Abstract
The Epidermal Growth Factor Receptor (EGFR) is the prototypical receptor tyrosine kinase (RTK). These cell surface receptors are integral membrane proteins that bind ligands on their extracellular domain and relay that information to within the cell. The activated EGFR regulates diverse cell fates such as growth, proliferation, differentiation, migration, and apoptosis. These signaling properties are important for the appropriate development and maintenance of an organism. However, when inappropriately controlled, due to EGFR overexpression or hyperactivation, these signaling events are characteristic of many cancers. It remains unclear whether the uncontrolled EGFR activity leads to cell transformation or is a consequence of cell transformation. Regardless of the cause, increased EGFR activity serves both as a biomarker in the diagnosis of some cancers and is a molecular target for anti-cancer therapies. The promising results with current anti-EGFR therapies suggest that the receptor is a viable molecular target for a limited number of applications. However, to become an effective therapeutic target for other cancers that have elevated levels of EGFR activity, current approaches for inhibiting EGFR signaling will need to be refined. Here we describe the molecular mechanisms that regulate EGFR inactivation and discuss their potential as therapeutic targets for inhibiting EGFR signaling.
Keywords: EGFR, endocytosis, degradation, MVB
The ErbB Family
The EGFR, or ErbB-1, is one of four members of the ErbB family that also includes ErbB-2, ErbB-3 and ErbB-4 (Carpenter, 2003; Citri et al. 2003). The EGFR is expressed as a monomeric 1186 amino acid transmembrane protein with approximately half of the protein extracellular (621 amino acids) (Ullrich et al. 1984). The receptor contains three important regions—the extracellular ligand binding domain, a transmembrane-anchoring domain, and an intracellular intrinsic kinase domain (Chinkers and Brugge, 1984; Downward et al. 1984a). The other ErbB family members are structurally similar, but differ in their preference for ligands, ability to associate with other family members, tissue distribution, and signaling properties (Wiley, 2003).
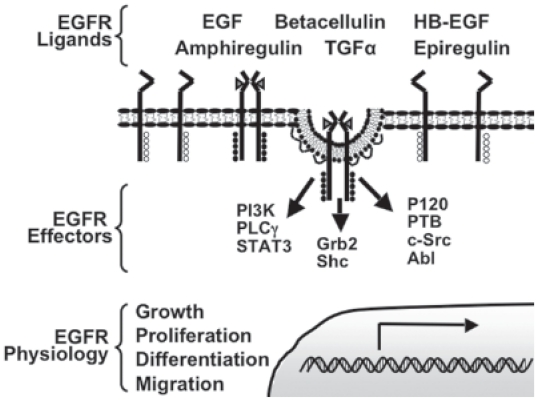
Activation of the EGFR is dependent on binding ligand in the extracellular space that has been secreted in either an autocrine or paracrine manner (Fig. 1). There are six unique endogenous ligands for the EGFR helping generate diverse signals: EGF, transforming growth factor α (TGF-α) heparin-binding EGF (HB-EGF), amphiregulin, betacellulin, and epireglin (Harris et al. 2003). These ligands differ from one another in their regulated secretion, tissue distribution, and binding properties. Studies in which specific ligands have been ablated, either surgically or genetically, indicate that often one ligand can compensate for another (Tsutsumi et al. 1987). When three different ligands (EGF, TGF-α, and amphiregulin) were simultaneously knocked out of C57 black mice, there were defects in the skin, eye development, mammary gland development, and coat hair. When only one or two of the three ligands were ablated, there were only subtle phenotypes. One of which was mammary gland development when amphiregulin was knocked out in conjunction with TGFα or EGF (Luetteke et al. 1999). These findings suggest there are complementary roles for these ligands and underscore the difficulty in understanding receptor physiology in the context of a whole animal.
Figure 1. Schematic of EGFR activation.
Inactive EGFR exist as monomers on the plasma membrane. Upon binding of one of six endogenous ligands, two monomers dimerize and activate the receptor’s intrinsic kinase domain. The active kinase domain of one EGFR monomer transphosphorylates tyrosine residues on carboxyl terminus of its receptor pair. Once activated, the phosphotyrosines serve as docking site for downstream effectors, which include enzymes, adaptor proteins, and other regulatory molecules. Signaling from effectors integrates to modulate cell physiology, some of which are indicated. Phosphatidyl inositol 3-kinase (PI3K), phospholipase Cγ (PLCγ), signal transducers and activators of transcription 3 (STAT3), Growth factor receptor-bound protein 2 (Grb2), Src homology containing protein (Shc), p120 ras GTPase activating protein (P120), phosphatase B (PTB), cellular sarcoma (c-Src), and Abl.
The EGFR is expressed in a variety of tissues (Yano et al. 2003). The EGFR is also critical to the development of tissues of epithelial, mesenchymal, and neuronal origin. EGFR knockout mice have been bred from different genetic backgrounds, showing either embryonic or postnatal lethality with multiple organ defects (Miettinen et al. 1995; Sibilia and Wagner, 1995). These findings point to the essential role of the EGF receptor in the development and tissue homeostasis of organisms in which it is expressed. Thus, the complete loss of EGFR function is deleterious to the organism.
EGFR Activation
Ligand binding initiates receptor activation by inducing a conformational change and allowing for dimerization of two EGF receptor monomers (Ferguson et al. 2003). Dimerization leads to transphosphorylation of tyrosine residues on the cytoplasmic tail of one receptor by the intracellular kinase domain of the corresponding dimer (Lammers et al. 1990). Tyrosine phosphorylation is the essential activation step in EGFR signal transduction as these residues serve as docking sites for downstream signaling molecules containing Src homology 2 (SH2) or phosphotyrosine binding (PTB) domains. Signaling pathways activated by the EGFR include the phosholipase C gamma (PLCγ), mitogen-activated protein kinase (MAPK), and phosphatidylinositol 3 kinase (PI3K), Signal Transducers and Activators of Transcription 3 (STAT3) and Akt pathways (Yarden and Sliwkowski, 2001). The activities and biochemical changes induced by these signaling pathways integrate to mediate the specific modulation in cell biology such as cell growth, proliferation, differentiation, migration, and regulation of apoptosis (Jorissen et al. 2003).
EGFR and Cancer
The first link between EGFR and malignant transformation came from studies that showed the EGFR is a homolog of the avian erythroblastosis virus v-erbB oncogene (Downward et al. 1984b). Several years later, a direct link between increased signaling from the EGFR and malignant growth was found in Epstein-Barr virus infected cells, a condition already known to lead to epithelial malignancies (Miller et al. 1995). Clinical studies provided supporting evidence that many human tumors and cell lines derived from human tumors overexpress EGFRs. Together, these data pointed to a potential role for the EGFR in tumor formation (Libermann et al. 1985; Merlino et al. 1984; Yamamoto et al. 1986). The hypothesized link between EGFR and cancer was strengthened when in vitro studies showed infection of NIH-3T3 cells with either retrovirus encoding EGFR, or a eukaryotic vector encoding EGFR cDNA, induced a transformed phenotype (Di Fiore et al. 1987; Velu et al. 1987).
EGFR overexpression and/or hyperactivation is associated with a number of cancers such as breast, ovary, renal, non-small cell lung, head and neck, colorectal, pancreatic, prostate, cervical and bladder (Sebastian et al. 2006). Overexpression is used as an indicator of poor prognosis in breast, ovarian, and head and neck cancers (Fischer-Colbrie et al. 1997; Ishitoya et al. 1989; Magne et al. 2001; Sebastian et al. 2006). In the clinic, increased EGFR expression has also been implicated with resistance to hormonal therapies in advanced stage breast cancers (Newby et al. 1997).
Although, it remains controversial as to whether EGFR overexpression is the cause of the cancer or a secondary consequence, the strong association between the EGFR and cancer has made it a natural candidate as an anti-cancer chemotherapeutic. Enhanced EGFR signaling can arise from a variety of mechanisms including receptor overexpression, mutations leading to constitutive activation, increased ligand production, or defective inactivation (Zandi et al. 2007). Early studies by Haigler and Carpenter indicated that in cultured cells, inhibition of ligand binding with an EGFR specific antibody could prevent DNA synthesis (Haigler and Carpenter, 1980). Since then, the EGFR has been a major molecular target in the treatment of cancer.
Current EGFR-Targeted Inhibition Strategies
From the work in tissue culture, two strategies have emerged for inhibiting uncontrolled cell growth arising from EGFR overexpression or hyperactivation—monoclonal antibodies (MAbs) and tyrosine kinase inhibitors (TKIs). These approaches share the same goal of inhibition of receptor activity but differ in their molecular mechanism. MAbs target the extracellular portion of the receptor whereas the TKIs inhibit the intracellular portion. Multiple drugs from each class have been approved by the FDA for the treatment of certain types of cancers.
There are two monoclonal antibodies that have approval from the Food and Drug Administration (FDA)—Cetuximab (Erbitux) [February 2004] and Vectibix (Panitumumab) [September 2006]. These antibodies are used therapeutically for the treatment of metastatic colorectal cancer and cancers of the head and neck. Both Cetuximab and Vectibix inhibit binding of ligands to the EGFR causing a decrease in basal and ligand mediated receptor activation (Harari et al. 2007; Huang et al. 1999; Moroni et al. 2005). The decreased receptor activity inhibits cell growth, induces apoptosis, and decrease the production of other cellular factors associated with cancer progression and metastasis, such as matrix metalloproteinases, and vascular endothelial growth factor (Astsaturov et al. 2006; Zhu, 2007). Currently, the MAbs are used in combination with other agents or alone when the cancer is refractory to standard therapy.
