Abstract
Skeletogenic heterochronies have gained much attention in comparative developmental biology. The temporal appearance of mineralized individual bones in a species – the species ossification sequence – is an excellent marker in this kind of study. Several publications describe interspecific variation, but only very few detail intraspecific variation. In this study, we describe and analyze the temporal order of ossification of skeletal elements in the zebra finch, Taeniopygia guttata, the Japanese quail, Coturnix coturnix japonica, and the White Pekin duck, a domestic race of the mallard Anas platyrhynchos, and explore patterns of intraspecific variation in these events. The overall sequences were found to be conserved. In the duck, variability is present in the relative timing of ossification in the occipital, the basisphenoid and the otic regions of the skull and the phalanges in the postcranium. This variation appears generally in close temporal proximity. Comparison with previously published data shows differences in ossification sequence in the skull, the feet, and the pelvis in the duck, and especially the pelvis in the quail. This clearly documents variability among different breeds.
Keywords: Aves, comparative embryology, evolution of development, intraspecific variation, ossification sequence, sequence heterochrony, skeletogenesis
INTRODUCTION
Species-specific developmental timing of skeletogenetic events has gained attention in comparative developmental biology. Studies range from descriptions of the timing of the appearance of molecular markers specific to skeletogenesis (i.e. Welten et al., 2005; Eames and Schneider, 2008; Kerney et al., 2010), to observations of morphogenetic movements of cranial neural crest cells (e.g., Olsson and Hanken, 1996; Vaglia and Smith, 2003; Tokita, 2006; Mitgutsch et al., 2008, 2009), to analyses of the temporal appearance of cartilaginous and skeletal elements. In particular, the temporal appearance of mineralized individual bones in a species – the ossification sequence of the species – has attracted much interest and the timing of the onset of ossification of skeletal elements has been investigated and analyzed in a wide variety of taxa throughout the Craniota, both extant and extinct, including teleosts, amphibians, sauropsids, and mammals (e.g., Trueb, 1985; Starck, 1989; Mabee and Trendler, 1996; Maisano, 2002a, b; Sánchez-Villagra, 2002; Rose, 2003; Sheil, 2003, 2005; Schoch, 2006; Fröbisch, 2008; Maxwell, 2008a, b, 2009; Maxwell and Harrison, 2008; Sánchez-Villagra, et al. 2008, 2009; Weisbecker et al., 2008; Werneburg et al., 2009; Hugi et al., 2010; Maxwell et al., 2010; Weisbecker and Mitgutsch, 2010).
Heterochronies have been found, but the overall ossification sequence pattern is relatively conserved when compared to variations in other developmental characters, although the variability is likely to differ among taxa and character sets (e.g., Prochel et al., 2008; Werneburg et al., 2009). Early developmental heterochronies could in some cases be linked to dramatic differences in life histories, for example when comparing species exhibiting an altricial condition at birth to precocial species (e.g., Vaglia and Smith, 2003; Weisbecker et al., 2008). Weisbecker et al. (2008), for example, found developmental heterochronies relating to ossification sequences when comparing marsupials to placentals. Specifically, relatively late ossification of hind limb long bones and early ossification of the anterior axial skeleton has been found in marsupials. Weisbecker et al. (2008) explained these results as a requirement of active movement in the altricial neonate marsupials. Detailed information on species-specific ossification sequences can thus not only serve taxonomic identification of embryonic skeletal specimens or reveal the identity of early skeletal elements, but can also give hints at life histories of fossil taxa and help in interpreting the developmental states of fossil embryonic specimens using an extant phylogenetic bracket (e.g., Balanoff and Rowe, 2007). Furthermore, as increasingly sophisticated methodological and conceptual frameworks for evaluating sequence data are being developed and discussed (e.g. Richardson et al., 2001; Jeffery et al., 2002a, b, 2005; Koenemann and Schram, 2002; Schulmeister and Wheeler, 2004; Harrison and Larsson, 2008; Maxwell and Harrison, 2009; Germain and Laurin, 2009; Maxwell et al., 2010), the use of data on developmental timing during skeletogenesis might provide a new and promising set of characters for craniate systematics (Maisano, 2002b; Maxwell and Harrison, 2008, 2009). Although early developmental heterochronies could be shown in some cases to characterize certain higher clades and to coincide with certain specific life history modes or anatomical peculiarities of the corresponding adults (e.g., Vaglia and Smith, 2003; Tokita, 2006; Weisbecker et al., 2008), such connections are not always easy to make (e.g. Chipman et al., 2000; Maxwell, 2008b; Mitgutsch et al., 2008, 2009; Werneburg and Sánchez-Villagra, in press), suggesting that characteristics in embryonic timing should rather be seen in connection with embryonic anatomy and demands, rather than with those of temporally distant developmental stages (Mitgutsch et al., 2008, 2009). Comparative embryological studies covering different taxa and different character complexes have gathered increasing evidence for intraspecific and, even among closely related taxa, interspecific variability in timing of early embryogenic events (Mabee and Trendler, 1996; Chipman et al., 2000; Moore and Townsend, 2003; Sheil and Greenbaum, 2005; Mitgutsch et al., 2008, 2009; Wilson et al., 2010). When analyzing the timing of such events among distantly related taxa (e.g., Bininda-Emonds et al., 2003; Werneburg and Sánchez-Villagra, 2009; Weisbecker and Mitgutsch, 2010), this intraspecific variability, is reflected in considerable homoplasy and consequently a “terminal branch”-placement of shifts in developmental timing when using algorithms such as event-pair cracking (Jeffery et al., 2002b) and Parsimov (Jeffery et al., 2005). To understand the evolution of the timing of early developmental events and the relevance of a phylogenetic signal contained in these data for certain taxonomic levels, it is vital to both sample closely related taxa and to further analyze individual variation.
Until recently, avian data were relatively scarce when compared to the number of studies from other major craniate clades, but the knowledge of ossification sequences in birds has been greatly extended (Starck, 1989, 1993; Nakane and Tsudzuki, 1999; Maxwell, 2008a, b; 2009; Maxwell and Harrison, 2008, 2009; Maxwell and Larsson, 2009); see Table 1. Reports covering the conditions in altricial birds are, however, still rare when compared to reports covering precocial species (but see Starck, 1989, 1993) owing to the difficulty in accessing embryonic specimens due to the demand in parental care, and the low reproduction rates of altricial forms. Nevertheless, the estrildid zebra finch, Taeniopygia guttata (Passeriformes), has become a well-established model in developmental neuroscience and is bred on a regular basis in a number of developmental biology laboratories (e.g., Tchernichovski et al., 2001; Bolhuis and Gahr, 2006). This species is thus a practical choice for the study of developmental timing in an altricial species. To address intraspecific variability, however, precocial species prove the far better choice. This is especially true since species belonging to the Galloanseres, such as the members of the phasianids (e.g., turkey, chicken) and anatids (e.g., duck) are bred for the food industry, and species such as the chick and the quail are well established “model species” in developmental biology. The White Pekin duck Anas platyrhynchos, a domestic race of the mallard and the Japanese quail, Coturnix coturnix japonica are particularly suited for such studies, for several reasons. First, previous reports covering the ossification sequence of this species are available for comparisons and second, interspecific comparison is less problematic within Galloanseres than in other avian taxa, due to the comparatively good sampling (see Table 1). Additionally, the Japanese quail and the White Pekin duck have been used in experiments involving the creation of chimeras, taking advantage of the different morphologies as well as the different developmental speeds of these two bird species (Lwigale and Schneider, 2008). These chimeras have been used intensively to investigate the evolution of developmental integration during craniofacial development with particular focus on the cranial neural crest, a potentially skeletogenic embryonic stem cell population (i.e., Schneider and Helms, 2003; Tucker and Lumsden, 2004; Eames and Schneider, 2008; Jheon and Schneider, 2009; Tokita and Schneider, 2009). Knowledge regarding the variation of developmental timing in both species will be an important background for the evaluation of such experimental designs.
Table 1.
