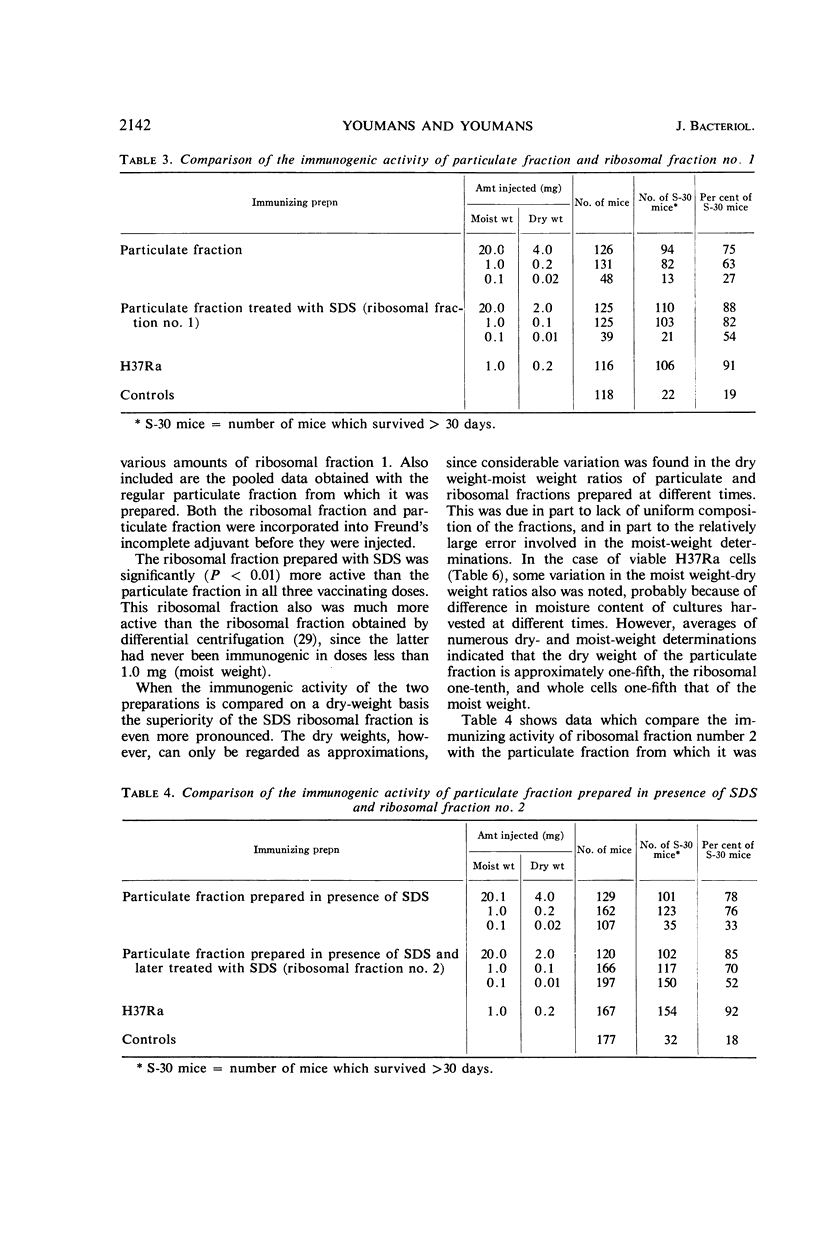
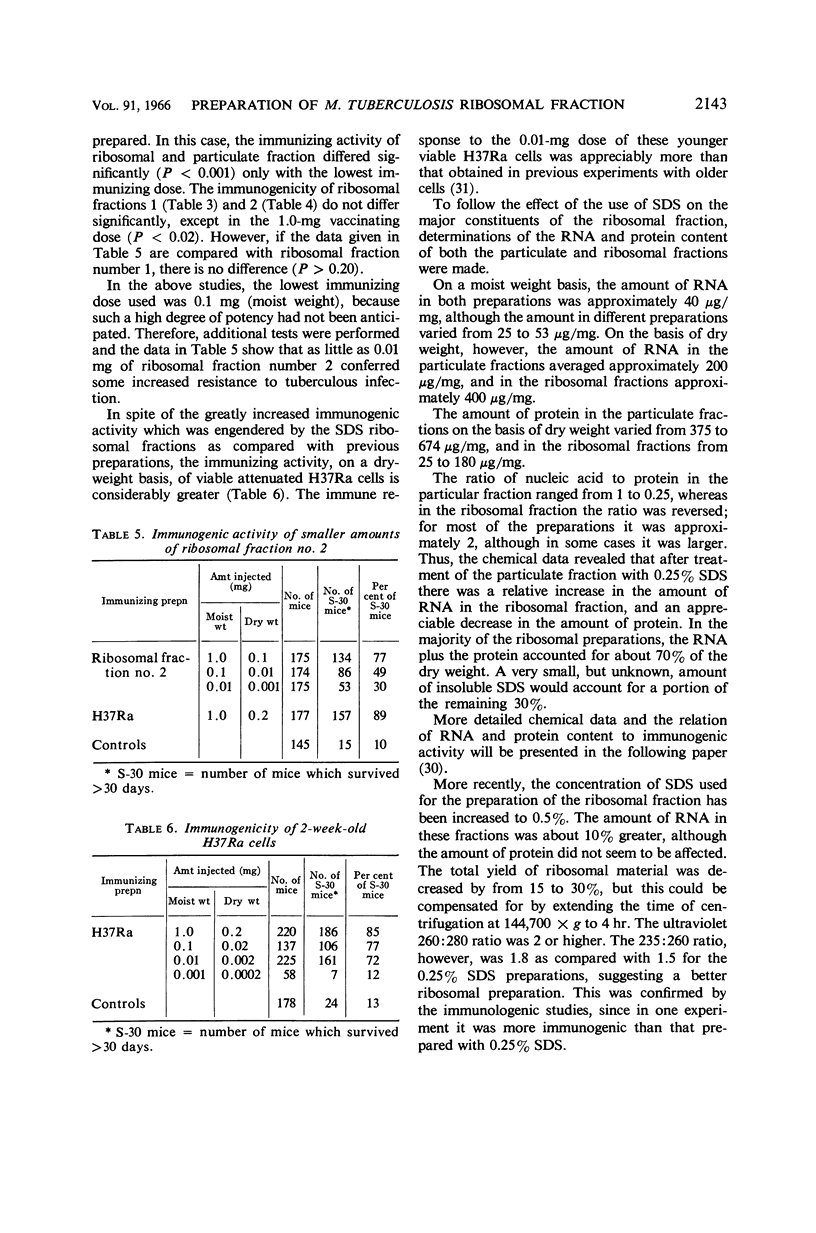
Abstract
Youmans, Anne S. (Northwestern University Medical School, Chicago, Ill.), and Guy P. Youmans. Preparation of highly immunogenic ribosomal fractions of Mycobacterium tuberculosis by use of sodium dodecyl sulfate. J. Bacteriol. 91:2139–2145. 1966.—Ribosomal fractions of Mycobacterium tuberculosis, strain H37Ra, were prepared by treatment of the intracellular particulate fraction with 0.25 or 0.5% sodium dodecylsulfate (SDS) followed by centrifugation at 144,700 × g for 3 hr. This procedure has greatly simplified the preparation of ribosomal fractions and has given fractions composed of approximately 50% ribonucleic acid (RNA) and 15 to 20% protein. When incorporated into Freund's incomplete adjuvant and injected intraperitoneally into CF-1 mice, the SDS ribosomal fractions were more immunogenic than the particulate fractions from which they were prepared. They were as much as 100 times more immunogenic than ribosomal fractions prepared by differential centrifugation, 1 μg (dry weight) per mouse being sufficient for the induction of some immunity. However, none of these ribosomal preparations, in comparable doses, was as immunogenic as the living cells from which they were prepared. It was also shown that the addition of 10−4m MgCl2 to the final diluent increased immunogenic activity, whereas larger concentrations (10−3m) reduced immunogenic activity. Preparation of the ribosomal fraction from ruptured cells in one continuous process during the course of 1 day increased the activity. Two-week-old H37Ra cells contained more RNA and were more immunogenic than the older cultures which have been used in the past.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATTARDI G., SMITH J. Virus specific protein and a ribo-nucleic acid associated with ribosomes in poliovirus infected HeLa cells. Cold Spring Harb Symp Quant Biol. 1962;27:271–292. doi: 10.1101/sqb.1962.027.001.026. [DOI] [PubMed] [Google Scholar]
- CHAO F. C. Dissociation of macromolecular ribonucleoprotein of yeast. Arch Biochem Biophys. 1957 Aug;70(2):426–431. doi: 10.1016/0003-9861(57)90130-3. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., SMITH K. C., ALLEN F. W. The preparation and characterization of ribonucleic acids from yeast. J Biol Chem. 1955 Sep;216(1):185–193. [PubMed] [Google Scholar]
