Abstract
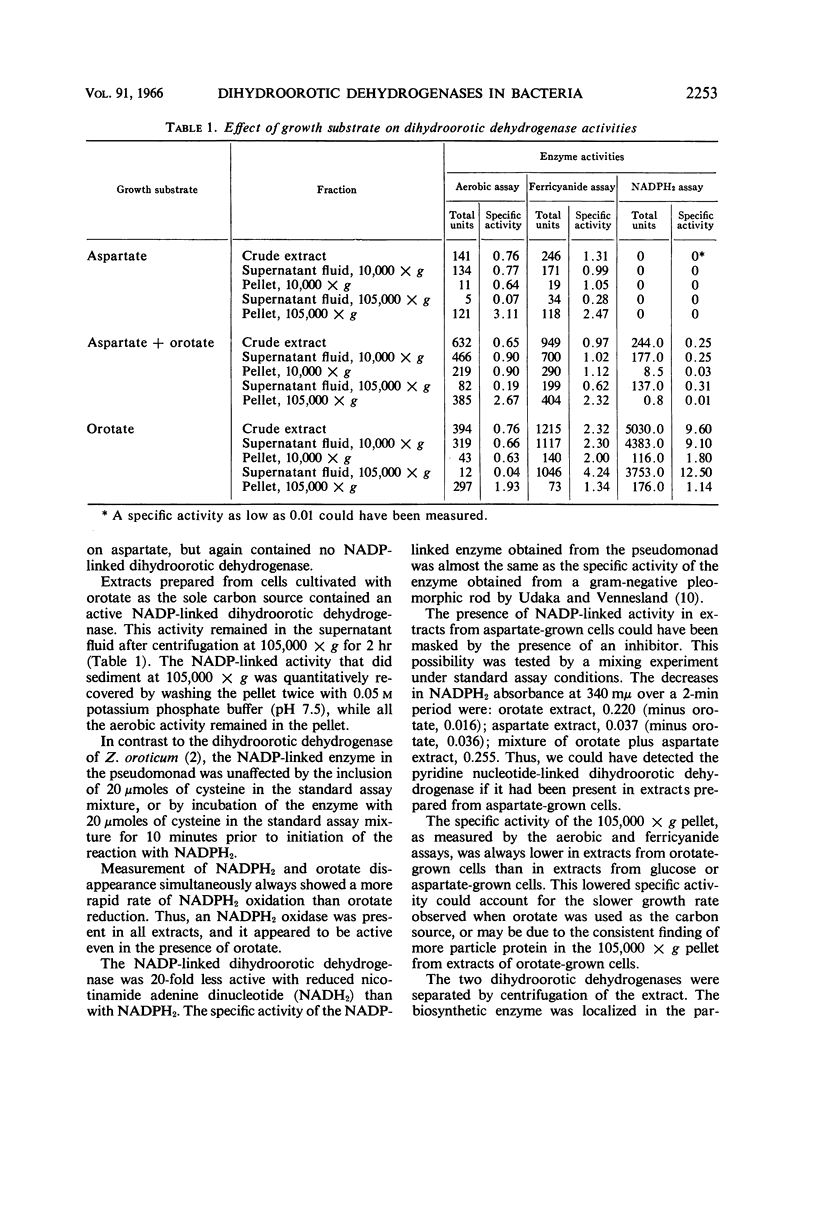
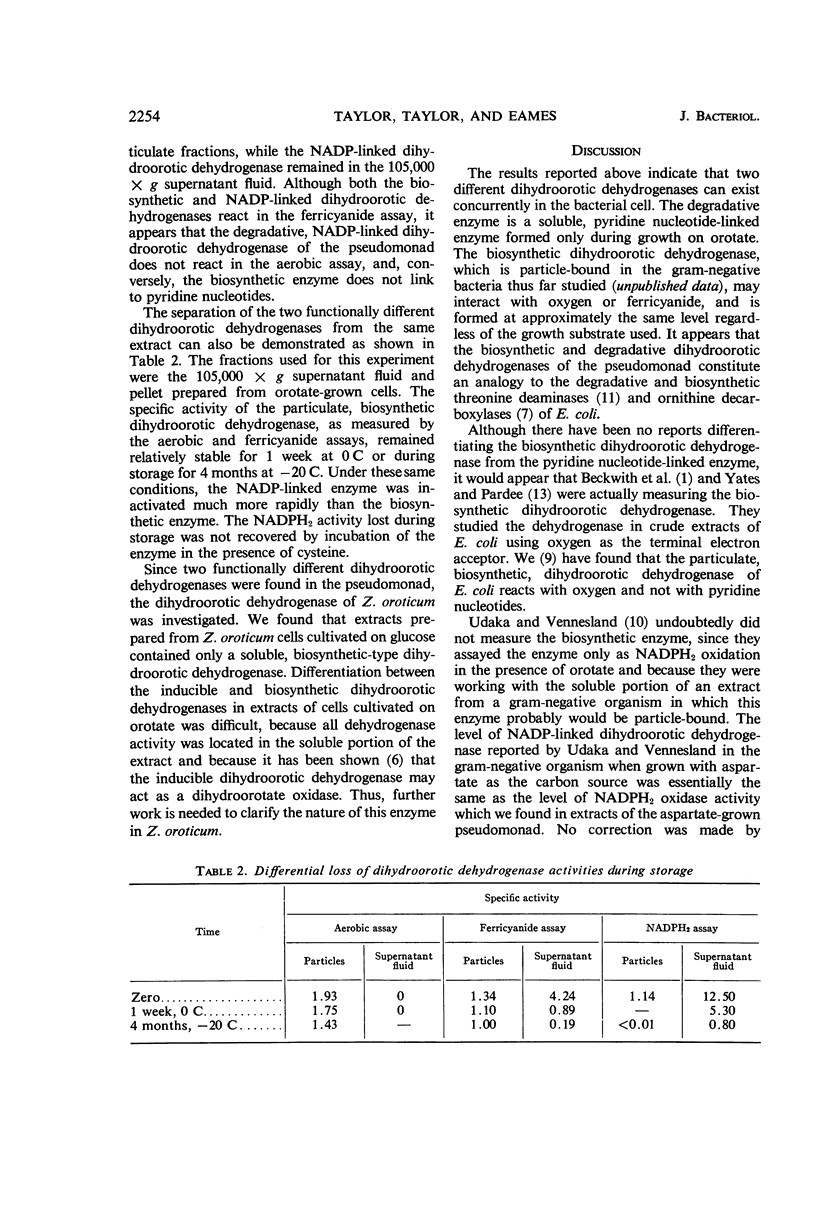
Taylor, W. H. (Portland State College, Portland, Ore.), M. L. Taylor, and D. F. Eames. Two functionally different dihydroorotic dehydrogenases in bacteria. J. Bacteriol. 91:2251–2256. 1966.—We have investigated the relationship between the two kinds of dihydroorotic dehydrogenases produced by bacteria. A pseudomonad, capable of growth on a salts medium with glucose, aspartate, glycerol, or orotate as the carbon source, was isolated from lake bank mud. A particle-bound dihydroorotic dehydrogenase, similar to the biosynthetic enzyme in Escherichia coli, was formed by the pseudomonad when the carbon source was orotate, glucose, glycerol, or aspartate. A soluble, degradative nicotinamide adenine dinucleotide phosphate-linked dihydroorotic dehydrogenase, as well as the particle-bound biosynthetic enzyme, was formed when the pseudomonad was cultivated on orotate. The biosynthetic enzyme links to oxygen or ferricyanide, but not to pyridine nucleotides. Zymobacterium oroticum, when cultivated on glucose, contained only the biosynthetic type of dihydroorotic dehydrogenase. The presence of two functionally different dihydroorotic dehydrogenases in the pseudomonad was suggested on the basis of the following observations: (i) the two enzyme activities were separated by centrifugation; (ii) the pyridine nucleotide-linked activity was formed only when orotate was present in the growth medium; and (iii) the biosynthetic enzyme was stable to storage at −20 C for 4 months, whereas the degradative enzyme activity was destroyed by storage under these conditions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKWITH J. R., PARDEE A. B., AUSTRIAN R., JACOB F. Coordination of the synthesis of the enzymes in the pyrimidine pathway of E. coli. J Mol Biol. 1962 Dec;5:618–634. doi: 10.1016/s0022-2836(62)80090-4. [DOI] [PubMed] [Google Scholar]
