Abstract
Kinesins are tetrameric motor molecules, consisting of two kinesin heavy chains (KHCs) and two kinesin light chains (KLCs) that are involved in transport of cargo along microtubules. The function of the light chain may be in cargo binding and regulation of kinesin activity. In the mouse, two KLC genes, KLC1 and KLC2, had been identified. KLC1 plays a role in neuronal transport, and KLC2 appears to be more widely expressed. We report the cloning from a testicular cDNA expression library of a mammalian light chain, KLC3. The KLC3 gene is located in close proximity to the ERCC2 gene. KLC3 can be classified as a genuine light chain: it interacts in vitro with the KHC, the interaction is mediated by a conserved heptade repeat sequence, and it associates in vitro with microtubules. In mouse and rat testis, KLC3 protein expression is restricted to round and elongating spermatids, and KLC3 is present in sperm tails. In contrast, KLC1 and KLC2 can only be detected before meiosis in testis. Interestingly, the expression profiles of the three known KHCs and KLC3 differ significantly: Kif5a and Kif5b are not expressed after meiosis, and Kif5c is expressed at an extremely low level in spermatids but is not detectable in sperm tails. Our characterization of the KLC3 gene suggests that it carries out a unique and specialized role in spermatids.
Keywords: gene regulation, meiosis, spermatid, spermatogenesis, testis
INTRODUCTION
Conventional kinesin protein is a mechanochemical enzyme involved in plus-end-directed translocation of membrane-bound organelles on microtubules (MTs). It consists of two heavy chains (KHCs) and two light chains (KLCs). The KHC has a tripartite structure based on functional domains. The N-terminus contains a globular head that hydrolyzes ATP, binds MT, and is responsible for translocation along MT. The middle to C-terminal segment of KHC consists of an α-helical coiled-coil, rod-like region that mediates dimerization. The KHC C-terminus consists of a small globular domain that is the KLC-binding site and is involved in cargo attachment [1].
Originally, KLC was cloned from rat [2], and subsequently, homologues were isolated from numerous organisms [3–7]. Analysis of KLC sequences revealed several conserved motifs in KLC family members as well as regions of divergence. The sequences at the N- and C-termini are highly variable, whereas the middle region contains highly conserved heptade repeats (HRs) and tandem tetra-tricopeptide repeats (TPRs). The HR region is predicted to form an α-helical coiled-coil, and it mediates binding to a similar region in KHC [6, 8]. The most highly conserved motif of KLC is the middle region, consisting of six long, imperfect, tandem TPR repeats [9]. In other proteins, TPRs are known to mediate protein-protein interactions [10] and are predicted to form amphipathic α-helices. The KLC also harbors a putative PEST motif, which is involved in protein degradation, between the HR and TPR [3].
Based on its location within kinesin, KLC was predicted to mediate either cargo binding or regulation of kinesin [2, 11]. Subsequent studies have largely borne out these predictions: KLC mediates binding of membrane-bound organelles to kinesin [12], and this may be mediated by the TPR [13]. Furthermore, different splice variants of KLC may specifically target kinesin to distinct molecular cargoes, such as the Golgi complex and mitochondria [14, 15]. Additionally, KLC phosphorylation and interaction with regulatory molecules, such as calmodulin, appear to regulate kinesin activity [16, 17]. In vitro, however, KLCs appear to be dispensable for both binding to membrane-bound vesicles [18] and regulation of kinesin activity [19–21]. One possibility is that KLC introduces plasticity to these aspects of kinesin function. For example, a correlation has been observed between animal complexity and kinesin gene number [22]: fungi express one KHC gene and no associated KLC, Caenorhabditis elegans expresses one of each, and mice encode three KHC (i.e., Kif5a, Kif5b, and Kif5c) [23–26] as well as KLC1 and KLC2 [27]. It has been proposed that the complexity of organisms correlates with that of their cargo transport requirements and, hence, diversity in kinesin gene function. This is supported by differences in expression patterns at the tissue, cellular, and subcellular level among different KHC and KLC isoforms, which suggests differences in function amongst kinesin genes. The function of KLC remains a matter of investigation.
We report here the cloning and characterization of a third mammalian KLC, KLC3, from a testicular expression library. Sequence analysis and molecular studies demonstrate that KLC3 is identical to a hypothetical gene that had previously been predicted to exist in the ERCC2 locus [28]. KLC3 has the characteristics of a genuine kinesin light chain and, thus, can be assigned to the family of mammalian KLC. We analyzed KLC3 mRNA expression in the testis and discovered that it is primarily expressed in postmeiotic, male germ cells, which is in contrast to the expressions of KLC1 and KLC2, which become undetectable in postmeiotic germ cells. This finding is intriguing, and it suggests that KLC3 acts to replace KLC1 and/or KLC2 in spermatids.
MATERIALS AND METHODS
Yeast-2-Hybrid Screening
The use and construction of the yeast-2-hybrid plasmids, expression cDNA library, and cell lines have been described elsewhere [29]. A kinase dead c-mos mutant was inserted as an EcoRI/SalI fragment in pGBT9, in frame with the GAL4 DNA-binding domain, and served as bait in the cDNA screening. Colonies that grew on His− plates were tested for β-galactosidase expression on membranes as described elsewhere [29]. Positive yeast colonies were further analyzed.
Cloning and Sequencing of cDNAs
The KLC3 cDNA was isolated from yeast cDNA plasmids using protocols recommended by the manufacturer (Clontech, Palo Alto, CA). Briefly, yeast cells were centrifuged and pellets resuspended in lysis solution (2% v/v Triton X-100, 1% w/v SDS, 100 mM NaCl, 10 mM Tris [pH 8.0], and 1.0 mM EDTA). To the cell suspension, acid-washed beads and phenol:chloroform:isoamylalcohol were added, and the cells were vigorously vortexed. After centrifugation, supernatant materials were transferred and ethanol precipitated. The pGAD-KLC3 DNA was resuspended and quantitated.
Following isolation, the DNA was transfected into competent XL1-Blue bacteria (Stratagene, La Jolla, CA) and plated onto LB-agar plates containing ampicillin. Inserts were subcloned into a modified pBluescript KSII+ vector containing an ATG codon in frame with the EcoRI site to allow in vitro translation [29]. The clones were digested using PstI, and KLC3 fragments were subcloned into p-Bluescript for sequence analysis in the University of Calgary DNA Core Facility. The resulting sequences were assembled and aligned to known genes in GenBank and EMBL sequence databases using GCG Wisconsin (Madison, WI) package programs SeqEd, Bestfit, and FastA. The KLC3 sequence comparisons and alignments were done using Omiga software (Oxford Molecular, Hunt Valley, MD). The GenBank accession number for KLC3 is AF166267. Omiga was used for the conceptual translation of KLC3. Sequences for all other organisms were obtained via BLAST searches. These protein sequences were then used to generate the alignments in Omiga using default parameters. For the HR, TPR, and PEST region analyses, the relevant regions of KLC3 were identified from regions of high homology in the sequence alignment. The mKLC1, mKLC2, rKLC1, and human hsKNS2 sequences have been published elsewhere [2, 27]. Predicted secondary structures were generated using the GOR II or Chou and Fassman algorithms in Omiga.
Antibody Production
Full-length KLC3 cDNA, digested with EcoRI/SalI, was subcloned into the pMAL-c2 vector (New England Biolabs, Beverly, MA), producing a maltose-binding protein (MBP)-KLC3 fusion protein. Recombinant MBP-KLC3 proteins were produced by inducing transfected TB1 bacteria (NEB) with isopropyl-β-D-thiogalactose as described elsewhere [29]. Bacteria were lysed by sonication. After centrifugation, the MBP-KLC3 fusion protein was purified from the extract using amylose-agarose columns and eluted using maltose. Eluted fusion protein was analyzed by SDS-PAGE.
For monoclonal antibody (mAb) production, 6-wk-old Balb/c mice were injected with 10 μg of MBP-KLC3 fusion protein. For hybridoma production, SP2/MIL-6 myeloma cells, grown in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum, were fused to spleen cells [30]. The mAb production was analyzed by immunofluorescence using frozen rat testis sections and Western blot assays using NitroPlus membranes (MSI, Inc., Westboro, MA) onto which MBP-KLC3 had been transferred.
For polyclonal antibody production, rabbits were injected with affinity-purified MBP-KLC3, initially with complete Freund adjuvant and subsequently with incomplete Freund adjuvant. Serum was collected and tested for KLC3 recognition by Western blot analysis as described above.
