in gray. Ranges of the three cutthroat lineages are indicated by colors.
Results
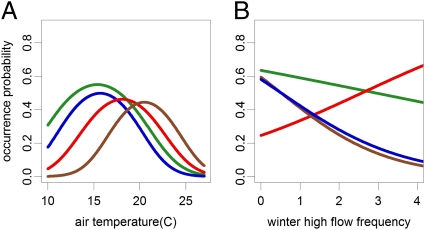
The four trout species differed substantially in their relationships with temperature, flood seasonality, and presence of other trout. Brook trout and cutthroat trout occurred in the coldest streams, whereas rainbow trout occupied warmer locations, and brown trout the warmest locations (Table 1 and Fig. 2A). Fall-spawning brook trout and brown trout showed a strong negative relationship with winter high flow frequency, as predicted; spring-spawning cutthroat displayed a weak negative relationship, and spring-spawning rainbow trout had a positive relationship (Table 1 and Fig. 2B). Cutthroat trout showed a negative relationship to the occurrence of all three other species at either the stream reach or subwatershed scale or both, with some variability among cutthroat trout lineages (Table S1). There was no evidence for biotic interactions among the other species. Other variables of importance to trout distributions were stream slope, mean flow (primarily an indicator of landscape position or stream size), road presence, and distance to the nearest unconfined valley bottom (UVBs, landscape features associated with high densities of fall-spawning trout; refs. 24 and 25). In-sample classification accuracy of models was 64–76%. See SI Text for additional model details.
Table 1.
Model-averaged parameter estimates (means ±1 SE) for temperature and flow predictor variables in the composite model for each species
| Trout | Variable |
| Cutthroat | |
| ptemp | −0.59 ± 0.12 |
| ptemp2 | −0.88 ± 0.13 |
| w99 | −0.20 ± 0.11 |
| Brook | |
| ptemp | −0.66 ± 0.12 |
| ptemp2 | −1.20 ± 0.12 |
| w2 | −0.98 ± 0.14 |
| Rainbow | |
| dtemp | −0.32 ± 0.16 |
| dtemp2 | −0.89 ± 0.18 |
| w99 | 0.58 ± 0.11 |
| Brown | |
| ptemp | 1.90 ± 0.28 |
| ptemp2 | −1.44 ± 0.26 |
| w95 | −1.15 ± 0.22 |
Variable abbreviations (e.g., “ptemp” and “wtemp”) are defined in Methods. Parameter estimates are weighted averages of multiple individual models. Variables have been standardized by subtracting the mean and dividing by 2 SD.
Fig. 2.
Occurrence probability of trout species as a function of air temperature (A) and winter high flow frequency (B). Green indicates cutthroat trout; blue indicates brook trout; red indicates rainbow trout; brown indicates brown trout. The different temperature and flow metrics for different species were standardized to a common x axis in each panel to facilitate comparisons; the figures are shown with original axes and with confidence intervals in Fig. S1.