The other class of EGFR inhibitors, TKIs, are small molecules that block EGFR activity by competing with ATP for use as a substrate by the receptor’s intrinsic kinase domain. The FDA has approved two EGFR-specifc TKIs, Iressa (Gefitinib) [December 2004] and Tarceva (Erlotinib) [November 2004], for the treatment of non-small cell lung carcinoma (Morgillo et al. 2007). A third FDA-approved drug, Lapatinib, can inhibit the kinase activity of both the EGFR and ErbB2. Lapatinib is used in combination with Herceptin for the treatment of HER2/neu positive breast cancers that are resistant to other therapies (Moy et al. 2007). Like the MAbs the mechanism of action for the TKIs is to block the activation of downstream signaling pathways. Interestingly, Gefitinib has been shown to be most effective in ~10% of non-small cell lung carcinoma patients with mutations around the ATP binding domain of the EGFR (Lynch et al. 2004).
Additional strategies targeting the EGFR focus on the rate at which new receptors are made and target the mRNA that encodes the EGFR. These approaches include EGFR-specific RNA interference (O’Grady et al. 2005), antisense oligonucleotides (Melisi et al. 2004), and ribozymes (Yamazaki et al. 1998). While these are effective approaches in tissue culture models, the methodology for delivering RNAi, oligonucleotides, and ribozymes to patients requires further development.
Limitations of the Current Therapies that Target the EGFR
It should be noted that there are some restrictions to the FDA approved drugs that inhibit EGFR signaling and cancer growth. First, despite the wide range of cancers that are characterized by EGFR overexpression and/or hyperactivation, these drugs have only been approved for a limited number of cancers. However, both classes of drugs are currently enrolling patients in Phase 2 and Phase 3 trials for the treatment of other EGFR positive cancers, such as cervical, skin, myelogenous leukemia, prostate and glioblastomas (ClinicalTrials.gov, 2007).
Second, MAb and TKI therapies have numerous unpleasant side effects. Dermatological (rash, light sensitivity, and acne) and gastrointestinal (diarrhea, loss of appetite, and nausea) side effects have been reported for both therapeutic approaches (Rocha-Lima et al. 2007). Since proper EGFR function is required for normal skin and gastrointestinal mucosal regeneration (Grazul-Bilska et al. 2003; Jones et al. 1999), these toxicities are in all likelihood due to EGFR inhibition and aberrant EGFR-mediated regulation of tissue homeostasis.
Novel Targets for Attenuating EGFR Signaling in Cancer
The treatment of patients with tumors that overexpress EGFRs could benefit from novel methods of inhibiting EGFR signaling. Alternative approaches to targeting the EGFR need to take into consideration the empirically determined strengths and weaknesses of the current therapies.
Rather than inhibit the activation of the EGFR, an alternative approach would be to accelerate the rate of receptor inactivation. Strategies to attenuate the activated EGFR in cancers by accelerating the endocytic process have been largely overlooked. Components of the endocytic pathway could be stimulated to accelerate the normal rate of signal attenuation. At this time, there are no agents under consideration, but that likely reflects the lack of molecular details regarding the EGFR signal termination and which molecules would be the best candidates.
Despite the incomplete understanding of this process, there are some obvious advantages to this strategy. By decreasing the duration of the active EGFR, the signaling necessary for cellular homeostasis would still be permitted while uncontrolled cell growth and replication would be inhibited. Second, those cells with the highest levels of receptor expression and/or activity would be affected the most. Third, by targeting receptor inactivation, there is no discrimination between uncontrolled cell growth that arises due to receptor overexpression versus receptor hyperactivation. This may allow such compounds have a broader applicability.
Below we discuss four potential mechanisms for signal termination of the activated EGFR from within the endocytic pathway: 1) dissociation of the ligand:receptor complex, 2) phosphatase-mediated receptor dephosphorylation 3) sequestration of the activated EGFR from effector molecules, and 4) lysosomal degradation of the receptor (Fig. 2). These molecular mechanisms have been shown to be effective ways of attenuating EGFR signaling in tissue culture models, but it remains unclear which ones are physiologically important. Nevertheless, at this point, they all remain as viable pharmacological targets. This discussion will include the pros and cons of each mechanism.
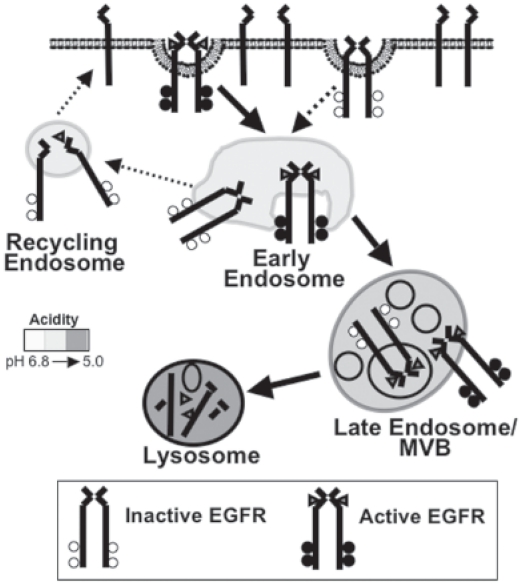
Figure 2. Ligand stimulated EGFR Endocytic trafficking (indicated by solid arrows).
Ligand stimulation accelerates the rate of EGFR internalization via clathrin-coated pits. Following invagination and pinching off, the resulting clathrin-coated vesicle sheds its clathrin and delivers its cargo to the early endosome. In the early endosome, the cargo is sorted for delivery to its appropriate cellular location. In most cases, the EGFR is delivered to the lysosome for degradation. Early endosome matures into a late endosome/multiviesicular body (MVB). The contents of the late endosome/MVB are delivered to a lysosome for degradation. Indicated with dashed arrows are other possible routes of endocytic trafficking. A small percentage of total EGFR internalizes via clathrin-coated pits in a ligand independent manner (~1%–2%/min). In addition, unliganded EGFR will traffic from the early endosome to the plasma membrane via a recycling endosome upon ligand dissociation.
EGFR Endocytic Trafficking
Ligand binding mediates two related functions. First, as mentioned above, is the initiation of downstream signaling pathways. Second, the ligand: receptor complex is internalized. This process, termed endocytosis, reduces the amount of receptor available for ligand binding on the cell surface as well as inactivates the receptor through dephosphorylation and/or receptor degradation. In addition, the internalization of the receptor physically moves the receptor through various endocytic compartments, and thereby changes the downstream effectors with which it has contact. Ligand-mediated receptor endocytosis has historically been overlooked as a molecular mechanism to attenuate the signaling of the EGFR.
There is a steady-state cycle in which the EGFR is slowly internalized (~1%–2% of the total receptor population/min) and rapidly recycled to the cell surface (Lund et al. 1990). However, ligand binding accelerates this process and induces a more dramatic redistribution of the receptor from the plasma membrane and directs the activated EGFR to the lysosome for degradation (Fig. 2). The EGFR is the only member of the ErbB family shown to undergo ligand-mediated internalization. In cells that express multiple ErbB family members, receptors that heterodimerize with the EGFR prevent endocytosis of the entire ligand:receptor complex (Baulida et al. 1996, Lenferink et al. 1998).
At the plasma membrane, ligand-bound EGFRs move laterally along the cell surface to a plasma membrane domain whose intracellular face is enriched with clathrin. The membrane domain invaginates to give rise to a clathrin-coated pit that eventually pinches off to form a clathrin-coated vesicle containing the ligand:receptor complex. Once inside the cell, the clathrin is shed from the vesicle and is now referred to as a primary endocytic vesicle. This vesicle fuses with the early endosome, and delivers the EGF:EGFR complex. Through endosomal maturation, the cargo arrives in a second endosomal compartment often referred to interchangeably as the multivesicular body (MVB) or late endosome. During this maturation, membrane structures form in the lumen, called intralumenal vesicles. In these internal vesicles, the EGFR is oriented such that the carboxyl terminal phosphotyrosines no longer have access to effector proteins. Finally, cargo is transferred to the lysosome by fusion of the endosome with lysosome, and degraded in the acidic, protease rich environment (Sorkin and von Zastrow, 2002).
Ligand-mediated endocytosis has always been recognized as a means of regulating EGFR signaling, but the mechanisms of regulation are still being discovered. Early studies by Wells et al. used truncation mutants of the EGFR that retained the ability to signal, but could not internalize. NIH 3T3 cells expressing these mutants underwent EGF-dependent cell transformation/mitogenesis at lower doses (Wells et al. 1990). From these data, it was concluded that internalization of the EGFR played a role in attenuating EGFR-mediated responses. This idea was challenged by Vieira et al. when they inhibited EGFR internalization using a dominant negative mutant of the large GTPase, dynamin. Dynamin regulates the internalization of clathrin-coated pits, and the expression of dominant negative dynamin allowed activation of the EGFR and retention at the plasma membrane. In these experiments, it was determined that full activation of MAPK and PI3-K could not be achieved if the receptor were retained on the cell surface. Conversely, the activity of some effectors (i.e., PLCγ and Shc) was enhanced by cell surface retention of the receptor. Thus, receptor internalization both positively and negatively regulates signaling (Vieira et al. 1996).