Taxonomic sampling of available studies of avian ossification sequences (Schinz and Zangerl, 1937; Erdmann, 1940; Rogulska, 1962; Schumacher and Wolff, 1966; Starck 1989; Nakane and Tsudzuki, 1999; Maxwell, 2008a, b, 2009; Maxwell and Harrison, 2008)
| Anatidae | Anas plathyhynchos, Carinia moschata, Somateria mollissima |
| Columbidae | Columba livia |
| Corvidae | Corvus frugilegus |
| Dromaidae | Dromaius novaehollandiae |
| Estrildidae | Padda oryzivora |
| Laridae | Larus argentatus, L. canus, L ridibundus |
| Phasianidae | Coturnix coturnix, Gallus gallus, Meleagris gallopavo |
| Podicipedidae | Podiceps cristatus |
| Psittacidae | Melopsittacus undulatus |
| Rheidae | Rhea americana |
| Stercorariidae | Stercorarius skua |
| Sternidae | Sterna hirundo |
| Struthionidae | Struthio camelus |
| Tinamidae | Eudromia elegans |
| Turnicidae | Turnix suscitator |
The present study aims at a better understanding of the skeletogenesis of the white Pekin duck, the Japanese quail, and the zebra finch, and addresses questions regarding intraspecific variation and potential links between ossification patterns and life history. How common is intraspecific variation in the temporal order of ossification and is it evenly distributed or limited to several parts of the skeleton? Which elements are involved and to what degree? Is variation more common to certain developmental periods than to in others? Do altricial birds show notable particularities in their ossification sequences when compared to precocial birds?
MATERIALS AND METHODS
Embryo maintenance and collection
Zebra finch eggs containing embryos at different developmental stages were collected from a captive breeding colony at the Institute of Neuroinformatics, University of Zurich/ETHZ Zurich, Switzerland and from a captive breeding colony at the Institute of Biology, Leiden University, Leiden, The Netherlands. Embryos were fixed in 4% neutral buffered formaldehyde. Some zebra finch specimens were afterwards stored in 70% ethanol. Fertilized quail and duck eggs were obtained from AA LAB (Westminster, CA) and stored at 14°C for up to two weeks. All further treatment was in accordance with NIH and UCSF guidelines. To initiate the continuation of embryonic development, eggs were transferred and incubated in HOVA BATOR incubators at 38°C in a humid environment. For fixation of the embryos, eggshells were removed and the specimens were transferred to phosphate buffered saline (PBS). After washing in PBS, the embryos were fixed in either Karnovsky’s fixative or 4% PFA in PBS and stored at room temperature. Specimens of Hamburger-Hamilton (HH) stage (Hamburger and Hamilton, 1951) 35 and older were decapitated prior to fixation.
Histological procedures
Specimens were washed in PBS and dehydrated through graded ethanol series. The embryos were skinned and eviscerated, and fat was removed when necessary. All specimens were cleared and double stained for cartilage (alcian blue) and mineralized bone (alizarin red) following standard laboratory protocols (Dingerkus and Uhler 1977) with slight modifications. The cleared and stained embryos were transferred to glycerin and stored in glycerin with an addition of Thymol for preservation.
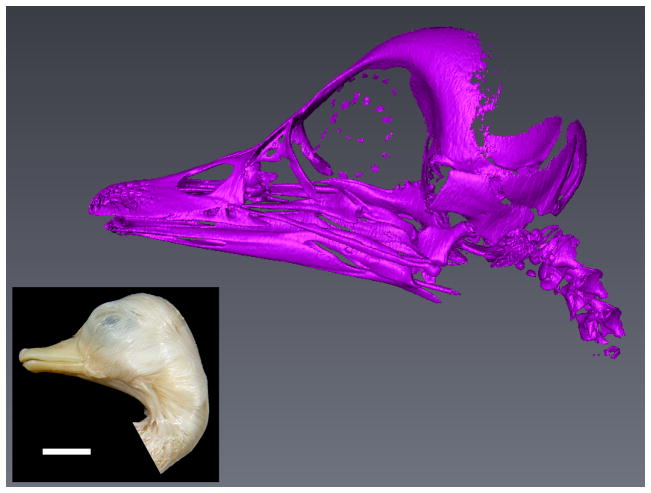
To illustrate further the ossification state of an older duck specimen, micro-computer tomography scanning was made at the Anthropological Institute of the University of Zürich.
Documentation and establishment of ossification sequences
Each head and body was treated as a discrete specimen. Data were collected by detailed personal observation on a stereo dissection microscope. Present ossified elements “B” were tabulated for each specimen (see Appendices 1–11). Onset of ossification was recorded based on the first presence of alizarin red staining. For the variation study (regarding A. platyrhynchos data), data were recorded separately for right and left half of the embryos. The vertebral column was divided into a cranial, middle, and caudal part of each group of cervical, thoracal, lumbal, and caudal vertebrae. In case of the lumbal vertebrae the middle and caudal part forms the synsacrum and the caudal part of caudal vertebrae, the pygostyle. The scleral ring was excluded from analysis since it was removed together with the eyes in most cases. Missing data are indicated in three different ways. “NA” means that some part of the body was lost by preparation, or could otherwise not be observed. Because of the variation of vertebral ribs, the tenth rib, when not present, was also labeled “NA”. If there was an obvious technical problem, i.e. no staining at all, it was marked as “NC”. In cases of apparently unmineralized bones it was impossible to decide whether this was due to technical issues, so the element was marked as “w” (white). In cases of dermal ossifying bones in the skull (frontal, parietal) visible but unstained structures were recorded as ossified. For the smaller zebra finch dataset, missing skeletal elements in incomplete specimens or elements that clearly showed insufficient staining and/or signs of demineralization were generally indicated by question marks. For each specimen, the numbers of missing (non-ossified), questionable, and present (ossified) skeletal elements were counted and the specimens were ordered according to number of present elements. For each bone, the numbers of specimens in which the element was present, the number of specimens in which its condition could not be determined and the number of specimens in which it was not present was counted. The elements were then sorted based on the number of specimens in which they were present. This representation of the data (ontogenetic states table) allows for easy recognition of elements variable in their relative temporal appearance as well as their overall temporal appearance.
In establishing ossification sequences, not stained but visible structures (w) of dermal bones were counted as ossified. In case of the frontal in the duck, which would get a different rank for right and left side, the lower rank was assigned.
Results
Information regarding the onset of ossification of bones in all specimens is presented in Appendices 1 to 11.
Anas platyrhynchos
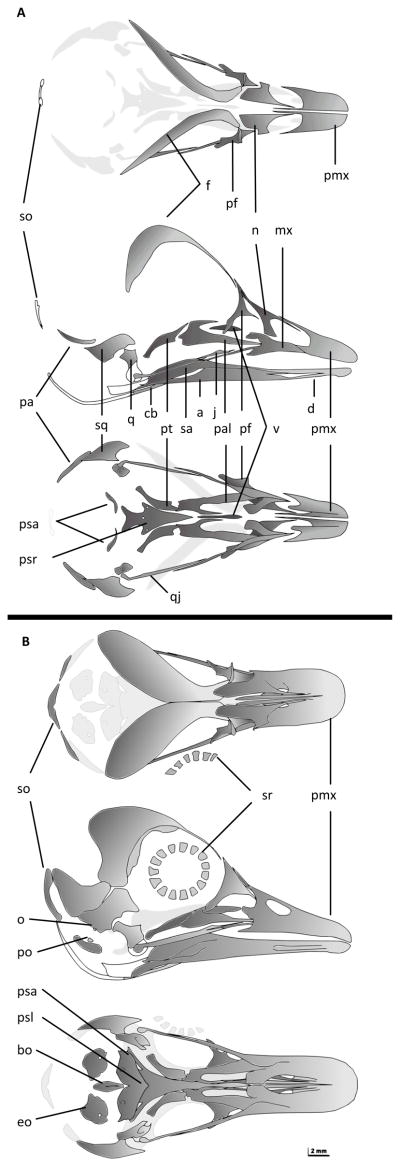
In earlier stages (i.e. PIMUZ lab#2008.301CM; approx. HH 35/36), most elements of the skull were still separated from each other (Fig. 1A) and easy to distinguish. In the oldest specimens (PIMUZ lab#2008.135CM, PIMUZ lab#2008.101CM), (HH40+; Maxwell, 2008b); beak length ~12–14mm; the bones of the facial skull were largely fused but the skull was still opened dorsally (Fig. 1B, Fig. 2); the oldest cleared and stained specimen showed 28 ossified elements in the skull and 66 in the postcranium.
Fig. 1.