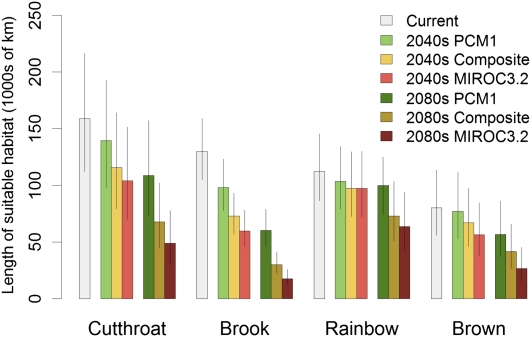
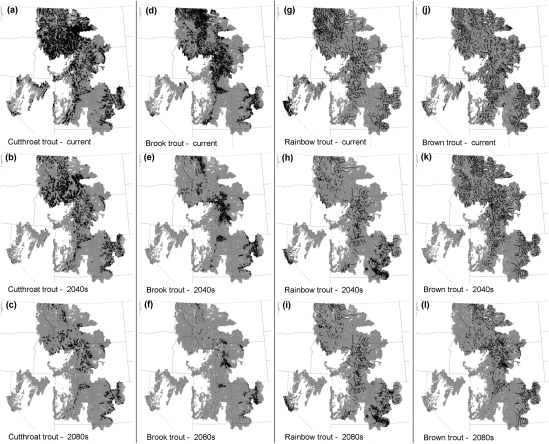
Under the climate projections, both native cutthroat trout and nonnative brook trout showed a strong decline in length of suitable habitat (Figs. 3 and 4). Cutthroat trout was projected to decline by 28% in the 2040s composite scenario and 58% in the 2080s scenario; brook trout was projected to lose 44% and 77% of its range, respectively, for these scenarios. Rainbow trout was projected to decrease modestly (13%) in length of suitable habitat in 2040s, with a moderate decrease (35%) in the 2080s (Figs. 3 and 4). Brown trout was projected to decline by 16% in the short term and 48% in the long term (Figs. 3 and 4). There were very large differences between the MIROC3.2 and the PCM1 model projections of suitable habitat. For example, cutthroat trout was projected to decline by 70% under the 2080s MIROC3.2 scenario but only 33% under the 2080s PCM1 scenario (Fig. 3). We projected that the total length of habitat suitable for one or more trout species would decline by ∼47% under the 2080s composite scenario. This was accompanied by a range shift from larger, low-elevation streams to smaller, high-elevation streams, so habitat volume and trout biomass could decline more than is indicated by the change in stream length.
Fig. 3.
Projected stream length of suitable habitat for trout under current conditions and climate change scenarios. Whiskers show 90% confidence intervals for projections.
Fig. 4.
Projected distribution of suitable habitat for trout under current conditions, 2040s A1B, and 2080s A1B climate change scenarios, based on the composite GCM. Black indicates mostly suitable; gray indicates mostly unsuitable.
The sensitivity analysis indicated that individual species declines were associated with different variables. A combination of increasing temperature and increasing winter high flow frequency drove the brook trout losses (Table 2), whereas the declines of brown trout (with much higher thermal tolerances) were attributable almost solely to increasing winter high flow frequency. The more modest declines of rainbow trout resulted from negative effects of temperature increases offset by positive effects of increasing winter high flow frequency. The projected cutthroat trout declines were associated most strongly with temperature increases, but additional sensitivity analyses also showed a substantial role of biotic interactions. Under current conditions, we projected that cutthroat trout would occupy 239,000 km of stream if none of the other trout species were present, rather than the 159,000 km, suggesting that nonnative species are responsible for a 33% reduction in cutthroat trout suitable stream length at present (classifying rainbow trout as nonnative). Similarly, under the 2080s composite model scenario we calculated that the length would be 92,000 km instead of 68,000 km, if the other species were absent. Thus, we projected that nonnative species would continue depressing cutthroat trout populations by about 26% in the future.
Table 2.
Results of sensitivity analysis, indicating importance of climate-related variables in determining suitable habitat for trout under climate change
| Condition | Cutthroat | Brook | Rainbow | Brown |
| Current suitable stream length | 159 | 130 | 112 | 80 |
| Projected suitable stream length under 2080s scenario | 68 | 30 | 73 | 42 |
| Projected in 2080s if no change in temperature | 187 (×2.8) | 92 (×3.1) | 162 (×2.2) | 45 (×1.1) |
| Projected in 2080s if no change in winter high flows | 77 (×1.1) | 45 (×1.5) | 46 (×.63) | 75 (×1.78) |
| Projected in 2080s if no change in temperature or winter high flows | 205 (×3.0) | 131 (×4.4) | 114 (×1.6) | 81 (×1.9) |
| Projected in 2080s if no change in mean flow | 69 (×1.0) | 30 (×1.0) | 72 (×0.99) | 41 (×0.98) |
Values indicate projected length of suitable habitat under current conditions (row 1), under projections for the 2080s A1B composite scenario (row 2), and under the 2080s A1B composite scenario with selected climate metrics held constant at current levels (rows 3–6). Units are km × 1,000. Values in parentheses indicate change relative to the 2080s A1B composite scenario (row 2).