- FENNER F. The enumeration of viable tubercle bacilli by surface plate counts. Am Rev Tuberc. 1951 Oct;64(4):353–380. doi: 10.1164/art.1951.64.4.353. [DOI] [PubMed] [Google Scholar]
- HENDLER R. W., TANI J. ON THE CYTOLOGICAL UNIT FOR PROTEIN SYNTHESIS IN VIVO IN E. COLI. II. STUDIES WITH INTACT CELLS OF TYPE B. Biochim Biophys Acta. 1964 Feb 17;80:294–306. doi: 10.1016/0926-6550(64)90101-x. [DOI] [PubMed] [Google Scholar]
- KEMP J. W., ALLEN F. W. Ribonucleic acids from pancreas which contain new components. Biochim Biophys Acta. 1958 Apr;28(1):51–58. doi: 10.1016/0006-3002(58)90426-8. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W., KELLER E. B., GROSS J., ZAMECNIK P. C. Studies on cytoplasmic ribonucleoprotein particles from the liver of the rat. J Biol Chem. 1955 Nov;217(1):111–123. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANDELSTAM J. The intracellular turnover of protein and nucleic acids and its role in biochemical differentiation. Bacteriol Rev. 1960 Sep;24(3):289–308. doi: 10.1128/br.24.3.289-308.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PALADE G. E., SIEKEVITZ P. Liver microsomes; an integrated morphological and biochemical study. J Biophys Biochem Cytol. 1956 Mar 25;2(2):171–200. doi: 10.1083/jcb.2.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENMAN S., BECKER Y., DARNELL J. E. A CYTOPLASMIC STRUCTURE INVOLVED IN THE SYNTHESIS AND ASSEMBLY OF POLIOVIRUS COMPONENTS. J Mol Biol. 1964 Apr;8:541–555. doi: 10.1016/s0022-2836(64)80010-3. [DOI] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. The survival of starved bacteria. J Gen Microbiol. 1962 Oct;29:233–263. doi: 10.1099/00221287-29-2-233. [DOI] [PubMed] [Google Scholar]
- ROBSON J. M., SMITH J. T. Immunizing effects of a lipopolysaccharide in mice. Am Rev Respir Dis. 1961 Jul;84:100–102. doi: 10.1164/arrd.1961.84.1.100. [DOI] [PubMed] [Google Scholar]
- STAEHELIN T., WETTSTEIN F. O., OURA H., NOLL H. DETERMINATION OF THE CODING RATIO BASED ON MOLECULAR WEIGHT OF MESSENGER RIBONUCLEIC ACID ASSOCIATED WITH ERGOSOMES OF DIFFERENT AGGREGATE SIZE. Nature. 1964 Jan 18;201:264–270. doi: 10.1038/201264a0. [DOI] [PubMed] [Google Scholar]