Anas platyrhynchos, schematic drawings of PIMUZ lab#2008.301CM (~HH35/36) (A) and PIMUZ lab#2008.135CM (HH40+, beak length ~12mm) (B), top to bottom in dorsal, lateral, and ventral view of the skull. Ossified parts are shown in grey, dark grey elements in the front, light grey elements on the opposite side. Abbreviations: a, angular; bo, basioccipital; cb, ceratobranchial; d, dentary; eo, exoccipital; f, frontal; j, jugal; mx, maxilla; n, nasal; o; opistotic pa, parietal; pal, palatine; pf, prefrontal; pmx, premaxilla; po, prootic; psa, parasphenoid ala; psl, parasphenoid lamina; psr, parasphenoid rostrum; pt, pterygoid; q, quadrate; qj, quadratojugal; sa, supra-angular; so, supraoccipital; sq, squamosal; sr, scleral ring; v, vomer.
Fig. 2.
Micro-CT image of a skull of Anas platyrhynchos close to hatching. Scale bar 1cm.
The first mineralized bone to appear in the skull was the squamosal (~HH35) followed by palatine, pterygoid, prefrontal, and the bones forming the beak such as maxilla, jugal, quadratojugal, splenial, angular, and supraangular, followed shortly by premaxilla and dentary. Afterwards (~HH36) the nasal and frontal bones ossified. These bones appeared in quick succession. Before the onset of ossification in the quadrate the sequence was found to be consistent for all specimens. From HH36 onwards, some variation in ossification sequence could be documented (see Appendix 1, 2 and below). Variation was found concerning the basisphenoid, otic, and occipital regions. Furthermore shifts were found involving the supraoccipital and the prootic, the parasphenoid lamina, the opisthotic and the basioccipital. Comparing left and right side of the skull (Appendix 3), we found variation in the appearance of the opisthotic (one specimen: PIMUZ lab#2009.146CM) and the parietal bones (two specimens: PIMUZ lab#2009.161CM, PIMUZ lab#2009.193CM).
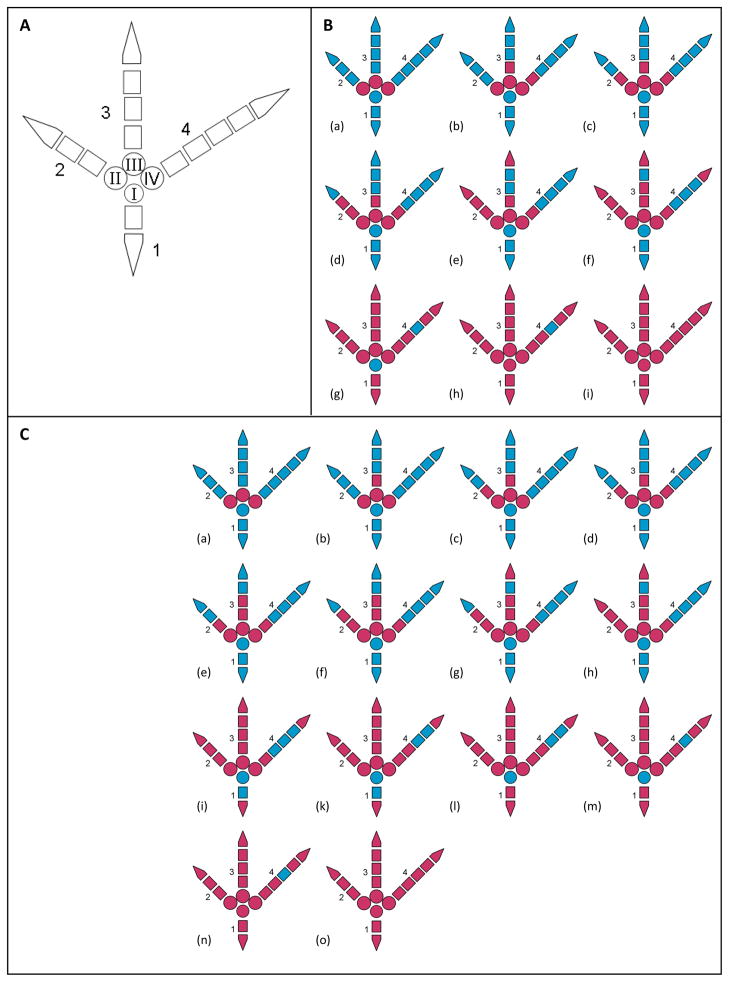
In the postcranium, the long bones were the first to mineralize, first humerus, femur, and tibiotarsus (~HH36/37), later radius, ulna and fibula. Ossification in the wing started earlier than in the foot. The metacarpals mineralized first, right after the furcula and before the metatarsals. With the ossification of the scapula (~HH36/37), that of the shoulder girdle was completed. Next, the vertebral ribs ossified almost simultaneously (~HH36). The number of vertebral ribs was found to vary from nine to 11. While ossification in the hand proceeded from proximal to distal, in the foot an additional process occurred in digits 1, 3 and 4 from proximal to distal. With the ossification of metatarsal I, and a bit later the 3. phalanx of digit 4, ossification in the foot was complete. The toes fully ossified from digits 2, 3, 1 to 4 (see Fig. 3). Although ossification of the hand started first, the 1. phalanx of digit minus was still cartilaginous in our oldest specimen. The vertebral column ossified from cranial to caudal; first the vertebral centra then the cervical ribs and at last the vertebral arches ossified. Exceptions were the relative order of ossification within the phalanges and among phalanges and other elements for example those belonging to the pelvic or axial skeleton (see Appendix 6 and Fig 3). Further variation involved the metatarsal I and the caudal part of cervical ribs. Little variation was found between right and left body part involving 1. phalanx of the 2. Digit, the 1. phalanx of the 4. digit, the 1. phalanx of digit majoris, and the 2. phalanx of the 2. Digit.
Fig. 3.
(A) Schematic drawings of a right foot of Anas platyrhynchos HH36–40+), dorsal view; 1–4 digits, I–IV metatarsals; (B) ossification sequence from Maxwell (2008b); (C) ossification sequence from our dataset; cartilaginous elements in blue, mineralized elements in red. The two datasets complete each other. Differences are a shift between the 2. phalanges of digits 2 and 4 (B (b), C (c)) and secondly the comparatively early appearance of the 2. phalanx of digit 3. In our data set the latter ossifies right after the second phalanges of digits 2, 3 and 4 (C (f)). The data of Maxwell (2008b) showed an earlier ossification of the whole digit 2 and the end phalanx of digit 3 (B (f))
Using the set of whole embryos (until approx. HH 37) and separated embryonic heads with attached cervical vertebrae, shifts between the exoccipital and a vertebral centrum were observed. For complete skeletons, ossification started postcranially with the long bones (Appendix 7). After radius and ulna, the metacarpals and at the same time the squamosal of the skull ossified. While fibular and metatarsals I–IV ossified, most bones of the skull (angular, supraangular, jugal, quadratojugal, maxilla, splenial, prefrontal, palatine, and pterygoid) appeared. The vertebral ribs, pelvic girdle, shoulder girdle, and the first phalanges (1.phal of dig. 2, 3 and 4; 2.phal of dig. 2 and 3; 1.phal. of dig. alulae and majoris) appeared, followed by the ossification of the bones from the occipital and otic regions. Only then the vertebral column started to ossify. Variability was observed between the exoccipital and cranial vertebral centra. Few other variations between cranium and postcranium involved vomer, parasphenoid ala, exoccipital, and prootic, as well as scapula, 2. phalanx of digit 3 and 2. phalanx of digit 2.
Coturnix coturnix japonica
A dataset of 11 specimens (one skull, three bodies, seven complete embryos, ranging from HH36 to 40+) was collected and 30 cranial and 31 postcranial elements were recorded (Appendix 8–10). In the first specimen, the bones forming the beak (premaxilla, maxilla, jugal, quadratojugal, dentary, angular and supraangular) as well as the squamosal, nasal, prefrontal, palatine and the pterygoid were already ossified. Consecutively the ceratobranchial, exoccipital, parasphenoid rostrum, frontal, quadrate, prootic, prearticular, basioccipital, parasphenoid lamina, and ala, parietal, vomer, opisthotic and finally the supraoccipital ossify. Postcranially, the ossification started with the long bones humerus, radius, ulna, femur and tibiotarsus and the furcula, followed by the fibula, scapula, illium, the vertebral ribs, coracoid, the phalanges of the hind limb, the pubis, the phalanges of the forelimb, and the ischium. Ossification in the vertebral column started with the cervical and thoracal centra, followed by the cervical vertebral arches and ribs, the thoracal vertebral arches, and synsacral centra. Finally the sternum and the sternal ribs ossified. Looking at complete skeletons, variation was found involving the prootic and the dorsal ribs (Appendices 8–10).