Germ Cell Extracts and Western Blot Analysis
Elutriated germ cells were prepared as described elsewhere [31]. Briefly, testes were isolated from mice. Seminiferous tubules were treated with collagenase/trypsin to release Sertoli cells and germ cells, which were fractionated by centrifugal elutriation as described elsewhere [31]. Fractions were obtained of 90% pure pachytene spermatocytes, round spermatids, and late spermatids. The elutriated cells were extracted in lysis buffer (50 mM Hepes [pH 7.0], 1% v/v Nonidet-P40 [Sigma, St. Louis, MO], 1 μg/ml of aprotinin, and 100 μg/ml of PMSF), and protein was quantified by spectrophotometry.
Mature spermatozoa were collected from rat epididymis and washed in PBS according to the method described by Oko [32]. Spermatozoa were pelleted and resuspended in demembranization buffer (5 mM 1,4-dithiothreitol [DTT], 50 mM Tris-HCl [pH 9.0], and 2% v/v Triton) and then gently mixed for 15 min at 4°C. Treated spermatozoa were pelleted and resuspended a second time in demembranization buffer. After the third centrifugation step, spermatozoa were washed twice in 50 mM Tris (pH 9.0) and either spread on coverslips treated with poly-L-lysine and processed for immunofluorescence or extracted in SDS sample buffer for Western blot analyses.
Western blot assays were done as described elsewhere [31] using the extracts to identify KLC3 as follows: Extracts were boiled in SDS sample buffer and loaded onto 10% w/v SDS-PAGE gels to separate proteins. After electrophoresis, proteins were transferred by semidry blotting (Hoefer Scientific Instruments, San Francisco, CA) onto NitroPlus membranes. The blots were blocked with 5% v/v calf serum and 0.1% v/v Tween in PBS and then incubated with polyclonal KLC3 or tubulin (Sigma) antisera overnight at 4°C. Primary antibodies were detected using horse radish peroxidase-conjugated goat anti-rabbit IgG (for KLC3) or sheep-anti-mouse IgG (for tubulin) (Boehringer Mannheim, Indianapolis, IN).
Immunofluorescence
Adult rat or mouse testes were isolated and frozen in embedding compound (Miles Inc., Elkhart, IN) in −80°C hexane. Ten-micrometer frozen sections were fixed in −20°C acetone for 5 min. Sections were incubated with indicated primary mAbs diluted in PBS at 37°C for 30 min in a humidified chamber. The sections were rinsed in 0.5% Tween-20/PBS and washed once for 10 min at room temperature in PBS containing 0.5% v/v Tween-20 with gentle shaking. The Cy2-labeled secondary rabbit anti-mouse antiserum or Cy3-labeled secondary goat anti-rabbit antiserum was added in PBS for 30 min at 37°C in the humidified chamber. After final washes, sections were rinsed in H2O, mounted, and examined. Deconvolution confocal immunofluorescence microscopy was done at the University of Calgary Electron Microscopy and Imaging Facility using rat and mouse spermatozoa.
Reverse Transcription-Polymerase Chain Reaction
Reverse transcription-polymerase chain reaction (RT-PCR) was carried out using random priming as follows: Reactions contained 5 μg of total RNA, 1 μl of random hexamers (500 μg/ml), and sterile water to 11 μl and were heated to 70°C, then chilled on ice. Next, 4 μl of first strand buffer was added (Gibco BRL, Burlington, ON, Canada), together with 2 μl of DTT (0.1 M), 1 μl of dNTPs (10 mM each of dATP, dCTP, dTTP, and dGTP), and 1 μl of Superscript RT (Gibco). Reactions were done for 1.5 h at 37°C. The resulting cDNA was used as a template for PCR as follows: All PCR reactions contained 1× Taq buffer (Gibco), 2.0 mM MgCl2, 20 pmol of 5′- and 3′-primers, 1% v/v dimethyl sulfoxide, 0.2 mM dNTPs, 1 ng of DNA template, and 1 U of Tsg polymerase. Five to 10 μl of the 25-μl PCR reactions were analyzed on 1.2% w/v agarose gels. All PCR programs were 94°C for 5 min; 40 cycles of 94°C for 60 sec, annealing for 30 sec, and extension at 72°C for 60 sec; 72°C for 10 min; and then cooling to 4°C.
Primers and annealing temperatures were as follows: KLC3 primers (5′-tgg tga acc act cgc tg-3′ and 5′-gtc gac g[c/g]t gag gat ctc ctt gta-3′) anneal at 54°C. ERCC-2 primers (5′-gct cag atc ttg tgt gct g-3′ and 5′-gtg tctc ctc tga ctg cag-3′) anneal at 60°C. KLC2 primers (5′-acg atg gtg ctt cct cga-3′ and 5′-a tct tca tcc agc ttt cgg-3′) anneal at 55°C. KLC1 primers (5′-atg cat gac aac atg tcc ac-3′ and 5′-cag cgg aca gca ctg gag-3′) anneal at 58°C. Kif5a primers (5′-agc tgg cgg tca act acg-3′ and 5′-gc ctt ctt cac c tcc tcc-3′) anneal at 56°C. Kif5b primers (5′-aac tgg ctg tta att atg-3′ and 5′-tc aac agc ttg ctt gact-3′) anneal at 50°C. Kif5c primers (5′-agc tgg ctg tca att acg-3′ and 5′-tc cag agc ttt ctt cac c-3′) anneal at 57°C.
MT-Binding Assays
The MTs were purified essentially according to the method described by Vallee and Collins [33] from rat and mouse brain or testes, resulting in MT preparations that contain MT-associated proteins, including multiple antigenic peptide and kinesins. In short, brain and testes samples were extracted in ice-cold BRB80 buffer containing the protease inhibitors pepstatin, leupeptin, and PMSF and centrifuged at 55 000 rpm for 15 min at 4°C. Supernatants containing depolymerized MT were removed and stored on ice. The MT extracts were next supplemented with 0.5 mM GTP, 15 U/ml of hexokinase, and 20 mM D-glucose to deplete ATP. Next, the extracts were warmed to 30°C, and 5 μM taxol was added. After 5 min of incubation at 30°C, 15 μM taxol was added (20 μM total), after which AMP-PNP (2 mM) was added to stabilize KHC on MTs. The extracts were layered onto sucrose cushions and spun at 40 000 rpm for 20 min at 22°C. Cushion was washed twice with BRB80 before being removed to avoid contamination of the MT pellet. The MT pellets were then resuspended in 100 μl of BRB80 and depolymerized on ice for 20 min. The GTP (0.5 mM) and AMP-PNP (2 mM) were added to the MT preparations. Extracts were warmed to 30°C, polymerized, and centrifuged as described above. The MT pellets were subjected to another round of depolymerization and polymerization as described above. Final pellets of polymerized MT were resuspended in 10% v/v glycerol/BRB80 and flash-frozen in liquid nitrogen.
To analyze binding of KLC3 to MT, KLC3 cDNA was transcribed in vitro and translated in the presence of 35S-cysteine using the TNT reticulocyte transcription and translation system (Promega, Madison, WI). Radiolabeled proteins were incubated with polymerized MT at 30°C for 15 min in the presence of AMP-PNP (2 mM), GTP (0.5 mM), and taxol (20 μM). The MTs were pelleted at 30 000 rpm for 15 min at 22°C. Supernatants were saved for SDS-PAGE analysis, and two subsequent pelleting reactions were performed. Aliquots of both supernatants and pellets were boiled in SDS sample buffer and analyzed by electrophoresis on 10% w/v SDS-PAGE gels, and the gels were dried and exposed to Kodak BIOMAX film (Rochester, NY). To analyze if the binding of KLC3 to MT was sensitive to ATP, KLC3 cDNA was in vitro transcribed and translated and incubated with MT as described above. However, the two final pelleting reactions were performed in the presence of either AMP-PNP (2 mM) as described above or ATP at a final concentration of 2.5, 10, or 20 mM. The MT-bound KLC3 was analyzed as described above. Gel strips containing radiolabeled MT-bound KLC3 were identified by autoradiography, isolated, and extracted, and the radioactivity was quantitated.
