Abstract
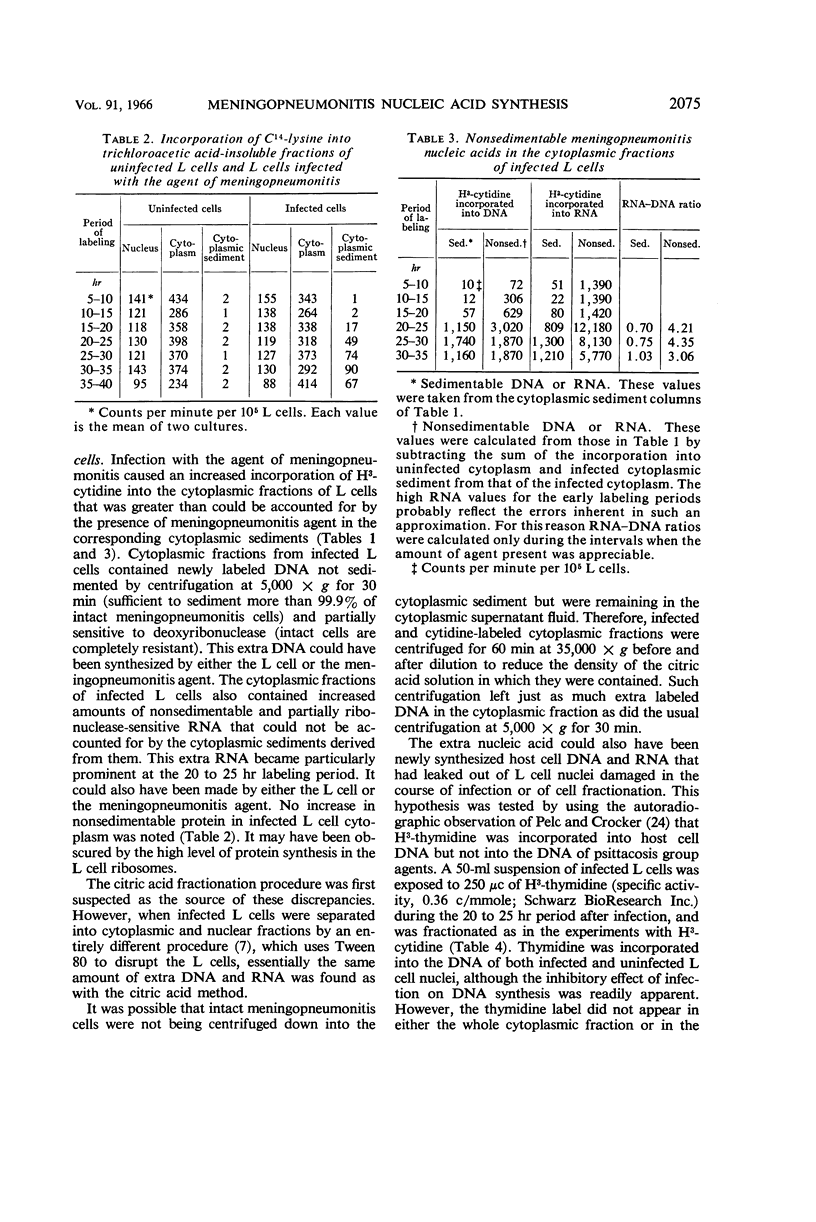
Schechter, Esther M. (The University of Chicago, Chicago, Ill.). Synthesis of nucleic acid and protein in L cells infected with the agent of meningopneumonitis. J. Bacteriol. 91:2069–2080. 1966.—Synthesis of deoxyribonucleic acid (DNA), ribonucleic acid (RNA), and protein in uninfected L cells and in L cells infected with the meningopneumonitis agent was compared by measuring rates of incorporation of H3-cytidine and C14-lysine into nuclear, cytoplasmic, and agent fractions in successive 5-hr periods during the meningopneumonitis growth cycle. Synthesis of meningopneumonitis DNA, RNA, and protein was first clearly evident in the labeling period 15 to 20 hr after infection, soon after initiation of agent multiplication. The rates of synthesis of agent DNA, RNA, and protein increased logarithmically for a brief period and then declined. However, rates of isotope incorporation into all three meningopneumonitis macromolecules were sustained at near maximal values throughout the remainder of the meningopneumonitis growth cycle. These data are most readily interpreted in terms of multiplication of the meningopneumonitis agent by binary fission. The L cell response to infection was a decreased rate of DNA and RNA synthesis and an accelerated rate of cell death. Host protein synthesis was unaffected. The inhibition of nucleic acid synthesis in infected L cells probably involved competition between host and parasite for nucleic acid precursors. Different sublines of L cells varied greatly in the degree to which their nucleic acid-synthesizing mechanisms were damaged by infection. The cytoplasm of infected L cells contained newly synthesized DNA and RNA that could not be accounted for as intact meningopneumonitis cells. This nucleic acid probably arose from disintegration of the fragile intracellular forms of the meningopneumonitis agent.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG J. A., REED S. E. NATURE AND ORIGIN OF INITIAL BODIES IN LYMPHOGRANULOMA VENEREUM. Nature. 1964 Jan 25;201:371–373. doi: 10.1038/201371a0. [DOI] [PubMed] [Google Scholar]