Taeniopygia guttata
The following order of mineralization of skeletal elements (example shown in Fig. 4, all specimens in Appendix 11, HH35–HH40+) was recorded: (1) femur, humerus, radius, tibia/fibula, ulna, metatarsalia, supraangular, pterygoid, dentary, premaxilla, parasphenoid lamina; (2) angular, maxilla, palatine, parasphenoid rostrum, quadratojugal, squamosal, ceratobranchial, metacarpalia, nasal bone, scapula, vomer (initially paired, fusing later on), coracoid, furcula, ribs, splenial; (3) exoccipital, parasphenoid ala, basioccipital, frontal, parietal, quadrate, phalanges, ilium, pubis, ischium, cervical and thoracal vertebrae; (4) synsacral and caudal vertebrae. Mineralization of ribs and vertebrae within the scored groups of elements happens sequential in anterior-posterior order. Mineralization of ischium and pubis precedes mineralization of ischium and pubis when comparing the progress of ossification.
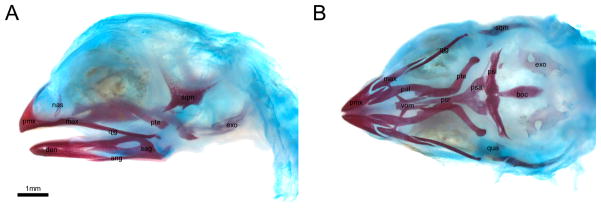
Fig. 4.
Cleared and stained embryo of Taeniopygia guttata (HH40+). Cartilage blue, mineralized bone red; (A) lateral view; (B) palate from ventral; extended focal images, background removed digitally. Abbreviations: ang, angular; boc, basioccipital; den, dentary; exo, exoccipital; max, maxilla; nas, nasal; pal, palatine; prm, premaxilla; psa, parasphenoid ala; psl, parasphenoid lamina; psr, parasphenoid rostrum; pte, pterygoid; qjg, quadratojugal; qua, quadrate; sag, supra-angular; sqm, squamosal; vom, vomer
The endochondral ossifications of the basal plates become also visible after the squamosal but – judging from the extent of ossification – probably precedes the appearance of frontal and parietal in the zebra finch. Intraspecific variation is obvious in relatively late ossifying elements.
DISCUSSION
Our results show a general pattern largely concordant with those reported for other birds (Starck, 1989; Maxwell, 2008a, b, 2009; Maxwell and Harrison, 2008, 2009; Maxwell and Larsson, 2009). The proximal longbones of the limbs are among the first elements to ossify, together with some jaw and palatal elements. Other visceral elements, and jugal arch and ribs (starting to ossify dorsally) follow. The more distal elements of the limbs ossify comparatively late. Shoulder girdle element ossification precedes the ossification of the pelvic elements. The more anterior parts of the axial skeleton ossify before the more caudal groups. Of the dermal bones of the braincase, the squamosal appears clearly before frontal and parietal bones.
Intraspecific variability
This study addresses the issue of individual variation in avian ossification sequences by individually scoring ossification states in the available specimens and, in the cases of duck and quail, by comparing obtained ossifications sequences to those reported from other sources for the same species; intraspecific variability can be potentially biased towards higher variation in studies of this kind because of additional factors, such as the fact that ossification sequences are usually reconstructed from different specimens that might originate from different sources, or that samples might have been exposed to different fixation times or react differently to staining/visualization techniques, for example due to their individual absolute size and tissue differentiation.
The sequence of ossification was found to be invariable during the earliest developmental stages investigated. While this might be interpreted with respect to the total number of elements available, it is also a sampling issue, due to limitations of selecting individuals based on a measure for “morphogenetic equidistance”. Variation found had largely been restricted to elements that would be neighbors in a sequence of ossification and thus would be plausible during early stages, depending on the resolution available. Especially the facial bones appear very early and nearly simultaneous in development. The rapid development during this period renders a better temporal resolution problematic, but at the same time hints at the possibility of higher variation. Postcranial results equal those from the skull. While in early stages, variability in ossification events was not detected, more variability became visible with the number of ossified elements increasing. For the duck, our sample provides also a good approximation of the complete order of ossification events during the earlier developmental period. Only very few asymmetries, meaning one element being found on one body side but not on the contralateral one, were found in the skull of the duck. Only three out of 71 specimens show deviation in only one of 28 characters. In fact, the variation could be much higher. Because only the beginning of ossification is tabulated, the exact point in time at which a bone begins ossifying would be ideal for recording. When mineralization proceeds very fast, asymmetry could only be expected during a very limited timeframe. During observations, differences in the progress of mineralization between left and right side could be observed. Since it is impossible to ensure identical conditions in one egg, the observed variation between left and right sides of the body might well be due to factors that affect on embryonic development such as temperature, humidity or gravitation.
In comparable studies covering non-avian species such as the Siamese fighting fish, Betta splendens (Mabee and Trendler, 1996) or the tailed frog, Ascaphus truei (Moore and Townsend, 2003) considerable amounts of intraspecific variation have been reported especially in skull bones. In B. splendens for example, prootic, exoccipital, hyoid, basioccipital and quadrate were found to be affected. Exoccipital, parasphenoid and frontoparietal were reported to show variation in A. truei (Moore and Townsend, 2003) a situation that can be confirmed for another frog species, the African bullfrog (Pyxicephalus adspersus) when comparing different reports of its sequence of ossification (Haas, 1999; Sheil, 1999). As in the duck, many variations in the sequences affect otic and occipital regions, and the parasphenoid. Plasticity in the development of the autopodia have been reported for other sauropsids such as the Chinese soft-shelled turtle Pelodiscus sinensis (Sánchez-Villagra et al., 2009) or the snapping turtle Chelydra serpentina (Rieppel, 1993; Sheil and Greenbaum, 2005) and at least one more homoeothermic species, the African striped mouse Rhabdomys pumilio (Wilson et al., 2010).
To resolve fully a sequence of ossification within a species, in vivo observation of ossification in high numbers of specimens would be ideal. However, so far ossifications sequences have to be reconstructed from observations in fixed developmental states of different individuals. Due to the rapid developmental progress especially during early ontogenesis, it is difficult to obtain a good resolution of the ossification events during these periods. Especially in the skull, several elements appear to ossify simultaneous, a finding that can have massive influences on analyses comparing differently resolved datasets using event-pair data (Harrison and Larsson, 2008).
Variation among datasets
The ossification of the quail Coturnix coturnix japonica has been documented in three previous studies (Starck, 1989; Nakane and Tsudzuki, 1999; Maxwell, 2008a). These studies all report the very early ossification of the long bones and of most of the bones in the skull. Looking at the reported sequences for the limbs, no differences were found, but the datasets themselves do reveal some discrepancies (see Maxwell, 2008a, Table 2) adding to the intraspecific variability reported by Maxwell (2008a).
Table 2.
Exemplified comparison of ossification sequences reported for Coturnix coturnix japonica
| Study | sequence |
|---|---|
| Starck (1989) | pubis → illium & exoccipitale → ischium |
| Nakane & Tsudzuki (1999) | ilium ischium → exoccipitale & pubis |
| Maxwell (2008a) | pubis → illium → ischium → exoccipitale |
| this study | exoccipitale & ilium → pubis → ischium |
When comparing our dataset to previously published data on duck ossification sequences by Maxwell (2008b) some differences became apparent (Table 3, Fig. 3), involving the parietal, quadrate, frontal, the vertebral ribs, and the phalanges, indicating generally even higher variation than indicated by our own dataset alone.
Table 3.