KHC-Binding Assays
A full-length brain KHC cDNA (Kif5c, gift of Dr. B. Schnapp) was used to analyze interaction of KLC3 and KHC. The haem agglutinin (HA)-tagged KLC3 and KHC were translated individually or cotranslated at different ratios using the TNT system (Promega, Madison, WI) as described above. All translation reactions were immunoprecipitated using anti-HA mAb. Immunoprecipitated proteins were analyzed by SDS-PAGE and autoradiography. An HR-deletion mutant of KLC3 was constructed by PCR. KLC1, KLC3, KLC3ΔHR, and Mos were fused to the HA-tag and then translated individually or cotranslated with KHC at indicated ratios of plasmid DNAs. Immunoprecipitation, SDS-PAGE, and autoradiography were conducted as described above.
RESULTS
A Predicted Gene in the ERCC-2 Locus Encodes KLC3
During the course of other investigations, full-length rat testicular cDNAs were cloned in a yeast-2-hybrid screen, using a kinase-dead mutant of the Mos oncoprotein as bait, to uncover potential interacting partners of Mos that contribute to its function in spermatogenesis. The clones were sequenced and compared to GenBank database entries. They showed significant homology to a putative gene that had previously been predicted in the mouse, hamster, and human ERCC-2 loci [28] and that has sequence characteristics of KLC genes. For all three species, the gene arrangement in the ERCC2 locus is similar, and the predicted KLC gene is in very close proximity to the ERCC2 gene. Based on the very high similarity in sequence and KLC characteristics (discussed below), we propose to name this gene KLC3.
To further substantiate this result, KLC3 cDNA was used to isolate KLC3 genomic clones from a mouse genomic library. If, indeed, the two genes are identical, then genomic KLC3 clones should harbor ERCC2 sequences, and the ERCC2 gene and KLC3 gene should be linked via their 3′-ends separated by a 196-base pair (bp) intragenic region. Resulting genomic clones were, therefore, analyzed by PCR for the presence of KLC3 and ERCC2 sequences in close proximity to each other. The KLC3-specific PCR primers were designed to amplify across the next-to-last intron of KLC3 (located between putative exons 10 and 11), and ERCC2-specific primers were designed that amplify across ERCC2 exons 19 and 20. Primer sequences were based on the KLC3 cDNA sequence and on the ERCC2 genomic DNA sequence [28], respectively. To verify the close proximity of the two genes, we also performed PCR using primers that spanned the 3′ exons, introns, and intergenic space of these two genes.
In all PCR reactions, high-molecular-weight mouse genomic DNA and cloned KLC3 genomic DNA were compared. The results are shown in Figure 1 and demonstrate 1) that genomic KLC3 clone contains both KLC3 (lane 1) and ERCC2 (lane 4) sequences, 2) that high-molecular-weight genomic DNA (Fig. 1A, lanes 2, 5, and 8) and genomic KLC3 clone (Fig. 1A, lanes 1, 4, and 7) generate identical PCR fragments for all primer combinations, 3) that ERCC2 and KLC3 genes are in close proximity as predicted (lanes 7 and 8), and 4) that arrangement of the KLC3 and ERCC2 genes is conserved in the rat compared to the mouse, hamster, and human (Fig. 1B, lanes 1 and 2). The authenticity of the bands was verified by restriction of the PCR products with enzymes that were predicted to cut based on the sequences obtained by us (data not shown) and by Lamerdin et al. [28]. These results demonstrate that the isolated cDNA derives from the predicted gene in the mouse ERCC2 locus, and based on the similarity in gene structure and arrangement in the ERCC2 locus between human and rodent, we infer that the KLC3 gene localizes to human chromosome 19q13.2, the location of the human ERCC2 gene.
FIG. 1.
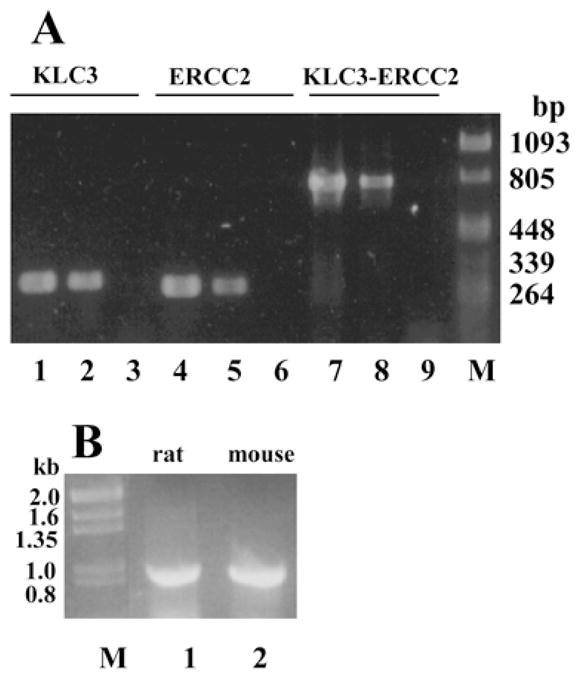
KLC3 and ERCC2 are in close proximity. A) To establish that KLC3 and ERCC2 genes are in close proximity in the genome, PCR was employed to detect the two gene sequences in a genomic KLC3 clone (lanes 1, 4, and 7) and in high molecular weight genomic DNA (lanes 2, 5, and 8). Controls lacking DNA were also included (lanes 3, 6, and 9). For reactions in lanes 1–3, KLC3-specific primers were used (predicted product size, 300 bp). For reactions in lanes 4–6, ERCC2-specific primers were used (predicted product size, 274 bp). For reactions in lanes 7–9, primers specific for both genes, such that they span the intragenic region, were employed (predicted product size, 813 bp). B) Rat and mouse genomic DNA was compared by PCR to determine if ERCC2 and KLC3 are in close proximity in rat (lane 1), as they are in mouse (lane 2). High molecular weight genomic DNA was used as a template using primers spanning ERCC2 and KLC3 sequences as described in A. Both samples generate a product identical in size (813 bp). M, Lambda and pBluescript molecular weight markers.
Sequence Analysis Reveals That KLC3 Is a Divergent Member of the KLC Family
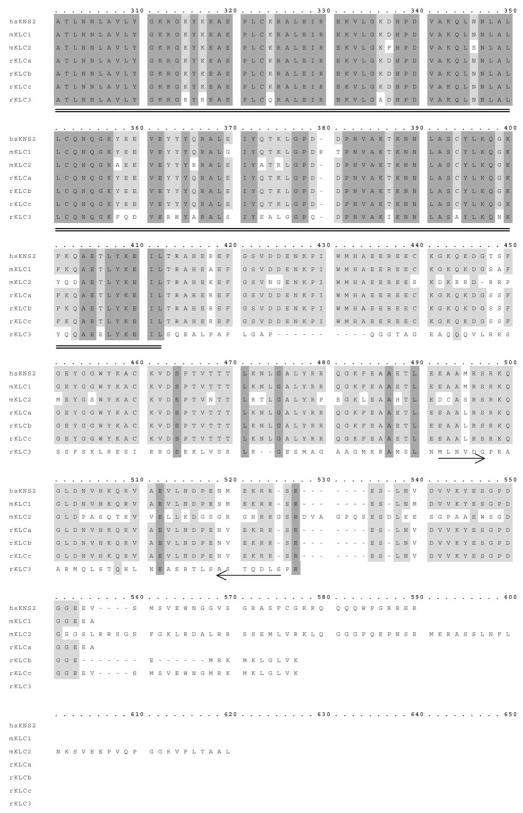
We analyzed the KLC3 cDNA sequence to gain insight regarding the structure and possible functional domains of the protein and to verify its assignment to the KLC family. Mammalian KLC protein sequences were aligned with KLC3. Results are shown in Figure 2 and reveal that, in general, KLC3 fits the KLC sequence homology pattern: high sequence conservation in the HR domain (amino acids 95–127, underlined), high degree of similarity in the TPR (amino acids 205–412, double-underlined), and lack of significant sequence similarity at the N- and C-termini. However, KLC3 is distinguished from the other mammalian KLC by a reduced degree of similarity in the conserved TPR (~76%) and HR regions (~55%). In addition, in areas of sequence divergence in the KLC family, KLC3 is also divergent: homology ranges from 10% to 30% in the N-and C-termini. Moreover, KLC3 is the shortest KLC family member, which may result, in part, from the absence of the sixth TPR domain [9]. The predicted secondary structure of all KLC genes, including KLC3, is almost identical in the HR and TPR regions and consists largely of helices interrupted by turns, the spacing of which is similar for the KLC proteins (data not shown). This cDNA and protein sequence analysis also demonstrated that one exon had not been predicted in the putative gene in the ERCC2 locus: amino acids 386–416 (Fig. 2) were predicted not to encode but, instead, to be an enhancer sequence [28], demonstrating the limitations of gene-finding algorithms used in that study.