The compartmentalization of the EGFR as it moves through the endocytic pathway provides additional mechanisms to regulate receptor:effector interactions. It remains to be seen whether the signaling from a given endocytic compartment can be attributed to a specific cell physiology. If this does prove to be the case, inhibition of EGFR signaling from distinct cellular locations may be a new way to modulate receptor response.
Ligand:Receptor Dissociation
Within 5–10 minutes of ligand stimulation, the ligand:receptor complex enters the cell and traffics to the early endosome. The early endosome, as well as all subsequent endosomes, is characterized by its increasingly acidic environment. The early endosome is recognized as a point of sorting in the cell where cargoes are directed to a variety of cellular locations such as the late endosome, endoplasmic reticulum, or to the plasma membrane. For the EGFR, it has been shown that all three routes are viable options and dependent on the cell type. However, ligand-stimulated EGFR degradation is a saturable process, and trafficking to the lysosome may be the primary destination of the ligand:receptor complex in cells with the physiologic levels of receptor and trafficking proteins (French et al. 1994; Mizuno et al. 2005). Similarly, recycling of the stimulated EGFR back to the plasma membrane may be the consequence of receptor overexpression (Masui et al. 1993). Targeting to the ER and onto the nucleus is a relatively new model, but has been shown in multiple cell lines and affects the transcription of cyclin D, and important regulator of cell cycle regulation (Liao and Carpenter, 2007).
The molecular mechanisms of some aspects of early endosomal sorting have been well-established. Binding of ligand to the EGFR is pH sensitive, with optimal binding occurring at physiological pH and dissociation occurring at lower pHs. The lower pH of the early endosome can cause dissociation of the receptor from the ligand. However, all ligand:EGFR interactions are not affected equally by pH. For instance, TGF-α is more sensitive to the early endosome pH and therefore dissociates more readily in the early endosome (Korc and Finman, 1989). Once free of ligand, the receptor becomes rapidly desphosphorylated, thereby inhibiting interactions with downstream effectors, such as c-Cbl (Lenferink et al. 1998). In the absence of c-Cbl association and receptor ubiquitylation, the receptor is not properly targeted to the lysosome for destruction and instead recycles to the plasma membrane. Thus, the TGF-α:EGFR dissociation induced in the early endosome has the immediate consequence of receptor inactivation, but the potential for multiple rounds of receptor activation and enhanced signaling.
If one considers the models of compartmentalized signaling, a strategy of ligand:receptor dissociation may be effective for inhibiting EGFR signals from the late endosome/MVB, as the activated complex would never enter that compartment. To date, it has not been shown that unique or significant signaling originates from the late endosomes, although this remains a formal possibility. Overall, ligand:receptor dissociation has limited potential as a mechanism for inhibiting EGFR signaling, unless additional measures could be taken to ensure the unbound receptor were targeted for degradation.
Inactivation by Protein Tyrosine Phosphatases
A second mechanism by which EGFRs are inactivated is the catalyzed dephosphorylation of the receptor by phosphatases. The discovery of protein-tyrosine phosphatases (PTP) came several years after that of tyrosine kinases, but they were immediately recognized as important regulatory components of signaling (Tonks et al. 1988). Disruption of the reciprocal interactions between kinases and phosphatases can result in dramatic changes in cell physiology (Tonks and Neel, 2001). All PTPs share a central catalytic domain, while the differences in their amino and carboxy terminus confer unique cellular locations and binding partners (Barford et al. 1995). PTPs dephosphorylate substrates through the formation of a covalent bond between a phosphatase cysteine residue and the substrate phosphate followed by hydrolysis (Cirri et al. 1993; Zhang and VanEtten, 1991).
There are approximately 100 members of the PTP superfamily. Thirty-eight (38) comprise the “classical” tyrosine-only specific subfamily, and the remainder belonging to the dual specificity phosphatases (DSPs) which dephosphorylate tyrsosine, threonine or serine residues (Tonks, 2006). To date, more is known about tyrosine phosphatase regulation of EGFR signaling than regulation by dual specificity phosphatases. The tyrosine-only phosphatases can be further divided into the transmembrane receptor-like PTPs and the intracellular non-receptor PTPs (Andersen et al. 2004). Both receptor and non-receptor tyrosine phosphatases have been shown to directly regulate EGFR activity (Suarez Pestana et al. 1999; Tomic et al. 1195). This regulation can occur at multiple levels: by choice of protein substrate (receptor), recognition sequence, and subcellular localization.
The development of “substrate-trapping” mutants of phosphatases has proved to be an invaluable tool for identifying specific substrates. Through mutation of either critical residues of the active site (cysteine) or the catalytic domain (asparigine), these mutants overcome the transient nature of the enzyme-substrate complex to retain high affinity binding of phosphatases to their substrates (Flint et al. 1997). These mutatants were key in the identification of the EGFR as a substrate for numerous phosphatases. Substrate trapping mutants were expressed in COS1 cells and used to co-immunoprecipitate the T-cell protein tryosine phosphatase (TCPTP) and the EGFR. Subsequently it was shown that overexpression of transfected TCPTP, but not the substrate-trapping mutant, dephophorylated the receptor in an EGF-dependent manner, indicating the presence of a regulatory feedback mechanism (Tiganis et al. 1998). In a subsequent study in COS1 cells, TC45, the nuclear form of TCPTP, was transiently overexpressed and examined for effects on EGF-induced activation of specific signaling pathways. Overexpression of TC45 caused a decrease in EGFR phosphorylation and reduced signaling to downstream effectors, namely PI3-K- mediated activation of Akt (Tiganis et al. 1999). Similarly, in U87MG cells, a glioblastoma-astrocytoma cell line that normally expresses low levels of TC45, stable overexpression of TC45 negatively regulates the receptor and decreased both Akt and MAPK signaling (Klingler-Hoffmann et al. 2001). Together, these studies illustrate how the targeted dephosphorylation of the EGFR can decrease the activity of effectors that lead to cell growth.
The specificity of a phosphatase can also be intramolecular. The existence of site-specific phosphatases that differentially dephosphorylate particular phospho-tyrosines on the carboxy terminus of the EGFR is well established. The loss of these phosphotyrosines allows for regulation of individual EGFR signaling pathways. Using the previously described substrate-trapping mutants, the direct association between receptor-type protein-tyrosine phosphatase-κ (RPTP-κ) and the EGFR was demonstrated when the two proteins were co-expressed in Chinese Hamster Ovary cells which do not normally express either protein. The RPTP-κ has been shown in vitro to rapidly dephosphorylate EGFR at phosphotyrosine residues 1068 and 1173, but not phosphotyrosine 992. While overexpression of RPTP-κ in human keratinocytes led to decreased levels of phosphorylated EGFR and growth, RNAi mediated knockdown had the reciprocal effect of increasing basal and EGF-mediated phosphorylation levels and MAPK activity (Xu et al. 2005).
Tyrosine-residue specific dephosphorylation has also been identified for the non-receptor SH2-domain containing PTPs, SHP-1, and SHP-2. SHP-1 associates directly with the EGFR in human mammary carcinoma cells through phosphotyrosine 1173 on the EGFR (Keilhack et al. 1998; Tomic et al. 1995), while SHP-2 mediates the dephosphorylation of tyrosine 992 (Agazie and Hayman, 2003). Overexpression of SHP-1 in human epithelial cells led to the downregulation of MAPK activity, while expression of the tyrosine 1173 to phenylalanine mutant (Y1173F) EGFR mutant, prevents SHP-1 binding to the receptor, and leads to enhanced EGFR-mediated MAPK signaling (Keilhack et al. 1998).
The third mechanism by which phosphatase activity is regulated is by restriction of its cellular distribution, thereby controlling where in the cell EGFR signaling is terminated. By adding back a fluorescently-tagged, catalytically inactive PTPB1 mutant to PTP1B−/− mouse fibroblasts, Haj et al. were able to determine the subcellular localization at which the modified phosphatase associated with a GFP-tagged EGFR (Haj et al. 2002). As expected, the ligand-stimulated GFP-EGFR internalized. Once inside the cell, the EGFR trafficked to the ER where PTPB1 association was determined by Fluorescence Energy Transfer (FRET). The authors propose this to be the site of dephosphorylation, and suggest this occurs prior to the EGFR being targeted to the lysosome. The authors speculate that there may be “dephosphorylation compartments” at other locations within the cell. Intriguingly, they propose that endocytosis of the receptor may potentiate signaling by removing the receptor from plasma membrane localized PTPs.