Rank order of element ossification in Anas platyrhynchos. Comparison between this study and Maxwell (2008b). Only elements appearing in both datasets are listed. Left: actual sequence ordered by rank for direct comparison; right: alphabetical ordered elements with its ranks. Abbreviations: c, cartilaginous; NA, not present
| Maxwell (2008b) | this study | Maxwell (2008b) | this study | |||
|---|---|---|---|---|---|---|
| 1 | Quadratojugal | Squamosal | 1 | Angular | 1 | 2 |
| 1 | Squamosal | Quadratojugal | 2 | Articular | 12 | c |
| 1 | Angular | Angular | 2 | Basihyal | c | c |
| 1 | Supraangular | Supraangular | 2 | Basioccipital | 10 | 13 |
| 2 | Prefrontal | Prefrontal | 2 | Ceratobranchial | 3 | 4 |
| 2 | Maxilla | Maxilla | 2 | Dentary | 3 | 2 |
| 2 | Nasal | Nasal | 2 | Entoglossal | c | c |
| 2 | Palatine | Palatine | 2 | Epibranchial | c | c |
| 2 | Pterygoid | Pterygoid | 2 | Epiotic | 13 | NA |
| 3 | Jugal | Jugal | 2 | Exoccipital | 8 | 8 |
| 3 | Premaxilla | Pre-maxilla | 2 | Frontal | 5 | 2 |
| 3 | Dentary | Dentary | 2 | Jugal | 3 | 2 |
| 3 | Ceratobranchial | Frontal | 2 | Maxilla | 2 | 2 |
| 4 | Parietal | Splenial | 2 | Nasal | 2 | 2 |
| 4 | Quadrate | Parietal | 3 | Opisthotic | 11 | 12 |
| 5 | Frontal | Cerathobranchial | 4 | Palatine | 2 | 2 |
| 6 | Vomer | Parasphenoid rostrum | 5 | Parasphenoid ala | 8 | 8 |
| 7 | Parasphenoid rostrum | Quadrate | 6 | Parasphenoid lamina | 8 | 11 |
| 7 | Splenial | Vomer | 7 | Parasphenoid rostrum | 7 | 5 |
| 8 | Exoccipital | Exoccipital | 8 | Parietal | 4 | 3 |
| 8 | Parasphenoid ala | Parasphenoid ala | 8 | Prearticular | 9 | 14 |
| 8 | Parasphenoid lamina | Supraoccipital | 9 | Prefrontal | 2 | 2 |
| 8 | Supraoccipital | Prootic | 10 | Premaxilla | 3 | 2 |
| 9 | Prearticular | Parasphenoid lamina | 11 | Prootic | 11 | 10 |
| 10 | Basioccipital | Opisthotic | 12 | Pterygoid | 2 | 2 |
| 11 | Opisthotic | Basiooccipital | 13 | Quadrate | 4 | 6 |
| 11 | Prootic | Prearticular | 14 | Quadratojugal | 1 | 2 |
| 12 | Articular | Articular | c | Splenial | 7 | 2 |
| 14 | Epiotic | Entoglossum | c | Squamosal | 1 | 1 |
| c | Basihyal | Basihyal | c | Supraangular | 1 | 2 |
| c | Entoglossal | Urohyal | c | Supraoccipital | 8 | 9 |
| c | Epibranchial | Epibranchial | c | Urohyal | c | c |
| c | Urohyal | Epiotic | NA | Vomer | 6 | 7 |
For comparisons of separate datasets, several factors should be taken into account as potential sources of variability, such as the possibility of effects from dissimilarly applied techniques and investigator bias, but more importantly, distinct temporal resolutions of ossification sequences resulting from different availability and preservation status. Different resolutions in temporal sequence data lead to divergent numbers in simultaneous events. Simultaneous events in sequences of developmental events can be the result of lack of resolution due to insufficient availability of developmental data. Otherwise, these can reflect a real occurrence of simultaneous developmental events or they can reflect temporal variation among developmental events when variable elements get grouped into one rank. In intraspecific comparisons utilizing “event-pairing” (Mabee and Trendler, 1996; Smith, 1997; Velhagen, 1997), simultaneous events will bias further analyses by supporting their own shifts as they will always occur grouped due to the non-independent nature of event-pair data. As an extreme example, comparing a taxon with minimal resolution (all developmental events have the same rank) to a taxon with full resolution (all developmental events have their own rank) would result in finding the maximum possible number of heterochronies.
The considerable numbers of heterochronies found on terminal branches in other studies, the branches leading to the terminal taxon (i.e. Bininda-Emonds et al., 2003; Wilson et al., 2010; Weisbecker and Mitgutsch, 2010), could, although they have been discussed as methodological artifacts (Harrison and Larsson, 2008), also hint at high rates of evolutionary changes and thus a sign that many shifts in developmental timing are convergent. Furthermore, given the existence of such high intraspecific variability of ossification, the terminal placements could also be the result of insufficient resolution in taxon sampling.
Avian ossification sequences and life history
Although the skeletons of precocial birds are considerably more ossified by the time of hatching than the skeletons of altricial birds (e.g. Blom and Lilja, 2004), the grand order of mineralization events seems largely conserved (Starck, 1989, Maxwell, 2008b). Among the bird species for which ossification sequences are available (Table 1), the estrildid species and Melopsittacus undulatus are the birds showing the highest degree of altriciality (altricial 2, following the classification of Starck, 1993) whereas in particular the anatids and phasianids rather belong to the opposite end of the altricial-precocial spectrum. Certain variations in mineralization events could be detected, especially in events from different body regions (e.g. mineralization of skull elements vs. limb elements or forelimb vs. hind limb elements). Also, the delayed integration of the skull roof relative to other ossified complexes could be interpreted as related to rapid postnatal growth. However, a functional explanation of the phylogenetic distribution of such characters remains problematic, given the varying available sequence resolution and the lack of information on individual variation.
Several bird species investigated here and in other previous related works are domesticated forms. The evolutionary aspects of domestication were already noted by Charles Darwin (Darwin, 1859). Since then, multiple studies have showed the influence of domestication on animal genetics, morphology and behavior (see i.e. Coppinger and Schneider, 1995; Dobney and Larson, 2006; Matsuzaki et al., 2009; Tamlin et al., 2009). Regarding the comparative aspect when looking at early developmental characters and life history evolution, Maxwell (2008b) pointed out the importance of accounting for the effects of artificial selection when studying races bred in captivity such as the White Pekin duck. Such races are oftentimes selected for reaching sizes to exceed the size of their wild forms and for rapid postnatal growth. Rapid postnatal growth is a trait often times connected to altriciality, and thus comparisons of developmental traits of the White Pekin duck to both precocial wild forms and altricial birds is a productive avenue for investigating life history evolution and for understanding potential impacts of postnatal selection on prenatal embryonic stages. While some studies suggest that selection for late ontogenetic characteristics can alter earlier embryonic development (i.e. Lilja et al., 2001), evidence for such selection causing developmental heterochronies in timing of ossification events is lacking. Egg size also does not seem to influence ossification sequences (Maxwell, 2008a). Altricial birds have a shorter incubation time relative to egg size than precocial species. But the incubation time itself does not explain all differences in the amount of ossification. Although the turkey has a considerably longer incubation period than either the chicken or the quail, it does not demonstrate a higher number of ossified elements (Maxwell, 2008a). Avian species with higher growth rates tend to have a higher proportion of cartilage in their skeletons (Starck, 1996; Blom and Lilja, 2004). The postnatal growth rate of Coturnix coturnix, Turnix suscitator and Carinia moschata is relatively low, whereas Melopsittacus undulatus, Padda oryzivora and Columba livia have a postnatal growth factor, which is about ten times higher (Starck, 1989). A high growth rate may also lead to a delay in the ossification of some skeletal elements (Arendt and Wilson, 2000). Nevertheless, a screening for more altricial developmental patterns in White Pekin duck than in wild ducks would thus be worthwhile, as our oldest specimens still show broad cartilaginous areas between the ossified zones in the postcranial elements. Also, sternum and sternal ribs do not ossify until hatching and although the bones of the facial skull are widely fused, the skull is still open in oldest prehatching specimens (Fig. 2).
That only the function of the bone influences the timing of ossification seems unlikely concerning birds. For example, in organisms with a feeding larval stage, such as many fish and amphibian species, it is important for maxillary and palatal elements to be ossified for efficient feeding and to prevent damage to the brain caused by large food items (Adriaens and Verraes, 1998). In birds, however, the palatal, maxillary, and mandibular elements ossify well before any feeding activity, so a purely functional hypothesis cannot explain their early formation (Maxwell, 2008b). The feeding apparatus is very well ossified before hatching in birds, as has been also recorded in dinosaur taxa (Delfino and Sánchez-Villagra, 2010).
With the renewed interest in comparative embryology, interspecific comparisons are increasingly based on developmental events instead of staging tables (for discussion see e.g., de Jong et al., 2009; Werneburg, 2009). Such approaches lack the bias towards finding similarity as opposed to the latter in both inter- and intraspecific comparisons. Since intraspecific variation provides diversity and the basis for selection, the analysis of intraspecific variation of developmental timing is vital to understand the appearance of interspecific heterochronies and to explore potential links among developmental characters (e.g., Schmidt and Starck, 2004; Colbert and Rowe, 2008).
High interspecific variability and the possibility of considerable convergent evolutionary changes in developmental timing of ossification sequences suggest that connections between changes in developmental timing and adult morphology or life history require very dense taxon sampling to be made with confidence. Also, informative phylogenetic signals that could be retrieved from ossification sequence data for certain taxonomic levels would need to be adjusted by further taxon sampling, by additional information on intraspecific variation in total ossification sequences, and by further data on the developmental appearance of individual bones and groups of bones in a number of species.