FIG. 2.
Sequence comparison of KLC3 with mammalian KLC by alignment of the reported mammalian KLC proteins with KLC3. Residues that are conserved in all species are shaded in dark gray. Residues conserved in several species are shaded in light gray. Hyphens indicate differences. The conserved HR region is underlined, and the TPR sequences are double-underlined. The location of primers used in PCR are indicated with arrows.
Overall, KLC3 has the KLC family signature motifs, and we propose that KLC3 is a novel member of the KLC family.
HR Mediates KLC3 In Vitro Interaction with KHC
In kinesin, the HR domain mediates the interaction of KLC with a KHC subunit. KLC3 contains the HR sequence, and we predicted that it could interact with KHC. To verify this functional interaction, HA-tagged KLC3 protein and Kif5c were translated individually or cotranslated at differing ratios, followed by immunoprecipitation using anti-HA mAb, and complexes were analyzed by SDS-PAGE. The results are shown in Figure 3A. Anti-HA antibodies recognize HA-KLC3 (lane 1); we detected the expected tagged-KLC3 protein as well as smaller peptides that most likely result from translation starts at internal AUGs. Additionally, anti-HA antibodies do not recognize Kif5c, as expected (lane 2). Importantly, Kif5c associates with KLC3 (lanes 3–5).
FIG. 3.
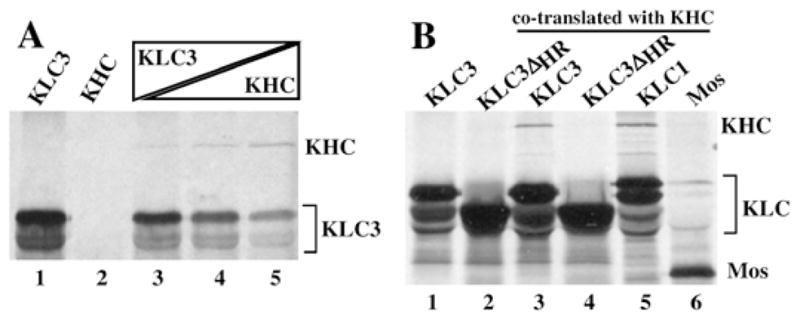
Interaction of KLC3 with KHC. A) To analyze KLC3 interaction with KHC, immunoprecipitations of HA-tagged KLC3 and Kif5c were conducted using anti-HA antibodies. Both proteins were in vitro transcribed and translated individually (lanes 1 and 2, respectively) or together at different ratios (lanes 3–5, increasing ratio of KHC vs. KLC3). Immunoprecipitated proteins were resolved by SDS-PAGE followed by autoradiography; KHC and KLC are indicated. B) The HR region of KLC3 was deleted, and the resulting construct, HA-tagged KLC3-ΔHR, was used in experiments similar to those described in A. The mobility of the KLC3-ΔHR mutant (lane 2) is higher than that of KLC3 (lane 1), as expected. KLC3, KLC3-ΔHR, KLC1 (positive control for association with KHC), and Mos (negative control for association with KHC) were expressed as fusion proteins with an HA-tag and co-translated with Kif5c (lanes 3–6, respectively). Complexes were immunoprecipitated with anti-HA antibodies and analyzed by SDS-PAGE.
To determine if the KLC3 HR sequence is involved in this association, an HA-KLC3 deletion mutant lacking the HR was constructed. Experiments were carried out as described above, with the addition of HA-KLC1 as a positive control and HA-Mos as a negative control (Mos associates with KLC, not KHC). The results in Figure 3B show that KLC3 and KLC1 can form a complex with Kif5c (lanes 3 and 5, respectively), but that the KLC3ΔHR deletion mutant lacks this ability (lane 4). We conclude that the association of KLC3 and Kif5c is mediated by the HR motif, a result consistent with those of other KLC family members.
KLC3 Binds to MT and Is Released by ATP
Kinesins bind to MT. The stabilization of kinesin on MT by the nonhydrolyzable ATP analogue AMP-PNP and its subsequent partial release by ATP is a distinguishing characteristic of kinesins. Therefore, we analyzed if KLC3 can bind to MT to lend further support to our classification of KLC3 into the KLC family.
Taxol-stabilized MTs were isolated from mouse brain and testes in the presence of AMP-PNP, thus ensuring co-purification of MT-associated proteins, including kinesins (results from brain and testes were identical; brain is shown). The MT preparations were incubated with in vitro-translated, radiolabeled KLC3 (in the presence of AMP-PNP) and then pelleted through sucrose cushions. The MT pellet was next subjected to two rounds of washing and pelleting. Aliquots of the MT pellet and the supernatants were analyzed by SDS-PAGE and autoradiography. The results are shown in Figure 4A. KLC3 (lower panel, lane 5) partitions efficiently with the MT (upper panel, lane 5). Little, if any, KLC3 is detected in the last two supernatants (lower panel, lanes 3 and 4), which also lack tubulins (upper panel, lanes 2–4). Next, the experiments were repeated, but washes of MT pellets were done in the presence of increasing amounts of ATP. For quantification, the MT pellet and aliquots of the supernatants were analyzed by SDS-PAGE and autoradiography, and the radioactivity in KLC3-containing gel slices was counted. The results are shown in Figure 4B. A 40% reduction was found in binding of KLC3 to MT in the presence of increasing amounts of ATP, with a concomitant increase in KLC3 in the soluble fraction.
FIG. 4.
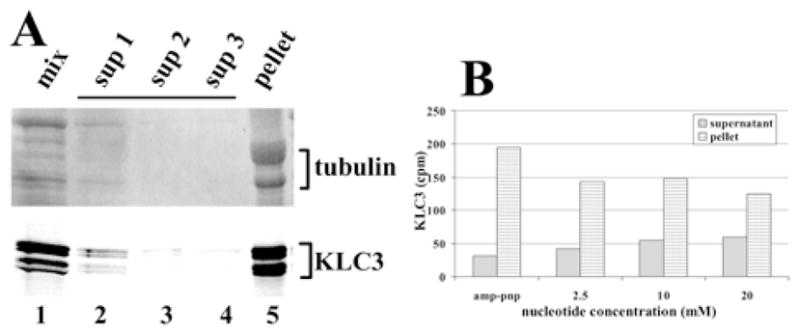
MT binding of KLC3. A) In vitro-translated, radiolabeled KLC3 was incubated with purified, taxol-stabilized MTs and pelleted. The MT pellets were washed and repelleted twice more, after which the pellet and the three supernatants were examined for tubulin (upper panel, coomassie-stained region of the gel) and KLC3 (lower panel, autoradiogram). Lane 1: aliquots of reaction mixture of translation mix and MT (mix); lanes 2–4: aliquots of supernatants from the first, second, and third pelleting reaction (sup1–sup3); lane 5: final pellet (pellet). Tubulin and KLC3 are indicated. B) To analyze ATP-dependency of the KLC3-MT association, the above protocol was repeated in the presence of either AMP-PNP or indicated amounts of ATP. Aliquots of the supernatant and the entire pellet were analyzed by SDS-PAGE. KLC3-containing gel slices were identified by autoradiography, excised from the gel, and counted. The amount recovered is shown. Supplanting AMP-PNP with ATP resulted in a shift of KLC3 from the MT pellet to the supernatant.
We conclude that KLC3 is stabilized on MT in the presence of AMP-PNP and is partially solubilized by the addition of ATP. This is consistent with previous observations of mKLC2-binding dynamics to MT [34]. Together with the demonstration that KLC3 can form a complex with KHC, our results suggest that, in agreement with results for other KLCs, KLC3 likely binds MT as a KLC3-KHC complex.
KLC3 Has a Unique mRNA Expression Pattern in Testis
We originally isolated KLC3 from a testicular cDNA expression library. To investigate its expression pattern in testis and compare it to that in other tissues and to those of other KLC family members, RT-PCRs of indicated KLC and KHC genes was carried out using gene-specific primers. The RNA was isolated from various mouse tissues and from pachytene spermatocytes and round spermatids and then quantified, and equal amounts were used for cDNA production using random priming. The cDNAs were characterized for suitability with β-actin-specific primers. As shown in Figure 5, lane A, β-actin-specific RT-PCR produced a product with an equal intensity for all samples.
FIG. 5.