Discussion
We found that multiple drivers—temperature, flow regime, and biotic interactions—determined the response of trout species to climate change in the western USA. For example, projected declines of one species (brown trout) were driven by increasing frequency of winter high flows, with minimal influence of temperature (except as mediated by flow changes). The projected shift in flow regime also negatively affected brook trout, which like brown trout is a fall-spawning species. However, spring-spawning cutthroat trout and rainbow trout showed a modest negative response and a strong positive response, respectively, to winter high flows. The positive response of rainbow trout likely reflects preadaptation to this flow regime, which is characteristic of much of the species’ native range (14). Other researchers have recognized that climate-driven shifts in flow regime will play a role in changes to stream biota and aquatic ecosystems (9, 26), and some have modeled climate-related flow effects on species at the stream scale (27). However, until now this has not been extended to broad scales due to a lack of quantitative estimates of hydrologic metrics under future conditions. Despite the important influence of flow regime, we nevertheless found that temperature increases themselves were likely to play a dominant role in driving future declines of cutthroat trout, brook trout, and rainbow trout. The actual mechanisms for temperature-driven extirpations are likely to be manifold and complex, involving growth rates, incubation times, competitive ability relative to other species, and asynchrony with prey, which can cause negative population growth rates even if temperatures never reach the lethal range for individuals (28).
Biotic interactions were an important driver of native cutthroat trout distributions, which, consistent with past observations, responded negatively to the presence of nonnative trout (24). Both currently and under future climate conditions, the presence of other species reduced the distribution of cutthroat trout by 26–33%, although the species primarily responsible for this shifted from brook trout to rainbow trout. This adds to the evidence (6, 19, 29) against the argument that biotic interactions are relatively unimportant in determining species distributions at broad scales (30). We did not find evidence of negative interactions among the nonnative species. We had hypothesized that westslope cutthroat trout, with a recent evolutionary history of sympatry with other trout, would have weaker biotic interactions than cutthroat trout lineages that evolved in isolation, but the evidence for this was mixed. Whereas westslope cutthroat trout did show a weaker response to brook trout and rainbow trout, its response to brown trout was stronger than that of the other lineages.
Modeling Notes.
We found that the predictive accuracy of our models was only moderate for most species. We attribute this in part to the inconsistent introduction of nonnative species across the study area, to the historical extirpation of cutthroat trout from portions of its range, and to our inability to include fine-scale variables such as fires and debris flows (31) and natural and artificial movement barriers (32), because the data were not consistently available. Consequently, we do not recommend using these models for fine-scale predictions without local validation. In addition, forecasting future distributions is inherently uncertain. Our results indicated that just one source of uncertainty, the choice of GCM, can account for >50% variability in estimates of suitable habitat. Therefore, our results are most useful for understanding the different trajectories and relative climate sensitivities of the different trout species, as well as the habitats that are most sensitive to change (e.g., those where winter flooding will increase along with temperatures), rather than making precise predictions of habitat losses.
Broad-scale species distributional modeling such as this complements results from laboratory studies and finer-scale analyses in advancing our understanding of species niches, because it can describe the full range of actual species relationships to environmental variables. For example, laboratory studies showed that brook trout in Wyoming outcompete cutthroat trout at warmer temperatures (33), but a recent analysis of trout distributions in the interior Columbia River Basin (a subset of the range studied here) indicated that warm temperatures were more limiting for brook trout than cutthroat trout (34). Our results help resolve this apparent contradiction by showing that cutthroat trout have a broader thermal niche than brook trout across the western United States, even though individual cutthroat trout populations could have thermal preferences higher or lower than sympatric brook trout due to local adaptations.
Broader Implications.