Conclusions and outlook
The overall sequence of ossification in the White Pekin duck Anas platyrhynchos was found to be invariable, but variability is present in the relative timing of ossification in bones belonging to certain cranial or postcranial regions. This variation appears generally in elements ossifying in close temporal proximity. The comparison with previously published data suggests additional variability among different breeds both in duck and quail; a screen for differences in developmental variation between domestic breeds and wild forms would give interesting further insights. Nevertheless, intraspecific variation in ossification sequence seems to be a general phenomenon, and must be accounted for in interspecific comparisons. Future studies of ossification sequences should ideally be designed so that intraspecific variation, if present, will be revealed.
Taking these considerations into account, it will be interesting to explore further whether interspecific variability of ossification sequences is equally shown by all events or if events associated with certain groups of bones are more likely to interchange their relative times, motivating screen for developmental modularity within ossification sequences. Goswami et al. (2009) have shown that such patterns of modules could be assigned to therian mammal clades whereas no heterochronies were discernable for the same data. Also, Schoch (2006) pointed out the existence of clusters of bones highly conserved in vertebrate evolution that do not seem to disintegrate over time.
Supplementary Material
Ontogenetic states for ossified elements in Anas platyrhynchos specimens, cranial elements, left side. Abbreviations: B, mineralized bone as detected by alizarin red staining; NA, not present; NC, no decision (technical problem); w, bone present, no alizarin uptake
Ontogenetic states for ossified elements in Coturnix coturnix japonica, complete skeletons. Abbreviations as in Appendix 1
Ontogenetic states for ossified elements in Taeniopygia guttata, complete skeletons. Abbreviations: B, mineralized bone as detected by alizarin red staining; ?, missing data
Ontogenetic states for ossified elements in Anas platyrhynchos specimens, cranial elements, right side. Abbreviations as in Appendix 1
Ontogenetic states for ossified elements in Anas platyrhynchos, cranial elements. Abbreviations as in Appendix 1
Ontogenetic states for ossified elements in Anas platyrhynchos, postcranial elements, left side. Abbreviations as in Appendix 1
Ontogenetic states for ossified elements in Anas platyrhynchos, postcranial elements, right side. Abbreviations as in Appendix 1
Ontogenetic states for ossified elements in Anas platyrhynchos, postcranial elements. Abbreviations as in Appendix 1
Ontogenetic states for ossified elements in Anas platyrhynchos, complete skeletons. Abbreviations as in Appendix 1
Ontogenetic states for ossified elements in Coturnix coturnix japonica, cranial elements. Abbreviations as in Appendix 1
Ontogenetic states for ossified elements in Coturnix coturnix japonica, postcranial elements. Abbreviations as in Appendix 1
Acknowledgments
This work was supported by the Swiss National Fond to MRS-V and by NIDCR R03 DE014795, R01 DE016402, and NIAMS R21 AR052513 to RAS. We thank the Palaeontological Department of the University of Zürich and the Department of Orthopaedic Surgery of the University of California at San Francisco for support and Jasmina Hugi, Daisuke Koyabu, Morana Mihaljevic, Laura B. Wilson, Christian Kolb, James Neenan, Torsten Scheyer, Ingmar Werneburg, and the members of the Schneider-lab for comments and discussion. Kristin Butcher is thanked for help in handling the duck and quail specimens. Michael K. Richardson and Merijn de Bakker, University of Leiden, The Netherlands, are thanked for generously making additional zebra finch embryos available. Christopher Zollikofer and Naoki Morimoto, University of Zürich, kindly made available the micro-CT facilities and supervised their use. Part of this study has been presented as a Master’s Thesis by CW.
References
- Adriaens D, Verraes W. Ontogeny of the osteocranium in the African catfish Clarias gariepinus Burchell (1822) (Siluriformes: Clariidae): Ossification sequence as a response to functional demands. J Morphol. 1998;235:183–237. doi: 10.1002/(SICI)1097-4687(199803)235:3<183::AID-JMOR2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Arendt JD, Wilson DS. Population differences in the onset of cranial ossification in pumpkinseed (Lepomis gibbosus), a potential cost of rapid growth. Can J Fish Aquat Sci. 2000;57:351–356. [Google Scholar]
- Balanoff AM, Rowe T. Osteological description of an embryonic skeleton of the extinct elephant bird, Aepyornis (Palaeognathae: Ratitae) J Vert Paleontol. 2007;27 (suppl):1–53. [Google Scholar]
- Bininda-Emonds ORP, Jeffery JE, Richardson MK. Is sequence heterochrony an important evolutionary mechanism in mammals? J Mammal Evol. 2003;10:335–361. [Google Scholar]
- Blom J, Lilja C. A comparative study of growth, skeletal development and eggshell composition in some species of birds. J Zool. 2004;262:361–369. [Google Scholar]
- Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nat Rev Neurosci. 2006;7:347–357. doi: 10.1038/nrn1904. [DOI] [PubMed] [Google Scholar]
- Chipman AD, Haas A, Tchernov E, Khaner O. Variation in anuran embryogenesis: differences in sequence and timing of early developmental events. J exp Zool (Mol Dev Evol) 2000;288:352–365. doi: 10.1002/1097-010X(20001215)288:4<352::AID-JEZ8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Colbert MW, Rowe T. Ontogenetic sequence analysis: using parsimony to characterize developmental sequences and sequence polymorphism. J exp Zool (Mol Dev Evol) 2008;310B:398–416. doi: 10.1002/jez.b.21212. [DOI] [PubMed] [Google Scholar]
- Coppinger R, Schneider RA. Evolution of working dogs. In: Serpell J, editor. The Domestic Dog. Cambridge University Press; Cambridge: 1995. pp. 21–47. [Google Scholar]
- Darwin C. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. London: John Murray; 1859. p. 515. [PMC free article] [PubMed] [Google Scholar]
- de Jong IML, Colbert MW, Witte F, Richardson MK. Polymorphism in developmental timing: intraspecific heterochrony in a Lake Victoria cichlid. Evol Dev. 2009;11:625–635. doi: 10.1111/j.1525-142X.2009.00370.x. [DOI] [PubMed] [Google Scholar]
- Delfino M, Sánchez-Villagra MR. A survey of the rock record of reptilian ontogeny. Seminars Cell Dev Biol. 2010;21:432–440. doi: 10.1016/j.semcdb.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Dingerkus G, Uhler LD. Enzyme clearing of alcian blue stained whole small vertebrates for demonstration of cartilage. Stain Technol. 1977;52:229–232. doi: 10.3109/10520297709116780. [DOI] [PubMed] [Google Scholar]
- Dobney K, Larson G. Genetics and animal domestication: New windows on an elusive process. J Zool. 2006;269:261–271. [Google Scholar]
- Eames BF, Schneider RA. The genesis of cartilage size and shape during development and evolution. Development. 2008;135:3947–3958. doi: 10.1242/dev.023309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann K. Zur Entwicklungsgeschichte der Knochen im Schädel des Huhnes bis zum Zeitpunkt des Ausschlüpfens aus dem Ei. Zeitschr Morphol Ökol Tiere. 1940;36:315–400. [Google Scholar]
- Fröbisch NB. Ossification patterns in the tetrapod limb – conservation and divergence from morphogenetic events. Biol Rev. 2008;83:571–600. doi: 10.1111/j.1469-185X.2008.00055.x. [DOI] [PubMed] [Google Scholar]
- Germain D, Laurin M. Evolution of ossification sequences in salamanders and urodele origins assessed through event-pairing and new methods. Evol Dev. 2009;11:170–190. doi: 10.1111/j.1525-142X.2009.00318.x. [DOI] [PubMed] [Google Scholar]