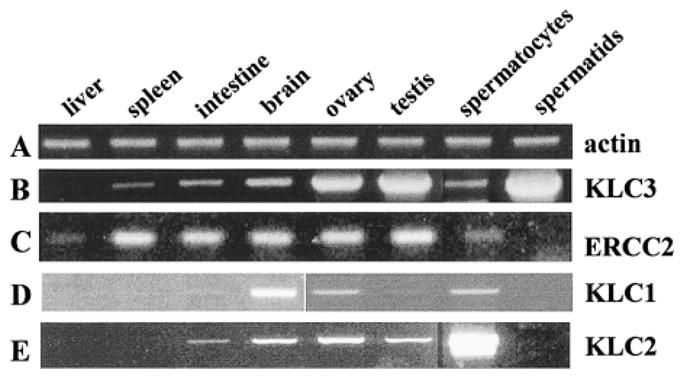
In testis, KLC3 is predominantly expressed in spermatids. Gene expression was analyzed by RT-PCR, and total RNA was isolated from indicated tissues and elutriated male germ cells, quantified, and standardized. The cDNA was produced by random priming and amplified with primers specific for the indicated genes. Lane A: β-actin (positive control for the procedures); lane B: KLC3 (note the widespread distribution of KLC3 in various tissues, with highest KLC3 RNA expression in spermatids); lane C: ERCC2; lane D: KLC1; lane E: KLC2.
Next, we used KLC3-specific primers to analyze KLC3 expression. Representative results of this semiquantitative assay are shown in Figure 5, lane B. KLC3 was detected in all tissues analyzed. However, the intensity of the bands varied significantly among the tissues, being barely detectable in liver, high in testis and ovary, and highest in round spermatids. Identical results were obtained with independent sets of primers. The authenticity of all PCR products was verified by sequencing or restriction analysis (data not shown). We conclude that, in testis, KLC3 is expressed predominantly in postmeiotic male germ cells.
Because KLC3 and ERCC2 genes are in close proximity, we next compared ERCC2 expression to that of KLC3. Figure 5, lane C, shows that KLC3 and ERCC2 expression profiles significantly differ from one another (lanes B and C, respectively) in spite of their close linkage indicative of differential gene regulation.
To our knowledge, the testicular expression patterns of KLC1, a predominantly neuronal KLC, and of KLC2, which is ubiquitously expressed, have not been reported previously. Their expression was also analyzed by RT-PCR. Our results extend those for the previously reported tissue expression patterns of KLC1 and KLC2 (Fig. 5, lanes D and E, respectively) [27]. KLC1 is most abundant in brain, but KLC1 RNA can also be detected in ovary, testis, and pachytene spermatocytes. KLC2 is expressed in several tissues, including testis, and in pachytene spermatocytes. Importantly, we demonstrate here that KLC1 and KLC2 are not expressed in spermatids. Thus, KLC3 appears to be the only known KLC to be expressed in postmeiotic male germ cells.
KLC3 Protein Expression in Spermatids
To detect KLC3 protein in male germ cells, Western blot analyses and indirect immunofluorescence assays were carried out. First, KLC3 polyclonal antibodies were used in Western blot analysis to detect KLC3 in various germ cell fractions and in epididymal spermatozoa (Fig. 6A). The antibodies detected KLC3 with an apparent molecular weight of 58 000 in elongating spermatids (lane 1), a mix of elongating spermatids and round spermatids (lane 2), round spermatids (lane 3), and epididymal sperm (lane 5), but not in pachytene spermatocytes (lane 4). As predicted from the sequence, KLC3 is smaller than KLC1 or KLC2 (61 and 66 kDa, respectively). The testicular KLC3 protein expression profile reflects that of the mRNA and demonstrates that KLC3 is a postmeiotic protein.
FIG. 6.
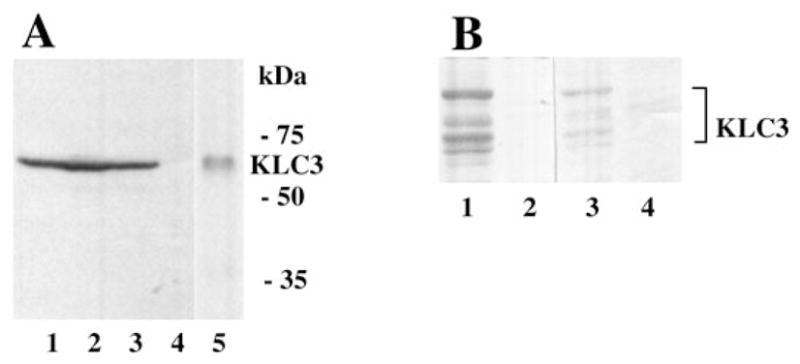
KLC3 protein expression. A) To confirm the KLC3 mRNA expression pattern in male germ cells and determine its apparent size, Western blot analysis was carried out using extracts from elongating spermatids, a mix of elongating spermatids and round spermatids, round spermatids, and pachytene spermatocytes (lanes 1–4, respectively) and epididymal spermatozoa (lane 5). KLC3 expression was probed using polyclonal antibodies. B) Monoclonal antibodies were raised against KLC3 for immunofluorescence studies. Specificities of the mAbs were analyzed by Western blots. Extracts from cells transfected with an HA-tagged KLC3 (lanes 1 and 3) or an unrelated control construct (lanes 2 and 4) were resolved by SDS-PAGE, transferred to membranes, and probed with anti-HA specific mAb (lanes 1 and 2) and anti-KLC3 mAb (lanes 3 and 4). Anti-KLC3 mAb specifically recognizes KLC3.
Because the polyclonal antiserum did not consistently give reproducible results in immunofluorescence assays, mAbs were generated. These antibodies work relatively in-efficiently in Western blots of sperm extracts, but they are specific, as demonstrated by their ability to specifically detect KLC3 in cells transiently transfected with HA-KLC3 using Western blot experiments (Fig. 6B), in which the KLC3 protein profiles produced by anti-HA mAb and anti-KLC3 mAb were indistinguishable (compare lanes 1 and 3). Cells transfected with an unrelated construct did not contain KLC3 (lanes 2 and 4).
This same mAb was next used in immunofluorescence assays of rat testicular cryosections. Representative series are shown in Figure 7, A and B. Several seminiferous tubule cross-sections are evident in the figure. The results show KLC3 protein in sperm tails, which protrude into the lumen of the seminiferous tubules. No staining is evident in premeiotic germ cells or any somatic cells in the testis. This pattern is, thus, similar to that of Odf1 and Odf2, two major outer, dense-fiber proteins with molecular weights of 27 000 and 84 000, respectively [29], that are first detected in step 10 spermatids and later during development in elongating spermatids and in sperm tails.
FIG. 7.
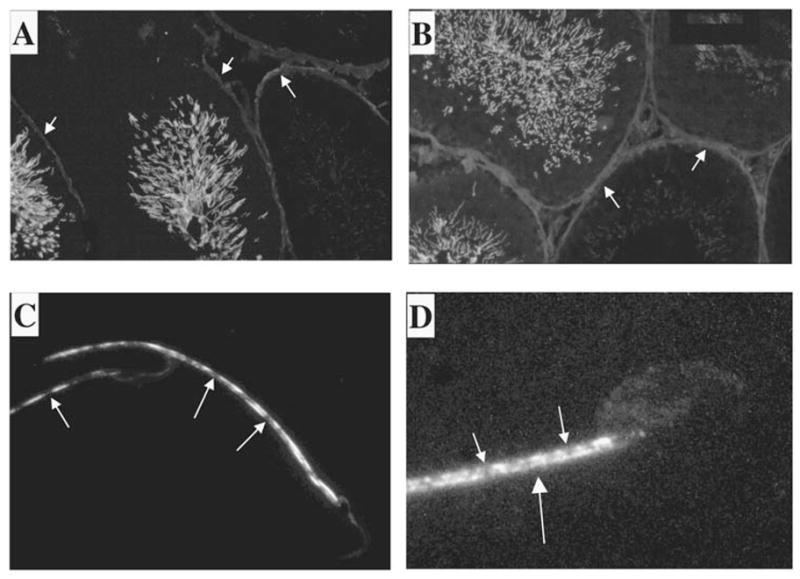
KLC3 localization in testis. The expression pattern of KLC3 protein in seminiferous tubules was examined by immunofluorescence using the mAb raised against KLC3. Frozen rat testicular sections were analyzed using anti-KLC3 mAb (A and B). Tails of KLC3-positive cells can be seen protruding into the lumen. A detailed image of KLC3 in sperm tails was obtained by deconvolution confocal microscopy of rat and mouse epididymal spermatozoa using anti-KLC3 mAb (C and D, respectively). Note that only the midpiece shows KLC3 staining, and that this pattern is not homogenous. Original magnification ×20 (A and B) and ×100 (C and D).