Our models forecast significant declines in trout habitat across the interior western United States in the 21st century, a result we expect will apply to much of the rest of the temperate world because three of our study species (rainbow, brown, and brook trout) are common on multiple continents. This decline will have significant socioeconomic consequences, as recreational trout fisheries are valued at hundreds of millions of dollars in the United States alone (35). To some extent, warm-water species are likely to replace trout in many streams, providing alternative recreational fishery opportunities, although it is unclear whether common introduced species such as smallmouth bass (Micropterus dolomieu) can fully exploit the range of habitats currently occupied by trout. In any case, there will be ecological consequences of a shift in dominant fish species that will affect such things as nutrient cycling and reciprocal terrestrial-stream subsidy balances (36) in ways that are difficult to foresee.
For management agencies charged with maintaining healthy trout populations, global change creates obvious challenges; these are exacerbated by the uncertainty inherent in climate forecasts and the complexity of the climate responses we describe here. We argue that efforts to understand this complexity, in terms of which climatic and biotic drivers are significant for different species in different locations, can point to management actions that are efficiently targeted and robust to uncertainty. For example, there is little that can be done to influence the predicted increase in winter high flows, so some declines in fall-spawning species (e.g., brook trout and brown trout) may be inevitable in regions where flows are likely to shift. In contrast, stream temperature is often influenced by anthropogenic activity and future increases can be offset by restoration measures such as maintenance of stream flows and reforestation (37). Thus, managers interested in conserving cutthroat trout or rainbow trout habitat may wish to focus on such restoration activities, which are likely to provide some benefits regardless of the precise climate trajectory. In selecting actions, managers should consider local conditions; for example, the response of cutthroat trout depends substantially on which nonnative species are present, and this varies from region to region. Overall, we argue that considering biotic interactions and variables other than temperature not only gives us a richer understanding of species-climate relationship, but also can inspire a more strategic portfolio of management alternatives.
Methods
Dataset.
The fish occurrence dataset was assembled from fish collections made by state and federal agencies (see Acknowledgments) and collectively represented a geographically extensive sample of stream habitats of the interior west of the United States (Fig. 1). As an assembled dataset, it did not have a formal sampling design and was subject to spatial autocorrelation, which was addressed in the analysis as described below. We included only sites sampled using electrofishing (n = 9,522) or snorkeling (n = 368) between 1985 and 2004. Cutthroat trout were detected at 5,055 sites, brook trout were detected at 2,820 sites, rainbow trout were detected at 1,031 sites, and brown trout were detected at 655 sites; 1,437 sites had none of these species. In developing models for the nonnative trout, we used subsets of the database that excluded sites in subbasins (watersheds delineated by 8-digit US Geological Survey hydrologic unit codes; http://water.usgs.gov/GIS/huc.html) where there was no evidence of species introductions. More details are in the SI Text.
We selected 12 abiotic variables as candidate predictors of trout species occurrence (see SI Text), based in part on an earlier study assessing the relative sensitivities of different trout species to climatic factors in the interior Columbia River Basin (34). We used air temperature as a surrogate for stream temperature because data for the latter were not widely available. Candidate temperature metrics included a point measurement for the site (ptemp) and the mean temperature in the drainage above a site (dtemp). We calculated four candidate metrics of winter high flow frequency: the probability of the 2-y recurrence interval flow occurring in the winter (w2), the probability of the 1.5-y flow occurring in winter (w1.5), the number of winter days with flows among the top 5% for the year (w95), and the number of winter days with flows among the top 1% (w99). Winter was defined as December 1st through February 28th. Two candidate mean flow metrics were mean annual flow (mflow) and mean summer flow (sflow), with summer defined as the period between the decline of the spring flood and September 30th (38). All flow metrics were derived from the Variable Infiltration Capacity (VIC) model (39) coupled with simple routing (38) to produce daily hydrographs for stream segments in the 1:100 K National Hydrography Database (NHD) Plus dataset (http://www.horizon-systems.com/nhdplus/). Metrics were selected based on performance in a validation study (38). Candidate metrics for UVBs included a binary measure of whether a site was within a UVB (vbpres) and a continuous measure of the distance in kilometers to the nearest UVB (vbdist). Slope was from the NHD Plus dataset. We used the occurrence of roads within 1 km of the stream segment on which the site was located (road) as a binary land use variable. Roads were from the 2000 TIGER/Line road database (www.census.gov/geo/www/tiger).