- Sved S. The metabolism of exogenous ribonucleic acids injected into mice. Can J Biochem. 1965 Jul;43(7):949–958. doi: 10.1139/o65-109. [DOI] [PubMed] [Google Scholar]
- TISSIERES A., WATSON J. D. Ribonucleoprotein particles from Escherichia coli. Nature. 1958 Sep 20;182(4638):778–780. doi: 10.1038/182778b0. [DOI] [PubMed] [Google Scholar]
- WADE H. E., MORGAN D. M. The nature of the fluctuating ribonucleic acid in Escherichia coli. Biochem J. 1957 Feb;65(2):321–331. doi: 10.1042/bj0650321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUMANS A. S., YOUMANS G. P. EFFECT OF MITOCHONDRIAL STABILIZERS ON THE IMMUNOGENICITY OF THE PARTICULATE FRACTION ISOLATED FROM MYCOBACTERIUM TUBERCULOSIS. J Bacteriol. 1964 Jun;87:1346–1354. doi: 10.1128/jb.87.6.1346-1354.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUMANS A. S., YOUMANS G. P. FURTHER STUDIES ON A LABILE IMMUNOGENIC PARTICULATE SUBSTANCE ISOLATED FROM MYCOBACTERIUM TUBERCULOSIS. J Bacteriol. 1964 Feb;87:278–285. doi: 10.1128/jb.87.2.278-285.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUMANS A. S., YOUMANS G. P. IMMUNOGENIC ACTIVITY OF A RIBOSOMAL FRACTION OBTAINED FROM MYCOBACTERIUM TUBERCULOSIS. J Bacteriol. 1965 May;89:1291–1298. doi: 10.1128/jb.89.5.1291-1298.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUMANS A. S., YOUMANS G. P. NATURE OF THE LABILE IMMUNOGENIC SUBSTANCE IN THE PARTICULATE FRACTION ISOLATED FROM MYCOBACTERIUM TUBERCULOSIS. J Bacteriol. 1964 Oct;88:1030–1037. doi: 10.1128/jb.88.4.1030-1037.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUMANS G. P., YOUMANS A. S. The measurement of the response of immunized mice to infection with Mycobacterium tuberculosis va. hominis. J Immunol. 1957 May;78(5):318–329. [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Effect of trypsin and ribonuclease on the immunogenic activity of ribosomes and ribonucleic acid isolated from Mycobacterium tuberculosis. J Bacteriol. 1966 Jun;91(6):2146–2154. doi: 10.1128/jb.91.6.2146-2154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans G. P., Youmans A. S. Nonspecific factors in resistance of mice to experimental tuberculosis. J Bacteriol. 1965 Dec;90(6):1675–1681. doi: 10.1128/jb.90.6.1675-1681.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]