- FRIEDMANN H. C., VENNESLAND B. Crystalline dihydroorotic dehydrogenase. J Biol Chem. 1960 May;235:1526–1532. [PubMed] [Google Scholar]
- GRAVES J. L., VENNESLAND B. The stereospecific hydrogen exchange in the dihydroorotic dehydrogenase reaction. J Biol Chem. 1957 May;226(1):307–316. [PubMed] [Google Scholar]
- LIEBERMAN I., KORNBERG A. Enzymic synthesis and breakdown of a pyrimidine, orotic acid. I. Dihydro-orotic dehydrogenase. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):223–234. doi: 10.1016/0006-3002(53)90141-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MILLER R. W., MASSEY V. DIHYDROOROTIC DEHYDROGENASE. I. SOME PROPERTIES OF THE ENZYME. J Biol Chem. 1965 Mar;240:1453–1465. [PubMed] [Google Scholar]
- Morris D. R., Pardee A. B. A biosynthetic ornithine decarboxylase in Escherichia coli. Biochem Biophys Res Commun. 1965 Sep 22;20(6):697–702. doi: 10.1016/0006-291x(65)90072-0. [DOI] [PubMed] [Google Scholar]
- PARDEE A. B., YATES R. A. Pyrimidine biosynthesis in Escherichia coli. J Biol Chem. 1956 Aug;221(2):743–756. [PubMed] [Google Scholar]
- REYNOLDS E. S., LIEBERMAN I., KORNBERG A. The metabolism of orotic acid in aerobic bacteria. J Bacteriol. 1955 Mar;69(3):250–255. doi: 10.1128/jb.69.3.250-255.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UDAKA S., VENNESLAND B. Properties of triphosphopyridine nucleotide-linked dihydroorotic dehydrogenase. J Biol Chem. 1962 Jun;237:2018–2024. [PubMed] [Google Scholar]
- UMBARGER H. E., BROWN B. Threonine deamination in Escherichia coli. II. Evidence for two L-threonine deaminases. J Bacteriol. 1957 Jan;73(1):105–112. doi: 10.1128/jb.73.1.105-112.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YATES R. A., PARDEE A. B. Control by uracil of formation of enzymes required for orotate synthesis. J Biol Chem. 1957 Aug;227(2):677–692. [PubMed] [Google Scholar]