The presence/absence data for nonnative species were tested as biotic predictor variables affecting presence/absence of other species. Cutthroat trout, which is not known to displace other trout species in the region, was not considered as a candidate biotic predictor. These biotic predictors were calculated at two scales: (i) the site and (ii) the subwatershed (12-digit US Geological Survey hydrologic unit code). The latter indicated whether there was one or more records of a species occurrence within the drainage. We also tested the hypothesis that westslope cutthroat trout was less affected by nonnative trout than the other cutthroat trout lineages by including an interaction term.
Model Building.
We used multilevel logistic regression to fit species distribution models for the four trout species. This method allowed us to specify potential relationships between candidate variables and species occurrences based on a priori hypotheses (SI Text; ref. 34), and to address spatial autocorrelation. Multilevel modeling reduces parameter bias associated with autocorrelation by modeling errors at multiple levels (40) and is well suited to the hierarchical structure of stream networks (41). We specified a multilevel model with groups at the subbasin level.
Our first step was to identify the best-supported metric from each group of correlated metrics of the same type (for example, ptemp and dtemp for temperature) for each species. For select variables (ptemp, dtemp, mflow, sflow, and vbdist), we tested both a linear and a quadratic effect; for example, mflow was tested by itself and as mflow plus mflow2. The highly skewed mflow and sflow variables were natural-log-transformed to improve model fit; preliminary tests showed no improvements from transforming other variables. Continuous predictor variables were standardized by subtracting the mean and dividing by 2 SD. Each competing model was fit using the glmer function in the lme4 package (42) using R 2.11 software (43). We used Akaike's Information Criterion (AIC) to identify the best metric from each group.
We used these predictors to construct a global model for each species. We fit the global model and all possible subsets using multilevel logistic regression. We ranked the resulting models for each species by AIC and retained all models within 6 points of the best overall model (i.e., AICmin) as a confidence set of parsimonious models (21), excluding those that were the same as a better-ranked model except for the addition of an uninformative parameter (i.e., one that improved the AIC score < 2; refs. 21 and 44). We constructed a composite model for each species using model averaging of this confidence set (21). We calculated the within-sample predictive performance of each composite model, using the area under the curve of the receiver-operator characteristic plot (AUC) and classification accuracy as performance metrics. Then we divided the data into five regions based on latitudinal bands and conducted fivefold cross-validation by withholding data from one region at a time, fitting the model with data from the other regions, and predicting occurrence probabilities for sites in the withheld region. By making projections for distinct geographical regions, each with different combinations of climatic and physical conditions, we hoped to gain insight into each model's performance under future, unobserved combinations of climatic and physical conditions. This was an estimate of model transferability (45, 46).
Projections.
We used the composite models to predict occurrence of each species throughout the NHD Plus stream network, excluding lakes and rivers larger than ∼2500 km2 drainage area (ref. 38; SI Text), under current conditions and climate scenarios for the 2040s and 2080s associated with the A1B greenhouse gas emissions trajectory (22). The A1B is a middle-of-the road scenario in terms of assumptions for accumulation of greenhouse gases (22). For each of the future time periods, we used projections from three models: (i) MIROC 3.2, which projects greater warming and less summer precipitation in the study region than other GCMs; (ii) PCM1, which projects less warming and more summer precipitation; and (iii) a mean of the 10 IPCC models with the lowest bias in simulating observed climate across the region (23). This resulted in six future scenarios, three for the 2040s and three for the 2080s. The MIROC3.2 and PCM1 models bracketed the range of possible future temperatures; the former projected a mean increase of 5.51 °C in mean summer temperature by the 2080s, whereas the latter projected a mean increase of 2.49 °C for the same period. The GCM model simulations were downscaled using a spatially explicit delta method (23). For each of the scenarios, we used the VIC model to generate hydrographs, from which we extracted the flow metrics described previously. We derived temperature metrics from the downscaled GCM projections. Because biotic predictors were only supported for cutthroat trout, we incorporated biotic data into projections by first modeling other species, and then using the projected biotic data in the forecasts for cutthroat trout (29). For each species, we set the predicted probability threshold to define presence equal to species prevalence in the fitting dataset (47). We mapped the predicted distributions, which we interpreted as suitable habitat, and calculated the total suitable stream length for each species under each scenario.