Deconvolution confocal microscopy of anti-KLC3-labeled rat and mouse epididymal sperm was next used to analyze the KLC3 pattern in more detail in epididymal spermatozoa. These results confirm the immunofluorescence results and further demonstrate, interestingly, that the KLC3 staining pattern in sperm tails is nonhomogenous in both rat and mouse. Figure 7C shows the head and mid-piece regions of two rat epididymal spermatozoa that have a discontinuous staining pattern in the midpiece. Figure 7D shows the head and midpiece area of a mouse epididymal spermatozoon that, as in rat, have the discontinuous KLC3 staining pattern. Sperm heads do not contain KLC3.
KHC Is Not Detectable in Spermatids and Sperm Tails
The above expression pattern indicates that, of the three known KLCs, only KLC3 is expressed after meiosis in spermatogenesis, which raises the question of the interacting KHC partner in these cells. To determine the KHC mRNA profile in testis, RT-PCR was conducted as described above with primers specific for each of the three known KHC isoforms (i.e., Kif5a, Kif5b, and Kif5c). Kif5a and Kif5c had previously been reported to be neuronal-specific genes. The PCR product authenticity was verified by restriction analysis (data not shown), and the results are shown in Figure 8. Surprisingly, all three KHC genes are expressed in testis at levels comparable to those in brain (Fig. 8, A–C). Kif5b and Kif5c are expressed in spermatocytes, but Kif5a is not. Importantly, only Kif5c appears to be expressed after meiosis in round spermatids, albeit at a very low level. These results suggest 1) that the Kif5a signal obtained for total testis derives from somatic cells in that organ and 2) that, in spermatids, Kif5c may interact with KLC3. Figure 8C shows that, in addition to the expected Kif5c PCR fragment, a smaller PCR Kif5c product can also be detected, the origin of which remains to be determined but which could result from a spliced mRNA species.
FIG. 8.
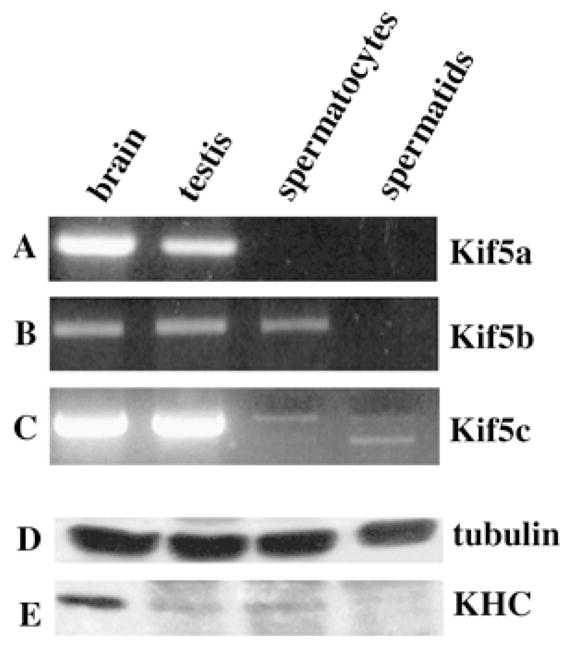
Expression pattern of the three known KHC genes in mouse testis. The KHC gene expression in brain, testis, spermatocytes, and spermatids (as indicated) was carried out as described previously by RT-PCR. Primer pairs were specific for Kif5a, Kif5b, and Kif5c (A–C, respectively). Note the absence of Kif5a in male germ cells and of Kif5b in spermatids. Kif5c is expressed at very low levels in spermatids. Western blot analysis of KHC expression in brain, testis, spermatocytes, and spermatids, as indicated. Samples and procedures were standardized for protein content, as indicated by the similar amount of tubulin in the various samples using anti-tubulin antisera (D). The expression of KHC was analyzed using H1 and H2 mAbs, which recognize Kif5b and Kif5c (E). This procedure cannot discriminate between the two proteins, which differ by only 1 kDa. Note the very low level of expression in spermatids.
Next, KHC isoform expression was analyzed in testis with two different mAbs that have been used extensively to detect Kif5b and also to recognize in vitro-translated Kif5c. Western blots probed with these antibodies show a single band in extracts from brain, total testis, and spermatocytes and a very light band in spermatids (Fig. 8E). Kif5c and Kif5b are practically identical in size (956 vs. 963 aa) and most likely account for the band in spermatocytes. In conjunction with our RT-PCR results, we conclude that the small amount of KHC protein detected in spermatids is Kif5c and, therefore, that only Kif5c can interact with KLC3 in spermatids. We cannot detect Kif5a expression in the Western blot assay. Our data also suggest that KLC3 protein may be more abundant in spermatids than Kif5c protein. Thus, a fraction of KLC3 likely interacts with another, uncharacterized partner in spermatids or acts independent of KHC.
The possible association of KLC3 and Kif5c suggested that we might detect Kif5c staining of elongating spermatid tails. To investigate this, immunofluorescence experiments were conducted using the two anti-KHC mAb, and colabeling experiments were done using frozen rat testicular sections. Figure 9 shows a portion of a section of seminiferous tubules stained for nuclear DNA with 4′,6′-diamidino-2-phenylindole (DAPI; Fig. 9A) for the 84-kDa major outer, dense-fiber protein Odf2, which visualizes spermatid tails protruding into the lumen (Fig. 9B) and for KHC (Fig. 9C). The KHC staining could not be detected in sperm tails in our assays. Anti-KHC antibodies revealed punctuate structures of unknown identity, which were confined largely to the perimeter of the seminiferous tubules. Colabeling experiments could not be carried out for KHC and KLC3, because both are detected by mAb.
FIG. 9.
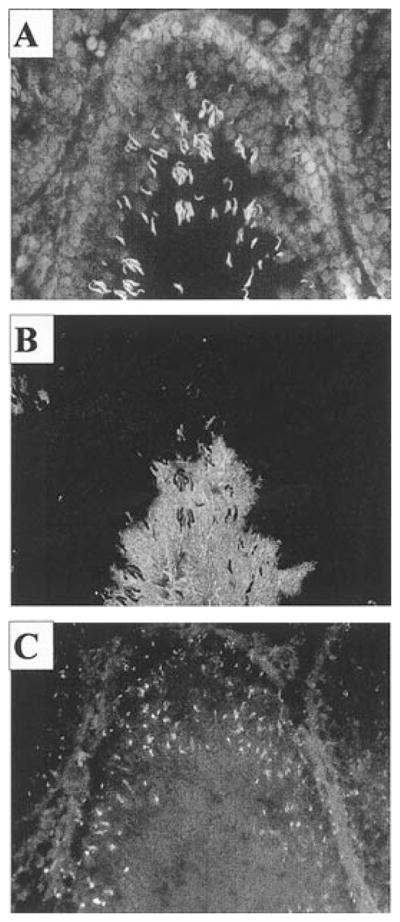
KHC is not detectable in sperm tails. The expression profile of KHC genes in seminiferous tubules was analyzed by immunofluorescence of rat frozen testicular sections using three different KHC-specific antibodies. All gave identical results. A) DAPI stain of nuclear DNA. B) Odf-2 staining of sperm tails using polyclonal anti-Odf2 antiserum. C) KHC staining using monoclonal anti-KHC antibodies. Note the punctuate pattern of KHC expression, and the absence of KHC staining in sperm tails. Original magnification ×20.
In conclusion, the majority of KLC3 does not associate with known KHC proteins in spermatids.
DISCUSSION
Here, we report the identification and characterization of a third KLC gene, KLC3, which is in close proximity to the ERCC2 gene and the protein product of which, in testis, is exclusively expressed in round and elongating spermatids. We show that KLC3 protein possesses all of the characteristics of a genuine KLC. This is based on the following observations: 1) the presence of significant sequence homology to other KLC genes; 2) the presence of conserved HR and TPR domains; 3) in vitro interaction with KHC, mediated, as expected for a true KLC, by the HR region; and 4) efficient in vitro association with MT in an ATP-dependent fashion. A surprising result is that, after meiosis, KLC3 appears to be the only KLC expressed. Both KLC1 and KLC2 are expressed in spermatocytes, but not in spermatids, as determined by RT-PCR. We also demonstrate that of the three known KHC genes, only Kif5c is expressed in spermatids, but at a very low level. We did not detect colocalization of Kif5c with KLC3 in sperm tails. We believe this is unlikely to result from a level of Kif5c less than the limit of detection, because three different antibodies failed to detect Kif5c in sperm tails and, in agreement with our observations, other groups investigating KHC localization within the testis have detected signal in the Golgi network of Sertoli cells [35] and the manchette [35], but not in sperm tails.