It is important to note that increases in phosphatase activity are not always associated with decreased EGFR signaling. Increased phosphatase activity can also positively regulate EGFR signaling. For instance it has been shown than SHP-2 specifically dephosphorylates phosphotyrosine 992 of the EGFR (Agazie and Hayman, 2003). Interestingly, the Drosophila homolog of SHP-2 dephosphorylates the binding site for the SH2 domain of the Ras GTPase activating protein (RasGAP), which hydrodylzes GTP bound Ras to GDP Ras. Therefore, positive regulation of EGFR through inhibiting the activity of a negative regulator of downstream EGFR signaling seems the most likely mechanism of SHP-2′s positive effect on EGFR signaling activation.
When considering strategies to attenuate EGFR signaling, there are a number of options including enhanced phosphatase recruitment, expression, and activation of receptor specific inactivating phosphatases and inhibition of phosphatases, such as SHP-2, that positively regulate signaling. However, prior to phosphatase becoming a therapeutic target, several important questions regarding molecular mechanism must be answered. For instance, is the phosphatase specific for the EGFR or will other receptors also be affected? Are the tyrosines that are dephosphorylated going to affect signaling pathways required for cell growth and/or survival? Are the changes in phosphatase activity going to positively or negative regulate signaling? Nevertheless, the fact the modulation of phosphatase activity and expression in tissue culture models causes measurable changes in cell physiology, strongly supports the idea that phosphatases are viable therapeutic targets.
Trafficking Mediated EGFR Inactivation
As mentioned previously, the EGFR remains active during its endocytic trafficking. Both the phosphorylated receptor and downstream effectors have been isolated from early endosomes (DiGuglielmo et al. 1994; Lai et al. 1989). There are two logical points in the late endocytic pathway in which receptor inactivation may occur. The first point is when the ligand receptor complex gets sequestered into the intralumenal vesicles of the MVB. This causes the physical separation of the receptor and downstream effectors. However, it is controversial as to whether this event functionally attenuates receptor-mediated signaling. The second potential point of signal termination is the lysosomal degradation of the receptor. While this will clearly terminate the signaling process, since it follows receptor sequestration it may not be physiologically relevant.
EGFR sequestration
The appearance of the ligand-stimulated EGFR in multivesicular endosomes has been reported by numerous groups in a variety of cell types (Carpentier et al. 1987; Dunn et al. 1986; Miller et al. 1986). The ultrastructural analysis of liver carcinoma cell lines revealed stimulation with EGF stimulates MVB biogenesis and increases the number of internal vesicles in MVBs (White et al. 2006). The data indicating the liganded EGFR facilitates receptor sequestration into MVBs allows for the generation of a model in which this process separates the receptor and effector, thereby limiting the duration of receptor signaling. Until recently, there were no data that indicated that the removal of the activated receptor from the cytosol to the intralumenal vesicle affected signaling. Thus, it was unclear whether entry into MVB was a regulatory mechanism for EGFR signaling or an intermediate step on the path to lysosomal degradation.
EGFRs destined for recycling are retained on the limiting membrane of the early endosome. Receptors that make it to the late endosome/MVB accumulate onto areas of the limiting membrane of the late endosome that invaginate and pinch off to form internal vesicles within the late endosome/MVB. Sequestration of the EGFR within MVBs not simply a passive event, as receptor activation mediates this lysosomal sorting through a series of highly conserved, regulated, and concerted steps (Felder et al. 1990). The molecular identity of many of the proteins involved in this sorting were first identified in yeast genetic studies and are referred to as the vacuolar protein sorting (Vps) proteins (Katzmann et al. 2002; Raymond et al. 1992). The role of an increasing number of mammalian homologs has been confirmed in tissue culture models (Babst, 2005).
Targeting of the EGFR for degradation begins with the phosphorylated receptor associating with the E3 ubiquitin ligase c-Cbl, ubiquitylation of the receptor, and targeting of the receptor for degradation. Ubiquitylation of the EGFR has an established role in sorting the receptor for lysosomal degradation, but the role of this process in internalization remains controversial (Duan et al. 2003; Huang et al. 2006; Jiang and Sorkin, 2003). The current model for sorting is recognition of the ubiquitinated receptor by the ubiquitin-interacting motif (UIM) of hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) in complex with STAM-1 (Bache et al. 2003; Urbe et al. 2003). Together, these proteins recruit related protein complexes, known as the ESCRT (Endosomal Sorting Complex Required for Transport) complexes I, II, and III (Hurley and Emr, 2006). These complexes, along with several other highly conserved proteins, are responsible for directing the ligand: receptor complex to the intralumenal vesicles and subsequent lysosomal degradation (Table 1).
Table 1.
Role of various proteins in EGFR trafficking and signaling.
| Complex/Protein | MVB | EGFR degradation with KD | EGFR signaling with KD | Citation |
|---|---|---|---|---|
| ESCRT- I | ||||
| Tsg101/Vps23 | Inhibits MVB biogenesis | ↓ ↓ ↓ | Sustained MAPK activation | (Babst et al. 2000; Bache et al. 2006b; Doyette et al. 2005; Malerod et al. 2007; Olabisi et al. 2006; Razi and Futter, 2006) |
| VPS28/Vps28 | N.D. | Inhibits degradation (antibody) | N.D. | (Bishop et al. 2002) |
| VPS37/Vps37 | N.D. | ↓ ↓ | N.D. | (Bache et al. 2006a) |
| ESCRT-II | ||||
| EAP30/Vps22 | Decrease in EGFR ILV sequestrastion | ↓ ↓ | MAPK—no change | (Malerod et al. 2007) |
| EAP20/Vps25 | N.D. | No change (Bowers) ↓ ↓ (Langelier et al.) |
N.D. | (Bowers et al. 2006; Langelier et al. 2006) |
| ESCRT-III | ||||
| CHMP6/Vps20 | N.D. | ↓ | N.D. | (Langelier et al. 2006) |
| CHMP3/Vps24 | Decreased size of MVBs | ↓ | MAPK—no change | (Bache et al. 2006b; Yan et al. 2005) |
| Other | ||||
| Hrs/Vps27 | Increased MVB size; decreased ILVs | ↓ | ↑ MAPK | (Bache et al. 2003; Lu et al. 2003; Malerod et al. 2007; Razi and Futter, 2006) |
| Rab7 | N.D. | ↓ ↓ (dominant neg) ↓ ↓ ↓ (knockdown) |
(Ceresa and Bahr, 2006) personal communication | |
| Vps4/Vps4 | Reduced # of ILVs (mutant) | N.D. | (Sachse et al. 2004) | |
| Vps34 | Decreased ILV formation | ↓ | N.D. | (Futter et al. 2001) |
| UBPY/Doa4 | Increased # and size of MVB; fewer ILVs | ↓ ↓ | N.D. | (Row et al. 2006) |
| LIP5/Vtal | N.D. | ↓ ↓ ↓ | N.D. | (Ward et al. 2005) |
Notes: Shown in a partial listing of proteins that have been shown to have a role in EGFR trafficking through the late endocytic pathway. N.D. = not determined. Downward arrows represent the change in EGFR degradation kinetics. ↓ ↓ ↓, ↓ ↓, and ↓ indicate an increase in the half-life of ligand-stimulated EGFR by >5-fold, 3–4 fold, and 1–2 fold, respectively. Unless otherwise noted, studies were done by knocking down protein expression. # refers to the number. ILV = Intralumenal vesicles.
ESCRT mediated EGFR sequestration
Through mediation of MVB biogenesis and lysosomal sorting, proteins implicated in the late endocytic trafficking of the EGFR can impact signaling. Multiple lines of evidence, in both mammalian and C. elegans models, indicate the Cbl-mediated ubiquitination of the EGFR negatively regulates EGFR signaling (Levkowitz et al. 1999; Yoon et al. 1995). The hrs deletion mutants in Drosophila reveal impairment in MVB formation leading to sustained EGFR phosphorylation and MAPK activity. The phenotype of hrs deletion mutants is the embryonic expansion of cells dependent on the EGFR during development. Therefore, the role of the earliest mediators of lysosomal sorting in signaling is well documented, whereas the role of the remaining mediators is only beginning to be understood.
Recent studies by Bache et al. examined the contribution of proteins from the ESCRT-I or ESCRT-III complex in EGFR trafficking and signaling. Using HeLa cells as a model, two ESCRT proteins, Tsg101 (ESCRT-I), and Vps24 (ESCRT-III) were independently knocked down by RNAi (Bache et al. 2006b). While the knockout of either protein caused significant delays in ligand-stimulated receptor degradation, only knockdown of Tsg101 sustained EGFR signaling to the effectors MEK and MAPK. Using high-resolution electron microscopy, the authors observed that knockdown of Vps24 caused the accumulation of the EGFR within internal vesicles of endosomes that appeared smaller than typical MVBs. A failure to sequester the EGFR and retain it on the limiting membrane of MVBs has been previously reported when TSG101 levels are depleted by RNAi (Razi and Futter, 2006). These results support the model that sorting to lumenal vesicles of late endocytic compartments sufficiently terminates receptor signaling. A link between Tsg101 function and the uncontrolled growth characteristic of cancer cells is suggested by a study in which Tsg101 was inactivated by mRNA antisense transcripts, which led to increased colony formation in soft agar. Further the Tsg101 antisense transcripts, when injected into nude mice, increased the appearance of metastatic tumors (Li and Cohen, 1996).