We conducted a sensitivity analysis to determine which variables contributed the most to species distribution changes under future conditions. We reran the projections using the composite scenario for the 2080s, iteratively holding the hydroclimatic variables (temperature, winter high flow frequency, temperature plus winter high flow frequency, and mean flow) unchanged from current conditions, and recorded the resulting change in total stream length of predicted suitable habitat for each species. To assess the role of biotic interactions, we calculated the total length of predicted suitable habitat for cutthroat trout if there were no other species present (i.e., all biotic interaction parameters set to 0), for the current and 2080s composite scenarios.
Supplementary Material
Acknowledgments
The fish data used in this study were compiled from multiple sources, including a previous database of sites in the range of westslope cutthroat trout (48), which included data from the Idaho Fish and Game's General Parr Monitoring database and other sources. Additional data were provided by Bart Gammett, James Capurso, Mark Novak, Steven Kujala, Daniel Abeyta, and Paul Cowley; Joseph Benjamin; Hilda Sexauer; Kevin Meyer; Brad Shepard; Dona Horan; and Harry Vermillion. David Nagel, Sharon Parkes, and Gwynne Chandler helped to develop the dataset and GIS layers. Expert advice over the course of the study was provided by Russ Thurow, John Buffington, and Jim McKean. This manuscript was substantially improved by comments from Robert Al-Chokhachy, Dana Warren, two anonymous reviewers and the editor. This work was funded by Grant 2008-0087-000 of the National Fish and Wildlife Foundation's Keystone Initiative for Freshwater Fishes, US Geological Survey Grant G09AC00050, and a contract from the US Forest Service Rocky Mountain Research Station (RMRS).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103097108/-/DCSupplemental.
References
- 1.Brown JH, Gillooly JF, Allen AP, Savage VM, West GB. Toward a metabolic theory of ecology. Ecology. 2004;85:1771–1789. [Google Scholar]
- 2.Poff NL, et al. The natural flow regime. Bioscience. 1997;47:769–784. [Google Scholar]
- 3.Lytle DA, Poff NL. Adaptation to natural flow regimes. Trends Ecol Evol. 2004;19:94–100. doi: 10.1016/j.tree.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Wiens JA, Stralberg D, Jongsomjit D, Howell CA, Snyder MA. Niches, models, and climate change: assessing the assumptions and uncertainties. Proc Natl Acad Sci USA. 2009;106(Suppl 2):19729–19736. doi: 10.1073/pnas.0901639106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van der Putten WH, Macel M, Visser ME. Predicting species distribution and abundance responses to climate change: why it is essential to include biotic interactions across trophic levels. Philos Trans R Soc Lond B Biol Sci. 2010;365:2025–2034. doi: 10.1098/rstb.2010.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araújo MB, Luoto M. The importance of biotic interactions for modelling species distributions under climate change. Glob Ecol Biogeogr. 2007;16:743–753. [Google Scholar]
- 7.Poloczanska ES, Hawkins SJ, Southward AJ, Burrows MT. Modeling the response of populations of competing species to climate change. Ecology. 2008;89:3138–3149. doi: 10.1890/07-1169.1. [DOI] [PubMed] [Google Scholar]
- 8.Rahel FJ, Olden JD. Assessing the effects of climate change on aquatic invasive species. Conserv Biol. 2008;22:521–533. doi: 10.1111/j.1523-1739.2008.00950.x. [DOI] [PubMed] [Google Scholar]