The question of KLC3’s function in spermatids remains to be addressed and is closely linked to its interaction with other molecules. Based on our data, several possibilities present themselves. One is that an unknown KHC interacts with KLC3 in sperm tails. Alternatively, KLC3 may associate with another member of the extended kinesin-like family. We consider these two possibilities to be less likely, because genomic localization experiments show only three KHC genes in the mouse [25] and no precedent exists for KLC interaction with any kinesin-like proteins other than KHC. However, related KHC genes of lower similarity would have been missed in the first assay. A third possibility is that the spermatid provides a unique environment in which the majority of KLC3 does not interact with a known KHC. In support of this possibility, coimmunoprecipitation Western blot experiments failed to detect known KHCs complexed to KLC3 in spermatids (unpublished results). Finally, based on its localization in sperm tails, KLC3 could be involved in intraflagellar transport. This is a distinct possibility that follows from the known functions of kinesins, but arguments exists against such a role. First, conventional kinesin has not been implicated in this process, which is carried out by members of the kinesin II family [36]. Members of the kinesin II family have been detected in sperm tails in both the rat [37] and sea urchin [38]. Second, interaction between KLC3 and a kinesin II family member involved in intraflagellar transport would be required, but, as mentioned above, no precedent exists for interaction of a KLC with any kinesin other than conventional kinesin. The function of KLC3 in spermatids remains to be resolved, and preliminary experiments suggest that KLC3 can associate with mitochondria in elongating spermatids (unpublished results). Thus, mitochondria may be one cargo for KLC3. Such a function would be in agreement with similar observations for rat KLCb, the C-terminus of which was demonstrated to associate with mitochondria [14]. Our current results do not rule out that, in other KLC3-positive tissues, including brain, KLC3 could have a role in transport in association with KHC and MT.
As mentioned in the Introduction, the number of KLC and KHC genes expressed by rodents as opposed to lower eukaryotes suggests that KLC genes have evolved to generate additional plasticity of kinesin function, with KLC2 and KLC3 being associated with higher eukaryotes [27]. KLC1, on the other hand, is expressed in all eukaryotes except fungi. This is supported by the fact that KLC3 sequences are less similar to mouse KLC1 than C. elegans and Drosophila KLC1 are to rat KLC1. Database searches for these organisms did not show any sequences with significant similarity to KLC3. It is theorized that KLC3 sequence idiosyncrasies may underlie specificity regarding KHC interaction, cargo binding, regulation by kinases, regulation of KHC function, and the novel putative functions for KLC3 in sperm tails. Thus, the presence of three KHC and three KLC genes, each with a distinct expression pattern, together with the diversity of KLC sequences in higher organisms, could reflect the unique needs of specialized and complex tissues in higher organisms.
Differential expression patterns have been reported for KLC1 and KLC2 genes in neuronal cells [27, 39], for KHC genes in developing [39, 40] and differentiated neuronal cells [24], and for KLC1 splice isoforms among various tissues [3, 41]. However, to our knowledge, the expression patterns in testis have not been reported before. Here, we find that the three mammalian KHC genes each have a unique testicular pattern. Kif5a is predominantly expressed in somatic testicular cells, and only Kif5c is expressed in spermatids, and at a very low level. An important result came from RT-PCR, which demonstrated that neither KLC1 nor KLC2 mRNAs could be detected in spermatids, even though both genes are expressed in pachytene spermatocytes. This suggests 1) that these two KLC genes become repressed during meiosis, 2) that their protein products are not required during spermatid differentiation, and 3) that KLC3 carries out the function of KLC1 and/or KLC2 in spermatids. Overall, distinct testicular cell types express different subsets of kinesin genes and/or express these genes to different extents, each with specialized, nonredundant functions. Thus, we propose that KLC gene duplication is not a consequence of a need for redundancy. Consistent with this is the predominantly neuronal phenotype of the KLC1 knockout mouse [42], which is in line with the largely neuronal expression pattern of this gene [27].
In conclusion, our characterization of the third KLC gene, KLC3, suggests that it carries out a unique role in spermatids.
Acknowledgments
We thank B. Schnapp for the gift of Kif5c cDNA and antibody and Dr. S. Goldstein for the gift of KLC2 cDNA. Anti-KLC3 mAb were generated with assistance from Dr. S. Robbins. We thank M. Hoeffer for contributions to the initial yeast-2-hybrid experiments and Dr. X. Shao for expert advice on the yeast-2-hybrid system.
Footnotes
Supported by grants from the Medical Research Council of Canada to F.A.v.d.H. A.J. and B.B. were supported in part by studentships from the Alberta Cancer Foundation.
References
- 1.Bloom GS, Endow SA. Motor proteins. 1: kinesins. Protein Profile. 1994;1:1059–1116. [PubMed] [Google Scholar]
- 2.Cyr JL, Pfister KK, Bloom GS, Slaughter CA, Brady ST. Molecular genetics of kinesin light chains: generation of isoforms by alternative splicing. Proc Natl Acad Sci U S A. 1991;88:10114–10118. doi: 10.1073/pnas.88.22.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beushausen S, Kladakis A, Jaffe H. Kinesin light chains: identification and characterization of a family of proteins from the optic lobe of the squid Loligo pealii. DNA Cell Biol. 1993;12:901–909. doi: 10.1089/dna.1993.12.901. [DOI] [PubMed] [Google Scholar]
- 4.Cabeza-Arvelaiz Y, Shih LC, Hardman N, Asselbergs F, Bilbe G, Schmitz A, White B, Siciliano MJ, Lachman LB. Cloning and genetic characterization of the human kinesin light-chain (KLC) gene. DNA Cell Biol. 1993;12:881–892. doi: 10.1089/dna.1993.12.881. [DOI] [PubMed] [Google Scholar]
- 5.Fan J, Amos LA. Kinesin light chain isoforms in Caenorhabditis elegans. J Mol Biol. 1994;240:507–512. doi: 10.1006/jmbi.1994.1465. [DOI] [PubMed] [Google Scholar]
- 6.Gauger AK, Goldstein LS. The Drosophila kinesin light chain. Primary structure and interaction with kinesin heavy chain. J Biol Chem. 1993;268:13657–13666. [PubMed] [Google Scholar]
- 7.Wedaman KP, Knight AE, Kendrick-Jones J, Scholey JM. Sequences of sea urchin kinesin light chain isoforms. J Mol Biol. 1993;231:155–158. doi: 10.1006/jmbi.1993.1267. [DOI] [PubMed] [Google Scholar]
- 8.Diefenbach RJ, Mackay JP, Armati PJ, Cunningham AL. The C-terminal region of the stalk domain of ubiquitous human kinesin heavy chain contains the binding site for kinesin light chain. Biochemistry. 1998;37:16663–16670. doi: 10.1021/bi981163r. [DOI] [PubMed] [Google Scholar]
- 9.Gindhart JG, Jr, Goldstein LS. Tetratrico peptide repeats are present in the kinesin light chain. Trends Biochem Sci. 1996;21:52–53. [PubMed] [Google Scholar]
- 10.Lamb JR, Tugendreich S, Hieter P. Tetratrico peptide repeat interactions: to TPR or not to TPR? Trends Biochem Sci. 1995;20:257–259. doi: 10.1016/s0968-0004(00)89037-4. [DOI] [PubMed] [Google Scholar]