While the work by Bache et al. provide a compelling argument that receptor sequestration is a key regulatory mechanism terminating EGFR signaling, there are data that contradict this model. Other studies suggest that signaling can be terminated despite defects in intralumenal vesicle sequestration (Malerod et al. 2007). Depletion of Vps22 by siRNA in HeLa and HEp2 cells causes a slowed EGFR degradation and accumulation of the receptor along the limiting membranes of the MVB as shown by electron microscopy and biochemical analysis. However, whereas this study, in accordance with previous reports, showed TSG101 knockdown enhances EGF-dependent MAPK phosphorylation, Vps22 knockdown did not prolong MAPK activity as compared to wild type cells. Interestingly, the authors also found that lysates from Vps22 siRNA treated cells have reduced levels of two other ESCRT-II proteins, Vps25 and Vps36. Therefore, Vps22 may contribute to the stability of the entire complex. The signaling data in Vps22 depleted cells highlight the controversy regarding whether receptor inactivation can occur upstream of intralumenal sequestration of the EGFR and the exact molecular role of each of the ESCRT complexes.
Non-ESCRT medicated EGFR sequestration
Proteins that are not part of the ESCRT complex also have been shown to play a role in EGFR intralumenal sequestration. For instance, the EGFR effector annexin-1 has been shown to be required for sequestration (Futter et al. 1993). Despite the failure of the EGFR to internalize into vesicles within the MVB in annexin-1 mouse knockout cells, the EGFR is still efficiently degraded, albeit with a minor delay in the rate of degradation (White et al. 2006). The authors suggest that internal vesicle formation is not necessary for EGFR degradation, but makes the degradation process more efficient. Thus, entry of receptors into limiting membranes of the MVB may in fact be to sequester the receptor rather than simply as an intermediate in the degradation pathway. Similar results have been seen with the knockdown of Vps34, a PI-3 kinase, in HEp-2 cells (Futter et al. 2001). Like the annexin-1 knockout, the active EGFR is retained on the limiting membrane of the late endocytic compartment and there is only a slight delay in the rate of delivery to lysosomes. Examination of the tyrosine-phosphorylation of downstream effectors in Vps34 knockdown cells revealed enhanced phosphorylation of several proteins. One implication of these findings is that there are two possible points of inactivation in the late endocytic pathway since, in the absence of sequestration-mediated inactivation, lysosomal delivery functions as the mechanism of receptor inactivation.
EGFR degradation
There is evidence that sequestration of the EGFR is not sufficient for terminating the signaling capability of the EGFR and lysosomal degradation is the rate-limiting step in receptor inactivation. Oksvold et al. prevented lysosomal degradation using inhibitors of lysosomal enzymes or blocked trafficking to lysosome using the lysosomotropic amine chloroquine (Oksvold et al. 2001). Immunoblots of subcellular fractions isolated over gradients revealed the accumulation of activated EGFR and MAPK within the same fraction. Immunoelectron microspcopy revealed the majority of phosphorylated EGFR accumulated intralumenally, with a small fraction present on the cytosolic membrane. Immunofluorescent staining of cells treated with chloroquine or lysosomal inhibitors showed co-localization of the adaptor proteins Shc, Grb2, and Cbl with phosphorylated EGFR indicating the potential for signaling existed. These proteins accumulated in MVB as defined by the presence of MVB markers CD63 and LAMP-1, and exclusion of the early endosome marker EEA1. Immunoblot analysis of phosphorylated EGFR and MAPK revealed cells stimulated with EGF and chased with chloroquine for 120′, but not untreated cells, sustained their activity at time points that electron microscopy revealed to be consistent with EGFR localization in MVBs. These data indicate that in the absence of lysosomal delivery, activated receptor can accumulate in MVBs and signal to downstream effectors.
In the pharmacologically treated cells, immunoelectron microscopy revealed the majority of phosphorylated receptors accumulated in internal vesicles of the MVB. It is a formal possibility that sustained signaling was due to the fraction remaining on the limiting membrane. EGF stimulation substantially increases the volume of cytosol containing MVBs (Futter et al. 1998; White et al. 2006); suggesting proteins other than the EGFR, such as downstream effectors, could be removed from the cytosol and delivered for degradation. Therefore, the presence of an activated effector is not definitive evidence that physiologic signaling is occurring. Further analysis of downstream signaling events, such as DNA transcription, cell viability, and cell motility is needed to establish whether the active effectors modulate cell biology.
The molecular regulation of EGFR signaling in the late endocytic pathway remains unclear. Specifically, determining whether the terminal step in EGFR signaling is mediated through receptor sequestration or degradation will aid in the design and use of therapeutic agents. To date, the majority of studies have focused on disrupting the sequestration/degradation process. As a strategy to terminate hyperactive EGFR signaling, it will be more useful to accelerate these trafficking events and examine the biochemical (effector activity) and physiological (cell biology) consequences. These findings will determine the potential of the late endocytic pathway as a therapeutic target.
Concluding Remarks
Clinical and experimental data indicate that the EGFR is a viable target to inhibit the growth of non-transformed and transformed cells. Monoclonal antibodies targeting the EGFR and tyrosine kinase inhibitors have both demonstrated success in inhibiting the growth of cancerous and non-cancerous cells. However, there still remain a number of cancers characterized by EGFR overexpression that are refractory to these therapies. Rather than strive to make better inhibitors of EGFR activity, we suggest the alternative approach of looking for ways to accelerate the inactivation of the EGFR once it becomes stimulated. This strategy would have the greatest effect on those cells with the highest levels of EGFR expression and EGFR activation. As the biochemical details of the pathways that lead to receptor inactivation are elucidated, it allows one to creatively think about how this information can be used in anti-cancer-therapies.
References
- Agazie YM, Hayman MJ. The phosphotyrosine phosphatase SHP2 is a critical mediator of transformation induced by the oncogenic fibroblast growth factor receptor 3. Oncogene. 2003;22:6909–18. doi: 10.1038/sj.onc.1206798. [DOI] [PubMed] [Google Scholar]
- Andersen JN, Jansen PG, Echwald SM, Mortensen OH, Fukada T, Del Vecchio R, Tonks NK, Moller NP. A genomic perspective on protein tyrosine phosphatases: gene structure, pseudogenes, and genetic disease linkage. FASEB J. 2004;18:8–30. doi: 10.1096/fj.02-1212rev. [DOI] [PubMed] [Google Scholar]
- Astsaturov I, Cohen RB, Harari PM. EGFR.-targeting monoclonal antibodies in head and neck cancer. Curr Cancer Drug Targets. 2006;6:691–710. doi: 10.2174/156800906779010191. [DOI] [PubMed] [Google Scholar]
- Babst M. A protein’s final ESCRT. Traffic. 2005;6:2–9. doi: 10.1111/j.1600-0854.2004.00246.x. [DOI] [PubMed] [Google Scholar]
- Babst M, Odorizzi G, Estepa EJ, Emr SD. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, function in late endosomal trafficking. Traffic. 2000;1:248–58. doi: 10.1034/j.1600-0854.2000.010307.x. [DOI] [PubMed] [Google Scholar]
- Bache KG, Brech A, Mehlum A, Stenmark H. Hrs regulates multivesicular body formation via ESCRT recruitment to endosomes. J Cell Biol. 2003;162:435–42. doi: 10.1083/jcb.200302131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bache KG, Slagsvold T, Cabezas A, Rosendal KR, Raiborg C, Stenmark H. The Growth-Regulatory Protein HCRP1/hVps37A Is a Subunit of Mammalian ESCRT-1 and mediates Receptor Down-Regulation. Mol Biol Cell. 2006a;15:4337–46. doi: 10.1091/mbc.E04-03-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bache KG, Stuffers S, Malerod L, Slagsvold T, Raiborg C, Lechardeur D, Walchli S, Luckacs GL, Brech A, Stenmark H. The ESCRT-III Subunit hVps24 Is Required for Degradation but Not Silencing of the Epidermal Growth Factor Receptor. Mol Biol Cell. 2006b;17:2513–23. doi: 10.1091/mbc.E05-10-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barford D, Jia Z, Tonks NK. Protein tyrosine phosphatases take off. Nat Struct Biol. 1995;2:1043–53. doi: 10.1038/nsb1295-1043. [DOI] [PubMed] [Google Scholar]