- 9.Palmer MA, et al. Climate change and river ecosystems: protection and adaptation options. Environ Manage. 2009;44:1053–1068. doi: 10.1007/s00267-009-9329-1. [DOI] [PubMed] [Google Scholar]
- 10.Arnell NW. The effect of climate change on hydrological regimes in Europe: a continental perspective. Glob Environ Change. 1999;9:5–23. [Google Scholar]
- 11.Mohseni O, Erickson TR, Stefan HG. Sensitivity of stream temperatures in the United States to air temperatures projected under a global warming scenario. Water Resour Res. 1999;35:3723–3733. [Google Scholar]
- 12.Selong JH, McMahon TE, Zale AV, Barrows FT. Effect of temperature on growth and survival of bull trout, with application of an improved method for determining thermal tolerance in fishes. Trans Am Fish Soc. 2001;130:1026–1037. [Google Scholar]
- 13.Seegrist DW, Gard R. Effects of floods on trout in Sagehen Creek, California. Trans Am Fish Soc. 1972;101:478–482. [Google Scholar]
- 14.Fausch KD, Taniguchi Y, Nakano S, Grossman GD, Townsend CR. Flood disturbance regimes influence rainbow trout invasion success among five holarctic regions. Ecol Appl. 2001;11:1438–1455. [Google Scholar]
- 15.Warren DR, Ernst AG, Baldigo BP. Influence of spring floods on year-class strength of fall- and spring-spawning salmonids in Catskill mountain streams. Trans Am Fish Soc. 2009;138:200–210. [Google Scholar]
- 16.Barnett TP, et al. Human-induced changes in the hydrology of the western United States. Science. 2008;319:1080–1083. doi: 10.1126/science.1152538. [DOI] [PubMed] [Google Scholar]
- 17.Ohlund G, Nordwall F, Degerman E, Eriksson T. Life history and large-scale habitat use of brown trout (Salmo trutta) and brook trout (Salvelinus fontinalis) - implications for species replacement patterns. Can J Fish Aquat Sci. 2008;65:633–644. [Google Scholar]
- 18.Fausch KD, White RJ. Competition between brook trout (Salvelinus fontinalis) and brown trout (Salmo trutta) for positions in a Michigan stream. Can J Fish Aquat Sci. 1981;38:1220–1227. [Google Scholar]
- 19.Bradley BA. Regional analysis of the impacts of climate change on cheatgrass invasion shows potential risk and opportunity. Glob Change Biol. 2009;15:196–208. [Google Scholar]
- 20.Behnke RJ. Trout and Salmon of North America. New York: The Free Press, Simon and Schuster; 2002. [Google Scholar]
- 21.Burnham KP, Anderson DR. Model Selection and Multimodel Inference. New York, NY: Springer; 2002. [Google Scholar]
- 22.IPCC . Climate Change 2007: Working Group 2: Impacts, Adaptation and Vulnerability. Geneva: Intergovernmental Panel on Climate Change; 2007. [Google Scholar]
- 23.Littell JS, et al. Regional Climate and Hydrologic Change in the Northern US Rockies and Pacific Northwest: Internally Consistent Projections of Future Climate for Resource Management. Seattle, WA: University of Washington; 2010. [Google Scholar]
- 24.Baxter CV, Hauer FR. Geomorphology, hyporheic exchange, and selection of spawning habitat by bull trout (Salvelinus confluentus) Can J Fish Aquat Sci. 2000;57:1470–1481. [Google Scholar]
- 25.Dunham JB, Adams SB, Schroeter RE, Novinger DC. Alien invasions in aquatic ecosystems: Toward an understanding of brook trout invasions and potential impacts on inland cutthroat trout in western North America. Rev Fish Biol Fish. 2002;12:373–391. [Google Scholar]
- 26.Beechie T, Buhle E, Ruckelshaus M, Fullerton A, Holsinger L. Hydrologic regime and the conservation of salmon life history diversity. Biol Conserv. 2006;130:560–572. [Google Scholar]
- 27.Jager HI, Van Winkle W, Holcomb BD. Would hydrologic climate changes in Sierra Nevada streams influence trout persistence? Trans Am Fish Soc. 1999;128:222–240. [Google Scholar]