- CROCKER T. T., PELC S. R., NIELSEN B. I., EASTWOOD J. M., BANKS J. POPULATION DYNAMICS AND DEOXYRIBONUCLEIC ACID SYNTHESIS IN HELA CELLS INFECTED WITH AN ORNITHOSIS AGENT. J Infect Dis. 1965 Apr;115:105–122. doi: 10.1093/infdis/115.2.105. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- EAGLE H. The specific amino acid requirements of a mammalian cell (strain L) in tissue culture. J Biol Chem. 1955 Jun;214(2):839–852. [PubMed] [Google Scholar]
- HIGASHI N. ELECTRON MICROSCOPIC STUDIES ON THE MODE OF REPRODUCTION OF TRACHOMA VIRUS AND PSITTACOSIS VIRUS IN CELL CULTURES. Exp Mol Pathol. 1965 Feb;76:24–39. doi: 10.1016/0014-4800(65)90021-3. [DOI] [PubMed] [Google Scholar]
- HIGASHI N., TAMURA A., IWANAGA M. Developmental cycle and reproductive mechanism of the meningopneumonitis virus in strain L cells. Ann N Y Acad Sci. 1962 Mar 5;98:100–121. doi: 10.1111/j.1749-6632.1962.tb30536.x. [DOI] [PubMed] [Google Scholar]
- KENNEDY E. P. Metabolism of lipides. Annu Rev Biochem. 1957;26:119–148. doi: 10.1146/annurev.bi.26.070157.001003. [DOI] [PubMed] [Google Scholar]
- LITWIN J., OFFICER J. E., BROWN A., MOULDER J. W. A comparative study of the growth cycles of different members of the psittacosis group in different host cells. J Infect Dis. 1961 Nov-Dec;109:251–279. doi: 10.1093/infdis/109.3.251. [DOI] [PubMed] [Google Scholar]
- MIKA L. A., PIRSCH J. B. Replication of variola virus in suspended cultures of mammalian cells. Appl Microbiol. 1961 Nov;9:545–548. doi: 10.1128/am.9.6.545-548.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN J. F., MORTON H. J., PARKER R. C. Nutrition of animal cells in tissue culture; initial studies on a synthetic medium. Proc Soc Exp Biol Med. 1950 Jan;73(1):1–8. doi: 10.3181/00379727-73-17557. [DOI] [PubMed] [Google Scholar]
- MOULDER J. W., GRISSO D. L., BRUBAKER R. R. ENZYMES OF GLUCOSE CATABOLISM IN A MEMBER OF THE PSITTACOSIS GROUP. J Bacteriol. 1965 Mar;89:810–812. doi: 10.1128/jb.89.3.810-812.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOULDER J. W., GRISSO D. L., CHO G. J. ENDOGENOUS METABOLISM OF PROTEIN AND RIBONUCLEIC ACID IN A MEMBER OF THE PSITTACOSIS GROUP. J Infect Dis. 1965 Jun;115:254–262. doi: 10.1093/infdis/115.3.254. [DOI] [PubMed] [Google Scholar]
- McLIMANS W. F., DAVIS E. V., GLOVER F. L., RAKE G. W. The submerged culture of mammalian cells; the spinner culture. J Immunol. 1957 Nov;79(5):428–433. [PubMed] [Google Scholar]