- Baulida J, Kraus MH, Alimandi M, DiFiore PP, Carpenter G. All ErbB. Receptors other than the epidermal growth factor receptor are endocytosis impaired. J Biol Chem. 1996;271:5251–7. doi: 10.1074/jbc.271.9.5251. [DOI] [PubMed] [Google Scholar]
- Bishop N, Horman A, woodman P. Mammalian class E vps proteins recognize ubiquitin and act in the removal of endosomal protein-ubiquitin conjugates. J Cell Biol. 2002;157:91–101. doi: 10.1083/jcb.200112080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers K, Piper SC, Edeling MA, Gray SR, Owen DJ, Lehner PJ, Luzio JP. Degradation of endocytosed epidermal growth factor and virally ubiquinated major histocompatibility complex class I is independent of mammalian ESCRTII. J Biol Chem. 2006;281:5094–105. doi: 10.1074/jbc.M508632200. [DOI] [PubMed] [Google Scholar]
- Carpenter G. ErbB-4: Mechanism of Action and Biology. Exp Cell Res. 2003;284:66–77. doi: 10.1016/s0014-4827(02)00100-3. [DOI] [PubMed] [Google Scholar]
- Carpentier JL, White MF, Orci L, Kahn RC. Direct visualization of the phosphorylated epidermal growth factor receptor during its internalization in A431 cells. J Cell Biol. 1987;105:2751–62. doi: 10.1083/jcb.105.6.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceresa BP, Bahr SJ. rab7 Activity Affects Epidermal Growth Factor: Epidermal Growth Factor Receptor Degradation by Regulating Endocytic Trafficking from the Late Endosome. J Biol Chem. 2006;281:1099–106. doi: 10.1074/jbc.M504175200. [DOI] [PubMed] [Google Scholar]
- Chinkers M, Brugge JS. Characterization of structural domains of the human epidermal growth factor receptor obtained by partial proteolysis. J Biol Chem. 1984;259:11534–42. [PubMed] [Google Scholar]
- Cirri P, Chiarugi P, Camici G, Manao G, Raugei G, Cappugi G, Pamponi G. The role of Cys12, Cys17 and Arg 18 in the catalytic mechanism of low M(r) cytosolic phosphotyrosine protein phosphatase. Eur J Biochem. 1993;214:647–57. doi: 10.1111/j.1432-1033.1993.tb17965.x. [DOI] [PubMed] [Google Scholar]
- Citri A, Skaria KB, Yarden Y. The Deaf and Dumb: the Biology of ErbB-2 and ErbB-3. Exp Cell Res. 2003;284:54–65. doi: 10.1016/s0014-4827(02)00101-5. [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov. U.S . National Institutes of Health; 2007. [Google Scholar]
- Di Fiore PP, Pierce JH, Fleming TP, Hazan R, Ullrich A, King CR, Schlessinger J, Aaronson SA. Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell. 1987;51:1063–70. doi: 10.1016/0092-8674(87)90592-7. [DOI] [PubMed] [Google Scholar]
- DiGuglielmo GM, Baass PC, Ou WJ, Posner BI, Bergeron JJ. Compartmentalization of Shc, Grb2 and mSOS, and hyper-phosphorylation of raf-1 by EGF but not insulin in liver parenchyma. EMBO J. 1994;13:4269–77. doi: 10.1002/j.1460-2075.1994.tb06747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J, Parker P, Waterfield MD. Autophosphorylation sites on the epidermal growth factor receptor. Nature. 1984a;311:483–5. doi: 10.1038/311483a0. [DOI] [PubMed] [Google Scholar]
- Downward J, Yarden Y, Mayes E, Scrace G, Totty N, Stockwell P, Ullrich A, Schlessinger J, Waterfield MD. Close similarity of epidermal growth factor receptor and v-erb-B. oncogene protein sequences. Nature. 1984b;307:521–7. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Doyette A, Russell MR, Hopkins CR, Woodman PG. Depletion of TSG101 forms a mammalian “Class E” compartment: a multicisternal early endosome with multiple sorting defects. J Cell Sci. 2005;118:3003–17. doi: 10.1242/jcs.02421. [DOI] [PubMed] [Google Scholar]
- Duan L, Miura Y, Dimri M, Majumder B, Dodge IL, Reddi AL, Ghosh A, Fernandes N, Zhou P, Mullane-Robsinson K, Rao N, Donoghue S, Rogers RA, Bowtell D, Naramura M, Gu H, Band V, Band H. Cbl-mediated ubiquitylation is required for lysosomal sorting of epidermal growth factor receptor but is dispensable for endocytosis. J Biol Chem. 2003;278:28950–60. doi: 10.1074/jbc.M304474200. [DOI] [PubMed] [Google Scholar]
- Dunn WA, Connolly TP, Hubbard AL. Receptor-mediated endocytosis of epidermal growth factor by rat hepatocytes:receptor pathway. J Cell Biol. 1986;102:24–36. doi: 10.1083/jcb.102.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder S, Miller K, Moehren G, Ullrich A, Schlessinger J, Hopkins CR. Kinase activity controls the sorting of the epidermal growth factor receptor with the multivesicular body. Cell. 1990;61:623–34. doi: 10.1016/0092-8674(90)90474-s. [DOI] [PubMed] [Google Scholar]
- Ferguson KM, Berger MB, Medrola JM, Cho HS, Leahy DJ, Lemmon MA. EGF activates its receptor by removing interactions that autoinhibit ectodomain dimerization. Mol. Cell. 2003;11:507. doi: 10.1016/s1097-2765(03)00047-9. [DOI] [PubMed] [Google Scholar]
- Fischer-Colbrie J, Witt A, Heinzl H, Speiser P, Czerwenka K, Sevelda P, Zeillinger R. EGFR. and steroid receptors in ovarian carcinoma: comparison with prognostic parameters and outcome of patients. Anticancer Res. 1997;17:613–9. [PubMed] [Google Scholar]
- Flint AJ, Tiganis T, Barford D, Tonks NK. Development of “substrate-trapping” mutants to identify substrates for phosphatases. Proc Natl Acad Sci USA. 1997;94:1680–5. doi: 10.1073/pnas.94.5.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French AR, Sudlow GP, Wiley HS, Lauffenburger DA. Postendocytic Trafficking of Epidermal Growth Factor-Receptor Complexes is Mediated through Saturable and Specific Endosomal Interactions. J Biol Chem. 1994;269:15749–55. [PubMed] [Google Scholar]
- Futter CE, Collinson LM, Backer JM, Hopkins CR. Human VPS34 is required for internal vesicle formation with multivesicular endosomes. J Cell Biol. 2001;155:1251–64. doi: 10.1083/jcb.200108152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter CE, Felder S, Schlessinger J, Ullrich A, Hopkins CR. Annexin I is phosphorylated in the multivesicular body during the processing of the epidermal growth factor receptor. J Cell Biol. 1993;120:77–83. doi: 10.1083/jcb.120.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter CE, Gibson A, Allchin EH, Maxwell S, Ruddock LJ, Odorizzi G, Domingo D, Trowbridge IS, Hopkins CR. In Polarized MDCK cells basolateral vesicles arise from clathrin-g-adaptin-coated domains on endosomal tubules. J Cell Biol. 1998;141:611–23. doi: 10.1083/jcb.141.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grazul-Bilska AT, Johnson ML, Bilski JJ, Redmer DA, Reynolds LP, Abdullah A, Abdullah KM. Wound Healing: The Role of Growth Factors. Drugs Today (Barc) 2003;39:787–800. doi: 10.1358/dot.2003.39.10.799472. [DOI] [PubMed] [Google Scholar]
- Haigler HT, Carpenter G. Production and characterization of antibody blocking epidermal growth factor:receptor interactions. Biochim Biophys Acta. 1980;598:314–25. doi: 10.1016/0005-2736(80)90009-7. [DOI] [PubMed] [Google Scholar]
- Haj FG, Verveer PJ, Squire A, Neel BG, Bastiaens PI. Imaging sites of receptor dephosphorylation by PTP1B. on the surface of the endoplasmic reticulum. Science. 2002;295:1701–11. doi: 10.1126/science.1067566. [DOI] [PubMed] [Google Scholar]
- Harari PM, Allen GW, Bonner JA. Biology of interactions; antiepidermal growth factor receptor agents. J Clin Oncol. 2007;25:4057–65. doi: 10.1200/JCO.2007.11.8984. [DOI] [PubMed] [Google Scholar]
- Harris RC, Chung E, Coffey RJ. EGF Receptor ligands. Exp Cell Res. 2003;284:2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- Huang F, Kirkpatrick D, Jiang X, Gygi S, Sorkin A. Differential Regulation of EGF Receptor Internalization and Degradation by Multiubiquitination within the Kinase Domain. Mol Cell. 2006;21:737–48. doi: 10.1016/j.molcel.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Huang SM, Bock JM, Harari PM. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:1935–40. [PubMed] [Google Scholar]
- Hurley JH, Emr SD. The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu Rev Biophys Biomol Struct. 2006;35:277–98. doi: 10.1146/annurev.biophys.35.040405.102126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitoya J, Toriyama M, Oguchi N, Kitamura K, Ohshima M, Asano K, Yamamoto T. Gene amplification and overexpression of EGF receptor in squamous cell carcinomas of the head and neck. Br. J. Cancer. 1989;59:559. doi: 10.1038/bjc.1989.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Sorkin A. Epidermal growth factor receptor internalization through clathrin-coated pits requires Cbl RING finger and pro-line-rich domains but not receptor polyubiquitylation. Traffic. 2003;4:529–43. doi: 10.1034/j.1600-0854.2003.t01-1-00109.x. [DOI] [PubMed] [Google Scholar]