- Goswami AV, Weisbecker V, Sánchez-Villagra MR. Developmental modularity and the marsupial-placental dichotomy. J exp Zool (Mol Dev Evol) 2009;312B:186–195. doi: 10.1002/jez.b.21283. [DOI] [PubMed] [Google Scholar]
- Haas A. Larval and metamorphic skeletal development in the fast-developing frog Pyxicephalus adspersus (Anura, Ranidae) Zoomorphol. 1999;119:23–35. [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Harrison LB, Larsson HCE. Estimating evolution of temporal sequence changes: a practical approach to inferring ancestral developmental sequences and sequence heterochrony. Syst Biol. 2008;57:378–387. doi: 10.1080/10635150802164421. [DOI] [PubMed] [Google Scholar]
- Hugi J, Mitgutsch C, Sánchez-Villagra MR. Chondrogenic and ossification patterns in White’s skink Liopholis whitii (Scincidae, Reptilia) Zoosyst Evol. 2010;86:21–32. [Google Scholar]
- Jeffery JE, Bininda-Emonds ORP, Coates MI, Richardson MK. Analyzing evolutionary patterns in amniote embryonic development. Evol Dev. 2002a;4:292–302. doi: 10.1046/j.1525-142x.2002.02018.x. [DOI] [PubMed] [Google Scholar]
- Jeffery JE, Richardson MK, Coates MI, Bininda-Emonds ORP. Analyzing developmental sequences within a phylogenetic framework. Syst Biol. 2002b;51:478–491. doi: 10.1080/10635150290069904. [DOI] [PubMed] [Google Scholar]
- Jeffery JE, Bininda-Emonds ORP, Coates MI, Richardson MK. A new technique for identifying sequence heterochrony. Syst Biol. 2005;54:230–240. doi: 10.1080/10635150590923227. [DOI] [PubMed] [Google Scholar]
- Jheon AH, Schneider RA. The cells that fill the bill: neural crest and the evolution of craniofacial development. J Dent Res. 2009;88:12–21. doi: 10.1177/0022034508327757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerney R, Gross JB, Hanken J. Early cranial patterning in the direct-developing frog Eleutherodactylus coqui revealed through gene expression. Evol Dev. 2010;12:373–382. doi: 10.1111/j.1525-142X.2010.00424.x. [DOI] [PubMed] [Google Scholar]
- Koenemann S, Schram FR. The limitations of ontogenetic data in phylogenetic analyses. Contrib Zool. 2002;71:47–65. [Google Scholar]
- Lilja C, Blom J, Marks HL. A comparative study of embryonic development of Japanese quail selected for different patterns of postnatal growth. Zoology. 2001;104:115–122. doi: 10.1078/0944-2006-00016. [DOI] [PubMed] [Google Scholar]
- Lwigale PY, Schneider RA. Other chimeras: quail-duck and mouse-chick. Methods Cell Biol. 2008;87:59–74. doi: 10.1016/S0091-679X(08)00203-3. [DOI] [PubMed] [Google Scholar]
- Mabee PM, Trendler TA. Development of the cranium and the paired fins in Betta splendens (Teleostei: Percomorpha): intraspecific variation and interspecific comparisons. J Morphol. 1996;227:249–287. doi: 10.1002/(SICI)1097-4687(199603)227:3<249::AID-JMOR1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Maisano JA. Postnatal skeletal ontogeny in Callisaurus draconoides and Uta stansburiana (Iguania: Phrynosomatidae) J Morphol. 2002a;251:114–139. doi: 10.1002/jmor.1078. [DOI] [PubMed] [Google Scholar]
- Maisano JA. The potential utility of postnatal skeletal developmental patterns in squamate phylogenetics. Zool J Linn Soc. 2002b;136:277–313. [Google Scholar]
- Matsuzaki SS, Mabuchi K, Takamura N, Nishida M. Behavioural and morphological differences between feral and domesticated strains of common carp Cyprinus carpio. J Fish Biol. 2009;75:1206–1220. doi: 10.1111/j.1095-8649.2009.02345.x. [DOI] [PubMed] [Google Scholar]
- Maxwell EE. Comparative embryonic development of the skeleton of the domestic turkey (Meleagris gallopavo) and other galliform birds. Zoology. 2008a;111:242–257. doi: 10.1016/j.zool.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Maxwell EE. Ossification sequence of the avian order Anseriformes, with comparison to other precocial birds. J Morphol. 2008b;269:1095–1113. doi: 10.1002/jmor.10644. [DOI] [PubMed] [Google Scholar]
- Maxwell EE. Comparative ossification and development of the skull in palaeognathous birds (Aves: Palaeognathae) Zool J Linn Soc. 2009;156:184–200. [Google Scholar]
- Maxwell EE, Harrison LB. Ossification sequence of the common Tern (Sterna hirundo) and its implications for the interrelationships of the Lari (Aves, Charadriiformes) J Morphol. 2008;269:1056–1072. doi: 10.1002/jmor.10633. [DOI] [PubMed] [Google Scholar]
- Maxwell EE, Harrison LB. Methods for the analysis of developmental sequence data. Evol Dev. 2009;11:109–119. doi: 10.1111/j.1525-142X.2008.00307.x. [DOI] [PubMed] [Google Scholar]
- Maxwell EE, Larsson HCE. Comparative ossification sequence and skeletal development of the postcranium of palaeognathous birds (Aves: Palaeognathae) Zool J Linn Soc. 2009;157:169–196. [Google Scholar]
- Maxwell EE, Harrison LB, Larsson HCE. Assessing the phylogenetic utility of sequence heterochrony: evolution of avian ossification sequences as a case study. Zoology. 2010;113:57–66. doi: 10.1016/j.zool.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Mitgutsch C, Piekarski N, Olsson L, Haas A. Heterochronic shifts during early cranial neural crest cell migration in two ranid frogs. Acta Zool. 2008;89:69–78. [Google Scholar]
- Mitgutsch C, Olsson L, Haas A. Early embryogenesis in discoglossoid frogs: a study of heterochrony at different taxonomic levels. J Zool Syst Evol Res. 2009;47:248–257. [Google Scholar]
- Moore MK, Townsend VR., Jr Intraspecific variation in cranial ossification in the tailed frog, Ascaphus truei. J Herpetol. 2003;37:714–717. [Google Scholar]
- Nakane Y, Tsudzuki M. Development of the skeleton in Japanese quail embryos. Develop Growth Differ. 1999;41:523–534. doi: 10.1046/j.1440-169x.1999.00454.x. [DOI] [PubMed] [Google Scholar]
- Olsson L, Hanken J. Cranial neural-crest migration and chondrogenic fate in the Oriental fire-bellied toad Bombina orientalis: defining the ancestral pattern of head development in anuran amphibians. J Morphol. 1996;229:105–120. doi: 10.1002/(SICI)1097-4687(199607)229:1<105::AID-JMOR7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Prochel J, Goswami A, Carmona FD, Jimenéz R. Ossification sequence in the mole Talpa occidentalis (Eulipotyphla, Talpidae) and comparison with other mammals. Mammal Biol. 2008;73:299–403. [Google Scholar]
- Richardson MK, Jeffery JE, Coates MI, Bininda-Emonds ORP. Comparative methods in developmental biology. Zoology. 2001;104:278–283. doi: 10.1078/0944-2006-00033. [DOI] [PubMed] [Google Scholar]
- Rieppel O. Studies on skeleton formation in reptiles: Patterns of ossification in the skeleton of Chelydra serpentina (Reptilia, Testudines) J Zool. 1993;231:487–509. [Google Scholar]
- Rogulska T. Differences in the process of ossification during the embryonic development of the chick (Gallus domesticus L.), rook (Corvus frugilegus L.) and black headed gull (Larus ridibundus L.) Zool Polon. 1962;12:223–233. [Google Scholar]
- Rose C. The developmental morphology of salamander skulls. In: Heatwole H, Davies M, editors. Amphibian Biology. Vol. 5. Surrey Beatty and Sons; Chipping Norton: 2003. pp. 1684–1781. [Google Scholar]
- Sánchez-Villagra MR. Comparative patterns of postcranial ontogeny in therian mammals: an analysis of relative timing of ossification events. J Exp Zool. 2002;294:264–273. doi: 10.1002/jez.10147. [DOI] [PubMed] [Google Scholar]
- Sánchez-Villagra MR, Goswami A, Weisbecker V, Mock O, Kuratani S. Conserved relative timing of cranial ossification patterns in early mammalian evolution. Evol Dev. 2008;10:519–530. doi: 10.1111/j.1525-142X.2008.00267.x. [DOI] [PubMed] [Google Scholar]