- 11.Hirokawa N, Pfister KK, Yorifuji H, Wagner MC, Brady ST, Bloom GS. Submolecular domains of bovine brain kinesin identified by electron microscopy and monoclonal antibody decoration. Cell. 1989;56:867–878. doi: 10.1016/0092-8674(89)90691-0. [DOI] [PubMed] [Google Scholar]
- 12.Yu H, Toyoshima I, Steuer ER, Sheetz MP. Kinesin and cytoplasmic dynein binding to brain microsomes. J Biol Chem. 1992;267:20457–20464. [PubMed] [Google Scholar]
- 13.Stenoien DL, Brady ST. Immunochemical analysis of kinesin light chain function. Mol Biol Cell. 1997;8:675–689. doi: 10.1091/mbc.8.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khodjakov A, Lizunova EM, Minin AA, Koonce MP, Gyoeva FK. A specific light chain of kinesin associates with mitochondria in cultured cells. Mol Biol Cell. 1998;9:333–343. doi: 10.1091/mbc.9.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gyoeva FK, Bybikova EM, Minin AA. An isoform of kinesin light chain specific for the Golgi complex. J Cell Sci. 2000;113:2047–2054. doi: 10.1242/jcs.113.11.2047. [DOI] [PubMed] [Google Scholar]
- 16.Lindesmith L, McIlvain JM, Jr, Argon Y, Sheetz MP. Phosphotransferases associated with the regulation of kinesin motor activity. J Biol Chem. 1997;272:22929–22933. doi: 10.1074/jbc.272.36.22929. [DOI] [PubMed] [Google Scholar]
- 17.Matthies HJ, Miller RJ, Palfrey HC. Calmodulin binding to and cAMP-dependent phosphorylation of kinesin light chains modulate kinesin ATPase activity. J Biol Chem. 1993;268:11176–11187. [PubMed] [Google Scholar]
- 18.Skoufias DA, Cole DG, Wedaman KP, Scholey JM. The carboxyl-terminal domain of kinesin heavy chain is important for membrane binding. J Biol Chem. 1994;269:1477–1485. [PubMed] [Google Scholar]
- 19.Stock MF, Guerrero J, Cobb B, Eggers CT, Huang TG, Li X, Hackney DD. Formation of the compact confomer of kinesin requires a COOH-terminal heavy chain domain and inhibits microtubule-stimulated ATPase activity. J Biol Chem. 1999;274:14617–14623. doi: 10.1074/jbc.274.21.14617. [DOI] [PubMed] [Google Scholar]
- 20.Coy DL, Hancock WO, Wagenbach M, Howard J. Kinesin’s tail domain is an inhibitory regulator of the motor domain. Nat Cell Biol. 1999;1:288–292. doi: 10.1038/13001. [DOI] [PubMed] [Google Scholar]
- 21.Friedman DS, Vale RD. Single-molecule analysis of kinesin motility reveals regulation by the cargo-binding tail domain. Nat Cell Biol. 1999;1:293–297. doi: 10.1038/13008. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein LS, Philp AV. The road less traveled: emerging principles of kinesin motor utilization. Annu Rev Cell Dev Biol. 1999;15:141–183. doi: 10.1146/annurev.cellbio.15.1.141. [DOI] [PubMed] [Google Scholar]
- 23.Navone F, Niclas J, Hom-Booher N, Sparks L, Bernstein HD, Mc-Caffrey G, Vale RD. Cloning and expression of a human kinesin heavy chain gene: interaction of the COOH-terminal domain with cytoplasmic microtubules in transfected CV-1 cells. J Cell Biol. 1992;117:1263–1275. doi: 10.1083/jcb.117.6.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niclas J, Navone F, Hom-Booher N, Vale RD. Cloning and localization of a conventional kinesin motor expressed exclusively in neurons. Neuron. 1994;12:1059–1072. doi: 10.1016/0896-6273(94)90314-x. [DOI] [PubMed] [Google Scholar]
- 25.Xia C, Rahman A, Yang Z, Goldstein LS. Chromosomal localization reveals three kinesin heavy chain genes in mouse. Genomics. 1998;52:209–213. doi: 10.1006/geno.1998.5427. [DOI] [PubMed] [Google Scholar]
- 26.Nakagawa T, Tanaka Y, Matsuoka E, Kondo S, Okada Y, Noda Y, Kanai Y, Hirokawa N. Identification and classification of 16 new kinesin superfamily (KIF) proteins in mouse genome. Proc Natl Acad Sci U S A. 1997;94:9654–9659. doi: 10.1073/pnas.94.18.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahman A, Friedman DS, Goldstein LS. Two kinesin light chain genes in mice. Identification and characterization of the encoded proteins. J Biol Chem. 1998;273:15395–15403. doi: 10.1074/jbc.273.25.15395. [DOI] [PubMed] [Google Scholar]
- 28.Lamerdin JE, Stilwagen SA, Ramirez MH, Stubbs L, Carrano AV. Sequence analysis of the ERCC2 gene regions in human, mouse, and hamster reveals three linked genes. Genomics. 1996;34:399–409. doi: 10.1006/geno.1996.0303. [DOI] [PubMed] [Google Scholar]
- 29.Shao X, Tarnasky HA, Schalles U, Oko R, van der Hoorn FA. Interactional cloning of the 84-kDa major outer dense fiber protein Odf84. Leucine zippers mediate associations of Odf84 and Odf27. J Biol Chem. 1997;272:6105–6113. doi: 10.1074/jbc.272.10.6105. [DOI] [PubMed] [Google Scholar]
- 30.Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 31.Higgy NA, Zackson SL, van der Hoorn FA. Cell interactions in testis development: overexpression of c-mos in spermatocytes leads to increased germ cell proliferation. Dev Genet. 1995;16:190–200. doi: 10.1002/dvg.1020160211. [DOI] [PubMed] [Google Scholar]
- 32.Oko R. Comparative analysis of proteins from the fibrous sheath and outer dense fibers of rat spermatozoa. Biol Reprod. 1988;39:169–182. doi: 10.1095/biolreprod39.1.169. [DOI] [PubMed] [Google Scholar]
- 33.Vallee RB, Collins CA. Purification of microtubules and microtubule-associated proteins from sea urchin eggs and cultured mammalian cells using taxol, and use of exogenous taxol-stabilized brain micro-tubules for purifying microtubule-associated proteins. Methods Enzymol. 1986;134:116–127. doi: 10.1016/0076-6879(86)34080-1. [DOI] [PubMed] [Google Scholar]
- 34.Rahman A, Friedman DS, Goldstein LS. Two kinesin light chain genes in mice. Identification and characterization of the encoded proteins. J Biol Chem. 1998;273:15395–15403. doi: 10.1074/jbc.273.25.15395. [DOI] [PubMed] [Google Scholar]
- 35.Johnson KJ, Hall ES, Boekelheide K. Kinesin localizes to the trans-Golgi network regardless of microtubule organization. Eur J Cell Biol. 1996;69:276–287. [PubMed] [Google Scholar]
- 36.Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller MG, Mulholland DJ, Vogl AW. Rat testis motor proteins associated with spermatid translocation (dynein) and spermatid flagella (kinesin-II) Biol Reprod. 1999;60:1047–1056. doi: 10.1095/biolreprod60.4.1047. [DOI] [PubMed] [Google Scholar]
- 38.Henson JH, Cole DG, Roesener CD, Capuano S, Mendola RJ, Scholey JM. The heterotrimeric motor protein kinesin-II localizes to the midpiece and flagellum of sea urchin and sand dollar sperm. Cell Motil Cytoskeleton. 1997;38:29–37. doi: 10.1002/(SICI)1097-0169(1997)38:1<29::AID-CM4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 39.Vignali G, Lizier C, Sprocati MT, Sirtori C, Battaglia G, Navone F. Expression of neuronal kinesin heavy chain is developmentally regulated in the central nervous system of the rat. J Neurochem. 1997;69:1840–1849. doi: 10.1046/j.1471-4159.1997.69051840.x. [DOI] [PubMed] [Google Scholar]
- 40.Vignali G, Niclas J, Sprocati MT, Vale RD, Sirtori C, Navone F. Differential expression of ubiquitous and neuronal kinesin heavy chains during differentiation of human neuroblastoma and PC12 cells. Eur J Neurosci. 1996;8:536–544. doi: 10.1111/j.1460-9568.1996.tb01238.x. [DOI] [PubMed] [Google Scholar]
- 41.Su QN, Namikawa K, Toki H, Kiyama H. Differential display reveals transcriptional up-regulation of the motor molecules for both antero-grade and retrograde axonal transport during nerve regeneration. Eur J Neurosci. 1997;9:1542–1547. doi: 10.1111/j.1460-9568.1997.tb01510.x. [DOI] [PubMed] [Google Scholar]
- 42.Rahman A, Kamal A, Roberts EA, Goldstein LS. Defective kinesin heavy chain behavior in mouse kinesin light chain mutants. J Cell Biol. 1999;146:1277–1288. doi: 10.1083/jcb.146.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]