- Jones MK, Tomikawa M, Mohajer B, Tarnawski AS. Gastrointestinal Mucosal Regeneration: Role of Growth Factors. Frontiers in Bioscience. 1999;4:d303–9. doi: 10.2741/a428. [DOI] [PubMed] [Google Scholar]
- Jorissen RN, Walker F, Pouliot N, Garrett TPJ, Ward CW, Burgess AW. Epidermal Growth Factor Receptor: Mechanisms of Activation and Signalling. Exp Cell Res. 2003;284:31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- Keilhack H, Tenev T, Nyakatura E, Godovac-Zimmermann J, Nielsen L, Seedorf K, Bohmer F. Phosphotyrosine 1173 Mediates Binding of the Protein-tyrosine Phosphatase SHP-1 to the Epidermal Growth Factor Receptor and Attenuation of Receptor Signaling. J Biol Chem. 1998;273:24839–246. doi: 10.1074/jbc.273.38.24839. [DOI] [PubMed] [Google Scholar]
- Klingler-Hoffmann M, Fodero-Tavoletti MT, Mishima K, Narita Y, Cavenee WK, Furnari FB, Huang HJ, Tiganis T. The protein tyrosine phosphatase TCPTP suppresses the tumorigenicity of glioblastoma cells expressing a mutant epidermal growth factor receptor. J Biol Chem. 2001;276:46313–8. doi: 10.1074/jbc.M106571200. [DOI] [PubMed] [Google Scholar]
- Korc M, Finman JE. Attenuated processing of epidermal growth factor in the face of marked degradation of transforming growth factor-alpha. J Biol Chem. 1989;264:14990–9. [PubMed] [Google Scholar]
- Lai WH, Cameron PH, Doherty JJ, 2nd, Posner BI, Bergeron JJ. Ligand-mediated autophosphorylation activity of the epidermal growth factor receptor during internalization. J Cell Biol. 1989;109:2751–60. doi: 10.1083/jcb.109.6.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers R, van Obberghen E, Ballotti R, Schlessinger J, Ullrich A. Transphosphorylation as a possible mechanism for insulin and epidermal growth factor receptor activation. J Biol Chem. 1990;265:16886–90. [PubMed] [Google Scholar]
- Langelier C, von Schwedler UK, Fisher RD, DeDomenico I, White PL, Hill CP, Kaplan J, Ward D, Sundquist WI. Human ESCRT-II complex and its role in human immunodeficiency virus type 1 release. J Virol. 2006;80:9465–80. doi: 10.1128/JVI.01049-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenferink AEG, Pinkas-Kramarski R, van de Poll MLM, van Vugt MJH, Klapper LN, Tzahar E, Waterman H, Sela M, van Zoelan EJJ, Yarden Y. Differential endocytic routing of homo-and hetero-dimeric ErbB. tyrosine kinases confers signaling superiority to receptor heterodimers. The EMBO J. 1998;17:3385–97. doi: 10.1093/emboj/17.12.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkowitz G, Waterman H, Ettenberg SA, Katz M, Tsygankov AY, Alroy I, Lavi S, Iwai K, Reiss Y, Ciechanover A, Lipkowitz S, Yarden Y. Ubiquitin ligase activity and tyrosine pho-phorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol Cell. 1999;4:1029–40. doi: 10.1016/s1097-2765(00)80231-2. [DOI] [PubMed] [Google Scholar]
- Li L, Cohen SN. Tsg101: a novel tumor susceptibility gene isolated by controlled homozygous functional knockout of allelic loci in mammalian cells. Cell. 1996;85:319–29. doi: 10.1016/s0092-8674(00)81111-3. [DOI] [PubMed] [Google Scholar]
- Liao HJ, Carpenter G. Role of the Sec61 translocon in EGF receptor trafficking to the nucleus and gene expression. Mol Biol Cell. 2007;18:1064–72. doi: 10.1091/mbc.E06-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libermann TA, Nusbaum HR, Razon N, Kris R, Lax I, Soreq H, Whittle N, Waterfield MD, Ullrich A, Schlessinger J. Amplication, enhanced expression and possible rearrangement of EGFR. gene in primary human brian tumours of glial origin. Nature. 1985;313:144–7. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- Lu Q, Hope LW, Brasch M, Reinhard C, Cohen SN. TSG101 interaction with HRS mediates endosomal trafficking and receptor down-regulation. Proc Natl Acad Sci USA. 2003;100:7626–31. doi: 10.1073/pnas.0932599100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, Lee DC. Targeted inactivation of the EGF and Amphi-regulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland developement. Development. 1999;126:2739–50. doi: 10.1242/dev.126.12.2739. [DOI] [PubMed] [Google Scholar]
- Lund KA, Opresko LK, Starbuck C, Walsh BJ, Wiley HS. Quantitative analysis of the endocytic system involved in hormone-induced receptor internalization. J Biol Chem. 1990;265:15713–23. [PubMed] [Google Scholar]
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA. Activating mutations in the epidermal growth factor receptor underlysing responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- Magne N, Pivot X, Bensadoun RJ, Guardiola E, Poissonnet G, Dassonville O, Francoual M, Formento JL, Demard F, Schneider M, Milano G. The relationship of epidermal growth factor receptor levels to the prognosis of unresectable pharyngeal cancer patients treated by chemo-radiotherapy. Eur J Cancer. 2001;37:2169–77. doi: 10.1016/s0959-8049(01)00280-5. [DOI] [PubMed] [Google Scholar]
- Malerod L, Stuffers S, Brech A, Stenmark H. Vps22/EAP30 in ESCRT-II Mediates Endosomal sorting of growth factor and chemokine receptors destined for lysosomal degradation. Traffic. 2007 doi: 10.1111/j.1600-0854.2007.00630.x. in press. [DOI] [PubMed] [Google Scholar]
- Masui H, Castro L, Mendelsohn J. Consumption of EGF by A431 cells: evidence for receptor recycling. J Cell Biol. 1993;120:85–93. doi: 10.1083/jcb.120.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melisi D, Troiani T, Damiano V, Tortora G, Ciardiello F. Therapeutic integration of signal transduction targeting agents and conventional anti-cancer treatments. Endocr Relat Cancer. 2004;11:51–68. doi: 10.1677/erc.0.0110051. [DOI] [PubMed] [Google Scholar]
- Merlino GT, Xu YH, Ishii S, Clark AJ, Semba K, Toyoshima K, Yamamoto T, Pastan I. Amplification and enhanced expression of the epidermal growth factor receptor gene in A431 human carcinoma cells. Science. 1984;224:417–9. doi: 10.1126/science.6200934. [DOI] [PubMed] [Google Scholar]
- Miettinen PJ, Berger JE, Meneses J, Phung Y, Pedersen RA, Werb Z, Derynck R. Epithelial immaturity and multi-organ failure in mice lacking epidermal growth factor receptor. Nature. 1995;376:337–41. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- Miller K, Beardmore J, Kanety H, Schlessinger J, Hopkins CR. Localization of the epidermal growth factor (EGF) receptor within the endosome of EGF-stimulated epidermoid carcinoma (A431) cells. J Cell Biol. 1986;102:500–9. doi: 10.1083/jcb.102.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WE, Earp HS, Raab-Traub N. The Epstein-Barr virus latent membrane protein 1 induces expression of the epidermal growth factor receptor. J Virol. 1995;69:4390–8. doi: 10.1128/jvi.69.7.4390-4398.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno E, Iura T, Mukai A, Yoshimori T, Kitamura N, Komada M. Regulation of epidermal growth factor receptor down-regulation by UBPY-mediated deubiquitination at endosomes. Mol Biol Cell. 2005;16:5163–74. doi: 10.1091/mbc.E05-06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgillo F, Bareschino MA, Bianco R, Tortora G, Ciardiello F. Primary and acquired resistance to anti-EGFR targeted drugs in cancer therapy. Differentiation. 2007 doi: 10.1111/j.1432-0436.2007.00200.x. in press. [DOI] [PubMed] [Google Scholar]
- Moroni M, Veronese S, Benvenuti S, Marrapese G, Sartore-Bianchi A, Di Nicolantonio F, Gambacorta M, Siena S, Bardelli A. Gene copy number for epidermal growth factor receptor (EGFR.) and clinical response to antiEGFR. treatment in colorectal cancer: a cohort study. Lancet Oncol. 2005;6:279–86. doi: 10.1016/S1470-2045(05)70102-9. [DOI] [PubMed] [Google Scholar]
- Moy B, Kirkpatrick P, Kar S, Goss P. Lapatinib. Nat Rev Drug Discov. 2007;6:431–2. doi: 10.1038/nrd2332. [DOI] [PubMed] [Google Scholar]
- Newby JS, Johnston SR, Smith IE, Dowsett M. Expression of epidermal growth factor receptor and c-erbB.2 during the development of tamoxifen resistance in human breast cancer. Clin Cancer Res. 1997;3:1643–51. [PubMed] [Google Scholar]
- O’Grady M, Raha D, Hanson BJ, Bunting M, Hanson GT. Combining RNA interference and kinase inhibitors against cell signalling components involved in cancer. BMC Cancer. 2005;5 doi: 10.1186/1471-2407-5-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksvold MP, Skarpen E, Wierod L, Paulsen RE, Huitfeldt HS. Re-localization of activated EGF receptor and its signal transducers to multivesicular compartments downstream of early endosomes in response to EGF. Eur J Cell Biol. 2001;80:285–94. doi: 10.1078/0171-9335-00160. [DOI] [PubMed] [Google Scholar]