- 28.McCullough DA, et al. Research in thermal biology: burning questions for coldwater stream fishes. Rev Fish Sci. 2009;17:90–115. [Google Scholar]
- 29.Heikkinen RK, Luoto M, Virkkala R, Pearson RG, Korber JH. Biotic interactions improve prediction of boreal bird distributions at macro-scales. Glob Ecol Biogeogr. 2007;16:754–763. [Google Scholar]
- 30.Pearson RG, Dawson TP. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Glob Ecol Biogeogr. 2003;12:361–371. [Google Scholar]
- 31.Rieman BE, Hessburg PF, Luce C, Dare MR. Wildfire and management of forests and native fishes: conflict or opportunity for convergent solutions? Bioscience. 2010;60:460–468. [Google Scholar]
- 32.Fausch KD, Rieman BE, Dunham JB, Young MK, Peterson DP. Invasion versus isolation: trade-offs in managing native salmonids with barriers to upstream movement. Conserv Biol. 2009;23:859–870. doi: 10.1111/j.1523-1739.2008.01159.x. [DOI] [PubMed] [Google Scholar]
- 33.DeStaso J, Rahel FJ. Influence of water temperature on interactions between juvenile Colorado River cutthroat trout and brook trout in a laboratory stream. Trans Am Fish Soc. 1994;123:289–297. [Google Scholar]
- 34.Wenger SJ, et al. Role of climate and invasive species in structuring trout distributions in the Interior Columbia Basin. Can J Fish Aquat Sci. 2011;68:988–1008. [Google Scholar]
- 35.Harris A. Trout Fishing in 2006: A Demographic Description and Economic Analysis. Arlington, VA: US Fish and Wildlife Service; 2010. [Google Scholar]
- 36.Baxter CV, Fausch KD, Murakami M, Chapman PL. Fish invasion restructures stream and forest food webs by interrupting reciprocal prey subsidies. Ecology. 2004;85:2656–2663. [Google Scholar]
- 37.Isaak DJ, et al. Effects of climate change and wildfire on stream temperatures and salmonid thermal habitat in a mountain river network. Ecol Appl. 2010;20:1350–1371. doi: 10.1890/09-0822.1. [DOI] [PubMed] [Google Scholar]
- 38.Wenger SJ, Luce CH, Hamlet AF, Isaak DJ, Neville HM. Macroscale hydrologic modeling of ecologically relevant flow metrics. Water Resour Res. 2010;46 W09513. [Google Scholar]
- 39.Elsner M, et al. Implications of 21st century climate change for the hydrology of Washington State. Clim Change. 2010;102:225–260. [Google Scholar]
- 40.Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analsyis Methods. London, UK: Sage Publications; 2002. [Google Scholar]
- 41.Wagner T, Hayes DB, Bremigan MT. Accounting for multilevel data structures in fisheries data using mixed models. Fisheries (Bethesda, MD) 2006;31:180–187. [Google Scholar]
- 42.Bates D, Maechler M. 2009. lme4: Linear mixed-effects models using S4 classes, R package version 0.999375-32. [Google Scholar]
- 43.R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. [Google Scholar]
- 44.Arnold T. Uninformative parameters and model selection using Akaike's Information Criterion. J Wildl Manage. 2010;74:1175–1178. [Google Scholar]
- 45.Randin CF, et al. Are niche-based species distribution models transferable in space? J Biogeogr. 2006;33:1689–1703. [Google Scholar]
- 46.Vaughan IP, Ormerod SJ. The continuing challenges of testing species distribution models. J Appl Ecol. 2005;42:720–730. [Google Scholar]
- 47.Liu CR, Berry PM, Dawson TP, Pearson RG. Selecting thresholds of occurrence in the prediction of species distributions. Ecography. 2005;28:385–393. [Google Scholar]
- 48.Rieman B, Dunham J, Peterson J. Development of a Database to Support a Multi-Scale Analysis of the Distribution of Westslope Cutthroat Trout. Boise, ID: USDA Forest Service Rocky Mountain Research Station; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.