- PODOLIAN V. Ia, MILIUTIN V. N., GUDIMA O. S., LUKINA R. N. MORFOGENEZ VIRUSA ORNITOZA. Vopr Virusol. 1964 Mar-Apr;68:208–212. [PubMed] [Google Scholar]
- POLLARD M., TANAMI Y. Cytochemistry of trachoma virus replication in tissue cultures. Ann N Y Acad Sci. 1962 Mar 5;98:50–61. doi: 10.1111/j.1749-6632.1962.tb30531.x. [DOI] [PubMed] [Google Scholar]
- SCHECHTER E. M., TRIBBY I. I., MOULDER J. W. NUCLEIC ACID METABOLISM IN L CELLS INFECTED WITH A MEMBER OF THE PSITTACOSIS GROUP. Science. 1964 Aug 21;145(3634):819–821. doi: 10.1126/science.145.3634.819. [DOI] [PubMed] [Google Scholar]
- SHARON N., POLLARD M. INHIBITION OF T'ANG TRACHOMA AGENT BY 5'-FLUORODEOXYCYTIDINE. Proc Soc Exp Biol Med. 1963 Nov;114:344–347. doi: 10.3181/00379727-114-28671. [DOI] [PubMed] [Google Scholar]
- STARR T. J., POLLARD M., TANAMI Y., MOORE R. W. Cytochemical studies with psittacosis virus by fluorescence microscopy. Tex Rep Biol Med. 1960;18:501–514. [PubMed] [Google Scholar]
- TAMURA A., HIGASHI N. PURIFICATION AND CHEMICAL COMPOSITION OF MENINGOPNEUMONITIS VIRUS. Virology. 1963 Aug;20:596–604. doi: 10.1016/0042-6822(63)90284-8. [DOI] [PubMed] [Google Scholar]
- TAMURA A., IWANAGA M. RNA SYNTHESIS IN CELLS INFECTED WITH THE MENINGOPNEUMONITIS AGENT. J Mol Biol. 1965 Jan;11:97–108. doi: 10.1016/s0022-2836(65)80175-9. [DOI] [PubMed] [Google Scholar]
- TANAMI Y., POLLARD M., STARR T. J. Replication pattern of psittacosis virus in a tissue culture system. Virology. 1961 Sep;15:22–29. doi: 10.1016/0042-6822(61)90072-1. [DOI] [PubMed] [Google Scholar]
- VOLKIN E., COHN W. E. Estimation of nucleic acids. Methods Biochem Anal. 1954;1:287–305. doi: 10.1002/9780470110171.ch11. [DOI] [PubMed] [Google Scholar]
- WEISS E., MYERS W. F., DRESSLER H. R., CHUN-HOON H. GLUCOSE METABOLISM BY AGENTS OF THE PSITTACOSIS-TRACHOMA GROUP. Virology. 1964 Apr;22:551–562. doi: 10.1016/0042-6822(64)90076-5. [DOI] [PubMed] [Google Scholar]
- Weiss E. Adenosine Triphosphate and Other Requirements for the Utilization of Glucose by Agents of the Psittacosis-Trachoma Group. J Bacteriol. 1965 Jul;90(1):243–253. doi: 10.1128/jb.90.1.243-253.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]