- Olabisi OO, Mahon GM, Kostenko EV, Liu Z, Ozer HL, White-head IP. Bcr interacts with components of the endosomal sorting complex required for transport-I and is required for epidermal growth factor receptor turnover. Cancer Res. 2006;66:6250–7. doi: 10.1158/0008-5472.CAN-06-0536. [DOI] [PubMed] [Google Scholar]
- Raymond CK, Howald-Stevenson I, Vater CA, Stevens TH. Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in E vps mutants. Mol Biol Cell. 1992;3:1389–402. doi: 10.1091/mbc.3.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razi M, Futter CE. Distinct roles for Tsg101 and Hrs in multi-vesicular body formation and inward vesiculation. Mol Biol Cell. 2006;17:3469–83. doi: 10.1091/mbc.E05-11-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Lima CM, Soares HP, Raez LE, Singal R. EGFR. Targeting of Solid tumors. Cancer Control. 2007;14:295–304. doi: 10.1177/107327480701400313. [DOI] [PubMed] [Google Scholar]
- Row PE, Prior IA, McCullough J, Clague MJ, Urbe S. The ubiquitin isopeptidase UBPY regulates endosomal ubiquitin dynamics and is essential for receptor down-regulation. J Biol Chem. 2006;281:12618–24. doi: 10.1074/jbc.M512615200. [DOI] [PubMed] [Google Scholar]
- Sachse M, Strous GJ, Klumperman J. ATPase-deficient hVPS4 impairs formation of internal endosomal vesicles and stabilizes bilayered clathrin coats on endosomal vacuoles. J Cell Sci. 2004;117:1699–1708. doi: 10.1242/jcs.00998. [DOI] [PubMed] [Google Scholar]
- Sebastian S, Settleman J, Reshkin SJ, Azzariti A, Bellizzi A, Paradiso A. The complexity of targeting EGFR. signalling in cancer: from expression to turnover. Biochim Biophys Acta. 2006;1766:120–39. doi: 10.1016/j.bbcan.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Sibilia M, Wagner EF. Strain-dependent epithelial defects in mice lacking the EGFR. Science. 1995;269:234–8. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- Sorkin A, von Zastrow M. Signal Transduction and Endocytosis: Close Encounters of Many Kinds. Nat Cell Biol Rev. 2002;3:600–14. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- Suarez Pestana E, Tenev T, Gross S, Stoyanov B, Ogata M, Bohmer FD. The transmembrane protein tyrosine phosphatase RPTPsigma modulates signaling of the epidermal growth factor receptor in A431 cells. Oncogene. 1999;18:4069–79. doi: 10.1038/sj.onc.1202794. [DOI] [PubMed] [Google Scholar]
- Tiganis T, Bennet AM, Ravicharndran KS, Tonks NK. Epidermal Growth Factor receptor and the adaptor protein p52Shc are specific substrates of T-cell protein tyrosine phosphatase. Mol Cell Biol. 1998;18:1622–34. doi: 10.1128/mcb.18.3.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiganis T, Kemp BE, Tonks NK. The protein-tyrosine phosphatase TCPTP regulates epidermal growth factor receptor-mediated and phosphatidylinositol 3-kinase-dependent signaling. J Biol Chem. 1999;274:27768–75. doi: 10.1074/jbc.274.39.27768. [DOI] [PubMed] [Google Scholar]
- Tomic S, Greiser U, Lammers R, Kharitonenkov A, Imyanitov E, Ullrich A, Bohmer FD. Assoication of SH2 domain protein tyrosine phosphatases with the epidermal growth factor receptor in human tumor cells. Phasphatidic acid activates receptor dephosphorylation by PTP1C. J Biol Chem. 1995;270:21277–84. doi: 10.1074/jbc.270.36.21277. [DOI] [PubMed] [Google Scholar]
- Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7:833–46. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- Tonks NK, Diltz CD, Fischer EH. Purification of the major protein-tyrosine-phosphatases of human placenta. J Bio Chem. 1988;263:6722–30. [PubMed] [Google Scholar]
- Tonks NK, Neel BG. Combinatorial control of the specificity of protein tyrosine phosphatases. Curr Opin Cell Biol. 2001;13:182–95. doi: 10.1016/s0955-0674(00)00196-4. [DOI] [PubMed] [Google Scholar]
- Tsutsumi O, Kubota Y, Oka T. Effect of sialoadenectomy, treatment with epidermal growth factor (EGF) antiserum and the replacement of EGF on the epidermis in mice. J Endocrinol. 1987;113:193–7. doi: 10.1677/joe.0.1130193. [DOI] [PubMed] [Google Scholar]
- Ullrich A, Coussens L, Hayflick JS, Dull TJ, Gray A, Tam AW, Lee J, Yarden Y, Libermann TA, Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature. 1984;309:418–25. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Urbe S, Sachse M, Row PE, Presisinger C, Barr FA, Strous G, Klumperman J, Clague MJ. The UIM domain of Hrs couples receptor sorting to vesicle formation. J Cell Sci. 2003;116:4169–79. doi: 10.1242/jcs.00723. [DOI] [PubMed] [Google Scholar]
- Velu TJ, Beguinot L, Vass WC, Willingham MC, Merlino GT, Pastan I, Lowry DR. Epidermal-growth-factor-dependent transformation by a human EGF receptor proto-oncogene. Science. 1987;238:1408–10. doi: 10.1126/science.3500513. [DOI] [PubMed] [Google Scholar]
- Vieira AV, Lamaze C, Schmid SL. Control of EGF Receptor Signaling by Clathrin-Mediated Endocytosis. Science. 1996;274:2086–9. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- Ward DM, Vaughn MB, Shiflett SL, White PL, Pollock AL, Hill J, Schnegelberger R, Sundquist WI, Kaplan J. The role of LIP5 and CHMP5 in multivesicular body formation and HIV-1 budding in mammalian cells. J Biol Chem. 2005;280:10548–55. doi: 10.1074/jbc.M413734200. [DOI] [PubMed] [Google Scholar]
- Wells A, Welsh JB, Lazar CS, Wiley HS, Gill GN, Rosenfeld MG. Ligand-Induced Transformation by a Noninternalizing Epidermal Growth Factor Receptor. Science. 1990;247:962–4. doi: 10.1126/science.2305263. [DOI] [PubMed] [Google Scholar]
- White IJ, Bailey LM, Aghakhani MR, Moss SE, Futter CE. EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J. 2006;25:1–12. doi: 10.1038/sj.emboj.7600759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley HS. Trafficking of the ErbB. receptors and its influence on signaling. Exp Cell Res. 2003;284:78–88. doi: 10.1016/s0014-4827(03)00002-8. [DOI] [PubMed] [Google Scholar]
- Xu Y, Tan LJ, Grachtchouk V, Voorhees JJ, Fisher GJ. Receptor-type protein-tyrosine phosphatase-kappa regulates epidermal growth factor receptor function. J Biol Chem. 2005;280:42694–700. doi: 10.1074/jbc.M507722200. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Kamata N, Kawano H, Shimizu S, Kuroki T, Toyoshima K, Rikimaru K, Nomura N, Ishizaki R, Pastan I. High incidence of amplification of the epidermal growth factor receptor gene in human squamous carcinoma cell lines. Cancer Res. 1986;46:414–6. [PubMed] [Google Scholar]
- Yamazaki H, Kijima H, Ohnishi Y, Abe Y, Oshika Y, Tsuchida T, Tokunaga T, Tsugu A, Ueyama Y, Tamaoki N, Nakamura M. Inhibition of tumor growth by ribozyme-mediate suppression of aberrant epidermal growth factor receptor gene expression. J Natl Cancer Inst. 1998;90:581–7. doi: 10.1093/jnci/90.8.581. [DOI] [PubMed] [Google Scholar]
- Yan Q, Hunt PR, Freliin L, Vida TA, Pevsner J, Bean AJ. mVps24p functions in EGF receptor sorting/trafficking from the early endosome. Exp Cell Res. 2005;304:265–73. doi: 10.1016/j.yexcr.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Yano S, Kondo K, Yamaguchi M, Richmond G, Hutchison M, Wakeling A, Averbuch S, Wadsworth P. Distribution and function of EGFR. in human tissue and the effect of EGFR. tyrosine kinase inhibition. Anticancer Res. 2003;23:3639–50. [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB. Signalling Network. Nat Rev Mol Cell Biol. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Yoon CH, Lee J, Jongeward GD, Sternberg PW. Similarity of sli-1, a regulator of vulval development in C. elegans, to the mammalian proto-oncogene c-cbl. Science. 1995;269:1102–5. doi: 10.1126/science.7652556. [DOI] [PubMed] [Google Scholar]
- Zandi R, Larsen AB, Andersen P, Stockhausen MT, Poulsen HS. Mechanisms for oncogenic activation of the epidermal growth factor receptor. Cell Signal. 2007;19:2013–23. doi: 10.1016/j.cellsig.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Zhang ZY, VanEtten RL. Pre-steady-state and steady-state kinetic analysis of the low molecular weight phosphotyrosyl protein phosphatase from bovine heart. J Biol Chem. 1991;266:1516–25. [PubMed] [Google Scholar]
- Zhu Z. Targeted cancer therapies based on antibodies directed against epidermal growth factor receptor: status and perspectives. Acta Pharmacol Sin. 2007;28:1476–93. doi: 10.1111/j.1745-7254.2007.00681.x. [DOI] [PubMed] [Google Scholar]