- Sánchez-Villagra MR, Müller H, Sheil CA, Scheyer TM, Nagashima H, Kuratani S. Skeletal development in the Chinese soft-shelled turtle Pelodiscus sinensis (Testudines: Trionychidae) J Morphol. 2009;270:1381–1399. doi: 10.1002/jmor.10766. [DOI] [PubMed] [Google Scholar]
- Schinz HR, Zangerl R. Beiträge zur Osteogenese des Knochensystems beim Haushuhn, bei der Haustaube und beim Haubensteissfuss. Denkschr Schweiz Naturforsch Ges. 1937;72:116–162. [Google Scholar]
- Schmidt K, Starck JM. Developmental variability during early embryonic development of the zebra fish, Danio rerio. J exp Zool (Mol Dev Evol) 2004;302B:446–457. doi: 10.1002/jez.b.21010. [DOI] [PubMed] [Google Scholar]
- Schneider RA, Helms JA. The cellular and molecular origins of beak morphology. Science. 2003;299:565–568. doi: 10.1126/science.1077827. [DOI] [PubMed] [Google Scholar]
- Schoch RR. Skull ontogeny: developmental patterns of fishes conserved across major tetrapod clades. Evol Dev. 2006;8:524–536. doi: 10.1111/j.1525-142X.2006.00125.x. [DOI] [PubMed] [Google Scholar]
- Schulmeister S, Wheeler WC. Comparative and phylogenetic analysis of developmental sequences. Evol Dev. 2004;6:50–57. doi: 10.1111/j.1525-142x.2004.04005.x. [DOI] [PubMed] [Google Scholar]
- Schumacher GH, Wolff E. Zur vergleichenden Osteogenese von Gallus domesticus L., Larus ridibundus L. und Larus canus L. II. Zeitliches Erscheinen der Ossifikationen bei Larus ridibundus L. und Larus canus L. Morph Jahrb. 1966;110:620–635. [PubMed] [Google Scholar]
- Sheil CA. Osteology and skeletal development of Pyxicephalus adspersus (Anura: Ranidae: Raninae) J Morphol. 1999;240:49–75. doi: 10.1002/(SICI)1097-4687(199904)240:1<49::AID-JMOR5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Sheil CA. Osteology and skeletal development of Apalone spinifera (Reptilia: Testudines: Trionychidae) J Morphol. 2003;256:42–78. doi: 10.1002/jmor.10074. [DOI] [PubMed] [Google Scholar]
- Sheil CA. Skeletal development of Macrochelys temminckii (Reptilia: Testudines: Chelydridae) J Morphol. 2005;263:71–106. doi: 10.1002/jmor.10290. [DOI] [PubMed] [Google Scholar]
- Sheil CA, Greenbaum E. Reconsideration of skeletal development of Chelydra serpentina (Reptilia: Testudinata: Chelydridae): evidence for intraspecific variation. J Zool. 2005;265:235–267. [Google Scholar]
- Smith KK. Comparative patterns of craniofacial development in eutherian and metatherian mammals. Evolution. 1997;51:63–1678. doi: 10.1111/j.1558-5646.1997.tb01489.x. [DOI] [PubMed] [Google Scholar]
- Starck JM. Zeitmuster der Ontogenesen bei nestflüchtenden und nesthockenden Vögeln. Cour Forsch-Inst Senckenberg. 1989;114:1–319. [Google Scholar]
- Starck JM. Evolution of avian ontogenies. Curr Ornithol. 1993;10:275–366. [Google Scholar]
- Starck JM. Comparative morphology and cytokinetics of skeletal growth in hatchlings of altricial and precocial birds. Zool Anz. 1996;235:53–75. [Google Scholar]
- Tamlin AL, Bowman J, Hackett DF. Separating wild from domestic American mink Neovison vison based on skull morphometrics. Wildlife Biology. 2009;15:266–277. [Google Scholar]
- Tchernichovski O, Mitra PP, Lints T, Nottebohm F. Dynamics of the vocal imitation process: how a zebra finch learns its song. Science. 2001;291:2564–2569. doi: 10.1126/science.1058522. [DOI] [PubMed] [Google Scholar]
- Tokita M. Cranial neural crest cell migration in cockatiel Nymphicus hollandicus (Aves: Psittaciformes) J Morphol. 2006;267:333–340. doi: 10.1002/jmor.10408. [DOI] [PubMed] [Google Scholar]
- Tokita M, Schneider RA. Developmental origins of species-specific muscle patterns. Dev Biol. 2009;331:311–325. doi: 10.1016/j.ydbio.2009.05.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueb L. A summary of osteocranial development in anurans with notes on the sequence of cranial ossification in Rhinophrynus dorsalis (Anura: Pipoidea: Rhinophrynidae) S Afr J Sci. 1985;81:181–185. [Google Scholar]
- Tucker AS, Lumsden A. Neural crest cells provide species-specific patterning information in the developing branchial skeleton. Evol Dev. 2004;6:32–40. doi: 10.1111/j.1525-142x.2004.04004.x. [DOI] [PubMed] [Google Scholar]
- Vaglia J, Smith KK. Early differentiation and migration of cranial neural crest in the opossum, Monodelphis domestica. Evol Dev. 2003;5:121–135. doi: 10.1046/j.1525-142x.2003.03019.x. [DOI] [PubMed] [Google Scholar]
- Velhagen WA. Analyzing developmental sequences using sequence units. Syst Biol. 1997;46:204–210. doi: 10.1093/sysbio/46.1.204. [DOI] [PubMed] [Google Scholar]
- Weisbecker V, Goswami A, Wroe S, Sánchez-Villagra MR. Ossification heterochrony in the therian postcranial skeleton and the marsupial-placental dichotomy. Evolution. 2008;62:2027–2041. doi: 10.1111/j.1558-5646.2008.00424.x. [DOI] [PubMed] [Google Scholar]
- Weisbecker V, Mitgutsch M. A large-scale survey of heterochrony in anuran cranial ossification patterns. J Zool Syst Evol Res 2010 [Google Scholar]
- Welten CMM, Verbeek FJ, Meijer AH, Richardson MK. Gene expression and digit homology in the chicken embryo wing. Evol Dev. 2005;7:18–28. doi: 10.1111/j.1525-142X.2005.05003.x. [DOI] [PubMed] [Google Scholar]
- Werneburg I. A standard system to study vertebrate embryos. Plos One. 2009;4(6):e5887. doi: 10.1371/journal.pone.0005887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneburg I, Hugi J, Müller J, Sánchez-Villagra MR. Embryogenesis and ossification of Emydura subgolobosa (Testudines, Pleurodira, Chelidae) and patterns of turtle development. Dev Dyn. 2009;238:2770–278. doi: 10.1002/dvdy.22104. [DOI] [PubMed] [Google Scholar]
- Werneburg I, Sánchez-Villagra MR. Timing of organogenesis support basal position of turtles in the amniote tree of life. BMC Evol Biol. 2009;9:82. doi: 10.1186/1471-2148-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneburg I, Sánchez-Villagra MR. The early development of the Echidna, Tachyglossus aculeatus (Mammalia: Monotremata) and the Grundmuster of mammalian development. Acta Zool in press. [Google Scholar]
- Wilson LAB, Schradin C, Mitgutsch C, Galliari FC, Mess A, Sánchez-Villagra MR. Skeletogenesis and sequence heterochrony in rodent evolution, with particular emphasis on the African striped mouse, Rhabdomys pumilio (Mammalia) Org Divers Evol. 2010;10:243–258. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ontogenetic states for ossified elements in Anas platyrhynchos specimens, cranial elements, left side. Abbreviations: B, mineralized bone as detected by alizarin red staining; NA, not present; NC, no decision (technical problem); w, bone present, no alizarin uptake
Ontogenetic states for ossified elements in Coturnix coturnix japonica, complete skeletons. Abbreviations as in Appendix 1
Ontogenetic states for ossified elements in Taeniopygia guttata, complete skeletons. Abbreviations: B, mineralized bone as detected by alizarin red staining; ?, missing data
Ontogenetic states for ossified elements in Anas platyrhynchos specimens, cranial elements, right side. Abbreviations as in Appendix 1
Ontogenetic states for ossified elements in Anas platyrhynchos, cranial elements. Abbreviations as in Appendix 1
Ontogenetic states for ossified elements in Anas platyrhynchos, postcranial elements, left side. Abbreviations as in Appendix 1
Ontogenetic states for ossified elements in Anas platyrhynchos, postcranial elements, right side. Abbreviations as in Appendix 1
Ontogenetic states for ossified elements in Anas platyrhynchos, postcranial elements. Abbreviations as in Appendix 1
Ontogenetic states for ossified elements in Anas platyrhynchos, complete skeletons. Abbreviations as in Appendix 1
Ontogenetic states for ossified elements in Coturnix coturnix japonica, cranial elements. Abbreviations as in Appendix 1
Ontogenetic states for ossified elements in Coturnix coturnix japonica, postcranial elements. Abbreviations as in Appendix 1